Abstract
Perforated patch clamp recordings were performed on cultured superficial neonatal rat dorsal horn (DH) spinal cord neurones in order to study the presynaptic modulation of GABA release at unitary synaptic connections. Since ATP can be coreleased with GABA at about two-thirds of GABAergic synapses between DH neurones, and can be rapidly metabolized to adenosine in the extracellular space, we investigated the potential role of A1 adenosine receptors and GABAB receptors which might function as inhibitory autoreceptors. Adenosine and GABAB receptor agonists reduced the amplitude of electrically evoked GABAergic inhibitory postsynaptic currents (eIPSCs) as well as the frequency of GABAergic miniature IPSCs, suggesting a presynaptic action of these substances. The actions of adenosine were blocked by the A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX). The effects of adenosine and GABAB agonists were occlusive, indicating a functional convergence of the signalling pathways engaged by A1 and GABAB receptors. A1 and GABAB antagonists increased the amplitude of eIPSCs in a supra-additive manner, suggesting a tonic activation of these receptors by ambient adenosine and GABA. Moreover, using trains of electrical stimulations, we were able to unravel a phasic (activity-dependent) activation of presynaptic A1 and GABAB autoreceptors only in the case of neurones coreleasing ATP and GABA, despite the presence of functional presynaptic A1 and GABAB receptors on all GABAergic DH neurones. This selective, convergent and activity-dependent inhibition of GABA release by A1 and GABAB autoreceptors might modulate the integrative properties of postsynaptic DH neurones under physiological conditions and/or during the development of pathological pain states.
Adenosine and γ-aminobutyric acid (GABA) play an important role as modulators of excitatory and inhibitory synaptic transmission in the central nervous system (CNS). Their inhibitory effects mainly involve the activation of metabotropic A1 and GABAB receptors, which are negatively coupled to adenylate cyclase through pertussis toxin-sensitive G proteins (Reppert et al. 1991; Knight & Bowery, 1996; Ralevic & Burnstock, 1998). Both types of receptors are present postsynaptically, but their major sites of action to control synaptic transmission appear to be presynaptic (Misgeld et al. 1995; Malcangio & Bowery, 1996; Wu & Saggau, 1997; Ralevic & Burnstock, 1998; Cunha, 2001). GABAB and A1 receptors are widely distributed in the brain and the spinal cord (Reppert et al. 1991; Malcangio & Bowery, 1996; Kaupmann et al. 1998; Ralevic & Burnstock, 1998; Towers et al. 2000) and colocalize in numerous structures of the CNS where they are likely to coexist in single neurones and participate in convergent modulatory effects on synaptic transmission. This hypothesis has however never been tested directly, in particular in the case of synaptic contacts between a single pair of neurones. In this context, an important issue is also to know if both presynaptic receptor types use separate or, at least partly, common/shared signalling pathways, because this situation will have fundamental consequences on the integrative properties of the presynaptic terminals and the dynamic control of transmitter release.
In order to address these questions, we have used a culture system of neonatal rat dorsal horn (DH) spinal cord neurones which allows recording of synaptic transmission between pairs of identified neurones under conditions of optimal control of the composition of extracellular medium and drug application (Jo & Schlichter, 1999; Hugel & Schlichter, 2000). We have already shown that the properties of the neurones and of the synaptic contacts formed in culture were similar to those which develop in situ (Jo et al. 1998b) and that about two-thirds of the GABAergic neurones in this system used adenosine 5′-triphosphate (ATP) as a cotransmitter (Jo & Schlichter, 1999). ATP is rapidly metabolized to adenosine in the extracellular space following the action of nucleotidases (Salter et al. 1993; Dunwiddie et al. 1997; Cunha et al. 1998; Zimmermann, 2000) and this is also the case in our DH cultures (Jo & Schlichter, 1999). In the hippocampus, it has been estimated that the generation of adenosine from ATP takes less than 200 ms (Dunwiddie et al. 1997) and this fast metabolism raises the possibility that adenosine rapidly generated from synaptically released ATP could in turn activate presynaptic P1/adenosine autoreceptors. Moreover, there is a dense expression of A1 and GABAB receptors in the DH of the spinal cord (Reppert et al. 1991; Towers et al. 2000; Fredholm et al. 2001). All these considerations suggest that our culture system of neonatal DH neurones is ideally suited to study the activation of presynaptic metabotropic GABAB and adenosine autoreceptors and to look for their functional interaction in the control of the synaptic release of GABA.
METHODS
The techniques for preparing primary cultures of neonatal rat DH neurones and for recording synaptic transmission between pairs of neurones were described in detail elsewhere (Jo et al. 1998a; Jo & Schlichter, 1999; Hugel & Schlichter, 2000) and are only briefly described below.
Tissue culture
Animal treatment was in compliance with European Communities Council directives (86/609/EEC). After decapitation of 3- to 4-day-old Wistar rats under deep diethyl-ether anaesthesia, the dorsal third of the spinal cord was cut with a razor blade and the tissue fragments were digested enzymatically for 45 min at 37 °C with papain (20 u ml−1, Sigma, France) in oxygenated divalent-free Earle's balanced salt solution (EBSS, Gibco, France) and mechanically dissociated with a 1 ml plastic pipette. The mechanical dissociation was performed after resuspending the tissue in culture medium, the composition of which was the following: minimum essential medium-alpha (MEM-alpha, Gibco, France), fetal calf serum (5 % v/v, Gibco, France), heat-inactivated horse serum (5 % v/v, Gibco, France), penicillin and streptomycin (50 i.u. ml−1 for each, Gibco, France), transferrin (10 mg ml−1, Sigma, France), insulin (5 mg ml−1, Sigma, France), putrescine (100 nm, Sigma, France) and progesterone (20 nm, Sigma, France). After additional trituration with a fire-polished pasteur pipette, the cells were plated in the collagen-coated central compartment (total volume: ≈250 μl) of 35 mm plastic culture dishes. This compartment was formed after heating the dishes in a press (BB-Form 2, Mecanex, Switzerland) (Bader et al. 1987). Cultures were maintained in a water-saturated atmosphere (95 % air, 5 % CO2) at 37 °C until use (10–15 days). One day after seeding the cells, cytosine arabinoside (10 μm, Sigma, France) was added to the culture medium for 12 h in order to reduce glial proliferation. It must be emphasized that this procedure did not suppress glial proliferation but only slowed it. This situation allowed us to perform recordings from neurones which had developed in culture for at least 10 days and therefore had formed synaptic contacts which were functionally similar to those observed in slices (Jo et al. 1998a; Jo & Schlichter, 1999; Hugel & Schlichter, 2000). All neurones recorded in this study were surrounded by a glial network, and this point is particularly important because glial cells express various types of neurotransmitter transporters, participate actively in the clearance and homeostasis of neurotransmitters in the synaptic cleft and extracellular space and are likely to release neuromodulatory substances (Cherubini & Conti, 2001; Haydon, 2001).
Electrophysiological recordings
Experiments were performed at room temperature (20–22 °C) after 10–15 days in culture. Perforated-patch clamp recordings were made with an Axopatch 200A amplifier (Axon Instruments, USA) and low resistance (3–4 MΩ) electrodes using amphotericin B as the pore-forming agent (Rae et al. 1991). The external solution contained (mm): NaCl 135, KCl 5, CaCl2 2.5, MgCl2 1, Hepes 5 and glucose 10, pH 7.35, and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μm), D-amino-phosphonovaleric acid (D-APV, 30 μm) and strychnine (1 μm) to block fast glutamatergic and glycinergic synaptic transmissions. In some experiments, tetrodotoxin (TTX, 0.5 μm) was added to this solution, in order to record miniature GABAergic inhibitory postsynaptic currents (mIPSCs) in isolation.
For the recording of GABAergic mIPSCs, the pipette solution contained (mm): CsCl 125, CaCl2 5, MgCl2 2, Hepes 10, EGTA 10, pH 7.35. With this solution, the equilibrium potentials for Cl− (ECl) and cations (Ecations) were both 0 mV. During electrical stimulation experiments (see below) we used a pipette solution containing (mm): Cs2SO4 75, CsCl 4 and Hepes 10, pH 7.35. Under these conditions, ECl and Ecations were −90 mV and 0 mV, respectively. All pipette solutions contained amphotericin B (150 μg ml−1, Sigma, France). Voltage and current traces were stored digitally on a video tape recorder (sampling rate 20 kHz) and/or on a personal computer after being filtered at 5 kHz by the Axopatch 200A. Acquisition and analysis were performed with the programs pCLAMP (Axon Instruments) and Mini Analysis (version 6.3, Synaptosoft, USA). The analysis of miniature synaptic currents was performed on sequences with at least 300 synaptic events each. The average value of membrane noise was 3–4 pA. The event detection level of the Mini Analysis software was set just above the membrane noise in order to detect a maximum of events, i.e. abrupt deflections of the baseline. These events included both channel openings and synaptic currents. Therefore, all events detected by the software were inspected visually and mIPSCs were selected on the basis of their rapid rise times (< 2 ms) and exponential decay kinetics. Only mIPSCs with amplitudes exceeding 1.5–2 times that of the noise level were considered. All other events were rejected. For kinetic analysis, only isolated mIPSCs were used and were fitted by exponential functions over the total time course of their decaying phases, i.e. until full return to baseline.
Electrical stimulation
The stimulation procedure was identical to that described previously for the same culture preparation (Jo et al. 1998a; Jo & Schlichter, 1999; Hugel & Schlichter, 2000). Extracellular electrical stimulation of the cell body of the presynaptic neurone triggered the synaptic release of GABA, which produced a fast inhibitory postsynaptic current (eIPSC) due to the activation of Cl−-permeable ionotropic GABAA receptors, and eventually the release of ATP, which activated postsynaptic cation-permeable ionotropic P2X receptors underlying a fast excitatory postsynaptic current (eEPSC) (Jo & Schlichter, 1999). Stimulation was performed with short pairs (interval 400 ms) of stimuli (0.1 ms in duration) delivered at 0.1 Hz and having amplitudes between −10 and −20 V. In some experiments, designed to reveal the activation of presynaptic autoreceptors by the phasic release of transmitter, trains of eight pulses having the same characteristics as those described above were applied to the presynaptic neurone. In this case the trains were delivered at a frequency of 0.05 Hz.
In all electrical stimulation experiments, we fixed ECl at −90 mV and ECations at 0 mV (see above) and the recordings were made at a holding potential (HP) of 0 mV in order to isolate GABAergic eIPSCs. At the beginning of each recording we determined if the presynaptic neurone was coreleasing GABA and ATP or releasing only GABA. This was done by checking for the presence of a fast P2X receptor-mediated eEPSC at a HP of −90 mV in the postsynaptic neurone following electrical stimulation of the presynaptic cell. For each recording, only isolated pairs of neurones with no visible physical contacts with fibres from other neurones were selected, and we carefully verified that the postsynaptic neurone received a monosynaptic input from the neurone which was extracellularly stimulated (Jo & Schlichter, 1999). The electrical stimuli were applied through two patch pipettes glued tip to tip and filled with extracellular solution. The tip of one of the stimulation electrodes was placed in contact with the cell body of the presynaptic neurone and the intensity of the electrical stimulus was progressively increased. Synaptic contacts were identified as monosynaptic unitary connections when the following criteria were satisfied: (1) all or none appearance of the eIPSC, (2) absence of increase in eIPSC amplitude when stimulus intensity was further increased, (3) disappearance of eIPSCs when the polarity of the stimulus was inverted, and (4) constant latency of the eIPSC.
For analysis and illustration in Fig. 1 and Fig. 3–5, we averaged on the one hand 20 traces recorded under control or washout conditions and on the other hand 10 traces recorded during the application of the substance(s) to test.
Figure 1. Effect of adenosine and baclofen on the amplitudes of electrically evoked GABAergic eIPSCs in isolated pairs of DH neurones.
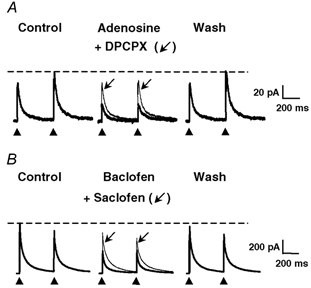
A paired-pulse stimulation protocol consisting of two identical pulses (0.1 ms duration) separated by 400 ms was applied at a frequency of 0.1 Hz. The GABAergic eIPSCs were recorded in isolation at a holding potential (HP) of 0 mV. A, adenosine (10 μm) reversibly decreased the amplitudes of GABAergic eIPSCs (thick traces). This phenomenon was accompanied by a change in the paired-pulse ratio, indicating a presynaptic site of action of adenosine. The effect of adenosine was strongly reduced in the presence of 1 μm DPCPX (thin traces, arrow), suggesting the involvement of A1 receptors. B, baclofen (10 μm) also reduced the amplitudes of GABAergic eIPSCs (thick traces) and changed the paired-pulse ratio of eIPSCs. The effect of baclofen was completely reversible and strongly attenuated by 100 μm saclofen (thin traces, arrow), suggesting the involvement of GABAB receptors. In this and following figures displaying electrically evoked synaptic currents, the control and wash traces are averages of 20 individual consecutive traces, and traces in the presence of pharmacological substances are averages of 10 individual and consecutive traces. The black arrowheads below the traces indicate the times of application of the electrical stimuli to the presynaptic neurone, and the dashed horizontal lines indicate the amplitude of the second (panel A) or first (panel B) eIPSC under control conditions.
Figure 3. Occlusion of the responses mediated by activation of GABAB and A1 receptors, indicating a convergence of the signalling pathways engaged by the two receptor types.
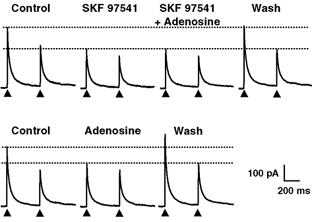
Top, SKF 97541 (1 μm) induced a marked reduction in the amplitude of GABAergic eIPSCs which was accompanied by a change in the paired-pulse ratio, indicating a presynaptic site of action. In the presence of SKF 97541, application of adenosine (10 μm) induced no further decrease in the amplitude of eIPSCs. Note that the amplitude of eIPSCs recovered fully after washing out SKF 97541 and adenosine. Bottom, when adenosine was then applied alone, there was a significant and reversible reduction of the amplitude of eIPSCs and an increase of the paired-pulse ratio indicating the presence of functional adenosine receptors on the presynaptic neurone. The HP was 0 mV. All recordings are from the same pair of neurones. For each panel, the upper dotted horizontal lines indicate the amplitude of the first eIPSC under control conditions and the lower dotted lines that of the first eIPSC in the presence of SKF 97541 (upper panel) or adenosine (lower panel).
Figure 5. Presynaptic A1 and GABAB autoreceptors were activated in a phasic (activity-dependent) manner in neurones coreleasing GABA and ATP but not in neurones releasing only GABA.
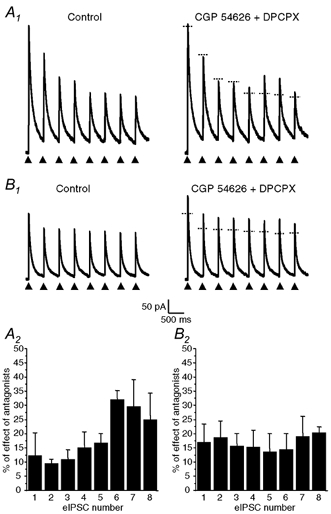
Trains of eight identical electrical pulses (0.1 ms duration) separated by 400 ms (2.5 Hz, arrow heads) were applied at a frequency of 0.05 Hz (i.e. every 20 s) to the cell body of presynaptic neurones coreleasing GABA and ATP (A) or of neurones releasing only GABA (B) as determined by the presence or absence of P2X receptor-mediated eEPSCs at a HP of −90 mV. A1 and B1, effects of coapplication of DPCPX (1 μm) and CGP 54626 (1 μm) on the amplitude of eIPSCs evoked by the train of eight pulses. Traces on the left were obtained under control conditions and traces recorded in the presence of CGP 54626 + DPCPX are represented on the right. The horizontal dotted line segments on the traces recorded in the presence of the antagonists indicate the peak amplitude of each corresponding control eIPSC (recorded in the absence of the antagonists). A2 and B2, histograms representing the mean ± s.e.m. of the increase in amplitude of each eIPSC within the train in the presence of CGP 54626 + DPCPX for four different neurones coreleasing ATP and GABA (A2) and three distinct neurones which released only GABA (B2). The amplitudes of the effect on the sixth and seventh eIPSCs in A2 were found to be significantly different from that on the other eIPSCs (one-way ANOVA with repeated measures, P < 0.05 and post hoc comparison with Duncan test, P < 0.05). There was no statistically significant difference in the case of neurones releasing only GABA (B2). The HP was 0 mV.
Drugs and application of substances
All substances were prepared as 1000 times concentrated stock solutions. Bicuculline (Sigma, France), strychnine (Sigma, France), TTX (Latoxan, France), 3-aminopropyl(methyl) phosphinic acid (SKF 97541, Tocris, distributed by Bioblock, France), adenosine (Sigma, France) and saclofen (Tocris, Bioblock, France) were prepared in distilled water. D-APV, Sigma, France) was prepared in NaOH (1 M) and 8-cyclopentyl-1,3-dipropylxanthine (DPCPX, Tocris, Bioblock, France) in ethanol. All these stock solutions and pertussis toxin (Sigma, France) were stored at −20 °C. CNQX (Tocris, Bioblock, France) and [S-(R*,R*)]-[3-[[1-(3,4-dichlorophenyl)ethyl] amino]-2-hydroxypropyl](cyclohexylmethyl)phosphinic acid (CGP 54626, Tocris, Bioblock, France) were prepared in dimethyl sulfoxide (DMSO) and stored at 4 °C.
The substances to be tested were dissolved at their final concentrations in extracellular solution just before the recording session and were applied by bath perfusion at a constant rate of 2 ml min−1. The exchange rate of the solutions was relatively rapid since the central chamber (of the culture dish) had a total volume of ≈250 μl.
All statistical results are given as means ± s.e.m. (standard error of the mean). Statistical differences between sets of data were determined using Student's t test or one-way ANOVA (Origin, OriginLab Corp., USA) by setting the confidence interval at 0.95. Differences in cumulative probability distributions of amplitudes or inter-event interval durations of mIPSCs were determined by a Kolmogorov-Smirnov test (program written and kindly supplied by Dr Jean-Luc Rodeau, Strasbourg). In the experiments using electrical stimulation with eight pulses in order to reveal the possible involvement of a phasic control by presynaptic A1 and GABAB receptors, the significance of the effect of DPCPX + CGP 54626 on the amplitude of the eIPSCs evoked by each pulse was determined with a one-way ANOVA with repeated measures (to determine an effect of the factor ‘pulse’) combined with a post hoc comparison (Duncan test) to localize the statistically different elements within the series of pulses (effect of rank order).
RESULTS
Exogenous application of adenosine and GABAB agonists
In order to check for the presence of functional adenosine and GABAB receptors, we first tested the effect of adenosine (10 μm) and of the GABAB receptor agonist baclofen (10 μm) on the amplitude of electrically evoked GABAergic inhibitory postsynaptic currents (eIPSCs, Fig. 1). In all cases tested, adenosine and baclofen reversibly reduced the amplitudes of eIPSCs. The mean percentage reduction of the amplitude of the first eIPSCs of the paired-pulse protocol by adenosine (10 μm) was 27 ± 4 % (n = 16). The response induced by baclofen was mimicked by the selective GABAB agonist SKF 97541 (1 μm) (Towers et al. 2000; Bowery et al. 2002). Since the effects of both agonists were not significantly different (t test, P > 0.05), they were pooled and the mean inhibition of the amplitude of the first eIPSC by the GABAB agonists was of 54 ± 6 % (n = 12). The stimulation by pairs of electrical stimuli induced paired-pulse inhibition (PPI, Fig. 1B) in the majority of neurones tested (84 %, 21 out of 25 cells), but paired-pulse facilitation (PPF, Fig. 1A) was occasionally observed (16 %, 4 out of 25 cells). In all cases, adenosine and GABAB agonists induced a change in the paired-pulse ratio, indicating the involvement of a presynaptic site of action of these substances. The mean changes in paired-pulse ratio (PPR) elicited by adenosine and GABAB agonists were 15 ± 3 % (n = 16) and 25 ± 5 % (n = 12), respectively. During the exogenous application of GABAB agonists we observed that the PPR was increased by 21 ± 3 % in 75 % of the cells (n = 12). This phenomenon is classically expected following the activation of presynaptic inhibitory receptors by exogenous application of a receptor agonist. In the remaining 25 % of the neurones the PPR was decreased by 44 ± 10 % (n = 12). Similarly, during the application of adenosine (10 μm) we observed that the PPR was either increased by 17 ± 1 % in 56 % of the cases (n = 16) or decreased by 12 ± 2 % in 44 % of the cases (n = 16). Our interpretation of the decrease in PPR is that, in those cases, during the application of exogenous agonists, the synaptic release of GABA occurring during the first eIPSC becomes able to modulate the amplitude of the second eIPSC by adding its effect to that of the exogenously applied agonist. This decrease could eventually also involve the action of another inhibitory transmitter such as adenosine. This phenomenon was probably not observed under control conditions because the ambient concentration of adenosine or GABA in the vicinity of the synaptic terminals was too low to reveal a significant effect of the transmitter(s) released synaptically during the first eIPSC on the amplitude of the second eIPSC (see below and Discussion). At present, it is difficult to draw conclusions about the exact mechanism involved in the decrease in PPR observed in some cases. However, the important observation in the context of the present study was that in all cases there was a change in the PPR indicating that both adenosine and GABAB receptor agonists probably acted at presynaptic receptors.
The decay phases of eIPSCs could be fitted by the sum of two exponential functions with time constants designated as τfast and τslow (Table 1). Adenosine or GABAB receptor agonists did not significantly modify either the values of these time constants or the relative contribution of the fast component of the synaptic current to the total amplitude of the eIPSC (Table 1, t test, P > 0.05).
Table 1.
Absence of effect of adenosine and GABAB receptor agonists on the deactivation kinetics of GABAergic elPSCs
| τfast (ms) | τslow (ms) | Afast/ATotal (%) | n | |
|---|---|---|---|---|
| Control | 26 ± 5 | 146 ± 20 | 50 ± 4 | 14 |
| Adensine (10 μm) | 28 ± 10 | 129 ± 8 | 49 ± 4 | 14 |
| Control | 23 ± 6 | 124 ± 6 | 46 ± 7 | 11 |
| Baclofen (10 μm) | 22 ± 8 | 122 ± 26 | 52 ± 7 | 11 |
eIPSCs were recorded at a HP of 0 mV. τfast and τslow represent the fast and the slow exponential decay time constants of eIPSCs amd Afast/ATotal the relative contribution of the amiplitude of the fast component to the total amplitude of the eIPSC. Results are expressed as means ± s.e.m.
Adenosine (10 μm), baclofen (10 μm) and SKF 97541 (1 μm) also reduced the frequency of miniature GABAA receptor-mediated IPSCs (mIPSCs) without affecting their amplitude or kinetic parameters significantly (Fig. 2 and Table 2; t test, P > 0.05), confirming the implication of a presynaptic site of action of these substances.
Figure 2. Effect of A1 and GABAB receptor activation on miniature GABAergic IPSCs (mIPSCs).
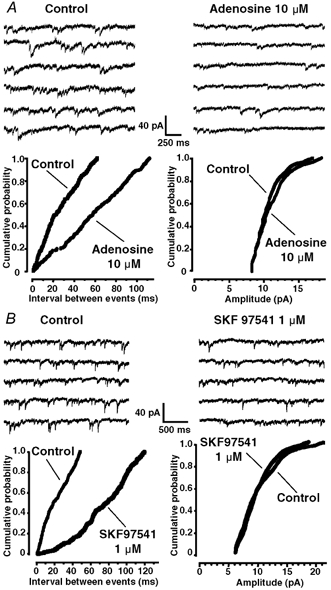
A top, representative traces showing mIPSC activity under control conditions (left) and in the presence of adenosine (10 μm, right). A bottom, cumulative probability histograms of time intervals between successive mIPSCs (left panel) and amplitudes of mIPSCs (right panel), before and during adenosine (10 μm) application. Adenosine shifted significantly the distribution of the inter-event intervals to the right (Kolmogorov-Smirnov test, P < 0.05), indicating a decrease in mIPSC frequency, but did not significantly alter the distribution of mIPSC amplitudes. B top, effect of the GABAB receptor agonist SKF 97541 (1 μm) on GABAergic mIPSCs. In a neurone displaying GABAergic mIPSCs under control conditions (left panel), SKF 97541 (1 μm) reduced the frequency of the synaptic currents (right panel). B bottom, cumulative probability histogram of time intervals between successive mIPSCs (left panel) and amplitudes of mIPSCs (right panel), before and during SKF 97541 (1 μm) application. SKF 97541 significantly changed the distribution of the inter-event intervals (Kolmogorov-Smirnov test, P < 0.05), indicating an decrease in mIPSC frequency, but had no significant effect on the distribution of mIPSC amplitudes. The recordings were performed at a HP of −60 mV and in the presence of 0.5 μm TTX.
Table 2.
Effects of adenosine and GABAB receptor agonists on miniature GABAergic IPSCs (mIPSCs)
| Rise time (ms) | τ (ms) | Amplitude (pA) | Reduction in frequency (%) | n | |
|---|---|---|---|---|---|
| Control | 1.4 ± 0.1 | 28 ± 1 | 11.0 ± 0.2 | ||
| Adenosine (10 μm) | 1.5 ± 0.1 | 33 ± 3 | 11.1 ± 0.2 | 40 ± 8 | 4 |
| Control | 1.4 ± 0.1 | 26.4 ± 4 | 17.5 ± 4 | ||
| Baclofen (10 μm) or SKF 97541 (1 μm) | 1.5 ± 0.2 | 24.6 ± 3 | 16.9 ± 4 | 59 ± 6 | 5 |
mIPSCs were recorded at a HP of –60 mV. τ represents the mono-exponential decay time constant of mIPSCs. The reduction in frequency is expressed as a change in percentage (%) of the frequency in the presence of the agonist with respect to the mean frequency under control conditions. Results are expressed as means ± s.e.m.
Effect of antagonists on responses evoked by exogenously applied agonists
The effect of adenosine (10 μm) on eIPSCs was inhibited by 85 ± 7 % (n = 3) in the presence of 1 μm DPCPX (Fig. 1A) suggesting the involvement of A1 type adenosine receptors (Ralevic & Burnstock, 1998; Fredholm et al. 2001). Similarly, the effect of baclofen (10 μm) was reduced by 85 ± 13 % (n = 3) in the presence of the GABAB receptor antagonist saclofen (100 μm) (Bowery et al. 2002) (Fig. 1B). These results suggested that all GABAergic neurones possess functional presynaptic metabotropic receptors for adenosine (A1) and GABA (GABAB), which inhibit the release of GABA.
Presynaptic A1 and GABAB receptors converge onto a same target
Simultaneous application of A1 and GABAB receptor agonists
We next tested the additivity of the effects mediated by A1 and GABAB receptor activation. SKF 97541 is a highly selective GABAB receptor agonist with a Kd around 20 nm (Bowery et al. 2002) and was applied at a concentration of 1 μm (a concentration of ≈50 times its Kd). As illustrated in Fig. 3, SKF97541 occluded the effect of adenosine in all cells tested (n = 3). SKF 97541 (1 μm) produced a marked inhibition of eIPSCs (59 ± 9 %, n = 3). When adenosine (10 μm) was then applied in the presence of SKF 97541, no significant further inhibition of eIPSCs was observed. The total inhibition observed in the presence of both SKF 97541 and adenosine was 60 ± 0 %, (n = 3) and was not significantly different from that observed with SKF 97541 alone (t test, P > 0.05). There was a full recovery of the amplitude of eIPSCs after washing out the two agonists. When adenosine (10 μm) was subsequently applied alone, it induced a reversible inhibition of eIPSCs (26 ± 1 %, n = 3) in all neurones tested (n = 3), indicating the presence of functional adenosine receptors capable of inhibiting the synaptic release of GABA on these neurones. DPCPX (1 μm) did not modify the effect of SKF 97541 (1 μm) on eIPSCs (mean inhibition: 4 ± 2 %, n = 3) and CGP 54626 (1 μm), a selective antagonist of GABAB receptors (Bowery et al. 2002), did not affect the response to adenosine (10 μm) (mean potentiation: 4 ± 2 % (n = 3).
These results indicated that SKF 97541 occluded the effect of adenosine and suggested that at least the final effector of the pathways activated by A1 and GABAB receptors was in common.
Involvement of a pertussis toxin-sensitive G protein
When the cultures were incubated overnight with pertussis toxin (150 ng ml−1), coapplication of adenosine (10 μm) and SKF 97541 (1 μm) did not significantly inhibit the amplitude of eIPSCs (mean inhibition: 3.6 ± 0.3 %, n = 8), suggesting that both A1 and GABAB receptors couple to a pertussis toxin-sensitive G protein of the Go/Gi type. This was in marked contrast with the situation in non-treated cultures in which an inhibitory effect of adenosine and GABAB receptor agonists on eIPSCs was systematically observed (see above).
Tonic activation of A1 and GABAB receptors
In order to check for the tonic activation of A1 and GABAB receptors, we examined the effect of GABAB receptor antagonist CGP 54626 (1 μm) (Bowery et al. 2002) and of the A1 receptor antagonist DPCPX (1 μm) (Ralevic & Burnstock, 1998; Fredholm et al. 2001) on the amplitude of eIPSCs (Fig. 4). In a total population of 16 cells, application of each antagonist alone or the combined application of both antagonists increased the amplitude of eIPSCs in a statistically significant manner (one-way ANOVA, P < 0.05). The increases in eIPSC amplitudes were as follows: DPCPX (+12 ± 3 %, n = 10), CGP 54626 (+10 ± 3 %, n = 9) and DPCPX+CGP 54626 (+31 ± 3 %, n = 5). The effect of DPCPX + CGP 54626 was significantly larger than the expected algebraic sum of the individual effects of each antagonist (one-way ANOVA, P < 0.05). Moreover, the effects of the antagonists were reversible and accompanied by a change in the paired-pulse ratio indicating a presynaptic action of these substances. The inhibitory effect of endogenous adenosine was probably mediated by the selective activation of A1 receptors, because, at a concentration of 0.1 μm at which it is absolutely selective for A1 receptors (Fredholm et al. 2001), DPCPX increased the amplitude of eIPSCs by 10 ± 2 % (n = 4). This value was comparable to and not significantly different from the increase in eIPSC amplitude induced by DPCPX at a concentration of 1 μm (+12 ± 3 %, n = 10) (t test, P > 0.05).
Figure 4. Supra-additive effect of the A1 receptor antagonist DPCPX and of the GABAB receptor antagonist CGP 54626 revealing a tonic and convergent activation of A1 and GABAB receptors by ambient adenosine and GABA levels.
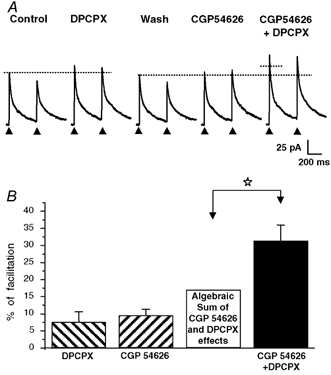
A, application of DPCPX (1 μm) or CGP 54626 (1 μm) alone (i.e. in the absence of exogenous agonists) induced a modest increase in the amplitude of eIPSCs, indicating, however, the presence of a tonic activation of A1 and GABAB under resting conditions. When applied together, DPCPX and CGP 54626 induced a pronounced increase in eIPSC amplitude, which was much larger than the theoretical algebraic sum of the individual effects of each antagonist alone (represented by the dotted horizontal line segment). All recordings were from the same neurone. B, histogram summarizing the effects of DPCPX (1 μm), CGP 54626 (1 μm) and DPCPX (1 μm) + CGP 54626 (1 μm) in four different neuronal pairs tested with the experimental protocol illustrated in A. The theoretical algebraic sum of the individual mean effects of DPCPX (1 μm) and CGP 54626 (1 μm) (open bar) was significantly different from the mean effect of DPCPX (1 μm) and CGP 54626 (1 μm) coapplication determined experimentally (filled bar; paired t test, P < 0.05, white star). The columns represent the mean values and the vertical bars are the s.e.m.s. The HP was 0 mV and the horizontal dotted lines in A indicate the amplitude of the first eIPSC under control conditions and after washing out DPCPX.
These results indicate that under our experimental conditions, synaptic GABA release was placed under a tonic inhibitory control mediated by endogenous adenosine and GABA acting respectively at A1 and GABAB receptors. Moreover, the effects of antagonists were found to be supra-additive, a phenomenon which was indicative of a convergent control of transmitter release involving shared or at least partly common transduction pathways.
Effects of A1 and GABAB receptor antagonists on miniature IPSCs
We next checked the effects of DPCPX (1 μm) and CGP 54626 (1 μm) applied alone or in combination on the frequency of mIPSCs, which are mainly independent of Ca2+ influx through voltage-dependent calcium channels in our preparation (Hugel & Schlichter, 2000). The average increases in mIPSC frequency induced by the antagonists were as follows: DPCPX (21 ± 5 %, n = 5), CGP 56626 (27 ± 6 %, n = 6) and DPCPX + CGP 54626 (613 ± 21 %, n = 3). As observed for eIPSCs (see above), the effect of DPCPX + CGP 54626 was significantly larger than the algebraic sum of the individual effect of each antagonist (one-way ANOVA, P < 0.05).
Phasic activation of A1 and GABAB receptors during repetitive stimulation of the presynaptic neurone
A major issue was to know if synaptically released GABA and adenosine, generated from the extracellular metabolism of synaptically released ATP, were capable of activating presynaptic GABAB and A1 receptors in a phasic (activity-dependent) manner. To test this possibility, we stimulated the presynaptic neurone with trains of eight identical electrical stimuli. The stimuli within the train were separated by 400 ms (2.5 Hz) and the trains were applied every 20 s (0.05 Hz). This protocol was first performed under control conditions and then in the presence of DPCPX (1 μm) plus CGP 54626 (1 μm) to block A1 and GABAB receptors. We then evaluated the relative change (percentage of control) in the amplitude of each eIPSC within the train in the presence of the antagonists. Figure 5 summarizes the results obtained for neuronal pairs in which we detected a corelease of ATP and GABA (n = 4, Fig. 5A) and neuronal pairs which displayed only GABAergic eIPSCs (n = 3, Fig 5B). For neurones coreleasing GABA and ATP, it clearly appeared that the amplitudes of the sixth and the seventh eIPSCs were increased in the presence of DPCPX and CGP 54626. This was confirmed on a statistical basis, since we found a clear effect of the factor ‘pulse number’ (one-way ANOVA with repeated measures, P < 0.05) which was localized to sixth and seventh eIPSCs (post hoc comparison with Duncan test, P < 0.05). Interestingly, when the same protocol was used in the case of pairs of neurones which displayed only GABAergic eIPSCs but no P2X receptor-mediated eEPSCs, we never observed a difference in the magnitude of the potentiation of the eIPSC amplitude as a function of the rank order of the eIPSCs in the presence of DPCPX and CGP 54626 (n = 3, Fig. 5B). In fact, an increase in the amplitude of eIPSCs was apparent, but this increase was proportionally the same for each individual eIPSC within the train and reflected the magnitude of the tonic inhibitory component due to ambient adenosine and GABA levels. On a statistical basis, there was no difference in effect of the antagonists on the different eIPSCs within the train, i.e. no effect of the factor ‘pulse number’ (one-way ANOVA, with repeated measures, P > 0.05).
DISCUSSION
Our experiments, performed on pairs of synaptically connected cultured DH neurones, indicate the presence of functional adenosine A1 and GABAB receptors on the terminals of all GABAergic neurones where their activation converges onto a common effector to inhibit neurotransmitter release. Interestingly, a tonic presynaptic inhibition by ambient GABA and adenosine was noted for all GABAergic neurones but a phasic (activity-dependent) activation of A1 and GABAB autoreceptors was detected only for neurones coreleasing ATP and GABA.
Before discussing our results in more detail, it is important to recall that our culture system uses neonatal rather than more immature embryonic DH neurones. In this model, we have previously shown that the electrical properties, the expression of neurotransmitters/neuropeptides and their receptors by the cultured neurones as well as the functional properties of fast excitatory (glutamatergic) and inhibitory (GABAergic) synaptic transmissions were similar to those described in the most superficial layers of acute spinal cord slices. Moreover, these properties remain stable, at least over the culture period examined (2–3 weeks) (Jo et al. 1998a,b; Jo & Schlichter, 1999; Hugel & Schlichter, 2000). Therefore, we believe that our model is well adapted to address issues concerning the properties of neurotransmitter release and its modulation at the level of synaptic contacts between pairs of isolated and identified superficial DH neurones, a point which remains difficult (problematic) to study in slice preparations. However, we are aware of the fact that the results obtained on synapses formed in culture cannot be directly extrapolated to native networks such as those encountered in slices.
Presence of functional presynaptic A1 and GABAB receptors
Our pharmacological analysis clearly indicated the presence of functional presynaptic A1 and GABAB receptors which were coupled to a pertussis toxin-sensitive G protein and were blocked by their selective antagonists DPCPX (Ralevic & Burnstock, 1998; Fredholm et al. 2001) and CGP 54626 (Bowery et al. 2002). The presence of postsynaptic A1 and GABAB receptors which are functionally coupled to inwardly rectifying potassium (GIRK) channels has been described in DH neurones (Kangrga et al. 1991; Salter et al. 1993; Li & Perl, 1994). However, our experimental conditions prevented the measurement of the consequences of their activation due to the presence of Cs+ in the pipette solution, a condition which blocks K+ currents in the postsynaptic cell from which we recorded. In a previous study, our laboratory has shown the presence of postsynaptic adenosine receptors which could be activated by adenosine generated from the extracellular hydrolysis of ATP and tonically inhibit GABAA receptors in a subset (two-thirds) of DH neurones. This is not in conflict or in contradiction with the present results, which demonstrate the existence of presynaptic adenosine receptors and might be explained by the use of very low concentrations of DPCX (50 nm) in our earlier study. Indeed, at the postsynaptic level, GABAA currents are modulated only by A1 receptors (Jo & Schlichter, 1999) but not by GABAB receptors (authors’ unpublished observations) whereas at the presynaptic level the release of GABA is placed under a convergent/overlapping inhibitory control mediated by both A1 and GABAB receptors (present study). Therefore, the use of a low concentration of DPCPX would efficiently reduce the postsynaptic modulation of GABAA receptors, which is solely mediated by adenosine receptors, whereas its effect at the presynaptic level would be very weak due to the persistence of the GABAB receptor-mediated inhibition in the presence of the A1 receptor antagonist (see below).
The effects of A1 and GABAB receptor activation that we describe here are very similar to those exerted on excitatory and inhibitory transmission in the brain (Scanziani et al. 1992; Thompson & Gahwiler, 1992; Thompson et al. 1992; Shen & Johnson, 1997; Oliet & Poulain, 1999) and the spinal cord (Li & Perl, 1994; Ataka et al. 2000; Chery & De Koninck, 2000; Iyadomi et al. 2000; Lao et al. 2001). We also clearly show the existence of an inhibitory effect of adenosine on GABAergic transmission between DH neurones, a phenomenon which had not been reported before. Moreover, our results indicate a systematic colocalization of A1 and GABAB receptors at least on the terminals of GABAergic neurones, a situation which is in agreement with the high density of expression of A1 and GABAB receptors in the superficial DH of the spinal cord (Reppert et al. 1991; Towers et al. 2000).
Interaction of presynaptic A1 and GABAB receptors
An original and novel finding of our study was the discovery, at the level of unitary synaptic connections, of a functional interaction between A1 and GABAB autoreceptors which was revealed under basal conditions (tonic inhibition) as well as during the action potential-dependent release of ATP and GABA (phasic inhibition). SKF 97541 is a highly selective GABAB receptor agonist with an affinity in the low nanomolar range (≈20 nm) (Bowery et al. 2002). When GABAB receptors were activated by the application of a high (close to saturation) concentration of SKF 97541 (1 μm), adenosine induced no further significant reduction of eIPSCs despite the presence of functional adenosine receptors (see Fig. 3). These observations suggested that both receptor types engaged at least partially common and/or convergent signalling pathways. A similar occlusive presynaptic effect has been reported in the retino-tectal pathway of the goldfish in the case of group II metabotropic glutamate receptors (mGluRs) and A1 receptors (Zhang & Schmidt, 1999) and was correlated with the convergent modulation of N-type voltage-dependent Ca2+ channels. In principle the two pathways engaged by A1 and GABAB receptors in our system could converge at several levels of the transduction cascade which include the receptors (Devi, 2001), the G proteins (Sodickson & Bean, 1998; Leaney & Tinker, 2000), the second messengers (Gerber & Gahwiler, 1994) and/or the target ion channels (Zhang & Schmidt, 1999). From our results, it is not possible to determine precisely at which level(s) this interaction occurs. However, our results clearly suggest that both receptor systems act on a common target channel population. This was attested by the fact that during exogenous application of high concentrations of A1 and GABAB agonists, we observed an occlusion of the effects mediated by these agonists. Similarly, an occlusive effect was detected at low concentrations of endogenous adenosine and GABA such as those responsible for the tonic inhibition of eIPSCs. In this case, the occlusion was revealed by the supra-additive effect of A1 and GABAB antagonists on the amplitude of eIPSCs. Indeed, if ambient adenosine and GABA accumulate close to synaptic terminals and activate A1 and GABAB receptors in a convergent manner and modulate a common population of effectors (e.g. the same population of Ca2+ or K+ channels), it is expected that the application of an antagonist of one receptor type (e.g. A1) will produce a modest effect because the common final effector channel population will still be modulated by the other tonically activated receptor pathway (i.e. GABAB). Therefore, the algebraic sum of the individual effects of each antagonist must be smaller than the effect of both antagonists applied together. This was precisely what we observed. By contrast, if a synergistic interaction of the two pathways occurred, then the algebraic sum of the effects of each antagonist applied alone should be larger than the magnitude of the effect induced by the coapplation of the two antagonists (Gee et al. 2003), a situation that was never observed in our experiments.
Low concentrations of extracellular GABA (in the micromolar range) and adenosine (≈0.1 μm) have been documented in brain slices and cultures of neurones from the CNS including the spinal cord (Prince & Stevens, 1992; Vautrin et al. 1993; Kaneda et al. 1995; Schlichter et al. 2000; Latini & Pedata, 2001; Mody, 2001) and are sufficient to activate significantly GABAB and A1 receptors (Dunwiddie & Masino, 2001; Fredholm et al. 2001; Bowery et al. 2002). Accumulation of GABA can result from the synaptic release of GABA and subsequent diffusion outside the synapse (spillover) or the reversal of a GABA transporter (Cherubini & Conti, 2001) whereas adenosine is generated mainly by the extracellular metabolism of ATP released from neurones or glial cells (Salter et al. 1993; Dunwiddie et al. 1997; Cunha et al. 1998; Zimmermann, 2000; Haydon, 2001). Adenosine can also be released by a transporter-mediated mechanism (Salter et al. 1993; Latini & Pedata, 2001) and there are some indications in favour of a synaptic release of adenosine in the hippocampus (Brager & Thompson, 2003).
Phasic inhibition of GABA-ATP corelease by A1 and GABAB autoreceptors
Using a pulse train stimulation protocol consisting of eight consecutive electrical pulses at 2.5 Hz, we were able to reveal the contribution of A1 and GABAB receptors to an activity-dependent modulation of transmitter release from neurones coreleasing ATP and GABA, but not from GABAergic neurones which apparently did not corelease ATP (absence of P2X receptor-mediated eEPSCs in the postsynaptic neurone). We are aware of the fact that the number of observations in this set of experiments was relatively small (n = 7 with n = 4 for neurones coreleasing ATP and GABA and n = 3 for neurones releasing only GABA). This was essentially due to the high technical difficulty of these experiments. However, it must be emphasized that, in the sample of cells from which we recorded (n = 7), the results were highly reproducible and strictly correlated with the presence or the absence of an ATP-GABA cotransmission. Indeed, an activity-dependent facilitation of eIPSCs by DPCPX and CGP 54626 was observed in all (four out of four) cases of ATP-GABA corelease whereas such an effect was never detected when only GABA was released from the presynaptic neurone (three out of three cases). In the case of neurones coreleasing ATP and GABA, the contribution of A1 and GABAB receptors was only apparent after the sixth electrical pulse, indicating the existence of a delay of about 2 s between the first release of ATP and GABA and the activation of A1 and GABAB autoreceptors. This delay is much longer than that expected for a direct coupling of receptors with ion channels via a G protein (< 1 s) even at non-saturating agonist concentrations (Sodickson & Bean, 1998). We suggest that the extra time (≈1 s) necessary for the A1 and GABAB effect to appear was due (1) in part to the time required for the transformation of ATP into adenosine by ectonucleotidases in the extracellular space (Dunwiddie et al. 1997) and (2) mainly to the time required to reach a significant change in the local concentrations of adenosine and GABA which would be sufficient to induce a detectable change in the convergent modulation of eIPSCs via the activation of A1 and GABAB autoreceptors. We speculate that this effect was not observed in neurones releasing only GABA, because the final concentrations of GABA reached close to GABAB autoreceptors at unitary synaptic connections were not sufficient to significantly alter transmitter release, at least under the prevailing experimental conditions.
Physiological significance
Under resting conditions, ATP and GABA coreleasing synapses have a prominent GABAergic component and can therefore be considered as globally inhibitory (Jo & Schlichter, 1999). Therefore, their selective activity-dependent suppression could favour a net shift of the integrative properties of the postsynaptic neurone towards an excitatory activity. This might be the case during central sensitization of nociceptive pathways and during the development of inflammatory or neuropathic pain states (Millan, 1999; Woolf & Costigan, 1999; Woolf & Salter, 2000). Under these conditions, the selective and activity-dependent suppression of GABAergic transmission and/or GABA-ATP cotransmission might facilitate the expression of long-term potentiation (LTP) in nociceptive pathways (Sandkuhler, 2000). This may occur in a manner comparable to the facilitation of LTP induction in the hippocampus by an activity-dependent recruitment of presynaptic GABAB receptors (Davies et al. 1991) or by the relief of a tonic inhibition on NMDA receptors imposed by Ca2+ influx through P2X receptors (Pankratov et al. 2002). Our results also indicate a strong interaction of GABAB and A1 receptors in the control of transmitter release. Such interactions are also likely to occur in the native tissue although this has to be clearly verified using spinal cord slices. If this were the case, one can speculate that any modulation in the extracelluar concentrations of adenosine and/or GABA could possibly fine-tune the degree of activation of these presynaptic autoreceptors and thereby change the efficacy of this presynaptic control system within the neuronal networks of the superficial dorsal horn. An important issue for further investigations is to know if a similar activity-dependent modulation also applies to the synaptic release of ATP, which acts as a cotransmitter in about two-thirds of GABAergic DH neurones (Jo & Schlichter, 1999; Hugel & Schlichter, 2000). A differential effect on both components could have important functional consequences on the integrative properties of the target neurones which include DH interneurones and eventually the terminals of primary afferent neurones, two cell types in which GABAA and P2X receptors have been shown to interact (Jo & Schlichter, 1999; Sokolova et al. 2001).
Acknowledgments
We thank Mrs Catherine Moreau and Mrs Francine Herzog for excellent technical assistance. This work was supported by grants from the Institut UPSA de la Douleur and the Institut Universitaire de France. We acknowledge additional support from Université Louis Pasteur (Strasbourg) and Centre National de la Recherche Scientifique (CNRS, France). Many thanks to Dr Jean-Luc Rodeau (UMR7519, CNRS, Strasbourg) for his Kolmogorov-Smirnov analysis software.
REFERENCES
- Ataka T, Kumamoto E, Shimoji K, Yoshimura M. Baclofen inhibits more effectively C-afferent than Adelta-afferent glutamatergic transmission in substantia gelatinosa neurons of adult rat spinal cord slices. Pain. 2000;86:273–282. doi: 10.1016/S0304-3959(00)00255-4. [DOI] [PubMed] [Google Scholar]
- Bader CR, Bertrand D, Schlichter R. Calcium-activated chloride current in cultured sensory and parasympathetic quail neurones. J Physiol. 1987;394:125–148. doi: 10.1113/jphysiol.1987.sp016863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, Bonner TI, Enna SJ. Mammalian gamma-aminobutyric acid(B) receptors: structure and function. Pharmacol Rev. 2002;54:247–264. doi: 10.1124/pr.54.2.247. [DOI] [PubMed] [Google Scholar]
- Brager DH, Thompson SM. Activity-dependent release of adenosine contributes to short-term depression at CA3-CA1 synapses in rat hippocampus. J Neurophysiol. 2003;89:22–26. doi: 10.1152/jn.00554.2002. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Conti F. Generating diversity at GABAergic synapses. Trends Neurosci. 2001;24:155–162. doi: 10.1016/s0166-2236(00)01724-0. [DOI] [PubMed] [Google Scholar]
- Chery N, De Koninck Y. GABA(B) receptors are the first target of released GABA at lamina I inhibitory synapses in the adult rat spinal cord. J Neurophysiol. 2000;84:1006–1011. doi: 10.1152/jn.2000.84.2.1006. [DOI] [PubMed] [Google Scholar]
- Cunha RA. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int. 2001;38:107–125. doi: 10.1016/s0197-0186(00)00034-6. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Sebastiao AM, Ribeiro JA. Inhibition by ATP of hippocampal synaptic transmission requires localized extracellular catabolism by ecto-nucleotidases into adenosine and channeling to adenosine A1 receptors. J Neurosci. 1998;18:1987–1995. doi: 10.1523/JNEUROSCI.18-06-01987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CH, Starkey SJ, Pozza MF, Collingridge GL. GABA autoreceptors regulate the induction of LTP. Nature. 1991;349:609–611. doi: 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
- Devi LA. Heterodimerization of G-protein-coupled receptors: pharmacology, signaling and trafficking. Trends Pharmacol Sci. 2001;22:532–537. doi: 10.1016/s0165-6147(00)01799-5. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Diao L, Proctor WR. Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J Neurosci. 1997;17:7673–7682. doi: 10.1523/JNEUROSCI.17-20-07673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Ap IJ, Jacobson KA, Klotz KN, Linden J. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Gee CE, Benquet P, Gerber U. Group I metabotropic glutamate receptors activate a calcium-sensitive transient receptor potential-like conductance in rat hippocampus. J Physiol. 2003;546:655–664. doi: 10.1113/jphysiol.2002.032961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber U, Gahwiler BH. GABAB and adenosine receptors mediate enhancement of the K+ current, IAHP, by reducing adenylyl cyclase activity in rat CA3 hippocampal neurons. J Neurophysiol. 1994;72:2360–2367. doi: 10.1152/jn.1994.72.5.2360. [DOI] [PubMed] [Google Scholar]
- Haydon PG. Glia: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Hugel S, Schlichter R. Presynaptic P2X receptors facilitate inhibitory GABAergic transmission between cultured rat spinal cord dorsal horn neurons. J Neurosci. 2000;20:2121–2130. doi: 10.1523/JNEUROSCI.20-06-02121.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyadomi M, Iyadomi I, Kumamoto E, Tomokuni K, Yoshimura M. Presynaptic inhibition by baclofen of miniature EPSCs and IPSCs in substantia gelatinosa neurons of the adult rat spinal dorsal horn. Pain. 2000;85:385–393. doi: 10.1016/S0304-3959(99)00285-7. [DOI] [PubMed] [Google Scholar]
- Jo YH, Schlichter R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nat Neurosci. 1999;2:241–245. doi: 10.1038/6344. [DOI] [PubMed] [Google Scholar]
- Jo YH, Stoeckel ME, Freund-Mercier MJ, Schlichter R. Oxytocin modulates glutamatergic synaptic transmission between cultured neonatal spinal cord dorsal horn neurons. J Neurosci. 1998a;18:2377–2386. doi: 10.1523/JNEUROSCI.18-07-02377.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YH, Stoeckel ME, Schlichter R. Electrophysiological properties of cultured neonatal rat dorsal horn neurons containing GABA and met-enkephalin-like immunoreactivity. J Neurophysiol. 1998b;79:1583–1586. doi: 10.1152/jn.1998.79.3.1583. [DOI] [PubMed] [Google Scholar]
- Kaneda M, Farrant M, Cull-Candy SG. Whole-cell and single-channel currents activated by GABA and glycine in granule cells of the rat cerebellum. J Physiol. 1995;485:419–435. doi: 10.1113/jphysiol.1995.sp020739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangrga I, Jiang MC, Randic M. Actions of (-)-baclofen on rat dorsal horn neurons. Brain Res. 1991;562:265–275. doi: 10.1016/0006-8993(91)90630-e. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, Karschin A, Bettler B. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- Knight AR, Bowery NG. The pharmacology of adenylyl cyclase modulation by GABAB receptors in rat brain slices. Neuropharmacology. 1996;35:703–712. doi: 10.1016/0028-3908(96)84642-9. [DOI] [PubMed] [Google Scholar]
- Lao LJ, Kumamoto E, Luo C, Furue H, Yoshimura M. Adenosine inhibits excitatory transmission to substantia gelatinosa neurons of the adult rat spinal cord through the activation of presynaptic A(1) adenosine receptor. Pain. 2001;94:315–324. doi: 10.1016/S0304-3959(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79:463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- Leaney JL, Tinker A. The role of members of the pertussis toxin-sensitive family of G proteins in coupling receptors to the activation of the G protein-gated inwardly rectifying potassium channel. Proc Natl Acad Sci U S A. 2000;97:5651–5656. doi: 10.1073/pnas.080572297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Perl ER. Adenosine inhibition of synaptic transmission in the substantia gelatinosa. J Neurophysiol. 1994;72:1611–1621. doi: 10.1152/jn.1994.72.4.1611. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Bowery NG. GABA and its receptors in the spinal cord. Trends Pharmacol Sci. 1996;17:457–462. doi: 10.1016/s0165-6147(96)01013-9. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Misgeld U, Bijak M, Jarolimek W. A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system. Prog Neurobiol. 1995;46:423–462. doi: 10.1016/0301-0082(95)00012-k. [DOI] [PubMed] [Google Scholar]
- Mody I. Distinguishing between GABA(A) receptors responsible for tonic and phasic conductances. Neurochem Res. 2001;26:907–913. doi: 10.1023/a:1012376215967. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Poulain DA. Adenosine-induced presynaptic inhibition of IPSCs and EPSCs in rat hypothalamic supraoptic nucleus neurones. J Physiol. 1999;520:815–825. doi: 10.1111/j.1469-7793.1999.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratov YV, Lalo UV, Krishtal OA. Role for P2X receptors in long-term potentiation. J Neurosci. 2002;22:8363–8369. doi: 10.1523/JNEUROSCI.22-19-08363.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince DA, Stevens CF. Adenosine decreases neurotransmitter release at central synapses. Proc Natl Acad Sci U S A. 1992;89:8586–8590. doi: 10.1073/pnas.89.18.8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Stehle JH, Rivkees SA. Molecular cloning and characterization of a rat A1-adenosine receptor that is widely expressed in brain and spinal cord. Mol Endocrinol. 1991;5:1037–1048. doi: 10.1210/mend-5-8-1037. [DOI] [PubMed] [Google Scholar]
- Salter MW, De Koninck Y, Henry JL. Physiological roles for adenosine and ATP in synaptic transmission in the spinal dorsal horn. Prog Neurobiol. 1993;41:125–156. doi: 10.1016/0301-0082(93)90006-e. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J. Learning and memory in pain pathways. Pain. 2000;88:113–118. doi: 10.1016/S0304-3959(00)00424-3. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Capogna M, Gahwiler BH, Thompson SM. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992;9:919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- Schlichter R, Rybalchenko V, Poisbeau P, Verleye M, Gillardin J. Modulation of GABAergic synaptic transmission by the non-benzodiazepine anxiolytic etifoxine. Neuropharmacology. 2000;39:1523–1535. doi: 10.1016/s0028-3908(99)00253-1. [DOI] [PubMed] [Google Scholar]
- Shen KZ, Johnson SW. Presynaptic GABAB and adenosine A1 receptors regulate synaptic transmission to rat substantia nigra reticulata neurones. J Physiol. 1997;505:153–163. doi: 10.1111/j.1469-7793.1997.153bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodickson DL, Bean BP. Neurotransmitter activation of inwardly rectifying potassium current in dissociated hippocampal CA3 neurons: interactions among multiple receptors. J Neurosci. 1998;18:8153–8162. doi: 10.1523/JNEUROSCI.18-20-08153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova E, Nistri A, Giniatullin R. Negative cross talk between anionic GABAA and cationic P2X ionotropic receptors of rat dorsal root ganglion neurons. J Neurosci. 2001;21:4958–4968. doi: 10.1523/JNEUROSCI.21-14-04958.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Gahwiler BH. Comparison of the actions of baclofen at pre- and postsynaptic receptors in the rat hippocampus in vitro. J Physiol. 1992;451:329–345. doi: 10.1113/jphysiol.1992.sp019167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Haas HL, Gahwiler BH. Comparison of the actions of adenosine at pre- and postsynaptic receptors in the rat hippocampus in vitro. J Physiol. 1992;451:347–363. doi: 10.1113/jphysiol.1992.sp019168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers S, Princivalle A, Billinton A, Edmunds M, Bettler B, Urban L, Castro-Lopes J, Bowery NG. GABAB receptor protein and mRNA distribution in rat spinal cord and dorsal root ganglia. Eur J Neurosci. 2000;12:3201–3210. doi: 10.1046/j.1460-9568.2000.00237.x. [DOI] [PubMed] [Google Scholar]
- Vautrin J, Schaffner AE, Barker JL. Tonic GABA secretion of cultured rat hippocampal neurons rapidly transformed by Zn2+ into quantal release. Neurosci Lett. 1993;158:125–129. doi: 10.1016/0304-3940(93)90245-g. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Costigan M. Transcriptional and posttranslational plasticity and the generation of inflammatory pain. Proc Natl Acad Sci U S A. 1999;96:7723–7730. doi: 10.1073/pnas.96.14.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Wu LG, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- Zhang C, Schmidt JT. Adenosine A1 and class II metabotropic glutamate receptors mediate shared presynaptic inhibition of retinotectal transmission. J Neurophysiol. 1999;82:2947–2955. doi: 10.1152/jn.1999.82.6.2947. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]


