Abstract
Somatic noxious stimulation can evoke profound cardiovascular responses by altering activity in the autonomic nervous system. This noxious stimulation attenuates the cardiac vagal baroreflex, a key cardiovascular homeostatic reflex. This attenuation is mediated via NK1 receptors expressed on GABAergic interneurones within the nucleus of the solitary tract (NTS). We have investigated the effect of noxious stimulation and exogenous substance P (SP) on the sympathetic component of the baroreflex. We recorded from the sympathetic chain in a decerebrate, artificially perfused rat preparation. Noxious hindlimb pinch was without effect on the sympathetic baroreflex although the cardiac vagal baroreflex gain was decreased (56%, P < 0.01). Bilateral NTS microinjection of SP (500 fmol) produced a similar selective attenuation of the cardiac vagal baroreflex gain (62%, P < 0.005) without effect on the sympathetic baroreflex. Recordings from the cardiac sympathetic and vagal nerves confirmed the selectivity of the SP inhibition. Control experiments using a GABAA receptor agonist, isoguvacine, indicated that both components of the baroreflex (parasympathetic and sympathetic) could be blocked from the NTS injection site. The NTS microinjection of a NK1 antagonist (CP-99,994) in vivo attenuated the tachycardic response to hindlimb pinch. Our data suggest that noxious pinch releases SP within the NTS to selectively attenuate the cardiac vagal, but not the sympathetic, component of the baroreflex. This selective withdrawal of the cardiac vagal baroreflex seems to underlie the pinch-evoked tachycardia seen in vivo. Further, these findings confirm that baroreflex sympathetic and parasympathetic pathways diverge, and can be independently controlled, within the NTS.
Somatic noxious stimulation is associated with a complex series of autonomic responses (see review by Janig, 1985). Prominent amongst these autonomic changes are an increase in cardiac output and a redistribution of blood flow to muscles at the expense of skin and viscera (Janig, 1985). The increase in cardiac output is mediated via an increase in both heart rate and ventricular contractility. The nucleus of the solitary tract (NTS) might be expected to play a role as it is a key brainstem site for cardiovascular homeostasis and is the first central locus for integration of the baroreflex (see Barraco, 1994). There is evidence for the involvement of the NTS in the autonomic responses to noxious stimulation (Boscan et al. 2002b); somatic noxious stimulation has been shown to induce c-fos expression in NTS neurones (Pezzone et al. 1993); recordings from NTS neurones have shown excitatory responses to noxious somatic stimuli (Toney & Mifflin, 2000); and the tachycardia evoked by somatic noxious pinch can be attenuated by pharmacological block of the NTS (Boscan & Paton, 2001). We have reported that somatic noxious stimulation can attenuate the cardiac vagal baroreflex gain (Boscan et al. 2002a). This reduction in cardiac vagal baroreflex gain is mediated via neurokinin-1 (NK1) receptors within the NTS (Boscan et al. 2002a). The microinjection of substance P (SP) into the NTS mimics this inhibition of the cardiac vagal baroreflex via an action on GABAergic interneurones (Boscan et al. 2002a). The attenuation of the cardiac vagal component of the baroreflex may either be solely responsible for, or may play a permissive role in, the characteristic tachycardic response to noxious stimuli.
In this study, we have assessed whether somatic noxious stimulation and microinjected SP would block the sympathetic (as well as parasympathetic) limb of the baroreflex. To facilitate these experiments we have employed a novel variant of the working heart-brainstem preparation (Paton, 1996): the decerebrate artificially perfused rat (Pickering et al. 2002). We demonstrate that both noxious pinch and microinjected SP selectively attenuate the cardiac vagal but not the sympathetic limb of the baroreflex by an action within the NTS. We also show that the in vivo microinjection of a NK1 antagonist to the NTS of an anaesthetized rat can significantly attenuate the tachycardic response to noxious pinch. Parts of this paper have been presented previously in abstract form (Pickering et al. 2003).
METHODS
All procedures conformed with the UK Animals (Scientific Procedures) Act 1986 and were approved by our institutional ethical review committee. Initial experiments were performed using decerebrated, un-anaesthetized, artificially perfused in situ preparations of Wistar rats (males 75–150 g, University of Bristol colony). The majority of these experiments employed a novel decerebrate, artificially perfused rat preparation (DAPR; see Pickering et al. 2003, Fig. 1). The decerebration involved the removal and destruction of the forebrain rendering the animal completely insentient. The working heart-brainstem preparation (WHBP; see Paton, 1996, for details) was employed for recordings of the inferior cardiac nerve (ICN) and of the cardiac vagal nerve (CVN) as it permits better access to these nerves in the mediastinum. A final series of experiments were performed in vivo, on halothane-anaesthetized rats, to examine the effect of NTS microinjection of an NK1 receptor antagonist on the response to hindpaw pinch.
Figure 1. Schematic diagram of the decerebrate artificially perfused rat, recording of the baroreflex and response to noxious hindpaw pinch.
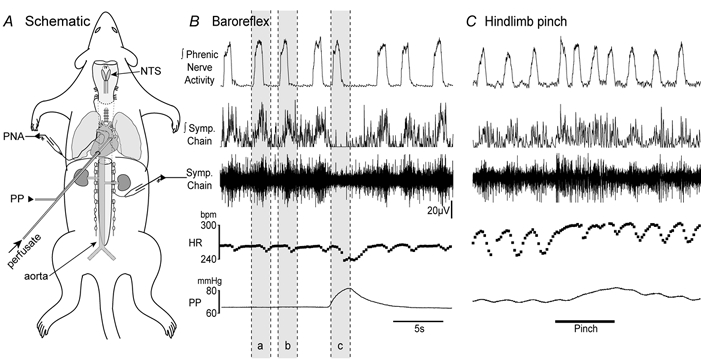
A, schematic diagram of the decerebrate artificially perfused rat. The rat is decerebrated and perfused with carbogenated Ringer solution containing Ficoll 70 at 32 °C via a double-lumen perfusion cannula inserted through the left ventricle into the ascending aorta. Perfusion pressure (PP) is monitored via the second lumen of the cannula. Recordings were made from the phrenic nerve (PNA) and sympathetic nerves using suction electrodes. The pump flow rate was adjusted to produce a eupnoeic pattern of phrenic discharge. Perfusion pressure ramps were generated by increasing pump flow. The dorsal surface of the brainstem was exposed to allow drug microinjection into the NTS. B, typical recordings of the baroreflex showing integrated phrenic nerve activity, sympathetic nerve activity (integrated and raw), instantaneous heart rate (HR) and perfusion pressure. The phrenic nerve recording exhibits rhythmic augmenting bursts of activity consistent with eupnoea. The heart rate trace shows respiratory sinus arrhythmia. The sympathetic activity is strongly respiratory modulated with peaks of activity during post-inspiration. A perfusion pressure ramp (c) triggered baroreflex bradycardia and sympathoinhibition. The degree of sympathoinhibition was quantified by ratioing the integrated sympathetic nerve activity during the ramp against the averaged activity from two preceding time periods (a and b) at equivalent stages of the respiratory cycle. C, the application of a noxious pinch (1.9 N mm−2, bar) to the hindpaw evoked marked increases in sympathetic nerve activity and consequent pressor and tachycardic responses. The changes were accompanied by a tachypnoea which was characteristically most pronounced at the beginning of the pinch. (Note sinus arrhythmia on the heart rate trace.)
For experiments using the WHBP, rats were anaesthetized with halothane until loss of the paw pinch withdrawal reflex. The rat was bisected sub-diaphragmatically, immersed in carbogenated Ringer solution at 10 °C, suction decerebrated pre-collicularly and cerebellectomized to expose the fourth ventricle. After transfer to the recording chamber the descending aorta was cannulated with a double-lumen cannula for retrograde perfusion.
For the DAPR preparation the rat was deeply anaesthetized with halothane. The stomach, intestines and spleen were ligated and removed via a midline laparotomy. The sternum was then split and the ribcage retracted to allow access to the mediastinum. The animal was cooled by immersion in carbogenated Ringer solution at 10 °C, decerebrated pre-collicularly and cerebellectomized to expose the fourth ventricle, and transferred to the recording chamber. A double-lumen perfusion cannula was introduced via an incision in the left ventricle into the ascending aorta.
Both preparations were perfused at flow rates of 28–35 ml min−1 using a roller pump (Watson Marlow 505S) with a Ringer solution containing 1.25 % Ficoll 70 (see below) gassed with carbogen at 32 °C. The second lumen of the cannula was used to monitor aortic perfusion pressure. The phrenic nerve was identified in the thorax and its activity recorded via a suction electrode. The perfusate flow was adjusted until the respiratory motor pattern consisted of an augmenting burst discharge indicating eupnoea (Paton, 1996). The presence of this distinctive burst pattern was used to gauge the viability of the preparation and allowed calculation of the respiratory rate. The electrocardiogram (ECG) was recorded simultaneously with the phrenic nerve signal which was amplified, filtered (80 Hz-3 kHz) and displayed on an oscilloscope. Instantaneous heart rate (HR) was triggered from the R wave of the ECG using a window discriminator.
Nerve recordings
Nerve recordings were made using suction electrodes and AC amplified (Neurolog NL104). Recordings were made from the sympathetic chain (at L2–3 and T8–9), ICN and CVN. The identity of the latter two nerves was confirmed by electrical stimulation (1–4 s train at 30–50 Hz, 1 ms pulses of 10–20 V) of the distal cut end of the nerve to obtain tachycardia (ICN) or bradycardia (CVN). The activity of all sympathetic nerves exhibited marked respiratory modulation and was profoundly attenuated by an increase in perfusion pressure (to stimulate arterial baroreceptors; Fig. 1B). The CVN also exhibited respiratory modulation with bursts of activity in the post-inspiratory period. However, by contrast with the sympathetic outflow, the CVN showed increased discharge in response to pressor stimuli.
Stimulation methods
Noxious pinch
The hindlimbs were stimulated at the level of the paw with either a manually operated calibrated forceps or a pneumatic pincher (both custom-built). These allowed control of the intensity of the pinch stimulus. In order to achieve a reflex tachycardia, the stimulus intensity and duration were 1.5 ± 0.2 N mm−2 and 10 ± 3 s, respectively (Fig. 1C). These parameters produced consistent cardiorespiratory reflex responses when a period of approximately 6 min was allowed between each stimulus. The intensity and duration of the stimulus, once determined for each preparation, remained constant throughout the experiment. The number of stimuli to a limb per experiment ranged from 6 to 10.
Baroreceptor
Perfusion pressure ramps were generated to stimulate the arterial baroreceptors by maximally increasing the pump flow for 2–4 s. The rate of rise of perfusion pressure in a preparation was consistent between trials. The evoked baroreflex bradycardia was blocked by methyl-atropine (30–60 μg) indicating that it was vagally mediated. Initially, a stimulus-response curve was constructed and all subsequent perfusion pressure challenges were on the linear part of the baroreflex response curve. To take account of the marked respiratory modulation of sympathetic nerve activity the perfusion pressure ramps were timed to peak during post-inspiration when sympathetic nerve activity should be maximal (see Fig. 1B and data analysis section below for method of quantification). When baroreceptor and mechanical noxious limb stimulation were co-applied, the increase in perfusion pressure was timed to occur when the evoked tachycardia had reached a plateau.
The perfusion pressure of the DAPR (50 ± 3 mmHg, n = 6) was lower than the WHBP (80 ± 6 mmHg, n = 7) reflecting the lower vascular resistance of the DAPR, which is presumably due to the larger vascular tree. This resulted in correspondingly smaller pressure ramps in the DAPR in response to increased perfusate flow. Hence, in some of the DAPR preparations it was necessary to increase the vascular resistance in order to provide an adequate baroreflex stimulus by adding a small dose of arginine-vasopressin to the circulating perfusate (0.1 i.u. (200 ml)−1≈ 1 nm).
NTS microinjection studies
In the WHBP and DAPR the surface landmarks of the dorsal medulla are seen clearly as the preparation is cerebellectomized. Calamus scriptorius was used as a landmark to direct micropipettes into sites known to contain both baroreceptive afferent terminals (Blessing, 1997) and baroreceptive neurones in the rat (Deuchars et al. 2000). A three-barrelled micropipette (external tip diameter between 10 and 30 μm) was placed into the NTS using a micromanipulator. The volume microinjected (50 nl) was assessed by viewing the movement of the meniscus through a binocular microscope fitted with a calibrated eyepiece graticule. Drugs were microinjected bilaterally into the NTS at 400–600 μm deep to the dorsal medullary surface and 250 μm rostral to calamus scriptorius, 300–500 μm lateral to the midline. All injections were made within a period of 60 s and the baroreflex was tested 60 s after the last injection and then examined repeatedly every 3 min for 15 min. In most experiments NaCl (0.9 %; 50 nl) was microinjected into the same NTS sites to control against volume-related artefacts. In all cases, saline failed to modify baseline heart rate, perfusion pressure, phrenic nerve activity or any of the reflexes tested.
In vivo studies
Our previous in situ experiments have shown NTS microinjection of a NK1 antagonist (CP-99,994) to prevent the noxious inhibition of the vagal baroreflex (Boscan et al. 2002a). We therefore hypothesized that injection of a NK1 antagonist to the NTS in vivo would attenuate the heart rate response to a noxious pinch stimulus. Experiments were performed on anaesthetized adult male Wistar rats (190–250 g, n = 4) to examine the effects of CP-99,994 injected into the NTS. Rats were anaesthetized for surgery with 2–3 % halothane in 100 % oxygen delivered via a nose cone. The left femoral artery was cannulated for arterial pressure monitoring. The atlanto-occipital ligament was incised and the dura reflected to expose the dorsal surface of the medulla. The ECG and respiratory EMG were recorded via subcutaneous electrodes placed over the chest wall. Rat body temperature was monitored (rectal probe) and maintained at 37 °C with a warming mattress and homeostatic feedback circuit.
Once the surgery had been completed the inspired halothane concentration was reduced to between 1 and 1.5 % to the point where only a slight flicker of hindpaw withdrawal was seen in response to pinch. A period of 15 min was allowed for equilibration after changing the inspired halothane concentration to ensure a stable level of anaesthesia. A calibrated pneumatic mechanical pincher (custom-built) was used to stimulate the right 4th toe. Pinches were applied of increasing intensity (1.1–1.9 N mm−2 for 5 s, every 6 min) until a reproducible cardiorespiratory response was obtained (typically a tachycardia, biphasic depressor/pressor arterial pressure response and tachypnoea). Once three consecutive pinches produced consistent cardiorespiratory responses then CP-99,994 (50 μm, 50 nl) was injected bilaterally to the NTS (0.5 mm rostral and lateral to calamus scriptorius and 3–600 μm below the medullary surface). The response to pinch was monitored at 6 min intervals until recovery. After recovery of the response the animal was killed with an overdose of pentobarbital.
Histological analysis
At the end of all experiments one barrel of the micropipette was used to microinject 2 % Pontamine Sky Blue (50 nl) to mark the drug injection sites. The brainstem was removed and fixed in 4 % paraformaldehyde in 0.1 M phosphate-buffered saline containing 30 % sucrose. Transverse sections (40 μm thick) were obtained using a freezing microtome and mounted on subbed glass slides. The sections were counterstained with neutral red and the data included in the statistical analysis were from experiments in which microinjections were confirmed to be within the caudal-intermediate NTS.
Data analysis
All data were relayed via a CED 1401 A-D interface (CED, Cambridge, UK) to a computer running Spike2 software (CED) with custom-written scripts for data acquisition and on- and off-line analysis. Measurements of peak reflex responses of heart rate, phrenic nerve activity cycle length and perfusion pressure were taken. The baroreceptor reflex gain was calculated from the ratio of the Δheart rate/Δ perfusion pressure (beats min−1 mmHg−1). The change in respiratory rate during a pinch was taken from the first complete respiratory cycle during the stimulus as this tended to be the most marked (Fig. 1C) and was uncontaminated by any subsequent application of a perfusion pressure ramp.
Nerve recordings were band-pass filtered (100 Hz to 2 kHz), rectified and integrated (time constant 50 ms). Both sympathetic and vagal recordings exhibited respiratory modulation. Sequential perfusion pressure ramps were therefore timed to peak during the same phase of the respiratory cycle. A custom script was used to ratio the change in nerve activity during the perfusion pressure ramp against the average of two control periods taken from the corresponding phase of preceding respiratory cycles (see Fig. 1B).
Significance of data was assessed using either Student's paired two-tailed t test or Wilcoxon's signed rank test as appropriate (Statview, SAS, Cary, NC, USA). All values quoted are the mean ± standard error of mean (s.e.m.) and ‘n’ is the number of preparations. Differences were taken as significant at the 95 % confidence limit.
Materials
The composition of the modified Ringer solution was (mm): NaCl 125; NaHCO3 24; KCl 5; CaCl2 2.5; MgSO4 1.25; KH2PO4 1.25; dextrose 10; pH 7.3 after carbogenation. Ficoll 70 (1.25 %) was added to the perfusion solution. The following drugs were used in the microinjection experiments: substance P (10 μm to 1 mm); isoguvacine (10 mm); CP-99,994 (50 μm) and Pontamine Sky Blue dye used to mark the sites of microinjection. All drugs were purchased from Sigma, UK, with the exception of CP-99,994 (a gift from Pfizer). The dose range of SP microinjections (500 fmol to 50 pmol) was based on a previous study in our laboratory which showed a 50 % inhibition of the baroreflex bradycardia to be achieved at a dose of ≈1 pmol (Boscan et al. 2002a). These doses permitted recovery of the baroreceptor reflex response following a 15 min washout period.
RESULTS
Experiments were performed using the DAPR preparation (n = 20), the WHBP (n = 9) and anaesthetized rats (n = 4). Using the DAPR preparation, recordings were made from the sympathetic chain. The sympathetic activity exhibited marked respiratory phase-linked modulation with bursting discharge peaking during early post-inspiration (Fig. 1B). Ramp increases in perfusion pressure demonstrated a baroreflex-mediated inhibition of the ongoing sympathetic activity (Fig. 1B) in all sympathetic outflows examined.
Responses to noxious hindlimb pinch in the DAPR
Calibrated hindlimb pinches increased sympathetic nerve activity (212 ± 32 %, n = 8, P < 0.005, Fig. 1C). This was accompanied by significant increases in perfusion pressure (7.6 ± 1.8 mmHg), heart rate (8.4 ± 2.4 beats min−1) and respiratory rate (16 ± 5 breaths min−1). In agreement with our previous observations (Boscan et al. 2002a), noxious pinch attenuated the cardiac vagal bradycardic component of the baroreflex (from a gain of −1.7 ± 0.33 to −0.74 ± 0.21 beats min−1 mmHg−1; P < 0.01, n = 6; Fig. 2). However, noxious pinch failed to significantly attenuate the baroreflex sympathoinhibition (-69 ± 4 % vs.−77 ± 6 %, ns, n = 6; Fig. 2) despite the fact that the level of sympathetic activity was elevated above control by the noxious stimulus.
Figure 2. Effect of noxious pinch on the baroreflex.
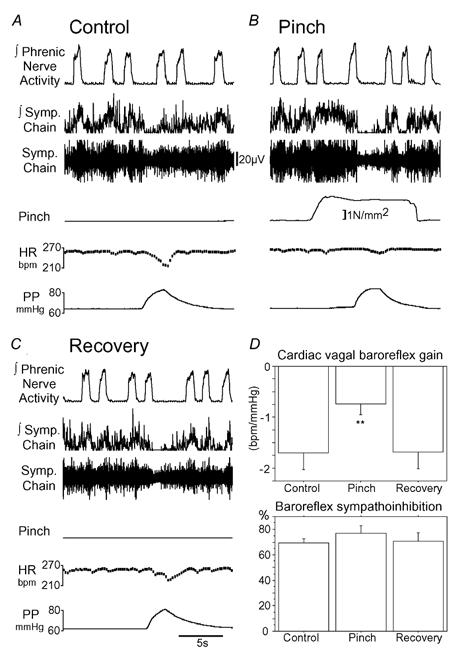
A, control baroreflex response to a perfusion pressure ramp with a bradycardia (gain −2.2 beats min−1 mmHg−1) and sympathoinhibition (87 %). B, noxious pinch of the hindlimb evoked an increase in sympathetic nerve activity. Application of a pressure ramp during the pinch showed that the baroreflex bradycardia was attenuated (gain −0.7 beats min−1 mmHg−1). However, the baroreflex sympathoinhibition (98 %) was unaffected by the noxious pinch. C, the baroreflex bradycardia recovered fully following the pinch. D, pooled data (means ± s.e.m., n = 6) showing that hindlimb pinch reversibly attenuates the baroreflex bradycardia (**P < 0.01) but has no effect on baroreflex sympathoinhibition.
Effect of microinjection of substance P into the NTS on the sympathetic and parasympathetic limbs of the baroreflex
To investigate a possible role for SP in this selective nociceptive effect on the components of the baroreflex we undertook a series of experiments employing microinjection to the NTS. The microinjection of SP (500 fmol, n = 6) was associated with transient increases in perfusion pressure (2.5 ± 0.8 mmHg, P < 0.05) and heart rate (6.0 ± 4.8 beats min−1, ns), and with a fall in respiratory rate (from 22 ± 5 to 18 ± 4 breaths min−1, P < 0.05) that recovered over a period of 5 min. Bilateral microinjection of SP (500 fmol) produced a reversible inhibition of the cardiac vagal baroreflex gain (from −1.81 ± 0.37 to −0.68 ± 0.2 beats min−1 mmHg−1, P < 0.005, n = 6, Fig. 3). In contrast, there was no significant change in the baroreflex inhibition of activity in the thoracic sympathetic chain (-78 ± 4 % vs.−76 ± 6 %, ns, n = 6, Fig. 3). Nor indeed was there any change in the sympathetic baroreflex to doses of SP up to 100-fold higher (50 pmol), which irreversibly attenuated the baroreflex bradycardia (n = 4, data not shown).
Figure 3. Effect of NTS microinjection of substance P on the baroreflex.
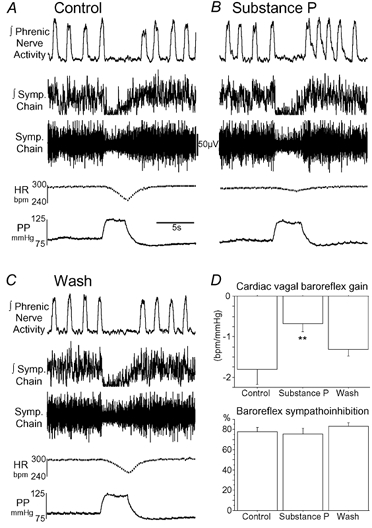
A, control baroreflex response to a perfusion pressure ramp with a bradycardia (gain −1.3 beats min−1 mmHg−1) and sympathoinhibition (96 %). B, after bilateral microinjection of SP to the NTS (500 fmol, 50 nl) the baroreflex bradycardia was attenuated (gain −0.35 beats min−1 mmHg−1) but the sympathoinhibition was unchanged (95 %). C, the baroreflex bradycardia recovered after 15 min of washing. D, pooled data (mean ± s.e.m., n = 6) showing microinjected SP to attenuate the cardiac vagal baroreflex gain (**P < 0.005) without effect on the baroreflex sympathoinhibition.
Does substance P in the NTS selectively affect the baroreflex sympathetic outflow to the heart?
We wished to assess whether the sparing of sympathetic baroreflex function by SP could reflect a differential effect on the baroreflex control of the heart versus the vasculature. Hence we made recordings from the ICN in the WHBP. The ICN displayed respiratory cycle-modulated bursting activity (e.g. Fig. 5). Bilateral NTS microinjection of SP (500 fmol) had no effect on the baroreflex inhibition of the ICN (-81 ± 5 % vs.−84 ± 5 %, ns, n = 6); by contrast the baroreflex bradycardia was attenuated (64 ± 10 % reduction, P = 0.001, n = 6).
Figure 5. Effect of NTS microinjection of isoguvacine on the baroreflex.
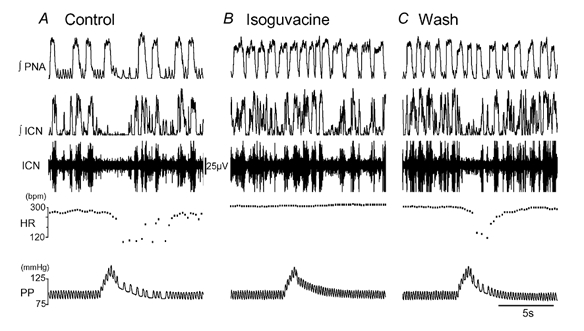
A, recording of the inferior cardiac nerve (ICN) in the WHBP. A perfusion pressure ramp evoked a baroreflex bradycardia and sympathoinhibition. B, NTS microinjection of isoguvacine (500 pmol, 50 nl) increased the respiratory rate and produced an increase in heart rate. The perfusion pressure ramp now failed to evoke either a bradycardia or sympathoinhibition. C, the isoguvacine baroreflex block reversed after 5 min washing. The increase in respiratory rate persisted for a further 10 min.
Does substance P attenuate baroreflex-evoked parasympathetic activity in the cardiac vagal nerve?
Recordings of the CVN showed post-inspiratory bursts of activity. Perfusion pressure ramps evoked graded (data not shown) increases in cardiac vagal activity (Fig. 4). Bilateral NTS microinjection of SP (500 fmol) reversibly attenuated the baroreflex increase in CVN activity (-49 ± 3 %, P = 0.01, n = 4, Fig. 4).
Figure 4. Effect of substance P on cardiac vagal nerve activity in the WHBP.
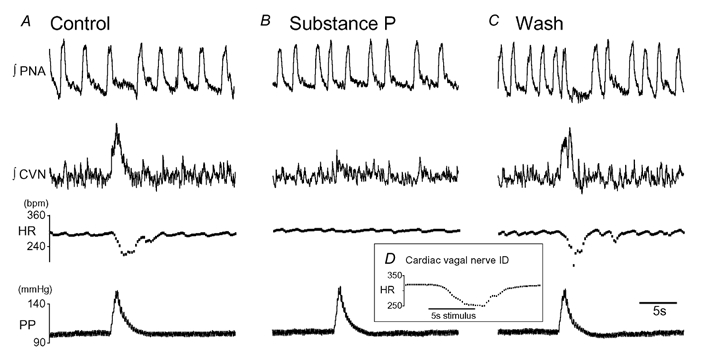
A, recording from cardiac branch of the vagus (CVN). A perfusion pressure ramp evoked a burst of activity in the cardiac vagus followed by a baroreflex bradycardia. B, bilateral NTS microinjection of SP (500 fmol, 50 nl) attenuated the baroreflex bradycardia and reduced the amplitude of the cardiac vagal burst. C, the baroreflex bradycardia and evoked vagal activity returned to control after 15 min wash. D, the cardiac vagal nerve was identified by electrical stimulation of the distal cut end of the cardiac vagus to evoke a bradycardia (5 s train at 10 Hz, pulses 0.2 ms × 40 V).
Is the substance P sparing of the sympathetic baroreflex locus specific within the NTS?
We hypothesized that the lack of effect of SP on the sympathoinhibition might be secondary to an anatomically differential distribution within the NTS of the baroreflex pathways controlling the sympathetic and parasympathetic outflows. However, bilateral microinjections of high-dose SP (50 pmol) at two distinct rostrocaudal sites within the NTS failed to have any effect on the baroreflex sympathoinhibition (n = 3).
We have previously shown that NTS injection of isoguvacine, a GABAA receptor agonist, can reversibly inhibit the baroreflex bradycardia (Boscan & Paton, 2001). We therefore injected isoguvacine (50 nl) into the same locus in the NTS as SP to examine its effect on baroreflex sympathoinhibition. The bilateral injection of isoguvacine (50 nl, 500 pmol) into the NTS (n = 5) produced an increase in heart rate (21.6 ± 8.4 beats min−1, P = 0.07) and a marked tachypnoea (from 22 ± 6 to 41 ± 9 breaths min−1, P < 0.005) but had no effect on the perfusion pressure. This was associated with a pronounced attenuation of both the cardiac vagal baroreflex gain (from −2.2 ± 0.56 to −0.16 ± 0.04 beats min−1 mmHg−1, P < 0.05, n = 4) and the sympathetic baroreflex (from −82 ± 5 % to −6 ± 8 %, P < 0.005, n = 5) recorded from both the ICN (Fig. 5, n = 3) and the thoracic sympathetic chain (n = 2). This attenuation was rapidly reversible after 5–10 min. In all of these experiments SP failed to affect the baroreflex sympathoinhibition when injected to the same location. These data indicate that the neuronal circuits mediating both autonomic components of the baroreflex are accessible to 50 nl injections to the intermediate NTS.
Effect of NTS microinjection of a NK1 receptor antagonist on noxious hindlimb pinch in vivo
In vivo recordings showed noxious pinch of the 4th toe (Fig. 6, n = 4) to evoke a tachycardia (25 ± 7 beats min−1, P < 0.04) associated with tachypnoea (increased by 21 ± 4 breaths min−1, P < 0.02) and a biphasic arterial pressure response with a transient depressor (-5 ± 2 mmHg, P = 0.09) followed by a pressor effect (-11.5 ± 3 mmHg, P = 0.03). The bilateral microinjection of CP-99,994 (50 μm× 50 nl: 2.5 pmol) to the NTS produced a small fall in resting heart rate (11.5 ± 3.8 beats min−1, P = 0.058) without change in arterial pressure or respiratory rate. The injection of the NK1 antagonist attenuated the pinch-evoked tachycardia (reduced to 13 ± 5 beats min−1, P < 0.05) and reduced the pressor component of the pinch response (to 7.3 ± 3 mmHg, P < 0.05). There was no significant change in the tachypnoea (24.5 ± 13.8 beats min−1) or the depressor component of the response (-7.3 ± 2.5 mmHg). The attenuation of respiratory sinus arrhythmia (50 ± 11 % inhibition, P < 0.03) seen during the pinch-induced tachycardia was reduced by CP-99,994 (to 30 ± 2 % inhibition). The effects of CP-99,994 on the pinch responses were reversible over 15–60 min.
Figure 6. Effect of NTS microinjection of CP-99,994 on noxious pinch in vivo.
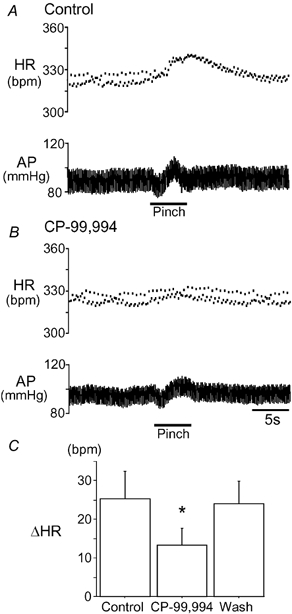
A, the cardiovascular response to mechanical noxious hindpaw pinch was examined in a halothane-anaesthetized rat. The pinch evoked a tachycardia (HR) and a biphasic arterial pressure (AP) response. Note the presence of respiratory sinus arrhythmia seen in the instantaneous heart rate trace as a rhythmic dispersion of consecutive points and its subsequent attenuation during the pinch. B, after the bilateral NTS microinjection of CP-99,994 (NK1 receptor antagonist) the tachycardia was attenuated. C, graph showing the pinch-evoked change in heart rate (mean ± s.e.m., n = 4). CP-99,994 caused a reversible reduction in the magnitude of the pinch-evoked tachycardia (*P < 0.05).
DISCUSSION
Using the decerebrate artificially perfused rat preparation we have been able to record sympathetic nerve activity from a number of outflows in the absence of anaesthetic agents. We have demonstrated that brief increases in arterial pressure are associated with inhibition of sympathetic nerve activity as well as with vagally mediated bradycardia, consistent with activation of the arterial baroreflex. Noxious stimulation of the hindlimb produced an increase in sympathetic activity associated with a pressor, tachycardic and tachypnoeic response. In examining the effect of noxious pinch stimulation (5–10 s) on the baroreflex components, we have been helped by the ability in our artificially perfused preparations to deliver temporally discrete (1–2 s) pressor challenges. This allowed us to follow dynamically any change in baroreflex function induced by noxious pinch stimulation or by the microinjection of neuroactive agents to the NTS.
Our data indicate that somatic nociceptive pinch stimuli selectively attenuate the cardiac parasympathetic but not the sympathetic limb of the baroreflex. We employed a hindlimb pinch to mimic a naturally occurring noxious stimulus. This stimulus is not selective and is likely to activate nociceptors in skin, muscle and perhaps joints and periosteum, and hence activate both Aδ and C fibres entering the spinal cord. Several previous studies have documented attenuation of the vagal component of the baroreflex following both noxious stimulation (Nosaka & Murata, 1989; Boscan et al. 2002a) and electrical stimulation of the sciatic nerve (Quest & Gebber, 1972). However, few studies have examined the effect of noxious stimulation on the sympathetic component of the baroreflex. One study of somatic stimuli on the response to aortic depressor nerve (ADN) stimulation showed that noxious heating of the hindlimb selectively attenuated the baroreflex bradycardia without affecting the depressor action (Nosaka & Murata, 1989). This apparent selective action of noxious heating was different to the effect of electrical stimulation of the sciatic nerve that attenuated both the bradycardia and the depressor response to ADN stimulation. It would therefore appear that the selective modulation of the baroreflex outflows seen with certain noxious stimulii can be different to the global baroreflex attenuation described for either the exercise pressor response (Mitchell et al. 1983) or indeed for the defence response (Hilton, 1982).
We have previously proposed a role for SP in mediating the nociception-evoked attenuation of cardiac vagal baroreflex activity (Boscan et al. 2002a). This suggestion is strengthened by our observation that SP microinjected to the NTS selectively attenuates the cardiac parasympathetic but not the sympathetic components of the baroreflex. The stimulation of hindlimb muscle afferents has been shown to release SP in the NTS (Potts et al. 1999). Furthermore, we show here that the microinjection of a NK1 antagonist to the NTS in vivo reversibly attenuated the tachycardic response to noxious pinch. This leads us to propose a functional role for SP within the NTS as its release appears to mediate the nociceptive modulation of the baroreflex bradycardia.
The action of the NK1 antagonist to attenuate the pinch-evoked tachycardia in vivo may solely reflect disinhibition of the vagal baroreflex during the noxious pinch. Indeed we have previously demonstrated such disinhibition of the vagal baroreflex by a NK1 antagonist in the working heart-brainstem preparation (Boscan et al. 2002a). However, we cannot exclude that there may also be a second effect of SP released by noxious stimulation in vivo upon NK1 receptors within the NTS that evokes a tachycardic and pressor effect independently of its inhibition of the vagal baroreflex.
A number of anatomical studies have suggested an important role for SP within the NTS. There is a high density of SP-immunoreactive nerve terminals within the NTS (Nakaya et al. 1994; Massari et al. 1998) and the NK1 receptor is prominently expressed by neurones within the NTS (Nakaya et al. 1994). There are SP-containing neurones within the NTS (Ljungdahl et al. 1978; Harlan et al. 1989) as well as SP projections from other CNS sites. From our studies we are unable to distinguish whether the SP released within the NTS by noxious pinch comes from NTS neurones or from projection neurones. However, it is worth noting that there are direct projections to the NTS from the dorsal horn (laminae I, V and X) and from the trigeminal nucleus caudalis. These substance P-containing projections have been proposed as the neuronal substrate for noxious somato-visceral interaction (Torvik, 1956; Menetrey & Basbaum, 1987; Esteves et al. 1993, 2001b). In particular the spinal lamina I neurones projecting to the NTS, which have been suggested to be collaterals of the spino-parabrachial projection (Gauriau & Bernard, 2002), may play a key role in somatic nociceptive autonomic responses. These projection neurones have been shown to have excitatory phenotypes containing either glutamate or SP immunoreactivity (Esteves et al. 2001a). A proportion of these lamina I neurones also express NK1 receptors, which has been suggested to indicate a role in the transmission of nociceptive information rostrally from the dorsal horn (Todd et al. 2000).
The role of SP in cardiovascular regulation within the NTS has been the subject of extensive scrutiny that has produced contradictory findings (see review by Sapru, 1994). Our observed SP attenuation of the baroreflex gain is supported by a report that SP in rats attenuates the response to carotid sinus nerve stimulation (Perez et al. 1992). However, in a study of anaesthetized, vagotomized dogs, NTS microinjection of SP was reported to increase baroreflex gain (Seagard et al. 2000). This study examined the depressor response evoked by increasing pressure in an isolated carotid sinus, i.e. the sympathetic limb of the baroreflex. We have been unable to demonstrate a potentiation of the baroreflex sympathoinhibition by SP. This may reflect a species difference or an effect of anaesthesia. Further observations in the dog on the effect of SP on the cardiac vagal component of the baroreflex are warranted to address this discrepancy.
The ability of SP to selectively attenuate the cardiac parasympathetic component of the baroreflex by an action within the NTS suggests that some neurones within the nucleus are already committed to cardiac parasympathetic versus sympathetic outflows. We have previously shown that both nociceptive and SP baroreflex attenuation can be blocked by the GABAA receptor antagonist bicuculline (Boscan et al. 2002a). A similar result has been observed where the SP attenuation of the response to carotid sinus nerve stimulation was prevented by bicuculline (Perez et al. 1992). We suggest that SP is acting to excite NTS GABAergic interneurones, which inhibit the NTS pathway to the cardiac vagal motoneurones but not the pathway to the cardiac sympathetic outflow (Fig. 7). Based on the selective inhibition of the cardiac parasympathetic but not sympathetic components of the baroreflex, we would postulate that there are two pools of GABAergic interneurones of which only one pool has NK1 receptors.
Figure 7. Schematic model of effect of noxious stimulation on baroreflex within the NTS.
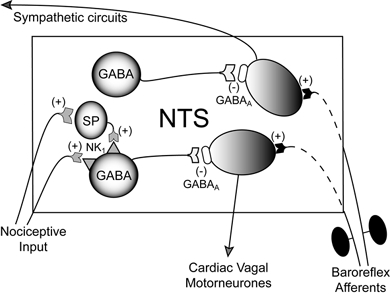
Based on our findings we propose that within the NTS there is a divergence in the baroreflex pathways to the sympathetic and parasympathetic centres. These pathways are capable of being independently regulated by GABAergic interneurones. One population of GABAergic interneurones are excited by SP (via NK1 receptors) released in response to noxious stimulation. These interneurones selectively inhibit (via GABAA receptors) the circuit projecting to the cardiac vagal motoneurones. There are also GABAA receptors within the NTS on the path to the sympathetic outflow. The source of the SP may be from either neurones intrinsic to the NTS or from projection neurones.
Our observation that NTS microinjections of isoguvacine, a selective GABAA agonist, attenuates both the sympathetic and parasympathetic components of the baroreflex indicates that the NTS baroreflex pathways to both the sympathetic and parasympathetic systems contain GABAA receptors. Indeed, we have previously demonstrated that microinjection of angiotensin II (AII) to the NTS attenuates both the vagal and cardiac sympathetic limbs of the baroreflex (Boscan et al. 2001) via the activation of GABAergic interneurones (Paton et al. 2001). These data indicate the vital role of NTS GABAergic interneurones in shaping the autonomic responses to afferent baroreflex information and they provide a means by which putative neuromodulators such as SP and AII can alter NTS processing.
There is some supporting evidence to indicate that the pathways within the NTS projecting to the parasympathetic and sympathetic centres may have differing pharmacological sensitivities. Using in vivo microinjections it has been suggested that the baroreflex bradycardia is particularly sensitive to ionotropic glutamate receptor antagonists whereas the baroreflex sympathoinhibition is relatively resistant (Machado et al. 2000). These data may indicate that there are different circuits for processing information destined for parasympathetic versus sympathetic integrating stations. Further, there are reported differences in the arterial pressure and heart rate responses to selective stimulation of C- and Aδ-fibres in the aortic depressor nerve of rats (Fan et al. 1999). This again suggests that the NTS is capable of differential processing of baroreflex inputs to separately influence sympathetic and parasympathetic outflows.
The differential modulation of the cardiac parasympathetic and sympathetic components of the baroreflex arc by noxious stimulation has not been reported previously. The attenuation of the cardiac vagal baroreflex permits noxious stimuli to cause an increase in heart rate and hence cardiac output. By contrast, the preservation of the sympathetic baroreflex may limit any pressor response to nociceptive stimulation and by causing vasodilatation assist the distribution of the increased blood flow to tissues such as the muscle vascular beds.
Acknowledgments
Thanks to Max Headley for his encouragement and critical input and to Simon Lishman for technical assistance. A.E.P. is supported by a British Journal of Anaesthesia/Royal College of Anaesthetists grant. J.Fp.R.P. is funded by the British Heart Foundation (BS/93003). P.B. was a University of Bristol Postgraduate Scholar.
REFERENCES
- Barraco IRA. Nucleus of the Solitary Tract. Boca Raton, FL, USA: CRC Press; 1994. [Google Scholar]
- Blessing WW. The Lower Brainstem and Bodily Homeostasis. New York: Oxford University Press; 1997. Anatomy of the lower brainstem; pp. 29–101. [Google Scholar]
- Boscan P, Allen AM, Paton JF. Baroreflex inhibition of cardiac sympathetic outflow is attenuated by angiotensin II in the nucleus of the solitary tract. Neuroscience. 2001;103:153–160. doi: 10.1016/s0306-4522(00)00559-5. [DOI] [PubMed] [Google Scholar]
- Boscan P, Kasparov S, Paton JFR. Somatic nociception activates NK1 receptors in the nucleus tractus solitarii to attenuate the baroreceptor cardiac reflex. Eur J Neurosci. 2002a;16:907–920. doi: 10.1046/j.1460-9568.2002.02131.x. [DOI] [PubMed] [Google Scholar]
- Boscan P, Paton JF. Role of the solitary tract nucleus in mediating nociceptive evoked cardiorespiratory responses. Auton Neurosci. 2001;86:170–182. doi: 10.1016/S1566-0702(00)00255-1. [DOI] [PubMed] [Google Scholar]
- Boscan P, Pickering AE, Paton JF. The nucleus of the solitary tract: an integrating station for nociceptive and cardiorespiratory afferents. Exp Physiol. 2002b;87:259–266. doi: 10.1113/eph8702353. [DOI] [PubMed] [Google Scholar]
- Deuchars J, Li YW, Kasparov S, Paton JF. Morphological and electrophysiological properties of neurones in the dorsal vagal complex of the rat activated by arterial baroreceptors. J Comp Neurol. 2000;417:233–249. [PubMed] [Google Scholar]
- Esteves FOG, Kaye JC, McWilliam PN, Lima D, Batten TF. Immunohistochemical profiles of spinal lamina I neurones retrogradely labelled from the nucleus tractus solitarii in rat suggest excitatory projections. Neuroscience. 2001a;104:523–538. doi: 10.1016/s0306-4522(01)00071-9. [DOI] [PubMed] [Google Scholar]
- Esteves F, Lima D, Coimbra A. Structural types of spinal cord marginal (lamina I) neurons projecting to the nucleus of the tractus solitarius in the rat. Somatosens Mot Res. 1993;10:203–216. doi: 10.3109/08990229309028832. [DOI] [PubMed] [Google Scholar]
- Esteves FOG, Tavares I, Almeida A, Batten TF, McWilliam PN, Lima D. Projection sites of superficial and deep spinal dorsal horn cells in the nucleus tractus solitarii of the rat. Brain Res. 2001b;921:195–205. doi: 10.1016/s0006-8993(01)03118-3. [DOI] [PubMed] [Google Scholar]
- Fan W, Schild JH, Andresen MC. Graded and dynamic reflex summation of myelinated and unmyelinated rat aortic baroreceptors. Am J Physiol. 1999;277:R748–756. doi: 10.1152/ajpregu.1999.277.3.R748. [DOI] [PubMed] [Google Scholar]
- Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 2002;87:251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- Harlan RE, Garcia MM, Krause JE. Cellular localization of substance P- and neurokinin A-encoding preprotachykinin mRNA in the female rat brain. J Comp Neurol. 1989;287:179–212. doi: 10.1002/cne.902870204. [DOI] [PubMed] [Google Scholar]
- Hilton SM. The defence-arousal system and its relevance for circulatory and respiratory control. J Exp Biol. 1982;100:159–174. doi: 10.1242/jeb.100.1.159. [DOI] [PubMed] [Google Scholar]
- Janig W. Systemic and specific autonomic reactions in pain: efferent, afferent and endocrine components. Eur J Anaesthesiol. 1985;2:319–346. [PubMed] [Google Scholar]
- Ljungdahl A, Hokfelt T, Nilsson G. Distribution of substance P-like immunoreactivity in the central nervous system of the rat – I. Cell bodies and nerve terminals. Neuroscience. 1978;3:861–943. doi: 10.1016/0306-4522(78)90116-1. [DOI] [PubMed] [Google Scholar]
- Machado BH, Castania JA, Bonagamba LG, Salgado HC. Neurotransmission of autonomic components of aortic baroreceptor afferents in the NTS of awake rats. Am J Physiol. 2000;279:H67–75. doi: 10.1152/ajpheart.2000.279.1.H67. [DOI] [PubMed] [Google Scholar]
- Massari VJ, Shirahata M, Johnson TA, Lauenstein JM, Gatti PJ. Substance P immunoreactive nerve terminals in the dorsolateral nucleus of the tractus solitarius: roles in the baroreceptor reflex. Brain Res. 1998;785:329–340. doi: 10.1016/s0006-8993(97)01335-8. [DOI] [PubMed] [Google Scholar]
- Menetrey D, Basbaum AI. Spinal and trigeminal projections to the nucleus of the solitary tract: a possible substrate for somatovisceral and viscerovisceral reflex activation. J Comp Neurol. 1987;255:439–450. doi: 10.1002/cne.902550310. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Nakaya Y, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat. J Comp Neurol. 1994;347:249–274. doi: 10.1002/cne.903470208. [DOI] [PubMed] [Google Scholar]
- Nosaka S, Murata K. Somatosensory inhibition of vagal baroreflex bradycardia: afferent nervous mechanisms. Am J Physiol. 1989;257:R829–838. doi: 10.1152/ajpregu.1989.257.4.R829. [DOI] [PubMed] [Google Scholar]
- Paton JFR. A working heart-brainstem preparation of the mouse. J Neurosci Methods. 1996;65:63–68. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- Paton JF, Boscan P, Murphy D, Kasparov S. Unravelling mechanisms of action of angiotensin II on cardiorespiratory function using in vivo gene transfer. Acta Physiol Scand. 2001;173:127–137. doi: 10.1046/j.1365-201X.2001.00898.x. [DOI] [PubMed] [Google Scholar]
- Perez H, Ruiz S, Inostroza H, Perretta M. Substance P depresses bioelectrical responses evoked in the nucleus tractus solitarii: interaction with gamma-aminobutyric acid-ergic neurons. Eur J Pharmacol. 1992;213:435–437. doi: 10.1016/0014-2999(92)90633-f. [DOI] [PubMed] [Google Scholar]
- Pezzone MA, Lee WS, Hoffman GE, Pezzone KM, Rabin BS. Activation of brainstem catecholaminergic neurons by conditioned and unconditioned aversive stimuli as revealed by c-Fos immunoreactivity. Brain Res. 1993;608:310–318. doi: 10.1016/0006-8993(93)91472-5. [DOI] [PubMed] [Google Scholar]
- Pickering AE, Boscan P, Paton JFR. Differential modulation of baroreflex autonomic outputs by noxious stimulation: a role for substance P in the nucleus of the solitary tract. J Physiol. 2003;547.P:C12. [Google Scholar]
- Pickering AE, Waki H, Headley PM, Paton JFR. Investigation of systemic bupivacaine toxicity using the in situ perfused working heart-brainstem preparation of the rat. Anesthesiology. 2002;97:1550–1556. doi: 10.1097/00000542-200212000-00030. [DOI] [PubMed] [Google Scholar]
- Potts JT, Fuchs IE, Li J, Leshnower B, Mitchell JH. Skeletal muscle afferent fibres release substance P in the nucleus tractus solitarii of anaesthetized cats. J Physiol. 1999;514:829–841. doi: 10.1111/j.1469-7793.1999.829ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quest JA, Gebber GL. Modulation of baroreceptor reflexes by somatic afferent nerve stimulation. Am J Physiol. 1972;222:1251–1259. doi: 10.1152/ajplegacy.1972.222.5.1251. [DOI] [PubMed] [Google Scholar]
- Sapru HN. Transmitter/receptor mechanisms in cardiovascular control by the NTS; excitatory amino acids, acetylcholine and substance P. In: Barraco ARA, editor. Nucleus of the Solitary Tract. Boca Raton, FL, USA: CRC Press; 1994. pp. 267–287. [Google Scholar]
- Seagard JL, Dean C, Hopp FA. Modulation of the carotid baroreceptor reflex by substance P in the nucleus tractus solitarius. J Auton Nerv Syst. 2000;78:77–85. doi: 10.1016/s0165-1838(99)00060-0. [DOI] [PubMed] [Google Scholar]
- Todd AJ, McGill MM, Shehab SA. Neurokinin 1 receptor expression by neurons in laminae I, III and IV of the rat spinal dorsal horn that project to the brainstem. Eur J Neurosci. 2000;12:689–700. doi: 10.1046/j.1460-9568.2000.00950.x. [DOI] [PubMed] [Google Scholar]
- Toney GM, Mifflin SW. Sensory modalities conveyed in the hindlimb somatic afferent input to nucleus tractus solitarius. J Appl Physiol. 2000;88:2062–2073. doi: 10.1152/jappl.2000.88.6.2062. [DOI] [PubMed] [Google Scholar]
- Torvik A. Afferent connections to the sensory trigeminal nuclei, the nucleus of the solitary tract and adjacent structures. An experimental study in the rat. J Comp Neurol. 1956;106:51–142. doi: 10.1002/cne.901060104. [DOI] [PubMed] [Google Scholar]


