Abstract
Repetitive transcranial magnetic stimulation (rTMS) has long lasting effects on cortical excitability at the site of stimulation, on interconnected sites at a distance and on the connections between them. In the present experiments we have used the technique of transcallosal inhibition between the motor cortices to examine all three effects in the same protocol. Ten healthy subjects received 900 rTMS stimuli at 1 Hz from a figure of eight coil over the left motor hand area. The intensity of rTMS was above the threshold for inducing short latency interhemispherical inhibition with a single stimulus (equivalent to 115–120 % resting motor threshold). Before and after the rTMS we evaluated: (1) in the left hemisphere, the amplitude of motor-evoked potentials (MEPs), and contralateral and ipsilateral cortical silent periods (CSP, ISP); (2) in the right hemisphere, MEP, CSP, ISP and short-interval intracortical inhibition and intracortical facilitation (SICI/ICF), and (3) interhemispherical inhibition (IHI) from the left-to-right hemisphere using a paired-pulse method. There were two main effects after rTMS to the left hemisphere: first, the amplitude of MEPs from the right hemisphere increased; second, there was a reduction in the IHI from the left-to-right hemisphere at interstimulus intervals of 7 and 10 ms but not at longer intervals (15–75 ms). Control experiments showed that these effects were not due to afferent inputs produced by the muscle twitches induced during the rTMS. The data are compatible with the notion that rTMS to the left hemisphere leads to reduced interhemispherical inhibition of the right hemisphere and a consequent increase in corticospinal excitability in that hemisphere.
Repetitive transcranial magnetic stimulation (rTMS) of the human cortex can induce lasting changes in cortical excitability at the site of stimulation as well as at interconnected sites at a distance (Paus et al. 2001; Strafella et al. 2001). These effects can be revealed by measuring brain activity before and after rTMS with EEG or metabolic imaging techniques (PET, fMRI), or by probing with single pulse TMS. At the present time, the best studied area is the hand representation of the primary motor cortex. Repetitive TMS here leads to after effects in the excitability of corticospinal and intracortical circuits that depend on the intensity, frequency and duration of stimulation (Pascual-Leone et al. 1994; Chen et al. 1997; Maeda et al. 2000a,b; Muellbacher et al. 2000; Touge et al. 2001; Tsuji & Rothwell, 2002). In general, rTMS at 1 Hz tends to decrease corticospinal excitability as assessed with single pulse TMS whereas stimulation at frequencies above 5 Hz may increase excitability.
Stimulation of motor cortex can also produce effects at a distance. For example, Wassermann et al. (1998a) described changes in the opposite motor cortex after 1 Hz stimulation, and Valero-Cabréet al. (2001) have described changes in spinal excitability. PET studies have shown lasting changes in metabolism in SMA as well as contralateral cortex (Siebner et al. 2000). Conversely, effects can be produced in the motor cortex after rTMS of other sites in the brain. For example, Gerschlager et al. (2001) and Munchau et al. (2002) showed that 1 Hz stimulation of the premotor cortex could decrease corticospinal excitability of motor cortex (M1) and change the time course of paired-pulse corticocortical inhibition and/or excitation.
Repetitive TMS can also change the activity in connections between two cortical areas. Jing & Takigawa (2000) used EEG measurements to reveal these changes. They applied short trains of threshold 10 Hz rTMS over the left prefrontal cortex and found that this produced an increase in directed coherence between the stimulated area and parietal sites. Strens et al. (2002) observed an increase in the ipsilateral corticocortical and interhemispherical coherence after 15 min of 1 Hz rTMS at 90 % of the active motor threshold.
Most of the previous studies using rTMS have concentrated on measuring effects at the site of stimulation or at sites at a distance or in the connectivity between them. The aim of the present study was to measure all three variables in order to try to understand the relation, if any, between them. We have concentrated on the interhemispherical connection between the two motor cortices first studied in a paired-pulse protocol by Ferbert et al. (1992). They found that a single conditioning pulse to the hand area of one motor cortex could reduce the excitability of the corticospinal projection from the opposite motor cortex. Although there may be a small subcortical component to this effect (Gerloff et al. 1998), the majority appears to involve transcallosal pathways. The present experiments involved giving rTMS to one hand area and testing the excitability of corticospinal and corticocortical connections in homologous areas of both hemispheres as well as the excitability of the interhemispheric connection between them. We used 1 Hz stimulation since this has been shown to be effective and safe on several previous studies on the hand area. The intensity of stimulation was set so that single stimuli were above the threshold for inducing interhemispherical inhibition. Since activation of interhemispherical projections has a higher threshold than activation of corticospinal projections, this meant that the intensity was about 115–120 % of the resting motor threshold.
METHODS
Subjects
Ten healthy right-handed volunteers (5 women; mean age of all 10, 30 years; range 20–39) were included in the study. All subjects gave their informed written consent before participation. Experimental procedures were approved by the joint ethical committee of the National Hospital for Neurology and Neurosurgery and performed according to the ethical standards laid down in the Declaration of Helsinki. Subjects were seated in a comfortable reclining chair with a mounted head rest during experiments.
Experimental design
The study design is illustrated in Fig. 1. All subjects participated in two experiments on two separate days. In both experiments, a single train of 900 suprathreshold pulses were given to the left M1HAND. The experiment performed on the first day was assigned randomly among subjects. In experiment 1 several measures of corticospinal excitability of the homologous right M1HAND were assessed prior to, and after, a conditioning session of suprathreshold 1 Hz rTMS. Measurements of cortical excitability were carried out at rest and intracortical excitability was assessed with paired-pulse TMS (Kujirai et al. 1993). Two ‘inhibitory’ and two ‘facilitatory’ intervals were tested to quantify the balance between intracortical inhibition and facilitation. In experiment 2 we studied the modulatory effects of suprathreshold 1 Hz rTMS on left-to-right interhemispherical inhibition (Ferbert et al. 1992).
Figure 1. Design of the main experiments and sites of stimulation.
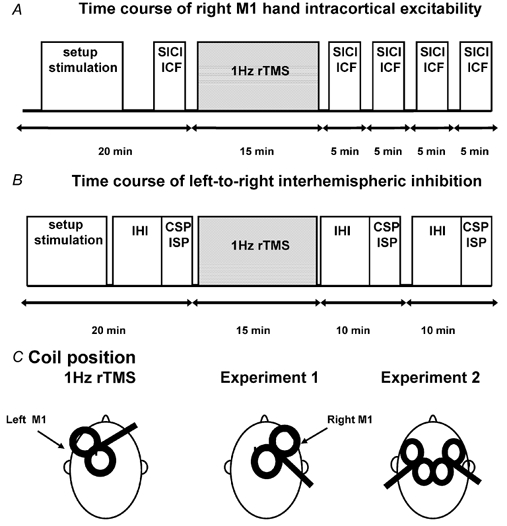
A 900-stimuli train of 1 Hz rTMS at about 120 % RMT was delivered over the left M1HAND using a handle-front orientation of the coil. The SICI/ICF of the right M1HAND (A, experiment 1) and the time course of left-to-right interhemispherical inhibition (B, experiment 2) were examined before and after 15 min rTMS.
Repetitive transcranial magnetic stimulation
Focal rTMS was applied over the left M1HAND with a Magstim Rapid stimulator and a flat figure-of-eight coil with mean loop diameter of 9 cm (The Magstim Company, Whitland, Dyfed, UK). The magnetic stimulus had a biphasic waveform with a pulse width of approximately 300 μs. The coil was placed tangentially to the scalp with the handle pointing antero-medially at a 45 deg angle from the midline. This orientation was chosen, based on the finding that the lowest motor threshold for the rapid Magstim stimulator is achieved when the first upstroke passed the motor cortex in the antero-posterior direction (Kammer et al. 2001). The same orientation is optimal for inducing a silent period in ipsilateral muscles (Meyer et al. 1995). The site of stimulation was optimal to elicit motor-evoked potentials (MEPs) in the right first dorsal interosseous (FDI) muscle. We determined the optimal position for stimulation by moving the coil in 0.5 cm steps around the presumed M1HAND. The site where stimuli of slightly suprathreshold intensity consistently produced the largest MEPs with the steepest negative slope in the right FDI muscle (referred to as ‘motor hot spot’) was marked with a red wax pen by drawing a semilunar line following the anterior bifurcation of the coil and a straight line indicating the orientation of the coil handle. Resting-motor threshold (RMT) was assessed using the Magstim Rapid stimulator as the lowest intensity able to evoke a MEP of more than 50 μV in at least five out of ten consecutive trials in the right FDI (Rothwell et al. 1999). Active-motor threshold (AMT) was defined as the stimulator intensity sufficient to elicit a reliable MEP of at least 200 μV in amplitude in the tonically contracting contralateral FDI muscle in at least five of ten consecutive stimuli.
Each rTMS session consisted of 900 stimuli at 1 Hz (15 min in total). The intensity of stimulation was adjusted to the individual threshold to elicit a left-to-right interhemispherical inhibition (i.e. stimulus intensity that induced an interhemispherical inhibition of about 70 % at an interstimulus interval of 10 ms). Mean stimulus intensity was 117.3 ± 1.5 % of individual RMT, ranging from 100 to 130 %. These stimulation parameters were in accordance with published safety recommendations (Wassermann, 1998b).
Experiment 1: assessment of intracortical excitability of the right M1HAND
The paired-pulse technique described by Kujirai et al. (1993) was employed to probe intracortical excitability of the right M1HAND. Paired-magnetic pulses were generated by two high power Magstim 200 stimulators connected by a Bistim module and delivered through a standard figure-of-eight coil with an outer diameter of each wing of 9 cm placed over the motor hot spot of the right M1HAND (Magstim Company, Whitland, Dyfed, UK). The magnetic stimulus had a nearly monophasic pulse configuration with a rise time of approximately 100 μs, decaying back to zero over approximately 0.8 ms. The coil current during the rising phase of the magnetic field flowed toward the handle. The coil was placed tangentially to the scalp with the handle pointing backwards and laterally at a 45 deg angle to the sagittal plane inducing a posterior-anterior current in the brain. This orientation was chosen based on the finding that the lowest motor threshold is achieved when the induced electrical current in the brain flows approximately perpendicular to the line of the central sulcus (Brasil-Neto et al. 1992; Mills et al. 1992). We determined the optimal position for activation of the left FDI muscles by moving the coil in 0.5 cm steps around the presumed motor hand area of the right motor cortex. The site at which stimuli of slightly suprathreshold intensity consistently produced the largest MEPs in the target muscle was marked as the ‘motor hot spot’ on the scalp with a wax pen. TMS was always given over the motor hot spot.
To avoid a floor effect for paired-pulse inhibition, we set the intensity of the conditioning stimulus to evoke an inhibition of the test MEP to about 50 % at an ISI of 2 ms. The test stimulus was set at an intensity that, when given alone, would evoke an EMG response of ≈1 mV peak to peak. In addition to the unconditioned test stimulus, paired pulses were given at four conditioning-test intervals. ISIs of 2 and 4 ms probed the magnitude of short-interval intracortical inhibition (SICI), whereas ISIs of 9 and 12 ms were used to assess the strength of intracortical facilitation (ICF).
We assessed paired-pulse excitability of the right M1HAND in separate blocks at baseline and four times after the conditioning rTMS session (Fig. 1). The first block started immediately after the end of the 15 min train of 1 Hz rTMS. The second block started approximately 5 min later and the subsequent blocks started approximately 10 and 15 min after the end of 1 Hz rTMS. In each block, 15 trials were recorded for each condition in a pseudorandom order (i.e. test-stimulus alone, paired stimuli at ISI of 2, 4, 9 and 12 ms). Inter-trial interval was set at 4 s.
To measure the relative strength of SICI and ICF, the amplitudes of the conditioned MEPs were measured from peak to peak (mV) and averaged for each condition. Mean peak-to-peak amplitude of the conditioned responses were expressed as a percentage of the unconditioned response (100 %). We averaged the data from the first-second block (i.e. post1-rTMS) and the third-fourth block (i.e. post2-rTMS) after rTMS to compare the time course of SICI/ICF with that of the left-to-right interhemispherical connection.
Experiment 2: assessment of interhemispherical inhibition of the right M1HAND
A conditioning-test protocol as described by Ferbert et al. (1992) was used to evaluate left-to-right interhemispherical inhibition of the right M1HAND. Two high power Magstim 200 stimulators were connected to specially designed figure-of-eight coils with a small outer diameter of each half wing (4 cm). Using the same coil orientation as in experiment 1, the motor hot spot for the small figure-of-eight coils was determined for both the right and left M1HAND. The small diameter of the coils allowed for an optimal placement of the coils over the motor hot spots of the right and left M1HAND.
A conditioning stimulus was applied to the left M1HAND, and the test stimulus was applied to the homologous right M1HAND. We set the intensity of the first (conditioning) stimulus to obtain an inhibition of the test MEP to about 50 % at an ISI of 10 ms. The second (test) stimulus was set at an intensity that, when given alone, would evoke an EMG response of ≈1 mV peak-to-peak amplitude. Left-right IHI was tested at eight conditioning-test intervals (3, 7, 10, 15, 30, 45, 60 and 75 ms). Measurements were carried out in two blocks of 110 trials with an inter-trial interval of 4 s. The various conditions (the test response given alone, or the test stimulus preceded by a conditioning stimulus) were intermixed in a pseudorandom manner in one block. For each block, 30 motor-evoked potentials (MEPs) evoked by the test response alone and 10 MEPs for each condition-test interval were recorded from the left FDI muscle. The amplitudes of the conditioned MEPs were measured peak to peak (mV) in each single trial and averaged for each condition. Peak-to-peak amplitudes of the conditioned responses were expressed as a percentage of the unconditioned response evoked by the test stimulus alone (100 %).
After each measurement of interhemispherical inhibition at rest, we measured the ipsilateral and contralateral silent period (ISP and CSP, respectively) during simultaneous isometric contractions of both FDI muscles at approximately 30 % of maximum force. The level of contraction was controlled by auditory and visual feed back of ongoing EMG activity of the ipsilateral FDI muscle. Single pulses were delivered using a small figure-of-eight coil and the intensity of stimulation was matched to equal the intensity of the test stimulus in the IHI protocol. Fifteen magnetic pulses were applied over the left and right M1HAND every 4 s. Fatigue was avoided by allowing for a break after the seventh trial. The time course of left-to-right IHI, as well as the CSP and ISP were evaluated once before 1 Hz rTMS and twice after rTMS over the left motor area. The first block was performed immediately at the end of the rTMS (post1-rTMS) and the second block started about 10 min after the end of rTMS (post2-rTMS; Fig. 1).
The onset and duration of CSP and ISP were determined in each individual rectified trial. The duration of CSP was measured from the end of the MEP elicited by the suprathreshold TMS pulse to the onset of continuous EMG activity after the period of EMG suppression. In the trials during contraction the mean area of the contralateral MEP was also measured from the onset to the end of the responses in each rectified trial. We also measured peak-to-peak amplitude of each MEP preceding the CSP and calculated the average MEP amplitude of the right FDI muscle during voluntary contraction.
ISP was analysed after averaging and rectifying all 15 trials. The onset of the ISP was defined as the point when EMG activity was reduced by more than one standard deviation of mean EMG amplitude at baseline. The end of the ISP was defined as the point when EMG activity returned back to the level of prestimulus EMG activity. The relative magnitude of EMG suppression during the ISP was expressed as the percentage of the mean area of 30 ms of EMG activity preceding the stimulus artifact.
Control experiment 1: effect of repetitive 1 Hz stimulation of the right ulnar nerve
Suprathreshold 1 Hz rTMS over the left M1HAND produced a twitch in the right FDI muscle. Therefore, we carried out a control experiment in four subjects to study the after effects of suprathreshold repetitive stimulation of the right ulnar nerve on left-to-right IHI. We measured left-right IHI at ISIs of 7 and 10 ms before and after a 15 min train of 1 Hz stimulation of the right ulnar nerve. The conditioning-test protocol to test IHI has been described above. Using a Digitimer D160 stimulator (Digitimer Ltd, Welyn, Herts, UK) we gave bipolar electric stimuli to the ulnar nerve at the wrist with the cathode located proximal to the anode. The intensity was gradually increased until ulnar nerve stimulation evoked a twitch in the right FDI muscle similar in size to that seen during 1 Hz rTMS of the left M1HAND.
Control experiment 2: influence of the amplitude of the test response on IHI evoked by a constant conditioning pulse
Since focal 1 Hz rTMS over the left M1HAND increased the amplitude of MEPs evoked by single-pulse TMS over the right M1HAND, it is conceivable that an increase in test pulse efficacy (i.e. increased size of the unconditioned test response) might have attenuated the magnitude of IHI. Therefore, we studied the impact of an increase in amplitude of the test response on the relative strength of IHI in four subjects. We used three Magstim 200 stimulators. Two stimulators were connected with a Bistim module to randomly deliver test stimuli at two different intensities over the right M1. Stimulus intensities were adjusted to evoke EMG responses of ≈1 mV or 1.5 mV peak-to-peak amplitude in the relaxed left FDI muscle. The intensity of the conditioning stimulus was adjusted to produce an interhemispherical inhibition of about 50 % at an ISI of 10 ms with the unconditioned test response having a peak-to-peak amplitude of about 1 mV. The conditioning stimuli were randomly delivered over the left M1 at 7 and 10 ms before the test stimuli.
Control experiment 3: effect of current direction and intensity of stimulation
To study the effects of the orientation of the coil on cortical excitability, we gave 900 rTMS stimuli at 1 Hz (15 min in total) over the left M1HAND with the coil held tangentially to the skull with the handle pointing 45 deg postero-laterally in three subjects. The intensity of stimulation was adjusted so that a single pulse would produce interhemispherical inhibition of test responses to at least 70 % of their control size at an ISI of 10 ms. Mean stimulus intensity was 115 ± 2.8 % of individual RMT, ranging from 110 to 120 % with RMT assessed using the Magstim Rapid stimulator with a backward orientation of the coil. Before and immediately after the end of the 15 min train an MEP recruitment curve of the right FDI muscle was made using a Magstim 200 stimulator connected to a figure-of-eight coil. The intensity of stimulation was increased with steps of 10 % starting from 110 to 140 % of the RMT. For each intensity, 10 trials were collected with inter-trial intervals of 5 s and averaged.
Control experiment 4: effect of repetitive 1 Hz stimulation on spinal cord excitability
To study the effects of 1 Hz rTMS on spinal cord excitability, we compared the amplitude of the H reflex and MEP in the forearm flexor (FF) muscles before and after 15 min rTMS at 1 Hz over the left M1HAND in two subjects. The intensity of stimulation was adjusted to the individual's threshold to elicit a left-to-right IHI of at least 70 % of the control. The H reflex was obtained by stimulating the median nerve at the elbow using a Digitimer D160 stimulator. Test MEPs were evoked using a Magstim 200 stimulator with a figure of eight coil. For both responses the intensity of stimulation was adjusted to obtain an amplitude of about 1 mV. The EMG activity was recorded from the left FF muscles with surface electrodes. Measurements were made before, immediately after the end and 10 min after the end of the rTMS. For each block, 30 trials were collected with inter-trial intervals of 4 s and averaged.
Recording muscle activity
In all subjects, surface electromyographic (EMG) recordings were made through a pair of Ag-AgCl surface electrodes (1 cm diameter) placed over the left and right FDI muscles, using a belly-tendon montage. Raw signals were amplified and band-pass filtered (3 Hz to 3 kHz) using Digitimer 360 amplifiers with a time constant of 3 ms and a low-pass filter of 3 kHz. Signals were digitized using a CED 1401 laboratory interface (Cambridge Electronic Design Ltd, Cambridge, UK) and stored on a personal computer for later analysis at a sample rate of 5 kHz (Signal 2.0, Cambridge Electronic Design Ltd). Auditory (speakers) and visual (oscilloscope) feedback of EMG activity was given to the subjects to ensure either complete relaxation or tonic voluntary contraction at a constant level.
Statistical analysis
Mean values of each measure of intra and intercortical excitability were entered in separate two-way repeated-measures analyses of variance (ANOVA) with time of measurement (i.e. block of trials: pre-rTMS, post1-rTMS, post2-rTMS) and stimulation condition (i.e. test response alone and conditioned test responses at various ISIs in the main experiments; intensity of stimulation in control experiment (3) as within-subject factors. Post hoc analyses were performed with a Student's paired-samples t test. The Greenhouse-Geisser correction was used when necessary to correct for non-sphericity. A P value of < 0.05 was considered significant for all statistical analysis. All results are given as means ± s.e.m.
RESULTS
None of the subjects reported adverse effects during and after rTMS.
Experiment 1: assessment of intracortical excitability of the right M1HAND
Resting motor threshold of the left M1HAND measured with the biphasic stimulator used to deliver rTMS was 44 ± 2 %. The intensity used for the 15 min, 1 Hz train was 52 ± 2 %, or 119 ± 1 % RMT.
In the right hemisphere the motor threshold measured with a monophasic stimulator was 41 ± 2 % at rest and 32 ± 2 % during contraction. The intensity used in the SICI/ICF protocol was 51 ± 3 % for the test stimulus and 25 ± 2 % for the conditioning stimulus.
Figure 2 illustrates the time course of SICI/ICF before and at the two time points after rTMS of the opposite M1HAND. The data are plotted both as absolute amplitudes in mV peak to peak (Fig. 2A) and as percentage changes relative to the unconditioned MEPs (Fig. 2B). Within-subjects two-way ANOVA on the absolute data values showed a significant main effect of time (F(1.1, 10.6) = 7.2, P = 0.018) and ISI (F(1.3, 12.1) = 15.3, P = 0.001) but there was no significant interaction between them (F(1.7, 15.4) = 1.2, P = 0.3). This indicates that the time course of SICI/ICF was the same before and after rTMS (see also Fig. 2B), but that there was an overall difference in MEP amplitude. Compared with the pre-rTMS values, the mean amplitude of the MEP (averaged across all SICI/ICF intervals) was larger at both time points after rTMS (pre-rTMS vs. post1: t = −2.8, P = 0.019; pre-rTMS vs. post2: t = −2.4, P = 0.035). The same was true if the unconditioned MEP only was measured (pre-rTMS vs. post1: t = −2.4, P = 0.036; pre-rTMS vs. second post2 t = −2.3, P = 0.044).
Figure 2. Effects of 15 min, 1 Hz rTMS delivered over the left motor cortex on the ICI-ICF of the right M1HAND.
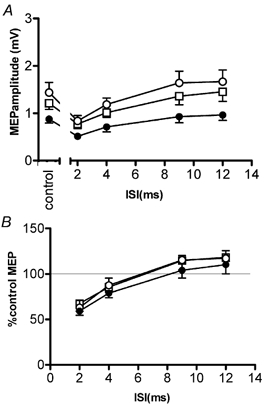
The time course of the test MEP inhibition and facilitation before rTMS (•), 1 and time course after rTMS (open symbols). Post 1 (○) averaged data from the first-second block performed immediately at the end of rTMS and post 2 (▪) averaged data from the third-fourth block performed 10 min after the end of rTMS. A, mean (± s.e.m.) amplitude of the test MEP measured in mV whilst B plots the same data as percentage inhibition.
Experiment 2: assessment of interhemispherical inhibition of the right M1HAND
Resting motor threshold of the left M1HAND measured with the biphasic stimulator used to deliver rTMS was 42 ± 2 %. The intensity used for the 15 min, 1 Hz train was 49 ± 3 %, or 115 ± 3 % RMT.
Resting motor threshold measured with the small coils used for IHI was 40 ± 2 % for the left M1HAND and 37 ± 1 % for the right M1HAND. In the interhemispherical conditioning-test protocol, the mean intensity of the conditioning stimulus applied to the left M1HAND was 47 ± 3 % whilst the test stimulus intensity for the right M1HAND was 47 ± 3 %.
Figure 3 shows the time course of IHI from left-to-right hemispheres. A plots the data in terms of absolute MEP amplitudes in mV peak-to-peak and B plots the same data in terms of the percentage of the unconditioned MEP amplitude. Two-way repeated-measures ANOVA on the absolute data revealed a significant main effect of time (pre- vs. post-rTMS) (F(7, 56) = 11.9, P < 0.0001) and a significant interaction between the two main factors of ISI and time (F(14, 112) = 1.8; P = 0.04). The time effect was due to the fact that MEPs were larger after rTMS than before. The interaction term indicates that the time course of IHI was different before and after rTMS. Post hoc analysis showed that this was because there was less inhibition at ISIs of 7 and 10 ms after rTMS. This can be seen most clearly in the percentage data of Fig. 3B and C. Post hoc paired-samples t tests show a decrease in interhemispherical inhibition at ISIs of 7 and 10 ms for both time points after rTMS (ISI = 7 ms: pre-rTMS vs. post1-rTMS: t = −4.21; P = 0.003; ISI = 10 ms: pre-rTMS vs. post1-rTMS: t = −4.23; P = 0.003. ISI = 7 ms: pre-rTMS vs. post2-rTMS: t = −2.7; P = 0.024; ISI = 10 ms: pre-rTMS vs. post2-rTMS: t = −3.5; P = 0.008).
Figure 3. Effects of 15 min, 1 Hz rTMS delivered over the left motor cortex on the left-to-right interhemispherical inhibition.
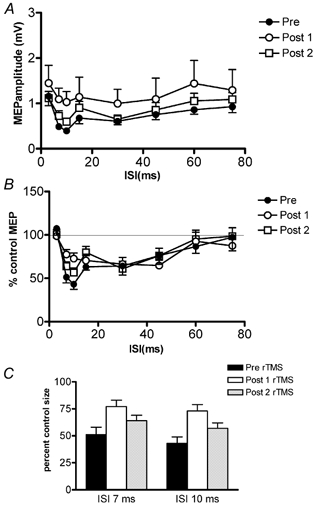
The time course of the test MEP inhibition before rTMS (filled symbols) and time course after rTMS (open symbols). Post 1 (○) was performed immediately at the end of rTMS and post 2 (▪) was performed 10 min after the end of rTMS. A, the mean (± s.e.m.) amplitude of the test MEP measured in mV whilst B plots the same data as percentage inhibition. C, summary of the amount of inhibition at ISI 7 and 10 ms. Note that the amount of inhibition was much greater before rTMS.
Since the conditioning stimulus to the left hemisphere was above RMT, it was possible to measure the amplitude of the contralateral MEP that it evoked. Effectively this meant that we could measure corticospinal excitability in the same hemisphere as received the rTMS. Corticospinal excitability was not significantly changed before and after rTMS (MEP amplitude right FDI: pre-rTMS = 0.9 ± 0.2 mV; post1-rTMS = 0.9 ± 0.2 mV; post2-rTMS = 1.0 ± 0.2 mV).
The ipsilateral silent period (ISP) also gives information about interhemispherical connections between the hemispheres. Figure 4A and B show data for both the left ISP (which is comparable to the direction of interhemispherical inhibition above, from left hemisphere to right hemisphere) and the right ISP (i.e. right-to-left hemisphere). The amount of inhibition in the ISP did not change significantly after rTMS, although there was a tendency for left-right inhibition to increase in the post 1 period. There was no effect on the duration of the CSP (Fig. 4C and D), nor on the amplitude of the MEP that preceded it (not shown).
Figure 4. Effects of rTMS on the ipsilateral (A and B) and contralateral (C and D) cortical SP.
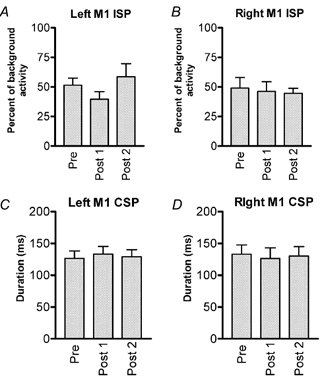
A, SP obtained in the left FDI muscle stimulating the left M1HAND and B, SP obtained in the right FDI muscle stimulating the left M1HAND. C, SP obtained in the right FDI stimulating the left M1HAND and D, SP obtained in the left FDI muscle stimulating the right M1HAND. Post 1 was performed immediately at the end of rTMS and post 2 was performed 10 min after the end of rTMS. Data are: A and B, the mean (± s.e.m.) values of the ipsilateral SP expressed as a percentage of baseline activity and C and D the duration of the contralateral SP expressed measured in ms.
Correlation between results in experiment 1 and experiment 2
Eight subjects performed both sets of experiments so that it was possible to correlate whether the increase in MEP measured in experiment 1 was related to the decrease in IHI in experiment 2. Even though the two experiments were carried out on different days, there was a tendency for subjects with the largest increase in MEP to have the largest decrease in IHI (linear regression: r = 0.68; P = 0.06), but this was not significant.
Control experiment 1: repetitive 1 Hz stimulation of the right ulnar nerve
Fifteen minutes of 1 Hz electrical nerve stimulation had no effect on the left-right IHI at 7 and 10 ms ISIs. A two-way repeated measures ANOVA showed a main effect of ISI (F(1, 3) = 35.4, P < 0.001), but no significant effects for the factor time (ISI = 7 ms: pre-nerve stimulation, 56.3 ± 8.4 %, post-nerve stimulation, 61.3 ± 9 %; ISI = 10 ms: pre-nerve stimulation, 57.6 ± 4.1 %, post-nerve stimulation, 59.7 ± 11.6 %).
Control experiment 2: influence of the amplitude of the test response on IHI evoked by a constant conditioning pulse
Figure 5 shows the left-right IHI at 7 and 10 ms using test MEPs of two different amplitudes (1 and 1.5 mV on average). A paired-sample t test showed that the amount of inhibition was the same for both test pulse amplitudes (P > 0.05).
Figure 5. Effects of test MEP amplitude on the left-right interhemispherical inhibition.
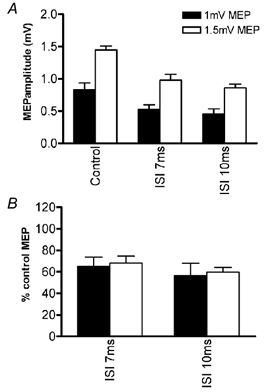
Data obtained with a control MEP amplitude of 1 mV (▪), and data obtained with a 1.5 mV control MEP (□). Data in A show means (± s.e.m.) amplitude of the control and conditioned (ISI 7 or 10 ms) measured in mV peak to peak. B plots the same data as the percentage inhibition at ISI 7 and 10 ms.
Control experiment 3: effect of rTMS coil orientation
In the experiments above, rTMS to the left M1HAND had no effect on the size of MEPs evoked from the same hemisphere even though it facilitated those from the opposite hemisphere. This was unexpected since 1 Hz stimulation of the motor cortex has been reported by many authors to reduce motorcortical excitability (Chen et al. 1997; Fitzgerald et al. 2002). However, in the present studies we were using rTMS with the opposite orientation to that used in many other studies. We examined the effect of reversing the orientation of the rTMS coil from the present position with the handle pointing forwards, to a position rotated through 180 deg so that the handle pointed backwards. The intensity of rTMS was adjusted so that a single pulse produced approximately 70 % IHI in a paired-pulse protocol. Since the threshold for producing transcallosal effects is higher with the backwards orientation, the absolute stimulus intensity was higher with this orientation (65 ± 5 %) than it had been in the original set of experiments (53 ± 4 %).
Figure 6 shows the MEP recruitment curve in the right FDI muscle after 15 min, 1 Hz rTMS over the left M1HAND area with the backwards coil orientation. Two-way ANOVA showed a main effect of the factor intensity of stimulation (F(1, 2) = 40; P = 0.02) and a significant interaction of the factor time and intensity of stimulation (F(13, 2.7) = 13.8; P = 0.03). Thus 1 Hz rTMS with reversed coil orientation inhibited MEPs evoked from the same hemisphere as reported by others (Touge et al. 2001).
Figure 6. Effects of current direction on the MEP recruitment curve over of the right FDI muscle.
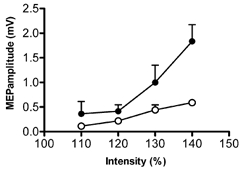
MEP recruitment curve (•) before 1 Hz rTMS with a backward orientation of the coil and MEP amplitude after 1 Hz train stimulation (○). Data correspond to the amplitude of the test MEP expressed as means ± s.e.m. mV.
Control experiment 4: effect of repetitive 1 Hz stimulation on spinal cord excitability
In both subjects, the amplitude of the MEP recorded from the left FF muscles increased after 15 min rTMS of the ipsilateral hemisphere (post1-rTMS, 115 ± 8 %; post2-rTMS, 123 ± 22 %; P < 0.05 for the data set of each individual) whilst the size of the H reflex remained unchanged (post1-rTMS, 93 ± 10 %; post2-rTMS, 106 ± 18 %).
DISCUSSION
The present results show that 15 min rTMS at 1 Hz over the left motor hand area can decrease interhemispherical inhibition (IHI) from the left-to-right hemisphere and increase the amplitude of MEPs evoked from the right hemisphere. Contralateral and ipsilateral silent periods from both hemispheres (CSP, ISP), and SICI/ICF in the right hemisphere were unaffected. The data are compatible with the idea that this form of stimulation reduces tonic IHI from the left-to-right hemisphere and that this secondarily leads to an increase in MEP amplitude after stimulation of the right motor hand area.
Decreased left-to-right IHI
It seems most probable that the changes in IHI were a direct result of repetitive stimulation of the left motor hand area. However, potentially two other factors could have contributed. First, the amplitude of the control MEP from the right hemisphere increased after left hemisphere rTMS, and this could have affected the response to the interhemispherical conditioning shock (Ferbert et al. 1992; Daskalakis et al. 2002). However, control experiments showed that the percentage IHI produced by a standard stimulus was unaffected over the range of MEP amplitudes seen in this experiment. In addition, there seems no reason why changes in control MEP amplitude should have affected IHI at ISIs of only 7 and 10 ms and not at other intervals.
The second factor that could have changed IHI is the afferent input from the twitches produced by the rTMS. Several authors have described persistent changes in cortical excitability after repetitive afferent input (Ridding et al. 2001), and it is conceivable that the input generated by muscle twitches during rTMS had a particular effect on interhemispherical pathways. However, this seems unlikely to have occurred in the present experiments since mimicking the twitches by direct electrical stimulation of the ulnar nerve did not result in any lasting changes in IHI. We conclude that most, if not all, of the effect on IHI at ISI of 7 and 10 ms was due to a direct effect of rTMS on the left motor hand area.
Two questions arise from this. First, why was IHI in the paired-pulse protocol reduced after rTMS only at ISIs of 7 and 10 ms and not at longer ISIs? Second, why was the ISP following stimulation of the left hemisphere unchanged since this is also thought to involve transcallosal pathways (Meyer et al. 1995)? A recent paper by Chen et al. (2003) may be very relevant. They used a variety of stimulus intensities and coil orientations to show that the ISP and the later part of IHI at ISI > 40 ms are probably mediated by the same circuits, whereas the early part of IHI at an ISI of 8 ms was produced by activity in a different circuit. Our results are therefore compatible with the idea that rTMS has a specific long-lasting effect on the IHI circuit active at short ISIs, but not on that which is active at longer ISIs.
Changes in excitability of responses evoked from the right motor hand area
The right motor cortex was in the hemisphere opposite to the side of rTMS. We evaluated the excitability of circuits involved in producing the MEP, SICI/ICF and CSP, ISP. The only effect was on the MEP amplitude, which was increased for up to 20 min after the end of rTMS. Although such selectivity is unusual, it is not incompatible with the known differences in mechanism of these various effects, all of which have separate neural circuits within the cortex. The implication is that rTMS preferentially targets one of them. It should also be noted that in the paired-pulse protocol we used a low conditioning stimulus intensity in order to obtain approximately 50 % SICI. Since the threshold for ICF is slightly higher than for SICI (Kujirai et al. 1993) this meant that the amount of ICF was small. Thus, we do not feel able to draw any definitive conclusion about the changes in ICF in the non-stimulated hemisphere.
Spinal mechanisms are unlikely to contribute to the changes in MEP since H reflexes in the arm were unchanged. However, some contribution cannot be ruled out completely since MEPs and H reflexes may sometimes recruit different fractions of the spinal motoneurone pool in some muscle groups (Morita et al. 2000).
The increase in amplitude of MEPs from the right hemisphere is similar to results reported in a recent report by Schambra et al. (2003). However Wassermann et al. (1998a) found the opposite: that 1 Hz rTMS reduced MEP amplitudes evoked from the opposite hemisphere. We think that this may be due mainly to differences in the intensity of rTMS. Wassermann et al. (1998a) used intensities that were around resting motor threshold. Our stimuli were individually set to be above the threshold for activating interhemispherical effects, averaging 117 % RMT, whilst Schambra et al. (2003) used 115 % RMT. Presumably the higher intensity stimulation activates additional neural circuitry that leads to predominant excitatory effects on the opposite hemisphere. A parallel observation was made recently by Fitzgerald et al. (2002) who noted that the inhibitory effect of 1 Hz rTMS on the stimulated hemisphere also was larger with higher intensities of rTMS.
The orientation of the rTMS was not specified by Wassermann et al. (1998a), but in a personal communication, Dr Wassermann has suggested that it was probably a biphasic pulse with the initial phase inducing posterior-anterior current in the brain. In the present case we used the opposite orientation since this has the lowest threshold for stimulating the corticospinal output of motor cortex (with the reversing phase, rather than the initial phase, of the biphasic-induced current flowing posterior-anterior across the central sulcus). A posterior- anterior induced current flow is also optimal for evoking the ipsilateral silent period with a monophasic pulse (Meyer et al. 1996). It may well be that if Wassermann et al. (1998a) had used the same orientation as we did then the sign or magnitude of the after effects may have been different.
Changes in excitability of the left motor hand area
The MEP amplitude and the silent period in the right FDI were unchanged by rTMS of the left hemisphere. We were surprised by this since 1 Hz stimulation usually decreases the amplitude of MEPs evoked from the same hemisphere for several minutes after the end of rTMS (Maeda et al. 2000a; Touge et al. 2001; Fitzgerald et al. 2002). However this may well have been because we used the opposite orientation of the rTMS coil to deliver the 1 Hz stimulation as that used in previous reports. In these studies, the initial current in the biphasic pulse flowed from posterior-anterior in the brain across the central sulcus whereas the present experiments and a study by Siebner et al. (1999) employed the opposite orientation since this is optimal for activating corticospinal (and by implication interhemispherical (Meyer et al. 1996)) output with a biphasic stimulus current (Kammer et al. 2001). In accordance with the study by Siebner et al. (1999), we found no lasting effects on the MEP amplitude with the initial current having an anterior-posterior direction. However, a control experiment showed that if the rTMS coil was reversed, there was the usual reduction in amplitude of MEPs evoked from the same hemisphere. It should be noted that under these conditions, the absolute intensity of the rTMS pulses is higher than the orientation used in the main experiments. Further studies will be needed to test whether the after effects of rTMS on motor cortex depend only on intensity (Fitzgerald et al. 2002) or also on orientation.
Possible mechanisms
Our results raise two questions. First, by what mechanism did rTMS over the left hemisphere reduce the excitability of short interval IHI from left to right hemispheres? Second, did the change in IHI cause the increase in excitability of MEPs evoked from the right hemisphere?
rTMS could theoretically influence IHI by modifying excitability on the side of stimulation (left hemisphere), or the side of MEP testing (right hemisphere), or on both. Since we used an intensity of rTMS that was above threshold for inducing activity in the pathway, either mechanism is possible. For example, repeated activation of the pathway may have decreased the efficacy of inhibitory synapses in the right hemisphere, with the result that a single conditioning pulse would cause less inhibition of test MEPs than before rTMS. Alternatively, if the interhemispherical fibres in the left hemisphere are normally activated trans-synaptically by TMS, like corticospinal neurones, repeated activation of these synapses could reduce their effectiveness and again decrease IHI when tested after rTMS. At the present time we do not have enough information to speculate further. However, it is interesting to note that the intensity of rTMS could be an important factor in determining the site of any after effect. For example, if conditioning involved using low intensity rTMS that was insufficient to activate impulses in the interhemispherical fibres, then any after effects would necessarily be limited to changes in the stimulated hemisphere. Changes in the excitability of the opposite hemisphere would be due to secondary effects on the ongoing activity of interhemispherical connections. This may help to explain the apparent contradiction between the present results and those reported by Wassermann et al. (1998), who found that rTMS to one hemisphere decreased MEP amplitudes in the opposite hemisphere rather than increasing them. The lower intensity of rTMS that they used may have been less efficient in activating interhemispherical fibres, resulting in a different distribution of interhemispherical effects.
It is also important to note that our effects were limited to the early phase of IHI at 7 and 10 ms ISI and did not affect later phases or the ISP. This is compatible with the suggestion of Chen et al. (2003) that early IHI has a different mechanism to both the later phases of inhibition at ISI = 40 ms and the ISP. The lack of effect of rTMS on these latter pathway(s) may indicate they have a different frequency response for prolonged after effects. Alternatively, it may be that the pathway has a different threshold. Although Chen et al. (2003) did not investigate this in detail the original data of Ferbert et al. (1992) seem to suggest that inhibition at short ISIs has a lower threshold than inhibition at longer ISIs.
We would like to suggest that the effect of rTMS on interhemispherical inhibition and on the excitability of the contralateral corticospinal projection were linked. Indeed there was a tendency for subjects who had the largest increase in MEPs to have the largest decrease in IHI. If there was any ongoing interhemispherical inhibition in the resting state, then reduced excitability following rTMS might cause decreased tonic inhibition and increase the excitability of MEPs from the opposite hemisphere. Some evidence of the existence of tonic interactions between the hemispheres comes from studies of patients after hemispheric stroke. Both Kimiskidis et al. (2002) and Liepert et al. (2000a) have examined patients in the first few weeks after a stroke and found a decrease in MEP threshold from the unaffected hemisphere. They suggested that the stroke had removed tonic IHI from the stroke hemisphere and increased the excitability of the unaffected hemisphere. Other studies have described changes in SICI/ICF (Liepert et al. 2000b; Manganotti et al. 2002; Shimizu et al. 2002), again invoking changes in tonic interactions between the hemispheres. Our hypothesis is that these interactions exist in healthy subjects and that they can be modified transiently by rTMS to one side of the brain.
Finally, why did rTMS to the left hemisphere only affect corticospinal excitability in the right hemisphere? As noted above, studies in stroke patients suggest that interhemispherical interactions can also affect circuitry other than that involved in producing the MEP. Our suggestion is that such effects will indeed occur, but that their threshold, frequency selectivity or other parameters may be different from the effect on MEP amplitude. Further studies are needed to address this important question in detail.
Acknowledgments
We would like to thank Mr Peter Asselman for his invaluable help in maintaining and building the equipment used in these experiments. Dr F. Gilio and Dr V. Rizzo were supported by a European Commission Improving Human Potential programme (ref. HPRI-1999-CT-00025). Dr H. Siebner was the recipient of a Deutsche Forschungsgemeinshaft (DFG) Travelling Fellowship. The work was supported by the Medical Research Council.
Francesca Gilio and Vincenzo Rizzo contributed equally to this work.
REFERENCES
- Brasil-Neto J, Cohen LG, Panizza M, Nilsson J, Roth BJ, Hallett M. Optimal focal transcranial magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J Clin Neurophysiol. 1992;9:132–136. [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89:1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol. 2002;543:317–326. doi: 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Brown TL, Daskalakis ZJ, Chen R, Kulkarni J. Intensity-dependent effects of 1 Hz rTMS on human corticospinal excitability. Clin Neurophysiol. 2002;113:1136–1141. doi: 10.1016/s1388-2457(02)00145-1. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Cohen LG, Floeter MK, Chen R, Corwell B, Hallet M. Inhibitory influence of the ipsilateral motor cortex on responses to stimulation of the human cortex and pyramidal tract. J Physiol. 1998;510:249–259. doi: 10.1111/j.1469-7793.1998.249bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschlager W, Siebner HR, Rothwell JC. Decreased corticospinal excitability after subthreshold 1 Hz rTMS over lateral premotor cortex. Neurology. 2001;57:449–455. doi: 10.1212/wnl.57.3.449. [DOI] [PubMed] [Google Scholar]
- Jing H, Takigawa M. Observation of EEG coherence after repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:1620–1631. doi: 10.1016/s1388-2457(00)00357-6. [DOI] [PubMed] [Google Scholar]
- Kammer T, Beck S, Thielscher A, Laubis-Herrmann U, Topka H. Motor thresholds in humans: a transcranial magnetic stimulation study comparing different pulse waveforms, current directions and stimulator types. Clin Neurophysiol. 2001;112:250–258. doi: 10.1016/s1388-2457(00)00513-7. [DOI] [PubMed] [Google Scholar]
- Kimiskidis VK, Dimopoulos G, Papagiannopoulos S, Kazis D, Kazis A, Mills KR. Corticomotor threshold in stroke: the unaffected hemisphere. Clin Neurophysiol. 2002;113(suppl. 1):S46. doi: 10.1016/j.neucli.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselmann P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J, Hamzei F, Weiller C. Motor cortex disinhibition of the unaffected hemisphere after acute stroke. Muscle Nerve. 2000a;23:1761–1763. doi: 10.1002/1097-4598(200011)23:11<1761::aid-mus14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Liepert J, Storch P, Fritsch A, Weiller C. Motor cortex disinhibition in acute stroke. Clin Neurophysiol. 2000b;111:671–676. doi: 10.1016/s1388-2457(99)00312-0. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res. 2000a;133:425–430. doi: 10.1007/s002210000432. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000b;111:800–805. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- Manganotti P, Patuzzo S, Cortese F, Palermo A, Smania N, Fiaschi A. Motor disinhibition in affected and unaffected hemisphere in the early period of recovery after stroke. Clin Neurophysiol. 2002;113:936–943. doi: 10.1016/s1388-2457(02)00062-7. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Kuehn A, Roericht S. Influence of the direction of induced currents on callosally and corticospinally mediated electromyographic responses following magnetic motor cortex stimulation in man. J Physiol. 1996;497•P:34–35P. [Google Scholar]
- Meyer BU, Roricht S, Grafin-Von EH, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 1995;118:429–440. doi: 10.1093/brain/118.2.429. [DOI] [PubMed] [Google Scholar]
- Mills KR, Boniface SJ, Schubert M. Magnetic brain stimulation with a double coil: the importance of coil orientation. Electroencephalogr Clin Neurophysiol. 1992;85:17–21. doi: 10.1016/0168-5597(92)90096-t. [DOI] [PubMed] [Google Scholar]
- Morita H, Olivier E, Baumgarten J, Petersen NT, Christensen LO, Nielsen JB. Differential changes in corticospinal and Ia input to tibialis anterior and soleus motor neurones during voluntary contraction in man. Acta Physiol Scand. 2000;170:65–76. doi: 10.1046/j.1365-201x.2000.00762.x. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Hallett M. Effects of low-frequency transcranial magnetic stimulation on motor excitability and basic motor behavior. Clin Neurophysiol. 2000;111:1002–1007. doi: 10.1016/s1388-2457(00)00284-4. [DOI] [PubMed] [Google Scholar]
- Munchau A, Bloem BR, Irlbacher K, Trimble MR, Rothwell JC. Functional connectivity of human premotor and motor cortex explored with repetitive transcranial magnetic stimulation. J Neurosci. 2002;22:554–561. doi: 10.1523/JNEUROSCI.22-02-00554.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Paus T, Castro-Alamancos MA, Petrides M. Cortico-cortical connectivity of the human mid-dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. Eur J Neurosci. 2001;14:1405–1411. doi: 10.1046/j.0953-816x.2001.01757.x. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Mckay DR, Thompson PD, Miles TS. Changes in corticomotor representations induced by prolonged peripheral nerve stimulation in humans. Clin Neurophysiol. 2001;112:1461–1469. doi: 10.1016/s1388-2457(01)00592-2. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W. Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology. Clin Neurophysiol. 1999;(suppl. 52):97–103. [PubMed] [Google Scholar]
- Schambra HM, Sawaki L, Cohen LG. Modulation of excitability of human motor cortex (M1) by 1 Hz transcranial magnetic stimulation of the contralateral M1. Clin Neurophysiol. 2003;114:130–133. doi: 10.1016/s1388-2457(02)00342-5. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, Rossini PM. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002;125:1896–1907. doi: 10.1093/brain/awf183. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Peller M, Willoch F, Minoshima S, Boecker H, Auer C, Drzezga A, Conrad B, Bartenstein P. Lasting cortical activation after repetitive TMS of the motor cortex: a glucose metabolic study. Neurology. 2000;54:956–963. doi: 10.1212/wnl.54.4.956. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Tormos JM, Ceballos BA, Auer C, Catala MD, Conrad B, Pascual-Leone A. Low-frequency repetitive transcranial magnetic stimulation of the motor cortex in writer's cramp. Neurology. 1999;52:529–537. doi: 10.1212/wnl.52.3.529. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21:RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strens LH, Oliviero A, Bloem BR, Gerschlager W, Rothwell JC, Brown P. The effects of subthreshold 1 Hz repetitive TMS on cortico-cortical and interhemispheric coherence. Clin Neurophysiol. 2002;113:1279–1285. doi: 10.1016/s1388-2457(02)00151-7. [DOI] [PubMed] [Google Scholar]
- Touge T, Gerschlager W, Brown P, Rothwell JC. Are the of low-frequency rTMS on motor cortex excitability due to changes in the efficacy of cortical synapses. Clin Neurophysiol. 2001;112:2138–2145. doi: 10.1016/s1388-2457(01)00651-4. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Rothwell JC. Long lasting effects of rTMS and associated peripheral sensory input on MEPs, SEPs and transcortical reflex excitability in humans. J Physiol. 2002;540:367–376. doi: 10.1113/jphysiol.2001.013504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero-Cabre A, Oliveri M, Gangitano M, Pascual-Leone A. Modulation of spinal cord excitability by subthreshold repetitive transcranial magnetic stimulation of the primary motor cortex in humans. Neuroreport. 2001;12:3845–3848. doi: 10.1097/00001756-200112040-00048. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7 1996. Clin Neurophysiol. 1998b;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Wedegaertner FR, Ziemann U, George MS, Chen R. Crossed reduction of human motor cortex excitability by 1-Hz transcranial magnetic stimulation. Neurosci Lett. 1998a;250:141–144. doi: 10.1016/s0304-3940(98)00437-6. [DOI] [PubMed] [Google Scholar]


