Abstract
The serum- and glucocorticoid-induced kinase-1 (sgk1) increases the activity of a number of epithelial ion channels and transporters. The present study examines the distribution and subcellular localization of sgk1 protein in the rat kidney and the regulation of levels of expression induced by steroids. The results indicate that the kidney expresses predominantly the sgk1 isoform with a distribution restricted to the thick ascending limb of Henle, distal convoluted, connecting and cortical collecting tubules. Within cells, sgk1 strongly associates with the microsomal fraction of homogenates and it colocalizes with the Na+,K+-ATPase to the basolateral membrane. Analysis of the levels of expression of sgk1 by Western blotting and immunohistochemistry indicates constitutive high expression under basal conditions. Approximately half of the basal level is maintained by glucocorticoids whereas physiological fluctuations of aldosterone produce minor changes in sgk1 abundance in adrenal-intact animals. These results do not support the notion that physiological changes of aldosterone concentration turn the expression of sgk1 ‘on and off’ in the mammalian kidney. Additionally, localization of sgk1 to the basolateral membrane indicates that the effects mediated by sgk1 do not require a direct interaction with the ion channels and transporters whose activity is modulated, since most of these proteins are located in the apical membrane of renal epithelial cells.
sgk1 is a serine and threonine kinase closely related to protein kinase B, also known as Akt, protein kinase C, ribosomal protein S6 kinase and cyclic AMP-dependent protein kinase (Webster et al. 1993). sgk1 is important in the kidney because it increases the activity of ion channels and transporters involved in Na+ reabsorption. The epithelial Na+ channel (ENaC) (Chen et al. 1999; Naray-Fejes-Toth et al. 1999; Alvarez de la Rosa et al. 1999; Shigaev et al. 2000; Alvarez de la Rosa & Canessa, 2003), the sodium-potassium-two chloride cotransporter (NKCC) (Lang et al. 2000) and the Na+,K+-ATPase (Setiawan et al. 2002) are activated by co-expression with sgk1 in cultured cells or in Xenopus oocytes.
sgk1 is regulated at two different levels: induction of mRNA transcription and activation of the protein by phosphorylation. Serum (Webster et al. 1993), glucocorticoids and aldosterone (Webster et al. 1993; Chen et al. 1999; Naray-Fejes-Toth et al. 1999, 2000), hypo- and hyperosmolarity (Waldegger et al. 1997, 1999; Rozansky et al. 2002), follicle stimulating hormone (Alliston et al. 1997) and various growth factors (fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), tetradecanoyl phorbol-13-acetate (TPA) and transforming growth factor β (TGF-β1)) (Waldegger et al. 1999; Mizumo & Nishida, 2001) enhance transcription of the gene. Increases in phosphatidylinositol (3,4,5)-trisphosphate (PtdInsP3) levels activate the 3-phosphoinositide-dependent kinases, PDK1 and PDK2, which phosphorylate sgk1 at positions Thr256 and Ser422, respectively (Kobayashi & Cohen, 1999; Park et al. 1999), rendering sgk1 active. Other pathways independent of PtdInsP3, such as cell-cell and matrix interactions and phosphorylation by PKA (Perrotti et al. 2001; Lang & Cohen, 2001; Shelly & Herrera, 2002), have also been reported to activate sgk1.
Most studies in the kidney have focused on the regulation of sgk1 mRNA expression by aldosterone and glucocorticoids. In situ hybridization experiments have revealed the presence of sgk1 mRNA in the cortex, including glomeruli and distal tubules, the medulla and, with the highest abundance, in the renal papilla (Chen et al. 1999; Lang et al. 2000; Bhargava et al. 2001; Hou et al. 2002). These studies and Northern blot analyses have also shown increases in mRNA abundance after administration of exogenous glucocorticoids or aldosterone. There is also evidence that aldosterone may promote sgk1 activation by directly increasing the cellular levels of PtdInsP3 (Blazer-Yost et al. 1999; Paunescu et al. 2000), though the mechanisms involved are still unknown.
When cultured cells are grown in the absence of serum and steroids the levels of sgk1 protein are undetectable, but addition of aldosterone (Chen et al. 1999) or dexamethasone (Webster et al. 1993; Kobayashi et al. 1999) rapidly induces expression. The above findings have prompted the hypothesis that aldosterone turns ‘on and off’ the expression of sgk1 in the kidney. sgk1 then mediates the early aldosterone response by increasing the abundance of ENaC in the apical membrane of distal tubules (Loffing et al. 2001). Several mechanisms have been proposed for the effects of sgk1: translocation and incorporation of channels into the plasma membrane (Alvarez de la Rosa et al. 1999; Loffing et al. 2001), reduction of the rate of retrieval (Debonneville et al. 2001; Snyder et al. 2002) and increases in channel open probability (Vuagniaux et al. 2002). Whether sgk1 modulates the activity of the NKCC and the Na+,K+-ATPase by the same mechanisms has not been explored.
The purposes of this work are to determine the distribution of sgk1 protein in the kidney and to examine whether physiological fluctuations of aldosterone concentrations turn ‘on and off’ expression of sgk1.
METHODS
Generation of sgk1 antibody
A glutathione-S-transferase (GST) fusion protein was generated in pGEX plasmid (Amersham Pharmacia Biotech) encompassing residues Ser301 to Ser404 from the mouse sgk1 sequence. The fusion protein was produced in Escherichia coli, purified by affinity chromatography with glutathione agarose beads (Sigma) and injected every 4 weeks in the subcutaneous tissue of white New Zealand rabbits. Sera were collected after the third injection. All the procedures involving animals in this study were approved by Yale University Committee for the Use and Care of Animals, protocol number 07724.
Other antibodies and reagents
Calbindin and FLAG monoclonal antibodies were purchased from Sigma and anti-actin monoclonal antibody from Chemicon. Anion exchanger (AE1) monoclonal antibody, MAb 12B11 (Alper et al. 1997) was a gift of Dr D. Biemesderfer, Department of Nephrology, Yale University, USA. NKCC monoclonal antibody (T9) was a gift of Dr B. Forbush (Lytle et al. 1995), Department of Physiology, Yale University, USA. Monoclonal antibody α5 against the α subunit of the Na+,K+-ATPase, developed by Dr D. Fambrough (Lebovitz et al. 1989), was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the Department of Biological Sciences, University of Iowa, USA. Antibody conjugates used were: AlexaFluor488 goat anti-rabbit IgG (H + L) (Molecular Probes, Eugene, OR, USA); anti-mouse IgG (whole molecule) Cy3 conjugate F(ab’) fragment of sheep antibody (Sigma). Fluorescent deoxyribonuclease I conjugated to Texas Red was from Molecular Probes.
Animal treatments
Adrenalectomy and dexamethasone replacements were performed as previously described (Stanton et al. 1985). Briefly, Sprague-Dawley rats (Charles River, Boston, MA, USA), weighing 200–250 g were anaesthetized by ether inhalation for surgical preparations. After the operation, animals were placed on a temperature-controlled table at 37 °C and carefully monitored until fully recovered from anaesthesia, after approximately 5 min. Animals were divided into six groups: (1) control: sham adrenalectomized rats served as adrenal-intact controls. (2) ADX: rats were bilaterally adrenalectomized. (3) ADX plus dexamethasone: rats were bilaterally adrenalectomized and osmotic minipumps (ALZET no. 2002; Alza Corp., Palo Alto, CA, USA) were inserted subcutaneously into the neck. Dexamethasone was supplied by minipump at a concentration of 0.012 mg kg−1 day−1 for 1 week. NaCl (0.9 %) was given in drinking water to all ADX rats. (4) 1 week of low-salt diet (ICN, Cleveland, OH, USA). (5) single subcutaneous injection of aldosterone (0.002 mg kg−1) (animals were killed 3 h after injection). (6) single subcutaneous injection of spironolactone (5 mg kg−1)(animals were killed 3 h after injection). After each treatment rats were anaesthetized by I.P. administration of pentobarbital (1000 mg kg−1), followed by cervical dislocation after which the kidneys were immediately removed and processed for Western blotting.
Immunofluorescence microscopy
Rats were anaesthetized with sodium pentobarbital (65 mg kg−1, I.P.) and perfused via the left ventricle with Hanks’ balanced solution, with drainage from the severed inferior vena cava, until the kidneys were thoroughly blanched. Rats were then perfusion-fixed with 2 % paraformaldehyde-75 mm lysine-10 mm sodium periodate (PLP). Kidneys were excised and further fixed in PLP overnight at 4 °C. Fixed tissue blocks were washed four times with PBS and were infiltrated with 30 % sucrose in PBS, frozen in liquid nitrogen, and cut into 10 μm-thick sections with a Leica CM3050S cryostat. For 1 μm-thick sections, fixed blocks were infiltrated with PVP (50 % polyvinylpyrrolidone in 2.3 M sucrose in phosphate buffer) for 1–2 h, then cut into 1–2 mm square pieces, which were frozen on nails in liquid nitrogen for processing with a Leica Ultracut UCT. Sections (1 μm) were cut at −80 °C and were picked up in a wire loop of 2.3 M sucrose and placed on microscope slides (Electron Microscopy Sciences). Slides were stored at −20 °C until needed.
For indirect immunofluorescence, sections were preincubated at room temperature in PBS for 10 min, in 1 % bovine serum albumin in PBS for 15 min, then incubated at room temperature for 1–2 h with primary antibody as indicated. Anti-sgk1 antibody (1:2000) required 0.1 % SDS in the incubation medium. In some cases peptide antigens were included in the incubation mix at 12 μg ml−1. After washes with PBS, sections were incubated for 1 h with fluorophore-conjugated secondary antibodies (10–15 μg ml−1), again washed three times for 5 min each in PBS, and mounted in 50 % glycerol in PBS, pH 7.5, containing 2 % n-propyl-gallate as an antiquenching agent. Sections were examined with a Zeiss Axiophot and digital images were acquired with an AxioCam HRm. Images were compiled with Adobe Photoshop 7.0.
cDNA constructs
Mouse sgk1, sgk2 and sgk3 cDNAs were cloned by RT-PCR from mouse kidney polyA+ using primers designed according to the sequences published in the GenBank database. The cDNAs were tagged at the carboxy termini with the FLAG epitope and were ligated to pCDNA3.1 TOPO (Invitrogen). All constructs were sequenced at the Yale Keck DNA Sequencing Facility.
Culture and transient transfection of HEK 293 cells
HEK 293 cells grown on 60 mm dishes to 90 % confluence were transfected with sgk1, sgk2 or sgk3 cDNAs with Lipofectamine 2000 Reagent following the instructions provided by the manufacturer (Invitrogen). After 24 h, cells were harvested in 1 ml of PBS, recovered by centrifugation (2000 r.p.m. (1000 g) for 1 min), and lysed in 50 mm Tris pH 7.4, 50 mm NaCl, 1 % Triton X-100 and protease inhibitors (Complete, Roche). Protein concentration was quantified using the BCA Protein Assay Kit (Pierce).
Electrophoresis and immunoblotting of membranes
Rats were killed as indicated previously, kidneys from control and treated rats were removed, the cortex and medulla were dissected, minced and each region was homogenized in 50 mm Hepes, pH 7.4, 1.7 % sucrose and protease inhibitors, and centrifuged at 1000 g for 10 min at 4 °C. After collection of the supernatants the sucrose concentration was brought to 8 % and the samples were centrifuged at 250 000 g for 30 min in an Optima-TLX ultracentrifuge (Beckman). The supernatant and pellet were assayed for protein concentration using the BCA Protein Assay Kit (Pierce). In the indicated experiments, the pellet was resuspended in buffers containing one of the following: (1) 0.3 M sucrose; (2) 0.1 M Na2CO3, pH 11.5; (3) 0.5 M NaCl; (3) 2 M urea; or (4) 1 % Triton X-100 (Okamoto et al. 2000). Equal amounts of proteins were resolved on 10 % SDS-polyacrylamide gels and transferred to poly(vinylidenefluoride) (PVDF) membranes (Immobilon-P, Millipore). Membranes were blocked with 5 % non-fat dry milk in TBST (20 mm Tris, pH 7.6, 120 mm NaCl, 0.1 % Tween) for 30 min and incubated with anti-sgk antibody at a dilution of 1:8000 for 2 h at RT. After three 15 min washes with TBST, membranes were incubated with a 1:10000 dilution of horseradish peroxidase-labelled anti-rabbit secondary antibody (Sigma) for 1 h at RT. Membranes were washed three times with TBST, signals were developed with ECL+ (Amersham Pharmacia Biotech) according to the manufacturer's protocol and exposed to BioMax MR film (Eastman Kodak). When indicated, PVDF membranes were reprobed with additional primary antibodies after stripping away the previous antibodies. This was accomplished by incubating the membranes in 2 % SDS, 50 mm Tris (pH 6.8) and 100 mmβ-mercaptoethanol for 30 min at 60 °C. The signals were quantified by scanning densitometry of X-ray films using a GS-800 Calibrated Densitometer (BioRad).
Isolation of RNA and Northern blotting
Total RNA was isolated from kidneys of rats from control, low-salt diet, ADX and single dose of aldosterone groups using Trizol Reagent (Invitrogen). Total RNA was quantified and 20 μg RNA of each condition was loaded onto 1 % agarose gels containing formaldehyde (Sambrook et al. 1989). After electrophoresis, RNA was transferred to nylon membranes (Amersham) by capillary transfer in 20 × SSC buffer. RNA was cross-linked to membranes with UV light (UV Stratalinker 2400, Stratagene) prior to hybridization. Hybridization was performed at 42 °C in 50 % formamide, 6 × SSC buffer, 2 × Denhardt's reagent, 0.1 % SDS, denatured salmon sperm DNA and denatured full length cDNA of sgk1, sgk2 or sgk3 radiolabelled with [32P]α-dCTP by random priming (specific activity > 109 c.p.m. μg−1). Membranes were washed sequentially with 1 × SSC buffer, 0.1 % SDS at 42 °C and 0.2 × SSC buffer, 0.1 % SDS at 68 °C. Membranes were exposed to X-ray film at −70 °C with an intensifying screen for 12 h.
RESULTS
Characterization of sgk antibody
The ability of the serum to recognize sgk was tested by Western blotting. Proteins were extracted from kidney of control rats, and from HEK 293 cells mock transfected or transiently transfected with mouse sgk1, sgk2 or sgk3 cDNAs tagged in the carboxy terminus with the FLAG epitope, and processed for Western blotting. Membrane blots were sequentially probed, first with our rabbit anti-sgk antibody followed by stripping and reprobing with anti-FLAG monoclonal antibody. The left and middle panels in Fig. 1 show that the anti-sgk antibody recognizes all three isoforms although it has a higher specificity for sgk1. Densitometric quantification of the intensity of the bands normalized to sgk1 indicated that the antibody preferentially recognizes sgk1 (1.0) over sgk2 (0.4) and sgk3 (0.6).
Figure 1. Specificity of the anti-sgk antibody and identification of sgk isoforms expressed in the kidney.

Western blots of proteins extracted from HEK 293 cells transfected with sgk1, sgk2 and sgk3 cDNAs, control (non-transfected cells), and renal tissue examined with anti-FLAG monoclonal and anti-sgk antibodies. The lane labelled kidney contains 5-fold more protein than the lanes loaded with HEK 293 cell lysates. The arrows on the left indicate molecular masses of standards.
To note in Fig. 1 are the different electrophoretic mobilities of the three isoforms. The right panel shows that in the kidney the molecular mass of the band corresponds to that of sgk1, thus the kidney expresses predominantly the sgk1 isoform.
Localization of sgk1 in the rat kidney
The distribution of sgk1 in control rat kidneys was examined by fluorescence immunohistochemistry of 10 μm-thick sections using a 1:2000 dilution of sgk rabbit serum as the primary antibody. Figure 2A shows a sagittal view of the whole kidney. Tubules in the cortex and in parallel arrays of the outer portion of the medulla exhibited strong immunoreactivity. The signal markedly decreased at the junction with the inner medulla and was undetectable in the papilla. A higher magnification of the cortex (Fig. 2B) showed fluorescence in distal tubules but not in proximal tubules or in glomeruli. A higher magnification of the outer medulla (Fig. 2C) showed a signal in tubules formed by tall cells consistent with the morphology of thick ascending limb (TAL) but other tubules were negative. Figure 2D shows the inner medulla, where the signal was very faint.
Figure 2. Distribution of sgk1 in kidneys from normal rats examined by immunohistochemistry with anti-sgk antibody.
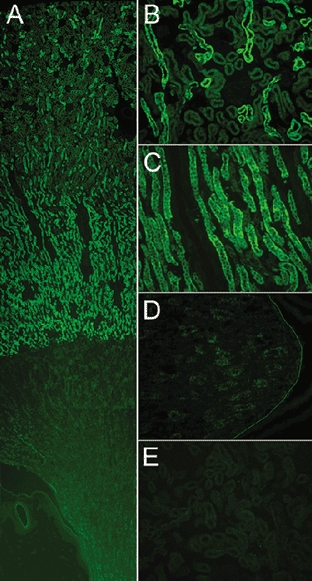
A, low magnification of a sagittal view of the kidney shows cortex, medulla and papilla. B, higher magnification of cortex; immunoreactivity is detected in distal tubules but not in proximal tubules or glomeruli. C, outer medulla; fluorescent signal localizes to arrays of tubules formed by tall cells, consistent with the thick ascending limb (TAL). There is a marked and sharp decrease in immunoreactivity in the transition from outer to inner medulla. D, papilla. A line was drawn to visualize the limit of the papilla. E, competition of primary antibody with the cognate peptide eliminates fluorescent signal from the renal cortex.
As a control for specificity, we pre-incubated the primary antibody with the cognate peptide for 3 h prior to addition of the antibody to the sections; all other steps in the procedure were identical. Competition of the primary antibody completely eliminated the fluorescence signal (Fig. 2E). Immunostaining with the pre-immune serum was also negative.
Double-staining of kidney with tubule-specific markers and anti-sgk antibodies
To identify the tubules expressing sgk1 we performed double-labelling with antibodies for sgk and markers specific for tubule segments. First, we used a monoclonal antibody against calbindin, a calcium-binding protein expressed primarily in the distal convoluted and connective tubules (DCT and CT, respectively). The images in the left column in Fig. 3 show double-labelling of renal cortex with anti-sgk rabbit antibody (A), calbindin monoclonal antibody (B) and the overlay of the these two images (C). Anti-calbindin antibody labelled some of the sgk1-positive tubules in the cortex, thus these tubule segments correspond to DCT and/or CT. In these tubules the apical side of the cells exhibited a stronger calbindin signal whereas the basolateral side was stronger for sgk1 (Fig. 3D). The arrow in Fig. 3C points to a region in a tubule where calbindin expression stops but the signal for sgk1 continues distally.
Figure 3. Double-labelling of sgk1 and calbidin, and sgk1 and NKCC2.
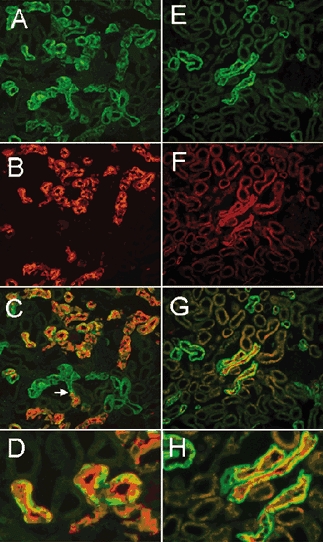
Left-hand panels show renal cortex sections labelled for sgk (green) and calbindin (red). A, sgk. B, calbindin monoclonal antibody as a marker for the distal convoluted tubule (DCT) and connective tubule (CT). C, overlay of A and B shows that all tubules expressing calbindin are also positive for sgk1. The arrow points to the transition of a tubule where immunoreactivity to calbindin becomes positive. D, higher magnification of C shows tubules with sgk labelling more intense toward the basolateral membrane whereas the signal of calbindin is more intense over the apical membrane. Right -hand panels show cortex sections double-labelled for sgk1 (green) and sodium-potassium-two chloride cotransporter monoclonal antibody (NKCC2) (red). E, sgk. F, NKCC2 monoclonal antibody. Staining is restricted to the apical membrane of a few tubules that correspond to cortical segments of TAL. G, overlay of E and F shows that sgk1 is expressed in all cortical tubules positive for NKCC2. H, higher magnification of G to show tubules with apical NKCC2 and basolateral sgk1 staining.
The images in the right-hand column of Fig. 3 show double-labelling of the renal cortex with anti-sgk1 (E), sodium- potassium-chloride cotransporter (NKCC) monoclonal antibody (F), and the overlay of the these two images (G). The NKCC antibody labelled the apical membrane of a subset of tubules, all of which were also positive for sgk1 (H). These results indicate that sgk1 is expressed in TALs.
Figure 4 shows double labelling with antibodies for sgk and the anion exchanger AE1. AE1 localizes exclusively to the basolateral membrane of acid-secreting intercalated cells and thus serves as a marker for the cortical collecting tubule (CCT) and medullary collecting tubule (MCT). The images in the left-hand column in Fig. 4 correspond to cortex stained with anti-sgk (A), anti-AE1 (B), and the overlay of these two images (C). Many cortical tubules were positive for both sgk1 and AE1 antibodies. But in these tubules, cells labelled with anti-AE1 did not react with the sgk antibody (D), indicating that intercalated cells do not express sgk1 or that they express it at much lower levels. In contrast, in the outer medulla, sgk1 and AE1 immunoreactivities did not colocalize. In this region there is complete segregation of the two proteins indicating that the collecting tubules of the outer medulla do not express sgk1 (Fig. 4E–H).
Figure 4. Double-labelling of sgk1 and AE1.
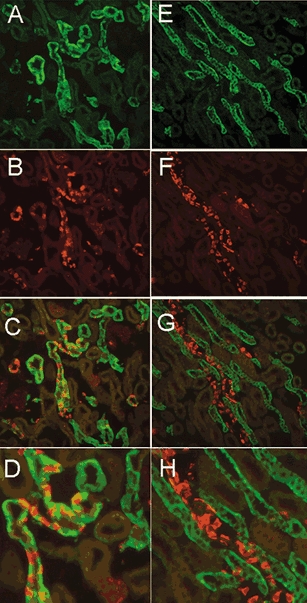
The left and right panels show images from renal cortex and outer medulla, respectively. A, cortex stained with sgk. B, AE1 shows staining of basolateral membranes of intercalated cells in CCT. C, overlay of A and B shows colocalization of sgk1 and AE1 in CNT and CCT. D, larger magnification of a CCT with intercalated cells stained with AE1 and principal cells with sgk1. Outer medulla stained with sgk1 (E), AE1 (F), overlay of E and F (G). H, higher magnification of G shows that sgk1 is not expressed in MCT.
These results indicate that sgk1 has a restricted distribution in the kidney. Tubule segments that express sgk1 are the TAL, starting in the outer medulla, the DCT, CT and CCT; expression stops in the cortical segments of the collecting tubule. Glomeruli, vessels and interstitial cells were devoid of sgk1 immunoreactivity.
Regulation of expression of sgk mRNA and protein by aldosterone and glucocorticosteroids
Previous studies have addressed the effects of cortisol and aldosterone on the levels of sgk1, sgk2 and sgk3 mRNA in the kidney. In situ hybridization and Northern blot analysis have revealed that the mRNA of sgk1 (Chen et al. 1999; Shigaev et al. 2000; Bhargava et al. 2001) but not sgk2 and sgk3 are upregulated by cortisol and aldosterone (Kobayashi et al. 1999). However, the levels of protein expression under basal conditions and after variations in steroid concentrations have not been examined in intact animals.
So far our results indicated that expression of sgk1 protein was high and readily detectable by Western blotting and by indirect immunofluorescence in kidneys of normal rats. To investigate the relative importance of cortisol and aldosterone in the basal levels of sgk1 we first examined the effect of adrenalectomy. This procedure suppresses cortisol and aldosterone but leaves intact other agents known to affect the transcription of sgk1, i.e. serum (Webster et al. 1993), growth factors (Waldegger et al. 1999; Mizumo & Nishida, 2001) and osmolarity (Waldegger et al. 1998). The upper panels of Fig. 5 show immunostaining of kidneys from adrenalectomized animals. To facilitate the identification of the aldosterone-responsive segments (DCT, connecting tubules (CNT), CCT and MCT) the sections were double-labelled with sgk and AE1 antibodies. ADX decreased the intensity of sgk1 signal in both cortex and outer medulla (Fig. 5A and B). Continuous infusion of dexamethasone to ADX rats restored the sgk1 signal to the level observed in normal animals (Fig. 6C and D). Treatment with low-salt diet, known to induce a chronic but physiological increase in the plasma levels of aldosterone, did not change the distribution or the intensity of the sgk1 in the cortex or in outer medulla. In particular, we did not observe increased expression in CCT (Fig. 5E and F).
Figure 5. Distribution and levels of sgk1 expression in animals with various levels of steroids.
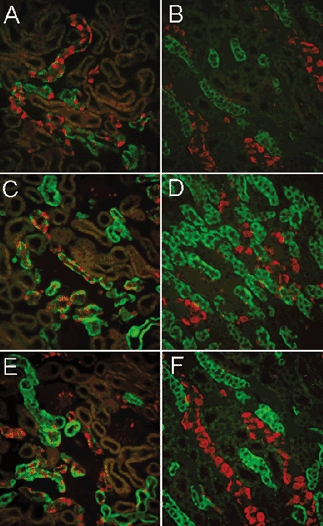
Left and right columns correspond to sections from cortex and outer medulla, respectively. All panels show double-labelling of sgk1 (green) and AE1 (red). A and B, bilateral adrenalectomy with high-salt diet; C and D, bilateral adrenalectomy with dexamethasone infusion by minipump. E and F, low-salt diet for 1 week. Immunoreactivity is reduced by adrenalectomy but levels return to baseline values with administration of dexamethasone. The reduction in sgk1 expression is not selective to the CCT but also is seen in the TAL. Sodium depletion did not increase the signal of sgk1.
Figure 6. Levels of expression of sgk1 analysed by Western blotting.
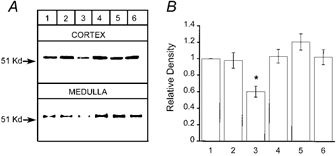
A, representative example of a Western blot. Equal amounts of proteins from renal cortex or medulla of rats subjected to various treatments to alter glucocorticoid and mineralocorticoid concentrations were examined by Western blotting with sgk antibody. B, quantification of the intensity of the sgk1 signal by scanning densitometry of gels (n = 4). Values were normalized to the intensity of the bands from normal rats. Lanes correspond to: (1) control rats, (2) low-salt diet, (3) adrenalectomy, (4) adrenalectomy and dexamethasone infusion by minipump, (5) single dose of aldosterone and (6) spironolactone, 3 h prior to harvesting kidneys. Values are reported as means ±s.d.* Significant statistical difference (Students's t test, P < 0.001).
To quantify the effects of adrenal steroids on the abundance of sgk1 protein we performed Western blot analysis. Using anti-sgk antibody we examined equal amounts of renal proteins from animals previously submitted to various treatments: (1) normal chow; (2) low-salt diet for 1 week; (3) bilateral adrenalectomy with high-salt in the diet for 1 week; (4) bilateral adrenalectomy with continuous dexamethasone infusion by minipump for 1 week; (5) subcutaneous injection of a single dose of aldosterone; or (6) spironolactone. Figure 6A shows representative Western blots from cortex and outer medulla probed with anti-sgk antibody. sgk1 was readily detected in kidneys from control rats but the intensity of the signal decreased after adrenalectomy. The other treatments did not produce statistically significant changes in sgk1 abundance. Figure 6B shows densitometric quantification of sgk1 from cortex normalized to the values obtained in control rats (n = 4). The levels decreased after adrenalectomy (0.6 ± 0.066) and returned to basal levels after dexamethasone supplementation (1.03 ± 0.082). Bilateral ADX was the only manoeuvre that produced a statistically significant change in the levels of expression of sgk1 (Students's t test, P < 0.001).
These results indicated that there was some degree of dissociation between the levels of protein and mRNA, which have been reported to change according to the levels of aldosterone. The results also raised the possibility that our treatments did not change the levels of circulating steroids. To address these issues we performed Northern blot analysis of RNA extracted from kidneys of control rats, treated with low-salt diet for 1 week, or 4 h after receiving a single injection of aldosterone. Similarly prepared blots were examined with probes for sgk1, sgk2, sgk3 and actin. Figure 7 shows that the intensity of sgk1 signal in the control animals is high, in agreement with the robust level of protein expression under basal conditions. sgk1 mRNA levels increased after low-salt treatment and decreased in ADX, hence, the treatments produced the expected changes in aldosterone concentration.
Figure 7. Northern blot analysis of sgk1, sgk2 and sgk3 mRNA from kidney.
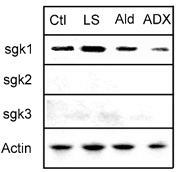
Equal amounts of total RNA (20 μg) extracted from kidneys of treated animals were analysed by Northern blotting using radiolabelled probes for sgk1, sgk2 and sgk3. For accurate quantification of the amount of RNA loaded, each blot was hybridized with an actin probe. All membranes were exposed to X-ray film for 12 h. Signals corresponding to sgk1 mRNA were the only signals detected. The levels changed according to the treatments. Ctl, control; LS, low-salt diet for 1 week; Ald, single injection of aldosterone; ADX, bilateral adrenalectomy.
On the other hand, the levels of sgk2 and sgk3 mRNAs were very low in all the conditions. Faint bands appeared after long exposure, amidst a significant increase in the background. These results are in agreement with our data from Western blots in which we did not detect sgk2 or sgk3 in kidney extracts.
Subcellular localization of sgk1 in renal epithelium
Immunostaining revealed that sgk1 is preferentially distributed toward the basolateral membrane in renal tubules (Figs 2, 3 and 4). Subcellular localization was further investigated with double-labelling of sgk and Na+,K+-ATPase on 1 μm-thick kidney sections. Figure 8A-E shows sections labelled with antibodies to sgk and the α subunit of the Na+,K+-ATPase. The anti- Na+,K+-ATPase antibody delineated basolateral membranes of cells from all tubular segments. The TAL exhibited the strongest signal. Deep and numerous infolds of the basolateral membrane can be identified in the higher-magnification images of the outer medulla (E). Staining with anti-sgk antibody produced a similar basolateral pattern (D). No apical staining was detected. The overlays of images from outer medulla show almost perfect colocalization of sgk1 and Na+,K+-ATPase (C).
Figure 8. Double-staining of kidney sections for sgk1, the Na+,K+-ATPase and actin.
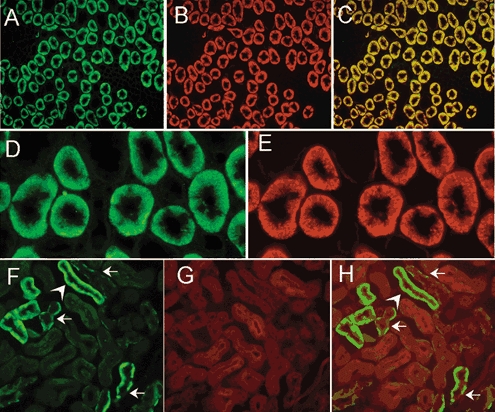
Staining of a transverse section of the outer medulla for sgk1 (A), the α subunit of the Na+,K+-ATPase (B), and overlay of these two images (C) showing colocalization of the two proteins. Higher magnification of the outer medulla stained for sgk1 (D), and the α subunit of the Na+,K+-ATPase (E) are also shown. Deep infolds of the basolateral membrane are delineated by the two antibodies whereas no fluorescent signal is apparent in the apical membrane. F, section of cortex stained with anti-sgk1. Arrows indicate the basolateral localization of the signal. These tubules correspond to CCT because the staining is restricted to principal cells and absent in intercalated cells. Actin labelled with DNase I conjugated with Texas red (G) distributes over the whole cytoplasm with enhancement of apical microvilli. Overlay shows sgk1 in the basolateral membrane but absent from the apical membrane (H).
Figure 8F–H shows sections of cortex labelled with anti-sgk1 antibody and deoxyribonuclease I conjugated to Texas Red, which labels unpolymerized G-actin (Haugland et al. 1994). The arrows point to cells where sgk1 is present only on the basolateral side. These tubules correspond to CCT because some of the cells express only actin, indicating that they are intercalated cells. As expected, actin is distributed over the whole cytoplasm and is concentrated in the microvilli of the apical side. In none of these tubules is there overlap of sgk1 and actin as shown in panel H.
To confirm the association of sgk1 with the basolateral plasma membrane, renal tissues were homogenized in the absence of detergents and centrifuged at high speed (250 000 g for 30 min) to separate the soluble material from membranes and cytoskeletal fraction. Western blotting of proteins from pellet and supernatant revealed that sgk1 was present only in the pellet (Fig. 9A). The pellet contained membranes, cytoskeleton and other non-integral membrane proteins that interact with transmembrane and/or cytoskeletal proteins. Many of the latter proteins can be stripped from the membrane fraction by high pH, high ionic strength or denaturing solutions (Okamoto et al. 2000). We resuspended the initial pellet in 200 μl of one of the following: (1) 0.3 M sucrose, (2) 0.1 M Na2CO3, pH 11.5, (3) 0.5 M NaCl, (4) 2 M urea or (5) 1 % TritonX-100 and the resuspension was centrifuged again at 250 000 g for 30 min. Equal amounts of protein from pellets and from each supernatant were loaded onto a gel and analysed by Western blotting with anti-sgk antibody (Fig. 9B). The first four treatments did not remove sgk1 from the pellet fraction; only 1 % Triton X-100 released sgk1. A similar blot was probed with an antibody for actin, a protein that forms part of the cytoskeleton. Figure 9C shows that treatment of the pellet with 0.1 M Na2CO3, pH 11.5, stripped actin from the pellet but the other treatments did not. Therefore, these results indicate that sgk1 is unlikely to bind to the cytoskeleton, but instead suggest strong interaction with an integral membrane protein.
Figure 9. sgk1 associates with the membrane fraction of kidney homogenates.
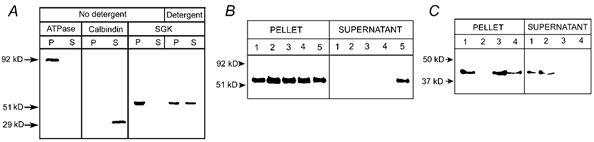
A, kidneys from normal rats were homogenized in the absence of detergent or in the presence of 1 % Triton X-100. The homogenates were centrifuged at 250 000 g for 30 min and equal amounts of proteins from pellet and supernatant were examined by Western blotting with antibodies for the α subunit of Na+,K+-ATPase, calbindin and sgk1. Na+,K+-ATPase (ATPase) and sgk1 were detected only in the pellet of non-detergent homogenates whereas calbindin was detected in the supernatant. After treatment of the pellet with 1 % Triton X-100, sgk1 was partially released to the supernatant. B, the pellet obtained after the first centrifugation (250 000 g for 30 min) was resuspended in 200 μl of: (1) 0.3 m sucrose, (2) 0.1 m Na2CO3, pH 11.5, (3) 0.5 m NaCl, (4) 2 m urea or (5) 1 % Triton X-100, and centrifuged again at 250 000 g for 30 min. Equal amounts of proteins from the pellets and 30 μl from the supernatant were loaded on a gel and analysed by Western blotting with anti-sgk1 antibody. C, Western blot similar to that shown in B, but probed with a monoclonal anti-actin antibody. Arrows indicate migration of molecular mass markers.
DISCUSSION
Relative expression of sgk isoforms in the rat kidney
It has previously been reported that the human kidney expresses mRNA of three sgk isoforms (Kobayashi et al. 1999). Our studies using Northern blot analysis revealed a strong signal corresponding to sgk1 mRNA after 12 h exposure of the membranes to film, whereas no signal was detected for sgk2 and sgk3 at this exposure. Evidence for the presence of small amounts of the latter two mRNAs was obtained by extending the time of exposure of the blot to film for more than 24 h, when faint bands could be resolved, and by recovering sgk2 and sgk3 products by RT-PCR.
These results were consistent with analysis of protein expression. As our anti-sgk antibody cross-reacts with the three isoforms, it was expected to detect all the isoforms, which can be distinguished by their different electrophoretic mobility in Western blots. However, as shown in Figs 1, 6 and 9, only sgk1 was detected in control and treated animals, indicating that sgk1 is the most abundant isoform in the kidney. We cannot rule out that by loading more protein onto gels or by increasing the time of exposure of the Western blot membranes to film we might have detected the other, less abundant isoforms. However, these conditions would have also saturated the sgk1 signal. Thus, we conclude that, in control conditions and in aldosterone-stimulated animals, sgk1 is the predominant isoform expressed by the kidney.
Regulation of sgk1 expression
Previous studies performed in cultured cells indicated that in serum-free medium and in the absence of steroids the basal levels of sgk1 mRNA and protein are very low, sometimes barely detectable (Chen et al. 1999; Faletti et al. 2002; Alvarez de la Rosa & Canessa, 2003). Addition of dexamethasone or aldosterone induced rapid (≈30 min) and large increases in the expression of sgk1. In addition, Loffing et al. (2001) showed that aldosterone triggered expression of sgk1 protein in the CNT and MCT of adrenalectomized rats. These findings have led to the view that in basal conditions the kidney does not express sgk1 and that physiological increases in aldosterone turn the expression from undetectable to high levels. Transient increases in sgk1 could then mediate the early aldosterone response by inducing translocation or activation of ENaC in the apical membrane of principal cells. Such a model could explain the results from microperfusion of isolated CCT (Reif et al. 1986) and patch clamp studies of principal cells (Frindt et al. 2001) that had shown little channel activity in rats fed a normal chow, despite expression of ENaC proteins (Masilamani et al. 1999).
The purpose of this study was to investigate whether physiological fluctuations of aldosterone change the abundance of sgk1 protein in an ‘on and off’ manner. We found that basal levels of sgk1 protein are robust and that manoeuvres that increase aldosterone concentration have little or no effect upon sgk1 abundance. At the transcriptional level, chronic sodium restriction increased mRNA levels as previously reported but this increase did not translate into an equivalent increase in protein. Therefore, the results do not support the current hypothesis of aldosterone as the chief stimulus for regulating sgk1 in the kidney. Moreover, it is difficult to conceive of a mechanism in which a small increase in the abundance of a kinase, from an already high basal level, can trigger the functional effects of the early aldosterone response, unless aldosterone also promotes phosphorylation of pre-existing sgk1.
We then investigated which additional factors sustain the basal levels of sgk1 expression. Suppression of glucocorticoids and aldosterone by adrenalectomy diminished but did not abolish expression of sgk1. However, sgk1 levels returned to normal after infusion of dexamethasone, thus normal levels of glucocorticoids alone are sufficient to induce a high level of sgk1 expression (Naray-Fejes-Toth et al. 2000). Therefore, we conclude that glucocorticoids induce sgk1 expression in vivo. Given the observation that adrenalectomy did not completely suppress expression of sgk1, as 60 % of basal levels remained, we have to postulate that other factors, in addition to steroids, participate in the maintenance of sgk1 levels. Cell adhesion, growth factors, insulin or other hormones present in the serum or in the interstitium surrounding the tubules could induce expression of sgk1 as it has been found in cultured cells (Buse et al. 1999; Mizumo & Nishida, 2001; Shelly & Herrera, 2002).
It is important to notice here that our results differ in the distribution, regulation of expression and subcellular localization of sgk1 from those reported by Loffing et al. (2001). The discrepancies may be explained, at least in part, by the different antibodies used in the two studies. Whereas we present extensive characterization by Western blotting and immunohistochemistry of the anti-sgk antibody, these controls were not provided in Loffing's studies (Loffing et al. 2001).
Together the data indicate that in vivo glucocorticoids, aldosterone and other factors induce sgk1 expression and maintain high constitutive levels of the kinase in the kidney. Most significantly, the results do not support the role of aldosterone as a switch that controls the abundance of sgk1 in the kidney.
sgk1 expression is not restricted to aldosterone-responsive tubule segments
We found sgk1 protein expressed in renal tubules, starting at the beginning of the TAL and ending in the CCT. Expression stopped where collecting ducts enter the outer medulla. sgk1 was absent or present at very low levels in MCT and papilla. This is a surprising result because the papilla is the region of highest tonicity, which was previously shown to be a potent stimulus for transcription of the sgk1 gene (Waldegger et al. 1997, 1998). Moreover, abundant expression of sgk1 mRNA has been documented in this area (Chen et al. 1999; Shigaev et al. 2000; Bhargava et al. 2001). The marked dissociation of mRNA and protein expression in the papilla suggests the presence of mechanisms that differentially suppress protein expression in this area of the kidney but not in the TAL, a nephron segment that is also exposed to a hyperosmolar milieu, but expresses high levels of sgk1.
The results also indicate no correlation between expression of sgk1 and proteins that confer aldosterone responsiveness such as mineralocorticoid receptors and 11β-hydroxysteroid dehydrogenase 2 (11β-HSD2) (Bostanjoglo et al. 1998; Reilly & Ellison, 2000). This is striking in the collecting tubule, where expression of sgk1 is limited only to the cortical segment even though the MCT is known to be a classical target for aldosterone action. On the other hand, expression was prominent in the TAL and the initial DCT although these tubule segments are not major sites of aldosterone action. The finding that sgk1 is not restricted to aldosterone-responsive tubule segments together with the previous result that expression responds to glucocorticoids, suggests that aldosterone is not the main regulator of sgk1 expression in vivo.
The presence of sgk1 in the TAL supports the notion that the sodium-potassium-two chloride cotransporter (NKCC2) may be a physiological target of sgk1 regulation as suggested by experiments in oocytes (Lang et al. 2000). In addition, the presence of sgk1 in the DCT makes it possible that the sodium-chloride cotransporter may also be under physiological regulation by sgk1.
Localization of sgk1 in the basolateral plasma membrane of renal tubule cells
Immnuhistochemical studies detected sgk1 in close proximity to the basolateral membrane whereas it was not detected in the apical membrane of any of the tubule segments. This subcellular localization was further confirmed by colocalization with the Na+,K+-ATPase. Cellular fractionation experiments corroborated association with the microsomal fraction.
A previous report by Brickley et al. (2002) showed that transfected sgk1 in HEK 293 cells, and in several other cell lines, localized to the plasma membrane (Brickley et al. 2002). Our findings extend this observation to the endogenous sgk1 expressed in the kidney and demonstrate sub-localization predominantly to the basolateral membrane of epithelial cells. In addition, we show that sgk1 does not interact with cytoskeletal proteins because these were removed by washing the membranes with a solution of high pH but this did not disrupt the association of sgk1.
What are the implications of these results to the basic mechanism(s) of sgk1 action? Some studies have suggested that sgk1 binds directly to the subunits of ENaC (Wang et al. 2001) or to proteins that interact with the channel, such as Nedd4–2 (Debonneville et al. 2001). However, these mechanisms require localization of sgk1 to the apical membrane. Although we cannot rule out that a small fraction of sgk1 is in close proximity to the apical membrane, the apical fraction must be very small because it was not detected by indirect immunofluorescence whereas the basolateral signal was very intense. Thus, the data favour an indirect mechanism for sgk1-mediated activation of ENaC in which sgk1 phosphorylates other molecules that then transduce the signal to apical channels and transporters.
On the other hand, two observations suggest that sgk1 might be required for maximal activity of ENaC in an aldosterone-independent manner. One observation is the current finding that the abundance of sgk1 does not fluctuate in an ‘on and off’ manner following aldosterone levels. The second is our previous finding that the increase in amiloride-sensitive current induced by constitutively active sgk1 is larger than the one induced by aldosterone in A6 cells (Alvarez de la Rosa & Canessa, 2003). This interpretation is supported by recent findings from mice with inactivation of the sgk1 gene (Wulff et al. 2002). Knockout mice fed a standard diet exhibit subtle changes in renal physiology but at the expense of significantly higher aldosterone levels than wild-type mice, indicating that an increase in aldosterone can maintain normal levels of sodium reabsorption as long as sodium is provided in the diet. On a low-sodium diet, knockout mice develop volume depletion probably because, in the absence of sgk1, sodium channels and transporters in the distal tubule cannot reach maximal activity even with high levels of aldosterone.
In summary, we show the following. (1) sgk1 is the predominant isoform expressed in the rat kidney. (2) sgk1 is restricted to a segment of the renal tubule that encompasses the TAL to the CCT. This distribution does not overlap with the classical aldosterone-responsive tubule segments. (3) sgk1 localizes to the basolateral membrane of epithelial cells. (4) Under basal conditions sgk1 is expressed heavily. Most of the constitutive expression is driven by glucocorticoids whereas increases in aldosterone concentration do not significantly change sgk1 expression.
Acknowledgments
This work was performed during the tenure of a Training Fellowship from the National Kidney Foundation to D. Alvarez de la Rosa and by a grant from the National Institutes of Health, RO1-DK54062, to C. Canessa.
REFERENCES
- Alliston TN, Maiyar AC, Buse P, Firestone GL, Richards JS. Follicle stimulating hormone-regulated expression of serum/glucocorticoid-inducible kinase in rat ovarian granulosa cells: a functional role for the Sp1 family in promoter activity. Mol Endocrinol. 1997;11:1934–1949. doi: 10.1210/mend.11.13.0033. [DOI] [PubMed] [Google Scholar]
- Alper SL, Stuart-Tilley AK, Biemesderfer D, Shmukler BE, Brown D. Immunolocalization of AE2 anion exchanger in rat kidney. Am J Physiol. 1997;274:F601–614. doi: 10.1152/ajprenal.1997.273.4.F601. [DOI] [PubMed] [Google Scholar]
- Alvarez de la Rosa D, Canessa CM. Role of SGK in hormonal regulation of the epithelial sodium channel in A6 cells. Am J Physiol Cell Physiol. 2003;284:C404–414. doi: 10.1152/ajpcell.00398.2002. [DOI] [PubMed] [Google Scholar]
- Alvarez de la Rosa D, Zhang P, Naray-Fejes-Toth A, Canessa CM. The serum and glucocorticoid kinase sgk increases the abundance of epithelial sodium channels in the plasma membrane of Xenopus oocytes. J Biol Chem. 1999;274:37834–37839. doi: 10.1074/jbc.274.53.37834. [DOI] [PubMed] [Google Scholar]
- Bhargava A, Fullerton MJ, Myles K, Purdy TM, Funder JW, Pearce D, Cole TJ. The serum- and glucocorticoid-induced kinase is a physiological mediator of aldosterone action. Endocrinology. 2001;142:1587–1594. doi: 10.1210/endo.142.4.8095. [DOI] [PubMed] [Google Scholar]
- Blazer-Yost B, Paunescu TG, Helman SI, Lee KD, Vlahos CL. Phosphoinositide 3-kinase is required for aldosterone-regulated sodium reabsorption. Am J Physiol. 1999;277:C531–536. doi: 10.1152/ajpcell.1999.277.3.C531. [DOI] [PubMed] [Google Scholar]
- Bostanjoglo M, Reeves WB, Reilly RF, Velazquez H, Robertson N, Litwack G, Morsing P, Dorup J, Bachmann S, Ellison DH, Bostonjoglo M. 11Beta-hydroxysteroid dehydrogenase, mineralocorticoid receptor, and thiazide-sensitive Na-Cl cotransporter expression by distal tubules. J Am Soc Nephrol. 1998;9:1347–1358. doi: 10.1681/ASN.V981347. [DOI] [PubMed] [Google Scholar]
- Brickley DR, Mikosz CA, Hagan CR, Conzen SD. Ubiquitin modification of serum and glucocorticoid-induced protein kinase-1 (SGK-1) J Biol Chem. 2002;277:43064–43070. doi: 10.1074/jbc.M207604200. [DOI] [PubMed] [Google Scholar]
- Buse P, Tran SH, Luther E, Phu PT, Aponte GW, Firestone GL. Cell cycle and hormonal control of nuclear-cytoplasmic localization of the serum- and glucocorticoid-inducible protein kinase, Sgk, in mammary tumor cells. A novel convergence point of anti-proliferative and proliferative cell signaling pathways. J Biol Chem. 1999;274:7253–7263. doi: 10.1074/jbc.274.11.7253. [DOI] [PubMed] [Google Scholar]
- Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, Firestone GL, Verrey F, Pearce D. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci U S A. 1999;96:2514–2519. doi: 10.1073/pnas.96.5.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraibi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Stau B. Phosphorylation of Nedd4–2 by Sgk1 regulates epithelial Na(+) channel cell surface expression. EMBO J. 2001;20:7052–7059. doi: 10.1093/emboj/20.24.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faletti CJ, Perrotti N, Taylor SI, Blazer-Yost BL. sgk: An essential convergence point for peptide and steroid hormone regulation of ENaC-mediated Na+ transport. Am J Physiol Cell Physiol. 2002;282:C494–500. doi: 10.1152/ajpcell.00408.2001. [DOI] [PubMed] [Google Scholar]
- Frindt G, Masilamani S, Knepper MA, Palmer LG. Activation of epithelial Na channels during short-term Na deprivation. Am J Physiol Renal Physiol. 2001;280:F112–118. doi: 10.1152/ajprenal.2001.280.1.F112. [DOI] [PubMed] [Google Scholar]
- Haugland RP, You W, Paragas VB, Wells KS, Dubose DA. Simultaneous visualization of G- and F-actin in endothelial cells. J Histochem Cytochem. 1994;42:345–350. doi: 10.1177/42.3.8308251. [DOI] [PubMed] [Google Scholar]
- Hou J, Speirs HJL, Seckl JR, Brown RW. Sgk1 gene expression in kidney and its regulation by aldosterone: spatio-temporal heterogeneity and quantitative analysis. J Am Soc Nephrol. 2002;13:1190–1198. [PubMed] [Google Scholar]
- Kobayashi T, Cohen P. Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem J. 1999;339:319–328. [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Deak M, Morrice N, Cohen P. Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem J. 1999;344:189–197. [PMC free article] [PubMed] [Google Scholar]
- Lang F, Cohen F. The regulation and physiological roles of serum and glucocorticoid-induced protein kinase. Sci STKE. 2001:RE17. doi: 10.1126/stke.2001.108.re17. [DOI] [PubMed] [Google Scholar]
- Lang F, Klingel K, Wagner CA, Stegen C, Warntges S, Friedrich B, Lanzendorfer M, Melzig J, Moschen I, Steuer S, Waldegger S, Sauter M, Paulmichl M, Gerke V, Risler T, Gamba G, Capasso G, Kandolf R, Hebert SC, Massry SG, Broer S. Deranged transcriptional regulation of cell-volume-sensitive kinase hSGK in diabetic nephropathy. Proc Natl Acad Sci U S A. 2000;97:8157–8162. doi: 10.1073/pnas.97.14.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovitz RM, Takeyasu K, Fambrough DM. Molecular characterization and expression of the (Na++ K+)-ATPase alpha-subunit in Drosophila melanogaster. EMBO J. 1989;8:193–202. doi: 10.1002/j.1460-2075.1989.tb03364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffing J, Zecevic M, Feraille E, Kaissling B, Asher C, Rossier BC, Firestone GL, Pearce D, Verrey F. Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK. Am J Physiol Renal Physiol. 2001;280:F675–682. doi: 10.1152/ajprenal.2001.280.4.F675. [DOI] [PubMed] [Google Scholar]
- Lytle C, Xu JC, Biemesderfer D, Forbush B., III Distribution and diversity of Na-K-Cl cotransport proteins: a study with monoclonal antibodies. Am J Physiol. 1995;269:C1496–505. doi: 10.1152/ajpcell.1995.269.6.C1496. [DOI] [PubMed] [Google Scholar]
- Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest. 1999;104:R19–23. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumo H, Nishida E. The ERK MAP kinase pathway mediates induction of SGK (serum- and glucocorticoid-inducible kinase) by growth factors. Genes Cells. 2001;6:261–268. doi: 10.1046/j.1365-2443.2001.00418.x. [DOI] [PubMed] [Google Scholar]
- Naray-Fejes-Toth A, Canessa C, Cleaveland ES, Aldrich G, Fejes-Toth G. sgk is an aldosterone-induced kinase in the renal collecting duct. Effects on epithelial Na+ channels. J Biol Chem. 1999;274:16973–16978. doi: 10.1074/jbc.274.24.16973. [DOI] [PubMed] [Google Scholar]
- Naray-Fejes-Toth A, Fejes-Toth G, Volk KA, Stokes JB. SGK is a primary glucocorticoid-induced gene in the human. J Steroid Biochem Mol Biol. 2000;75:51–56. doi: 10.1016/s0960-0760(00)00136-9. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Schwab RB, Scherer PE, Lisanti . Analysis of the association of proteins with membranes. In: Bonifacino JS, Dasso M, Harford JB, Lippicott-Schwartz J, Yamada K, editors. Current Protocols in Cell Biology. New York: John Wiley & Sons; 2000. [DOI] [PubMed] [Google Scholar]
- Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, Hemmings BA. Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. EMBO J. 1999;18:3024–333. doi: 10.1093/emboj/18.11.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paunescu TG, Blazer-Yost BL, Vlahos CJ, Helman SI. LY-294002-inhibitable PI3-kinase and regulation of baseline rates of Na+ transport in A6 epithelia. Am J Physiol Cell Physiol. 2000;279:C236–247. doi: 10.1152/ajpcell.2000.279.1.C236. [DOI] [PubMed] [Google Scholar]
- Perrotti N, He RA, Phillips SA, Haft CR, Taylor SI. Activation of serum- and glucocorticoid-induced protein kinase (Sgk) by cyclic AMP and insulin. J Biol Chem. 2001;276:9406–9412. doi: 10.1074/jbc.M007052200. [DOI] [PubMed] [Google Scholar]
- Reif MC, Troutman SL, Schafer JA. Sodium transport by rat cortical collecting tubule. Effects of vasopressin and desoxycorticosterone. J Clin Invest. 1986;77:1291–1298. doi: 10.1172/JCI112433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly R, Ellison DH. Mammalian distal tubule: physiology, pathophysiology, and molecular anatomy. Physiol Rev. 2000;80:277–313. doi: 10.1152/physrev.2000.80.1.277. [DOI] [PubMed] [Google Scholar]
- Rozansky DJ, Wang J, Doan N, Purdy T, Faulk T, Bhargava A, Dawson K, Pearce D. Hypotonic induction of SGK1 and Na+ transport in A6 cells. Am J Physiol Renal Physiol. 2002;283:F105–113. doi: 10.1152/ajprenal.00176.2001. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. New York: Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Setiawan I, Henke G, Feng Y, Bohmer C, Vasilets LA, Schwarz W, Lang F. Stimulation of Xenopus oocyte Na(+), K(+)ATPase by the serum and glucocorticoid-dependent kinase sgk1. Pflugers Arch. 2002;444:426–431. doi: 10.1007/s00424-002-0823-z. [DOI] [PubMed] [Google Scholar]
- Shelly C, Herrera R. Activation of SGK by HGF, RaC1 and integrin-mediated cell adhesion in MDCK cells: PI-3K-dependent and -independent pathways. J Cell Sci. 2002;115:1985–1993. doi: 10.1242/jcs.115.9.1985. [DOI] [PubMed] [Google Scholar]
- Shigaev A, Asher C, Latter H, Garty H, Reuveny E. Regulation of sgk by aldosterone and its effects on the epithelial Na+ channel. Am J Physiol Renal Physiol. 2000;278:F613–619. doi: 10.1152/ajprenal.2000.278.4.F613. [DOI] [PubMed] [Google Scholar]
- Snyder PM, Olson DR, Thomas BC. Serum and glucocorticoid-regulated kinase modulates Nedd4–2-mediated inhibition of the epithelial Na+ channel. J Biol Chem. 2002;277:5–8. doi: 10.1074/jbc.C100623200. [DOI] [PubMed] [Google Scholar]
- Stanton B, Giebisch G, Klein-Robbenhaar G, Wade J, Defronzo RA. Effects of adrenalectomy and chronic adrenal corticosteroid replacement on potassium transport in rat kidney. J Clin Invest. 1985;75:1317–1326. doi: 10.1172/JCI111832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuagniaux G, Vallet V, Jaeger NF, Hummler E, Rossier BC. Synergistic Activation of ENaC by three membrane-bound channel-activating serine proteases (mCAP1, mCAP2, and mCAP3) and serum- and glucocorticoid-regulated kinase (Sgk1) in Xenopus oocytes. J Gen Physiol. 2002;120:191–201. doi: 10.1085/jgp.20028598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldegger S, Barth P, Forrest JN, Jr, Greger R, Lang F. Cloning of sgk serine-threonine protein kinase from shark rectal gland-a gene induced by hypertonicity and secretagogues. Pflugers Arch. 1998;436:575–580. doi: 10.1007/s004240050674. [DOI] [PubMed] [Google Scholar]
- Waldegger S, Barth P, Raber G, Lang F. Cloning and characterization of a putative human serine/threonine protein kinase transcriptionally modified during anisotonic and isotonic alterations of cell volume. Proc Natl Acad Sci U S A. 1997;94:4440–4445. doi: 10.1073/pnas.94.9.4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldegger S, Klingel K, Barth P, Sauter M, Rfer ML, Kandolf R, Lang F. h-sgk Serine-threonine protein kinase gene as transcriptional target of transforming growth factor in human intestine. Gastroenterology. 1999;116:1081–1088. doi: 10.1016/s0016-5085(99)70011-9. [DOI] [PubMed] [Google Scholar]
- Wang J, Barbry P, Maiyar AC, Rozansky DJ, Bhargava A, Leong M, Firestone GL, Pearce D. SGK integrates insulin and mineralocorticoid regulation of epithelial sodium transport. Am J Physiol Renal Physiol. 2001;280:F303–313. doi: 10.1152/ajprenal.2001.280.2.F303. [DOI] [PubMed] [Google Scholar]
- Webster MK, Goya L, Ge Y, Maiyar AC, Firestone GL. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol Cell Biol. 1993;13:2031–2040. doi: 10.1128/mcb.13.4.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff P, Vallon V, Huang DY, Volkl H, Yu F, Richter K, Jansen M, Schlunz M, Klingel K, Loffing J, Kauselmann G, Bosl MR, Lang F, Kuhl D. Impaired renal Na(+) retention in the sgk1-knockout mouse. J Clin Invest. 2002;110:1263–1268. doi: 10.1172/JCI15696. [DOI] [PMC free article] [PubMed] [Google Scholar]


