Abstract
Clustering of cardiovascular risk factors is thought to occur early in life. The endothelium is an important regulator of microvascular function. We investigated the relationship between microvascular function and cardiovascular risk factors in 145 normal, healthy children aged 11–14 years. Skin microvascular responses, measured using laser Doppler imaging, to iontophoresis of acetylcholine (ACh) and sodium nitroprusside (SNP), were negatively correlated with percentage body fat (r = −0.20, P < 0.05 and r = −0.18, P < 0.05, respectively). Subjects were stratified into quintiles based on 2-h, post-feeding glucose levels. Subjects in the upper glucose quintile (range 7.4–11.4 mmol l−1) showed significantly lower vasodilatation to both ACh (P < 0.005) and SNP (P < 0.02) than those in the lower quintile (range 3.9–4.9 mmol l−1). Waist-to-hip ratio and the fasting insulin resistance index were significantly greater in subjects in the upper quintile than those in the lower quintile (P < 0.001 and P < 0.05, respectively). Additionally, in subjects in the upper glucose quintile, fasting triglyceride correlated with fasting insulin (r = 0.59, P < 0.001) and with the fasting insulin resistance index (r = 0.49, P < 0.009), and plasma levels of cholesterol and 2-h glucose were also correlated (r = 0.40, P < 0.05). In a cross-section of normal children, microvascular function was negatively associated with adiposity. Additionally, in a subgroup of subjects, there was a clustering of high post-feeding glucose, impaired microvascular function, increased insulin resistance and higher central fat distribution. These findings suggest that risk factors for adult cardiovascular disease begin to cluster in normal children, which might have important consequences for development of atherosclerosis later in life.
Individual risk factors for adult cardiovascular disease (hypertension, dyslipidaemia, glucose intolerance, hyperinsulinaemia, obesity) are present at a young age and are associated with atherosclerotic disease (Wilson et al. 1998; Berenson et al. 1998). It is also believed that clustering of cardiovascular risk factors, termed metabolic syndrome, might occur early in life and persist into adulthood (Bao et al. 1994; Chen et al. 2000). Changes in endothelial function occur early in the development of cardiovascular disease and are found in asymptomatic subjects with cardiovascular risk factors (Celemajer et al. 1992; Sorensen et al. 1994). A recent study in severely obese children showed evidence of increased arterial stiffness and impaired endothelium-dependent and endothelium-independent brachial artery vasodilatation (Tounian et al. 2001). In the metabolic syndrome however, early, subtle changes in endothelial function might be specifically related to capillary and arteriolar beds (Pinkney et al. 1997). An association between impaired microvascular function and blood pressure and insulin resistance has been shown in adults (Sernéet al. 1999). While information is available relating established risk factors to vascular dysfunction in adults, there is relatively little data regarding these relationships in normal, healthy children who have no established risk factors. The hypothesis tested in the present study was that changes in microvascular function occur early in a population of normal, healthy children and are related to cardiovascular risk factors.
METHODS
Study population
One hundred and fifty-eight children, aged 11–14 years, were recruited from participants of the Dundee Infant Feeding Study (Wilson et al. 1998), which was originally set up to examine the effects of infant feeding practices on health. Subjects were selected from a cross-section of the Dundee population in order to limit selection bias in terms of social class and cardiovascular risk variables. Both the child and parent gave written, informed consent to participate, and the protocol was approved by the Committee on Medical Research Ethics. The investigation conformed with the principles outlined in the Declaration of Helsinki. Three children gave a history of smoking more than one cigarette per week.
Anthropometric and laboratory measurements
Height, weight, waist-to-hip ratio, skinfold thickness and blood pressure were all measured by a single trained observer (F.G.) and are shown in Table 1. Body mass index was calculated (weight/height2) and percentage body fat was determined using the method described by Brook (1971). Pubertal status was determined using a self-assessment questionnaire and categorised from 1–5 according to the Tanner classification.
Table 1.
Characteristics of all subjects and subjects in the lower and upper 2-h glucose quintiles
| All subjects n = 145 | Lower quintile n = 29 | Upper quintile n = 29 | |
|---|---|---|---|
| Age (years) | 13.2 ± 0.9 | 13.3 ± 0.9 | 13.0 ± 1.0 |
| Male/female | 57/88 | 8/21 | 15/14 |
| Height (m) | 1.58 ± 0.08 | 1.59 ± 0.08 | 1.57 ± 0.08 |
| Weight (kg) | 50.3 ± 9.5 | 50.6 ± 8.9 | 50.2 ± 10.4 |
| BMI | 20.1 ± 2.9 | 20.0 ± 2.4 | 20.4 ± 3.7 |
| Waist-to-hip ratio | 0.78 ± 0.06 | 0.76 ± 0.04 | 0.82 ± 0.06** |
| Body fat (%) | 23.3 ± 3.6 | 23.1 ± 3.2 | 24.1 ± 4.4 |
| Systolic BP (mmHg) | 98 ± 8 | 101 ± 9 | 97 ± 8 |
| Diastolic BP (mmHg) | 62 ± 8 | 63 ± 10 | 61 ± 8 |
| Total cholesterol (mmol l−1) | 4.0 ± 0.6 | 3.9 ± 0.5 | 4.1 ± 0.7 |
| LDL (mmol l−1) | 2.14 ± 0.56 | 2.00 ± 0.46 | 2.26 ± 0.64 |
| HDL (mmol l−1) | 1.54 ± 0.34 | 1.55 ± 0.34 | 1.48 ± 0.31 |
| Triglyceride (mmol l−1) | 0.74 ± 0.31 | 0.69 ± 0.18 | 0.79 ± 0.26 |
| Post-feeding triglyceride (mmol l−1) | 1.18 ± 0.51 | 1.01 ± 0.26 | 1.20 ± 0.57 |
| Fasting glucose (mmol l−1) | 4.9 ± 0.7 | 4.3 ± 0.5 | 5.8 ± 0.7* |
| 2-h glucose (mmol l−1) | 6.4 ± 1.5 | 4.6 ± 0.3 | 8.7 ± 1.1* |
| Fasting insulin (pmol l−1) | 40.3 ± 19.4 | 39.0 ± 19.6 | 41.3 ± 19.0 |
| 2-h insulin (pmol l−1) | 388.9 ± 289.9 | 302.7 ± 151.5 | 484.8 ± 381.0* |
| Fasting insulin resistance index | 8.1 ± 4.3 | 6.8 ± 3.7 | 9.6 ± 4.6* |
Values are means ±s.d. BP, blood pressure; HDL, high density lipoprotein.
P < 0.05,
P < 0.0001 comparing lower and upper quintiles.
Serum lipid profiles were measured using a Hitachi 917 fast random analyser. Plasma glucose was measured by the glucose oxidase method with a Beckman Analyser. Plasma insulin was measured using an ELISA technique (DAKO). The fasting insulin resistance index (FIRI) was calculated from the (fasting glucose × fasting insulin)/25. This indirect marker of insulin resistance has been validated against the euglycaemic clamp method (Cleland et al. 1996). Details of these measurements are shown in Table 1. Glucose and insulin levels were measured before and after a physiological feeding challenge. Subjects were asked to eat a specially prepared muffin and milkshake containing the nutritional value of a ‘fast food’ convenience meal (e.g. burger, fries and milkshake): 34 g protein, 160 g carbohydrate, 51.3 g fat and 8.5 g fibre (energy = 1219 kcal, 5100 kJ).
Assessment of microvascular function
Subjects arrived between 08.45 and 10.00 h following an overnight fast. Experiments were conducted in a temperature-controlled laboratory (22 ± 1 °C). Subjects were seated comfortably with their arms supported at heart level. Skin microvascular responses to iontophoresis of 1 % acetylcholine (ACh, Sigma Chemical, St Louis, MO, USA) and sodium nitroprusside (SNP, David Bull Laboratories, Warwick, UK) were measured using laser Doppler imaging as described previously by us (Spence et al. 2000; Newton et al. 2001).
Drug delivery
Iontophoresis allows the non-invasive delivery of drugs to a small area of skin without inducing systemic effects (Sloan & Soltani, 1986). Two measurement sites (ACh and SNP) were prepared on the volar surface of the forearm by gently removing surface keratinocytes with adhesive tape and cleaning the area with alcohol. The iontophoresis chamber (Moor Instruments Ltd, Devon, UK) consisted of a Perspex ring of internal diameter 20 mm with a wire electrode running round the inner surface. The chamber was fixed to the skin with adhesive tape and filled with 2 ml of solution. A conductive hydro-gel pad was attached to the wrist, which served as a reference electrode. The leads from the electrodes were connected to the iontophoresis controller.
When an electrical potential difference is established, ions of the drug migrate across the skin. Using a current of 100 μA and larger electrodes, we do not elicit any vasodilatation via non-specific, galvanic effects because a much lower charge density (maximum of 2.5 × 10−2 mC mm−2) is generated (Berghoff et al. 2002). Each drug was administered as a successive accumulation of doses, each dose defined by the duration of current × time. ACh was applied iontophoretically using an anodal current for 20, 40 and 80 s (i.e. charges of 2, 4, 8 mC, respectively), with skin perfusion recorded for 100 s between doses. A cathodal current was used to delivery SNP iontophoretically at charges of 2, 4 and 8 mC. As the SNP response took longer to develop and plateau, perfusion was measured for 240 s between doses. The order of ACh and SNP iontophoresis was randomised.
Laser Doppler perfusion imaging
Skin microvascular perfusion was measured at the drug delivery site using a laser Doppler imager (MoorLDI, Moor Instruments Ltd, Devon, UK). A 2 mW helium-neon laser scans the surface of the skin, and light back-scattered from moving erythrocytes is shifted in frequency by an amount proportional to their velocity, according to the Doppler principle. For each scan, the computer builds up a colour-coded image representing skin perfusion in two dimensions. This relative measure of volume flow is expressed in arbitrary perfusion units (PU). The scan region was 8 cm × 8 cm. The recorded images were analysed using dedicated software (MoorLDI 3.1, Moor Instruments Ltd, Devon, UK). For each dose response, the stable perfusion value was taken and divided by the baseline measurement to give a ratio representing the change in flow.
Statistics
Analyses were computed using the SPSS statistical package. Data for microvascular responses were not normally distributed and were log transformed to achieve normality. Differences for responses to ACh and SNP between groups were compared using analysis of variance (ANOVA), followed by a modified post hoc Student's unpaired t test at each dose when a significant difference was found. Non serial differences between groups were compared using a t test or Mann-Whitney test depending on whether data were normally distributed or not. Multiple regression analysis was used to determine confounding variables on microvascular responses. Correlations between variables were tested using linear regression analysis. In all cases significance was acknowledged if the probability of a type-1 error was less than 5 % (i.e. P < 0.05).
RESULTS
Of the 158 children enrolled, 145 completed the study and therefore data are presented for these subjects only. Failure to complete the study was due to subjects feeling faint or being unable to complete the feeding challenge.
Females were more advanced in pubertal stage than males. There were 14, 37 and 37 females in Tanner stages 2, 3 and 4 compared with 21, 14 and 22 males in Tanner stages 2, 3 and 4, respectively. Females had significantly greater microvascular responses to ACh (P < 0.01) and SNP (P < 0.05) than males (Fig. 1).
Figure 1. Comparison of microvascular responses between males (n = 57) (•) and females (n = 88) (▪).

The figure shows significantly greater responses in females to A, acetylcholine (P < 0.01, ANOVA) and B, sodium nitroprusside (P < 0.05, ANOVA). Values are means ± s.e.m. *P < 0.05, **P < 0.01 (post hoc tests).
There were weak negative correlations between percentage body fat and peak responses to ACh (r = −0.20, P < 0.05) and SNP (r = −0.18, P < 0.05) responses. There were no significant correlations between microvascular responses and fasting glucose, lipid profiles or any other parameter listed in Table 1.
Plasma glucose levels 2 h after the feeding challenge varied considerably amongst the subjects, ranging from 3.9 to 11.4 mmol l−1. Over this range we found no significant correlations between glucose levels and microvascular responses. However, stratifying subjects into quintiles according to the 2 h glucose level did show significant differences in microvascular responses to ACh and SNP amongst the groups (Table 2). In a subgroup analysis, comparison of microvascular responses between subjects in the lower quintile of 2 h glucose (3.9–4.9 mmol l−1, n = 29) with subjects from the upper quintile (7.4– 11.4 mmol l−1, n = 29) showed a similar baseline skin perfusion in the two groups (15.5 ± 0.9 PU versus 14.2 ± 1.1 PU) but significantly reduced microvascular responses to ACh (P < 0.005) and SNP (P < 0.02) (Fig. 2).
Table 2.
Baseline skin perfusion (in arbitrary perfusion units) and microvascular responses to acetylcholine and sodium nitroprusside in groups (n = 29 in each) separated according to quintiles of 2-h glucose levels
| Acetylcholine | Sodium nitroprusside | |||||||
|---|---|---|---|---|---|---|---|---|
| Quintile (mm) | Baseline | 2 mC | 4 mC | 8 mC | Baseline | 2 mC | 4 mC | 8 mC |
| 1 (3.9–5.0) | 15.5 ± 0.91 | 5.07 ± 0.38 | 7.41 ± 0.39 | 8.85 ± 0.40 | 15.5 ± 0.98 | 2.48 ± 0.36 | 4.63 ± 0.33 | 6.44 ± 0.35 |
| 2 (5.0–5.8) | 13.9 ± 0.67 | 5.17 ± 0.26 | 7.48 ± 0.31 | 8.98 ± 0.32 | 15.5 ± 0.68 | 2.69 ± 0.14 | 4.85 ± 0.28 | 6.85 ± 0.50 |
| 3 (5.9–6.6) | 14.0 ± 1.15 | 5.65 ± 0.44 | 8.36 ± 0.48 | 9.60 ± 0.47 | 14.8 ± 1.40 | 2.87 ± 0.25 | 5.25 ± 0.48 | 6.85 ± 0.50 |
| 4 (6.7–7.3) | 14.9 ± 0.81 | 5.23 ± 0.39 | 7.13 ± 0.36 | 8.13 ± 0.35* | 15.7 ± 1.09 | 3.03 ± 0.27 | 4.54 ± 0.36 | 7.05 ± 0.44 |
| 5 (7.3–11.4) | 14.2 ± 1.07 | 4.75 ± 0.32 | 6.94 ± 0.35 | 8.15 ± 0.39** | 16.2 ± 1.26 | 2.60 ± 0.30 | 4.15 ± 0.40 | 5.29 ± 0.45† |
Glucose ranges for each quintile are shown in parentheses. There were significant differences amongst the groups for microvascular responses to acetylcholine (P < 0.001, ANOVA) and sodium nitroprusside (P < 0.005, ANOVA). Values are means ± s.e.m.
P < 0.001 comparing ACh responses between quintiles 1 and 4.
P < 0.005 comparing ACh responses between quintiles 1 and 5.
P < 0.02 comparing SNP responses between quintiles 1 and 5.
Figure 2. Comparison of microvascular responses to A, acetylcholine and B, sodium nitroprusside between subjects in the lower (n = 29) (♦) and upper (n = 29) (▪) glucose quintiles.
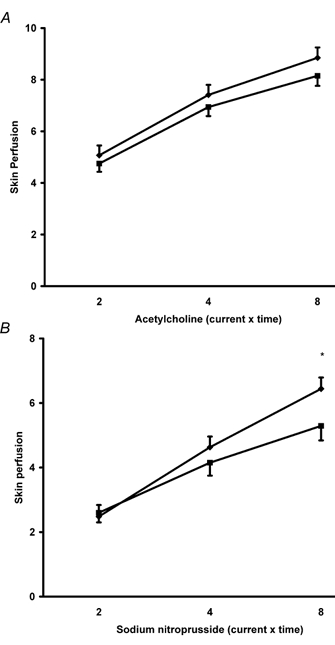
Subjects in the upper quintile showed significantly reduced responses to acetylcholine (P < 0.005, ANOVA) and sodium nitroprusside (P < 0.02, ANOVA). Values are means ± s.e.m. *P < 0.05 (post hoc test).
We found a highly significant correlation between ACh and SNP responses in all subjects (r = 0.77, P < 0.001) and also within subjects in the upper quintile of 2 h glucose (r = 0.78, P < 0.001).
Subjects in the upper glucose quintile had significantly elevated 2 h insulin levels (P < 0.05), a higher fasting insulin resistance index (P < 0.05), a greater waist-to-hip ratio (P < 0.001), and a trend towards a greater low density lipoprotein (LDL) cholesterol level (P = 0.09) than subjects in the lower glucose quintile (Table 1). In subjects in the upper glucose quintile, fasting triglyceride levels correlated with fasting insulin levels (r = 0.59, P < 0.001) and with the fasting insulin resistance index (r = 0.49, P < 0.009), and total cholesterol correlated with 2 h glucose (r = 0.40, P < 0.05).
DISCUSSION
The findings from the current cross-sectional study in healthy children show that microvascular function is negatively associated with percentage body fat and is impaired in subjects with elevated post-feeding glucose levels. Additionally, subjects with high 2 h glucose levels had a combination of greater insulin resistance, more central fat distribution and reduced microvascular vasodilatation. These results show that abnormal microvascular function is already present in healthy children and is associated with cardiovascular risk factors.
In the present study, we measured microvascular responses over a large area of skin, which we have shown produces more reproducible measurements than using single point laser Doppler flowmetry (Newton et al. 2001). Importantly, using relatively low iontophoretic currents over a large area of skin produces a smaller charge density, which means that there is no involvement of sensory nerves (Morris & Shore, 1996) and no axon reflex-mediated vasodilatation (Katz et al. 2001; Berghoff et al. 2002). It is reasonable to believe therefore, that the ACh and SNP microvascular responses measured in the skin in this study are primarily related to non-neurogenic, endothelium-dependent and NO-mediated smooth muscle vasodilatation, respectively. However, the precise mediators involved in the endothelium-dependent response to iontophoresis of ACh in skin are uncertain. We (Khan et al. 1997) and others (Noon et al. 1998) have shown that vasodilator prostanoids might be one of the mediators involved, but this has not been a consistent finding (Morris & Shore, 1996; Berghoff et al. 2002). A role for NO has also been shown in the skin microvascular response to intradermal injections of ACh (Warren, 1994).
An advantage of the techniques we have used in the present study is that they measure vascular responses specifically in the microcirculation, which is more likely to be a target of endothelial dysfunction associated with insulin resistance and other features of the metabolic syndrome (Pinkney et al. 1997). We feel that, for the questions addressed in this study, these techniques have an advantage over assessments of large vessel endothelial function, determined for example by measuring flow-mediated dilatation, which provide important information relating to atherogenesis but not to the possible consequences of metabolic disturbance. Moreover, in a recent study it was shown that flow-mediated dilatation measurements did not correlate with other techniques for measuring endothelial function (Lind et al. 2002), indicating that endothelial function differs between conduit and resistance vessels.
Vascular responses to ACh were weakly associated with percentage body fat. This is similar to the finding in adults (Perticone et al. 2001) where body mass index (BMI) and waist-to-hip ratio correlated negatively with ACh responses. It is important to note that many other studies that report a relationship between adiposity and cardiovascular function relate to individuals with a much greater body mass (BMI > 25) than those in the present study. The relationship between ACh responses and percentage body fat was only a weak one in our study perhaps because nearly all the subjects were not overweight or obese. Only 9 out of 145 (6 %) subjects had a BMI > 25. Additionally, it is recognised that obesity differs with sex, and the association between ACh-mediated vasodilatation and obesity is stronger in males than in females (Perticone et al. 2001). Thus, inclusion of more males in our study might have shown a stronger relationship between adiposity and ACh-mediated microvascular responses. Nevertheless, the weak association within this relatively normal range of body fat percentage does point to adiposity in childhood as being an important factor in endothelial function. In support of this, we have previously shown in these same children that E-selectin, a marker of endothelial dysfunction, correlates with BMI and waist-to-hip ratio (Kennedy et al. 1999). These findings are consistent with those from a recent study in which severely obese children showed evidence of endothelial dysfunction and increased arterial stiffness (Tounian et al. 2001). It has been suggested that obesity and fat distribution might induce endothelial dysfunction via an increase in oxidative stress (Perticone et al. 2001).
The impact of increasing adiposity not only has its effect in childhood but also later in life where annual changes in adiposity show a correlation with cholesterol (Siervogel et al. 1998). Childhood adiposity has an unfavourable impact on lipoprotein profiles at 35–45 years (Guo et al. 2000), and weight gain over 5 years is correlated with adverse changes in cardiovascular risk factors such as lipid profiles, insulin levels and blood pressure, especially in males (Rainwater et al. 2000). Moreover, obesity has been shown to be independently associated with coronary endothelial function in normal or mildly diseased coronary arteries (Al Suwaidi et al. 2001). Thus, current adiposity and changes in adiposity are detrimental to cardiovascular risk. In light of these findings and the recent reports of the increasing prevalence of obesity in children (Rudolf et al. 2001), such that 20 % of 9-year olds and 33 % of 11-year-old girls are overweight, it is of major concern that these factors might have detrimental impacts on the development of diabetes (Hyppönen et al. 2000; Ehtisham et al. 2000) and mortality and morbidity in adulthood.
We stratified our subjects into two groups based on 2-h glucose levels following a feeding challenge. Quintiles were chosen, which meant that all subjects in the upper quintile had a 2 h glucose level that would have been considered as ‘impaired’ glucose tolerance following a formal glucose tolerance test. The 2-h glucose level was chosen rather than the fasting glucose level as this appears to be a better predictor of cardiovascular mortality and morbidity (Smith et al. 2000; DECODE Study Group, 2001). It has also been suggested that fasting hyperglycaemia is indicative of a more advanced stage of clinical diabetes and is not a sensitive method for detecting impaired glucose tolerance (Sinha et al. 2002). Interestingly, the finding that 20 % of our normal children had poor glucose handling is very similar to that reported in a recent study in which 21–25 % of obese children showed glucose intolerance (Sinha et al. 2002).
When examining microvascular responses amongst the glucose quintiles, we found that ACh and SNP responses were significantly impaired in subjects with high 2 h glucose levels. These findings in normal children are consistent with our previous study in young children who had established diabetes (Khan et al. 2000), and with other studies in adults with diabetes (Morris et al. 1995). Although the level of impairment in microvascular responses was greater in our study of children with diabetes (Khan et al. 2000), it is important to remember that none of the children in the present study had diabetes. It is possible that these changes in microvascular function might become prominent in later life, especially since it is known that risk factors for cardiovascular disease track from childhood into adulthood. We have shown previously in children with diabetes that the passage through puberty into young adulthood results in a diminution in microvascular responses (Elhadd et al. 1998) and therefore, the impairment in ACh and SNP microvascular responses seen in the current study might become greater as these children pass through adolescence into adulthood.
The reasons for a decrease in microvascular responses to ACh and SNP are not known. However, elevated glucose is associated with increased oxidative stress in normal subjects (Title et al. 2000) and in children with diabetes (Elhadd et al. 1998), and it is possible that nitric oxide (NO) activity is diminished by oxygen-derived free radicals. Of interest was the highly significant correlation between ACh and SNP in all subjects, and within subjects in the upper quintiles of 2 h glucose, suggesting that a common factor/pathway might be affected.
Other differences between subjects in the lower and upper quintiles of 2 h glucose, in terms of cardiovascular risk, were a significantly higher waist-to-hip ratio and a tendency towards elevated low density lipoprotein levels in subjects with high 2 h glucose levels. There were also significant correlations between triglycerides and insulin resistance and between cholesterol and 2 h glucose levels. Collectively, the clustering of these cardiovascular risk factors is consistent with the metabolic syndrome (Chen et al. 2000) and confirms that risk factors for adult cardiovascular disease occur in childhood.
It is recognised that confounding factors can influence microvascular responses. Of the variables measured and included in the multiple regression model, sex and body fat percentage were found to be associated with microvascular responses, and these were corrected for in the statistical analyses. Other variables, not described in this study, such as birth weight, level of physical activity and social class, were found to have no associations with microvascular responses.
Our findings in this study might have important implications for the development of cardiovascular disease in later life. There is evidence from epidemiological and clinical studies that individuals with multiple risk factors have a much greater risk of developing cardiovascular disease compared with those who have a single risk factor (Berenson et al. 1998). As we found a tendency toward clustering of poor glucose handling, increased insulin resistance, increased central body fat and impaired microvascular responses, the children showing these associations are potentially at higher risk of developing cardiovascular disease in later life, especially if no intervention is implemented. In support of this, a recent American Heart Association Scientific Statement points out that there is an increasing amount of data to show that being overweight during childhood and adolescence is significantly associated with insulin resistance, dyslipidaemia and elevated blood pressure in young adulthood (Steinberger & Daniels 2003). Furthermore, the presence of atherosclerotic lesions in children and young adults correlates positively and significantly with established risk factors such as LDL cholesterol, BMI and blood pressure (Kavey et al. 2003), pointing to the possibility that our subjects with a poorer cardiovascular risk profile might already be at risk of having atherosclerotic lesions. Long-term follow-up studies have demonstrated tracking of obesity, hypercholesterolaemia and hypertension from childhood into adult life (Whittaker et al. 1997).
With regard to our findings of reduced microvascular responses with poor glucose handling and with increased adiposity, recent data suggest that the onset of impaired glucose tolerance in obese children and adolescents is clearly associated with severe insulin resistance (Sinha et al. 2002). Also recently, insulin resistance and blood pressure have been shown to be associated with impaired skin microvascular function in adults (Sernéet al. 1999).
A question that follows from this then is when should cardiovascular disease prevention begin? Since behavioural and lifestyle patterns learned in childhood tend to continue into adulthood, it would seem advisable to intervene early in childhood. It has been recommended that the focus of attention, with respect to children, should be on the identification of unhealthy lifestyle factors, with the promotion of physical activity, weight management and a healthy diet.
Acknowledgments
We acknowledge support from the Medical Research Council UK and TENOVUS Scotland.
REFERENCES
- Al Suwaidi J, Higano ST, Holmes DR, Lennon R, Lerman A. Obesity is independently associated with coronary endothelial dysfunction in patients with normal or mildly diseased coronary arteries. J Am Coll Cardiol. 2001;37:1523–1528. doi: 10.1016/s0735-1097(01)01212-8. [DOI] [PubMed] [Google Scholar]
- Bao W, Srinivasan SR, Wattigney WA, Berenson GS. Persistence of multiple cardiovascular risk clustering related to Syndrome X from childhood to young adulthood: the Bogalusa Heart Study. Arch Intern Med. 1994;154:1842–1847. [PubMed] [Google Scholar]
- Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattingney WA. Association between multiple risk factors and atherosclerosis in children and young adults. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- Berghoff M, Kathpal M, Kilo S, Hilz MJ, Freeman R. Vascular and neural mechanisms of ACh-mediated vasodilation in the forearm cutaneous microcirculation. J Appl Physiol. 2002;92:780–788. doi: 10.1152/japplphysiol.01167.2000. [DOI] [PubMed] [Google Scholar]
- Brook CGD. Determination of body composition of children from skinfold measurements. Arch Dis Child. 1971;46:182–184. doi: 10.1136/adc.46.246.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- Chen W, Bao W, Begum S, Elkasabany A, Srinivasan SR, Berenson GS. Age-related patterns of the clustering of cardiovascular risk variables of Syndrome X from childhood to young adulthood in a population made up of black and white subjects. Diabetes. 2000;49:1042–1048. doi: 10.2337/diabetes.49.6.1042. [DOI] [PubMed] [Google Scholar]
- Cleland S, Petrie J, Morris AD. FIRI: a fair insulin resistance index. Lancet. 1996;347:770. doi: 10.1016/s0140-6736(96)90126-9. [DOI] [PubMed] [Google Scholar]
- Ehtisham S, Barrett TG, Shaw NJ. Type 2 diabetes mellitus in UK children – an emerging problem. Diabetic Med. 2000;17:867–871. doi: 10.1046/j.1464-5491.2000.00409.x. [DOI] [PubMed] [Google Scholar]
- Elhadd TA, Khan F, Kirk G, McLaren M, Newton RW, Greene SA, Belch JJ. Influence of puberty on endothelial dysfunction and oxidative stress in young patients with Type-1 diabetes mellitus. Diabetes Care. 1998;21:1990–1996. doi: 10.2337/diacare.21.11.1990. [DOI] [PubMed] [Google Scholar]
- Guo SS, Chumlea CWM, Maynard ML, Siervogel RM. Adiposity during childhood, adolescence, and young adulthood in relation to adult cardiovascular risk factors. Circulation. 2000;102(suppl. 2):872. (abstract) [Google Scholar]
- Hyppönen E, Virtanen SM, Kenward MG, Knip M, Åkerblom HK. Obesity, increased linear growth, and risk of Type 1 diabetes in children. Diabetes Care. 2000;23:1755–1760. doi: 10.2337/diacare.23.12.1755. [DOI] [PubMed] [Google Scholar]
- Katz A, Ekberg K, Johansson B, Wahren J. Diminished skin blood flow in type I diabetes: evidence for non-endothelium-dependent dysfunction. Clin Sci. 2001;101:59–64. [PubMed] [Google Scholar]
- Kavey REW, Daniels SR, Lauer RM, Atkins DL, Hayman LL, Taubert K. American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. Circulation. 2003;107:1562–1566. doi: 10.1161/01.cir.0000061521.15730.6e. [DOI] [PubMed] [Google Scholar]
- Kennedy G, Green FC, McLaren M, Belch JJF. sE-selectin and cardiovascular risk factors in children. Thromb Haemost. 1999;225(Aug suppl.) (abstract) [Google Scholar]
- Khan F, Davidson NC, Littleford RC, Litchfield SJ, Struthers AD, Belch JJF. Cutaneous vascular responses to acetylcholine are mediated by a prostacyclin-dependent mechanism in man. Vasc Med. 1997;2:82–86. doi: 10.1177/1358863X9700200202. [DOI] [PubMed] [Google Scholar]
- Khan F, Elhadd TA, Greene SA, Belch JJF. Impaired skin microvascular function in children, adolescents and young adults with Type-1 insulin-dependent; diabetes mellitus. Diabetes Care. 2000;23:215–219. doi: 10.2337/diacare.23.2.215. [DOI] [PubMed] [Google Scholar]
- Lind L, Hall J, Johansson K. Evaluation of four different methods to measure endothelium-dependent vasodilation in the human forearm circulation. Clin Sci. 2002;102:561–567. [PubMed] [Google Scholar]
- Morris SJ, Shore AC. Skin blood flow responses to the iontophoresis of acetylcholine and sodium nitroprusside in man: possible mechanisms. J Physiol. 1996;496:531–542. doi: 10.1113/jphysiol.1996.sp021704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SJ, Shore AC, Tooke JE. Responses of the skin microcirculation to acetylcholine and nitroprusside in patients with in patients with NIDDM. Diabetologia. 1995;38:1337–1344. doi: 10.1007/BF00401767. [DOI] [PubMed] [Google Scholar]
- Newton DJ, Khan F, Belch JJF. Assessment of microvascular endothelial function in human skin. Clin Sci. 2001;101:567–572. [PubMed] [Google Scholar]
- Noon JP, Walker BR, Hand MF, Webb DJ. Studies with iontophoretic administration of drugs to human dermal vessels in vivo: cholinergic vasodilatation is mediated by dilator prostanoids rather than nitric oxide. Br J Clin Pharmacol. 1998;45:545–550. doi: 10.1046/j.1365-2125.1998.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perticone F, Ceravolo R, Candigliota M, Ventura G, Iacopino S, Sinopoli F, Mattioli PL. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: protective effect of vitamin C. Diabetes. 2001;50:159–165. doi: 10.2337/diabetes.50.1.159. [DOI] [PubMed] [Google Scholar]
- Pinkney JH, Stehouwer CDA, Coppack SW, Yudkin JS. Endothelial dysfunction: cause of the insulin resistance syndrome. Diabetes. 1997;46(suppl. 2):S9–13. doi: 10.2337/diab.46.2.s9. [DOI] [PubMed] [Google Scholar]
- Rainwater DL, Mitchell BD, Comuzzie AG, Vandeberg JL, Stern MP, MacCluer JW. Associations among 5-year changes in weight, physical activity, and cardiovascular disease risk factors in Mexican Americans. Am J Epidemiol. 2000;152:974–982. doi: 10.1093/aje/152.10.974. [DOI] [PubMed] [Google Scholar]
- Rudolf MC, Sahota P, Barth JH, Walker J. Increasing prevalence of obesity in primary school children. BMJ. 2001;322:1094–1095. doi: 10.1136/bmj.322.7294.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serné EH, Stehouwer CDA, ter Maatten JC, ter Wee PT, Rauwerda JA, Donker AJ, Gans RO. Microvascular function relates to insulin sensitivity and blood pressure in normal subjects. Circulation. 1999;99:896–902. doi: 10.1161/01.cir.99.7.896. [DOI] [PubMed] [Google Scholar]
- Siervogel RM, Wisemandle W, Maynard LM, Guo SS, Roche AF, Chumlea WC, Towne B. Serial changes in body composition throughout adulthood and their relationships to changes in lipid and lipoprotein levels – The Fels longitudinal study. Arterioscler Thromb Vasc Biol. 1998;8:1759–1764. doi: 10.1161/01.atv.18.11.1759. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, Savoye M, Rieger V, Taksali S, Barbetta G, Sherwin RS, Caprio S. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med. 2002;346:802–810. doi: 10.1056/NEJMoa012578. [DOI] [PubMed] [Google Scholar]
- Sloan JB, Soltani K. Iontophoresis in dermatology. J Am Acad Derm. 1986;15:671–684. doi: 10.1016/s0190-9622(86)70223-5. [DOI] [PubMed] [Google Scholar]
- Smith NL, Barzilay JI, Permanente K, Savage PJ, Heckbert SR, Kuller LH, Kromel RA, Resnick HE, Psaty BN. Fasting and 2-hour post-challenge glucose measures and risk of cardiovascular disease in the elderly: the cardiovascular health study. Circulation. 2000;102(suppl. 2):872. (abstract) [Google Scholar]
- Sorensen KE, Celermajer DS, Georgakopoulos D, Hatcher G, Betteridge DJ, Deanfield JE. Impairment of endothelium-dependent dilation is an early event in children with familial hypercholesterolaemia and is related to the lipoprotein(a) level. J Clin Invest. 1994;93:50–55. doi: 10.1172/JCI116983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence VA, Khan F, Belch JJF. Enhanced sensitivity of the peripheral cholinergic vascular response in patients with chronic fatigue syndrome. Am J Med. 2000;108:736–739. doi: 10.1016/s0002-9343(00)00407-1. [DOI] [PubMed] [Google Scholar]
- Steineberger J, Daniels SR. Obesity, insulin resistance, diabetes and cardiovascular risk in children. Circulation. 2003;107:1448–1453. doi: 10.1161/01.cir.0000060923.07573.f2. [DOI] [PubMed] [Google Scholar]
- Title LM, Cummings PM, Giddens K, Nassar BA. Oral glucose loading acutely attenuates endothelium-dependent vasodilation in healthy adults without diabetes: an effect prevented by vitamins C and E. J Am Coll Cardiol. 2000;36:2185–2191. doi: 10.1016/s0735-1097(00)00980-3. [DOI] [PubMed] [Google Scholar]
- Tounian P, Aggoun Y, Dubern B, Varille V, Guy Grand B, Sidi D, Girardet JP, Bonnet D. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–1404. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- Warren JB. Nitric oxide mediates and human skin blood flow to acetylcholine and ultraviolet light. FASEB J. 1994;8:247–251. doi: 10.1096/fasebj.8.2.7509761. [DOI] [PubMed] [Google Scholar]
- Whittaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- Wilson AC, Forsyth JS, Greene SA, Irvine L, Hau C, Howie PW. Relation of infant diet to childhood health: seven year follow up of children in the Dundee infant feeding study. BMJ. 1998;316:21–25. doi: 10.1136/bmj.316.7124.21. [DOI] [PMC free article] [PubMed] [Google Scholar]


