Abstract
Recently, transcranial magnetic stimulation of the motor cortex (TMS) revealed impaired voluntary activation of muscles during maximal efforts. Hence, we evaluated its use as a measure of voluntary activation over a range of contraction strengths in both fresh and fatigued muscles, and compared it with standard twitch interpolation using nerve stimulation. Subjects contracted the elbow flexors isometrically while force and EMG from biceps and triceps were recorded. In one study, eight subjects made submaximal and maximal test contractions with rests to minimise fatigue. In the second study, eight subjects made sustained maximal contractions to reduce force to 60 % of the initial value, followed by brief test contractions. Force responses were recorded following TMS or electrical stimulation of the biceps motor nerve. In other contractions, EMG responses to TMS (motor evoked potentials, MEPs) or to stimulation at the brachial plexus (maximal M waves, Mmax) were recorded. During contractions of 50 % maximum, TMS elicited large MEPs in biceps (> 90 % Mmax) which decreased in size (to ≈70 % Mmax) with maximal efforts. This suggests that faster firing rates made some motor units effectively refractory. With fatigue, MEPs were also smaller but remained > 70 % Mmax for contractions of 50–100 % maximum. For fresh and fatigued muscle, the superimposed twitch evoked by motor nerve and motor cortex stimulation decreased with increasing contraction strength. For nerve stimulation the relation was curvilinear, and for TMS it was linear for contractions of 50–100 % maximum (r2 = 1.00). Voluntary activation was derived using the expression: (1 – superimposed twitch/resting twitch) × 100. The resting twitch was measured directly for nerve stimulation and for TMS, it was estimated by extrapolation of the linear regression between the twitch and voluntary force. For cortical stimulation, this resulted in a highly linear relation between voluntary activation and force. Furthermore, the estimated activation corresponded well with contraction strength. Using TMS or nerve stimulation, voluntary activation was high during maximal efforts of fresh muscle. With fatigue, both measures revealed reduced voluntary activation (i.e. central fatigue) during maximal efforts. Measured with TMS, this central fatigue accounted for one-quarter of the fall in maximal voluntary force. We conclude that TMS can quantify voluntary activation for fresh or fatigued muscles at forces of 50–100 % maximum. Unlike standard twitch interpolation of the elbow flexors, voluntary activation measured with TMS varies in proportion to voluntary force, it reveals when extra output is available from the motor cortex to increase force, and it elicits force from all relevant synergist muscles.
The level of neural drive to muscle during exercise is termed voluntary activation (Gandevia et al. 1995) and is commonly estimated by interpolation of a single supramaximal electrical stimulus to the motor nerve during an isometric voluntary contraction (‘twitch interpolation’; Merton, 1954). If extra force is evoked at an appropriate latency by the ‘superimposed’ stimulus then either the stimulated axons were not all recruited voluntarily or they were discharging at sub-tetanic rates. Hence, voluntary activation must have been less than maximal (Merton, 1954; Belanger & McComas, 1981; Herbert & Gandevia, 1999; for review see Gandevia, 2001). To quantify voluntary activation, the amplitude of the superimposed twitch is expressed as a fraction of the twitch evoked by the same stimulus in the potentiated relaxed muscle (Thomas et al. 1989).
The amplitude of the superimposed twitch decreases with increasing voluntary force (e.g. Merton, 1954; Belanger & McComas, 1981; Allen et al. 1998). During brief maximal voluntary contractions (MVCs), the superimposed twitch is small or absent, suggesting that it is possible to drive motoneurones voluntarily to produce maximal force from appropriate muscles (e.g. Belanger & McComas, 1981; McKenzie et al. 1988; Gandevia et al. 1990; Herbert & Gandevia, 1996; Lyons et al. 1996; Thomas et al. 1997; Allen et al. 1998; Behm et al. 2002). Herbert & Gandevia (1999) developed, for adductor pollicis, a realistic model of twitch interpolation which incorporated axonal factors such as refractoriness and antidromic collision. The model revealed a linear relation between voluntary force and the superimposed twitch, and it accurately predicted small superimposed twitches during maximal voluntary efforts. In the elbow flexor muscles, the relationship between the amplitude of the superimposed twitch and voluntary force appears linear until high effort where increases in voluntary force occur with little or no change in size of the superimposed twitch (e.g. Dowling et al. 1994; Allen et al. 1998; De Serres & Enoka, 1998). This non-linearity may be due to differential voluntary activation of the synergistic muscles and lengthening of active muscles at high voluntary forces (Allen et al. 1998). Furthermore, excessive currents used to stimulate nerves to the biceps brachii and brachialis muscles may inadvertently contract antagonist elbow extensor muscles and reduce the superimposed twitch (Awiszus et al. 1997).
Muscle fatigue, the decline in voluntary force during sustained maximal efforts, is caused by both central and peripheral mechanisms (Bigland-Ritchie et al. 1978, 1995; Gandevia et al. 1995). Much of the fatigue arises from processes occurring within the muscle such as disturbances in the excitation-contraction coupling, depletion of muscle glycogen and accumulation of metabolites (for review see Fitts, 1994). These changes reduce the resting muscle twitch. However, during fatiguing exercise, changes in the central nervous system also reduce force output (e.g. Reid, 1928; Bigland-Ritchie et al. 1978; for review see Gandevia, 2001). For instance, during a sustained maximal effort, or a series of brief maximal efforts, the amplitude of the superimposed twitch evoked by motor nerve stimulation progressively increases when expressed relative to the amplitude of the resting muscle twitch evoked by the same stimulus (e.g. Thomas et al. 1989; Lloyd et al. 1991). This decline in voluntary activation in maximal efforts is the hallmark of ‘central fatigue’ (Gandevia et al. 1995). Its development is accompanied by changes within the motor cortex, which increase the size of the motor evoked potential (MEP) and lengthen the EMG silence (‘silent period’) to transcranial magnetic stimulation of the motor cortex (TMS) (Taylor et al. 1996, 1999; for review see Gandevia, 2001).
Stimulation of the motor cortex has also revealed submaximal activation of muscles during maximal voluntary efforts of respiratory muscles (Gandevia et al. 1990) and subsequently of thenar (Herbert & Gandevia, 1996) and elbow extensor muscles (Thomas et al. 1997). It has also revealed central fatigue of the elbow flexor muscles (Gandevia et al. 1996; Taylor et al. 2000). However, quantification of voluntary activation with motor cortical stimulation is difficult. It is inappropriate to normalise the superimposed twitch evoked by TMS to the resting twitch evoked by the same stimulus because the motoneuronal output evoked by the cortical stimulus at rest is not the same as during a contraction. This reflects the increase in motor cortical and motoneuronal ‘excitability’ with activity (Hess et al. 1987; Ugawa et al. 1995; Di Lazzaro et al. 1998; for review see Rothwell et al. 1991).
The present study was designed to compare the superimposed twitches evoked with motor cortical stimulation and motor nerve stimulation across the full range of voluntary force, and uses a new method for normalisation of the superimposed twitch evoked by TMS to quantify voluntary activation. In addition, comparison of the MEP during strong voluntary contractions with its evoked superimposed twitch gives insight into the extent to which motoneurones are driven by volition. With the muscle fatigued, both TMS and motor nerve stimulation demonstrate central fatigue but they provide different information about its cause. Preliminary data has been previously published in abstract form (Russell et al. 2001, 2003).
METHODS
Two studies, which comprised three experimental sessions, were performed to assess voluntary activation in the unfatigued and fatigued elbow flexor muscles. Fourteen subjects each took part in up to three of the experiments. Subjects sat with the right arm held firmly at the wrist in an isometric myograph which measured elbow flexion torque (termed force; Fig. 1A). Subjects were positioned with the shoulder and elbow flexed at 90 deg with the forearm vertical and fully supinated. All experimental procedures were approved by the institutional ethics committee and conducted according to the Declaration of Helsinki. Written informed consent was obtained.
Figure 1. Experimental apparatus and study protocols.
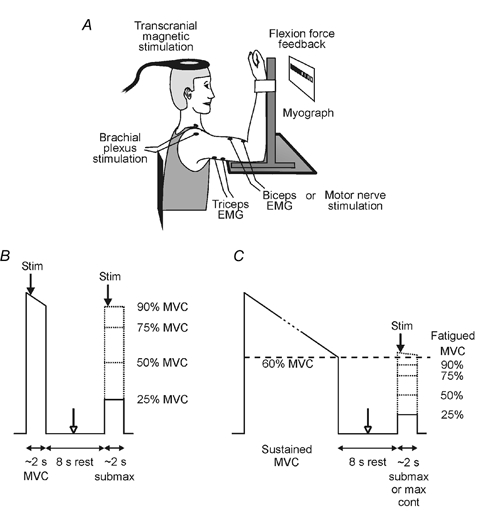
A, experimental apparatus. B, study 1 protocol. Subjects performed pairs of contractions involving a brief maximal voluntary contraction (MVC) followed by a brief submaximal contraction without fatigue. Transcranial magnetic stimulation of the motor cortex (TMS), electrical stimulation of the brachial plexus, or electrical stimulation of the biceps brachii motor nerve was delivered during each contraction (filled arrows). In some contraction pairs, electrical stimulation of the biceps brachii motor nerve was also delivered at rest between contractions (open arrow). C, study 2 protocol. Subjects performed pairs of contractions of the fatigued elbow flexor muscles. Each contraction pair involved a sustained MVC (maintained until maximal force had decreased to 60 % of maximal force without fatigue) followed by a brief maximal or submaximal contraction. Arrows indicate the timing of the stimuli.
Force and EMG recordings
Isometric elbow flexion force was measured using a linear strain gauge (Xtran, Melbourne, Australia: linear to 2 kN). Electromyographic activity (EMG) was recorded with surface electrodes (Ag-AgCl, 10 mm diameter) over the biceps brachii and triceps brachii muscles. Surface EMG signals were amplified (× 100–300), filtered (16–1000 Hz) and sampled (2000 Hz) for later analysis using a data acquisition system (CED 1401 interface with Signal software, Cambridge Electronic Design, Cambridge, UK).
Stimulation
Three forms of stimulation were used. These included stimulation of the brachial plexus, stimulation of intramuscular nerve fibres of the biceps and brachialis muscles (‘motor nerve’ stimulation) and TMS over the motor cortex. The evoked compound muscle action potentials were recorded using surface EMG.
Stimulation of the brachial plexus
Single electrical stimuli of 100 μs duration were delivered to the brachial plexus via a cathode in the supraclavicular fossa (Erb's point) and an anode on the acromion (constant current, DS7, Digitimer Ltd, Welwyn Garden City, Hertfordshire, UK, modified to deliver up to 1 A). The stimulation intensity (50–225 mA) was at least 30 % above the level required to produce a resting maximal compound muscle action potential (Mmax) in the biceps and triceps brachii muscles. On average, the amplitude of the resting Mmax was 20.3 ± 6.8 mV (mean ± S.D.) for biceps and 10.4 ± 3.4 mV for triceps.
Stimulation of the biceps brachii/brachialis intramuscular nerve fibres
Single electrical stimuli of 100 μs duration were delivered (constant current, DS7, Digitimer) to intramuscular nerve fibres innervating biceps and brachialis via a surface cathode located midway between the anterior edge of the deltoid and the elbow crease with the elbow flexed at 90 deg, and a surface anode positioned over the bicipital tendon (2–3 cm proximal to the elbow). The stimulation intensity (120–264 mA) was set 20 % above the level required to produce a resting twitch of maximal amplitude.
Transcranial magnetic stimulation of the motor cortex (TMS)
A circular coil (13.5 cm outside diameter) positioned over the vertex evoked MEPs recorded from biceps and triceps (Magstim 200, Magstim Co., Dyfed, UK). The direction of current flow in the coil preferentially activated the left motor cortex. The stimulator output (50–90 % of maximum) produced a large MEP in the biceps (minimum amplitude 50–60 % of Mmax) during brief MVCs of the elbow flexor muscles and only a small MEP in the triceps. Stimulus intensity remained constant throughout the protocol.
Protocol
The first study involved two experiments, the first of which assessed voluntary activation in the unfatigued elbow flexor muscles with motor cortical and motor nerve stimulation (n = 8). Subjects performed three brief (1–2 s) control MVCs. The peak force of each MVC was measured and four submaximal target forces of 25, 50, 75 and 90 % of the mean of these peak forces were displayed on a visual feedback device. Subjects then performed 40 pairs of test contractions in random order, with 1–2 min rest between pairs to avoid fatigue. Each pair involved a brief MVC (1–2 s) followed 8 s later by a brief submaximal contraction of the same duration (Fig. 1B). During each pair, stimulation of the motor cortex or biceps motor nerve was delivered and force responses were recorded. When motor nerve stimulation was delivered during contractions, an additional motor nerve stimulus was delivered with the muscle at rest between the two contractions (4 s after completing the MVC). Twenty pairs of contractions were performed for each type of stimulation with five pairs of contractions performed for each of the four submaximal target forces.
In the second experiment, subjects (n = 8; four subjects participated in both experiments) performed a similar protocol during which the EMG responses to stimulation of the motor cortex or brachial plexus were recorded during each contraction. To assess the effect of stimulus strength on the size of the TMS-evoked superimposed twitch and on the size of the MEP in the biceps and triceps muscles, three subjects repeated the second experiment involving motor cortical and brachial plexus stimulation on a separate day with a 45–65 % higher stimulus intensity for TMS (75–90 % of maximum stimulator output).
In the second study, voluntary activation was assessed in the fatigued elbow flexor muscles with motor cortical and motor nerve stimulation in the same experiment. Eight subjects were tested, six of whom had participated in one or both experiments with the unfatigued muscle. The experiment began in a similar way to the first study with subjects performing three brief control MVCs of the unfatigued elbow flexors. Subjects then performed 50 pairs of contractions of the fatigued elbow flexors, with minimal rest between pairs to maintain fatigue. Each pair was performed in random order and consisted of a sustained MVC followed 8 s later by a brief maximal or submaximal test contraction (1–2 s duration; Fig. 1C). The sole purpose of the sustained MVC was to fatigue the muscle and only ongoing voluntary force was recorded. The MVC was maintained until force had decreased to 60 % of the maximal force without fatigue. The submaximal target forces were set at 25, 50, 75 and 90 % of the force produced by a maximal contraction of the fatigued muscle (i.e. of 60 % of the MVC force without fatigue). During each brief test contraction, a stimulus was delivered to the motor cortex or the motor nerve. Stimulation of the motor nerve was also delivered at rest, 4 s after completing the sustained MVC. Twenty-five pairs of contractions were performed for each type of stimulus with five pairs performed for each of the four brief submaximal target forces and the brief maximal efforts. After a break of 1–2 min, subjects performed another 30 pairs of similar contractions in which stimuli were given to the motor cortex or brachial plexus so that EMG responses could be recorded. Fifteen pairs were performed for each type of stimulus with three pairs performed for each target force.
Data analysis
In the sets of contractions in which stimulation of either the motor cortex or biceps brachii motor nerve were given, voluntary activation was quantified by measurement of the force responses. For stimulation of the motor nerve (twitch interpolation), any increment in elbow flexion force evoked during a contraction (superimposed twitch; a in Fig. 2A) was expressed as a fraction of the amplitude of the maximal response evoked by the same stimulus in the potentiated relaxed muscle (resting twitch; b in Fig. 2A). The level of drive was then quantified as a percentage using this equation: voluntary activation (%) = (1 –a/b) × 100. For stimulation of the motor cortex, this equation was also used for quantifying voluntary activation with one exception. The amplitude of the ‘resting twitch’ evoked by motor cortical stimulation was estimated rather than measured directly. For each subject, a linear regression of the amplitude of the superimposed twitch evoked by motor cortical stimulation against voluntary force was performed for forces between 50 and 100 % of maximum. The y-intercept was taken as the amplitude of the ‘resting twitch’ evoked by motor cortical stimulation (Fig. 2B).
Figure 2. Methods used for calculation of voluntary activation with motor nerve or motor cortex stimulation.
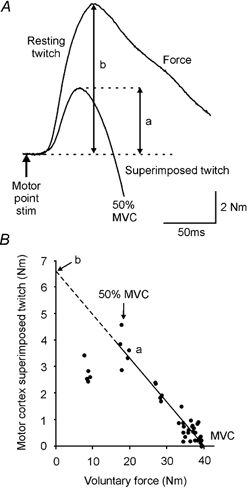
A, schematic representation of elbow flexion force following motor nerve stimulation. Arrow indicates the timing of the stimulus. Voluntary force trace with a resting muscle twitch (b) and superimposed twitch (a) evoked by motor nerve stimulation. Background forces have been offset to allow comparison of the twitches. B, single subject data displaying the linear correlation between the amplitude of the superimposed twitch (a) evoked by motor cortical stimulation between 50 and 100 % of maximal voluntary force (MVC). The linear regression was extrapolated to the y-axis and the y-intercept was taken as the estimated amplitude of the resting twitch (b) evoked by motor cortical stimulation.
In the trials in which motor cortical stimulation and brachial plexus stimulation were interspersed, the area of MEPs and Mmax were measured between set cursors for the biceps and triceps muscles. To account for the activity-dependent changes in muscle fibre action potentials, the area of each MEP was normalised to the average area of Mmax elicited during contractions of the same strength (e.g. Taylor et al. 1999; Gandevia et al. 1999). For each subject, force was normalised to the largest MVC force recorded during the experiment.
In the text, group data are presented as the mean ± standard deviation, whereas in figures, mean ± standard error of the mean (s.e.m.) are shown. Statistical analysis involved one-way analysis of variance (ANOVA) for comparisons between contraction strengths and two-way ANOVA for comparisons between the unfatigued and fatigued muscle. Post hoc discrimination between means was made with the Student-Newman-Keuls procedure. Unpaired t tests were used for comparison between stimuli and variables with and without fatigue. χ2 tests were used to compare the number of superimposed twitches evoked by motor cortical and motor nerve stimulation during submaximal and maximal contractions. Statistical significance was set at the 5 % level.
RESULTS
Force and EMG responses to stimulation of the motor cortex were measured in the elbow flexor muscles with and without fatigue during brief contractions of varying strengths. The amplitude of the superimposed twitch evoked by motor cortical stimulation was used to calculate voluntary activation and the results were compared with those obtained with motor nerve stimulation.
Force
During brief maximal and submaximal contractions without fatigue, motor cortical stimulation evoked larger superimposed twitches than motor nerve stimulation and they occurred in a greater number of efforts. Following motor cortical stimulation, superimposed twitches were present in 91.8 % of maximal efforts and in all submaximal contractions. In contrast, motor nerve stimulation evoked superimposed twitches in only 67.5 % of maximal efforts and in 97.5 % of submaximal contractions (χ2 = 27.88; P < 0.001). In each subject, in the unfatigued muscle, the amplitude of the superimposed twitch decreased with an increase in voluntary force when evoked by either motor cortical or motor nerve stimulation. Figure 3 shows the average twitch evoked in one subject at each contraction strength (see also Fig. 4A). For motor nerve stimulation, the relationship between twitch amplitude and contraction strength was curvilinear (polynomial r2 = 0.99). With motor cortical stimulation, the relationship was linear for contraction strengths between 50 and 100 % MVC (linear r2 = 1.00; Fig. 4). At weaker contraction strengths, the twitch was disproportionately small.
Figure 3. Responses to motor nerve and motor cortex stimulation, with and without muscle fatigue.
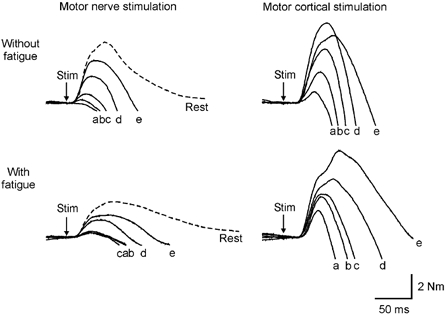
Average traces of elbow flexion force from one subject following a single motor nerve stimulus or transcranial magnetic stimulus to the motor cortex (TMS) at varying contraction strengths (a, 100 % MVC; b, 90 % MVC; c, 75 % MVC; d, 50 % MVC; e, 25 % MVC), with and without fatigue. Twitches to motor nerve stimulation were also recorded with the muscle at rest (dashed lines). Traces have been offset to allow comparison of the twitches. MVC, maximal voluntary contraction.
Figure 4. Single subject and group data.
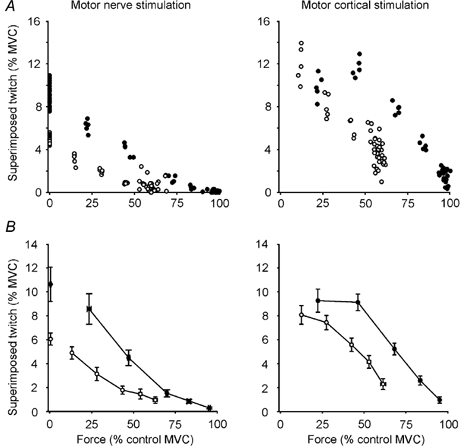
Single subject (A) and group data (B; means ± s.e.m.) showing the amplitude of the superimposed twitch evoked by motor nerve stimulation or TMS (motor cortical stimulation) at varying contraction strengths with fatigue (○) and without fatigue (•). All forces are shown as percentages of maximal voluntary force (MVC) of the unfatigued muscle. With fatigue, maximal voluntary force was reduced to 60 % MVC and submaximal contractions were targeted to 90, 75, 50 and 25 % of the reduced maximal force.
Between 50 and 100 % MVC, the superimposed twitch evoked by motor cortical stimulation was significantly larger than that evoked by motor nerve stimulation (Fig. 3 and Fig. 4, Table 1, P < 0.001, F1,7 = 41.51). However, the time-to-peak amplitude (TTP) for the superimposed twitches were similar for the two stimuli (Table 1, P = 0.08). For both types of stimulation, TTP decreased with an increase in voluntary force (motor cortex: P < 0.001, F3,21 = 35.46, 50–100 % MVC; motor nerve: P < 0.001, F5,35 = 118.88, 0–100 % MVC).
Table 1.
Summary of the mean amplitude and time-to-speak amplitude of the superimposed twitch evoked by motor corticl and motor nerve stimulation at varying voluntary contraction strengths with and without fatigue
| Without fatigue | With fatigue | |||||||
|---|---|---|---|---|---|---|---|---|
| Amplitude (%MVC) | TTP (ms) | Amplitude (%MVC) | TTP (ms) | |||||
| Cortex | Nerve | Cortex | Nerve | Cortex | Nerve | Cortex | Nerve | |
| Rest | 16.6 ± 3.5* | 10.6 ± 4.0 | — | 54.2 ± 6.1 | 11.8 ± 2.8* | 6.1 ± 1.4 | — | 56.6 ± 8.7 |
| 25% MVC | 9.3 ± 2.7 | 8.6 ± 3.6 | 41.1 ± 2.7 | 43.1 ± 5.9 | 8.1 ± 2.2 | 4.9 ± 1.5 | 49.6 ± 6.3 | 47.9 ± 5.8 |
| 50% MVC | 9.1 ± 2.0 | 4.5 ± 1.8 | 32.1 ± 7.0 | 32.6 ± 5.5 | 7.4 ± 1.7 | 3.1 ± 1.6 | 40.9 ± 5.4 | 33.4 ± 8.7 |
| 75% MVC | 5.2 ± 1.4 | 1.5 ± 0.9 | 19.4 ± 8.3 | 20.6 ± 6.6 | 5.6 ± 1.6 | 1.8 ± 1.0 | 33.8 ± 3.2 | 25.5 ± 8.2 |
| 90% MVC | 2.6 ± 1.1 | 0.8 ± 0.5 | 13.0 ± 1.3 | 16.8 ± 8.7 | 4.2 ± 1.5 | 1.5 ± 1.2 | 30.2 ± 3.9 | 24.1 ± 8.5 |
| 100% MVC | 1.0 ± 0.8 | 0.3 ± 0.2 | 10.5 ± 2.6 | 11.3 ± 7.0 | 2.3 ± 1.3 | 1.0 ± 0.8 | 21.5 ± 6.3 | 20.6 ± 8.3 |
MVC, maximal voluntary contraction; TTP, time-to-peak amplitude.
For motor cortical stimulation, the amplitude of the resting twitch was estimated through linear regression.
In the second study, subjects made repeated sustained MVCs to fatigue the elbow flexor muscles. Twitches evoked in the resting muscle by motor nerve stimulation in this fatigued condition were reduced to 57 % on average of that in the unfatigued condition (P < 0.001; Table 1). The TTP and half-relaxation time (RT1/2) lengthened in four of the five subjects who participated in both studies (e.g. Fig. 3, left panels, dashed traces) although this was not significant for the group. These changes signify peripheral fatigue. During brief maximal efforts, the absolute size of the superimposed twitch increased (Table 1, 100 % MVC) which indicates that central fatigue also occurred. The increase was significant both for twitches evoked by motor nerve (P = 0.033) and motor cortical stimulation (P = 0.025).
As for the unfatigued muscle, during fatigue the size of the superimposed twitch declined with increasing strength of voluntary contraction for both motor nerve and motor cortical stimulation. With fatigue, the correlation between voluntary activation and force was similar to the unfatigued condition (motor nerve, polynomial r2 = 0.99; motor cortex, linear r2 = 0.98). Figure 4 (open circles) shows amplitudes of the superimposed twitch for a single subject (panel A) and for the group (panel B). Despite both peripheral and central fatigue, the superimposed twitch evoked by motor cortical stimulation remained significantly larger than those evoked by motor nerve stimulation for contractions between 50 and 100 % MVC (P < 0.001, F1,7 = 90.01, Table 1). Time-to-peak amplitude was prolonged for twitches evoked by motor cortical or motor nerve stimulation.
Motor evoked potential (MEP)
Figure 5A shows, for one subject, averaged EMG traces of the Mmax and MEP in biceps and the MEP in triceps evoked during contractions of the unfatigued elbow flexors. The MEP in biceps was large in relation to Mmax. Mmax tended to decrease with high contraction strengths but the MEP was reduced even more. Figure 5B shows the mean area of MEPs in the biceps and triceps at varying contraction strengths. For each subject, MEPs were normalised to Mmax recorded during contractions of similar strength. In biceps when the muscle was not fatigued, the normalised MEP was largest during the 50 % MVC and decreased during maximal efforts (P < 0.001, F4,28 = 7.53). In 15 % of contractions made at 50 % MVC, the MEP was larger than the biggest Mmax recorded at the same contraction strength, whereas during maximal efforts, only 2 % of MEPs were larger than Mmax. With fatigue, the MEP during the 25 % MVC and 50 % MVC was significantly smaller than without fatigue while the MEP at higher contraction strengths remained unchanged (P < 0.001).
Figure 5. EMG responses to motor nerve and motor cortical stimulation.
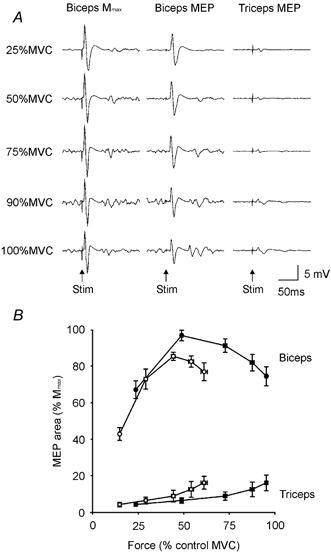
A, single-subject data showing averaged EMG responses following peripheral nerve stimulation (Mmax) and motor cortical stimulation (MEP) for the unfatigued biceps and following motor cortical stimulation (MEP) for the triceps. B, group data (means ± s.e.m.) showing the area of the motor evoked potential (MEP; expressed as a percentage of Mmax, see Methods) in the biceps (circles) and triceps (squares) muscles at varying contraction strengths with fatigue (open symbols) and without fatigue (filled symbols). MVC, maximal voluntary contraction.
In contrast to the large MEP in the agonist, the MEP was significantly smaller in the antagonist triceps (P < 0.001). The average areas of the MEPs in triceps across all of the contraction strengths were 11 ± 9 and 10 ± 9 % Mmax with and without fatigue, respectively. The MEP in triceps was largest during the maximal efforts (without fatigue: 16 ± 12 % Mmax; with fatigue: 17 ± 10 % Mmax) and increased in size with increasing contraction strength (P = 0.002, F4,28 = 5.38).
In a separate study of three subjects, a higher stimulus intensity of TMS elicited a much larger MEP in the triceps during strong contractions without fatigue (e.g. 76 ± 20 % Mmax during maximal efforts). This additional antagonist muscle contribution reduced the amplitude of the superimposed twitch evoked by motor cortical stimulation by approximately two-thirds during maximal efforts.
Voluntary activation
Figure 6 shows the average voluntary activation calculated for motor cortical (see Methods, 50–100 % MVC) and motor nerve stimulation (25–100 % MVC), with (open circles) and without fatigue (filled circles). The shape of the voluntary activation curves was different for motor cortical and motor nerve stimulation. For motor cortical stimulation, a linear relationship was present (r2 = 1.00). In contrast, for motor nerve stimulation the shape was curvilinear and was biased towards higher levels of voluntary activation. The shape of the voluntary activation curve was similar with and without fatigue for both forms of stimulation even though voluntary activation was lower with fatigue.
Figure 6. Relationship between voluntary force and measures of voluntary activation.
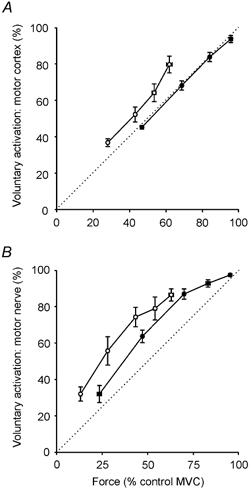
Voluntary muscle activation (%; see Methods), calculated with the use of motor cortical (A) and motor nerve stimulation (B) at varying contraction strengths with fatigue (○) and without fatigue (•). For motor cortical stimulation, data are plotted for contractions of 50, 75, 90 and 100 % maximal voluntary force (MVC). For motor nerve stimulation, contractions of 25, 50, 75, 90 and 100 % MVC are plotted. All forces are plotted as percentages of the MVC of the unfatigued muscle although with fatigue, contraction targets were set in relation to the fatigued maximal voluntary force. The dotted line is the line of identity.
Without fatigue, voluntary activation measured with motor cortical stimulation was high during maximal efforts (93.6 ± 5.6 %) and was not significantly different from that measured with motor nerve stimulation (97.4 ± 2.1 %; P = 0.09). However, with fatigue, voluntary activation was less during maximal efforts indicating the presence of central fatigue (motor cortex: 79.6 ± 13.0 %; motor nerve: 86.5 ± 9.4 %). During a 50 % MVC, voluntary activation measured with TMS was approximately half that during a maximal effort for both unfatigued and fatigued muscles. In contrast, voluntary activation measured with nerve stimulation was much higher during a 50 % MVC, approximately 65 % of that during a maximal effort, both with and without fatigue.
DISCUSSION
The present study has used motor cortical stimulation to define the extent to which the CNS has activated a muscle group across a wide range of contraction strengths. Results with this new method have been compared with those obtained with twitch interpolation which has long been the conventional method to calculate voluntary activation (Merton, 1954; Belanger & McComas, 1981; Thomas et al. 1989; see also Gandevia et al. 1996; Herbert & Gandevia, 1999). Three aspects of the results will be discussed in detail. First, the estimates of voluntary activation obtained with cortical stimulation follow the line of identity when plotted against voluntary force between 50 and 100 % MVC. Second, at 50 % MVC, the MEP evoked by cortical stimulation is near maximal compared to Mmax, and it diminishes progressively as force increases towards 100 % MVC. This finding may provide insight into the extent to which motoneurones are driven by volition. Third, when maximal voluntary force is reduced by 40 % by fatigue, about one-quarter of this reduction is due to a failure to drive the muscle optimally (i.e. central fatigue develops). Both types of stimulation (motor cortex and motor nerve) reveal central fatigue but they provide different information about its causes.
Voluntary activation in the unfatigued muscles
Between 50 and 100 % of maximal effort, voluntary activation increases linearly with increasing voluntary contraction strength when derived using cortical stimulation (see Fig. 6A). This is consistent with the idea that the superimposed twitch evoked by cortical stimulation reflects the ‘extra’ force obtained from motor units that voluntary effort did not recruit or did not discharge at a sufficiently fast rate. To allow direct comparison with results obtained with motor nerve stimulation, we derived the ‘resting twitch’ evoked by cortical stimulation by extrapolation and used this value in the conventional formula for voluntary activation (see Methods and Fig. 2B). This approach seems justified given the strong linear relation between voluntary force and voluntary activation measured with cortical stimulation.
In contrast to motor cortical stimulation, there is a non-linear relationship between voluntary activation estimated by motor nerve stimulation and voluntary force. At high forces, smaller changes in activation occur for a given change in voluntary force. Previous studies have also identified this non-linearity with stimulation over the biceps/brachialis muscles (Allen et al. 1998; see also De Serres & Enoka, 1998). One reason for the non-linearity is that the elbow flexor muscles innervated by the radial nerve (brachioradialis) are less well activated during maximal voluntary efforts (based on twitch interpolation) than those innervated by the musculocutaneous nerve (biceps and brachialis, Allen et al. 1998; for review Gandevia, 2001). Thus, the balance of force generated by the different elbow flexor muscles varies over the range of voluntary forces. In contrast, voluntary activation measured with cortical stimulation reveals the net output from all muscles that generate elbow flexion force. Consistent with this, the size of the superimposed twitch evoked by motor cortical stimulation is more than double that produced by motor nerve stimulation in the studies described here.
During brief efforts at 50 % MVC, the MEP was about the same size as a maximal motor response evoked by peripheral nerve stimulation. This suggests that under these conditions most motoneurones were activated by the motor cortical stimulus. In some contractions, the size of the MEP was slightly greater than Mmax suggesting that some motoneurones may have fired more than once as a result of the motor cortical stimulus. Similar conclusions have been reached for intrinsic muscles of the hand (Merton et al. 1982; Day et al. 1989). At lower voluntary forces, the MEP was much smaller than at 50 % MVC, presumably because of reduced ‘excitability’ at cortical and spinal sites (Kischka et al. 1993; Taylor et al. 1997). At forces above 50 % MVC, the MEP decreased progressively to 77 ± 14 % of Mmax during maximal efforts. As the MEP was normalised to Mmax recorded during a contraction of similar strength, our method accounts for any activity-dependent changes in the muscle fibre action potential (Taylor et al. 1999). Mmax did decrease slightly with increasing force (Fig. 5A), presumably more axons and muscle fibres were refractory as their firing rates increased. Thus, the decrease in MEP size implies a decrease in motoneuronal output in response to the stimulus. We suggest that the reduction reflects the inability of some motoneurones to fire in response to the excitatory input. They may be effectively ‘refractory’ due to intrinsic motoneuronal factors and the trajectory of the after-hyperpolarisation (see Matthews, 1999). Consistent with this we have found that the response to transmastoid stimulation (which activates corticospinal axons) is smaller during maximal than submaximal contractions (J. L. Taylor, J. E. Butler, N. T. Petersen and S. C. Gandevia, unpublished observations).
Although the MEP is reduced above 50 % MVC it is still more than 70 % of Mmax. This implies that many motoneurones are not firing ‘maximally’ during brief maximal efforts because they can fire in response to the synaptic input from the descending corticospinal volleys. Furthermore, the firing rate of motoneurones is not ‘optimal’ because the increased activity brought about by the descending volleys produces a small increase in muscle force.
Behaviour during muscle fatigue
When the elbow flexor muscles were fatigued by prior sustained maximal contractions so that maximal voluntary force dropped by 40 %, there was evidence of peripheral fatigue. The resting twitch evoked by motor nerve stimulation declined. There was also evidence of central fatigue. During brief maximal efforts, motor nerve stimulation evoked larger superimposed twitches than when the muscles were not fatigued. Thus, the ability of subjects to drive the muscle voluntarily was impaired. Increases also occurred in the superimposed twitches evoked by motor cortical stimulation during brief maximal efforts. While this also indicates development of central fatigue, it adds information on the site of impairment within the central nervous system. A larger superimposed twitch after motor nerve stimulation implies that even though the axons of the motoneurones are capable of increased firing rates and the muscle fibres could produce more force, motoneurone firing has slowed, or some motor units have been derecruited (Peters & Fuglevand, 1999). With motor cortical stimulation, the larger superimposed twitch is produced through synaptic activation of the motoneurones and demonstrates that the fall in motoneurone activity is not because the motoneurones are unresponsive to extra input. Motor cortical stimulation does not identify the mechanisms for the decrease in motoneurone activity with fatigue. Such mechanisms include decreased responsiveness of the motoneurones through changes in their intrinsic properties or through inhibitory afferent input, or disfacilitation through decreased excitatory afferent or descending input. Despite this, stimulation of the motor cortex produces extra output and this is sufficient to evoke a MEP, which is similar in size to that in the unfatigued muscle. There is presumably some as yet untapped cortical drive that can increase motoneurone firing and produce additional force. When fatigue had reduced the maximal voluntary force by 40 %, voluntary activation (measured with responses to motor cortical stimulation) fell by 14 % (in absolute terms). If we calculate the force that could have been produced by the fatigued muscle if voluntary activation had not fallen, the difference between the calculated force and the measured force is about 10 % MVC. This indicates that central fatigue accounts for approximately one-quarter of the 40 % fall in maximal voluntary force produced by sustained maximal contractions.
Although unchanged at higher contraction strengths, the size of the MEP in biceps was reduced at 25 and 50 % MVC when the muscle was fatigued. This decrease is difficult to interpret but may be due in part to the lower levels of voluntary activation, and consequent decreased excitability of motor cortical neurones and/or the motoneurones, needed to reach these target forces during fatigue. The activation needed to reach the target forces is low because the targets are set as percentages of a maximal effort in which there is central fatigue and poor voluntary activation. Other mechanisms including changes in the intrinsic properties of motoneurones or alterations in afferent input could also decrease the gain of the motoneurone pool, while a decreased response to stimulation of the motor cortex could also reduce MEP size. In contrast to the current findings, we have previously reported that MEPs in biceps get larger during fatiguing maximal efforts and have attributed this growth to increased cortical excitability (Taylor et al. 1996, 1999). However, the growth of MEPs occurred during sustained contractions and had recovered in brief MVCs performed after 15 s rest. Here, brief MVCs were performed 8 s after sustained MVCs of various durations and this period may be sufficient to allow recovery from any increase in excitability of the motor cortex.
For motor cortical stimulation, the plot of voluntary activation against force production remained linear when the elbow flexors were fatigued so the method of estimation of voluntary activation remained valid, and may remain so for other changes in peripheral force-generating capacity. Thus, a contraction to the target of 50 % of the fatigued MVC has a voluntary activation of approximately half that of a maximal effort. For nerve stimulation with the unfatigued and the fatigued muscles, the curves were also similar in shape. Hence, if there is preferential activation of biceps and brachialis rather than other elbow flexors, this does not appear to be changed by fatigue during maximal contractions. It is superficially attractive to compare the measures of voluntary activation produced by cortical and nerve stimulation, to identify the ‘site’ of failure of voluntary drive. Unfortunately, direct quantitative comparison is problematic because the two types of stimuli can activate different muscles and the shapes of the voluntary activation-voluntary force curves for motor cortical and motor nerve stimulation are different.
Estimation of voluntary activation of the elbow flexor muscles through superimposed twitches evoked by motor cortical stimulation provides a measure which is linearly related to the strength of contraction, whether of unfatigued or fatigued muscles. It can demonstrate central fatigue and shows that, when central fatigue is present after sustained MVCs, it is not due to a lack of responsiveness of the motoneurones to input or to an inability of motor cortical neurones to produce additional output.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (3206). We are grateful to Dr Jane E. Butler and Professor David Burke for helpful discussion of the manuscript.
REFERENCES
- Allen GE, McKenzie DK, Gandevia SC. Twitch interpolation of the elbow flexor muscles at high forces. Muscle Nerve. 1998;21:318–328. doi: 10.1002/(sici)1097-4598(199803)21:3<318::aid-mus5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Awiszus F, Wahl B, Meinecke I. Influence of stimulus cross talk on results of the twitch-interpolation technique at the biceps brachii muscle. Muscle Nerve. 1997;20:1187–1190. doi: 10.1002/(sici)1097-4598(199709)20:9<1187::aid-mus17>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Behm DG, Whittle J, Button D, Power K. Intermuscle differences in activation. Muscle Nerve. 2002;25:236–243. doi: 10.1002/mus.10008. [DOI] [PubMed] [Google Scholar]
- Belanger AY, McComas AJ. Extent of motor unit activation during effort. J Appl Physiol. 1981;51:1131–1135. doi: 10.1152/jappl.1981.51.5.1131. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Jones DA, Hosking GP, Edwards RHT. Central and peripheral fatigue in sustained maximum voluntary contractions of human quadriceps muscle. Clin Sci Mol Med. 1978;54:609–614. doi: 10.1042/cs0540609. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Rice CL, Garland SJ, Walsh ML. Task-dependent factors in fatigue of human voluntary contractions. Adv Exp Med Biol. 1995;384:361–380. doi: 10.1007/978-1-4899-1016-5_29. [DOI] [PubMed] [Google Scholar]
- Day BL, Dressler D, Maertens De Noordhout A, Marsden CD, Nakashima K, Rothwell JC, Thompson PD. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol. 1989;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Serres SJ, Enoka RM. Older adults can maximally activate the biceps brachii muscle by voluntary command. J Appl Physiol. 1998;84:284–291. doi: 10.1152/jappl.1998.84.1.284. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Effects of voluntary contraction on descending volleys evoked by transcranial stimulation in conscious humans. J Physiol. 1998;508:625–633. doi: 10.1111/j.1469-7793.1998.625bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JJ, Konert E, Ljucovic P, Andrews DM. Are humans able to voluntarily elicit maximum muscle force. Neurosci Lett. 1994;179:25–28. doi: 10.1016/0304-3940(94)90926-1. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Allen GM, Butler JE, Taylor JL. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol. 1996;490:529–536. doi: 10.1113/jphysiol.1996.sp021164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Allen GM, McKenzie DK. Central fatigue. Critical issues, quantification and practical implications. Adv Exp Med Biol. 1995;384:281–294. [PubMed] [Google Scholar]
- Gandevia SC, McKenzie DK, Plassman BL. Activation of human respiratory muscles during different voluntary manoeuvres. J Physiol. 1990;428:387–403. doi: 10.1113/jphysiol.1990.sp018218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Petersen N, Butler JE, Taylor JL. Impaired response of human motoneurones to corticospinal stimulation after voluntary exercise. J Physiol. 1999;521:749–759. doi: 10.1111/j.1469-7793.1999.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert RD, Gandevia SC. Muscle activation in unilateral and bilateral efforts assessed by motor nerve and cortical stimulation. J Appl Physiol. 1996;80:1351–1356. doi: 10.1152/jappl.1996.80.4.1351. [DOI] [PubMed] [Google Scholar]
- Herbert RD, Gandevia SC. Twitch interpolation in human muscles: mechanisms and implications for measurement of voluntary activation. J Neurophysiol. 1999;82:2271–2283. doi: 10.1152/jn.1999.82.5.2271. [DOI] [PubMed] [Google Scholar]
- Hess CW, Mills KR, Murray NMF. Responses in small hand muscles from magnetic stimulation of the human brain. J Physiol. 1987;388:397–419. doi: 10.1113/jphysiol.1987.sp016621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kischka U, Fajfr R, Fellenberg T, Hess CW. Facilitation of motor evoked potentials from magnetic brain stimulation in man: a comparative study of different target muscles. J Clin Neurophysiol. 1993;10:505–512. doi: 10.1097/00004691-199310000-00008. [DOI] [PubMed] [Google Scholar]
- Lloyd AR, Gandevia SC, Hales JP. Muscle performance, voluntary activation, twitch properties and perceived effort in normal subjects and patients with the chronic fatigue syndrome. Brain. 1991;114:85–98. [PubMed] [Google Scholar]
- Lyons MF, Cadden SW, Baxendale RH, Yemm R. Twitch interpolation in the assessment of the maximum force-generating capacity of the jaw-closing muscles in man. Arch Oral Biol. 1996;41:1161–1168. doi: 10.1016/s0003-9969(96)00086-6. [DOI] [PubMed] [Google Scholar]
- McKenzie DK, Plassman BL, Gandevia SC. Maximal activation of the human diaphragm but not inspiratory intercostal muscles during static inspiratory efforts. Neurosci Lett. 1988;89:63–68. doi: 10.1016/0304-3940(88)90481-8. [DOI] [PubMed] [Google Scholar]
- Matthews PB. The effect of firing on the excitability of a model motoneurone and its implications for cortical stimulation. J Physiol. 1999;518:867–882. doi: 10.1111/j.1469-7793.1999.0867p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merton PA. Voluntary strength and fatigue. J Physiol. 1954;123:553–564. doi: 10.1113/jphysiol.1954.sp005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merton PA, Hill DK, Morton HB, Marsden CD. Scope of a technique for electrical stimulation of human brain, spinal cord, and muscle. Lancet. 1982;2:597–600. doi: 10.1016/s0140-6736(82)90670-5. [DOI] [PubMed] [Google Scholar]
- Peters EJD, Fuglevand AJ. Cessation of human motor unit discharge during sustained maximal voluntary contraction. Neurosci Lett. 1999;274:66–70. doi: 10.1016/s0304-3940(99)00666-7. [DOI] [PubMed] [Google Scholar]
- Reid C. The mechanism of voluntary muscular fatigue. J Physiol. 1928;29:17–42. doi: 10.1136/bmj.2.3481.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC, Thompson PD, Day BL, Boyd S, Marsden CD. Stimulation of the human motor cortex through the scalp. Exp Physiol. 1991;76:159–200. doi: 10.1113/expphysiol.1991.sp003485. [DOI] [PubMed] [Google Scholar]
- Russell G, Taylor JL, Gandevia SC. Measurement of voluntary activation in elbow flexor muscles using transcranial magnetic stimulation. Proceedings of IUPS Satellite: Movement and Sensation. p. 95.
- Russell G, Taylor JL, Gandevia SC. Measurement of voluntary activation using transcranial magnetic stimulation. Proceedings of the Australian Neuroscience Society ORAL-06–05.
- Taylor JL, Allen GM, Butler JE, Gandevia SC. Effect of contraction strength on responses in biceps brachii and adductor pollicis to transcranial magnetic stimulation. Exp Brain Res. 1997;117:472–478. doi: 10.1007/s002210050243. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Allen GM, Butler JE, Gandevia SC. Supraspinal fatigue during intermittent maximal voluntary contractions of the human elbow flexors. J Appl Physiol. 2000;89:305–313. doi: 10.1152/jappl.2000.89.1.305. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Butler JE, Allen GM, Gandevia SC. Changes in motor cortical excitability during human muscle fatigue. J Physiol. 1996;490:519–528. doi: 10.1113/jphysiol.1996.sp021163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Butler JE, Gandevia SC. Altered responses of human elbow flexors to peripheral-nerve and cortical stimulation during a sustained maximal voluntary contraction. Exp Brain Res. 1999;127:108–115. doi: 10.1007/s002210050779. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Woods JJ, Bigland-Ritchie B. Impulse propagation and muscle activation in long maximal voluntary contractions. J Appl Physiol. 1989;67:1835–1842. doi: 10.1152/jappl.1989.67.5.1835. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Zaidner EY, Calancie B, Broton JG, Bigland-Ritchie B. Muscle weakness, paralysis, and atrophy after human cervical spinal cord injury. Exp Neurol. 1997;148:414–423. doi: 10.1006/exnr.1997.6690. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Terao Y, Hanajima R, Sakai K, Kanazawa I. Facilitatory effect of tonic voluntary contraction on responses to motor cortex stimulation. Electroencephalogr Clin Neurophysiol. 1995;97:451–454. doi: 10.1016/0924-980x(95)00214-6. [DOI] [PubMed] [Google Scholar]


