Abstract
Previous studies have suggested that melatonin alters sympathetic outflow in humans. The purpose of the present study was to determine in humans the effect of melatonin on sympathetic nerve activity and arterial blood pressure during orthostatic stress. Fifty minutes after receiving a 3 mg tablet of melatonin or placebo (different days), muscle sympathetic nerve activity (MSNA), arterial blood pressure, heart rate, forearm blood flow and thoracic impedance were measured for 10 min at rest and during 5 min of lower body negative pressure (LBNP) at −10 and −40 mmHg (n = 11). During LBNP, MSNA responses were attenuated after melatonin at both −10 and −40 mmHg (P < 0.03). Specifically, during the placebo trial, MSNA increased by 33 ± 8 and 251 ± 70 % during −10 and −40 mmHg, respectively, but increased by only 8 ± 7 and 111 ± 35 % during −10 and −40 mmHg with melatonin, respectively. However, arterial blood pressure and forearm vascular resistance responses were unchanged by melatonin during LBNP. MSNA responses were not affected by melatonin during an isometric handgrip test (30 % maximum voluntary contraction) and a cold pressor test. Plasma melatonin concentration was measured at 25 min intervals for 125 min in six subjects. Melatonin concentration was 14 ± 11 pg ml−1 before ingestion and was significantly increased at each time point (peaking at 75 min; 1830 ± 848 pg ml−1). These findings indicate that in humans, a high concentration of melatonin can attenuate the reflex sympathetic increases that occur in response to orthostatic stress. These alterations appear to be mediated by melatonin-induced changes to the baroreflexes.
Melatonin is secreted by the pineal gland in a circadian fashion at night (Reiter, 1991). The use of exogenous melatonin is widespread in the general public. Melatonin use is reported to be greater than the consumption of vitamin C tablets (Bonn, 1996). Melatonin has been linked to improved sleep, prevention of jet lag, enhanced immune response, antioxidant actions, and prevention of tumour growth (Brzezinski, 1997). In addition, data suggests that melatonin affects cardiovascular function (Krause et al. 1999). Melatonin has been reported to reduce arterial blood pressure in normal and hypertensive rats (Kawashima et al. 1987; Chuang et al. 1993; K-Laflamme et al. 1998). Studies in humans indicate that 1 mg of melatonin decreases arterial pressure and the plasma levels of noradrenaline during standing (Cagnacci et al. 1998; Arangino et al. 1999). Melatonin has been taken by astronauts to help improve sleep during spaceflight. However, because up to two-thirds of astronauts have orthostatic intolerance or hypotension after spaceflight (Buckey et al. 1996), the use of melatonin may exacerbate this serious physiological problem, and that which occurs in the large number of individuals who experience orthostatic intolerance in the general population (Robertson, 1999).
It has been reported that there is a close association between sympathetic nerve innervation and melatonin release (Bruce et al. 1991). It is not yet known whether the reported melatonin-mediated reductions in plasma noradrenaline observed during standing are the result of decreased central sympathetic outflow. The purpose of the present study was to determine the effect of melatonin on muscle sympathetic nerve activity (MSNA) and arterial blood pressure during orthostatic stress in humans. Based on previous studies, it was hypothesized that melatonin would attenuate MSNA responses during an orthostatic challenge induced by lower body negative pressure (LBNP). The findings of the current study support the concept that melatonin attenuates sympathetic nerve responses to orthostatic stress.
METHODS
Subjects
Twelve healthy subjects (five men, seven women) aged 26 ± 1 years, weighing 70 ± 4 kg and of average height 167 ± 3 cm, volunteered to participate in the study. These subjects were normotensive and not taking any medication. The testing protocols were explained verbally to each subject, and written informed consent was obtained from all participants. The experimental protocol was conducted according to the Declaration of Helsinki and was approved by the Institutional Review Board of the Pennsylvania State University College of Medicine.
Experimental design
Upon arrival at the laboratory, tympanic temperature was recorded in each of the subjects, who were then given a tablet of either 3 mg of melatonin or placebo (lactose) to ingest. The subjects were then prepared for the experimental protocol. After being positioned in a LBNP chamber in the supine posture, a finger blood-pressure-measuring device was placed on one hand, and measurement of leg MSNA (via microneurography) was prepared. Fifty minutes after ingestion of melatonin or placebo, a 10 min baseline recording period ensued for MSNA, arterial blood pressure and heart rate. Tympanic temperature was measured once again at the end of the baseline period. LBNP was then applied for 5 min at −10 mmHg and then increased to −40 mmHg for an additional 5 min.
To determine if melatonin had affects on MSNA responses to other physical stressors, subjects performed isometric handgrip at 30 % of maximum voluntary contraction for 2 min, and a 2 min cold pressor test. Ample time was allowed between tests such that all variables returned to baseline levels. Tympanic temperature was recorded at the end of these tests. This experimental protocol was repeated on a separate day at the same time using the opposite drug. The order of the drug testing was counterbalanced.
The LBNP protocol was repeated in its entirety on seven of the subjects to determine changes in central blood volume during LBNP. Changes in thoracic impedance provide an indication as to whether changes in central cardiac filling were affected by melatonin during the experimental protocol.
An additional study was performed on six of the subjects. MSNA, arterial blood pressure and heart rate were recorded continuously, and venous blood samples were obtained every 25 min for 125 min to determine plasma melatonin concentrations after taking 3 mg of melatonin. In addition, this study allowed us to re-examine more directly whether acute melatonin intake alters resting MSNA.
Measurements
Multifibre MSNA recordings were obtained using a tungsten microelectrode inserted into the peroneal nerve at the position of the popliteal fossa or head of the fibula of a resting leg. A reference electrode was placed subcutaneously 2–3 cm from the recording electrode. The recording electrode was then adjusted until a site was located where spontaneously occurring muscle sympathetic bursts were observed. The criteria for an acceptable MSNA recording site have been described previously (Ray, 2001). The nerve signal was filtered with a bandwidth of 700–2000 Hz and passed through a resistance capacitance integrating network with a time constant of 0.1 s to obtain a mean-voltage display of the nerve recording.
Arterial blood pressure and heart rate were measured continuously using a finger blood pressure monitoring unit (Portapres, TNO Biomedical Instrumentation, Amsterdam, The Netherlands) on the contralateral, non-exercising hand. Forearm blood flow was measured using venous occlusion plethysmography (Hokanson, Bellevue, WA, USA), with mercury-in-Silastic strain gauges placed around the maximal circumference of the forearm. Forearm blood flow was obtained every 15 s during the last 2 min of rest and each level of LBNP. During this measurement, a wrist cuff was inflated to 220 mmHg; this arrested circulation to the hand. During blood-flow determinations, a venous collecting cuff was applied to the arm, proximal to the elbow, and inflated to a pressure of 50 mmHg. Vascular resistance was calculated as mean arterial blood pressure divided by limb blood flow.
Thoracic impedance was measured by bioelectric impedance (HIC-2000, Bio-Impedance Technology, Chapel Hill, NC, USA) using electrode tape (Instrumentation for Medicine, Greenwich, CT, USA) placed around the top and base of the neck at the level of the xiphoid process, and 3 cm below. Thoracic impedance has been demonstrated to be a reliable indicator of changes in central blood volume (Ebert et al. 1986).
Mean voltage neurograms, arterial blood pressure, heart rate, forearm blood flows and thoracic impedance were collected on-line (MacLab 8E, ADInstruments, Milford, MA, USA) for later analysis, and displayed continuously to allow these parameters to be monitored.
Venous blood samples were obtained for measurement of plasma melatonin, via a brachial vein catheter. The blood sample was stored on ice and subsequently spun to separate the plasma. Plasma melatonin was measured in duplicate by radioimmunoassay (Buhlmann Laboratories, Switzerland). All samples were analysed together after the completion of all tests. The lower limit of detection was 0.3 pg ml−1 and the intra- and interassay coefficient of variation was 13 and 11 %, respectively.
Data analysis
All measurements were divided into 1 min bins for the purposes of analysis. Bursts of sympathetic activity were identified by inspection of the mean voltage neurogram. Total MSNA was determined by summing the area of the bursts. The values obtained over the last 3 min of LBNP at both −10 and −40 mmHg were averaged together, with the exception of forearm blood flow and vascular resistance, for which the last 2 min were averaged. Statistical analysis of the data during the LBNP trials was performed using a two-within-factors (intervention and drug trial) repeated-measures ANOVA. A one-within-factor (time) repeated-measures ANOVA was used for the second study in which responses were examined during 125 min of rest. A significance level of P < 0.05 was employed for all tests. All values are presented as mean ± S.E.M.
RESULTS
Study 1
Rest
Mean arterial pressure and tympanic temperature were not different between the two drug trials during the 10 min baseline period. Heart rate was lower (60 ± 2 vs. 64 ± 3 beats min−1) and MSNA burst frequency was slightly higher (25 ± 3 vs. 20 ± 2 bursts min−1) during the melatonin than the placebo trial. Tympanic temperature was not altered throughout the entire protocol with either melatonin or the placebo.
LBNP
MSNA responses were attenuated during LBNP at both −10 and −40 mmHg (P < 0.03; Fig. 1). For the placebo trial, MSNA increased by 33 ± 8 and 251 ± 70 % during LBNP at −10 and −40 mmHg, respectively, but only increased by 8 ± 7 and 111 ± 35 % during −10 and −40 mmHg with melatonin, respectively. However, there were no significant differences in mean arterial pressure and heart rate responses during LBNP between the melatonin and placebo trials. Mean arterial pressure was unchanged from baseline during both levels of LBNP. Heart rate was not significantly changed at −10 mmHg for both trials, but was significantly increased during −40 mmHg for both trials (Δ16 ± 2 and Δ17 ± 3 beats min−1 for melatonin and placebo trials, respectively; P < 0.001). Forearm blood flow decreased and forearm vascular resistance increased during LBNP, with the greater changes occurring at −40 mmHg. However, melatonin had no affect on either forearm blood flow or vascular resistance during these trials (Fig. 2).
Figure 1. The change in MSNA (total activity and burst frequency) during LBNP at −10 and −40 mmHg with and without melatonin.
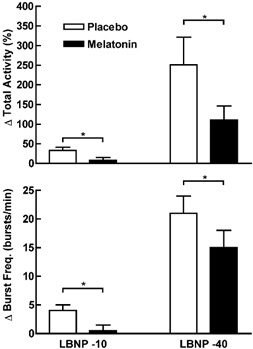
MSNA responses were attenuated during LBNP with melatonin. *Trials were significantly different from each other; P < 0.03.
Figure 2. Changes in forearm blood flow (FBF) and vascular resistance (FVR) during the last 2 min of LBNP at −10 and −40 mmHg with and without melatonin.
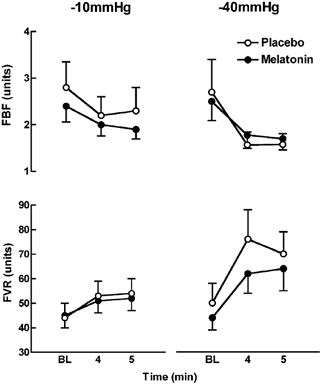
Changes were greater during the −40 mmHg trial. However, there were no significant main effects (drug, intervention) or interaction (drug × intervention) for either variable.
During the follow-up study, thoracic impedance was increased similarly during LBNP for both the placebo and melatonin trials (Table 1). However, resting and LBNP thoracic impedance was lower during the melatonin compared to the placebo trial.
Table 1.
Thoracic impedance at rest and during LBNP at –10 and –40 mmHg with melatonln and placebo
| LBNP | |||
|---|---|---|---|
| Baseline | −10 mmHg | −40 mmHg | |
| Placebo | 20.07 ± 1.36 | 20.30 ± 1.38* | 21.38 ± 1.52** |
| Melatonin | 19.63 ± 1.25 | 19.90 ± 1.28* | 20.88 ± 1.40** |
P < 0.001 vs. Baseline
P < 0.001 vs. Baseline and –10 mmHg.
Isometric handgrip and cold pressor test
MSNA, mean arterial pressure and heart rate increased significantly during the isometric handgrip test. There were no significant differences in the responses between the drug trials (Fig. 3). During the melatonin and placebo trials, MSNA increased by 78 ± 25 and 81 ± 29 %, mean arterial pressure increased by 21 ± 8 and 17 ± 7 mmHg and heart rate increased by and 18 ± 8 and 8 ± 3 beats min−1, respectively.
Figure 3. MSNA responses to 2 min of isometric handgrip (30 % maximum voluntary contraction) and a cold pressor test with and without melatonin.
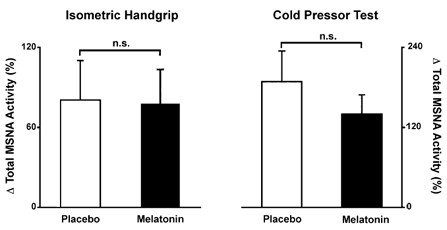
MSNA responses to these stressors were not altered by melatonin. n.s., not significant.
The cold pressor test elicited significant increases in MSNA (Δ140 ± 29 and Δ189 ± 46 % for the melatonin and placebo trials, respectively; Fig. 3) and mean arterial pressure (Δ23 ± 6 and Δ23 ± 6 mmHg for the melatonin and placebo trials, respectively). Heart rate was unchanged during the cold stimulus (Δ7 ± 5 and Δ7 ± 5 beats min−1 for the melatonin and placebo trials, respectively). MSNA, mean arterial pressure and heart rate responses were not different across the drug trials.
Study 2
After ingestion of 3 mg of melatonin, resting MSNA, mean arterial pressure and heart rate were not significantly different during the subsequent 125 min. The plasma concentration of melatonin increased following ingestion of melatonin (Fig. 4). Plasma melatonin was significantly increased by minute 25 (14 ± 11 and 405 ± 260 pg ml−1 for baseline and 25 min, respectively) and peaked by minute 75 (1830 ± 848 pg ml−1), remaining significantly elevated for the remaining time period.
Figure 4. Plasma melatonin concentration (pg ml−1) after ingestion of a 3 mg tablet of melatonin.
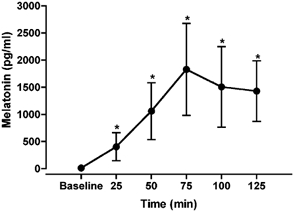
Plasma melatonin was significantly increased over the 125 min period. *Significantly different from baseline; P < 0.01.
DISCUSSION
There were two major findings from the current study. First, MSNA responses to LBNP were significantly attenuated following melatonin ingestion. In contrast, MSNA responses to the isometric handgrip and cold pressor test, two other sympathoexcitatory stimuli, were not altered by melatonin. Together these findings suggest that melatonin has specific physiological effects during baroreflex unloading. Second, despite reductions in MSNA during LBNP with melatonin, forearm vascular resistance remained unchanged. This finding suggests that forearm vascular control is altered by melatonin.
It is clear from study 2 that ingestion of 3 mg of melatonin elicits marked increases in plasma melatonin concentration. The plasma levels observed in this study are certainly unphysiological (1830 pg ml−1) as compared to peak normal physiological levels 60 pg ml−1 (Brzezinski, 1997). However, the use of this amount of melatonin (3 mg) as a supplement is not uncommon in the population. In addition, this dose is not beyond the levels taken by astronauts during spaceflight to improve sleep. Therefore, it is apparent that the responses observed in this study do have physiological relevance.
It has been reported that increases in plasma noradrenaline during standing are attenuated in both men and women after ingesting 1 mg of melatonin (Cagnacci et al. 1998; Arangino et al. 1999). The present results indicate that attenuated increases in MSNA during baroreceptor unloading contribute to this response. Whether changes in noradrenaline kinetics at the sympathetic nerve junction also contribute to reductions in plasma noradrenaline remain untested.
What is responsible for the attenuated increase in MSNA during LBNP after melatonin? Both the isometric handgrip and cold pressor responses were unaffected by melatonin. Thus, the effect of melatonin on MSNA was specific to LBNP and baroreceptor unloading. This finding suggests that melatonin alters the baroreflex control of MSNA. Because the MSNA responsiveness to −10 and −40 mmHg LBNP was reduced, this suggests that both the cardiopulmonary and arterial baroreflexes are affected. The aspect of the baroreflex arc that is affected by melatonin (i.e. afferent and efferent limb or central integration) remains unclear. In birds, melatonin receptors have been identified in the atria, ventricles and septum of the heart (Pang et al. 1996). Thus, modulation of cardiac mechanoreceptor stimulation by melatonin might occur. However, thoracic impedance data indicate that altered loading of the low-pressure cardiac mechanoreceptors is an unlikely explanation, because thoracic impedance increased similarly during LBNP with and without melatonin. Further studies are needed to identify the exact mechanism by which melatonin exerts its influence on the baroreflexes.
Despite reductions in MSNA responsiveness to LBNP after melatonin, mean arterial blood pressure was not reduced. This finding is in contrast to those obtained in studies in which subjects were made to stand, which reported a reduction in mean arterial pressure with melatonin (Cagnacci et al. 1998; Arangino et al. 1999). One explanation for this difference in arterial blood pressure response is that the orthostatic stress induced by LBNP at −40 mmHg is not as strong as that induced by standing. The lack of change in forearm vascular resistance with melatonin is consistent with the observation of no change in arterial blood pressure.
The lack of a reduction in arterial blood pressure and forearm vascular resistance during LBNP could be the result of the melatonin-induced augmentation of noradrenaline-induced vasoconstriction (Vandeputte et al. 2001). Thus, the lower sympathetic outflow during LBNP could be compensated by an augmented vascular sensitivity to noradrenaline. In addition, two different melatonin receptors have been identified with opposite vascular effects: MT1 receptors mediate vasoconstriction and MT2 receptors mediate vasodilatation (Doolen et al. 1998). It could be that in humans, melatonin preferentially activates MT1 receptors to elicit vasoconstriction to compensate for the diminished MSNA that occurs during LBNP. Finally, because MSNA was recorded from the leg, it is possible that MSNA responses in the leg do not reflect changes in the arm during LBNP with melatonin. Future studies are needed in humans to determine whether melatonin elicits differential vascular responses across various vascular beds (e.g. those of the limbs, kidneys or viscera).
MSNA at rest was not affected by melatonin. This finding was associated with no reductions in resting arterial blood pressure in both of the studies reported here. Thus, our studies do not support the concept of a strong depressor effect of melatonin in humans at rest. These findings together question whether the vasodilatation induced by melatonin in isolated vessels represents physiological responses in the intact organism.
The attenuated increase in MSNA during LBNP suggests that melatonin impacts negatively on orthostatic tolerance. MSNA has an important role in regulating peripheral resistance in humans. Failure of MSNA to increase appropriately during an orthostatic challenge renders an individual susceptible to reduced perfusion pressure. In astronauts, reductions in total peripheral resistance contribute importantly to post-spaceflight orthostatic intolerance (Buckey et al. 1996). Therefore, melatonin use may precipitate orthostatic intolerance in astronauts and in the general population. However, this study does not determine how long the effect of melatonin on MSNA persists. This information would be critical in determining when melatonin consumption should be stopped in order to prevent any untoward effects upon return from microgravity for astronauts and whether bedtime consumption of melatonin in the general population affects orthostasis during the daytime.
Heart rate variability studies in humans have indicated that melatonin increases cardiac vagal tone (Nishiyama et al. 2001). However, in Study 2 of the current report, no changes in heart rate were observed for 125 min following the ingestion of 3 mg of melatonin. Thus, the data from the present study do not provide support for this concept in humans, and are in agreement with earlier studies employing spectral analysis of respiratory sinus arrhythmias (Burgess et al. 2001; Harris et al. 2001).
Summary
A high concentration of melatonin can alter sympathetic outflow and forearm vascular control in humans during orthostatic stress. During cardiovascular challenges, the effects of melatonin appear to be specific to baroreceptor unloading, because MSNA responses were attenuated during LBNP but not during either exercise or a cold pressor test. The attenuation of MSNA during LBNP supports the concept that melatonin might have a negative impact on orthostatic tolerance in the general population and may be deleterious to astronauts who are susceptible to post-spaceflight orthostatic intolerance.
Acknowledgments
The project was supported by grants to Dr Ray from the National Institutes of Health (HL58503), National Aeronautics and Space Administration (NAG 9–1034), National Space and Biomedical Research Institute (CA00207), Established Investigator Award from the American Heart Association (0140035N) and a NIH-sponsored General Clinical Research Center with National Center for Research Resources Grant M01 RR10732.
REFERENCES
- Arangino S, Cagnacci A, Angiolucci M, Vacca AM, Longu G, Volpe A, Melis GB. Effects of melatonin on vascular reactivity, catecholamine levels, and blood pressure in healthy men. Am J Cardiol. 1999;83:1417–1419. doi: 10.1016/s0002-9149(99)00112-5. [DOI] [PubMed] [Google Scholar]
- Bonn D. Melatonin's multifarious marvels: miracle or myth. Lancet. 1996;347:184. [Google Scholar]
- Bruce J, Tamarkin L, Riedel C, Markey S, Oldfield E. Sequential cerebrospinal fluid and plasma sampling in humans:24-hour melatonin measurements in normal subjects and after peripheral sympathectomy. J Clin Endocrinol Metab. 1991;72:819–823. doi: 10.1210/jcem-72-4-819. [DOI] [PubMed] [Google Scholar]
- Brzezinski A. Melatonin in humans. N Engl J Med. 1997;336:186–195. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- Buckey JC, Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Moore WE, Gafney FA, Blomqvist CG. Orthostatic intolerance after spaceflight. J Appl Physiol. 1996;81:7–18. doi: 10.1152/jappl.1996.81.1.7. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Sletten T, Savic N, Gilbert SS, Dawson D. Effects of bright light and melatonin on sleep propensity, temperature, and cardiac activity at night. J Appl Physiol. 2001;91:1214–1222. doi: 10.1152/jappl.2001.91.3.1214. [DOI] [PubMed] [Google Scholar]
- Cagnacci A, Arangino S, Angiolucci M, Maschio E, Melis GB. Influences of melatonin administration on the circulation of women. Am J Physiol. 1998;274:R335–338. doi: 10.1152/ajpregu.1998.274.2.R335. [DOI] [PubMed] [Google Scholar]
- Chuang JI, Chen SS, Lin MT. Melatonin decreases brain serotonin release, arterial pressure and heart rate in rats. Pharmacology. 1993;47:91–97. doi: 10.1159/000139083. [DOI] [PubMed] [Google Scholar]
- Doolen S, Krause DN, Dubocovich ML, Duckles SP. Melatonin mediates two distinct responses in vascular smooth muscle. Eur J Pharmacol. 1998;345:67–69. doi: 10.1016/s0014-2999(98)00064-8. [DOI] [PubMed] [Google Scholar]
- Ebert TJ, Smith JJ, Barney JA, Merrill DC, Smith GK. The use of thoracic impedance for determining thoracic blood volume changes in man. Aviat Space Environ Med. 1986;57:49–53. [PubMed] [Google Scholar]
- Harris AS, Burgess HJ, Dawson D. The effects of day-time exogenous melatonin administration on cardiac autonomic activity. J Pineal Res. 2001;31:199–205. doi: 10.1034/j.1600-079x.2001.310302.x. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Miwa Y, Fujimoto K, Oohata H, Nishino H, Koike H. Antihypertensive action of melatonin in the spontaneously hypertensive rat. Clin Exp Hypertens A. 1987;9:1121–1131. doi: 10.3109/10641968709160037. [DOI] [PubMed] [Google Scholar]
- K-Laflamme A, Wu L, Foucart S, De Champlain J. Impaired basal sympathetic tone and alpha1-adrenergic responsiveness in association with the hypotensive effect of melatonin in spontaneously hypertensive rats. Am J Hypertens. 1998;11:219–229. doi: 10.1016/s0895-7061(97)00401-9. [DOI] [PubMed] [Google Scholar]
- Krause DN, Geary GG, Doolen S, Duckles SP. Melatonin and cardiovascular function. Adv Exp Med Biol. 1999;460:299–310. [PubMed] [Google Scholar]
- Nishiyama K, Yasue H, Moriyama Y, Tsunoda R, Ogawa H, Yoshimura M, Kugiyama K. Acute effects of melatonin administration on cardiovascular autonomic regulation in healthy men. Am Heart J. 2001;141:E9. doi: 10.1067/mhj.2001.114368. [DOI] [PubMed] [Google Scholar]
- Pang CS, Tang PL, Song Y, Brown GM, Pang SF. 2-[125I]Iodomelatonin binding sites in the quail heart: characteristics, distribution and modulation by guanine nucleotides and cations. Life Sci. 1996;58:1047–1057. doi: 10.1016/0024-3205(96)00058-6. [DOI] [PubMed] [Google Scholar]
- Ray CA. Interaction between vestibulosympathetic and skeletal muscle reflexes on sympathetic activity in humans. J Appl Physiol. 2001;90:242–247. doi: 10.1152/jappl.2001.90.1.242. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev. 1991;12:151–180. doi: 10.1210/edrv-12-2-151. [DOI] [PubMed] [Google Scholar]
- Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci. 1999;317:75–77. doi: 10.1097/00000441-199902000-00001. [DOI] [PubMed] [Google Scholar]
- Vandeputte C, Giummelly P, Atkinson J, Delagrange P, Scalbert E, Capdeville-Atkinson C. Melatonin potentiates NE-induced vasoconstriction without augmenting cytosolic calcium concentration. Am J Physiol Heart Circ Physiol. 2001;280:H420–425. doi: 10.1152/ajpheart.2001.280.1.H420. [DOI] [PubMed] [Google Scholar]


