Abstract
In guinea-pig ventricular myocytes, in which the deactivation of slowly activating delayed rectifier potassium current (IKs) is slow, IKs can be increased by rapid pacing as a result of incomplete deactivation and subsequent current accumulation. Whether accumulation of IKs occurs in dogs, in which the deactivation is much faster, is still unclear. In this study the conditions under which accumulation occurs in canine ventricular myocytes were studied with regard to its physiological relevance in controlling action potential duration (APD). At baseline, square pulse voltage clamp experiments revealed that the accumulation of canine IKs could occur, but only at rather short interpulse intervals (< 100 ms). With action potential (AP) clamp commands of constant duration (originally recorded at rate of 2 Hz), an accumulation was only found at interpulse intervals close to 0 ms. Transmembrane potential recordings with high-resistance microelectrodes revealed, however, that at the fastest stimulation rates with normally captured APs (5 Hz) the interpulse interval exceeded 50 ms. This suggested that no IKs accumulation occurs, which was supported by the lack of effect of an IKs blocker, HMR 1556 (500 nM), on APD. In the presence of the β-adrenergic receptor agonist isoproterenol (isoprenaline, 100 nM) the accumulation with AP clamp commands of constant duration was much more pronounced and a significant accumulating current was found at a relevant interpulse interval of 100 ms. HMR 1556 prolonged APD, but this lengthening was reverse rate dependent. AP clamp experiments in a physiologically relevant setting (short, high rate APs delivered at a corresponding rate) revealed a limited accumulation of IKs in the presence of isoproterenol. In conclusion, a physiologically relevant accumulation of IKs was only observed in the presence of isoproterenol. Block of IKs, however, led to a reverse rate-dependent prolongation of APD indicating that IKs does not have a dominant role at short cycle lengths.
Among the many ionic currents contributing to cardiac ventricular repolarization, the slowly activating delayed rectifier potassium current, IKs, is unique in its extremely slow activation kinetics. The activation occurs in the order of seconds and is quite constant in various species: guinea-pig (Bosch et al. 1998), rabbit (Lengyel et al. 2001), dog (Varro et al. 2000) as well as human (Virag et al. 2001). The deactivation, on the other hand, is species specific, slow in guinea-pig (time constant of 800 ms, Chinn 1993), but relatively fast in rabbit (Lengyel et al. 2001), dog (Volders et al. 1999a; Varro et al. 2000) and human (Virag et al. 2001; time constant of ˜100 ms for all).
Such kinetics potentially allow accumulation of the current during rapid pacing. Slow activation on the one hand ensures that IKs activated by a single pulse (action potential, AP) does not reach the maximal value and can be further increased. Short interpulse intervals during rapid pacing on the other hand allow only an incomplete deactivation. Therefore some of the channels remain in active, open state for the next beat and the current can gradually, from beat to beat, increase.
Increased levels of current at high rates due to this accumulation contribute, at least in guinea-pig, to the rate-dependent decrease in AP duration (APD; Jurkiewicz & Sanguinetti, 1993). Block of IKs could, therefore, result in a prolongation of APD that will be most pronounced at these high rates, which is considered an ideal protection against re-entry-based tachyarrhythmias and against the potentially dangerous reverse rate-dependent effects (greater APD prolongation at slower rates) of IKr blockers (Hondeghem & Snyders, 1990).
In guinea-pig ventricular myocytes, in which the deactivation of IKs is slow, accumulation was shown at physiologically relevant rates of 3 Hz (Lu et al. 2001) or 4 Hz (Jurkiewicz & Sanguinetti, 1993) and it was attributed to the shortening of the diastolic interval (Rocchetti et al. 2001). In large mammals including humans, however, the deactivation is much faster and accumulation at physiologically relevant rates (1–4 Hz) is therefore questionable. No significant accumulation was found in rabbit at a rate of 3 Hz (Lu et al. 2001). In dogs the accumulation was suggested to be unlikely at physiologically relevant rates (Gintant, 1996). These experiments, however, were performed in the absence of β-adrenergic stimulation, which is of crucial importance for the role of IKs in repolarization (Walsh & Kass, 1988; Volders et al. 2003).
In this study, the accumulation of IKs in canine ventricular myocytes was studied in detail and particular attention was paid to the physiological relevance of this phenomenon in controlling APD in different circumstances.
METHODS
Animal handling was in accordance with the Dutch Law on Animal Experimentation and the European Directive for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (86/609/EU). The experiments were approved by the Maastricht University Committee for Experiments on Animals.
Seventeen adult mongrel dogs of either sex (28 ± 1 kg) were used for the experiments. To induce anaesthesia, dogs received premedication (1 ml (5 kg)−1: 10 mg oxycodone HCl, 1 mg acepromazine and 0.5 mg atropine I.M.) and sodium pentobarbital (20 mg kg−1 I.V.), and subsequently they were artificially ventilated with a mixture of oxygen, nitrous oxide (40:60 %) and halothane (0.5–1 % vapour concentration). Upon thoracotomy heparin was administered I.V. The hearts (229 ± 9 g) were quickly excised and washed in cold cardioplegic solution. Left anterior descending and right coronary arteries were cannulated and simultaneously perfused. After ˜30 min of collagenase perfusion and subsequent washout of the enzyme, the digested part of the free ventricular wall was cut out and myocytes were isolated from most of it except a rim (< 1.5 mm) of epicardial and endocardial tissue. Myocytes were stored at room temperature in standard buffer solution and only quiescent rod-shaped cells with clear cross-striations were used for experiments.
Transmembrane potentials were recorded using the glass microelectrode technique and the microelectrodes had resistances of 30–60 MΩ when filled with 3 M KCl. Intracellular pacing was carried out at various cycle lengths (CL). Whole-cell currents were measured using patch pipettes with resistances of 1.0–3.0 MΩ when filled with internal solution. The square pulse voltage clamp protocols used are shown in the Results section. In AP clamp experiments, APs recorded with high resistance microelectrodes, either at baseline or in the presence of isoproterenol, were used as the command waveforms and interpulse intervals were determined as periods from the time point of 95 % completion of repolarization to the upstroke of the next AP. Experiments were performed at 37 ± 1 °C. Cell capacitance was 190 ± 6 pF (n = 54).
The standard buffer solution used for the experiments was of the following composition (mM): NaCl 145, KCl 4.0, CaCl2 1.8, MgCl2 1.0, glucose 11 and Hepes 10, pH 7.4 with NaOH at 37 °C. In most patch clamp experiments KCl was omitted from the external solution to increase IKs amplitude. Zero external K+ increases IKs magnitude (in our conditions by 129 ± 24 %, n = 12) indirectly through changes in the concentration gradient. The kinetics of IKs activation and deactivation are not influenced (Sanguinetti & Jurkiewicz, 1992). The patch-pipette solution contained (mM): potassium aspartate 125, KCl 20, MgCl2 1.0, MgATP 5, Hepes 5 and EGTA 10, pH 7.2 with KOH. L-type Ca2+ current was blocked with nifedipine (5 μM). Almokalant was dissolved in the superfusate (2 μM) to block IKr. Isoproterenol was first dissolved in distilled water containing 30 μM ascorbic acid and then kept in the dark at 5 °C until use. HMR 1556 ((3R,4S)-(+)-N-[3-hydroxy-2,2-dimethyl-6-(4,4,4-trifluorobutoxy)chroman-4-yl]-N-methylmethanesulfonamide, Aventis Pharma Deutschland GmbH, Frankfurt am Main, Germany) was used to identify IKs. This chromanol is highly selective for IKs; at a concentration of 500 nM, IKs is completely blocked (Volders et al. 2003) and other currents are unaffected (Gögelein et al. 2000; Thomas et al. 2003). HMR 1556 was dissolved in dimethyl sulfoxide (DMSO) as a 100 mM stock solution and diluted in the superfusate to achieve a final concentration immediately before each application. The final concentrations of DMSO (maximally 0.1 %) in the superfusate had no measurable effects on ion currents and AP.
The data are expressed as means ±s.e.m. Intergroup comparisons were made with Student's t test for unpaired and paired data groups, after testing for the normality of distribution. Multiple groups were analysed by one-way ANOVA. Differences were considered significant if P < 0.05.
RESULTS
IKs accumulation at baseline assessed with square voltage clamp pulses
Figure 1A shows the fundamental properties of IKs: slow activation occurring in the order of seconds, β-adrenergic stimulation of the current and block by a selective IKs blocker, HMR 1556. Repetitive depolarizing pulses (200 ms long, from −80 to +20 mV) with a short coupling interval (20 ms) revealed an accumulating outward current (Fig. 1B). The accumulating current was markedly increased in the presence of isoproterenol (100 nM), the β-adrenergic agonist, and completely blocked by HMR 1556 (500 nM; Fig. 1B) indicating that IKs is the underlying current.
Figure 1. The accumulating current is IKs.
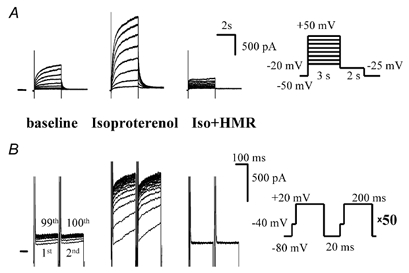
A, IKs activated by conventional seconds-long (3 s) depolarizations was increased by isoproterenol (Iso, 100 nM); administration of HMR 1556 (HMR, 500 nM) completely suppressed the current. The right panel shows the voltage protocol (applied at 0.1 Hz). B, repetitive depolarizations (voltage protocol on right) induced accumulation of an outward current that shows the same properties (increase by isoproterenol, block by HMR 1556) as IKs. The numbers in the baseline panel mark the 1st, 2nd, 99th and 100th depolarizations.
Increased IKs accumulation in the presence of isoproterenol could simply be a result of the β-adrenergic enhancement of IKs; however, changes in IKs kinetics could also contribute. Therefore the kinetics of IKs activation and deactivation were investigated in the absence and presence of isoproterenol (100 nM). The time course of full IKs activation could be measured during a 5 s pulse to +20 mV (Fig. 2C, inset). The half-maximal activation time at baseline was 511 ± 63 ms, and in the presence of isoproterenol it decreased to 412 ± 53 ms (n = 9, P < 0.05). Half-times for deactivation were voltage dependent, being, at baseline, 128 ± 13 ms on repolarization to −25 mV and 29 ± 2 ms at −80 mV. In the presence of isoproterenol, the deactivation half-times increased to 185 ± 15 ms at −25 mV and 46 ± 4 ms at −80 mV (P < 0.05 for both).
Figure 2. IKs accumulation is dependent on interpulse interval and on depolarization duration.
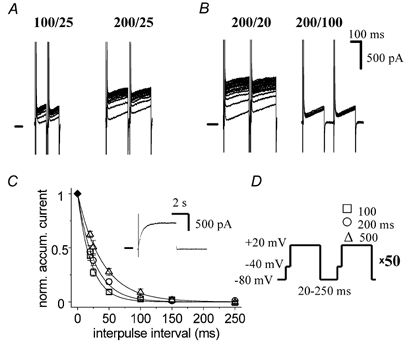
A, increasing the depolarization duration increases current accumulation. B, increasing the interpulse interval diminishes current accumulation. C, dependence of accumulation on interpulse interval and depolarization duration. Accumulated IKs was measured as the difference between currents at the end of the 1st and 100th pulses. Data (n = 6) are expressed relative to the IKs fully activated at +20 mV (measured as IKs activated during a 5 s-long pulse to +20 mV, example shown in inset). Y-axis label in full: normalized accumulated current. D, voltage protocol; interpulse interval varied from 20 to 250 ms, depolarization duration being 100, 200 or 500 ms.
Prolonging the depolarization duration (from 100 to 200 or 500 ms) increased current accumulation (Fig. 2A and C, accumulated IKs measured as the difference between currents at the end of the 1st and 100th pulses). On the other hand, prolongation of the interpulse interval (from 20 up to 250 ms) decreased the accumulation (Fig. 2B and C). Accumulation only occurred at interpulse intervals shorter than 100 ms (Fig. 2C); at longer interpulse intervals there was no accumulation irrespective of depolarization duration. The maximal level of the accumulated current (IKs) was always smaller than the amplitude of fully activated IKs (486 ± 88 pA, n = 13, activated by a sustained 5 s-long depolarization from −80 to +20 mV, Fig. 2C, inset).
The current accumulation was relatively fast and the time course was dependent on the interpulse interval (Fig. 3A). Irrespective of depolarization duration, the exponential time constant was 20 depolarization pulses for an interpulse interval of 20 ms and four depolarization pulses for an interval of 50 ms. As already mentioned in the Introduction, the accumulation is determined by deactivation kinetics. With slower deactivation more accumulation is expected, since fewer channels deactivate during a given interpulse interval and therefore more channels remain open for the next depolarization. To verify this hypothesis, we employed the voltage dependence of IKs deactivation, which is faster at more negative membrane potentials. The half-times of IKs deactivation at −50 and −80 mV were 94 ± 14 and 29 ± 2 ms (n = 9), respectively (Fig. 3B). The accumulation was measured at holding potentials of −80 and −50 mV and, indeed, a larger accumulation was found at −50 mV (Fig. 3B), when the deactivation was slower.
Figure 3. Time course and voltage dependence of IKs accumulation.
A, time course of accumulation. Upper panel, time course of accumulation at an interpulse interval of 20 ms (open symbols) and 50 ms (filled symbols). Only odd depolarization pulses are shown. Insets, 1st, 5th, 20th and 100th traces of the accumulating IKs at interpulse intervals of 20 ms (upper inset) and 50 ms (lower inset). Bottom panel, exponential time constant (expressed in numbers of depolarization pulses) of accumulation at various interpulse intervals (20–50 ms) and depolarization durations (100, 200 and 500 ms). Pooled data (n = 6). B, the magnitude of accumulation is dependent on the rate of deactivation. Upper panel, deactivation of IKs at −50 mV is slower than at −80 mV. Inset, voltage protocol. Bottom panel, accumulation of IKs, is larger at a holding potential of −50 mV than at −80 mV, a consequence of the slower deactivation kinetics at less negative potentials.
IKs accumulation assessed with AP clamp pulses at baseline and during β-adrenergic stimulation
The physiological pulse, i.e. the AP, is a much more complicated waveform than the square pulses used in our experiments described so far. To gain more insight into the physiological relevance of accumulation, the technique of AP clamp was employed. An AP recorded originally with high resistance glass microelectrodes at a CL of 500 ms (Fig. 4C) was used as the command waveform and current accumulation was measured under various conditions. With an interpulse interval of 20 ms, an accumulating outward current was found, which was again increased by isoproterenol (100 nM) and completely blocked by HMR 1556 (500 nM) as expected for IKs. The accumulated current measured as the difference current between the last (100th) and the first AP pulse is shown in Fig. 4B.
Figure 4. IKs accumulates with AP-like pulses.
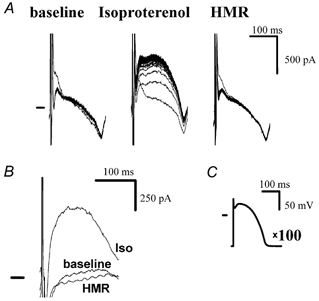
A, with AP pulses (interpulse interval of 20 ms) an accumulating current appeared at baseline, was increased by isoproterenol (100 nM) and was blocked by HMR 1556 (500 nM), consistent with IKs. B, accumulated current (obtained as the difference current of the 100th and the 1st traces shown in A) at baseline, in the presence of isoproterenol and in the presence of HMR 1556. C, command waveform AP.
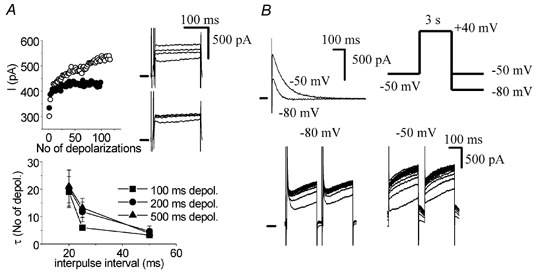
When considering the physiological relevance of IKs (accumulation), the adrenergic regulation of IKs is of crucial importance (Volders et al. 2003). β-Adrenergic agonists increase IKs directly (Marx et al. 2002), but also indirectly by changing the AP waveform (Volders et al. 2003). Plateau membrane potential (Vm) is increased and APD at Vm > 0 mV is prolonged. APD measured at 95 % completion of repolarization (APD95), however, shortens (Fig. 5B). Such changes of the AP profile are favourable for IKs activation, since longer periods of time are spent at potentials relevant for IKs activation (> 0 mV). Therefore, in the AP clamp experiments we used two different command waveforms: (1) baseline AP and (2) isoproterenol-shaped AP (shown, together with the 200 ms-long square pulse, in Fig. 5B). The accumulation accomplished using these AP clamp protocols (Fig. 5D and E) is shown and compared with a square pulse experiment (Fig. 5C) in Fig. 5 (interpulse interval of 20 ms for all). With regard to AP clamp experiments, at baseline the accumulation was negligible regardless of the AP shape. To reach a significant accumulation, isoproterenol (100 nM) had to be present. Both at baseline and with isoproterenol present, the accumulation was most prominent with a square pulse protocol.
Figure 5. Dependence of IKs accumulation on the shape of the depolarization pulse and on isoproterenol.
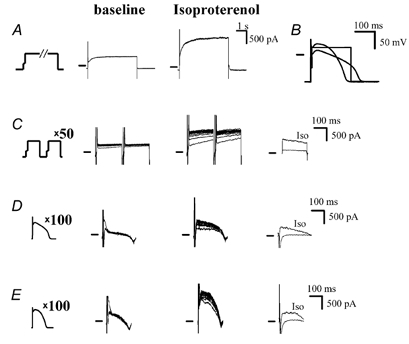
A, IKs activated by a 5 s-long pulse to +20 mV (1st panel) at baseline (2nd panel) and in the presence of isoproterenol (100 nM, 3rd panel). B, comparison of command waveform pulses: square 200 ms-long pulse to +20 mV, baseline AP (long, low plateau), isoproterenol-shaped AP (short, high plateau). The APs were originally recorded with high-resistance 3 M KCl-filled microelectrodes at a cycle length (CL) of 500 ms, at baseline and in the presence of isoproterenol. C, accumulation with square pulses (1st panel, interpulse interval of 20 mV), at baseline (2nd panel) and in the presence of isoproterenol (3rd panel). Accumulated currents (measured as difference currents between the 100th and 1st traces in previous panels) at baseline and with isoproterenol (4th panel). D, accumulation with baseline AP pulses (1st panel, interpulse interval of 20 ms), at baseline (2nd panel) and in the presence of isoproterenol (3rd panel). Accumulated currents (measured as the difference currents between the 100th and 1st traces in previous panels) at baseline and with isoproterenol (4th panel). E, accumulation with isoproterenol-shaped AP pulses (1st panel, interpulse interval of 20 ms), at baseline (2nd panel) and in the presence of isoproterenol (3rd panel). Accumulated currents (measured as the difference currents between the 100th and 1st traces in previous panels) at baseline and with isoproterenol (4th panel).
AP clamp experiments with varying interpulse intervals are summarized in Fig. 6. The accumulated current (IKs) was determined as the difference between the last (100th) and first traces at 100 ms from the start of the pulse (Fig. 6, inset). The analysis was also performed at 50 and 150 ms with qualitatively similar results. At baseline, regardless of AP shape, accumulation occurred only at interpulse intervals < 0 ms, i.e. the next AP started earlier than the time at which 95 % repolarization of the previous AP was reached. With isoproterenol (100 nM) present an accumulation was observed at interpulse intervals up to 100 ms and it was more pronounced with isoproterenol-shaped APs. The column graph allows comparison with fully activated IKs and IKs accumulated by square pulses (same voltage protocols as in Fig. 5, first column) in the absence and the presence of isoproterenol.
Figure 6. IKs accumulation with AP pulses is dependent on interpulse interval, shape of AP pulse and isoproterenol.
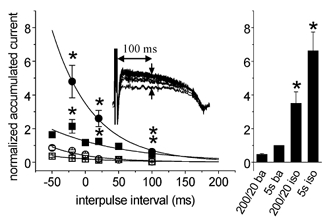
The magnitude of accumulation was determined as the difference between the 100th and the 1st traces at 100 ms from the start of the pulse (see inset) and normalized to the IKs activated during a 5 s-long pulse to +20 mV (see Fig. 2C). Open symbols represent values at baseline, filled symbols represent values in the presence of isoproterenol (100 nM), squares correspond to values obtained with baseline AP pulses, circles correspond to values recorded with isoproterenol-shaped AP pulses. The right panel enables comparisons to be made with fully activated IKs (IKs activated during a 5 s-long pulse to +20 mV) at baseline (5s ba) or in the presence of isoproterenol (5s iso) as well as with IKs accumulated with 200 ms-long square pulses (interpulse interval of 20 ms) at baseline (200/20 ba) or in the presence of isoproterenol (200/20 iso). n = 7, *P < 0.05, isoproterenol vs. baseline.
Physiological relevance of IKs accumulation
To understand the physiological relevance, we examined APD and interpulse interval at relevant (high) stimulation rates. This was done with high-resistance microelectrodes to prevent the cell dialysis and Ca2+ buffering. In each cell we tried to find the highest rate (shortest CL) with normal capturing: CL was gradually decreased in 50 ms steps, until capturing was lost (not all stimuli followed by an AP) or electrical alternans (pattern of a long AP followed by a short AP with APD95 difference > 10 ms) was encountered, then the CL was increased by 25 ms. This allowed us to determine the shortest CL captured with a resolution of 25 ms. At baseline the shortest CL captured was 200 ms (reached in 5 of 12 cells). APD95 at this CL was 149 ± 10 ms (Fig. 7A) leaving an interpulse interval of ˜50 ms (Fig. 7B). It should be realized, however, that IKs deactivation does not start at the level of 95 % repolarization (approximately −75 mV in our experiments), but much earlier (at approximately −10 mV, though at these less negative potentials the deactivation is slower). Therefore we also measured APD50 (50 % repolarization corresponds to approximately −20 mV), and it was 85 ± 7 ms (Fig. 7A) leaving ˜115 ms for IKs deactivation. Comparison of these values with data presented in Fig. 6 suggests that, at baseline, IKs accumulation is very unlikely. To verify this, HMR 1556 (500 nM) was administered and, regardless of CL, it showed no effect on APD (Fig. 7C), which, besides confirming lack of accumulation, strongly suggests that IKs does not significantly contribute to baseline APD (Volders et al. 2003).
Figure 7. APD at baseline.
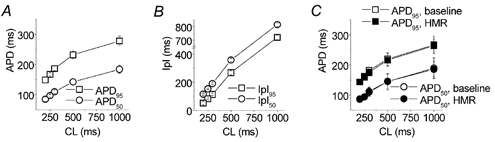
A, APD95 (squares) and APD50 (circles), n = 12. AP was measured with high resistance 3 M KCl-filled microelectrodes. B, interpulse intervals corresponding to APD in A. C, HMR 1556 (500 nM) failed to prolong baseline APD95 and APD50.
Isoproterenol (100 nM) induced shortening of APD95 (Fig. 8A, left panel), most notably at long CL (e.g. 1000 ms). At short CLs (e.g. 200 ms) the effect became negligible. In contrast, APD50 was prolonged at most CLs except the longest one (1000 ms; Fig. 8B, left panel), consistent with the change in AP configuration described above. Besides changes in AP duration and shape, isoproterenol also decreased the shortest CL with normal capturing (Fig. 8D), on average from 242 ± 14 ms to 175 ± 13 ms (n = 6, P < 0.05). Application of HMR 1556 (500 nM) in the presence of isoproterenol prolonged both APD95 and APD50 (Fig. 8A and B, right panels). The effect of HMR 1556 was CL dependent, being most pronounced at long CLs (Fig. 8C). The AP-prolonging effects were manifested at the end of the plateau and the overall AP shape was not changed. Consistent with the diminished effect on APD at short CLs, HMR 1556 did not influence the shortest CL with normal capturing (Fig. 8D).
Figure 8. APD in the presence of isoproterenol.
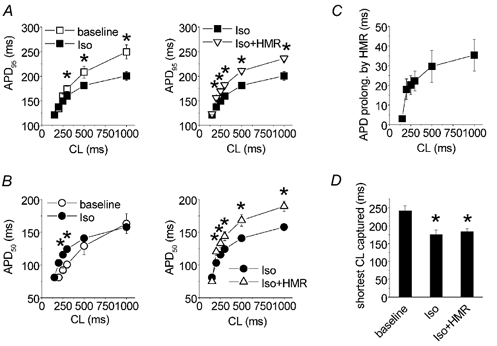
A, isoproterenol (100 nM) shortened APD95 at most CLs tested, except at short CLs (left panel). Addition of HMR 1556 (500 nM) on top of isoproterenol prolonged APD95 (right panel), n = 6, *P < 0.05. B, isoproterenol prolonged APD50 at short CLs (left panel). Addition of HMR 1556 (500 nM) on top of isoproterenol prolonged APD50 (right panel), n = 6, *P < 0.05. C, HMR 1556-induced prolongation of APD95 in the presence of isoproterenol. D, the shortest CL with normal capturing at baseline, in the presence of isoproterenol and after addition of HMR 1556, *P < 0.05 vs. baseline.
A diminished effect of HMR 1556 at short CLs (high rates) argues against accumulation in the presence of isoproterenol. The interpulse intervals (˜60 ms at CL of 200 ms, ˜30 ms at CL of 150 ms, Fig. 8A), however, are, according to AP clamp data (Fig. 6), short enough to ensure a substantial accumulation. One reason for this discrepancy could be that the AP clamp data were obtained with a constant AP (originally recorded at a CL of 500 ms) and only the interpulse interval varied. Normally, however, shortening of CL is associated with a shortening of the AP. Therefore at short CLs the AP could become so short that a sufficient activation of IKs (and subsequently accumulation) is precluded despite the presence of isoproterenol. To address this issue, we performed an AP clamp experiment, where APs recorded in the presence of isoproterenol at a CL of 175 ms served as the command waveform. Such an AP is significantly shorter than the 500 ms CL isoproterenol-shaped AP used in previous experiments (APD95 121 vs. 188 ms, Fig. 9A). In the presence of isoproterenol (100 nM), experiments with the 500 ms CL AP revealed a substantial accumulation at an interpulse interval of 20 ms (Fig. 9B). A significant IKs (identified as HMR 1556-sensitive current) was detected as early as during the first pulse (Fig. 9B, bottom panel, lower trace). When the 175 ms CL APs were delivered at a CL of 175 ms, accumulation, although much smaller than in the previous case, was also observed (Fig. 9C). HMR 1556-sensitive IKs detected during the first pulse (40 pA, Fig. 9C, bottom panel, lower trace) was smaller than IKs activated during the first 500 ms CL AP (94 pA). Nevertheless, the steady state IKs at a CL of 175 ms (205 pA, determined as HMR 1556-sensitive current during the 100th AP) was still twice the magnitude of IKs activated during the first 500 ms CL AP (steady state IKs at a CL of 500 ms). Similar results showing a significant accumulation at CLs of 175 and 150 ms were obtained in four other cells. This demonstrates that a significant accumulation of IKs is present at the shortest CL, even if HMR 1556 has a negligible effect on APD.
Figure 9. IKs accumulation in the presence of isoproterenol at a CL of 175 ms.
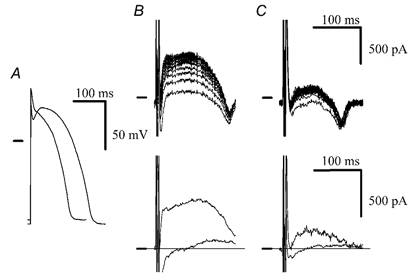
A, comparison of the isoproterenol-shaped AP used in the preceding AP clamp experiments (originally recorded at a CL of 500 ms) with an AP recorded in the presence of isoproterenol at a CL of 175 ms (the shorter AP). B, upper panel, accumulation with 500 ms CL AP as command waveform and interpulse interval of 20 ms in the presence of isoproterenol (100 nM). Lower panel, HMR 1556 (500 nM)-sensitive currents during the 1st and 100th AP pulses. C, upper panel, accumulation with 175 ms CL AP as command waveform at CL of 175 ms in the presence of isoproterenol (100 nM). Lower panel, HMR 1556 (500 nM)-sensitive currents during the 1st and 100th AP pulses.
DISCUSSION
The results of the present study show that a physiologically relevant accumulation of IKs in canine ventricular myocytes only occurs in the presence of a β-adrenergic agonist, isoproterenol (100 nM in our experiments). Block of IKs by HMR 1556 in the presence of isoproterenol led, however, to a reverse rate-dependent prolongation of APD. This indicates that despite the accumulative increase the relative functional contribution of IKs to the repolarization at fast rates decreases, probably as a result of the involvement of other repolarizing currents.
Role of IKs accumulation in repolarization
Experiments with conventional glass microelectrodes and a selective IKs blocker, HMR 1556, revealed that block of IKs in the presence of isoproterenol in canine ventricular cells led to a prolongation of APD, which was most pronounced at low stimulation rates and decreased with increasing the rate. The impact of block of IKs (or of any other current) on APD depends on the magnitude of the current blocked and also on the magnitude of the total repolarization current. On the one hand, if the total (net) repolarization current is much larger than IKs, the relative change of the net current by block of IKs will be negligible and consequently APD prolongation will also be minor. On the other hand, if the net current is of similar amplitude to IKs, block of IKs will shift the net repolarization current close to zero, which will result in a considerable prolongation of APD. Thus, the proportion of total repolarization current accounted for by IKs will determine the degree of APD prolongation.
Based on these arguments, it may be suggested that the relative contribution of total IKs (single AP IKs plus accumulated IKs) to the repolarization at (physiologically relevant) high rates is smaller than at low rates, where no accumulation occurs. Fast rate APs are much shorter than APs at low rates (Fig. 8 and Fig. 9), therefore IKs activated by single fast rate AP is significantly smaller than IKs during low rate APs (Fig. 9). However, because of the accumulation, the total (or steady-state) IKs was larger at fast rates (Fig. 9). For the relative contribution of total IKs to the repolarization to be smaller at fast than at low rates, the net outward current during repolarization at fast rates must increase more than IKs alone. In other words, outward currents other than IKs have to be increased (or inward currents decreased). Boyett and Fedida investigated the rate-dependent changes of membrane currents in dog (Boyett & Fedida, 1984) and sheep (Boyett & Fedida, 1988) Purkinje fibres and, interestingly, their results are consistent with this hypothesis: at high rates (above 1 Hz) there was an increase in the background outward current, which was attributed to the Na+-K+ pump, and simultaneously, there was a decrease in the inward Ca2+ current. In our experimental setting we were unable to verify these results, since in order to maximally visualize IKs in the voltage clamp experiments (both square pulses and AP clamp), K+ was omitted from extracellular solutions (i.e. the Na+-K+ pump was inhibited) and Ca2+ current was blocked by nifedipine (and IKr blocked by almokalant). Another rate-dependent current is the transient outward current (Ito). In contrast to IKs, Ito decreases at fast rates because of incomplete recovery from inactivation. In our experiments, Ito could be detected during the first pulse only (e.g. Fig. 4 and Fig. 5). In subsequent pulses it remained completely inactivated. Ito did not interfere with accumulation measurements (measured at 100 ms from the start of an AP), since in canine ventricular myocytes it inactivates with a time constant of ˜10 ms (Volders et al. 1999b).
A possible intrinsic reverse rate dependency of IKs block by HMR 1556 could also explain the discrepancy between AP clamp data and transmembrane potential recordings. However, such a reverse rate dependency is very unlikely, since in our square pulse patch clamp experiments the stimulation rates varied between 0.1 and > 5 Hz and HMR 1556 (500 nM) always fully suppressed IKs (both time-dependent outward current during depolarization and tail current upon repolarization; e.g. Fig. 1).
Mechanism of isoproterenol-induced increase of IKs accumulation
Isoproterenol enhances IKs conductance and negatively shifts the voltage dependence of IKs activation (Walsh & Kass, 1988; Sanguinetti et al. 1991; Han et al. 2001; Volders et al. 2003). In addition, changes in IKs kinetics, such as acceleration of IKs activation (Han et al. 2001; Volders et al. 2003; this study) and slowing down of deactivation (this study), were found in the presence of isoproterenol. These changes can significantly add to the accumulation. Accelerated activation results in an increased number of activated channels during a single pulse and subsequently can lead to a higher level of the total (steady-state) current. Analogous to this, when an increased activation was reached by prolongation of the depolarization pulse in our experiments (Fig. 2), increased accumulation occurred. Slower deactivation results in more accumulation, since fewer channels deactivate during a given interpulse interval, thus leaving more channels in an open state for the next depolarization. The experiments in Fig. 3B show that slower deactivation (at −50 versus −80 mV) is indeed associated with increased accumulation.
Reverse rate-dependent prolongation of APD by IKr blockers
IKr blockers prolong APD more at low than at high stimulation rates. In guinea-pig ventricular myocytes this reverse rate dependence was attributed to incomplete deactivation of IKs at fast rates, which partially offsets the rate-independent block of IKr (Jurkiewicz & Sanguinetti, 1993). In guinea-pigs, however, the deactivation of IKs is very slow. In other species including dog it is much faster (see Introduction). Despite the rapid deactivation of canine IKs, block of IKr still causes reverse rate-dependent prolongation of APD, which, according to Gintant (1996), is inconsistent with the postulated role of IKs in reverse rate dependence. Our data further argue against this role by showing that the IKs block itself (in the presence of isoproterenol) causes a reverse rate-dependent prolongation of APD (Fig. 8).
Role of IKs in canine ventricular repolarization
This issue remains rather controversial. On the one hand, IKs is suggested to be an important contributor, since congenital and acquired IKs deficiencies in both experimental and clinical conditions are often associated with abnormally long QT intervals. Some studies show AP prolongation after the application of IKs-blocking drugs such as chromanol 293B in a ventricular wedge preparation (Shimizu & Antzelevitch, 1998). On the other hand, some reports indicate that pharmacological IKs block does not prolong APD under baseline conditions (Varro et al. 2000).
Recent studies in various cardiac preparations (canine cardiac Purkinje cells, Han et al. 2001; rabbit sinoatrial node cells, Lei et al. 2002; canine ventricular cells, Volders et al. 2003) have shown that despite the lack of a contribution of IKs to APD at baseline, this current becomes important during β-adrenergic stimulation. Consistently, in conscious dogs, in which some level of adrenergic stimulation must be assumed, ventricular repolarization was found to be importantly dependent on IKs (Volders et al. 2003). These findings are in good agreement with the present data.
Methodological considerations
The concentration of isoproterenol in most of our experiments was 100 nM. IC50 for isoproterenol-induced stimulation of IKs was found to be in the nanomolar range (0.4–18 nM, Yazawa & Kameyama, 1990; Kathöfer et al. 2000) in guinea-pig ventricular myocytes and in Xenopus oocytes coexpressing KvLQT1/MinK channels and the human β3-adrenoreceptor subunit (8 nM, Kathöfer et al. 2000). In preliminary experiments we also found similar values for canine ventricular myocytes. Therefore, the stimulation of IKs in our experiments is maximal or close to maximal.
In most AP clamp experiments only the interpulse (diastolic) interval varied, whereas AP waveform was kept constant. This is an obvious limitation, since normal behaviour is much more dynamic and a change in diastolic interval is associated with a change in AP shape and duration. Because of this, our AP clamp experiments with a constant AP waveform will overestimate IKs accumulation. Even so, IKs accumulation only occurred in the presence of isoproterenol; unstimulated IKs did not accumulate at all. To account for the changes in AP waveform at fast rates in the presence of isoproterenol, we repeated the IKs accumulation experiments with rate-related short AP command waveforms, and some accumulation was found (Fig. 9).
In vivo implications
Translation of our cellular data to the in vivo situation is not straightforward. A serious limitation is that in cellular experiments the concentration of the β-adrenergic agonist was constant and the rate was changed artificially by pacing, whereas in vivo the rate changes in response to changing concentrations of catecholamines. This makes the in vivo situation much more complex and it would be very difficult to predict the effects of IKs block in vivo. On the one hand, with increasing rate, the APD-prolonging effects of IKs block decrease. On the other hand, in vivo, the transition from a low to a high rate is associated with increased β-adrenergic stimulation, during which the importance of IKs (and also the APD-prolonging effect of IKs block) increases. From the point of view of arrhythmology and if IKs blockers are considered as potential antiarrhythmic drugs it will be very important to determine which mechanism prevails and subsequently what kind of response (rate-dependent, reverse rate-dependent, rate-independent APD prolongation) can be expected for IKs block in vivo. For this, in vivo experiments employing the application of IKs blockers together with interventions to the adrenergic system (exercise, β-blocking therapy, etc.) will be necessary.
Conclusions
Our data indicate that at baseline (i.e. unstimulated conditions) IKs (accumulation) does not contribute to repolarization, and it only becomes important during β-adrenergic stimulation.
The prolongation of APD by IKs block during β-adrenergic stimulation was reverse rate-dependent, indicating that IKs accumulation does not have a dominant role at short CLs.
Acknowledgments
Paul G. A. Volders was supported by The Netherlands Organization for Health Research and Development (ZonMw 906–02–068). Morten B. Thomsen was supported by H. Lundbeck A/S, Denmark. The authors wish to thank Dr Uwe Gerlach (Aventis Pharma Deutschland GmbH, Frankfurt/Main, Germany) for providing HMR 1556 and Jet D. M. Beekman for expert technical assistance.
REFERENCES
- Bosch RF, Gaspo R, Busch AE, Lang HJ, Li GR, Nattel S. Effects of the chromanol 293B, a selective blocker of the slow, component of the delayed rectifier K+ current, on repolarization in human and guinea pig ventricular myocytes. Cardiovasc Res. 1998;38:441–450. doi: 10.1016/s0008-6363(98)00021-2. [DOI] [PubMed] [Google Scholar]
- Boyett MR, Fedida D. Changes in the electrical activity of dog cardiac Purkinje fibres at high heart rates. J Physiol. 1984;350:361–369. doi: 10.1113/jphysiol.1984.sp015206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett MR, Fedida D. The effect of heart rate on the membrane currents of isolated sheep Purkinje fibres. J Physiol. 1988;399:467–491. doi: 10.1113/jphysiol.1988.sp017092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinn K. Two delayed rectifiers in guinea pig ventricular myocytes distinguished by tail current kinetics. J Pharmacol Exp Ther. 1993;264:553–560. [PubMed] [Google Scholar]
- Gintant GA. Two components of delayed rectifier current in canine atrium and ventricle. Does IKs play a role in the reverse rate dependence of class III agents. Circ Res. 1996;78:26–37. doi: 10.1161/01.res.78.1.26. [DOI] [PubMed] [Google Scholar]
- Gögelein H, Brüggemann A, Gerlach U, Brendel J, Busch AE. Inhibition of IKs channels by HMR (1556) Naunyn Schmiedebergs Arch Pharmacol. 2000;362:480–488. doi: 10.1007/s002100000284. [DOI] [PubMed] [Google Scholar]
- Han W, Wang Z, Nattel S. Slow delayed rectifier current and repolarization in canine cardiac Purkinje cells. Am J Physiol Heart Circ Physiol. 2001;280:H1075–1080. doi: 10.1152/ajpheart.2001.280.3.H1075. [DOI] [PubMed] [Google Scholar]
- Hondeghem LM, Snyders DJ. Class III antiarrhythmic agents have a lot of potential but a long way to go. Reduced effectiveness and dangers of reverse use dependence. Circulation. 1990;81:686–690. doi: 10.1161/01.cir.81.2.686. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz NK, Sanguinetti MC. Rate-dependent prolongation of cardiac action potentials by a methanesulfonanilide class III antiarrhythmic agent. Specific block of rapidly activating delayed rectifier K+ current by dofetilide. Circ Res. 1993;72:75–83. doi: 10.1161/01.res.72.1.75. [DOI] [PubMed] [Google Scholar]
- Kathöfer S, Zhang W, Karle C, Thomas D, Schoels W, Kiehn J. Functional coupling of human beta 3-adrenoreceptors to the KvLQT1/MinK potassium channel. J Biol Chem. 2000;275:26743–26747. doi: 10.1074/jbc.M003331200. [DOI] [PubMed] [Google Scholar]
- Lei M, Cooper PJ, Camelliti P, Kohl P. Role of the 293b-sensitive, slowly activating delayed rectifier potassium current, IKs, in pacemaker activity of rabbit isolated sino-atrial node cells. Cardiovasc Res. 2002;53:68–79. doi: 10.1016/s0008-6363(01)00459-x. [DOI] [PubMed] [Google Scholar]
- Lengyel C, Iost N, Virag L, Varro A, Lathrop DA, Papp JG. Pharmacological block of the slow component of the outward delayed rectifier current (IKs) fails to lengthen rabbit ventricular muscle QTc and action potential duration. Br J Pharmacol. 2001;132:101–110. doi: 10.1038/sj.bjp.0703777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Kamiya K, Opthof T, Yasui K, Kodama I. Density and kinetics of IKr and IKs in guinea pig and rabbit ventricular myocytes explain different efficacy of IKs blockade at high heart rate in guinea pig and rabbit. Circulation. 2001;104:951–956. doi: 10.1161/hc3401.093151. [DOI] [PubMed] [Google Scholar]
- Marx SO, Kurokawa J, Reiken S, Motoike H, D'Armiento J, Marks AR, Kass RS. Requirement of a macromolecular signaling complex for β-adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295:496–499. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- Rocchetti M, Besana A, Gurrola GB, Possani LD, Zaza A. Rate dependency of delayed rectifier currents during the guinea-pig ventricular action potential. J Physiol. 2001;534:721–732. doi: 10.1111/j.1469-7793.2001.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti MC, Jurkiewicz NK. Role of external Ca2+ and K+ in gating of cardiac delayed rectifier K+ currents. Pflugers Arch. 1992;420:180–186. doi: 10.1007/BF00374988. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Jurkiewicz NK, Scott A, Siegl PK. Isoproterenol antagonizes prolongation of refractory period by the class III antiarrhythmic agent E-4031 in guinea pig myocytes. Mechanism of action. Circ Res. 1991;68:77–84. doi: 10.1161/01.res.68.1.77. [DOI] [PubMed] [Google Scholar]
- Shimizu W, Antzelevitch C. Cellular basis for the ECG features of the LQT1 form of the long QT syndrome: effects of β-adrenergic agonists and antagonists and sodium channel blockers on transmural dispersion of repolarization and torsade de pointes. Circulation. 1998;98:2314–2322. doi: 10.1161/01.cir.98.21.2314. [DOI] [PubMed] [Google Scholar]
- Thomas GP, Gerlach U, Antzelevitch C. HMR 1556, a potent and selective blocker of slowly activating delayed rectifier potassium current. J Cardiovasc Pharmacol. 2003;41:140–147. doi: 10.1097/00005344-200301000-00018. [DOI] [PubMed] [Google Scholar]
- Varro A, Balati B, Iost N, Takacs J, Virag L, Lathrop DA, Lengyel C, Talosi L, Papp JG. The role of the delayed rectifier component IKs in dog ventricular muscle and Purkinje fibre repolarization. J Physiol. 2000;523:67–81. doi: 10.1111/j.1469-7793.2000.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virag L, Iost N, Opincariu M, Szolnoky J, Szecsi J, Bogats G, Szenohradszky P, Varro A, Papp JG. The slow component of the delayed rectifier potassium current in undiseased human ventricular myocytes. Cardiovasc Res. 2001;49:790–797. doi: 10.1016/s0008-6363(00)00306-0. [DOI] [PubMed] [Google Scholar]
- Volders PGA, Sipido KR, Carmeliet E, Spätjens RLHMG, Wellens HJJ, Vos MA. Repolarizing K+ currents Ito1 and IKs are larger in right than left canine ventricular midmyocardium. Circulation. 1999a;99:206–210. doi: 10.1161/01.cir.99.2.206. [DOI] [PubMed] [Google Scholar]
- Volders PGA, Sipido KR, Vos MA, Spätjens RLHMG, Leunissen JD, Carmeliet E, Wellens HJ. Downregulation of delayed rectifier K+ currents in dogs with chronic complete atrioventricular block and acquired torsades de pointes. Circulation. 1999b;100:2455–2461. doi: 10.1161/01.cir.100.24.2455. [DOI] [PubMed] [Google Scholar]
- Volders PGA, Stengl M, van Opstal JM, Gerlach U, Spätjens RLHMG, Beekman JDM, Sipido KR, Vos MA. Probing the contribution of IKs to canine ventricular repolarization: Key role for β-adrenergic receptor stimulation. Circulation. 2003;107:2753–2760. doi: 10.1161/01.CIR.0000068344.54010.B3. [DOI] [PubMed] [Google Scholar]
- Walsh KB, Kass RS. Regulation of a heart potassium channel by protein kinase A and C. Science. 1988;242:67–69. doi: 10.1126/science.2845575. [DOI] [PubMed] [Google Scholar]
- Yazawa K, Kameyama M. Mechanism of receptor-mediated modulation of the delayed outward potassium current in guinea-pig ventricular myocytes. J Physiol. 1990;421:135–150. doi: 10.1113/jphysiol.1990.sp017937. [DOI] [PMC free article] [PubMed] [Google Scholar]


