Abstract
The involvement of cyclic guanosine 3′,5′-monophosphate (cGMP) and cGMP-dependent protein kinase (PKG) and their interaction with the Ca2+-dependent mechanisms in the regulation of ciliary activity are not well understood. To investigate how cGMP regulates ciliary activity, changes in ciliary beat frequency (CBF) and intracellular calcium concentration ([Ca2+]i) of rabbit tracheal ciliated cells in response to 8-bromo-cGMP (Br-cGMP) were simultaneously quantified using digital, high-speed phase-contrast and fluorescence imaging. Br-cGMP induced a response in ciliary activity that could be separated into two parts. Firstly, Br-cGMP induced a concentration-dependent increase in the basal CBF that occurred without increasing the [Ca2+]i. This response was not affected by excessively buffering the [Ca2+]i with BAPTA but was abolished by KT5823, a PKG inhibitor. Secondly, Br-cGMP induced a series of transient increases in CBF that were superimposed on the sustained increases in CBF. These transient increases in CBF correlated with the stimulation of a series of transient increases in [Ca2+]i and were abolished by BAPTA, but were unaffected by KT5823. The magnitude of the transient increases in CBF and [Ca2+]i were not dependent on the concentration of Br-cGMP. The Ca2+-dependent changes in CBF induced by ionomycin or ATP were not affected by KT5823. From these results, we propose that cGMP increases CBF in two ways: firstly through a Ca2+-independent mechanism involving PKG, and secondly through a Ca2+-dependent mechanism following the stimulation of changes in [Ca2+]i. In addition, we suggest that the Ca2+-dependent stimulation of rabbit airway ciliary activity does not initially require PKG activation.
Changes in ciliary beat frequency (CBF) are believed to be a key factor in the regulation of mucociliary transport and the defence mechanisms of the respiratory tract (Satir & Sleigh, 1990; Wanner et al. 1996). For example, a relatively small increase in CBF (16 %) can result in a large increase (56 %) in surface liquid velocity (Seybold et al. 1990), a response that is likely to enhance mucus clearance. It has been well established that airway CBF is strongly regulated by second messengers, such as Ca2+ and cAMP, and substantial evidence now exists for a regulatory role of cyclic guanosine 3′,5′-monophosphate (cGMP) (Tamaoki et al. 1991; Jain et al. 1993; Geary et al. 1995; Yang et al. 1997; Wyatt et al. 1998; Runer & Lindberg, 1999; Uzlaner & Priel, 1999; Li et al. 2000; Shirakami et al. 2000; Braiman et al. 2001; Zagoory et al. 2002). However, the effects of cGMP on ciliary activity remain controversial.
In other cells, cGMP has been found to modulate many cellular functions including smooth muscle cell contraction, cardiac function and platelet aggregation (Lincoln & Cornwell, 1993; Hobbs & Ignarro, 1996; Murad, 1996; Vaandrager & de Jonge, 1996) and is formed by activation of either soluble or membrane-bound guanylate cyclase (GC). While the membrane-bound or receptor form of GC is stimulated by ligands such as atrial natriuretic peptide (ANP), soluble GC is stimulated by nitric oxide (NO) (Schmidt & Walter, 1994; McDonald & Murad, 1995; Vaandrager & de Jonge, 1996). Increases in cGMP generally lead to the activation of cGMP-dependent protein kinase (PKG) (McDonald & Murad, 1995) and phosphorylation of target proteins (Bonini & Nelson, 1990; Walczak & Nelson, 1994; Porter & Sale, 2000).
The involvement of cGMP-PKG-mediated phosphorylation in ciliary motility is suggested by the immunoreactivity of rat tracheal ciliated cells for PKG Iβ (Zhan et al. 1999), the presence of a PKG substrate in the cilia of Paramecium (Bonini & Nelson, 1990) and a cGMP-stimulated PKG activity in bovine airway epithelial cells (Wyatt et al. 1998). However, cGMP has been reported to either inhibit (Tamaoki et al. 1991) or have no effect on the CBF (Uzlaner & Priel, 1999; Braiman et al. 2001) of rabbit tracheal cells or to stimulate CBF in rat (Li et al. 2000), bovine (Wyatt et al. 1998) and human (Geary et al. 1995; Runer & Lindberg, 1999) airway cells.
One possibility that may contribute to these inconsistencies is the relationship between Ca2+ and cGMP-PKG regulation of ciliary activity. It has been postulated, for rabbit airway and frog palate cells, that Ca2+ is incapable of increasing CBF without the activation of PKG (Uzlaner & Priel, 1999; Braiman et al. 2001; Ma et al. 2002; Zagoory et al. 2002). One implication of this idea is that there should be a significant delay between the increase in [Ca2+]i and the increase in CBF to accommodate the activation process of PKG and the phosphorylation of specific targets. However, by using high-speed phase-contrast (240 frames s−1) and fast fluorescence imaging (30 frames s−1), combined with a beat-by-beat analysis, we have found that, in response to mechanical stimulation or ATP, the changes in [Ca2+]i and CBF during Ca2+ waves or oscillations were very tightly coupled in rabbit airway ciliated cells (Evans & Sanderson, 1999; Lansley & Sanderson, 1999; Zhang & Sanderson, 2003). While it is possible that PKG activation may precede the onset of ATP-induced Ca2+ oscillations, it does not seem likely that PKG activation occurs during the propagation of intercellular Ca2+ waves through unstimulated adjacent cells (Lansley & Sanderson, 1999). In these cells, the latency between increases in [Ca2+]i and increases in CBF was very brief (˜100 ms at 37 °C). A similar dependency of the CBF on the [Ca2+]i, which was not influenced by PKG inhibitors, was also found in ovine airway epithelium (Salathe & Bookman, 1999; Salathe et al. 2000).
In view of the uncertainties of cGMP in the control of CBF, the aim of this study was to use our high-speed digital recording techniques (Zhang & Sanderson, 2003) to resolve the changes in CBF and [Ca2+]i during the activation of cGMP-PKG. From the data obtained, we suggest that cGMP regulates rabbit airway CBF in both a Ca2+-dependent manner and a Ca2+-independent manner and that the cGMP-PKG signalling pathway is not essential for Ca2+-dependent increases in CBF.
METHODS
Materials
Hanks' balanced salt solution (HBSS) without Phenol Red and Dulbecco's modified Eagle's Medium (DMEM) were obtained from Invitrogen Life Technologies (Carlsbad, CA, USA). HBSS was supplemented with 25 mM Hepes (sHBSS, pH 7.4). Fura-2 AM, Oregon Green 488 BAPTA-1 AM, calcium calibration buffers and fluorescent microspheres were obtained from Molecular Probes (Eugene, OR, USA). Br-cGMP (8-bromo-guanosine 3′,5′-cyclic monophosphate, sodium salt), KT5823, ionomycin, BAPTA AM and Pluronic F-127 were obtained from Calbiochem-Novabiochem Corp. (La Jolla, CA, USA). ATP, sulfobromophthalein, L-ascorbic acid and DMSO were obtained from Sigma-Aldrich Corporation (St Louis, MO, USA). Oregon Green 488 BAPTA-1 AM, BAPTA AM, fura-2 AM, KT5823 and ionomycin were dissolved in DMSO and diluted in sHBSS to the final working concentrations with DMSO concentrations of 0.8, 0.2, 0.1, 0.04 and 0.002 %, respectively.
Cell culture
Primary cultures of rabbit tracheal epithelial cells were prepared as previously described (Dirksen et al. 1995; Evans & Sanderson, 1999). Adult New Zealand White rabbits (˜1.5 kg) were initially sedated with intramuscular xylazine (0.375 ml of 20 mg ml−1; 5 mg kg−1) and ketamine HCl (0.7 ml of 100 mg ml−1; 35 mg kg−1) and killed with intravenous pentobarbital sodium (3 ml of 50 mg ml−1, 100 mg kg−1) according to the protocol approved by Institutional Animal Care and Use Committee of the University of Massachusetts Medical School. After removal by dissection, the tracheal mucosa was cut into ˜0.5 mm squares, plated on collagen-coated glass coverslips, and cultured in DMEM supplemented with 10 % fetal bovine serum and penicillin-streptomycin at 37 °C in 10 % CO2 for 7–11 days.
Measurement of CBF with high-speed digital microscopy
Rabbit airway CBF was detected and quantified with a high-speed digital imaging system combined with a beat-by-beat analysis as previously described (Zhang & Sanderson, 2003). In general, phase-contrast images (648 pixels × 200 lines), formed with red light from a tungsten-halogen bulb, were collected at 240 frames s−1 with a high-speed CCD (charge-coupled device) camera (TM-6710, Pulnix America, Sunnyvale, CA, USA) in conjunction with a frame grabber (‘Road Runner’, BitFlow Inc., Woburn, MA, USA) and recording-software called ‘Video Savant’ (IO Industries, London, ON, Canada). CBF was determined from the variation in the light intensity of the phase-contrast image that resulted from the repetitive motion of cilia. The mean grey-intensity of a region of interest (ROI, area of 0.87 μm × 0.80 μm, 3 pixels × 3 pixels) positioned over the cilia of interest was calculated for each image and plotted with respect to time (i.e. frame number) to form a grey-intensity waveform. The frequency of each ciliary beat cycle was determined from the period of each cycle of the grey-intensity waveform by using a beat-by-beat analysis (Zhang & Sanderson, 2003).
Measurement of [Ca2+]i with imaging of fura-2
The details of [Ca2+]i measurement have been published elsewhere (Leybaert et al. 1998; Zhang & Sanderson, 2003). Briefly, cells were incubated in 1 μM fura-2 AM in sHBSS containing 100 μM sulfobromophthalein for 1 h at 37 °C, washed in sHBSS containing 100 μM sulfobromophthalein and allowed at least 30 min for de-esterification of the fura-2 AM. The coverslip bearing the cells was mounted on a Nikon Diaphot 300 inverted microscope equipped with a × 40, 1.3 NA, Ph 4, oil-immersion objective. The cells were equilibrated in sHBSS for at least 10 min to reach the warm working temperature of ˜29.5 ± 1 °C. At higher temperatures, evaporation artifacts were difficult to control.
Fluorescence (at 510 nm), generated by exciting the fura-2-loaded cells with 340 or 380 nm light, was detected with a silicon-intensified target (SIT) camera (Cohu, San Diego, USA). An optical memory disc recorder (OMDR, Panasonic TQ3031F) was used to record the images in time-lapse (4 frames s−1) without frame averaging. Calcium measurements were made by determining the normalized change in fluorescence (Ft/F0 where Ft is the measured fluorescence and F0 is the starting fluorescence) from a ROI (area of 1.8 μm × 1.6 μm, 6 pixels × 6 pixels) at the base of the cilia. The [Ca2+]i was calculated from this value using the original [Ca2+]i, the starting fluorescence and reference [Ca2+]i as described previously (Leybaert et al. 1998).
The temporal alignment of the phase-contrast and fluorescence images was achieved by simultaneously recording a time marker consisting of a fluorescent glass micropipette with each camera. The spatial alignment of the two image sets was achieved by recording a phase-contrast sequence of ciliary movement with the SIT camera. As previously described, an in vitro calibration was performed using a thin glass chamber to determine the Kd (132 ± 14 nM, n = 8) of fura-2 and the fluorescence ratio in the absence of Ca2+ (Rmin), the fluorescence ratio in the presence of saturating Ca2+ (Rmax) and the fluorescence ratio at 380 nm (F0/Fs) in the absence (F0) and presence of saturating (Fs) Ca2+ for the dye (Lansley & Sanderson, 1999; Zhang & Sanderson, 2003). The Rmin and Rmax measured with our imaging system on separate occasions over 11 months were 0.20 ± 0.01 and 5.19 ± 0.14, respectively (n = 8).
Estimation of the [Ca2+]i with imaging of Oregon Green
When cells were treated with KT5823, they became sensitive to UV light (see later) and, as a result, fura-2 could not be used for [Ca2+]i measurement. As an alternative Oregon green, which is excited by longer wavelength light, was used to monitor changes in [Ca2+]i. Cells were loaded with 20 μM Oregon Green 488 BAPTA-1 AM in sHBSS containing 0.2 % Pluronic F-127 for 1 h at 37 °C followed by 30 min of de-esterification in sHBSS. The solutions contained 100 μM sulfobromophthalein to inhibit ion pump activity and 3 mg ml−1 ascorbic acid to reduce bleaching (Bergner & Sanderson, 2002). Fluorescence (at 523 nm), generated by exciting Oregon Green-loaded cells with 485 nm light, was detected with the SIT camera and recorded at 4 frames s−1. The change in [Ca2+]i was represented by the normalized change in fluorescence by dividing the measured fluorescence by starting fluorescence (Ft/F0).
Simultaneous measurement of CBF and [Ca2+]i
Simultaneous imaging of CBF and [Ca2+]i was achieved by directing the different wavelengths of light forming the phase-contrast and fluorescence images to the respective cameras (CCD, SIT) with a dichroic beam splitter (655 nm) (Sanderson, 2000). When fluorescence images were not required, the excitation light was turned off. To replace the solution in the experimental chamber (300 μl), 1 ml of experimental solution, warmed to the working temperature, was drawn through the chamber with suction.
Data analysis and statistics
The basal CBF and [Ca2+]i were calculated from the first 10 s of data. The change in [Ca2+]i (Δ[Ca2+]i) (using fura-2) was calculated by subtracting the basal [Ca2+]i from the observed [Ca2+]i. Normalized CBF was obtained by dividing the measured CBF by the starting basal CBF. Normalized CBF and Δ[Ca2+] were used to facilitate the comparison of data from cells with slightly different starting conditions and to be compatible with other studies in the literature. All data were expressed as means ±s.e.m. Statistical analysis was performed with Student's paired or unpaired t test, or one-way analysis of variance (ANOVA) (Student-Newman-Keuls method for further multiple pair-wise comparisons). A value of P < 0.05 was considered statistically significant.
RESULTS
Changes in CBF and [Ca2+]i induced by Br-cGMP
In general, the changes in CBF induced by Br-cGMP could be divided into two responses. These consisted of (1) a moderate but sustained increase in CBF and (2) a series of transient increases in CBF that were superimposed on the sustained increase in CBF (Fig. 1, black traces). These transient increases in CBF were considerably larger than the sustained increases in CBF. By contrast, the changes in [Ca2+]i induced by Br-cGMP consisted only of a series of transient increases arising from a steady basal level (Fig. 1, grey traces).
Figure 1. The effects of 8-Bromo-cGMP (Br-cGMP) on the ciliary beat frequency (CBF) and [Ca2+]i of rabbit airway ciliated cells.
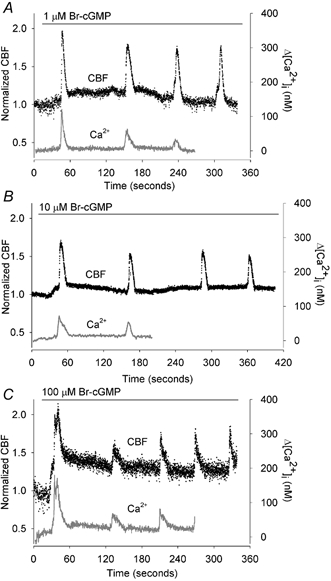
Data for both CBF and [Ca2+]i are normalized with the initial basal value and are representative of multiple cells. A, 1 μM Br-cGMP induced a two-part response in the CBF consisting of a small increase in the basal CBF (basal CBF = 10.4 Hz) that reached a maximum level within 60 s and declined thereafter, and larger transient increases in CBF that were superimposed on the basal CBF. By contrast, Br-cGMP only induced a series of transient increases in the [Ca2+]i without affecting the basal [Ca2+]i (n = 10). B, 10 μM Br-cGMP induced a higher sustained increase in the basal CBF (basal CBF = 15.9 Hz) but the superimposed transient increases in CBF, as well as the transient increases in [Ca2+]i, were unchanged (n = 11). C, 100 μM Br-cGMP only induced a further increase in the sustained CBF increase (basal CBF = 14.1 Hz) (n = 11). A-C, in all cases, the sustained increases in CBF occurred in the absence of changes in [Ca2+]i while the transient changes in CBF and [Ca2+]i were tightly coupled.
The correlation between the CBF and [Ca2+]i changes induced by Br-cGMP
With the high-temporal resolution data provided by our recording technique, it is possible to accurately correlate the changes in CBF with the changes in [Ca2+]i induced by Br-cGMP. In all cases, at either low or high concentrations of Br-cGMP, we found that the sustained increases in CBF (Fig. 2, blue dots) were initiated without a substantial change in [Ca2+]i. In addition, the sustained increases in CBF always occurred before the stimulation of the transient increases in CBF (Fig. 2, red dots). By contrast, these secondary transient increases in CBF were always tightly coupled with the transient increases in [Ca2+]i (Fig. 2); the increase in [Ca2+]i always preceded the increase in CBF. The change in CBF with respect to the [Ca2+]i during each phase of the Br-cGMP-induced response is clearly indicated in Fig. 2B and D. During the initial stages, the CBF increases (blue dots, vertical arrow) without a change in [Ca2+]i whereas as the subsequent increases in [Ca2+]i are associated with increases in CBF (red dots, diagonal arrow). These results imply that the sustained and moderate increases in CBF were induced directly by Br-cGMP whereas the transient increases in CBF were induced indirectly via changes in [Ca2+]i induced by Br-cGMP.
Figure 2. The correlation of the initial changes in CBF (coloured dots) with changes in [Ca2+]i (grey line) induced by 10 (A and B) or 100 μM (C and D) Br-cGMP.
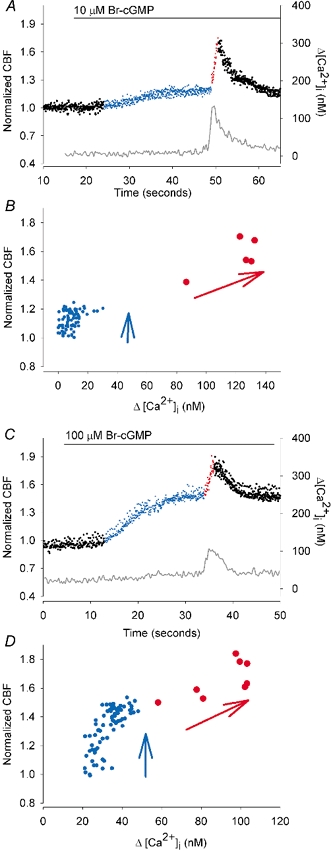
A, after the addition of 10 μM Br-cGMP, the CBF (normalized with basal CBF) slowly increased (blue dots) from the basal CBF (17.9 Hz, left black dots) while the [Ca2+]i remained unchanged. Approximately 30 s after the addition of Br-cGMP, the first transient increase in [Ca2+]i occurred and this was strongly correlated with a transient increase in CBF (red dots). B, the normalized CBF plotted against the corresponding [Ca2+]i for the time period illustrated in A. The vertical distribution of the blue dots, which represent the initial CBF increase induced by Br-cGMP, indicate that this phase of the response occurs independently of the [Ca2+]i. The inclined distribution of the red dots, which represent the CBF during the transient increase in [Ca2+]i, indicate that the CBF is dependent on the [Ca2+]i during this phase of the response. C and D, similar temporal and correlative representations of the changes in CBF and [Ca2+]i induced by 100 μM Br-cGMP. The initial increase in CBF (blue dots) is larger (basal CBF = 14.8 Hz) but still independent of the [Ca2+]i. The transient changes in CBF (red dots) are dependent on the [Ca2+]i.
Br-cGMP induces a concentration-dependent increase in CBF
The sustained increase in CBF induced directly by Br-cGMP was concentration dependent (Fig. 1 and Fig. 3). For example, 1 μM Br-cGMP induced a small but significant increase in CBF (relative increase of 1.13 ± 0.02, n = 10, P < 0.001) that usually reached a maximum level within 60 s. This was followed by a gradual decline in CBF to the basal rate after about 240–420 s (1.02 ± 0.02, n = 10, P > 0.05) (Fig. 1A and Fig. 3A). At higher concentrations, Br-cGMP induced significantly higher sustained increases in CBF that did not decline. For example, 10 μM Br-cGMP induced an initial relative increase in CBF of 1.23 ± 0.04 (n = 11, P < 0.001) that was sustained, after 240–420 s, at 1.17 ± 0.04 (n = 11, P < 0.001) (Fig. 1B and Fig. 3A). Similarly, 100 μM Br-cGMP induced an initial relative increase of 1.28 ± 0.05, (n = 11, P < 0.001) and this was sustained, after 240–420 s, at 1.24 ± 0.04, (n = 11, P < 0.001) (Fig. 1C and Fig. 3A). The initial increases in CBF induced by 100 μM Br-cGMP were also significantly greater than the increases in CBF induced by 1 μM Br-cGMP (P < 0.05). Similarly, the sustained increases in CBF induced by 10 and 100 μM Br-cGMP were significantly higher than the sustained increases in CBF induced by 1 μM Br-cGMP (P < 0.01 and P < 0.001, respectively). In control experiments, the application of sHBSS had no significant effect on either CBF or [Ca2+]i (n = 6).
Figure 3. The concentration-response relationship of CBF and [Ca2+]i in airway epithelial cells to Br-cGMP.
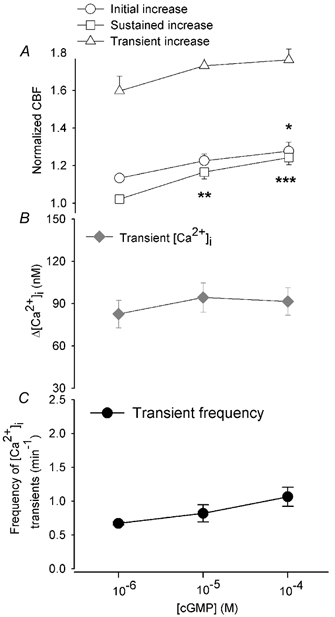
A, the initial increase in CBF (○) in response to 1, 10 and 100 μM Br-cGMP was 1.13 ± 0.02 (n = 10), 1.23 ± 0.04 (n = 11) and 1.28 ± 0.05, (n = 11), respectively, and was significantly greater (all P < 0.001) with respect to the basal rate with the increasing Br-cGMP concentration. The CBF increase induced by 100 μM Br-cGMP was also significantly greater than that induced by 1 μM Br-cGMP (*P < 0.05). The sustained increase in CBF (□) in response to 1, 10 and 100 μM Br-cGMP was 1.02 ± 0.02 (n = 10), 1.17 ± 0.04 (n = 11) and 1.24 ± 0.04 (n = 11), respectively, and this was also significantly greater (all P < 0.001) with the increasing Br-cGMP concentration. The sustained increase in CBF induced by 10 and 100 μM Br-cGMP was also significantly greater (**P < 0.01, ***P < 0.001) than that induced by 1 μM Br-cGMP. The transient increases in CBF (▵) in response to 1, 10 and 100 μM Br-cGMP were 1.60 ± 0.08 (n = 10), 1.73 ± 0.08 (n = 10) and 1.77 ± 0.06 (n = 11), respectively, but these were not significantly increased with increasing Br-cGMP concentration. The starting basal CBF (12.8 ± 0.9 Hz, n = 10; 11.9 ± 0.9 Hz, n = 11; 12.4 ± 0.6 Hz, n = 11) for each concentration of Br-cGMP was similar (P > 0.05). B, the concentration-response relationship of the transient change in [Ca2+]i to Br-cGMP. The transient changes in [Ca2+]i (♦) in response to 1, 10 and 100 μM Br-cGMP were 83 ± 10 nM, (n = 10), 94 ± 10 nM (n = 9) and 92 ± 10 nM (n = 10), respectively, but were not significantly different with increasing Br-cGMP concentration (P > 0.05). The basal [Ca2+]i (30 ± 4 nM, n = 10; 36 ± 4 nM, n = 11; 32 ± 5 nM, n = 11) for each concentration was similar (P > 0.05). C, the concentration-response relationship of the frequency of the transient changes in [Ca2+]i or CBF oscillations (•) to Br-cGMP. The frequency in response to 1, 10 and 100 μM Br-cGMP was 0.67 ± 0.02 min−1 (n = 5), 0.82 ± 0.13 min−1 (n = 5) and 1.07 ± 0.14 min−1 (n = 4), and not significantly different with the increasing Br-cGMP concentration (P > 0.05).
Br-cGMP-induced changes in Ca2+ signalling
As indicated previously, Br-cGMP also initiates a series of transient increases in [Ca2+]i. In response to 1 μM Br-cGMP, the Ca2+ transients had a mean magnitude of 83 ± 10 nM (n = 10) and occurred at a frequency of 0.67 ± 0.02 min−1 (n = 5) (Fig. 1A and Fig. 3B and C). The frequency was calculated from the duration of at least three sequential oscillations. However, not all cells (5 of 10 cells) displayed three oscillations during the relatively long recording period (up to 420 s) indicating that these values may represent the strongest response. Importantly, in all cases, these Ca2+ transients were accompanied by large transient changes in CBF (relative change = 1.60 ± 0.08, n = 10, Fig. 3A).
In response to 10 μM Br-cGMP, the [Ca2+]i transients had a mean magnitude of 94 ± 10 nM (n = 9) and an associated relative increase in CBF of 1.73 ± 0.08 (n = 10) at a frequency of 0.82 ± 0.13 min−1 (n = 5) (Fig. 1B and Fig. 3). In response to 100 μM Br-cGMP, the [Ca2+]i transients had a mean magnitude of 92 ± 10 nM (n = 10) and an associated relative increase in CBF of 1.77 ± 0.06 (n = 11) at a frequency of 1.07 ± 0.14 min−1 (n = 4) (Fig. 1C and Fig. 3). Although both the magnitude and the frequency of the [Ca2+]i and CBF transients had a tendency to increase with the increasing Br-cGMP concentrations, no statistical difference was found (Fig. 3).
The effect of buffering [Ca2+]i on the response to Br-cGMP
To confirm that Br-cGMP was capable of increasing CBF independently of changes in [Ca2+]i, the [Ca2+]i was buffered with the Ca2+ chelator BAPTA. After loading with fura-2, the cells were subsequently loaded with 20 μM BAPTA AM to establish a high intracellular concentration of BAPTA (Zhang & Sanderson, 2003). Upon treatment with 100 μM Br-cGMP, an increase in CBF was observed (Fig. 4) that consisted of an initial increase of 1.32 ± 0.08 (n = 6) that was followed by a sustained increase in CBF of 1.23 ± 0.05 (n = 6). These changes in CBF were indistinguishable from sustained CBF changes induced by 100 μM Br-cGMP in the absence of [Ca2+]i buffering (P > 0.05, respectively) (Fig. 4B and C). However, as expected in the presence of intracellular BAPTA, 100 μM Br-cGMP was unable to induce observable changes in the [Ca2+]i. These results further support the idea that the sustained increases in CBF are mediated by Br-cGMP whereas the transient increases in CBF are dependent on transient changes in [Ca2+]i.
Figure 4. The effect of buffering the [Ca2+]i with BAPTA in response to 100 μM Br-cGMP.
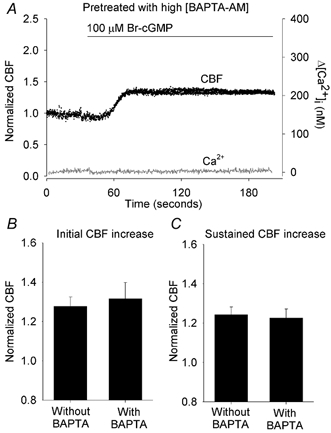
A, a representative response of a ciliated cell pretreated with 20 μM BAPTA AM and exposed to Br-cGMP (bar). The BAPTA buffering prevented any changes in [Ca2+]i (grey line). However, the CBF (black dots) still increased (to an initial normalized CBF = 1.36) and remained sustained (at a normalized CBF = 1.32). Transient increases in [Ca2+]i or CBF did not occur. The basal CBF was 12.1 Hz. B and C, the effect of buffering the [Ca2+]i with BAPTA on the initial (B) and sustained (C) changes in CBF induced by Br-cGMP. In control experiments, without BAPTA treatment, the initial and sustained increases in CBF induced by Br-cGMP were 1.28 ± 0.05 (n = 11) and 1.24 ± 0.04 (n = 11) respectively. These changes were indistinguishable (P > 0.05) from the initial (1.32 ± 0.08, n = 6) and sustained (1.23 ± 0.05, n = 6) increases in CBF induced by Br-cGMP in the presence of BAPTA. The basal CBFs for cells with (12.4 ± 1.0, n = 6) or without BAPTA treatment (12.4 ± 0.6, n = 11) were similar (P > 0.05).
The effect of KT5823 on the response to Br-cGMP with buffering [Ca2+]i
Since it is widely accepted that cGMP activates PKG, we tested the hypothesis that Br-cGMP-induced changes in CBF are induced by PKG activity by examining the effects of the PKG inhibitor KT5823 on the Br-cGMP-induced increases in CBF. Unfortunately, we initially found that, following treatment with KT5832 (< 0.5 μM) and exposure to 380 nm excitation light for simultaneous [Ca2+]i measurements, the CBF would gradually slow to a stop (after about 2 min). A similar phenomenon has been reported when the calmodulin inhibitors trifluoperazine and calmidazolium were applied to ovine airway ciliated cells (Salathe & Bookman, 1999). These results imply that the combination of exposure to 380 nm light and KT5823 is toxic to airway ciliated cells. To circumvent this problem, we used the Ca2+ indicator Oregon Green 488 BAPTA-1, which requires excitation light of 485 nm, to perform experiments with KT5832. A disadvantage of this approach is that the absolute value of the [Ca2+]i cannot be obtained with this dye. However, the normalized changes in fluorescence of the dye have been well accepted to represent the changes in [Ca2+]i.
After the cells were loaded with Oregon Green 488 BAPTA-1 AM to monitor [Ca2+]i, the cells were also loaded with the Ca2+ buffer BAPTA AM to prevent changes in [Ca2+]i. The cells were then pretreated with 2 μM KT5823 before exposure to 100 μM Br-cGMP. Under these conditions, Br-cGMP did not induce an increase in either CBF or [Ca2+]i (Fig. 5A and B, n = 7). A comparison of the Br-cGMP-induced initial or sustained changes in CBF in the presence or absence of KT5823 confirmed that the KT5823 abolished the effects of Br-cGMP (Fig. 5C and D). These results imply that Br-cGMP can induce changes in CBF via PKG.
Figure 5. The combined effect of buffering [Ca2+]i with BAPTA and inhibiting PKG activity with KT5823 on the response to Br-cGMP.
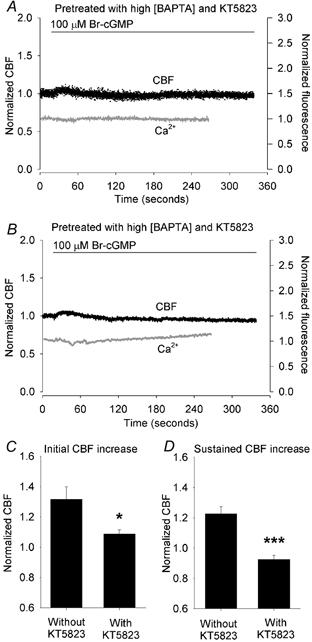
A and B, two representative cells illustrate the combined effect of the pretreatment of cells with KT5823 and BAPTA AM on the changes in CBF (black dots) and [Ca2+]i (grey lines) induced by 100 μM Br-cGMP (bar). KT5823 and BAPTA AM treatment prevented any significant increases in the [Ca2+]i and CBF in response to Br-cGMP. The basal CBF of the cells was 17.7 Hz (A) and 12.5 Hz (B). C and D, summaries of the combined effects of KT5823 and BAPTA AM on the initial (C) and sustained (D) increases in CBF induced by 100 μM Br-cGMP. The increase in the initial CBF (1.09 ± 0.03, n = 7) induced by Br-cGMP in the presence of KT5823 and BAPTA AM was significantly lower than the increase induced in cells without treatment (1.32 ± 0.08, n = 6, *P < 0.05). Similarly, the sustained increase in CBF (0.93 ± 0.03, n = 7) was significantly lower compared to cells without treatment (1.23 ± 0.05, n = 6, ***P < 0.001). The basal CBFs from each group were similar (12.4 ± 1.0 Hz, n = 6 and 15.3 ± 1.2 Hz, n = 7, P > 0.05).
The effects of KT5823 on the [Ca2+]i responses to cGMP
Because Br-cGMP induced both changes in CBF and [Ca2+]i, KT5823 was also used to assess whether PKG was involved in the stimulation of the Ca2+ transients. After cells were pretreated with KT5823, we found that exposure to Br-cGMP failed to increase the basal CBF, but a series of [Ca2+]i transients, with associated increases in CBF, still occurred (Fig. 6A). Neither the frequency of the [Ca2+]i transients (0.74 ± 0.09 min−1, n = 4) nor the mean peak CBF associated with the transients (1.56 ± 0.08, n = 6) were statistically different from those of cells without KT5823 treatment ([Ca2+]i transient frequency: 1.07 ± 0.14 min−1, n = 4; peak CBF: 1.77 ± 0.06, n = 11) (Fig. 6B and C). In accordance with our previous experiments with KT5823, the ability of Br-cAMP to induce initial and sustained changes in CBF (not associated with [Ca2+]i transients) was suppressed in comparison to those of cells not treated with KT5823 (Fig. 6D and E). These results suggest that Br-cGMP directly invokes changes in Ca2+ signalling and that increases in CBF can be mediated by increases in [Ca2+]i independently of PKG activity.
Figure 6. The effect of KT5823 treatment on changes in CBF and [Ca2+]i induced by 100 μM Br-cGMP.
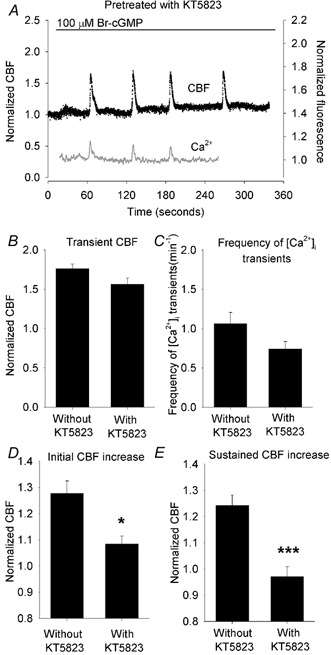
A, Br-cGMP (bar) induced a very small initial increase in CBF (black dots) that rapidly returned to the basal rate without inducing significant increases in [Ca2+]i (grey line). However, Br-cGMP still induced a series of transients in [Ca2+]i accompanied with transient increases in CBF. The basal CBF was 15.2 Hz. B and C, summaries of the effects of KT5823 treatment on Br-cGMP-induced transient changes in CBF (B) and the frequency of [Ca2+]i transients (C). KT5823 had no significant effect on the transient CBF (1.56 ± 0.08, n = 6 versus 1.77 ± 0.06, n = 11, P > 0.05) or the frequency of [Ca2+]i transients (0.74 ± 0.09, n = 4 versus 1.07 ± 0.14, n = 4, P > 0.05) as compared to controls. The basal CBFs from each group were similar (14.1 ± 0.9 Hz, n = 6 and 12.4 ± 0.6 Hz, n = 11, P > 0.05). D and E, summaries of the effects of KT5823 on initial (D) and sustained (E) CBF induced by Br-cGMP. Br-cGMP at 100 μM induced a significantly lower initial increase in CBF (1.08 ± 0.03, n = 6) compared to cells without KT5823 treatment (1.28 ± 0.05, n = 11, P < 0.05) and a significantly lower sustained CBF increase (0.97 ± 0.04, n = 6) compared to cells without KT5823 treatment (1.24 ± 0.04, n = 11, P < 0.001). *P < 0.05, ***P < 0.001.
Response of CBF and [Ca2+]i to ionomycin in the presence of KT5823
To confirm that CBF could be increased by increases in [Ca2+]i when the cGMP-PKG signalling pathway was inhibited, cells were pretreated with 2 μM KT5823 and then stimulated with ionomycin to increase the [Ca2+]i. In control experiments without KT5823, 1 μM ionomycin induced a fast increase in [Ca2+]i (normalized value of 1.18 ± 0.05, n = 5) and CBF (maximal rate: 2.43 ± 0.16, n = 5) (Fig. 7A). Subsequently, the [Ca2+]i returned to the basal level while the CBF decreased to a lower but sustained level of 1.50 ± 0.08 (n = 5). However, the changes in the [Ca2+]i and the initial maximum and sustained CBFs induced by ionomycin were unaffected by the pretreatment of the cells with KT5823 (Table 1, Fig. 7B). These results also suggest that the activation of PKG pathway was not necessary for the changes in CBF induced by increases in [Ca2+]i.
Figure 7. The effect of KT5823 treatment on ionomycin-induced increases in CBF and [Ca2+]i.
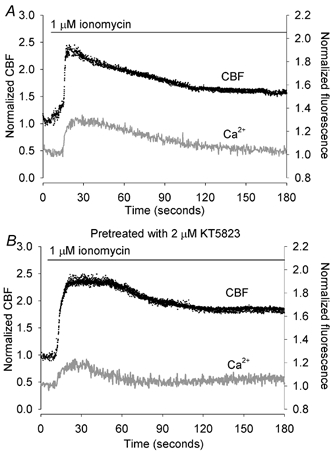
A, a representative trace (n = 5) of the simultaneous changes in CBF (black dots) and [Ca2+]i (grey line) in a ciliated epithelial cell in response to 1 μM ionomycin (bar). Ionomycin induced a rapid increase in CBF and [Ca2+]i. While the [Ca2+]i gradually returned to the basal level, the CBF remained sustained at an elevated level. B, following the pretreatment of cells with 2 μM KT5823, the response induced by 1 μM ionomycin was indistinguishable from the control response (n = 5).
Table 1.
The effects pre-treatment of cells with KT5823 on the changes in CBF and [Ca2+]i induced by 1 μM ionomyin
| Pre-treatment | Basal CBF | Normalized CBF (CBF1/CBF0) | Normalized maximum fluorescence | |
|---|---|---|---|---|
| KT5823 (n) | (Hz) | Maximum CBF | Sustained CBF | (Ft/F0) |
| Without (5) | a10.1 ± 1.2 | b2.43 ± 0.16 | c1.50 ± 0.08 | d1.18 ± 0.05 |
| With (5) | a12.2 ± 2.2 | b2.28 ± 0.27 | c1.74 ± 0.21 | d1.13 ± 0.03 |
CBF, ciliary beat frequeny.
No significant difference between groups (P > 0.05). Data values are represented as means ±s.e.m.
Response of CBF and [Ca2+]i to ATP in the presence of KT5823
To further explore whether PKG signalling is a prerequisite for the Ca2+-dependent increases in CBF induced by other agonists, we examined the effects of KT5823 on the oscillations in both CBF and [Ca2+]i induced by ATP (Zhang & Sanderson, 2003). In brief, we found that all aspects of the oscillatory changes in CBF and [Ca2+]i induced by ATP were unaffected by the pretreatment of cells with KT5823 (Table 2 and Fig. 8A and B). A similar initial increase in both CBF and [Ca2+]i and stimulation of sustained oscillations in CBF and [Ca2+]i, was observed in cells with (Fig. 8B) and without (Fig. 8A) KT5823 treatment. As previously described (Zhang & Sanderson, 2003), the CBF oscillations could be characterized with the following parameters: (i) the mean maximum (peak) CBF of the CBF oscillations, (ii) the mean minimum CBF of the CBF oscillations and (iii) the frequency of the oscillations in CBF. None of these parameters were significantly altered by pretreatment with KT5823. These results indicate that the PKG signalling pathway has no role in Ca2+-mediated changes in CBF or the mechanisms generating Ca2+ oscillations.
Table 2.
The effects pre-treatment of cells with KT5823 on the changes in CBF and [Ca2+]i induced by 2 μm ATP
| Pre-treatment | Basal CBF | Oscillations in CBF | Normalized CBF (CBFt/CBF0) | Normalized maximum fluorescence | |
|---|---|---|---|---|---|
| KT5823 (n) | (Hz) | (min−1) | Min CBF | Max CBF | (Ft/F0) |
| Without (5) | a12.8 ± 0.9 | b1.40 ± 2 | c1.24 ± 0.05 | d1.96 ± 0.020 | e1.16 ± 0.03 |
| With (5) | a11.8 ± 1.9 | b1.4 ± 0.5 | c1.13 ± 0.03 | d1.91 ± 0.12 | e1.18 ± 0.03 |
tmeasured fluoresence; F0, starting fluorence.
No significant difference between groups (P > 0.05). Data values are represented as means ±s.e.m.
Figure 8. The effect of KT5823 treatment on ATP-induced changes in CBF and [Ca2+]i.
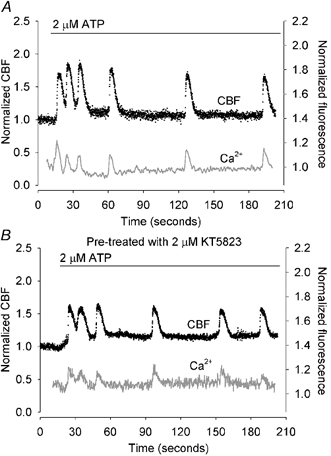
A, a representative trace of the simultaneous changes in CBF (black dots) and [Ca2+]i (grey line) in ciliated epithelial cells in response to 2 μM ATP (bar). ATP induced a rapid increases in CBF and [Ca2+]i which was followed by oscillations in both CBF and [Ca2+]i. The CBF oscillations occurred from an elevated minimum CBF while the [Ca2+]i oscillations occurred from a baseline that declined to the basal level. B, ATP induced a similar response in CBF and [Ca2+]i in cells that were pretreated with 2 μM KT5823 (n = 5).
DISCUSSION
In previous studies, cGMP (Br-cGMP or dibutyryl cGMP (db-cGMP)) has been reported to increase CBF in human (Geary et al. 1995; Runer & Lindberg, 1999), rat (Li et al. 2000) and bovine (Wyatt et al. 1998) airway ciliated cells. However, Tamaoki et al. (1991) reported that cGMP inhibited CBF in rabbit cells. By using high-speed digital recording to continuously sample CBF at 240 Hz and a beat-by-beat analysis to determine the duration or frequency of each ciliary beat cycle (Zhang & Sanderson, 2003), we have found, in keeping with the other studies, which cGMP increased CBF (Geary et al. 1995; Wyatt et al. 1998; Runer & Lindberg, 1999; Li et al. 2000) (Fig. 1 and Fig. 3A). But, unlike other studies, we have also found, by simultaneously measuring [Ca2+]i with CBF, that the CBF responses induced by cGMP consisted of a Ca2+-dependent response and a Ca2+-independent response.
Since a variety of factors may potentially influence the magnitude of the CBF, we chose to use Br-cGMP as the PKG activator because it is a potent, membrane-permeant activator that does not affect cGMP-stimulated or cGMP-inhibited phosphodiesterases (cGS-PDEs and cGI-PDEs) and is relatively resistant to hydrolysis by phosphodiesterases (Smolenski et al. 1998). Temperature also affects the response; Wyatt et al. (1998) and Li et al. (2000) reported a CBF increase of ˜10 % at room temperature, while Geary et al. (1995) and Runer et al. (1999) reported higher increases in CBF at warmer temperatures (˜25 % at 33 °C; ˜50 % at 36–37 °C). Our studies were conducted at ˜30 °C. Higher temperatures led to difficulties with evaporation artifacts. We only investigated the effects of 1–100 μM Br-cGMP on CBF and [Ca2+]i since higher concentrations did not further increase CBF (Wyatt et al. 1998; Li et al. 2000) and had the potential to activate PKA (Forte et al. 1992; Lincoln et al. 1996; Carvajal et al. 2000).
The Ca2+-independent increase in CBF induced by Br-cGMP was relatively small but was sustained at a constant rate and was dependent on the concentration of Br-cGMP (Fig. 3A). The sustained increase in CBF appeared to be mediated by the cGMP-PKG signalling pathway because the inhibition of PKG with KT5823 (in the presence or absence of the Ca2+ buffer, BAPTA) virtually abolished the Ca2+-independent changes in CBF (Fig. 5 and Fig. 6). Furthermore, when the Ca2+ buffering of the cell was increased by BAPTA, the sustained increases in CBF were still induced by Br-cGMP (Fig. 4).
The second major CBF response to cGMP was the large transient increases in CBF that were superimposed on the sustained elevation of CBF (Fig. 3). In all cases, we found that these transient increases in CBF were tightly-coupled with transient increases [Ca2+]i (Fig. 1 and Fig. 2) and that both the transient CBF and [Ca2+]i changes were abolished by increased [Ca2+]i buffering with BAPTA. In addition, the increases in [Ca2+]i always occurred prior to the increases in CBF as has been previously described (Evans & Sanderson, 1999; Lansley & Sanderson, 1999). From these results, we conclude that the transient increases in CBF are dependent on the transient increases in [Ca2+]i that are induced by Br-cGMP.
The mechanism by which [Ca2+]i transients are induced by Br-cGMP is not fully understood. However, the transients in [Ca2+]i still occurred in the presence of the PKG inhibitor KT5823 and this supports the possibility that cGMP acts directly, but independently of PKG, on the Ca2+ signalling machinery of the cell. We also found in this study that the frequency of Ca2+ transients induced by cGMP showed little or no change over the range of cGMP concentrations tested and this suggests that the sensitization of the Ca2+ signalling system may be readily saturated by small cGMP concentrations. The stimulation of the Ca2+ transients may result from increasing the sensitivity of the inositol 1,4,5-trisphosphate (IP3) receptor or ryanodine receptor (RyR) to endogenous second messengers such as IP3 or cyclic-adenosine diphosphate ribose (Berridge et al. 2000). However, contrary to these suggestions are other studies that have reported that cGMP can inhibit [Ca2+]i transients (Ruth et al. 1993) and oscillations (Pauvert et al. 2000; Kwan et al. 2001) by targeting the IP3 receptor (Ruth et al. 1993; Kwan et al. 2001) in epithelial cells and smooth muscle cells. The independence of the magnitude of the [Ca2+]i transients (and therefore the CBF transients) from the cGMP concentration is readily attributable to the Ca2+ release properties of the IP3 receptor that are inhibited by increasing [Ca2+]i (Berridge et al. 2000). As a result, the release of Ca2+ by the cell is a self-limiting response. An alternative mechanism through which cGMP may act is the stimulation of cGS-PDEs, cGI-PDEs or cGMP-gated ion channels (McDonald & Murad, 1995; Leinders-Zufall et al. 1997; Lohmann et al. 1997; Carvajal et al. 2000) and, under certain circumstances, PKA to phosphorylate other target proteins (McCann et al. 1989; Forte et al. 1992; Lincoln et al. 1996; Braiman et al. 1998; Sisson et al. 1999; Carvajal et al. 2000). The fact that the Ca2+ transients were always induced later than the sustained increases in CBF by cGMP suggests the action of cGMP may require additional intermediate steps. However, the relevance of these mechanisms to the Ca2+ oscillations observed in this report requires further investigation.
Although the regulation of CBF appears to involve both cGMP-PKG and Ca2+ signalling, the interaction of these two signalling pathways is not well understood. A number of studies have suggested that Ca2+ could not alter CBF by itself, but required the prerequisite activation of PKG (Uzlaner & Priel, 1999; Braiman et al. 2000, 2001; Ma et al. 2002; Zagoory et al. 2002). For example, the inhibition of PKG with KT5823 was reported to abolish agonist-induced increase in CBF (e.g. acetylcholine (Zagoory et al. 2002) or ATP (Uzlaner & Priel, 1999)) even though the [Ca2+]i responses were not significantly influenced. The proposed mechanism underlying this response was that PKG activation was stimulated by a cascade of signalling events associated with NO production. These included the Ca2+ activation of NO synthase to produce NO, which in turn activates GC to produce an elevation of cGMP. The subsequent increase in cGMP-dependent phosphorylation is proposed, together with the increased Ca2+, to stimulate a large increase in CBF (Uzlaner & Priel, 1999; Braiman et al. 2000, 2001; Ma et al. 2002; Zagoory et al. 2002). Unfortunately, in these previous studies, no significant increases in either CBF or Ca2+ were induced by exposure to db-cGMP (100 μM) (Uzlaner & Priel, 1999; Braiman et al. 2001). As a result, it was not clear that the PKG activation could induce an increase in CBF. Similarly, it was not possible to distinguish between the increases in both CBF and [Ca2+]i induced by ATP in the presence or absence db-cGMP (Braiman et al. 2001). Therefore, it was also unclear whether CBF increases induced by ATP were due to the direct effects of Ca2+ or the Ca2+-activation of PKG. However, we show here that the Ca2+ and CBF responses induced by Ca2+ ionophore, ionomycin or by ATP were unaffected by the PKG inhibitor. This result also underscores the idea that stimulation by ATP, which is believed to act via the activation of PLC, does not rely on PKG activity. Similarly, the non-specific entrance of Ca2+ into the cell also does not need to activate PKG before it can elevate the CBF.
Support for the direct action of Ca2+ on the CBF has also been provided by Salathe & Bookman (1999) and Salathe et al. (2000) who found that tightly coupled changes in CBF and [Ca2+]i were not influenced by inhibitors of NO synthase in ovine airway epithelium. Similarly, we have previously reported that Ca2+ waves are able to rapidly increase CBF in the absence of any prior stimulation (Lansley & Sanderson, 1992). The idea that Ca2+ and cGMP regulate ciliary activity independently is also supported by studies at the axonemal level in which Ca2+-dependent (Gundersen & Nelson, 1987; Son et al. 1993) and cyclic nucleotide-dependent (Miglietta & Nelson, 1988; Hochstrasser & Nelson, 1989) protein kinases were found to phosphorylate different axonemal proteins (Travis & Nelson, 1988; Bonini & Nelson, 1988, 1990; Hamasaki et al. 1989, 1991; Salathe et al. 1993; Walczak & Nelson, 1993, 1994; Satir et al. 1995; Porter & Sale, 2000).
In summary, we have found that Br-cGMP stimulates sustained increases in rabbit airway CBF in a PKG-dependent but Ca2+-independent manner. By contrast, Br-cGMP also initiates transient increases in [Ca2+]i that, in turn, lead to transient increases in CBF, which are independent of PKG. The inhibition of PKG had no significant influence on either CBF and [Ca2+]i transients induced by ATP, or the Ca2+-coupled CBF increase induced by ionomycin. As a result, we propose that cGMP can induce increases in CBF in a Ca2+-independent and Ca2+-dependent manner and that the stimulation of CBF by Ca2+ does not require the activation of PKG.
Acknowledgments
This work was supported by NIH grants HL49288 and HL70882 to M.J.S.
REFERENCES
- Bergner A, Sanderson MJ. Acetylcholine-induced calcium signaling and contraction of airway smooth muscle cells in lung slices. J Gen Physiol. 2002;119:187–198. doi: 10.1085/jgp.119.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Bonini NM, Nelson DL. Differential regulation of Paramecium ciliary motility by cAMP and cGMP. J Cell Biol. 1988;106:1615–1623. doi: 10.1083/jcb.106.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini NM, Nelson DL. Phosphoproteins associated with cyclic nucleotide stimulation of ciliary motility in Paramecium. J Cell Sci. 1990;95:219–230. doi: 10.1242/jcs.95.2.219. [DOI] [PubMed] [Google Scholar]
- Braiman A, Silberberg SD, Priel Z. Purinergic stimulation of ciliary activity. Drug Dev Res. 2000;50:550–554. [Google Scholar]
- Braiman A, Uzlaner N, Priel Z. Enhancement of CBF is dominantly controlled by PKG and/or PKA. In: Salathe M, editor. Cilia and Mucus, from Development to Respiratory Defense. New York: Blackwell Science Inc; 2001. pp. 67–79. [Google Scholar]
- Braiman A, Zagoory O, Priel Z. PKA induces Ca2+ release and enhances ciliary beat frequency in a Ca2+-dependent and -independent manner. Am J Physiol. 1998;275:C790–797. doi: 10.1152/ajpcell.1998.275.3.C790. [DOI] [PubMed] [Google Scholar]
- Carvajal JA, Germain AM, Huidobro-Toro JP, Weiner CP. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J Cell Physiol. 2000;184:409–420. doi: 10.1002/1097-4652(200009)184:3<409::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Dirksen ER, Felix JA, Sanderson MJ. Preparation of explant and organ cultures and single cells from airway epithelium. Methods Cell Biol. 1995;47:65–74. doi: 10.1016/s0091-679x(08)60792-x. [DOI] [PubMed] [Google Scholar]
- Evans JH, Sanderson MJ. Intracellular calcium oscillations regulate ciliary beat frequency of airway epithelial cells. Cell Calcium. 1999;26:103–110. doi: 10.1054/ceca.1999.0060. [DOI] [PubMed] [Google Scholar]
- Forte LR, Thorne PK, Eber SL, Krause WJ, Freeman RH, Francis SH, Corbin JD. Stimulation of intestinal Cl− transport by heat-stable enterotoxin: activation of cAMP-dependent protein kinase by cGMP. Am J Physiol. 1992;263:C607–615. doi: 10.1152/ajpcell.1992.263.3.C607. [DOI] [PubMed] [Google Scholar]
- Geary CA, Davis CW, Paradiso AM, Boucher RC. Role of CNP in human airways: cGMP-mediated stimulation of ciliary beat frequency. Am J Physiol. 1995;268:L1021–1028. doi: 10.1152/ajplung.1995.268.6.L1021. [DOI] [PubMed] [Google Scholar]
- Gundersen RE, Nelson DL. A novel Ca2+-dependent protein kinase from Paramecium tetraurelia. J Biol Chem. 1987;262:4602–4609. [PubMed] [Google Scholar]
- Hamasaki T, Barkalow K, Richmond J, Satir P. cAMP-stimulated phosphorylation of an axonemal polypeptide that copurifies with the 22S dynein arm regulates microtubule translocation velocity and swimming speed in Paramecium. Proc Natl Acad Sci U S A. 1991;88:7918–7922. doi: 10.1073/pnas.88.18.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki T, Murtaugh TJ, Satir BH, Satir P. In vitro phosphorylation of Paramecium axonemes and permeabilized cells. Cell Motil Cytoskeleton. 1989;12:1–11. doi: 10.1002/cm.970120102. [DOI] [PubMed] [Google Scholar]
- Hobbs AJ, Ignarro LJ. Nitric oxide-cyclic GMP signal transduction system. Methods Enzymol. 1996;269:134–148. doi: 10.1016/s0076-6879(96)69016-8. [DOI] [PubMed] [Google Scholar]
- Hochstrasser M, Nelson DL. Cyclic AMP-dependent protein kinase in Paramecium tetraurelia. Its purification and the production of monoclonal antibodies against both subunits. J Biol Chem. 1989;264:14510–14518. [PubMed] [Google Scholar]
- Jain B, Rubinstein I, Robbins RA, Leise KL, Sisson JH. Modulation of airway epithelial cell ciliary beat frequency by nitric oxide. Biochem Biophys Res Commun. 1993;191:83–88. doi: 10.1006/bbrc.1993.1187. [DOI] [PubMed] [Google Scholar]
- Kwan HY, Huang Y, Kong SK, Yao X. cGMP abolishes agonist-induced [Ca(2+)](i) oscillations in human bladder epithelial cells. Am J Physiol Renal Physiol. 2001;281:F1067–1074. doi: 10.1152/ajprenal.0031.2001. [DOI] [PubMed] [Google Scholar]
- Lansley AB, Sanderson MJ. Regulation of airway ciliary activity by Ca2+: simultaneous measurement of beat frequency and intracellular Ca2+ Biophys J. 1999;77:629–638. doi: 10.1016/S0006-3495(99)76919-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansley AB, Sanderson MJ, Dirksen ER. Control of the beat cycle of respiratory tract cilia by Ca2+ and cAMP. Am J Physiol. 1992;263:L232–242. doi: 10.1152/ajplung.1992.263.2.L232. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Rand MN, Shepherd GM, Greer CA, Zufall F. Calcium entry through cyclic nucleotide-gated channels in individual cilia of olfactory receptor cells: spatiotemporal dynamics. J Neurosci. 1997;17:4136–4148. doi: 10.1523/JNEUROSCI.17-11-04136.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leybaert L, Sneyd J, Sanderson MJ. A simple method for high temporal resolution calcium imaging with dual excitation dyes. Biophys J. 1998;75:2025–2029. doi: 10.1016/S0006-3495(98)77644-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Shirakami G, Zhan X, Johns RA. Regulation of ciliary beat frequency by the nitric oxide-cyclic guanosine monophosphate signaling pathway in rat airway epithelial cells. Am J Respir Cell Mol Biol. 2000;23:175–181. doi: 10.1165/ajrcmb.23.2.4022. [DOI] [PubMed] [Google Scholar]
- Lincoln TM, Cornwell TL. Intracellular cyclic GMP receptor proteins. FASEB J. 1993;7:328–338. doi: 10.1096/fasebj.7.2.7680013. [DOI] [PubMed] [Google Scholar]
- Lincoln TM, Cornwell TL, Komalavilas P, Boerth N. Cyclic GMP-dependent protein kinase in nitric oxide signaling. Methods Enzymol. 1996;269:149–166. doi: 10.1016/s0076-6879(96)69017-x. [DOI] [PubMed] [Google Scholar]
- Lohmann SM, Vaandrager AB, Smolenski A, Walter U, De Jonge HR. Distinct and specific functions of cGMP-dependent protein kinases. Trends Biochem Sci. 1997;22:307–312. doi: 10.1016/s0968-0004(97)01086-4. [DOI] [PubMed] [Google Scholar]
- Ma W, Silberberg SD, Priel Z. Distinct axonemal processes underlie spontaneous and stimulated airway ciliary activity. J Gen Physiol. 2002;120:875–885. doi: 10.1085/jgp.20028695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann JD, Bhalla RC, Welsh MJ. Release of intracellular calcium by two different second messengers in airway epithelium. Am J Physiol. 1989;257:L116–224. doi: 10.1152/ajplung.1989.257.2.L116. [DOI] [PubMed] [Google Scholar]
- McDonald LJ, Murad F. Nitric oxide and cGMP signaling. Adv Pharmacol. 1995;34:263–275. doi: 10.1016/s1054-3589(08)61091-1. [DOI] [PubMed] [Google Scholar]
- Miglietta LA, Nelson DL. A novel cGMP-dependent protein kinase from Paramecium. J Biol Chem. 1988;263:16096–16105. [PubMed] [Google Scholar]
- Murad F. The 1996 Albert Lasker Medical Research Awards. Signal transduction using nitric oxide and cyclic guanosine monophosphate. JAMA. 1996;276:1189–1192. [PubMed] [Google Scholar]
- Pauvert O, Marthan R, Savineau J. NO-induced modulation of calcium-oscillations in pulmonary vascular smooth muscle. Cell Calcium. 2000;27:329–338. doi: 10.1054/ceca.2000.0123. [DOI] [PubMed] [Google Scholar]
- Porter ME, Sale WS. The 9 + 2 axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility. J Cell Biol. 2000;151:F37–42. doi: 10.1083/jcb.151.5.f37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runer T, Lindberg S. Ciliostimulatory effects mediated by nitric oxide. Acta Otolaryngol. 1999;119:821–825. doi: 10.1080/00016489950180487. [DOI] [PubMed] [Google Scholar]
- Ruth P, Wang GX, Boekhoff I, May B, Pfeifer A, Penner R, Korth M, Breer H, Hofmann F. Transfected cGMP-dependent protein kinase suppresses calcium transients by inhibition of inositol 1,4,5-trisphosphate production. Proc Natl Acad Sci U S A. 1993;90:2623–2627. doi: 10.1073/pnas.90.7.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salathe M, Bookman RJ. Mode of Ca2+ action on ciliary beat frequency in single ovine airway epithelial cells. J Physiol. 1999;520:851–865. doi: 10.1111/j.1469-7793.1999.00851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salathe M, Lieb T, Bookman RJ. Lack of nitric oxide involvement in cholinergic modulation of ovine ciliary beat frequency. J Aerosol Med. 2000;13:219–229. doi: 10.1089/jam.2000.13.219. [DOI] [PubMed] [Google Scholar]
- Salathe M, Pratt MM, Wanner A. Cyclic AMP-dependent phosphorylation of a 26 kD axonemal protein in ovine cilia isolated from small tissue pieces. Am J Respir Cell Mol Biol. 1993;9:306–314. doi: 10.1165/ajrcmb/9.3.306. [DOI] [PubMed] [Google Scholar]
- Sanderson MJ. High-speed digital microscopy. Methods. 2000;21:325–334. doi: 10.1006/meth.2000.1022. [DOI] [PubMed] [Google Scholar]
- Satir P, Barkalow K, Hamasaki T. Ciliary beat frequency is controlled by a dynein light chain phosphorylation. Biophys J. 1995;68:222S. [PMC free article] [PubMed] [Google Scholar]
- Satir P, Sleigh MA. The physiology of cilia and mucociliary interactions. Annu Rev Physiol. 1990;52:137–155. doi: 10.1146/annurev.ph.52.030190.001033. [DOI] [PubMed] [Google Scholar]
- Schmidt HH, Walter U. NO at work. Cell. 1994;78:919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Seybold ZV, Mariassy AT, Stroh D, Kim CS, Gazeroglu H, Wanner A. Mucociliary interaction in vitro: effects of physiological and inflammatory stimuli. J Appl Physiol. 1990;68:1421–6. doi: 10.1152/jappl.1990.68.4.1421. [DOI] [PubMed] [Google Scholar]
- Shirakami G, Li D, Zhan X, Johns RA. Propofol stimulates ciliary motility via the nitric oxide-cyclic GMP pathway in cultured rat tracheal epithelial cells. Anesthesiology. 2000;93:482–488. doi: 10.1097/00000542-200008000-00028. [DOI] [PubMed] [Google Scholar]
- Sisson JH, May K, Wyatt TA. Nitric oxide-dependent ethanol stimulation of ciliary motility is linked to cAMP-dependent protein kinase (PKA) activation in bovine bronchial epithelium. Alcohol Clin Exp Res. 1999;23:1528–1533. [PubMed] [Google Scholar]
- Smolenski A, Burkhardt AM, Eigenthaler M, Butt E, Gambaryan S, Lohmann SM, Walter U. Functional analysis of cGMP-dependent protein kinases I and II as mediators of NO/cGMP effects. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:134–149. doi: 10.1007/pl00005234. [DOI] [PubMed] [Google Scholar]
- Son M, Gundersen RE, Nelson DL. A second member of the novel Ca(2+)-dependent protein kinase family from Paramecium tetraurelia. Purification and characterization. J Biol Chem. 1993;268:5940–5948. [PubMed] [Google Scholar]
- Tamaoki J, Kobayashi K, Sakai N, Kanemura T, Horii S, Isono K, Takeuchi S, Chiyotani A, Yamawaki I, Takizawa T. Atrial natriuretic factor inhibits ciliary motility in cultured rabbit tracheal epithelium. Am J Physiol. 1991;260:C201–205. doi: 10.1152/ajpcell.1991.260.2.C201. [DOI] [PubMed] [Google Scholar]
- Travis SM, Nelson DL. Regulation of axonemal Mg2+-ATPase from Paramecium cilia: effects of Ca2+ and cyclic nucleotides. Biochim Biophys Acta. 1988;966:84–93. doi: 10.1016/0304-4165(88)90131-6. [DOI] [PubMed] [Google Scholar]
- Uzlaner N, Priel Z. Interplay between the NO pathway and elevated [Ca2+]i enhances ciliary activity in rabbit trachea. J Physiol. 1999;516:179–190. doi: 10.1111/j.1469-7793.1999.179aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaandrager AB, de Jonge HR. Signalling by cGMP-dependent protein kinases. Mol Cell Biochem. 1996;157:23–30. doi: 10.1007/BF00227877. [DOI] [PubMed] [Google Scholar]
- Walczak CE, Nelson DL. In vitro phosphorylation of ciliary dyneins by protein kinases from Paramecium. J Cell Sci. 1993;106:1369–1376. doi: 10.1242/jcs.106.4.1369. [DOI] [PubMed] [Google Scholar]
- Walczak CE, Nelson DL. Regulation of dynein-driven motility in cilia and flagella. Cell Motil Cytoskeleton. 1994;27:101–107. doi: 10.1002/cm.970270202. [DOI] [PubMed] [Google Scholar]
- Wanner A, Salathe M, O'Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996;154:1868–1902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Spurzem JR, May K, Sisson JH. Regulation of ciliary beat frequency by both PKA and PKG in bovine airway epithelial cells. Am J Physiol. 1998;275:L827–835. doi: 10.1152/ajplung.1998.275.4.L827. [DOI] [PubMed] [Google Scholar]
- Yang B, Schlosser RJ, McCaffrey TV. Signal transduction pathways in modulation of ciliary beat frequency by methacholine. Ann Otol Rhinol Laryngol. 1997;106:230–236. doi: 10.1177/000348949710600309. [DOI] [PubMed] [Google Scholar]
- Zagoory O, Braiman A, Priel Z. The mechanism of ciliary stimulation by acetylcholine: Roles of calcium, PKA, and PKG. J Gen Physiol. 2002;119:329–339. doi: 10.1085/jgp.20028519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Li D, Johns RA. Immunohistochemical evidence for the NO cGMP signaling pathway in respiratory ciliated epithelia of rat. J Histochem Cytochem. 1999;47:1369–1374. doi: 10.1177/002215549904701103. [DOI] [PubMed] [Google Scholar]
- Zhang L, Sanderson MJ. Oscillations in ciliary beat frequency and intracellular calcium concentration in rabbit tracheal epithelial cells induced by ATP. J Physiol. 2003;546:733–749. doi: 10.1113/jphysiol.2002.028704. [DOI] [PMC free article] [PubMed] [Google Scholar]


