Abstract
During exercise, the carotid baroreflex is reset to operate around the higher arterial pressures evoked by physical exertion. The purpose of this investigation was to evaluate the contribution of somatosensory input from the exercise pressor reflex to this resetting during exercise. Nine subjects performed seven minutes of dynamic cycling at 30 % of maximal work load and three minutes of static one-legged contraction at 25 % maximal voluntary contraction before (control) and after partial blockade of skeletal muscle afferents with epidural anaesthesia. Carotid baroreflex function was assessed by applying rapid pulses of hyper- and hypotensive stimuli to the neck via a customised collar. Using a logistic model, heart rate (HR) and mean arterial pressure (MAP) responses to carotid sinus stimulation were used to develop reflex function stimulus-response curves. Compared with rest, control dynamic and static exercise reset carotid baroreflex-HR and carotid baroreflex-MAP curves vertically upward on the response arm and laterally rightward to higher operating pressures. Inhibition of exercise pressor reflex input by epidural anaesthesia attenuated the bi-directional resetting of the carotid baroreflex-MAP curve during both exercise protocols. In contrast, the effect of epidural anaesthesia on the resetting of the carotid baroreflex-HR curve was negligible during dynamic cycling whereas it relocated the curve in a laterally leftward direction during static contraction. The data suggest that afferent input from skeletal muscle is requisite for the complete resetting of the carotid baroreflex during exercise. However, this neural input appears to modify baroreflex control of blood pressure to a greater extent than heart rate.
Reflexes originating from carotid sinus baroreceptors are intimately involved in the moment-to-moment regulation of the cardiovascular system such that changes in blood pressure and heart rate (HR) occur in an inverse manner (Eckberg, 1977; Sagawa, 1983). Initially, the carotid baroreflex was considered to be relatively quiescent during exercise because both HR and blood pressure increase concomitantly with physical activity (Bristow et al. 1971; McRitchie et al. 1976; Mancia et al. 1978). In contrast, further experimentation has elucidated that the carotid baroreflex is not only operative during exercise maintaining its sensitivity, but is progressively ‘reset’ to operate at the higher arterial pressures produced by physical activity (Bevegard & Shepherd, 1966; Melcher & Donald, 1981; DiCarlo & Bishop, 1992; Potts et al. 1993; Norton et al. 1999). However, the mechanism by which this resetting occurs remains incompletely understood.
In addition to the baroreflexes, autonomic cardiovascular control during exercise is mediated by central command (a central neural drive originating in higher brain centres associated with the volitional component of exercise) (Krogh & Lindhard, 1913; Goodwin et al. 1972) and the exercise pressor reflex (a peripheral neural drive originating from skeletal muscle somatosensory afferents) (Coote et al. 1971; McCloskey & Mitchell, 1972; Mitchell et al. 1983). In humans, emerging evidence implicates each as a possible mechanism by which the carotid baroreflex is reset with exercise. For example, selective augmentation of central command via whole-body (Gallagher et al. 2001b) or axillary (Querry et al. 2001a) neuromuscular blockade has been shown to potentiate the resetting of the carotid baroreflex during both dynamic and static exercise. Likewise, it has been demonstrated that inhibition of central command during physical exertion attenuates baroreflex resetting (Ogoh et al. 2002). In contrast, the role of the exercise pressor reflex in modulating baroreflex activity during exercise is less clear. Several studies in humans have shown that perturbation of the exercise pressor reflex mediates a rapid resetting of the baroreflex at the onset and throughout the duration of physical activity (Eiken et al. 1992; Iellamo et al. 1997; Papelier et al. 1997; Gallagher et al. 2001a). However, these studies were designed to selectively manipulate muscle somatosensory input by supra-stimulation of the exercise pressor reflex (i.e. increasing the magnitude to which the reflex was activated at any given level of work). The limitations of this approach are: (i) it may excite the exercise pressor reflex beyond the range in which it normally operates and (ii) it may evoke activation of peripheral nociceptors that could contribute to the cardiovascular response elicited. These limitations potentially confound the interpretation of results derived using such methodology. Further, the effects of reducing skeletal muscle reflex input on baroreflex resetting are currently unknown.
Cognisant of these limitations, the present investigation was designed to determine the magnitude of carotid baroreflex resetting before and after partial blockade of skeletal muscle afferent input. Adoption of this experimental strategy allowed assessment of exercise pressor reflex function within its normal range of operation without concern for inadvertent stimulation of nociceptor afferents. We hypothesised that inhibition of the exercise pressor reflex would attenuate, but not eliminate, carotid baroreflex resetting during exercise. In order to test this hypothesis, subjects performed both dynamic and static leg exercise before and after epidural anaesthesia.
METHODS
Subjects
Nine men from the greater Copenhagen area participated in the investigation. Their mean (± S.E.M.) age, height and weight were 26 ± 1 years, 185 ± 2 cm and 78 ± 2 kg, respectively. Subjects were advised of the details of the study and signed an informed consent approved by the Municipal Ethical Committee of Copenhagen, Denmark. All experiments were performed in accordance with the Declaration of Helsinki. All subjects were asymptomatic for cardiovascular or respiratory disease and were currently not taking any medications. Subjects were familiarised with testing procedures and asked to abstain from caffeinated beverages for at least 12 h and from strenuous physical activity for a minimum of 24 h prior to any scheduled testing.
Measurements
The subject's heart rate was monitored using a five-lead electrocardiogram. Arterial blood pressure was measured with a Teflon catheter (20 g) placed in the brachial artery. The catheter was connected to a disposable pressure transducer (Baxter, Uden, The Netherlands) interfaced with a recording monitor (Danica Electronic-Dialogue 2000, Denmark). The transducer was placed at heart level with the subject in a semi-recumbent position enabling the beat-to-beat collection of systolic, diastolic and mean arterial pressure (MAP). Haemodynamic variables were monitored and recorded before and during dynamic and static exercise via a personal computer equipped with customised software. Respiratory measurements to assess the rate of oxygen uptake (V̇O2) were collected using breath-by-breath open circuit spirometry (MedGraphics CPX/D, St Paul, MN, USA). In addition, ratings of perceived exertion (RPE) were assessed using the Borg scale (range: 6 (light work) to 20 (hard work)) to quantify the intensity of effort during each experimental exercise bout (Borg, 1970).
Carotid baroreflex control of HR and MAP was assessed using a rapid neck pressure/neck suction (NP/NS) technique (Pawelczyk & Raven, 1989). Briefly, after a normal expiration and end-expiratory breath hold, 12 consecutive pulses (range: +40 to −80 Torr, 500 ms duration) were delivered precisely 50 ms after the R wave of the cardiac cycle via a malleable lead neck collar enclosing the anterior two-thirds of the neck (Eckberg et al. 1975). A pressure transducer was connected to a port on the collar in order to accurately quantify the level of stimulus applied. The carotid sinus pressure resulting from the pulses was estimated by subtracting the recorded pressure or suction from the prevailing MAP obtained two beats prior to the beginning of the manoeuvre. This estimated carotid sinus pressure (ECSP) was based on work that documented 85–100 % transmission of the externally applied pressure to the carotid sinus (Querry et al. 2001b). The HR and MAP responses elicited by the pulses were paired with calculated changes in ECSP to derive individual stimulus-response curves using a logistic model (Kent et al. 1972).
Epidural anaesthesia
Lidocaine (lignocaine; 5 ml of 1 %−2 % Xylocain, Astra) and Bupivacain (15–30 ml of 0.25 % Marcain, Astra) were administered epidurally through the intervertebral space of the 2nd and 3rd lumbar vertebrae using a 20 g spinal needle and the ‘loss of resistance’ technique. These doses reliably induce partial blockade of skeletal muscle somatosensory afferents (Mitchell et al. 1989a). The presence of afferent neural blockade was verified by loss of light touch sensation (assessed by stroking the skin with a cotton ball) and a decrease in motor function as described previously (Mitchell et al. 1989a; Strange et al. 1993; Williamson et al. 1994). In addition, sharp and deep pain were evaluated by pin pricking and squeezing the Achilles tendon, respectively (Mitchell et al. 1989a).
Exercise protocols
Dynamic exercise
During initial subject screening, each study participant performed incremental exercise to volitional fatigue (e.g. a 30 W increase each minute) on a cycle ergometer while seated in a semi-recumbent position to determine maximal work rate. On the day of experimentation, subjects performed cycling exercise for 7 min at an intensity equal to 30 % of maximal work rate. During exercise, subjects maintained a pedal cadence of 60 r.p.m. established by a metronome. From 4–7 min of cycling, carotid baroreflex function was evaluated (× 3) using the pulsed NP/NS technique. The V̇O2 was determined at rest and during the 3rd to 4th minute of exercise. In addition, RPE was assessed 3 min after exercise began and 15 s prior to completion of the cycling bout.
Static exercise
Seated in a semi-recumbent position, subjects were asked to perform two sets of static leg contractions for 3 min each. Using a strain gauge placed superior to the ankle, the force of each contraction was monitored continuously. To quantify the intensity of exercise performed in each bout, three preliminary maximal voluntary contractions (MVC) were performed with the mean taken to indicate leg strength. Subsequently, sustained static contractions of the knee extensors (quadriceps femoris muscles) were performed in the dominant leg at 25 % of MVC with the knee at a 90 deg angle. In order for the subject to maintain the desired intensity, visual feedback of force development was provided via a display monitor. After 1.5 min of contraction, carotid baroreflex function was evaluated on two separate occasions using the NP/NS technique. The V̇O2 was assessed during each minute of exercise. In addition, RPE was obtained 15 s prior to the cessation of exercise.
Dynamic and static exercise protocols were repeated after the induction of epidural anaesthesia. The same exercise load (i.e. 30 % of maximal work rate for dynamic exercise; 25 % MVC for static exercise) determined under control conditions was performed before and after epidural anaesthesia. These intensity levels were carefully chosen, allowing subjects to perform the same absolute work during both experimental conditions (i.e. control exercise and epidural anaesthesia exercise). Within a condition, the order of dynamic and static exercise bouts was randomised and separated by a minimum of 30 min.
Data and statistical analyses
The dependent variables HR and MAP were used to create carotid baroreflex-HR and carotid baroreflex-MAP stimulus-response curves when plotted against the independent variable ECSP. The data were individually fitted to a four-parameter logistic function (Kent et al. 1972) using the following equation:
where A1 is the HR or MAP response range (maximum to minimum), A2 is the gain coefficient (i.e. slope), A3 is the ECSP required to elicit equal pressor or depressor responses (i.e. centring point) and A4 is the minimum HR or MAP response. Data were fitted to this model by a non-linear least-squares regression minimizing the sum-of-squares error term to predict a curve of ‘best fit’ to each set of raw data. The gains of the carotid baroreflex-HR and carotid baroreflex-MAP stimulus-response curves were determined from the first derivative of the logistic function, whereas the maximal gains were calculated as the gain values located at the centring points (A3). Values for threshold (i.e. where no further increases in HR or MAP were elicited by reductions in ECSP) and saturation (i.e. where no further decreases in HR or MAP were elicited by increases in ECSP) were calculated as the maximum and minimum second derivatives, respectively, of the logistic function for the sigmoid curve. The operating point of each curve was defined as the pre-stimulus ECSP. All parameters were averaged and presented as group means.
When appropriate, paired t tests and one- and two-way analyses of variance (ANOVA) with repeated measures were used to determine significant differences for all variables before and after induction of epidural anaesthesia. For ANOVA statistical tests, a Student-Newman-Keuls analysis was employed post hoc when main effects were determined significant. Statistical significance was set at P < 0.05. Results are presented as means ± S.E.M. Statistical analyses were conducted using SigmaStat (Jandel Scienctific Software, SPSS Inc., Chicago, IL, USA).
RESULTS
Cardiorespiratory responses to dynamic exercise
Preliminary cycling determined the maximal work rate to be 259 ± 23 W for the subject group. The induction of epidural anaesthesia produced no significant changes in resting HR or MAP from control conditions. Compared with rest, dynamic exercise evoked significant increases in HR, MAP and V̇O2. During cycling, HR was slightly augmented (5–9 beats min−1) after epidural anaesthesia, diverging significantly from control exercise between the 2nd and 6th minute (Fig. 1A). In contrast, the blood pressure response after epidural anaesthesia was not significantly different from control although MAP tended to be less from the 4th minute to the cessation of epidural anaesthesia exercise (Fig. 1B). The V̇O2 was similar with and without epidural anaesthesia under both resting conditions and during dynamic exercise (Fig. 1C). Further, no significant differences existed in the reported RPE at either the 3rd or 7th minute of cycling (Fig. 1D).
Figure 1. Cardiorespiratory responses to dynamic exercise.
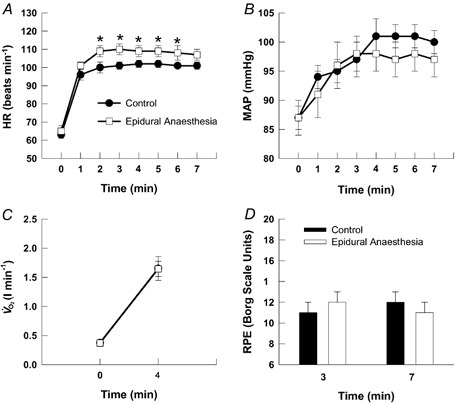
Changes in HR (A), MAP (B), V̇O2 (C) and RPE (D) in response to 7 min of dynamic two-legged cycling before (control) and after the induction of epidural anaesthesia. In both control and epidural anaesthesia exercise, cycling was performed at the same work rate (i.e. 30 % of control maximal work rate). * Significantly different from control exercise, P < 0.05.
Cardiorespiratory responses to static exercise
The cardiac and pressor responses to static exercise are presented in Fig. 2. Resting HR and MAP were not affected by the administration of epidural anaesthesia. During static exercise, HR, MAP and V̇O2 were significantly increased from resting values. The HR response to static contraction was not different between control and epidural anaesthesia exercise (Fig. 2A). In contrast, the pressor response to contraction was markedly attenuated after epidural anaesthesia (Fig. 2B). For example, at minute three of exercise the disparity culminated in a maximal difference of approximately 21 ± 5 mmHg between the two conditions. The V̇O2 and RPE responses to static contraction were unchanged between control and epidural anaesthesia exercise (Fig. 2C and D).
Figure 2. Cardiorespiratory responses to static exercise.
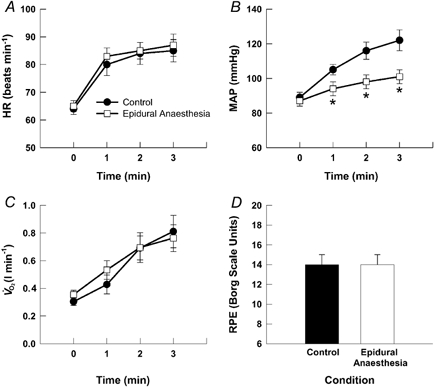
Changes in HR (A), MAP (B), V̇O2 (C) and RPE (D) in response to 3 min of one-legged static contraction of the quadriceps femoris muscles before (control) and after the induction of epidural anaesthesia. In both control and epidural anaesthesia exercise, contractions were performed at the same force (i.e. 25 % of control maximal voluntary contraction). * Significantly different from control exercise, P < 0.05.
Carotid baroreflex control of heart rate
Parameters delineated for resting carotid baroreflex-HR function curves were not significantly different before (i.e. control) or after induction of epidural anaesthesia. For clarity, only data collected under control resting conditions are presented in Table 1 and Fig. 3. During control dynamic exercise, the minimum HR response and the carotid sinus pressures at the centring point, threshold and saturation characterising the carotid baroreflex-HR stimulus-response curve were significantly greater than at rest (Table 1). Compared with rest, these alterations resulted, during dynamic exercise, in a resetting of the baroreflex function curve in both a laterally rightward and vertically upward direction that was unaffected by epidural anaesthesia (Fig. 3, upper panel). During control static exercise, each of these variables in addition to operating point pressure was significantly augmented from rest during muscle contraction relocating the reflex curve in a rightward and upward fashion (Table 1; Fig. 3, lower panel). However, in contrast to dynamic exercise, epidural anaesthesia induced significant reductions in the pressures at which the operating point, centring point, threshold and saturation were elicited without affecting the minimum HR response. This resulted in a unidirectional leftward relocation of the exercise stimulus- response curve (Fig. 3, lower panel).Within an exercise modality (i.e. dynamic or static exercise), there were no significant differences in the gains or response ranges generated for carotid baroreflex-HR stimulus- response curves at rest or during exercise under any condition.
Table 1.
Carotid baroreflex–HR stimulus–response curve parameters
| Derived variable | Rest | Control exercise | Epidural anaesthesia exercise |
|---|---|---|---|
| Dynamic exercise | |||
| Response range (beats min−1) | 15 ± 1 | 14 ± 1 | 15 ± 3 |
| Max. gain (beats min−1 mmHg−1) | −0.4 ± 0.1 | −0.3 ± 0.1 | −0.4 ± 0.1 |
| Centring point (mmHg) | 92 ± 6 | 122 ± 8† | 111 ± 7† |
| Minimum response (beats min−1) | 56 ± 4 | 93 ± 3† | 95 ± 7† |
| Threshold (mmHg) | 66 ± 6 | 92 ± 9† | 87 ± 11† |
| Saturation (mmHg) | 112 ± 6 | 146 ± 8† | 135 ± 5† |
| Operating point (mmHg) | 87 ± 2 | 94 ± 3 | 94 ± 3 |
| Static exercise | |||
| Response range (beats min−1) | 17 ± 3 | 23 ± 3 | 22 ± 3 |
| Max. gain (beats min−1 mmHg−1) | −0.5 ± 0.1 | −0.7 ± 0.1 | −0.6 ± 0.1 |
| Centring point (mmHg) | 99 ± 9 | 122 ± 10† | 107 ± 9* |
| Minimum response (beats min−1) | 50 ± 2 | 68 ± 4† | 69 ± 2† |
| Threshold (mmHg) | 64 ± 6 | 100 ± 9† | 87 ± 9*† |
| Saturation (mmHg) | 118 ± 11 | 145 ± 12† | 127 ± 9* |
| Operating point (mmHg) | 89 ± 3 | 118 ± 9† | 97 ± 5* |
Values are means ± S.E.M.
Significantly different from control exercise
significantly different from rest.
Figure 3. Carotid baroreflex control of HR.
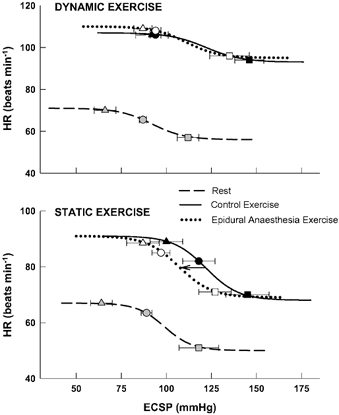
Reflex changes in HR elicited by perturbation of carotid sinus baroreceptors at rest and during dynamic (upper panel) and static (lower panel) leg exercise before (control) and after the induction of epidural anaesthesia. Lines represent fitted logistic functions developed from mean baroreflex curve parameters. Arrow indicates the directional effect of epidural anaesthesia on baroreflex resetting compared with control exercise. Triangles, estimated carotid sinus pressure (ECSP) at which response threshold occurred; squares, ECSP at which response saturation occurred; circles, ECSP at which the operating point was located. Grey (rest), black (control exercise) and open (epidural anaesthesia exercise) symbols denote the location of these parameters on each curve.
Carotid baroreflex control of blood pressure
The logistic parameters characterizing carotid baroreflex- MAP function curves at rest and during exercise are presented in Table 2 and Fig. 4. Resting parameters generated before and after the induction of epidural anaesthesia were not different and, for clarity, only control resting data are presented. During dynamic and static exercise, control carotid baroreflex-MAP reflex function curves exhibited significant augmentations in the minimum MAP response as well as the operating point, centring point, threshold and saturation pressures compared with resting values (Table 2). This resulted in a rightward and upward resetting of the stimulus-response curves during exercise (Fig. 4). After induction of epidural anaesthesia, these same parameters were consistently reduced during both dynamic cycling and static contraction. The effects of the epidural anaesthesia-induced alterations in carotid baroreflex function manifested in a bi-directional relocation of the stimulus-response curves laterally to the left and vertically downward as compared with control exercise. Under all conditions and exercise modalities, the gains and response ranges of the carotid baroreflex-MAP curves were similar.
Table 2.
Carotid baroreflex–MAP stimulus–response curve parameters
| Derived variable | Rest | Control exercise | Epidural anaesthesia exercise |
|---|---|---|---|
| Dynamic exercise | |||
| Response range (mmHg) | 12 ± 1 | 17 ± 2 | 12 ± 1 |
| Max. gain(mmHg mmHg−1) | −0.3 ± 0.1 | −0.3 ± 0.1 | −0.3 ± 0.1 |
| Centring point (mmHg) | 80 ± 4 | 108 ± 9† | 101 ± 8† |
| Minimum response (mmHg) | 80 ± 2 | 91 ± 4† | 87 ± 2† |
| Threshold (mmHg) | 52 ± 6 | 82 ± 7† | 70 ± 8 |
| Saturation (mmHg) | 108 ± 7 | 140 ± 10† | 125 ± 7*; |
| Operating point (mmHg) | 86 ± 2 | 98 ± 5† | 94 ± 4 |
| Static exercise | |||
| Response range (mmHg) | 12 ± 2 | 21 ± 3 | 14 ± 3 |
| Max. gain (mmHg mmHg−1) | −0.3 ± 0.1 | −0.4 ± 0.1 | −0.5 ± 0.1 |
| Centring point (mmHg) | 83 ± 4 | 119 ± 11† | 99 ± 10*; |
| Minimum response (mmHg) | 78 ± 2 | 106 ± 8† | 88 ± 5*; |
| Threshold (mmHg) | 55 ± 8 | 95 ± 8† | 78 ± 9†*; |
| Saturation (mmHg) | 110 ± 4 | 151 ± 12† | 119 ± 11*; |
| Operating point (mmHg) | 89 ± 3 | 117 ± 8† | 95 ± 5*; |
Values are means ± S.E.M.
Significantly different from control exercise
significantly different from rest.
Figure 4. Carotid baroreflex control of MAP.
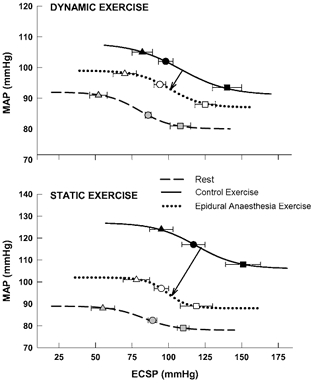
Reflex alterations in MAP elicited by perturbation of carotid sinus baroreceptors at rest and during dynamic (upper panel) and static (lower panel) leg exercise before (control) and after the induction of epidural anaesthesia. Lines represent fitted logistic functions developed from mean baroreflex curve parameters. Arrows indicate the directional effect of epidural anaesthesia on baroreflex resetting compared with control exercise. Triangles, estimated carotid sinus pressure (ECSP) at which response threshold occurred; squares, ECSP at which response saturation occurred; circles, ECSP at which the operating point was located. Grey (rest), black (control exercise) and open (epidural anaesthesia exercise) symbols denote the location of these parameters on each curve.
Efficacy of epidural anaesthesia
In all subjects, epidural anaesthesia induced a general sensory paresis (e.g. cutaneous sensory anaesthesia) between 1 and 5 cm below the umbilicus as well as partial motor paralysis of both legs consistent with previous reports (Mitchell et al. 1989a; Strange et al. 1993; Williamson et al. 1994). In addition, neurological examination of each leg determined that light touch and sharp pain senses were moderately diminished whereas the perception of deep pain was markedly reduced.
DISCUSSION
The major new finding from this investigation was that partial blockade of skeletal muscle somatosensory afferent nerve traffic, essential to the exercise pressor reflex, reduced the magnitude of carotid baroreflex resetting during both static and dynamic exercise. The consequence of epidural anaesthesia was not absolute, however, as it appeared reductions in exercise pressor reflex input affected baroreflex control of HR to a lesser extent than MAP. Further, the magnitude of the attenuation in resetting tended to be greater during static than dynamic exercise. For the carotid baroreflex-MAP stimulus- response curve, the effect of epidural anaesthesia during exercise manifested as a bi-directional relocation downward on the response arm and leftward to lower operating pressures during both dynamic cycling and static contraction. During static exercise only, the carotid baroreflex-HR stimulus response curve was likewise relocated, albeit only exhibiting a leftward shift on the response arm. These changes occurred without alterations in either the sensitivity (i.e. gain) or response range of the carotid baroreflex. This is the first time in normal healthy humans that prevention of baroreflex resetting has been exhibited after pharmacological blockade of the exercise pressor reflex. Collectively, the data suggest that the exercise pressor reflex actively participates in the resetting of the carotid baroreflex during exercise and is requisite for the complete expression of this phenomenon. Further, the finding that epidural anaesthesia reduced baroreflex resetting suggests that skeletal muscle afferents are tonically active even at low to moderate levels of exercise.
To establish that skeletal muscle afferent neural signals were necessary for complete carotid baroreflex resetting during exercise, this study was designed to minimise alterations in central command between control and blockade conditions. This was clearly achieved during static exercise protocols as the V̇O2, RPE and HR responses to muscle contraction (traditionally used as indices of central command activity; Eldridge et al. 1985; Mitchell et al. 1989a) remained unchanged before and after epidural anaesthesia. Nevertheless, although attenuated, carotid baroreflex resetting was not completely eliminated during static epidural anaesthesia exercise. This suggests that in the absence or reduction of input from the exercise pressor reflex, central command maintains the ability to modulate the baroreflex. Likewise, the MAP response to static contraction was reduced but not abolished by epidural anaesthesia while the HR response was completely unaffected by the manoeuvre. This supports the concept that during static exercise, the HR response can be entirely mediated via central command whereas input from skeletal muscle afferents is requisite for the full expression of the pressor response (Mark et al. 1985; Victor et al. 1989). Seemingly, this conclusion is in contrast to studies using partial neuromuscular blockade (a strategy used to augment central command activity) in which both the MAP and HR responses to static contraction were determined to be in direct proportion to the intensity of central effort (Mitchell et al. 1989b; Gandevia et al. 1993). However, taken together, findings from this and previous studies complement one another supporting the concept that both central and reflex mechanisms mediate the cardiovascular adjustments to exercise in a somewhat redundant fashion.
During dynamic exercise, V̇O2 and RPE values were not different between control and epidural anaesthesia. However, the HR response to cycling was slightly greater after epidural anaesthesia suggesting central command activity may have been elevated during this exercise protocol, possibly as a result of an anaesthesia-induced muscle weakness. Hence, maintenance of the same work rate may have required greater central effort (Ogoh et al. 2002). Compared with static contraction, this could account for the smaller epidural anaesthesia-induced reductions in the resetting of the carotid baroreflex-MAP curve during dynamic exercise and the lack of any appreciable effect of epidural anaesthesia on the magnitude of resetting characterising the carotid baroreflex-HR curve. Alternatively, compared with static exercise the moderate level of dynamic cycling chosen for this investigation may have limited activation of the exercise pressor reflex explaining the minimal effects of afferent blockade on baroreflex resetting.
Several animal investigations support the concept that the exercise pressor reflex alters or modifies carotid baroreflex function during exercise. Functionally, in both chloralose-anaesthetised and decerebrate cats, electrically induced static muscle contraction and, in some cases, passive muscle stretch (both potent stimuli to skeletal muscle receptors) have been shown to induce a rapid resetting of the baroreflex (Coote & Dodds, 1976; Potts & Mitchell, 1998; McIlveen et al. 2001). Anatomically, skeletal muscle and carotid sinus afferents have been found to share similar terminal pathways in several species including the dog, cat, rabbit and rat. For example, studies using nerve degeneration (Menetry & Basbaum, 1987), anterograde tracing with biotinylated dextran amine (Potts et al. 2002), and markers of synaptic stimulation such as c-Fos protein (Li & Dampney, 1992; Dean & Seagard, 1995; Li et al. 1998) have mapped baroreceptor and skeletal muscle somatosensory afferents to the nucleus tractus solitarius, a region within the medulla oblongata essential to cardiovascular regulation. Collectively, these studies in animals provide a physiological and anatomical basis for baroreflex resetting by the exercise pressor reflex.
In 1990, Rowell and O' Leary described a testable hypothesis for the mechanism(s) of baroreflex resetting during exercise (Rowell & O'Leary, 1990). Using hypothetical baroreflex-function curves, similar to those generated in the current investigation, it was suggested that during exercise central command relocates the initial operating point of the reflex (i.e. the prevailing blood pressure) from lower to higher arterial pressures; the consequence being a resetting of the entire stimulus- response curve around the newly established operating pressure. Functionally, this would result in a laterally rightward relocation of the response curve, presumably due to central command's ability to alter the function of neurons receiving baroreceptor afferents within the brainstem. In addition, vertical shifts in the stimulus- response curves could be produced via activation of the exercise pressor reflex, raising the dependent variable (e.g. HR, MAP, sympathetic nerve activity), without changing the initial operating point of the reflex. This possibility was theorised to be due to the exercise pressor reflex's ability to act on the efferent arm of the reflex rather than the central neuronal pool mediating baroreflex function. Together, the concerted actions of both central command and the exercise pressor reflex would produce a rightward and upward relocation of the baroreflex stimulus-response relationship during exercise preserving the functionality of the reflex.
Our laboratory has recently completed a series of studies to test this general hypothesis, culminating with the current investigation. Employing several different experimental protocols, we have determined that selective augmentation of central command activity increases the magnitude of carotid baroreflex resetting during exercise (Gallagher et al. 2001b; Querry et al. 2001a; Ogoh et al. 2002) producing not only a laterally rightward relocation in the stimulus- response curve (e.g. carotid baroreflex-HR and carotid baroreflex-MAP curves), as suggested previously (Rowell & O'Leary, 1990), but also a relocation of the curve vertically upward. Complementing these findings, reductions in central command activity were shown to attenuate carotid baroreflex resetting (Ogoh et al. 2002). Potentiated activation of the exercise pressor reflex by mechanical compression of the legs during exercise has also been shown to modulate carotid baroreflex activity (Gallagher et al. 2001a) with one caveat. Although the carotid baroreflex-MAP curve was reset bi-directionally in a manner similar to that elicited by augmentation of central command, the carotid baroreflex-HR curve was relocated laterally rightward only, operating at higher arterial pressures. The current investigation produced similar results but in a directionally opposite fashion under conditions that (i) prevented activation of the exercise pressor reflex beyond its normal operating range and (ii) reduced the probability of stimulating nociceptors that potentially could confound interpretation of results. Combined, the latter findings are not congruent with the concept that activation of the exercise pressor reflex solely induces vertical shifts on the response arm of the stimulus-response curve as previously theorised. Rather, depending upon the variable of interest, the exercise pressor reflex can evoke a bi-directional relocation of the curve (i.e. MAP) or a relocation of the curve in a primarily lateral direction (i.e. HR).
Perspectives
Data from the current study in combination with previous reports in animal and human investigations support the concept that both the exercise pressor reflex and central command actively reset the baroreflex during static and dynamic exercise. Further, neuroanatomical evidence suggests that the baroreflex and the exercise pressor reflex exhibit some commonality in their respective neural pathways. Likewise, putative cerebral cortical and subcortical structures implicated in the origin of central command have also been shown to possess neural connections within brainstem nuclei overlapping the terminal fields of baroreceptor afferents (Yasui et al. 1991; Williamson et al. 2001). Given these shared anatomical connections, it is likely that the preservation of baroreflex function during exercise is due to the ability of the exercise pressor reflex and central command to alter the activity of neurons receiving synaptic input from baroreceptor afferents within the central nervous system. As central command is characterized as a feed-forward mechanism it is probably the primary regulator of baroreflex function with the exercise pressor reflex, a feed-back mechanism, subserving a modulatory role in the resetting of the reflex. Further, it appears that both inputs are requisite for the complete resetting of the baroreflex during exercise.
Summary
Partial blockade of skeletal muscle afferent input by induction of epidural anaesthesia significantly attenuated the magnitude of baroreflex resetting during both dynamic and static exercise. However, the extent of the reduction in resetting evoked by inhibition of the exercise pressor reflex was dependent on both the response variable examined and the modality of exercise. In conclusion, the data indicate that the exercise pressor reflex is actively involved in baroreflex resetting during exercise.
Acknowledgments
The authors would like to thank Jere Mitchell, MD, for his critical input into the scientific development of this project. This work was supported by the Danish National Research Foundation (Grant 504–14), the National Aeronautics and Space Administration of the United States of America (Grant NAG5–4668), and the National Institutes of Health of the United States of America, National Heart, Lung and Blood Institute (Grant HL-45547).
REFERENCES
- Bevegard BS, Shepherd JT. Circulatory effects of stimulating the carotid arterial stretch receptors in man at rest and during exercise. J Clin Invest. 1966;45:132–142. doi: 10.1172/JCI105317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehab Med. 1970;2:92–98. [PubMed] [Google Scholar]
- Bristow JD, Brown EB, Cunningham DJC, Howsen MG, Petersen ES, Pickering TG, Sleight P. Effect of bicycling on the baroreflex regulation of pulse interval. Circ Res. 1971;28:582–592. [Google Scholar]
- Coote J, Hilton S, Perez-Gonzalez J. The reflex nature of the pressor response to muscular exercise. J Physiol. 1971;215:789–804. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote JH, Dodds WN. The baroreceptor reflex and the cardiovascular changes associated with sustained muscular contraction in the cat. Pflugers Arch. 1976;363:167–173. doi: 10.1007/BF01062286. [DOI] [PubMed] [Google Scholar]
- Dean C, Seagard JL. Expression of c-Fos protein in the nucleus tractus solitarius in response to physiological activation of carotid baroreceptors. Neurosci. 1995;69:249–257. doi: 10.1016/0306-4522(95)00217-7. [DOI] [PubMed] [Google Scholar]
- Dicarlo SE, Bishop VS. Onset of exercise shifts the operating point of the arterial baroreflex to higher pressures. Am J Physiol. 1992;262:H303–307. doi: 10.1152/ajpheart.1992.262.1.H303. [DOI] [PubMed] [Google Scholar]
- Eckberg DL. Baroreflex inhibition of the human sinus node: importance of stimulus intensity, duration, and rate of pressure change. J Physiol. 1977;269:561–577. doi: 10.1113/jphysiol.1977.sp011915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL, Cavanaugh MS, Mark AL, Abboud FM. A simplified neck suction device for activation of carotid baroreceptors. J Lab Clin Med. 1975;85:167–173. [PubMed] [Google Scholar]
- Eiken O, Convertino VA, Doerr DF, Morariu GA, Mekjavic I. Characteristics of the carotid baroreflex in man during normal and flow-restricted exercise. Acta Physiol Scand. 1992;144:325–331. doi: 10.1111/j.1748-1716.1992.tb09301.x. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Kiley JP, Waldrop TG. Stimulation by central command of locomotion, respiration, and circulation during exercise. Resp Physiol. 1985;59:313–337. doi: 10.1016/0034-5687(85)90136-7. [DOI] [PubMed] [Google Scholar]
- Gallagher KM, Fadel PJ, Stromstad M, Ide K, Smith SA, Querry RG, Raven PB, Secher NH. Effects of exercise pressor reflex activation on carotid baroreflex function during exercise in humans. J Physiol. 2001a;533:871–880. doi: 10.1111/j.1469-7793.2001.t01-2-00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher KM, Fadel PJ, Stromstad M, Ide K, Smith SA, Querry RG, Raven PB, Secher NH. Effects of partial neuromuscular blockade on carotid baroreflex function during exercise in humans. J Physiol. 2001b;533:861–870. doi: 10.1111/j.1469-7793.2001.t01-1-00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Killian K, McKenzie DK, Crawford M, Allen GM, Gorman RB, Hales JP. Respiratory sensations, cardiovascular control, kinaesthesia and transcranial stimulation during paralysis in humans. J Physiol. 1993;470:85–107. doi: 10.1113/jphysiol.1993.sp019849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol. 1972;226:173–190. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iellamo F, Legramante JM, Raimondi G, Peruzzi G. Baroreflex control of sinus node during dynamic exercise in humans: effects of central command and muscle reflexes. Am J Physiol. 1997;272:H1157–1164. doi: 10.1152/ajpheart.1997.272.3.H1157. [DOI] [PubMed] [Google Scholar]
- Kent BB, Drane JW, Blumenstein B, Manning JW. A mathematical model to assess changes in the baroreceptor reflex. Cardiology. 1972;57:295–310. doi: 10.1159/000169528. [DOI] [PubMed] [Google Scholar]
- Krogh A, Lindhard J. The regulation of respiration and circulation during the initial stages of muscle work. J Physiol. 1913;47:112–136. doi: 10.1113/jphysiol.1913.sp001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Potts JT, Mitchell JH. Effect of barodenervation on c-Fos expression in the medulla induced by static muscle contraction in cats. Am J Physiol. 1998;274:H901–908. doi: 10.1152/ajpheart.1998.274.3.H901. [DOI] [PubMed] [Google Scholar]
- Li YW, Dampney RAL. Expression of c-Fos protein in the medulla oblongata of conscious rabbits in response to baroreceptor activation. Neurosci Lett. 1992;144:70–74. doi: 10.1016/0304-3940(92)90718-m. [DOI] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory response originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlveen SA, Hayes SG, Kaufman MP. Both central command and exercise pressor reflex reset carotid sinus baroreflex. Am J Physiol. 2001;280:H1454–1463. doi: 10.1152/ajpheart.2001.280.4.H1454. [DOI] [PubMed] [Google Scholar]
- McRitchie RJ, Vatner SF, Boettcher D, Heyndrickx GR, Patrick TA, Braunwald E. Role of arterial baroreceptors in mediating cardiovascular reponse to exercise. Am J Physiol. 1976;230:85–89. doi: 10.1152/ajplegacy.1976.230.1.85. [DOI] [PubMed] [Google Scholar]
- Mancia G, Iannos J, Jamieson GG, Lawrence RH, Sharman PR, Ludbrook J. Effect of isometric handgrip exercise on the carotid sinus baroreceptor reflex in man. Clin Sci Mol Med. 1978;5:33–37. doi: 10.1042/cs0540033. [DOI] [PubMed] [Google Scholar]
- Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57:461–469. doi: 10.1161/01.res.57.3.461. [DOI] [PubMed] [Google Scholar]
- Melcher A, Donald DE. Maintained ability of carotid baroreflex to regulate arterial pressure during exercise. Am J Physiol. 1981;241:H838–849. doi: 10.1152/ajpheart.1981.241.6.H838. [DOI] [PubMed] [Google Scholar]
- Menetry D, Basbaum A. Spinal and trigeminal projections to the nucleus of the solitary tract: a possible substrate for somatovisceral and viscerovisceral reflex activation. J Comp Neur. 1987;255:439–450. doi: 10.1002/cne.902550310. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Ann Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Reeves DR, Rogers HB, Secher NH. Epidural anesthesia and cardiovascular responses to static exercise in man. J Physiol. 1989a;417:13–24. doi: 10.1113/jphysiol.1989.sp017787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JH, Reeves DR, Rogers HB, Secher NH, Victor RG. Autonomic blockade and cardiovascular responses to static exercise in partially curarized man. J Physiol. 1989b;413:433–445. doi: 10.1113/jphysiol.1989.sp017662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton KH, Boushel R, Strange S, Saltin B, Raven PB. Resetting of the carotid arterial baroreflex during dynamic exercise in humans. J Appl Physiol. 1999;87:332–338. doi: 10.1152/jappl.1999.87.1.332. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Wasmund WL, Keller DM, O-Yurvati A, Gallagher KM, Mitchell JH, Raven PB. Role of central command in carotid baroreflex resetting in humans during static exercise. J Physiol. 2002;543:349–364. doi: 10.1113/jphysiol.2002.019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papelier Y, Escourrou P, Helloco F, Rowell LB. Muscle chemoreflex alters carotid sinus baroreflex response in humans. J Appl Physiol. 1997;82:577–583. doi: 10.1152/jappl.1997.82.2.577. [DOI] [PubMed] [Google Scholar]
- Pawelczyk JA, Raven PB. Reductions in central venous pressure improve carotid baroreflex responses in conscious men. Am J Physiol. 1989;257:H1389–1395. doi: 10.1152/ajpheart.1989.257.5.H1389. [DOI] [PubMed] [Google Scholar]
- Potts JT, Lee SM, Anguelov PI. Tracing of projection neurons from the cervical dorsal horn to the medulla with the anterograde tracer biotinylated dextran amine. Auton Neurosci. 2002;98:64–69. doi: 10.1016/s1566-0702(02)00034-6. [DOI] [PubMed] [Google Scholar]
- Potts JT, Mitchell JH. Rapid resetting of carotid baroreceptor reflex by afferent input from skeletal muscle receptors. Am J Physiol. 1998;275:H2000–2008. doi: 10.1152/ajpheart.1998.275.6.H2000. [DOI] [PubMed] [Google Scholar]
- Potts JT, Shi X, Raven PB. Carotid baroreflex responsiveness during dynamic exercise in humans. Am J Physiol. 1993;265:H1928–1938. doi: 10.1152/ajpheart.1993.265.6.H1928. [DOI] [PubMed] [Google Scholar]
- Querry RG, Smith SA, Strømstad M, Ide K, Raven PB, Secher NH. Neural blockade during exercise augments central command's contribution to carotid baroreflex resetting. Am J Physiol. 2001a;280:H1635–1644. doi: 10.1152/ajpheart.2001.280.4.H1635. [DOI] [PubMed] [Google Scholar]
- Querry RG, Smith SA, Strømstad M, Ide K, Secher NH, Raven PB. Anatomical and functional characteristics of carotid sinus stimulation in humans. Am J Physiol. 2001b;280:H2390–2398. doi: 10.1152/ajpheart.2001.280.5.H2390. [DOI] [PubMed] [Google Scholar]
- Rowell LB, O'Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol. 1990;69:407–418. doi: 10.1152/jappl.1990.69.2.407. [DOI] [PubMed] [Google Scholar]
- Sagawa K. Handbook of Physiology. The Cardiovascular System. Vol. 3. Bethesda: Blackwell Science Inc; 1983. Baroreflex control of systemic arterial pressure and vascular bed; pp. 453–496. [Google Scholar]
- Strange S, Secher NH, Pawelczyk JA, Karpakka J, Christensen NJ, Mitchell JH, Saltin B. Neural control of cardiovascular responses and of ventilation during dynamic exercise in man. J Physiol. 1993;470:693–704. doi: 10.1113/jphysiol.1993.sp019883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor RG, Pryor SL, Secher NH, Mitchell JH. Effects of partial neuromuscular blockade on sympathetic nerve responses to static exercise in humans. Circ Res. 1989;65:468–476. doi: 10.1161/01.res.65.2.468. [DOI] [PubMed] [Google Scholar]
- Williamson JW, McColl R, Mathews D, Mitchell JH, Raven PB, Morgan WP. Hypnotic manipulation of effort sense during dynamic exercise: cardiovascular responses and brain activation. J Appl Physiol. 2001;90:1392–1399. doi: 10.1152/jappl.2001.90.4.1392. [DOI] [PubMed] [Google Scholar]
- Williamson JW, Mitchell JH, Olesen HL, Raven PB, Secher NH. Reflex increase in blood pressure induced by leg compression in man. J Physiol. 1994;475:351–357. doi: 10.1113/jphysiol.1994.sp020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y, Breder CD, Saper CB, Cechetto DF. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol. 1991;303:355–374. doi: 10.1002/cne.903030303. [DOI] [PubMed] [Google Scholar]


