Abstract
Mechanical loading is known to increase connective tissue blood flow of human tendons and to cause local release of vasodilatory substances. The present study investigated the importance of prostaglandins (PG) formed by cyclo-oxygenase isoforms (COX-1 and 2) for the exercise-related increase in blood flow in connective tissue. Healthy individuals (n = 24, age: 23–31 years) underwent 30 min of intermittent, isometric, plantarflexion with both calf muscles either without (n = 6, Control, C) or with blockade of PG formation, either COX-2 specific (n = 10, Celecoxib 2 × 100 mg day−1 for 3 days prior to the experiment) or COX unspecific (n = 8, indomethacin 100 mg (12 and 1 h pre-experiment) and acetyl salicylic acid 500 mg day−1 for 3 days pre-experiment). Prostaglandin E2 (PGE2) concentration was determined by microdialysis and blood flow by 133Xe washout. In C, interstitial PGE2 rose from (0.8 ± 0.2 (rest) to 1.4 ± 0.5 ng ml−1 (exercise), P < 0.05), whereas during unspecific COX inhibition, tissue PGE2 was completely inhibited at rest and during exercise. COX-2 specific blockade did not inhibit tissue PGE2 at rest, but totally abolished the exercise induced increase. Blood flow was similar in the three groups at rest (P > 0.05), whereas the increase in flow with exercise was reduced by 35 and 43 % with COX-2 specific blockade (3.2 ± 0.7 to 6.1 ± 1.5 ml (100 g tissue)−1 min−1 or COX unspecific blockade (3.0 ± 0.8 to 7.6 ± 1.6), respectively, compared to C (2.7 ± 0.8 to 10.2 ± 2.0)(P < 0.05). The findings indicate that COX-2 specific mechanisms are responsible for the exercise-induced increase in prostaglandin synthesis, and that increase in tissue prostaglandin plays an important role for blood flow in peritendinous connective tissue during physical loading in vivo.
Eicosanoids such as prostaglandins and thromboxanes are important mediators of several biological responses such as inflammation, platelet activation and tissue perfusion (Ostrom et al. 2001; Ouellet et al. 2001), and formation of these is dependent on the presence of cyclo-oxygenase (COX; O'Banion, 1999). Isoforms of COX expressed in several cell types are considered to be either constitutive (COX-1) or predominantly inducible e.g. by inflammation (COX-2), although some overlap and especially redundancy has been demonstrated (Vane et al. 1994, 1998; Ballou et al. 2000). The use of selective COX-2 inhibitors has been suggested to result in an increased vascular tone (McAdam et al. 1999; Catella-Lawson et al. 1999; Muscara et al. 2000). Thus, prostanoids may play a major role for blood flow regulation in various local regions of the musculo-skeletal system that is subjected to mechanical loading in humans. Despite being clearly expressed in both peripheral and central tissues (Samad et al. 2001), as well as evident in circulating blood, cyclo-oxygenase mediated formation of prostaglandins and resultant tissue concentrations has been difficult to assess in vivo in humans during stressful stimuli.
Muscular activity is associated with marked increases in blood flow of the contracting extremity, and it has been demonstrated that this includes not only contracting muscle but also the flow through adjacent tendon-related connective tissue, which rises up to 7-fold with exercise (Langberg et al. 1998; Boushel et al. 2000). Whereas in skeletal muscle tissue a significant vasodilatory role of prostanoids during exercise is debatable, other factors may play a larger role (Wilson & Kapoor, 1993; Davy et al. 1993; Duffy et al. 1998). The regulation of blood flow in tendon-related connective tissue with exercise remains unexplained and the role of prostaglandins in relation to this has never been studied. Interestingly, formation of inflammatory mediators presents a major problem in overuse injuries of the locomotor system, and aims to reduce prostanoid levels pharmacologically with non-steroidal anti-inflammatory drugs (NSAIDs) is widely used in clinical practice (Kurumbail et al. 1996).
The present study evaluated the importance of prostaglandin for connective tissue blood flow during physical stress. This was done by using differential blockade of cyclo-oxygenase in human subjects exercising with their calf muscles, and by determining interstitial concentrations of prostaglandin E2 in the peritendinous tissue of the Achilles tendon together with measurements of tissue blood flow using 133Xe-washout technique. It is hypothesized that blockade of cyclo-oxygenase activity will decrease tissue blood flow both at rest and during exercise.
METHODS
Subjects and medication
Twenty-four healthy young males (age: 23–31 years (range)) participated after informed written consent in this study was given and approved by the Ethical Committee of Copenhagen (KF 11–043/02) and performed in accordance with the Declaration of Helsinki. Subjects were randomised to three groups either receiving placebo (control, n = 6), unspecific blockade of cyclo-oxygenase by indomethacin (100 mg at 12 and 1 h prior to the experiment) and acetylsalicylic acid (500 mg per day, taken for 3 days prior to experiment; COX-1 and COX-2, n = 8) or receiving a selective COX-2 inhibitor Celebra (Celecoxib, Pfizer Inc. Groton, USA; Gierse et al. 1999; 100 mg twice daily for 3 days prior to the experiment; COX-2, n = 10). Subjects were blinded to the type of medication they received and were carefully instructed to take the medication as prescribed. Prior to the study the subjects were questioned to ensure that prescriptions were followed. None of the individuals took any other medicine regularly, and all were free from diseases and complaints of the musculo-skeletal system. Specifically, none of the subjects had signs of any overuse injuries in their muscles or tendons, nor did any have a previous history of Achilles tendon symptoms. The three experimental groups resembled each other with regard to age, anthropometric data and level of physical activity. All subjects were non-smokers.
In vivo microdialysis
A microdialysis catheter (CMA, Solna, Sweden) under ultra-sonography guidance was placed adjacent to the anterior border of the Achilles tendon (Fig. 1) as previously described with details in Langberg et al. 1999c,d. The microdialysis catheter (CMA 60; CMA/Microdialysis AB; 20 kDa molecular cut off, 0.5 mm outer diameter; length 30 mm) was perfused with Ringer-acetate solution and radioactive labelled with [15-3H(N)]PGE2 (specific activity 3.7 GBq (mmol)−1; NEN, Boston, MA, USA) at 2 μl min−1 using a high-precision syringe pump (CMA 100, Carnegie Medicine, Solna, Sweden). Units of 30 μl were sampled at the outlet of the microdialysis catheter for determination of PGE2 concentrations.
Figure 1. Microdialysis in human peritendinous connective tissue.
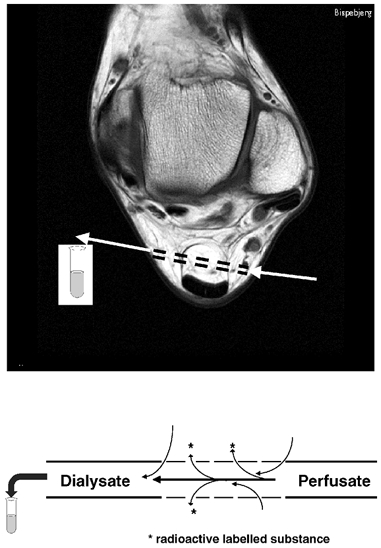
Illustration of the positioning of the microdialysis catheter in the peritendinous Achilles region as depicted on a magnetic resonance cross section image at the malleoli ankle level. Below, a schematic representation of microdialysis tubes used to determine interstitial concentrations of prostaglandin E2.
The interstitial concentrations (Ci) were calculated using the internal reference calibration method (Scheller & Kolb, 1991). Three microlitres of perfusate were added to 3 ml of liquid scintillation medium (Ultima Gold, Packard, Gronningen, Netherlands) and measured in a β -counter (Wallac 1409, Wallac, Turko, Finland). The relative recovery (RR) was calculated for each microcatheter as: (Cp–Cd)/Cp, where Cp is disintegration min−1 in the perfusate and Cd is disintegration min−1 in the dialysate. It is assumed that RR from interstitial fluid to perfusate of unlabelled procollagen molecule equals relative loss from perfusate to interstitial fluid of labelled collagen molecule.
Blood flow
Peritendinous blood flow was determined by administration of 133Xe (in isotonic saline, ≈10 MBq ml−1, 0.1 ml) injected directly ventrally to the Achilles tendon. Great care was taken not to inject any gas bubbles. The injection was made with a fine needle (outer diameter 0.4 mm) from the medial side at a depth of 1–2 cm. The depot was placed 5 cm proximal to the upper medial portion of the Achilles tendon insertion on the calcaneus on both right and left side. The needle was withdrawn from the tissue 0.5 min after the injection had been given to ensure that no leak appeared. The 133Xe washout was measured via portable scintillation detectors strapped to the skin above the 133Xe depots. The detectors were connected to a multichannel analyser system (Oakfield Instruments, Oxford, UK). The initial counting rate was ≈1.5 × 103 s−1. Counts were collected in 30 s periods.
Calculations of blood flow
From the clearance rate of 133Xe (Fig. 2) it is possible to calculate the blood flow (b.f.) in millilitres per 100 g tissue per minute, when the tissue-blood blood partition coefficient λ (being 5–10 μC g tissue−1/μC ml blood−1 in adipose tissue) is known (Kety, 1951):
Figure 2. An example of the 133Xe clearance curve for one subject.
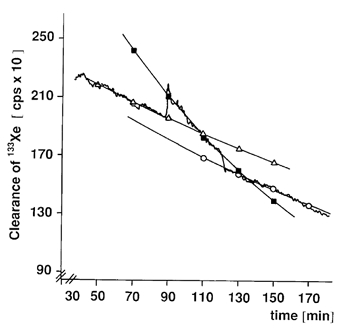
The clearance rate was measured during a resting period (0–90 min). The resting period was followed by a series of intermittent isometric exercises of the calf muscles (contraction 1.5 s-rest 1.5 s; 90–120 min). The study was terminated by a recovery phase of rest (120–180 min). For determining blood flow elimination rate the constant κ for the three mono-exponential curves fitting the various 133Xe washout curves were used. The various elimination-rate constants used were: rest (▵) κ = −4.4 × 10−5 (R = −0.98); exercise (▪) κ = −11.6 × 10−5 (R = −0.99); and recovery (○) κ = −6.4 × 10−5 (R = −0.99) respectively. Reprinted with permission from Clinical Physiology (Langberg et al. 1999a).
b. f. = −100 λκ,
where κ (ml (100 g tissue)−1 min−1) is the elimination rate constant for the mono-exponential washout of 133Xe (Lassen et al. 1964).
Previous studies have excluded any influence of lymph drainage on peritendinous blood flow during exercise (Langberg et al. 1999a).
Experimental protocol
After insertion of the microdialysis catheters and positioning of the xenon depots the subjects rested 120 min before starting the experiment to minimize the influence of the insertion procedure (Langberg et al. 1999c). Following this pre-period the subjects rested for additional 90 min during which the resting blood flow and resting tissue concentration was measured (Langberg et al. 1999a). The resting period was followed by an exercising period. During exercise the subjects were seated in a specially constructed experimental set up (Fig. 3), with the trunk perpendicular to the seat and the knees extended as previously described (Langberg et al. 1999a). The study was terminated by a recovery phase of 60 min rest. All subjects performed 30 min of intermittent static plantar flexion exercise (1.5 s contraction, 1.5 s relaxation in time with a metronome) at a torque output of 800 ± 50 N m per contraction (equivalent to individual body weight). The load imitated the workload of the triceps muscles during normal walking. Visual feedback ensured a constant force output during contraction.
Figure 3. A schematical drawing of the experimental set up.
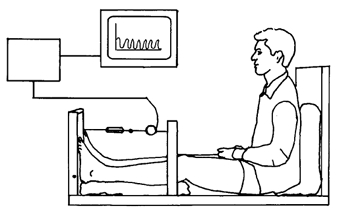
The subject is seated with the trunk perpendicular to the seat, the knee extended and both feet positioned on the vertical sheet with the axis of the sheet and the axis of plantar-dorsal flexion in the ankle joint aligned. The torque moment developed by m. triceps surae of both legs in the plantar direction is registered by a precalibrated (range: 0–2000 N) strain gauge (lever arm: 28 cm). The torque is amplified by a costume-build instrumental AC-amplifier and displayed on-line to the subject. Reprinted with permission from Clinical Physiology (Langberg et al. 1999a).
Immunohistochemistry
Blood samples were all added to indomethacin (10 μg ml−1, Sigma) and 4.5 mm EDTA, centrifuged at 2000 g for 10 min, and the plasma used for analysis.
Prostaglandin E2 concentrations both in plasma and in microdialysate were analysed using a commercially available PGE2 radioimmuno-assay kit (RIA KIT catalogue number NEK-020; NEN Research Products, Du Pont, Boston, USA). Samples or standards, together with 125I-PGE2 as the tracer, were incubated with rabbit anti-PGE2 antibodies overnight at 4 °C. The samples were precipitated by polyethylene glycol, centrifuged, decanted and the quantity of radioactivity in the pellet was determined in a gamma counter.
Statistics
All data are means ± s.e.m. or the range. Mann-Whitney's or Wilcoxon's non-parametric summed rank tests were used to test differences between groups or within groups, respectively. P < 0.05 (two-tailed test) was considered significant.
RESULTS
It was shown that the relative recovery for PGE2 was 44 ± 5 % at rest and 47 ± 5 during muscle contraction, and these results were used for determination of interstitial concentrations of PGE2 in peritendinous connective tissue using the internal reference calibration method (Scheller & Kolb, 1991).
In the control group, the interstitial tissue PGE2 concentration rose in response to exercise (0.8 ± 0.2 (rest) to 1.4 ± 0.5 ng ml−1 (exercise), P < 0.05; Fig. 4), whereas during unspecific cyclo-oxygenase inhibition (COX-1 and COX-2) tissue PGE2 was inhibited by 96 % and no increase in PGE2 was observed during exercise (0.03 ± 0.01 (rest) and 0.03 ± 0.01 ng ml−1 (exercise; Fig. 4)). COX-2 specific blockade did not inhibit tissue PGE2 at rest, but totally abolished the exercise-induced increase (0.8 ± 0.1 (rest) to 0.7 ± 0.2 ng ml−1 (exercise; Fig. 4)).
Figure 4. Tissue prostaglandin concentrations around the human Achilles tendon, and the effect of cyclo-oxygenase blockade at rest and during physical activity.
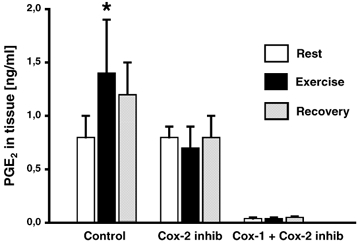
Interstitial tissue concentrations of prostaglandin E2 (PGE2) in human connective tissue around the Achilles tendon was determined using microdialysis in vivo. Intermittent, isometric plantar flexion was performed for 30 min by healthy males, either without (Control) or with blockade of cyclo-oxygenase 1 and 2 (indomethacin and acetyl salicylic acid) (COX-1and COX-2) or cyclo-oxygenase-2 (Celecoxib) (COX-2). *P < 0.05 vs. resting values.
Blood flow was similar in the three groups at rest (3.2 ± 0.7 (COX-2), 3.0 ± 0.8 (COX-1 and −2), and 2.7 ± 0.8 ml (100 g tissue)−1 min−1 (C), P > 0.05; Fig. 5), whereas during exercise the increase in flow was significantly reduced (P < 0.05) in the COX-2 (to 6.1 ± 1.5 ml (100 g tissue)−1 min−1) and COX unspecific inhibited group (to 7.6 ± 1.6 ml (100 g tissue)−1 min−1), respectively, compared with control (to 10.2 ± 2.0 ml (100 g tissue)−1 min−1) (Fig. 5).
Figure 5. The effect of cyclo-oxygenase blockade on the connective tissue blood flow aound the human Achilles tendon at rest and during physical activity.
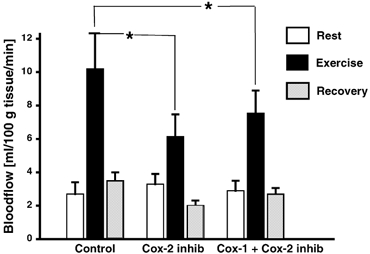
Blood flow in human connective tissue around the Achilles tendon determined using the 133 Xe wash out method. Intermittent, isometric plantar flexion was performed for 30 min by healthy males, either without (Control) or with blockade of cyclo-oxygenase 1+2 (indomethacin and acetylsalicylic acid) (COX-1and COX-2) or cyclo-oxygenase-2 (Celecoxib) (COX-2). *P < 0.05 vs. resting values.
DISCUSSION
The present study demonstrates several findings. First, it is possible to detect tissue concentrations of prostaglandins in humans both during rest and exercise in humans (Fig. 4). Second, it is shown that a blockade of cyclo-oxygenase inhibits the exercise-induced increase in prostaglandin synthesis, and that a cyclo-oxygenase-2 specific mechanism is responsible for this inhibition (Fig. 4). Third, and maybe most importantly, the present study shows that the cyclo-oxygenase-2 mediated inhibition of tissue prostaglandin synthesis during exercise plays an important role for the increased tissue blood flow in peritendinous connective tissue during muscular contractions and physical loading of human tendons in vivo (Fig. 5).
The findings of the present study suggest a differentiated role of the vasodilatory agents in the human peritendinous area, depending on whether the tissue is at rest or metabolic stressed as during muscular activity (Boushel et al. 2000) by demonstrating a separate and pronounced role of prostaglandins in exercise vasodilatation of connective tissue (Fig. 4 and Fig. 5). In support of this notion, it has recently been shown that IL-1β-induced COX-2, but not COX-1 formation in isolated human tendon fibroblasts, and that this was followed by PGE2 synthesis (Tsuzaki et al. 2003). Somewhat in contrast, the regulation of vasodilation in skeletal muscle at rest, as well as during exercise, has previously proved redundancy with regards to the interplay between PGE2, nitric oxide (NO) and endothelial-derived hyperpolarising factor (EDHF; Pohl et al. 2000). Despite the fact that some studies have been able to identify a separate role for prostaglandin in the regulation of flow in muscle in the resting state (Duffy et al. 1998), it has been questionable as to whether there is an effect of prostaglandin-synthesis blockade on skeletal muscle blood flow in humans during exercise and reactive hyperaemia (Wilson & Kapoor, 1993; Davy et al. 1993; Engelke et al. 1996). This indicates that regulation of blood flow in tissue regions dominated by connective tissue in close proximity to the tendon differ from that of contracting skeletal muscle itself. In this respect, it is interesting that earlier studies have found that the site of formation of prostaglandins in skeletal muscle was not located within the muscle cell, but rather originated from connective tissue of fascia and mysial sheets as well as from the vascular endothelium, and also that tendon connective tissue was the major source for prostaglandin formation in mature tendon and/or skeletal muscle preparations (McLennan & Macdonald, 1991). This also fits with a previous demonstration of a local prostaglandin release from peritendinous tissue in response to prolonged running exercise (Langberg et al. 1999d). In that study it was shown, that the microdialysis technique in itself created a short lasting insertion trauma-related increase in tissue prostaglandin and thromboxane for 90–120 min, and therefore all determinations in the present study are always performed more than 2 h after catheter insertion (Langberg et al. 1999c).
Although the present findings indicate that prostanoid formation is important for an increase in tendon blood flow during exercise and that blockade of prostaglandin formation is associated with a ≈40 % reduction of this increase, it remains clear that a large part of the blood flow increase during muscular contractions remains present even in the absence of changes in tissue prostaglandin concentrations. What factors are responsible for the remaining response can only be speculated upon, but NO and EDHF cannot be excluded. Such a hypothesis is supported by the finding of increased connective tissue concentrations of bradykinin in response to exercise in humans (Langberg et al. 2002). A purely mechanical phenomenon similar to the muscle pump pulling blood through the connective tendon region cannot be excluded from contributing to exercise-induced increase in blood flow, as it has been found that a pronounced negative interstitial fluid pressure in the Achilles peritendinous region occurs during exercise in parallel with increased regional blood flow (Langberg et al. 1999b).
The fact that COX-2 inhibition abolished the exercise-induced rise in prostaglandin, whereas unspecific cyclo-oxygenase resulted in an almost total inhibition of the entire prostaglandin formation (Fig. 4), fits very well with animal models in which prostaglandin production was induced by carrageenan-induced paw inflammation in rats (Anderson et al. 1996; Portanova et al. 1996; Zhang et al. 1997) or by a subcutaneous air pouch in mice (Vane et al. 1994). Local tissue concentrations cannot be directly compared between those models and the findings in the present study, but it is interesting to note that the relative changes from basal level with stimulation is of the same order of magnitude whether done with mechanical loading or with pharmacological intervention (Anderson et al. 1996; Portanova et al. 1996; Smith et al. 1998), and that indomethacin was able to block tissue concentrations of PGE2 almost completely (Seibert et al. 1994; Anderson et al. 1996; Zhang et al. 1997).
The findings of this work suggest that cyclo-oxygenase-2 is a key contributor to the increase in connective tissue levels of PGE2 in response to muscular contraction. The blockade with COX-2 specific inhibitors that result in a lack of increase in tissue PGE2 during exercise indicates that at least under physical stress, COX-2 acts as an inducible isoform. Anti-inflammatory medication (NSAID) is often considered the drug of choice in the treatment of chronic overused human tendons (Fredberg, 1997) although investigations on inflammatory markers within the chronic overused human Achilles and patella tendons have lacked the ability to show elevated levels of prostaglandins during rest (Alfredson et al. 1999, 2001). However, data obtained during exercise in the area around chronic overloaded human Achilles tendons have indicated that prostaglandin levels increase to a level significantly higher than around the contralateral healthy tendon with loading (H. Langberg, unpublished data). This could imply that the injured tendon represents a vulnerable structure that due to adherences in the peritendinous region (Abrahamsson et al. 1989) displays inflammatory reactions more easily upon loading. Whether such a rise in inflammation plays an important role either a stimulatory or a detrimental role in the tissue regeneration or in the nociceptive processes has not been widely addressed (Zhang et al. 1997). This (Mun-Bryce & Rosenberg, 1998) makes it difficult to judge whether inhibition of the elevation of prostaglandins by anti-inflammatory medication is of advantage or harm to the tendon. Cyclo-oxygenase unspecific NSAID, piroxicam, has been found to increase the strength of healing rat ligaments, but did not have any influence on the ultimate strength once the healing was completed (Dahners et al. 1988). Somewhat in contrast, recent studies on cyclo-oxygenase specific (COX-2) inhibitors (Celecoxib) indicated a small reduction in ligament strength early during healing, but no long-term results were provided (Elder et al. 2001). The positive effect of anti-inflammatory medication could also be a result of blockade of the inducible form of prostaglandins as found in the present study, thus reducing the hypervascularization that often accompanies an overloading of the tendon (Astrom & Westlin, 1994; Astrom & Rausing, 1995). A new study on the use of ultrasound guided sclerosis of neovessels in painful chronic Achilles tendinosis (Ohberg & Alfredson, 2002) supports the findings in the present study as it showed a good effect pointing towards hypervascularization as being part of the problem in chronic overused tendons. This would explain the rational behind NSAID blockade of PGE2 release and decreasing the abnormal resting blood flow.
In conclusion, the present study demonstrated a cyclo-oxygenase-2 specific mechanism, inducible during mechanical tissue stress, being responsible for the increase in tissue synthesis of prostaglandin, and that increase in tissue prostaglandin plays a major role in the increased tissue blood flow in peritendinous connective tissue observed during muscular contractions and physical loading of human tendons. This observation may have implications for the mechanism by which cyclo-oxygenase-blocking drugs influence both healthy and diseased tendon tissue in humans.
Acknowledgments
Annie Høj and Birgitte Lillethorup are thanked for skilled technical assistance. This study was supported by the Team Denmark Research Council, the Danish Sports Science Foundation, the Novo Nordisk Foundation, the Pfizer Foundation, the Danish Medical Research Council (22–01-0154), Copenhagen University Hospital Research Foundation, the Danish National Research Foundation (504–14) and Ministry of Culture Sports Research Council and the Natural Science and Engineering Research Council of Canada (NSERC).
REFERENCES
- Abrahamsson SO, Lundborg G, Lohmander LS. Segmental variation in microstructure, matrix synthesis and cell proliferation in rabbit flexor tendon. Scand J Plast Reconstr Surg Hand Surg. 1989;23:191–198. doi: 10.3109/02844318909075117. [DOI] [PubMed] [Google Scholar]
- Alfredson H, Forsgren S, Thorsen K, Lorentzon R. In vivo microdialysis and immunohistochemical analyses of tendon tissue demonstrated high amounts of free glutamate and glutamate NMDAR1 receptors, but no signs of inflammation, in Jumper's knee. J Orthop Res. 2001;19:881–886. doi: 10.1016/S0736-0266(01)00016-X. [DOI] [PubMed] [Google Scholar]
- Alfredson H, Thorsen K, Lorentzon R. In situ microdialysis in tendon tissue: high levels of glutamate, but not prostaglandin E2 in chronic Achilles tendon pain. Knee Surg Sports Traumatol Arthrosc. 1999;7:378–381. doi: 10.1007/s001670050184. [DOI] [PubMed] [Google Scholar]
- Anderson GD, Hauser SD, McGarity KL, Bremer ME, Isakson PC, Gregory SA. Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. J Clin Invest. 1996;97:2672–2679. doi: 10.1172/JCI118717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrom M, Rausing A. Chronic Achilles tendinopathy. A survey of surgical and histopathologic findings. Clin Orthop. 1995;316:151–164. [PubMed] [Google Scholar]
- Astrom M, Westlin N. Blood flow in chronic Achilles tendinopathy. Clin Orthop. 1994;308:166–172. [PubMed] [Google Scholar]
- Ballou LR, Botting RM, Goorha S, Zhang J, Vane JR. Nociception in cyclooxygenase isozyme-deficient mice. Proc Natl Acad Sci U S A. 2000;97:10272–10276. doi: 10.1073/pnas.180319297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boushel R, Langberg H, Green S, Skovgaard D, Bulow J, Kjaer M. Blood flow and oxygenation in peritendinous tissue and calf muscle during dynamic exercise in humans. J Physiol. 2000;524:305–313. doi: 10.1111/j.1469-7793.2000.t01-2-00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catella-Lawson F, McAdam B, Morrison BW, Kapoor S, Kujubu D, Antes L, Lasseter KC, Quan H, Gertz BJ, Fitzgerald GA. Effects of specific inhibition of cyclooxygenase-2 on sodium balance, hemodynamics, and vasoactive eicosanoids. J Pharmacol Exp Ther. 1999;289:735–741. [PubMed] [Google Scholar]
- Dahners LE, Gilbert JA, Lester GE, Taft TN, Payne LZ. The effect of a nonsteroidal antiinflammatory drug on the healing of ligaments. Am J Sports Med. 1988;16:641–646. doi: 10.1177/036354658801600615. [DOI] [PubMed] [Google Scholar]
- Davy KP, Herbert WG, Williams JH. Effect of indomethacin on the pressor responses to sustained isometric contraction in humans. J Appl Physiol. 1993;75:273–278. doi: 10.1152/jappl.1993.75.1.273. [DOI] [PubMed] [Google Scholar]
- Duffy SJ, Tran BT, New G, Tudball RN, Esler MD, Harper RW, Meredith IT. Continuous release of vasodilator prostanoids contributes to regulation of resting forearm blood flow in humans. Am J Physiol. 1998;74:H1174–1183. doi: 10.1152/ajpheart.1998.274.4.H1174. [DOI] [PubMed] [Google Scholar]
- Elder CL, Dahners LE, Weinhold PS. A cyclooxygenase-2 inhibitor impairs ligament healing in the rat. Am J Sports Med. 2001;29:801–805. doi: 10.1177/03635465010290062101. [DOI] [PubMed] [Google Scholar]
- Engelke KA, Halliwill JR, Proctor DN, Dietz NM, Joyner MJ. Contribution of nitric oxide and prostaglandins to reactive hyperemia in human forearm. J Appl Physiol. 1996;81:1807–1814. doi: 10.1152/jappl.1996.81.4.1807. [DOI] [PubMed] [Google Scholar]
- Fredberg U. Local corticosteroid injection in sports: review of literature and guidelines for treatment. Scand J Med Sci Sport. 1997;7:131–139. doi: 10.1111/j.1600-0838.1997.tb00129.x. [DOI] [PubMed] [Google Scholar]
- Gierse JK, Koboldt CM, Walker MC, Seibert K, Isakson PC. Kinetic basis for selective inhibition of cyclo-oxygenases. Biochem J. 1999;339:607–614. [PMC free article] [PubMed] [Google Scholar]
- Kety SS. The theory and applications of the exchange of inert gas at the lungs and tissues. Pharmacol Rev. 1951;3:1–41. [PubMed] [Google Scholar]
- Kurumbail RG, Stevens AM, Gierse JK, McDonald JJ, Stegeman RA, Pak JY, Gildehaus D, Miyashiro JM, Penning TD, Seibert K, Isakson PC, Stallings WC. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996;384:644–648. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- Langberg H, Bjorn C, Boushel R, Hellsten Y, Kjaer M. Exercise-induced increase in interstitial bradykinin and adenosine concentrations in skeletal muscle and peritendinous tissue in humans. J Physiol. 2002;542:977–983. doi: 10.1113/jphysiol.2002.018077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H, Bulow J, Kjaer M. Blood flow in the peritendinous space of the human Achilles tendon during exercise. Acta Physiol Scand. 1998;163:149–153. doi: 10.1046/j.1365-201X.1998.00361.x. [DOI] [PubMed] [Google Scholar]
- Langberg H, Bulow J, Kjaer M. Standardized intermittent static exercise increases peritendinous blood flow in human leg. Clin Physiol. 1999a;19:89–93. doi: 10.1046/j.1365-2281.1999.00148.x. [DOI] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Bulow J, Kjaer M. Negative interstitial pressure in the peritendinous region during exercise. J Appl Physiol. 1999b;87:999–1002. doi: 10.1152/jappl.1999.87.3.999. [DOI] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Karamouzis M, Bulow J, Kjaer M. Metabolism and inflammatory mediators in the peritendinous space measured by microdialysis during intermittent isometric exercise in humans. J Physiol. 1999c;515:919–927. doi: 10.1111/j.1469-7793.1999.919ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Petersen LJ, Bulow J, Kjaer M. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J Physiol. 1999d;521:299–306. doi: 10.1111/j.1469-7793.1999.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen NA, Lindbjerg J, Munck O. Measurement of blood-flow through skeletal muscle by intramuscular injection of Xenon-133. Lancet. 1964;i(2):686–689. doi: 10.1016/s0140-6736(64)91518-1. [DOI] [PubMed] [Google Scholar]
- McAdam BF, Catella-Lawson F, Mardini IA, Kapoor S, Lawson JA, Fitzgerald GA. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci U S A. 1999;96:272–277. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan IS, Macdonald RE. Prostaglandin synthetase and prostacyclin synthetase in mature rat skeletal muscles: immunohistochemical localisation to arterioles, tendons and connective tissues. J Anat. 1991;178:243–253. [PMC free article] [PubMed] [Google Scholar]
- Mun-Bryce S, Rosenberg GA. Gelatinase B modulates selective opening of the blood-brain barrier during inflammation. Am J Physiol. 1998;274:R1203–1211. doi: 10.1152/ajpregu.1998.274.5.R1203. [DOI] [PubMed] [Google Scholar]
- Muscara MN, Vergnolle N, Lovren F, Triggle CR, Elliott SN, Asfaha S, Wallace JL. Selective cyclo-oxygenase-2 inhibition with celecoxib elevates blood pressure and promotes leukocyte adherence. Br J Pharmacol. 2000;129:1423–1430. doi: 10.1038/sj.bjp.0703232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Banion MK. Cyclooxygenase-2: molecular biology, pharmacology, and neurobiology. Crit Rev Neurobiol. 1999;13:45–82. doi: 10.1615/critrevneurobiol.v13.i1.30. [DOI] [PubMed] [Google Scholar]
- Ohberg L, Alfredson H. Ultrasound guided sclerosis of neovessels in painful chronic Achilles tendinosis: pilot study of a new treatment. Br J Sports Med. 2002;36:173–175. doi: 10.1136/bjsm.36.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom RS, Gregorian C, Drenan RM, Gabot K, Rana BK, Insel PA. Key role for constitutive cyclooxygenase-2 of MDCK cells in basal signaling and response to released ATP. Am J Physiol Cell Physiol. 2001;281:C524–531. doi: 10.1152/ajpcell.2001.281.2.C524. [DOI] [PubMed] [Google Scholar]
- Ouellet M, Riendeau D, Percival MD. A high level of cyclooxygenase-2 inhibitor selectivity is associated with a reduced interference of platelet cyclooxygenase-1 inactivation by aspirin. Proc Natl Acad Sci U S A. 2001;98:14583–14588. doi: 10.1073/pnas.251543298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl U, De Wit C, Gloe T. Large arterioles in the control of blood flow: role of endothelium-dependent dilation. Acta Physiol Scand. 2000;168:505–510. doi: 10.1046/j.1365-201x.2000.00702.x. [DOI] [PubMed] [Google Scholar]
- Portanova JP, Zhang Y, Anderson GD, Hauser SD, Masferrer JL, Seibert K, Gregory SA, Isakson PC. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J Exp Med. 1996;184:883–891. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Scheller D, Kolb J. The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialysate samples. J Neurosci Methods. 1991;40:31–38. doi: 10.1016/0165-0270(91)90114-f. [DOI] [PubMed] [Google Scholar]
- Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Perkins W, Lee L, Isakson P. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci U S A. 1994;91:12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JK, Naylor HL, Pozeg ZI, Hughson RL. Failure of prostaglandins to modulate the time course of blood flow during dynamic forearm exercise in humans. J Appl Physiol. 1996;81:1516–1521. doi: 10.1152/jappl.1996.81.4.1516. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Zhang Y, Koboldt CM, Muhammad J, Zweifel BS, Shaffer A, Talley JJ, Masferrer JL, Seibert K, Isakson PC. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc Natl Acad Sci U S A. 1998;95:13313–13318. doi: 10.1073/pnas.95.22.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzaki M, Guyton G, Garrett W, Archambault JM, Herzog W, Almekinders L, Bynum D, Yang X, Banes AJ. Il-1 beta induces COX2, MMP-1, −3 and −13, ADAMTS-4, Il-1 beta and Il-6 in human tendon cells. J Orthop Res. 2003;21:256–264. doi: 10.1016/S0736-0266(02)00141-9. [DOI] [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Vane JR, Mitchell JA, Appleton I, Tomlinson A, Bishop-Bailey D, Croxtall J, Willoughby DA. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc Natl Acad Sci U S A. 1994;91:2046–2050. doi: 10.1073/pnas.91.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JR, Kapoor SC. Contribution of prostaglandins to exercise-induced vasodilation in humans. Am J Physiol. 1993;265:H171–175. doi: 10.1152/ajpheart.1993.265.1.H171. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Shaffer A, Portanova J, Seibert K, Isakson PC. Inhibition of cyclooxygenase-2 rapidly reverses inflammatory hyperalgesia and prostaglandin E2 production. J Pharmacol Exp Ther. 1997;283:1069–1075. [PubMed] [Google Scholar]


