Abstract
Voltage-gated potassium (KV) channels represent an important dilator influence in the cerebral circulation, but the composition of these tetrameric ion channels remains unclear. The goals of the present study were to evaluate the contribution of KV1 family channels to the resting membrane potential and diameter of small rat cerebral arteries, and to identify the α-subunit composition of these channels using patch-clamp, molecular and immunological techniques. Initial studies indicated that 1 μmol l−1 correolide (COR), a specific antagonist of KV1 channels, depolarized vascular smooth muscle cells (VSMCs) in pressurized (60 mmHg) cerebral arteries from −55 ± 1 mV to −34 ± 1 mV, and reduced the resting diameter from 152 ± 15 μm to 103 ± 20 μm. In patch clamped VSMCs from these arteries, COR-sensitive KV1 current accounted for 65 % of total outward KV current and was observed at physiological membrane potentials. RT-PCR identified mRNA encoding each of the six classical KV1 α-subunits, KV1.1–1.6, in rat cerebral arteries. However, only the KV1.2 and 1.5 proteins were detected by Western blot. The expression of these proteins in VSMCs was confirmed by immunocytochemistry and co-immunoprecipitation of KV1.2 and 1.5 from VSMC membranes suggested KV1.2/1.5 channel assembly. Subsequently, the pharmacological and voltage-sensitive properties of KV1 current in VSMCs were found to be consistent with a predominant expression of KV1.2/1.5 heterotetrameric channels. The findings of this study suggest that KV1.2/1.5 heterotetramers are preferentially expressed in rat cerebral VSMCs, and that these channels contribute to the resting membrane potential and diameter of rat small cerebral arteries.
Recently, efforts have intensified to identify the types of voltage-gated K+ (KV) channels in the cerebral circulation based on findings that vascular smooth muscle cells (VSMCs) rely on KV channels for vasodilatation (Cheong et al. 2001a 2001b). Several studies have demonstrated that KV channels contribute to the resting membrane potential (Vm) and diameter of cerebral microvessels, and that pharmacological inhibition of these channels depolarizes and constricts small cerebral arteries and arterioles, both in vitro and in vivo (Knot & Nelson, 1995; Sobey & Faraci, 1999; Horuichi et al. 2001). Strong evidence also indicates that KV channels in VSMCs are regulated by intracellular calcium, intravascular pressure and vasoconstrictor agents (Knot & Nelson, 1995; Faraci & Heistad, 1998; Cox & Petrou, 1999), and alterations in these factors have been implicated in pathological conditions including cerebral vasospasm and stroke (Faraci & Heistad, 1998). Thus, identifying the molecular composition of the functionally significant KV channels in the cerebral circulation has immediate implications for determining the ionic mechanisms that regulate blood flow to the brain.
Unfortunately, efforts to identify the composition of KV channels have been hampered by the highly diverse nature of KV channels, whose pore-forming α-subunits arise from at least 11 different gene families (KV1-KV11), each composed of several subfamily members (Coetzee et al. 1999; Robertson, 2001; Ottschytsch et al. 2002). Four similar or different α-subunits emanating from a single gene assemble as homo- or heterotetramers, respectively, to form the K+-selective pore. Many channels show overlap in their properties of unitary conductance, voltage-dependent activation and inactivation, and sensitivity to pharmacological antagonists (Coetzee et al. 1999; Robertson, 2001). For example, 4-aminopyridine (4-AP), the classical pharmacological antagonist of KV channels, shows only about a 10-fold difference in blocking potency between most KV gene families. Much is known about its blocking actions on cloned KV channel homotetramers (Grissmer et al. 1994; Coetzee et al. 1999), but its blocking effect on heterotetrameric KV channels whose heterogeneous composition appears to resemble most native KV channels (Scott et al. 1994; Koch et al. 1997; Wang et al. 1999; Kerr et al. 2001; Thorneloe et al. 2001), remains poorly understood. For this reason, 4-AP is of limited use in defining which KV channels are expressed in a particular vascular tissue. Although some toxins have been identified that preferentially block certain KV channel subfamilies (Garcia-Calvo et al. 1993; Dufton & Harvey, 1998; Kaczorowski & Garcia, 1999; Robertson, 2001), the incorporation of a single toxin-insensitive α-subunit into a KV channel tetramer can confer insensitivity to the toxin peptide, thereby masking the presence of toxin-susceptible α-subunits in the channel and confounding efforts to define its molecular components (Russell et al. 1994; Hulme et al. 1999). The homotetrameric or heterotetrameric KV channels may further associate with four ancillary β-subunits to form oligomultimeric structures that show additional diversity (Coetzee et al. 1999; Robertson, 2001).
In this regard, the novel nortriterpene drug correolide (COR), which was extracted from the Costa Rican tree Spachea correa and recently shown to selectively inhibit KV1 channels, represents the first specific antagonist of a single KV gene family (Kaczorowski & Garcia, 1999; Hanner et al. 1999; Felix et al. 1999). COR is most potent as a blocker of KV1.3 homotetramers, but it blocks tetramers assembled from each of the classical KV1-family members (KV1.1–1.6) in a specific, saturable and reversible fashion with a dissociation constant (Kd) of 10–100 nM (Felix et al. 1999). Recently, Cheong et al. (2001a) reported that COR constricted fragments of rabbit cerebral arterioles and blocked a large fraction of KV current in the cerebral VSMCs. Immunohistochemistry detected KV1.5 and 1.6 in the rabbit arteriolar VSMC layer, implicating these proteins in channel formation. The same authors (Cheong et al. 2001b) detected KV1.3 and 1.6 in pial arteriolar fragments of the mouse. The fragments constricted in response to agitoxin-2 and margatoxin (MgTX), toxin blockers that can bind to tetramers assembled from KV1.3 and 1.6 (Garcia-Calvo et al. 1993; Dufton & Harvey, 1998; Coetzee et al. 1999; Kaczorowski & Garcia, 1999). These findings suggest that KV1.3, 1.5 and 1.6 are potential candidates for assembly of KV1 channels in the cerebral VSMCs of these two animal species (Cheong et al. 2001a 2001b).
The goal of the present study was to determine if COR-sensitive KV1 channels regulate the resting membrane potential (Vm) and diameter of small rat cerebral arteries exposed to physiological intraluminal pressures. The second goal was to identify the α-subunit composition of these channels using patch-clamp, molecular and immunological approaches. Notably, the rat is the most common animal model used to study the physiological regulation of cerebral blood flow, and it also is used to study abnormal blood flow responses during pathological conditions including hypoxia, hypertension, diabetes and stroke (Faraci & Heistad, 1998). Potassium channels are thought to participate in many of these responses, and defining their molecular composition will be necessary to understand the ionic mechanisms that alter cerebrovascular reactivity in these disorders.
METHODS
Animals
Male Sprague-Dawley rats aged 10–16 weeks were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, IN, USA). Rats were heavily anaesthetized using ketamine (100 mg kg−1) and acepromazine (1 mg kg−1 I.M.), and decapitated for removal of the brain. The brain was placed briefly in ice-cold physiological salt solution (PSS) of the following composition (mM): 145 NaCl, 4 KCl, 1 MgCl2, 10 Hepes, 1.8 CaCl2, 10 glucose (pH = 7.4). Middle cerebral arteries were removed for vascular reactivity and patch-clamp studies. To obtain enough vascular tissue for Western and co-immunoprecipitation studies, the entire circle of Willis and its branches were removed. Procedures involving animals were approved by the Medical College of Wisconsin Animal Care and Use Committee.
Patch-clamp recording of KV currents
Single VSMCs were enzymatically isolated from rat middle cerebral arteries for analysis on a patch-clamp station as previously described (Jackson et al. 1996; Liu et al. 1998). Outward currents were elicited by progressive 8 mV voltage steps (500 ms duration, 5 s intervals) from a holding potential of −70 mV to +58 mV. Contaminating current through high-conductance, Ca2+-activated K+ channels was minimized in all experiments by including 100 nM iberiotoxin in a low-calcium bath solution, and dialysing cells with pipette solution containing 10 nM free calcium. The pipette solution contained (mM): 145 glutamate, 1 MgCl2, 10 Hepes, 1 EGTA, 1 Na2ATP. The pH was buffered to 7.1 with KOH (final K+ concentration of ˜145 mM). The bath solution was composed of (mM): 140 NaCl, 4 KCl, 0.1 CaCl2, 1 MgCl2, 10 Hepes, 10 glucose. The solution was buffered using NaOH to a final pH of 7.4.
To evaluate drug effects, K+ current was first elicited in drug-free bath solution and then in bath solution containing the drug of interest. In each cell, peak current amplitudes under each condition were averaged from three trials. Peak current was expressed as current density (pA pF−1) to normalize for differences between cell membrane areas. Membrane capacitance (in pF) was obtained by integrating the average charge elicited by hyperpolarizing pulses and dividing this value by input voltage (Liu et al. 1998).
Voltage dependence of activation and inactivation were analysed using standard double-pulse protocols to assess the level of KV window current, as outlined by Leblanc et al. (1994). Briefly, activation curves were constructed by applying 500 ms voltage steps from −70 mV to +58 mV in 8 mV increments, to VSMCs exposed to symmetrical 145 mM KCl. The peak amplitudes of inward tail currents were recorded upon return to a holding potential of −70 mV and normalized against maximal tail current amplitude. Inactivation curves were constructed in physiological K+ gradients from a holding potential of −70 mV by applying 30 s preconditioning steps (increments of 8 mV) from −86 mV to +18 mV. Following a 5 ms return to −70 mV, voltage was stepped to +58 mV for 500 ms to determine available K+ current. The peak outward current during the test pulse was normalized against the amplitude of the fully available current at the preconditioning pulse of −86 mV.
Measurement of arterial Vm and diameter
Rat middle cerebral arteries were cannulated and pressurized (60 mmHg) on glass micropipettes using a standard perfusion system and solutions described previously for this preparation (Madden & Christman, 1999). Internal diameter was measured by videomicroscopy previously calibrated for micron resolution, and drugs were added to the superfusate. At the start of each study, the intact function of VSMCs and endothelial cells was confirmed by observing a vasoconstrictor response to 1 μM 5-hydroxytryptamine and a subsequent dilator response to 1 μM acetylcholine. In a subset of arteries, the endothelium was removed by exposure of the vessel lumen to an air bolus for 1 h. Successful removal was defined as the loss of the dilator response to acetylcholine (Gauthier & Rusch, 2001). Membrane potential (Vm) was measured using glass microelectrodes filled with 3 M KCl that had tip resistances of 50–80 MΩ, and were fitted over a silver chloride wire connected to a preamplifier probe mounted on a micromanipulator. The probe was connected to a high-impedance amplifier, a storage oscilloscope, and a thermal paper recorder. Criteria for a successful impalement were defined as an abrupt negative drop in voltage upon cell entry, maintenance of a stable value for a minimum of 5 s and an immediate return to baseline upon withdrawal.
PCR gene amplification
Total RNA was isolated from middle cerebral arteries (RNeasy minikit, Qiagen), and reverse transcription (RT) was performed according to commercial instructions using 1 μg of total RNA (You-Prime First Strand Beads, Amersham-Pharmacia). Polymerase chain reaction (PCR) employed α-subunit-specific primers for KV1.1–1.6, after determining the optimal temperature for each primer pair. Amplification was performed in parallel using cDNAs from rat cerebral arteries and rat brain. The latter tissue expresses transcripts for each of the KV1.1–1.6 α-subunits, and served as a positive control (Thorneloe et al. 2001; Cox et al. 2001). The primer target sequences, the expected product sizes and relevant Genebank entries were: KV1.1 (1042–1325, 284 bp, no. NM000217); KV1.2 (1635–1999, 364 bp, no. J04731); KV1.3 (2350–2930, 581 bp, no. X16001); KV1.4 (1320–1962, 643 bp, no. L02751); KV1.5 (1548–2646, 1099 bp, no. M27158); KV1.6 (2634–3211, 578 bp, no. NM013568). Contamination of amplification reactions by genomic DNA was evaluated using primers that spanned three introns within the coding sequence of α-actin (Pratt et al. 2002). The expected product size from DNA amplification was 1506 bp, and the size of the intronless cDNA product was 637 bp. All products were resolved on 2 % agarose gels, and captured on film under ultraviolet light.
Western immunoblotting
Membrane protein was isolated from arteries of the circle of Willis and its branches, and Western immunoblotting was performed as recently described (Gauthier et al. 2002; Pratt et al. 2002). Membrane protein also was isolated from rat brain and used as a positive control for expression of five members (KV1.1–1.4 and Kv1.6) of the KV1 gene family. Only KV1.5 is reported to be absent in the rat brain (Beckh & Pongs, 1990; Wang et al. 1999). Subsequently, equal amounts of brain and cerebrovascular proteins (10–20 μg) were loaded into adjacent lanes, subjected to SDS-PAGE, and transferred to a nitrocellulose membrane. Polyclonal antibodies developed against amino acid sequences located in the cytoplasmic termini of KV1 channels were kindly provided by Dr Hans-Guenther Knaus (Koch et al. 1997). These included: anti-KV1.1 (residues 458–475), anti-KV1.3 (residues 456–474), anti-KV1.4 (residues 605–624), anti-KV1.5 (residues 578–598) and anti-KV 1.6 (residues 509–526). Monoclonal anti-KV1.2 (amino acids 428–499) was purchased from Upstate Biotechnology (Lake Placid, NY, USA). Immunoreactive bands corresponding to the molecular mass of KV1.1–1.6 were detected by chemiluminescence. The influence of N-linked oligosaccharides on the apparent molecular mass of KV1.2 on immunoblots was evaluated using peptide-N-glycosidase F according to commercial instructions (Prozyme, San Leandro, CA, USA).
Immunocytochemistry
Single VSMCs were enzymatically isolated (Jackson et al. 1996; Liu et al. 1998) and fixed on glass slides using either 95 % ethanol, a methanol-acetone mixture (1:1), or freshly made 4 % paraformaldehyde. After the slides were air-dried, immunostaining was performed in a manner similar to previous methods (Gauthier et al. 2002; Pratt et al. 2002). Briefly, cells were incubated for 45 min at 25 °C with monoclonal anti-KV1.2 (Upstate Biotechnology), polyclonal anti-KV1.5 (Dr H.-G. Knaus, University of Innsbruck), or a monoclonal antibody directed against smooth muscle-specific α-actin (BioGenex, San Ramon, CA, USA). Antibodies were diluted 1:100 in phosphate-buffered saline containing 5 % normal goat serum and 0.2 % Tween 20 (PBST-NGS). After washing, preparations were incubated for 30 min at 25 °C with a 1:400 dilution of the secondary AlexaFluor 594 goat anti-rabbit or goat-anti-mouse antibodies (A-11012, A-11005; Molecular Probes, Eugene, OR, USA) in 5 % PBST-NGS. Finally, nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) obtained from Sigma Chemical Company (St Louis, MO, USA). Fluorescent signals were analysed at × 400 magnification using fluorescent microscopy with a Nikon Eclipse Model E600 fluorescence microscope. The intensities of fluorescent signals were analysed using NIH software (Scion Image).
Immunoprecipitation
Rat cerebral arteries were homogenized using a Wheaton overhead stirrer on ice in homogenizing buffer containing (mM): 2 EDTA, 2 EGTA, 250 sucrose and 50 Mops, pH 7.4. To guard against proteolysis, additional protease inhibitors including leupeptin, antipain, aprotinin and PMSF were added at a concentration of 5 μg ml−1. The homogenate was sedimented at 1000 g for 10 min at 4 °C and the resulting supernatant was then centrifuged at 7000 g for 10 min at the same temperature. The protein content of the final membrane preparation was measured using the Pierce BSA Protein Assay (Rockford, IL, USA).
Membrane protein (35–200 μg reaction−1) was solubilized in 1 ml Triton Lysis Buffer (50 mM Hepes, 150 mM NaCl, 1 mM EGTA, 1.5 mM MgCl2, 10 % w/v glycerol, 1 % w/v Triton-X 100). Three micrograms of anti-KV1.5 (Alomone Labs, Jerusalem, Israel) were added as the precipitating antibody, and the sample was incubated at 4 °C for 2 h on a rocking platform. Protein A/G Plus beads (50 μl) pre-blocked with BSA (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were added and the incubation continued for an additional 1 h at 4 °C. Beads were then sedimented and washed three times with Triton lysis buffer. The bound proteins were eluted from the beads by boiling for 2 min in SDS sample buffer. Proteins were submitted to SDS-PAGE and probed with anti-KV1.2 or anti-KV1.5 (Upstate Biotechnology).
Drugs
Correolide (COR) was a kind gift from Dr Maria Garcia and Dr Gregory Kaczorowski of Merck Research Laboratories, Rahway, NJ, USA. COR was prepared as a 20 mM stock in dimethylsulfoxide and stored at 4 °C. 4-Aminopyridine (4-AP) was obtained from Sigma Chemical Company, prepared as a 1 M stock buffered using HCl to a final pH of 7.4 in distilled water, and stored at −20 °C. Iberiotoxin, α-dendrotoxin and margatoxin were obtained from Sigma Chemical Company, reconstituted as 100 μM stocks in PSS, and stored in aliquots at −20 °C.
Statistics
Data are expressed as means ±s.e.m. Significant differences between control and experimental preparations were determined by one-way ANOVA with repeated measures. A probability value of < 0.05 was considered statistically significant.
RESULTS
COR depolarizes and constricts small cerebral arteries
Knot & Nelson (1995) initially reported that 1 mM 4-AP depolarized isolated, pressurized rat cerebral arteries by 19 mV and triggered vasoconstriction. These findings implied a large contribution of KV channels to the resting Vm of cerebral VSMCs, although the identity of these channels has remained in doubt. In our studies designed to isolate the contribution of COR-sensitive KV1 channels to the resting Vm of cerebral VSMCs, superfusion of pressurized (60 mm Hg) rat middle cerebral arteries with 1 μM COR profoundly depolarized and induced action potentials in VSMCs indicative of a reduced membrane K+ conductance (Fig. 1A and B). The final addition of 1 mM 4-AP had no further effect on arteries depolarized by COR (Fig. 1C). In six arteries, COR depolarized the VSMCs by an average of 21 mV (39 %) from −55 ± 1 to −34 ± 1 mV. This Vm level was unchanged (−33 ± 1 mV) after addition of 1 mM 4-AP (Fig. 1D), implicating COR-sensitive KV1 channels as the functional KV channels in the VSMCs. In the same arteries, COR reduced the resting diameter from 152 ± 15 to 103 ± 20 μm (n = 6). The latter value was not different from the diameter of 99 ± 19 μm measured after the final addition of 1 mM 4-AP (Fig. 1E). COR also depolarized the VSMC in three endothelial-denuded arteries from −56 ± 1 to −36 ± 2 mV (n = 3), and induced vasoconstriction of these vessels, inferring a direct action of COR on the VSMCs (data not shown).
Figure 1. Effects of correolide (COR) and 4-aminopyridine (4-AP) on the resting Vm and diameter of rat small cerebral arteries.

A, microelectrode recordings of Vm in a rat middle cerebral artery indicate a resting Vm level of about −57 mV. B, the addition of 1 μM correolide (COR) induced a pronounced depolarization to about −34 mV (top trace). COR induced action potentials in another artery (bottom trace). C, the final addition of 1 mM 4-AP did not induce further depolarization. D and E, average Vm and diameter values for cerebral arteries from six rats exposed to 1 μM COR, and subsequently, exposed to both 1 μM COR and 1 mM 4-AP. Data are expressed as means ±s.e.m. (n = 6). * Significant difference (P < 0.05) between control and experimental values.
Whole-cell K+ current is correolide sensitive
Patch-clamp studies were designed to identify the properties of the COR-sensitive KV1 channels. Progressive 8 mV voltage steps from a holding potential of −70 mV to +58 mV elicited families of outward current in rat cerebral VSMCs exposed to physiological K+ gradients (Fig. 2A). To confirm that the charge carrier for the currents was primarily K+, outward currents were elicited by a depolarizing pulse from −70 to +58 mV, and then the amplitudes of tail currents resulting from a return pulse to potentials ranging from −100 to −4 mV were measured (Fig. 2B). The reversal potentials (Vrev) of 79 ± 2, −47 ± 3 and −18 ± 2 mV observed at external K+ concentrations of 4, 20 and 70 mM (Fig 2C-E; n = 4–9), respectively, were near the calculated reversal potential for a K+-selective pore of −90, −50 and −18 mV (Goldman, 1943).
Figure 2. Verification of K+ selectivity of outward current.

A, outward currents in rat cerebral VSMCs elicited by progressive 8 mV depolarizing steps from a holding potential of −70 mV to +58 mV. B, tail currents were generated by hyperpolarizing steps to between −100 and −4 mV (8 mV increments), following an initial activating pre-pulse from −70 to +58 mV. C-E, reversal potentials of VSMCs dialysed with pipette solution containing 145 mM K+, and bathed in external solution containing 4, 20 and 70 mM K+ (n = 4–6 cells averaged from 4–5 rats).
The addition of 0.1 μM and 1 μM COR to the bath progressively reduced voltage-gated K+ (KV) current at drug concentrations consistent with incremental and selective block of KV1 channels (Fig. 3A). The blocking effect of COR slowly developed during 10–15 min, consistent with its slow onset kinetics (Felix et al. 1999). In nine cerebral VSMCs, 0.1 and 1 μM COR blocked the maximal current elicited at +58 mV by 26 % and 65 %, respectively (Fig. 3B and C). After block was fully established, the further addition of 1 mM 4-AP to six cells only reduced maximal current by an additional 10 % (Fig. 3A and D). These findings inferred that the majority of KV current in rat cerebral VSMCs originated from COR-sensitive KV1 channels. The basis of the unblocked current was not investigated, but may have represented residual KV1 current or current from COR-insensitive KV channels from other gene families. Solvent controls using dimethylsulfoxide (DMSO) did not alter K+ current density or voltage sensitivity (n = 3).
Figure 3. Effects of correolide (COR) and 4-aminopyridine (4-AP) on voltage-gated K+ currents in rat cerebral VSMCs.

A, whole-cell K+ currents elicited in physiological K+ gradients were dose-dependently blocked by 0.1 and 1 μM correolide (COR), a selective blocker of KV1 channels. The further addition of 4-AP had little effect. Cell capacitance was 15 pF. B-D, averaged I-V relations demonstrating the effect of 0.1 μM COR, 1 μM COR, and the combined effect of 1 μM COR and 1 mM 4-AP on KV currents. Data are expressed as means ±s.e.m. (n = 6–9). * Significant difference (P < 0.05) between control and experimental values.
Cerebral arteries express mRNAs for KV 1.1–1.6
To identify the tetramer composition of KV1 channels in rat cerebral VSMCs, RT-PCR was used to screen for mRNAs encoding each of the six classical KV1 subfamilies (KV1.1-KV1.6). Using the rat brain cortex (Br) as a positive control (Thorneloe et al. 2001; Cox et al. 2001), all six KV1 subfamilies were detected in lanes loaded with amplified cDNA originating from rat brain (Br) RNA (Fig. 4A, left lanes) or rat cerebral (C) artery RNA (Fig. 4A, right lanes). To verify that the products in Fig. 4A were not artifactually amplified from genomic DNA rather than from mRNA, α-actin primers were used that spanned three introns within the genomic coding sequence of α-actin (Fig. 4B). Amplification of cDNA (70 nmol) revealed only the expected product of 637 bp, indicating amplification of intronless RNA. In contrast, amplification of intron-containing rat DNA (70 nmol) resulted in the expected 1506 bp product. In control reactions, amplification in the absence of cDNA or DNA provided no products.
Figure 4. Detection of amplified products corresponding to KV1.1–1.6 mRNA in rat brain and cerebral arteries.

A, RT-PCR screening detected mRNA encoding KV1.1–1.6 α-subunits in lanes loaded with amplified cDNA originating from rat brain (Br, left lanes) or rat middle cerebral arteries (C, right lanes). Detection of each product was verified in cDNA reverse transcribed from 7–12 different RNA isolations. Each isolation was prepared from tissues of two rats. The brain was used as a positive control. Expected product sizes (bp) were: 284 (1.1), 374 (1.2), 581 (1.3), 643 (1.4), 1111 (1.5), 578 (1.6). B, screening for genomic contamination of cDNA using primers for smooth muscle-specific α-actin that included three introns in the amplified region. Amplification reactions including cDNA (70 nmol) resulted in a 637 bp product consistent with the mRNA template, whereas reactions including DNA (70 nmol) resulted in the predicted 1506 bp product amplified from the intron-containing region. Control reactions included amplifications without cDNA or without DNA. Results were verified in at least three different preparations. Each preparation was obtained from tissues of two rats.
Only KV1.2 and 1.5 proteins are expressed in small cerebral arteries
Using subunit-specific antibodies, Western blots confirmed immunoreactive bands corresponding to KV1.1, 1.3, 1.4 and 1.6 in lanes loaded with 10 to 20 μg of rat brain (Br) protein (Fig. 5A, left lanes). In contrast, these α-subunits were not detected in adjacent lanes loaded with similar or higher concentrations of cerebrovascular (C) protein (Fig. 5A, right lanes). At least three separate Western blots using proteins isolated from 4–10 rats confirmed these findings. Only KV1.2 and KV1.5 appeared to be prominently expressed in cerebral arteries. KV1.2 was evident in rat brain and cerebral arteries as a prominent immunoreactive band at ˜80 kDa protein (Fig. 5B). Preadsorption of primary anti-KV1.2 with 1 μM of its antigenic competing peptide (+CP) abolished the immunoreactive band in the arterial tissue, indicating specificity of the antibody for its intended epitope (Fig. 5B). Notably, the predicted molecular mass for KV1.2 of 57 kDa is considerably lower than the ˜80 kDa immunoreactive band detected by anti-KV1.2 on Western analysis. In the brain, this discrepancy reportedly results from N-glycosylation of the S1-S2 extracellular linker of KV1.2 (Scott et al. 1994; Shi & Trimmer, 1999; Manganas & Trimmer, 2000). To determine if the KV1.2 protein is subjected to similar post-translational processing in cerebral arteries, brain and cerebral arterial proteins were treated with peptide-N-glycosidase F (PNGase F), a protease that catalyses the release of N-linked oligosaccharides. Reactions evaluated on Western blots revealed a characteristic faster mobility of KV1.2 (Manganas & Trimmer, 2000), confirming N-glycosylation of KV1.2 in rat brain and cerebral arterial membranes (Fig. 5C).
Figure 5. Screening by Western blots for KV1.1–1.6 proteins in rat brain and cerebral arteries.

A, Western blots showed immunoreactive bands corresponding to KV1.1, 1.3, 1.4 and 1.6 α-subunits in rat brain (Br, left lanes), but not in rat cerebral arteries (C, right lanes). The apparent molecular mass values of the brain proteins were approximately: 60 kDa (1.1), 90 kDa (1.3), 85 kDa (1.4) and 60 kDa (1.6). B, KV1.2 was detected in rat brain (Br) and cerebral arteries (C) at a molecular mass of ˜80 kDa. The immunoreactive band in the arterial proteins was eliminated by an antigenic competing peptide (+CP). C, brain (Br) or cerebral artery (C) membrane proteins were prepared in control buffer or were enzymatically treated with PNGase F (+P) before analysis by electrophoresis. Treatment with PNGase F reduced the apparent molecular mass of KV1.2 in both types of tissue, implying N-glycosylation of the mature protein. D, the KV1.5 α-subunit was detected as a ˜75 kDa band in cerebral arteries (C), but was absent in the brain. The immunoreactive band was eliminated by a competing peptide (+CP). Western results were verified in 3 (KV1.1, 1.3, 1.4, 1.6), 16 (KV1.2), 4 (KV1.2 PNGase F) and 6 (KV1.5) different protein preparations isolated from 4–10 rats.
In contrast, a distinct band corresponding to KV1.5 was not detected in rat brain (Fig. 5D), which is thought to lack expression of this protein (Beckh & Pongs, 1990; Wang et al. 1999). However, lanes loaded with cerebrovascular protein revealed a ˜75 kDa-immunoreactive band that was susceptible to competing peptide (+CP) inhibition. Although KV1.5 also contains a consensus site for N-linked glycosylation, the apparent molecular mass of ˜75 kDa seen on Western blots is only slightly higher than the predicted molecular mass of 67 kDa suggesting that other post-translational events may be involved in its processing. Notably, the apparent molecular mass of ˜75 kDa concurs with the migration of immunoreactive bands corresponding to KV1.5 in other vascular tissues subjected to Western analysis (Yuan et al. 1998; Xu et al. 1999).
Subsequently, rat cerebral VSMCs were freshly isolated for immunocytochemical analysis, and anti-KV1.2 and anti-KV1.5 were used to detect α-subunit expression. The VSMCs showed well-delineated membranes and stained positive for α-actin, a smooth muscle-specific protein (Fig. 6A). Immunofluorescent signals associated with KV1.2 and KV1.5 were also detected (Fig. 6B and C, respectively). The intensity of these signals was reduced when primary antibodies were preadsorbed by their respective competing peptides (+CP), although the presence of residual signal after preadsorption of anti-KV1.5 suggested partial nonspecific binding in nondenaturing conditions. Immunofluorescence was nearly absent in VSMCs exposed to only secondary antibodies (2° control) in the absence of anti-KV1.2 or anti-KV1.5.
Figure 6. Normarski and corresponding fluorescent images of freshly isolated rat cerebral VSMCs.
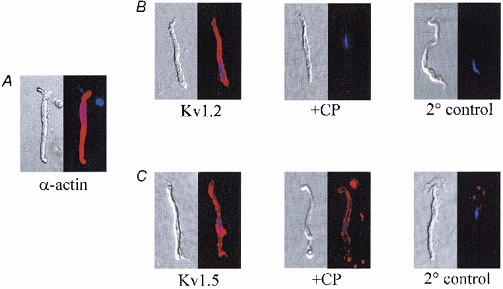
A, expression of smooth muscle-specific α-actin. Cells were visualized using Alexa Fluor 594-conjugated antibody (in red). Nuclei were labelled with DAPI (in blue). B, left to right, VSMCs labelled with anti-KV1.2 (Kv1.2), after adsorption of anti-KV1.2 with a competing peptide (+CP) or after incubation with secondary antibody only (2 ° control). C, left to right, VSMCs labelled with anti-KV1.5 (Kv1.5), after adsorption of anti-KV1.5 by a competing peptide (+CP), and after incubation with secondary antibody only (2 ° control). Within each panel, fluorescent images were acquired from VSMCs exposed for equivalent times. Results were verified in 3 (α-actin), 9 (Kv1.2) and 5 (Kv1.5) cells from different preparations.
KV1.2/1.5 complexes are expressed in cerebral VSMCs
To determine if KV1.2 and KV1.5 associate in cerebral VSMCs, anti-KV1.5 was used to immunoprecipitate proteins from cerebral arterial membranes and the precipitated proteins were detected by Western blotting (Fig. 7A-G). Microdissection of the circle of Willis and all its branches from 4–10 rats was required to obtain an adequate amount of protein for a single immunoprecipitation reaction. Due to the scarcity of tissue, and because initial screening indicated that anti-KV1.5, but not anti-KV 1.2, immunoprecipitated its appropriate cloned protein target, immunoprecipitation reactions were limited to anti-KV1.5. Cell membranes from human embyronic kidney (HEK) cells stably transfected with KV1.5 and KV1.2 served as positive controls (Fig. 7A and C, respectively), and revealed immunoreactive bands near the predicted molecular mass of 67 and 57 kDa for these subunits, respectively. Cerebral arterial membranes immunoprecipitated with anti-KV1.5, subjected to SDS-PAGE, and immunoblotted with anti-KV1.5 antibody also revealed a ˜75 kDa band, indicating successful precipitation of KV1.5 (Fig. 7B). The KV1.2 protein in cerebral arterial membranes was revealed as a band of ˜80 kDa on anti-KV1.2 blots (Fig. 7D). The higher apparent molecular mass of KV1.2 in cerebral arteries compared to stably expressed KV1.2 probably reflects the absence of N-glycosylation of this protein in HEK cells. Notably, when proteins from two different samples of cerebral arterial membranes were immunoprecipitated using anti-KV1.5 and blotted with anti-KV1.2, similar 80 kDa bands corresponding to KV1.2 were detected in the adjacent lanes on Western blot, inferring the presence of KV1.2/1.5 complexes in cerebral VSMCs (Fig. 7E and F). Preadsorption of anti-KV1.2 used for Western blotting with 1 μM of its antigenic competing peptide (+CP) abolished the 80 kDa-immunoreactive band detected in the anti-KV1.5 immunoprecipitate (Fig. 7G), providing further evidence for KV1.2/1.5 complexes in cerebral arterial membranes.
Figure 7. Co-immunoprecipitation of KV1.2 and 1.5 from rat cerebral arteries.

A, stably expressed KV1.5 in HEK cell membranes (1 μg) detected by anti-KV1.5 blot as a positive control. B, anti-KV1.5 immunoprecipitate from cerebral arterial membranes (200 μg) blotted with anti-KV1.5 revealed a 75 kDa band, indicating successful immunoprecipitation of KV1.5. C, stably expressed KV1.2 in HEK cell membranes (1 μg) detected by anti-KV1.2 blot as a positive control. D, native KV1.2 in cerebral arterial membranes (8 μg) detected by anti-KV1.2 blot as an ˜80 kDa band. E and F, anti-KV1.5 immunoprecipitate from 50 μg and 30 μg of two different cerebral arterial membrane preparations. An anti-KV1.2 blot revealed the same ˜80 kDa band in both arterial preparations, indicating successful co-immunoprecipitation of KV1.2 and 1.5. G, preadsorption of anti-KV1.2 with 1 μM of its antigenic competing peptide (+CP) abolished the 80 kDa-immunoreactive band detected in anti-KV1.5 immunoprecipitate. Anti-KV1.5 immunoprecipitate was from 30 μg of the same pool of arterial proteins as used in F. Results using cerebral arteries were verified in a minimum of four different protein preparations isolated from ˜60 rats.
KV current properties are consistent with KV1.2/1.5 heterotetramers
To determine if COR-sensitive KV1 current in rat cerebral VSMCs showed the expected properties of KV1.2/1.5 heterotetramers, the sensitivity of KV current to combined block by margatoxin and α-dendrotoxin was assessed. It was recently shown that incorporation of even a single toxin-insensitive KV1.5 renders KV1-family tetramers impervious to blocking toxins that otherwise would bind to KV1.2 homotetrameric channels (Hulme et al. 1999). In our preparation, combined superfusion with α-dendrotoxin (100 nM) and margatoxin (100 nM) only significantly reduced outward current at voltages positive to +42 mV (Fig. 8A and B). The subsequent addition of 1 μM COR profoundly reduced the remaining K+ current, indicating the presence of toxin-resistant KV1 channels. These data imply that functional KV1 channels appear to be composed of at least one toxin-insensitive KV1.5, a finding consistent with the immunoprecipitation studies (Fig. 7) indicating expression of KV1.2/1.5 complexes in cerebral VSMCs.
Figure 8. KV1 current is insensitive to α-dendrotoxin and margatoxin in rat cerebral VSMCs.

A, potassium currents generated by 8 mV depolarizing steps from −70 to +58 mV were only slightly reduced by α-dendrotoxin (α-DTX, 100 nM) and margatoxin (MgTX, 100 nM). Both drugs block KV1.2 homotetramers, but not KV1.5 homotetramers or KV1.2/1.5 heterotetrameric channels. The addition of 1 μM correolide (COR) reduced the same current. B, I-V relations averaged from six cells demonstrate the relative toxin-insensitivity of K+ currents in cerebral VSMCs, but the sensitivity of the same currents to block by COR. Data are expressed as means ±s.e.m. (n = 6). * Significant difference (P < 0.05) between control and experimental values at the same voltage.
Subsequently, patch-clamp studies were performed to determine if the voltage-dependent properties of KV1 current were consistent with KV1.2/1.5 channel formation. These experiments took advantage of the fact that KV1.2 channels activate at more positive potentials than KV1.5 channels whereas KV1.2/1.5 heterotetramers demonstrate ‘hybrid’ voltage sensitivity. For example, Grissmer et al. (1994) reported activation midpoints (V0.5) of +27 mV and −14 mV for KV1.2 and 1.5 channels stably transfected in B82 mouse fibroblasts, respectively. Similarly, Hulme et al. (1999) showed V0.5 values of +22 mV and −13 mV, respectively, for KV1.2 and 1.5 channels expressed in mouse L cells. KV1.2/1.5 heterotetramers demonstrated a ‘hybrid’V0.5 value of −5 mV in the same preparation. To determine the V0.5 value for activation of COR-sensitive KV1 channels in cerebral VSMCs, tail currents were generated by hyperpolarizing pulses to −70 mV in symmetrical K+ (140 mM) solutions, following 8 mV pre-pulse steps from −70 to +58 mV (Fig. 9A). The COR-sensitive tail current component was obtained by digital subtraction of COR-resistant current from total outward current. The current-voltage (I-V) relationship for activation of COR-sensitive KV1 current revealed a V0.5 value of −1 mV and a slope factor (k) of 14 (n = 6), consistent with the presence of functional KV1.2/1.5 channels (Fig. 9B and E). The voltage-dependent inactivation of COR-sensitive KV1 channels also was evaluated by exposing the channels to inactivating 8 mV pre-pulses from −86 to +18 mV (Fig. 9C), and then plotting the K+ current elicited by a voltage step from −86 to +58 mV as a function of pre-pulse voltage (Fig. 9D). COR-sensitive K+ current showed a V0.5 value for inactivation of −37 mV (n = 7) and the corresponding k was 12 (Fig. 9E). The ‘window current’ for COR-sensitive KV1 current, defined as the area under the intersection of the activation and inactivation relationships and thought to represent steady-state K+ current, indicated the availability of KV1 current within the range of negative Vm values observed in cerebral VSMCs (Fig. 9E).
Figure 9. Analysis of the voltage sensitivity of correolide (COR)-sensitive KV1 current.

A, pulse protocol used to assess the effect of 1 μM correolide (COR) on K+ channel activation in symmetrical K+ solutions. The two superimposed tail currents evoked by a voltage step from −70 to +58 mV demonstrate COR-sensitive tail currents. B, the effect of 1 μM COR on steady-state activation (n = 6). Values were provided by tail current analysis. C, pulse protocol used to assess the effect of 1 μM COR on inactivation in physiological K+ gradients. Superimposed traces were evoked by inactivating pre-pulses of −86, −14 and +18 mV, followed by a depolarizing step to +58 mV after a 5 ms delay at −70 mV to evaluate available current. D, the effect of 1 μM COR on steady-state inactivation (n = 7). Values were provided by experiments detailed in C. E, activation and inactivation relationships for COR-sensitive current. Values were obtained by subtracting COR-insensitive current from total K+ current, and were fitted with a Boltzmann function. The V0.5 value for activation was −1 mV and k was 14, whereas the V0.5 value for inactivation was −37 mV and k was 12. Data are expressed as means ±s.e.m. (n = 6–7). * Significant difference (P < 0.05) between control and experimental values at the same voltage.
DISCUSSION
In the present study, several lines of evidence implicate KV1.2/1.5 channels as the molecular basis of a physiological KV current in rat cerebral VSMCs. First, pressurized rat cerebral arteries profoundly depolarized and contracted in response to the KV1 channel blocker correolide (COR), indicating that KV1 channels contribute to the resting Vm and diameter of these small vessels. Second, although mRNAs encoding each of the KV1.1–1.6 subtypes were detected in cerebral arteries, Western blots using rat brain as a positive control only revealed the KV1.2 and 1.5 proteins in cerebrovascular tissue. The expression of KV1.2 and 1.5 was confirmed by immunocytochemistry in single VSMCs. Third, the co-immunoprecipitation of KV1.2 and 1.5 inferred that these subunits co-assemble to form channel tetramers in cerebral VSMC membranes. Fourth, a prominent COR-sensitive KV current was observed in the patch clamped VSMCs, and the toxin- and voltage-sensitive profiles of this current were consistent with the known properties of cloned KV 1.2/1.5 heterotetrameric channels.
KV channel expression in VSMCs
Numerous studies have documented an important contribution of KV channels to the resting tone of several vascular beds, and this contribution appears to be a critical dilator influence in the cerebral circulation. For example, block of KV channels by 4-AP profoundly depolarizes and constricts isolated rabbit and rat cerebral arteries, constricts arterioles isolated from the rat cerebral cortex and cerebellum, and reduces the diameter of rat basilar arteries in vivo (Knot & Nelson, 1995; Sobey & Faraci, 1999; Horuichi et al. 2001). However, in spite of their central role in regulating vascular tone, the heterogeneous nature of KV channels has hindered efforts to define their composition in small arteries and arterioles involved in blood flow regulation. At the transcript level, this diversity has been revealed by gene expression studies, which have detected a large number of mRNAs encoding the KV1-KV4 gene families and multiple subfamilies, and three β-subunit genes (KVβ1–3) in rat pulmonary, mesenteric and tail arteries (Yuan et al. 1998; Xu et al. 1999, 2000). However, although transcript number is large, several recent studies indicate that the composition of functional KV channels may be considerably less diverse. For example, whereas more than a dozen transcripts encoding KV gene families and subfamilies have been detected in rat pulmonary VSMCs, only some protein products including KV1.2 and 1.5, KV2.1 and KV3.1 have been implicated as channel components that contribute to the resting tone of these vessels, or participate in hypoxia-induced vasoconstriction in small pulmonary arteries (Archer et al. 1998; Hulme et al. 1999; Coppock et al. 2001; Smirnov et al. 2002). Additionally, Kerr et al. (2001) and Thorneloe et al. (2001) recently demonstrated a limited expression of KV1 proteins in the rabbit portal vein, and reported that the pharmacological and voltage-sensitive properties of the native channels were consistent with a predominant expression of KV1.2/1.5 channels that co-immunoprecipitated with KVβ1.2. Finally, Fergus et al. (2003) proposed that the expression of KV1.2/1.4 heteromultimers in rat renal arteries mediates the inactivating currents found in these VSMCs. To our knowledge, only the latter two studies have provided co-immunoprecipitation data to directly implicate distinct KV channel complexes in VSMCs.
The findings of the present study that KV1.2/1.5 complexes are expressed in small rat cerebral arteries complement those of Thorneloe et al. (2001) in the rabbit portal vein. These authors recently provided the first direct evidence for KV1.2/1.5 heterotetramers in a vascular tissue. Their findings of KV1.2/1.5 channels in a large systemic vein, and our findings indicating that similar channels are expressed in small cerebral arteries, raise the question of whether KV1.2/1.5 heterotetramers are preferentially assembled in the VSMC membranes of at least several different vascular beds. The finding of the present study that mRNAs encoding each of the classical KV1.1–1.6 α-subunits are expressed in rat cerebral arteries indicate that the potential exists for translation of multiple KV1 subfamilies, and concur with earlier reports of a large number of KV channel transcripts in VSMCs (Yuan et al. 1998; Xu et al. 1999, 2000; Cox et al. 2001). However, Western blotting indicated a preferential expression of KV1.2 and 1.5, inferring that cerebral VSMCs preferentially translate, assemble and express these proteins. In contrast, KV1.1, 1.3, 1.4 and 1.6 were not detected using antibodies that recognized these proteins in the brain. Similar studies in the rat and bovine brain have demonstrated a cell-specific pattern of KV1 channel expression in different types of neuronal cells (Scott et al. 1994; Koch et al. 1997; Wang et al. 1999), and indicated that addition of a single type of α-subunit to a KV1 channel assembly can target that channel for cell surface expression (Manganas & Trimmer, 2000).
Notably, our results and those of Thorneloe et al. (2001) indicating that KV1.2/1.5 channels are densely expressed in VSMCs differ from the recent findings of Cheong et al. (2001b) in mouse cerebral arterioles. In their studies, immunohistochemical analysis detected KV1.2 and 1.5 in the endothelium and perivascular nerves, respectively, and not in the VSMC layer of these vessels. Instead, KV1.3 and 1.6 appeared to predominate in the VSMC layer, and the arteriolar fragments constricted in response to agitoxin-2 and MgTX that inhibit KV1 channels assembled from KV1.3 and 1.6. The same authors (Cheong et al. 2001a) detected KV1.5 and 1.6 in the VSMC layer of rabbit pial arterioles, and the toxin resistance of a COR-sensitive KV1 current in the VSMCs inferred that most functional KV1 channels contained at least one KV1.5. These latter findings concur with our results that predict a predominance of KV1.5-containing channels in rat cerebral VSMCs, although we did not detect KV1.6 in our arteries. Instead, co-immunoprecipitation studies indicated that channel complexes minimally composed of KV1.2 and 1.5 were expressed, and COR-sensitive KV current showed a voltage-sensitive profile markedly similar to that described earlier for ‘hybrid’ KV1.2/1.5 heterotetramers. The basis for the differences between our study and those of Cheong et al. (2001a 2001b) is not readily apparent, although the different animal species and vessel sizes that were used could underlie some of the apparent differences in KV1 channel expression. Notably, an important point of agreement is that the cerebral VSMCs of mouse, rabbit and rat appear to contain a large component of functional KV1 current.
Limitations of the study
Several limitations of the present study should be acknowledged. First, whereas it appears that KV1.2 and 1.5 channels are expressed in rat cerebral VSMC, the present study cannot rule out the possibility that Western blotting failed to detect low expression levels of other KV1 α-subunits (i.e. KV1.1, 1.3, 1.4 and 1.6) that could potentially contribute to tetramer formation. Thus, our findings of KV1.2/1.5 channels should be interpreted as the minimal complexity for KV1 channels expressed in these cells. Second, because COR is the only KV1-specific blocking drug available to date, our studies relied solely on COR to block KV1 channels in isolated cerebral VSMCs and cerebral arteries. In this regard, it should be noted that COR has been tested for activity against many other ion channels that show sequence homology to KV1 channels, and found to be devoid of activity at concentrations up to 10 μM (Felix et al. 1999). COR has no effect on T- or L-type Ca2+ channels, Ca2+-activated K+ channels, or KV channels from other gene families (KV2, KV3, KV4) at concentrations less than 1 μM; block of some KV2 channels can be detected at higher concentrations (Felix et al. 1999). Thus, COR appears to be a useful tool for selectively blocking KV1 channels, but additional approaches including antisense, interfering RNA or gene knockout technologies will be needed to fully validate the findings of this study. Third, our study did not examine which KVβ-subunits (KVβ1–3) are expressed in rat cerebral VSMCs, and these subunits are known to alter the expression and kinetics of KV1 channels. Co-immunoprecipitation of KV1.2/1.5 and KVβ1.2 has been reported in the rabbit portal vein (Thorneloe et al. 2001), but the detection of mRNAs encoding all three β-subunits (KVβ1–3) in several types of VSMCs indicates the potential for heterogeneity that requires further consideration (Yuan et al. 1998; Xu et al. 1999, 2000; Cox et al. 2001).
Physiological significance
As reviewed by Robertson (1997), the synthesis and assembly of KV1 channels appear to be carefully directed to yield unique channels that regulate excitability in different cell types. In this regard, the findings of the present study provide initial insight into the molecular components of the vascular KV1 channels that may regulate blood flow to the rat brain, and suggest that KV1.2/1.5 heterotetramers may represent an important dilator influence in the cerebral circulation, since pharmacological block of these channels profoundly depolarizes and constricts small cerebral arteries. Indeed, KV1.2 and 1.5 have binding domains for protein kinases and other intracellular signalling molecules that regulate cerebrovascular reactivity (Attali et al. 1993; Aiello et al. 1995; Mason et al. 2002). Cerebral arteries may be able to adjust their reactivity responses to a variety of physiological factors by fine-tuning their relative expression levels of KV1.2/1.5 channels, as well as by relying on other K+ channel types as parallel pathways for vasodilatation. Furthermore, the observation that resting K+ conductance appears to be reduced in VSMCs of cerebral arteries exposed to cardiovascular diseases (Faraci & Heistad, 1998) infers that changes in KV1 channel expression or function may disrupt normal patterns of cerebral blood flow during pathological conditions including hypoxia, ischaemia, diabetes and hypertension.
Acknowledgments
S.A. was a Fulbright Exchange Scholar sponsored by the USA State Department. Research was supported by BGIA 9730168N from the American Heart Association (S.K.E.) and R01 HL-59238 from the USA National Institutes of Health (N.J.R.). The authors thank Mr Miodrag Pesic for graphics assistance and technical support. The gift of correolide from Dr Maria Garcia and Dr Gregory Kaczorowski of Merck Research Laboratories was greatly appreciated, as were the antibodies kindly supplied by Dr Hans-Guenther Knaus of the University of Innsbruck.
REFERENCES
- Aiello EA, Walsh MP, Cole WC. Phosphorylation by protein kinase A enhances delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am J Physiol. 1995;268:H926–934. doi: 10.1152/ajpheart.1995.268.2.H926. [DOI] [PubMed] [Google Scholar]
- Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercier J-C, El Yaagoubi A, Nguyen-Huu L, Reeve HL, Hampl V. Molecular identification of the role of voltage-gated K+ channels, Kv1. 5 and Kv2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest. 1998;101:2319–2330. doi: 10.1172/JCI333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attali B, Lesage F, Ziliani P, Guillemare E, Honore E, Waldmann R, Hugnot J-P, Mattel M-G, Lazdunski M, Barhanin J. Multiple mRNA isoforms encoding the mouse cardiac Kv1–5 delayed rectifier K+ channel. J Biol Chem. 1993;268:24283–24289. [PubMed] [Google Scholar]
- Beckh S, Pongs O. Members of the RCK potassium channel family are differentially expressed in the rat nervous system. EMBO J. 1990;9:777–782. doi: 10.1002/j.1460-2075.1990.tb08173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong A, Dedman AM, Beech DJ. Expression and function of native potassium channel (KVα1) subunits in terminal arterioles of rabbits. J Physiol. 2001a;534:691–700. doi: 10.1111/j.1469-7793.2001.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong A, Dedman AM, Xu SZ, Beech DJ. KVα1 channels in murine arterioles: differential cellular expression and regulation of diameter. Am J Physiol Heart Circ Physiol. 2001b;281:H1057–1065. doi: 10.1152/ajpheart.2001.281.3.H1057. [DOI] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chio J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz De Miera E, Rudy B. Molecular diversity of K+ channels. Ann NY Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Coppock EA, Tamkun MM. Differential expression of KV channel α-and β-subunits in the bovine pulmonary arterial circulation. Am J Physiol Lung Physiol. 2001;281:L1350–1360. doi: 10.1152/ajplung.2001.281.6.L1350. [DOI] [PubMed] [Google Scholar]
- Cox RH, Petrou S. Calcium influx inhibits voltage-dependent and augments Ca2+ -dependent K+ currents in arterial myocytes. Am J Physiol. 1999;277:C51–63. doi: 10.1152/ajpcell.1999.277.1.C51. [DOI] [PubMed] [Google Scholar]
- Cox RH, Folander K, Swanson R. Differential expression of voltage-gated K+ channel genes in arteries from spontaneously hypertensive and Wistar-Kyoto rats. Hypertension. 2001;37:1315–1322. doi: 10.1161/01.hyp.37.5.1315. [DOI] [PubMed] [Google Scholar]
- Dufton MJ, Harvey AL. Dendrotoxins: how does structure determine function. J Toxicol Toxin Rev. 1998;17:161–182. [Google Scholar]
- Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78:53–89. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- Felix JP, Bugianesi RM, Schmalhofer WA, Borris R, Goetz MA, Hensens OD, Bao J-M, Kayser F, Parsons WH, Rupprecht K, Garcia ML, Kaczorowski GJ, Slaughter RS. Identification and biochemical characterization of a novel nortriterpene inhibitor of the human lymphocyte voltage-gated potassium channel, KV1. 3. Biochem. 1999;38:4922–4930. doi: 10.1021/bi982954w. [DOI] [PubMed] [Google Scholar]
- Fergus DJ, Martens JR, England SK. Kv channel subunits that contribute to voltage-gated K+ channel current in renal vascular smooth muscle. Pflugers Arch. 2003;445:695–704. doi: 10.1007/s00424-002-0994-7. [DOI] [PubMed] [Google Scholar]
- Garcia-Calvo M, Leonard RJ, Novick J, Stevens SP, Schmalhofer WA, Kaczorowski GJ, Garcia ML. Purification, characterization, and biosynthesis of margatoxin, a component of Centruroides margaritatus venom that selectively inhibits voltage-dependent potassium channels. J Biol Chem. 1993;268:18866–18874. [PubMed] [Google Scholar]
- Gauthier KM, Liu C, Popovic A, Albarwani S, Rusch NJ. Freshly isolated bovine coronary endothelial cells do not express the BKCa channel gene. J Physiol. 2002;545:829–836. doi: 10.1113/jphysiol.2002.029843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier KM, Rusch NJ. Rat coronary endothelial cell membrane potential responses during hypertension. Hypertension. 2001;37:66–71. doi: 10.1161/01.hyp.37.1.66. [DOI] [PubMed] [Google Scholar]
- Goldman DE. Potential, impedance, and rectification in membrane. J Gen Physiol. 1943;27:37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissmer S, Nguyen AN, Aiyar J, Hanson DC, Mather RJ, Gutman GA, Karmilowicz MJ, Auperin DD, Chandy KG. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1. 1, 1.2, 1.3, 1.5 and 3.1, stably expressed in mammalian cell lines. Mol Pharmacol. 1994;45:1227–1234. [PubMed] [Google Scholar]
- Hanner M, Schmalhofer WA, Green B, Bordallo C, Liu J, Slaughter RS, Kaczorowski GJ, Garcia ML. Binding of correolide to KV1 family potassium channels. J Biol Chem. 1999;274:25237–25244. doi: 10.1074/jbc.274.36.25237. [DOI] [PubMed] [Google Scholar]
- Horuichi T, Dietrich HH, Tsugane S, Dacey RG. Role of potassium channels in regulation of brain arteriolar tone. Stroke. 2001;32:218–224. doi: 10.1161/01.str.32.1.218. [DOI] [PubMed] [Google Scholar]
- Hulme JT, Coppock EA, Felipe A, Martens JR, Tamkun MM. Oxygen sensitivity of cloned voltage-gated K+ channels expressed in the pulmonary vasculature. Circ Res. 1999;85:489–497. doi: 10.1161/01.res.85.6.489. [DOI] [PubMed] [Google Scholar]
- Jackson WF, Huebner JM, Rusch NJ. Enzymatic isolation and characterization of single vascular smooth muscle cells from cremaster arteries. Microcirc. 1996;3:313–328. doi: 10.3109/10739689609148305. [DOI] [PubMed] [Google Scholar]
- Kaczorowski GJ, Garcia ML. Pharmacology of voltage-gated and calcium-activated potassium channels. Cur Opin Chem Biol. 1999;3:448–458. doi: 10.1016/S1367-5931(99)80066-0. [DOI] [PubMed] [Google Scholar]
- Kerr PM, Clement-Chomienne O, Thorneloe KS, Chen TT, Ishii K, Sontag DP, Walsh MP, Cole WC. Heteromultimeric Kv1. 2-Kv1.5 channels underlie 4-aminopyridine-sensitive delayed rectifier K+ current of rabbit vascular myocytes. Circ Res. 2001;89:1038–1044. doi: 10.1161/hh2301.100803. [DOI] [PubMed] [Google Scholar]
- Knot HJ, Nelson MT. Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. Am J Physiol Heart Circ Physiol. 1995;269:H348–355. doi: 10.1152/ajpheart.1995.269.1.H348. [DOI] [PubMed] [Google Scholar]
- Koch RO, Wanner SG, Koschak A, Hanner M, Schwarzer C, Kaczorowski GJ, Slaughter RS, Garcia ML, Knaus H-G. Complex subunit assembly of neuronal voltage-gated K+ channels. J Biol Chem. 1997;272:27577–27581. doi: 10.1074/jbc.272.44.27577. [DOI] [PubMed] [Google Scholar]
- Leblanc N, Wan X, Leung PM. Physiological role of Ca2+-activated and voltage-dependent K+ currents in rabbit coronary myocytes. Am J Physiol Cell Physiol. 1994;266:C1523–1537. doi: 10.1152/ajpcell.1994.266.6.C1523. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hudetz AG, Knaus H-G, Rusch NJ. Increased expression of Ca2+-sensitive K+ channels in the cerebral microcirculation of genetically hypertensive rats. Evidence for their protection against cerebral vasospasm. Circ Res. 1998;82:729–737. doi: 10.1161/01.res.82.6.729. [DOI] [PubMed] [Google Scholar]
- Madden JA, Christman NjT. Integrin signaling, free radicals and tyrosine kinase mediate flow constriction in isolated cerebral arteries. Am J Physiol Heart Circ Physiol. 1999;277:H2264–2271. doi: 10.1152/ajpheart.1999.277.6.H2264. [DOI] [PubMed] [Google Scholar]
- Manganas LN, Trimmer JS. Subunit composition determines Kv1 potassium channel surface expression. J Biol Chem. 2000;38:29685–29693. doi: 10.1074/jbc.M005010200. [DOI] [PubMed] [Google Scholar]
- Mason HS, Latten MJ, Godoy LD, Horowitz B, Kenyon JL. Modulation of Kv1. 5 currents by protein kinase A, tyrosine kinase, and protein tyrosine phosphatase requires an intact cytoskeleton. Mol Pharmacol. 2002;61:285–293. doi: 10.1124/mol.61.2.285. [DOI] [PubMed] [Google Scholar]
- Ottschytsch N, Raes A, Van Hoorick D, Snyders DJ. Obligatory heterotetramerization of three previously uncharacterized Kv channel alpha-subunits identified in the human genome. Proc Natl Acad Sci U S A. 2002;99:7986–7991. doi: 10.1073/pnas.122617999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt PF, Bonnet S, Ludwig LM, Bonnet P, Rusch NJ. Upregulation of L-type Ca2+ channels in mesenteric and skeletal arteries of SHR. Hypertension. 2002;40:214–219. doi: 10.1161/01.hyp.0000025877.23309.36. [DOI] [PubMed] [Google Scholar]
- Robertson B. The real life of voltage-gated K+ channels: more than model behavior. Trends Pharmacol Sci. 1997;18:474–483. doi: 10.1016/s0165-6147(97)01140-1. [DOI] [PubMed] [Google Scholar]
- Robertson B. Pharmacology of voltage-gated K+ channels. In: Archer SA, Rusch NJ, editors. Potassium Channels in Cardiovascular Biology. New York: Blackwell Science Inc; 2001. pp. 195–218. [Google Scholar]
- Russell SN, Overturf KE, Horowitz B. Heteromultimeric formation and charybdotoxin sensitivity of two K+ channels cloned from smooth muscle. Am J Physiol. 1994;267:C1729–1733. doi: 10.1152/ajpcell.1994.267.6.C1729. [DOI] [PubMed] [Google Scholar]
- Scott VES, Muniz SM, Sewing S, Lichtinghagen R, Parcej DN, Pongs O, Dolly JO. Antibodies specific for distinct Kv subunits unveil a heterooligomeric basis for subtypes of dendrotoxin-sensitive K+ channels in bovine brain. Biochem. 1994;33:1617–1623. doi: 10.1021/bi00173a001. [DOI] [PubMed] [Google Scholar]
- Shi G, Trimmer JS. Differential asparagines-linked glycosylation of voltage-gated K+ channels in mammalian brain and in transfected cells. J Membr Biol. 1999;168:265–273. doi: 10.1007/s002329900515. [DOI] [PubMed] [Google Scholar]
- Smirnov SV, Beck R, Tammaro P, Ishii T, Aaronson PI. Electrophysiologically distinct smooth muscle cell subtypes in rat conduit and resistance pulmonary arteries. J Physiol. 2002;538:867–878. doi: 10.1113/jphysiol.2001.013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobey CG, Faraci FM. Inhibitory effect of 4-aminopyridine on responses of the basilar artery to nitric oxide. Br J Pharmacol. 1999;126:1437–1443. doi: 10.1038/sj.bjp.0702439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneloe KS, Chen TT, Kerr PM, Grier EF, Horowitz B, Cole WC, Walsh MP. Molecular composition of 4-aminopyridine-sensitive voltage-gated K+ channels of vascular smooth muscle. Circ Res. 2001;89:1030–1037. doi: 10.1161/hh2301.100817. [DOI] [PubMed] [Google Scholar]
- Wang FC, Parcej DN, Dolly JO. α-Subunit compositions of Kv1. 1-containing K+ channel subtypes fractionated from rat brain using dendrotoxins. Eur J Biochem. 1999;263:230–237. doi: 10.1046/j.1432-1327.1999.00493.x. [DOI] [PubMed] [Google Scholar]
- Xu C, Lu Y, Tang G, Wang R. Expression of voltage-dependent K+ channel genes in mesenteric artery smooth muscle cells. Am J Physiol. 1999;277:G1055–1063. doi: 10.1152/ajpgi.1999.277.5.G1055. [DOI] [PubMed] [Google Scholar]
- Xu C, Tan G, Lu Y, Wang R. Molecular basis of voltage-dependent delayed rectifier K+ channels in smooth muscle cells from rat tail artery. Life Sci. 2000;66:2023–2033. doi: 10.1016/s0024-3205(00)00529-4. [DOI] [PubMed] [Google Scholar]
- Yuan X-J, Wang J, Juhaszova M, Golovina VA, Rubin LJ. Molecular basis and function of voltage-gated K+ channels in pulmonary arterial smooth muscle cells. Am J Physiol. 1998;274:L621–635. doi: 10.1152/ajplung.1998.274.4.L621. [DOI] [PubMed] [Google Scholar]


