Abstract
Aerobic endurance training increases insulin action in skeletal muscle, but the effect of resistance training has not been well described. Controversy exists about whether the effect of resistance training is merely due to an increase in muscle mass. We studied the effect of cessation of resistance training in young, healthy subjects by taking muscle biopsies and measuring insulin-mediated whole body and leg glucose uptake rates after 90 days of heavy resistance training (T) and again after 90 days of de-training (dT). Data on leg glucose uptake were expressed relative to accurate measures of leg muscle mass by MRI scanning. Muscle strength (239 ± 43 vs. 208 ± 33 N m), quadriceps area (8463 ± 453 vs. 7763 ± 329 mm2) and glycogen content (458 ± 22 vs. 400 ± 26 mmol (kg dry weight muscle)−1) decreased, while myosin heavy chain isoform IIX increased 4-fold in dT vs. T, respectively (all P < 0.05). GLUT4 mRNA levels and enzyme activities and mRNA levels of glycolytic, lipolytic and glyconeogenic enzymes did not change with de-training. Likewise, capillary density did not change. Whole body glucose uptake decreased 11 % and leg glucose uptake decreased from 75 ± 11 (T) to 50 ± 6 (dT) nmol min−1 (mm muscle)−2 (P < 0.05) at maximal insulin, the latter decrease being due to decreased arterio-femoral venous glucose extraction. The decrease was mainly due to reduced non-oxidative glucose disposal. We have thus shown that 90 days after the termination of heavy resistance training, insulin-mediated glucose uptake rates per unit of skeletal muscle have decreased significantly.
Increased daily physical activity is now known to be of importance in the prevention of type 2 diabetes (Tuomilehto et al. 2001; Heled et al. 2002; Diabetes Prevention Program Research Group, 2002), but in the actual treatment of type 2 diabetes there is also an important role for exercise prescription. Physical training improves insulin action predominantly in skeletal muscle, where as much as a 2- to 3–fold increase in insulin-stimulated glucose uptake in the physiological range of plasma insulin concentrations can be seen (Dela et al. 1998). The mechanisms behind this phenomenon include several adaptations, e.g. increased capillary density and GLUT4 content, a shift towards more insulin-sensitive fibre types, possibly changes in the phospholipid composition of the sarcolemma, increases in glycolytic and oxidative enzymatic activity, and increases in glycogen synthase activity (for review see Ivy et al. 1999).
The vast majority of studies on the effect of training on insulin action, in the whole body and specifically in the leg muscles, have used endurance training as the prescribed exercise modality. Many of the above-mentioned changes within the muscles are also specific for endurance training, e.g. increased capillary density.
Resistance training is very different from endurance training. During the exercise sessions, emphasis is put on developing strength by short, but intensive, repeated bouts of isotonic (concentric and/or eccentric) muscle contractions. Depending on the tailoring of the exercise programme, a substantial degree of muscle hypertrophy can be achieved. In contrast to endurance training, resistance training usually does not elicit major increases in maximal oxygen uptake (V̇O2,max), and increases in skeletal muscle capillary density are not predominantly found following a resistance training programme.
Some studies (Szczypaczewska et al. 1989; Houmard et al. 1993; Smutok et al. 1993, 1994), but not all (Miller et al. 1984, 1994; Craig et al. 1989; Zachwieja et al. 1996), have shown improvements in glucose tolerance after resistance training. However, plasma insulin concentrations are decreased in some studies during the oral glucose tolerance test (OGTT) (Miller et al. 1984; Craig et al. 1989; Szczypaczewska et al. 1989; Houmard et al. 1993; Smutok et al. 1993, 1994), indicating an increased peripheral insulin sensitivity.
Specific measurements of insulin sensitivity require methods other than the non-steady-state OGTT, e.g. the euglycaemic, hyperinsulinaemic clamp technique. In three prospective resistance training studies this has been used in healthy subjects (Miller et al. 1994) and subjects with impaired glucose tolerance (Eriksson et al. 1998) or type 2 diabetes (Ishii et al. 1998), and in these 22–48 % improvements in the glucose infusion rates were found, which were generally attributable to increases in non-oxidative glucose metabolism. In two of these studies (Miller et al. 1994; Ishii et al. 1998) data were expressed relative to the fat-free mass, in an attempt to correct for the effect of a larger muscle mass in resistance-trained individuals. In two cross-sectional studies no effect of resistance training on insulin-stimulated glucose uptake per kilogram of muscle mass was found, and the positive effect of resistance training on the whole body was solely attributed to the larger muscle mass (Yki-Järvinen & Koivisto, 1983; Takala et al. 1999).
Thus, it remains to be determined whether resistance-trained muscle is qualitatively different from skeletal muscle, which is not resistance trained. Therefore we applied the leg balance technique in combination with a euglycaemic, hyperinsulinaemic clamp in order to measure glucose uptake rates in the leg in the trained and de-trained states. By expressing data relative to accurate (MRI scans) measurements of the muscle mass it is possible to determine if cessation of resistance training decreases insulin sensitivity in skeletal muscle. Furthermore, muscle biopsies were analysed for changes in capillary density, fibre type, GLUT4 mRNA content, and glycogen in order to provide explanations for any change in insulin sensitivity.
The majority of people with impaired glucose tolerance or type 2 diabetes are overweight, and for many reasons (psychological and sociological) they are not likely to take up endurance training. For these people resistance training probably represents an attractive exercise modality, but if this form of training is to be included in the general recommendations for an exercise prescription for patients with type 2 diabetes, further characterisation of resistance-trained muscle is necessary. The present study of changes in response to de-training is one step towards this.
METHODS
Subjects and experimental protocol
Seven young (26 ± 1 years (mean ± S.E.M.)), healthy men gave their informed consent to participate in the study, which was approved by the ethical committee of Copenhagen and Frederiksberg and conducted in accordance with the standards set by the Declaration of Helsinki. All subjects were not engaged in any organised sports activities, and had not performed any heavy resistance training at least 1 year prior to entering the study. None were taking any medication. The characteristics of the subjects are shown in Table 1.
Table 1.
Subject characteristics
| Trained state | De-trained state | |
|---|---|---|
| Weight (kg) | 74.3 ± 1.3 | 72.9 ± 1.2 |
| BMI (kg m−2) | 24.2 ± 0.6 | 23.8 ± 0.5 |
| Quadriceps area (mm−2) | 8463 ± 453 | 7763 ± 329* |
| Isometric quadriceps strength (N m) | 239 ± 43 | 208 ± 33* |
| Glucose (mmol I−1) | 5.4 ± 0.1 | 5.3 ± 0.1 |
| Insulin (pmol I−1) | 45 ± 6 | 48 ± 10 |
| C-peptide (pmol I−1) | 514 ± 48 | 559 ± 62 |
Seven young, healthy resistance-trained men were studied in their trained state, and after 6 months without training. Quadriceps areas were obtained from MRI scans at 50% femur length. Blood biochemistry values are fasting values. BMI, body mass index. Data are means ± S.E.M.
P < 0.05.
The subjects engaged in a heavy resistance training programme for 3 months. The training programme was progressive; that is, loading levels were monitored continuously and adjusted throughout the entire training period to maintain muscle loading as muscle strength increased. The initial resistance used for each exercise was based on the subject's one-repetition maximum (1 RM) strength, which was determined prior to training. The training programme was essentially as described earlier (Andersen & Aagaard, 2000). The subjects undertook the training programme 3 times a week (38 training sessions with a 90 day period). Four different resistance-training exercises for the legs were performed: hack squat, incline leg press, knee extensions and hamstring curl. Additionally, a number of upper body exercises were performed. The various exercises were conducted in four or five sets of 6–15 repetitions (corresponding to a 6–15 RM loading). The recovery period between sets was 2–3 min. In general, in the first 2 weeks (training sessions 0–5) exercises involved 10–15 RM loads, followed by 10 RM loads in training sessions 6–15, heavier loads of 6–10 RM in training sessions 16–30, and very heavy loads of 6–8 RM in the final weeks. All training sessions were surveyed and supervised. After the training period the subjects entered a 3 month de-training period, in which they returned to their everyday lifestyle with the same activity level as prior to entering the study, i.e. the subjects did not perform any resistance (or endurance) exercises during the de-training period.
The isometric strength of the quadriceps muscle was measured using a custom-built setup. The subjects were seated in a rigid chair with a 90 deg hip flexion and a 90 deg knee flexion. A steel cuff was strapped around the lower right leg and connected via a rigid steel bar to a strain gauge (Bofors KRG–4, Bofors, Sweden). Three maximal voluntary contractions of 3 s were performed, separated by rest periods of 60 s. Isometric strength was defined as the highest peak moment of force value obtained.
In both the trained and de-trained state, needle muscle biopsies were obtained from the mid-portion of the vastus lateralis muscle. In the trained state, biopsies were obtained 24 h after the last training session and 48 h before the clamp experiment. In the de-trained state, biopsies were obtained 48 h before the clamp experiment. The biopsy samples were quickly freed from visible blood and separated into two pieces. One piece was frozen directly in liquid nitrogen, stored at −80 °C and used later to determine enzyme activity and glycogen content. The other piece was mounted in an embedding medium and frozen in isopentane cooled by liquid nitrogen and stored at −80 °C for analysis of capillary density and myosin heavy chain (MHC) isoform composition.
Experimental procedure
Identical euglycaemic, hyperinsulinaemic clamps combined with arterio-venous catheterisation of the right leg were carried out with the subjects in the trained state (i.e. after 3 months of heavy resistance training) and after 3 months without resistance training (i.e. the de-trained state). The subjects were always studied in the fasting state; for subjects in the trained state this was 48 h after the last training session. On the experimental day the subjects arrived in the laboratory in the morning, where they were weighed and then lay on a bed. Electrocardiogram (ECG) and heart rate were monitored by precordial electrodes. A catheter was inserted in a medial cubital vein for infusions of insulin and glucose (20 %), and an arterial cannula was inserted in the radial or brachial artery for sampling of blood and continuous monitoring of blood pressure. In the right femoral vein, Teflon catheters were inserted for blood sampling and measurements of leg blood flow (thermodilution technique) as previously described (Dela et al. 1995).
After basal measurements, a two-step, sequential euglycaemic, hyperinsulinaemic clamp was started. For each subject a 50 ml insulin infusate had been prepared for each clamp step from insulin (Actrapid, Novo, Copenhagen, Denmark; 100 i.u. ml−1), saline, with 2.5 ml of the subjects own plasma. At each clamp step, insulin was given as a 2 ml bolus followed by constant infusion (rates of 28 and 480 mU min−1 m−2) for 90 min each. The infusion rates were chosen in order to elicit insulin concentrations in the physiological range as well as at a level that is maximally effective. Plasma glucose was maintained at euglycaemia by frequent arterial blood samples, analysed on an automatic glucose analyser (YSI 2300, Yellow Springs Instruments, USA), with subsequent adjustment of the glucose infusion rate. Arterial and femoral venous blood samples were drawn at time points of −30, −15, 70, 80 and 90 min in each clamp step.
Calculations and analytical procedures
Uptake and release of glucose, lactate, O2 and CO2 were calculated as arterio-venous whole blood concentration differences multiplied by blood flow. Concentrations of insulin and C-peptide were measured in arterial plasma. All blood samples were kept at − 20 °C until analysis, except for C-peptide which was kept at −80 °C. Detailed descriptions of the procedures used for stabilisation of blood samples and analysis of hormones, metabolites and gases have been described previously (Dela, 1996). Calculations of substrate oxidation and storage in the legs were based on measurements of O2 and CO2 in arterial and venous whole blood. O2 used for and CO2 produced from protein oxidation was not accounted for. Glycogenesis in the legs was indirectly determined as (leg glucose uptake – [glycosyl units oxidised + glucose converted to lipid + lactate (in glucose equivalents) released from legs]) (Mikines et al. 1988a).
Before the analysis of glycogen content, the muscle was freeze-dried and dissected free of connective tissue and blood under a microscope. The glycogen content in the muscle powder was analysed as previously described (Dela, 1996).
Quadriceps muscle anatomical cross-sectional area (CSA) was measured by magnetic resonance imaging (MRI). The length of the femur was determined in coronary scout scans as the distance from the greater trochanter to the lateral femur condyle. Axial images of the thigh were obtained at 50 % femur length, corresponding to the site of maximum quadriceps CSA (Narici et al. 1996). During analysis, the CSAs of individual muscles of the thigh were determined using the computer software of the MR scanner. A mask was drawn manually around each muscle, and the CSA was calculated automatically by the computer software. The CSA of the quadriceps muscle was defined as the sum of the CSAs of the vastus lateralis (VL), rectus femoris (RF), vastus medialis (VM) and vastus intermedius (VI) muscles.
Capillary density was determined using the double staining method combining Ulex europaeus agglutinin I lectin (UEA–I) and a collagen IV antibody, as previously described (Qu et al. 1997).
Determination of MHC isoform composition in muscle homogenates from the individual biopsies was performed as previously described (Andersen & Aagaard, 2000).
Enzyme activities
Muscle tissue (10–20 mg) was freeze-dried and dissected free of all visible blood, adipose and connective tissue under a stereomicroscope. Approximately 2 mg of the dissected tissue was homogenised in 800 μl 0.3 M K2 HPO4, 0.05 % BSA, pH 7.7 and stored at −80 °C for later analysis. Spectrometric determination of NADH changes at 340 nm (Lowry & Passonneau, 1972) was used for measuring citrate synthase (CS), short chain β-hydroxyacyl-CoA dehydrogenase (HAD), phosphofructokinase (PFK) and lactate dehydrogenase (LDH) activity. LDH activity was measured at 25 °C by 50 times dilution in a solution containing 1 mM pyruvate, 172 μM NADH, 0.02 % BSA, 0.02 M imidazole (pH 7.0). CS activity was measured at 25 °C by 50 times dilution in a solution containing 100 μM acetyl-CoA, 0.5 mM NAD (free acid), 1 mM sodium malate, 8 μg ml−1 malate dehydrogenase (1200 U mg−1, Boehringer Mannheim), 2.5 mM EDTA, 10 mM Tris–HCl (pH 8.0). HAD activity was measured at 25 °C by 50 times dilution in a solution containing 50 μM acetoacetyl–CoA, 35 μM NADH, 0.06 mM EDTA, 40 mM imidazole (pH 7.0). PFK activity was measured at 25 °C by 20 times dilution in a solution containing 0.9 mM fructose–6–phosphate, 0.3 mM NADH, 0.9 mM ATP, 0.9 mM AMP, 18 μg ml−1 aldolase (20 U mg−1, Boehringer Mannheim), 7 μg ml−1 glycerol–3–P–dehydrogenase (170 U mg−1, Boehringer Mannheim), 7 μg ml−1 triosephosphate isomerase (5000 U mg−1, Boehringer Mannheim), 0.9 mM Na2HPO4, 1.8 mM MgCl2, 0.9 mM mercaptoethanol, 0.045 % BSA, 45 mM Tris–HCl (pH 8.1). Enzyme activities are expressed as micromoles substrate per minute per gram dry weight muscle tissue.
Northern analysis
Total RNA was isolated from ˜10 mg muscle biopsies by phenol extraction using 500 μl TriReagent (Molecular Research Center Cincinnati, OH, USA) as described by (Chomczynski & Sacchi, 1987). Northern analysis was performed essentially as described by (Ingelbrecht et al. 1998). Briefly, 200 ng total RNA was separated on a 1 % denaturing formaldehyde agarose gel and blotted to a positively charged nylon membrane using alkaline transfer. The membrane was then hybridised with the specific single-stranded DNA probe (see below) at 50 °C (42 °C for 28S) overnight in UltraHyb (Ambion, Austin, TX, USA) followed by washing in 0.1 × SSPE and 0.1 % SDS at 60 °C (42 °C) to remove excess probe. The signals were detected and quantified on an ImagerFX (Bio-Rad Laboratories, Hercules, CA, USA). The membranes were stripped for probe and reused for successive hybridisations. All membranes were hybridised at the end with GAPDH for normalisation. The mRNA level was calculated by dividing the specific signal by the GAPDH signal. 28S rRNA was also measured, but no change was observed in the ratio between 28S rRNA and GAPDH mRNA before and after de-training, validating the use of GAPDH for normalisation (Schjerling, 2001). Samples from the same subject were loaded together. For GLUT4 mRNA one de-training value was removed as an outlier (more than 9 standard deviations from mean). For one subject the RNA from the biopsy taken in the de-trained state was degraded. For the purpose of maintaining paired statistical analysis, the mRNA measurements in the trained state for this subject were also excluded; thus for mRNA n = 6.
Single-stranded probes
The Pfu polymerase (Stratagene, La Jolla, CA, USA) was used to amplify PCR products from human muscle cDNA, using the primers listed in Table 2. The PCR products were cloned into the Sma I site of pBlueScript II SK(+) (Jonsdottir et al. 2000), resulting in the plasmids pCM160 (GLUT4, orientation opposite to lacZ) and pCM5 (GAPDH, orientation as lacZ). Templates for probes were made by PCR on the plasmids with M13 standard primers. The primers gave rise to the sense strand, which was 5′–biotinylated. Radioactive single-stranded probes were made from these PCR products using the biotinylated strand as template (Higginson et al. 2002). The 28S probe was made by 5′ phosphorylation of an oligonucleotide complementary to 28S rRNA (TCG CCG TTA CTG AGG GAA TCC TGG TTA GTT TCT TT), using T4 polynucleotide kinase and γ-32P-ATP.
Table 2.
Plasmids and primers used for Northern analysis
| Probe | ID* | Orient.† | Sense primer 5′→ 3′ | Antisense primer 5′→ 3′ | GenBank‡ |
|---|---|---|---|---|---|
| GLUT4 | pCM160 | Z- | CTGTGCCATCCTGATGACTG | GGGTTTCACCTCCTGCTCTA | NM-001042 |
| CS | pCM126 | Z+ | CTTGTCTTGTTCTTGCAGCC | CTCTTTGCCCACTCTTTTGA | NM-004077 |
| HAD | pCM297 | z− | GAACGAGGTGACGCATCCAA | AAAGAGCATTCCCACAGGCA | NM-005327 |
| GS | pCM148 | z+ | GTCCTGCACCTCCTCCACCA | CCCCAGACCTGAAAGCACCA | NM-002103 |
| LDH-M | pCM132 | z+ | CAACAGGATTCTAGGTGGAGG | CTTGGATAGTTGGTTGCATTG | NM-005566 |
| LDH-H | pCM306 | z+ | GCAGGAGTCCGTCAGCAAGA | CACCACTCCACACAGCCACA | NM-002300 |
| GAPDH | pCM5 | z+ | GAACATCATCCCTGCCTCTACT | GTCTACATGGCAACTGTGAGGA | NM-002046 |
Plasmid ID
Orientation in the vector (Z+ same as lacZ, Z− opposite to lacZ)
Genbank accession number
Statistics
Results are presented as means ± S.E.M. Analysis of variance for repeated measures was used for detection of differences between the trained and the de-trained state in comparisons where series of measurements were performed (e.g. leg glucose uptake at basal insulin levels and the two succeeding clamps steps). If significant differences were demonstrated, these were located by the Student-Newman-Keuls method. In comparisons with a single measurement (e.g. glycogen content in the trained and de-trained states) the Wilcoxon signed rank test was used. SigmaStat version 2.03 software package was used for all statistical calculations. P < 0.05 was considered significant in two-tailed testing.
RESULTS
Isometric strength increased 19 % during the heavy resistance training period (from 200 ± 20 to 239 ± 43 N m; P < 0.05) and decreased to pre-training levels after the period of de-training (208 ± 33 N m; P < 0.05). After de-training, muscle mass decreased (Table 1).
The MHC isoform composition showed some changes with de-training. MHC IIX increased approximately 4-fold with de-training, which was counteracted by a tendency for MHC IIA to decrease (P = 0.109). MHC I did not change (Fig. 1). The reason for the marked increase in MHC IIX was the fact that in four subjects no MHC IIX could be detected in the trained state (the last three subjects all had below 2 % MHC IIX), while all subjects expressed MHC IIX after de-training.
Figure 1. Fibre types and gene expression.
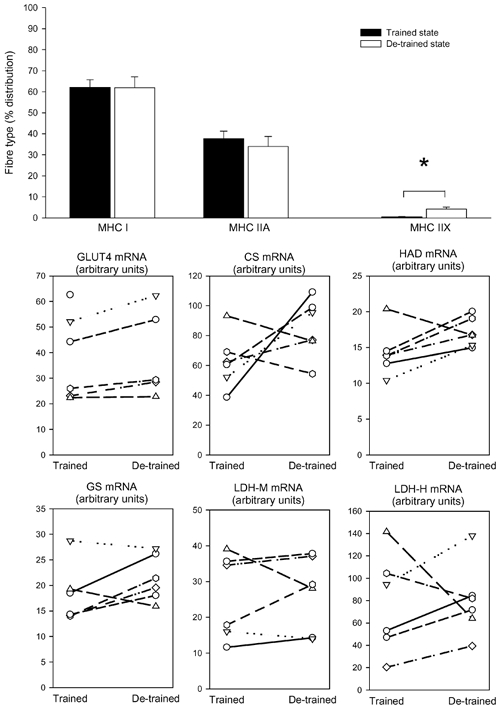
Seven young, healthy men carried out a heavy resistance training programme for 90 days (trained state) and thereafter abstained from training for a further 90 days (de-trained state). Skeletal muscle biopsies from vastus lateralis were taken in the trained and de-trained states and were analysed for myosin heavy chain (MHC) isoform composition (mean ± S.E.M.) (top graph) and mRNA levels (individual values are shown) of glucose transporter 4 (GLUT4), citrate synthase (CS), short chain β-hydroxyacyl-CoA dehydrogenase (HAD), glycogen synthase (GS) and lactate dehydrogenase in the muscle (LDH-M) and heart (LDH-H) forms. * Significant difference between trained and de-trained state (P < 0.05).
After de-training we could not detect significant changes in mRNA for GLUT4 (mean change +16 ± 4 %), CS (+52 ± 11 %), HAD (+24 ± 10 %), GS (+23 ± 11 %), LDH-M (+10 ± 3 %), or LDH-H (+29 ± 23 %) (Fig. 1).
Overall capillary density, expressed as number of capillaries per mm2 (Fig. 2) or as capillaries per fibre (data not shown) did not change with de-training.
Figure 2. Enzyme activities, glycogen content and capillary density in skeletal muscle biopsies.
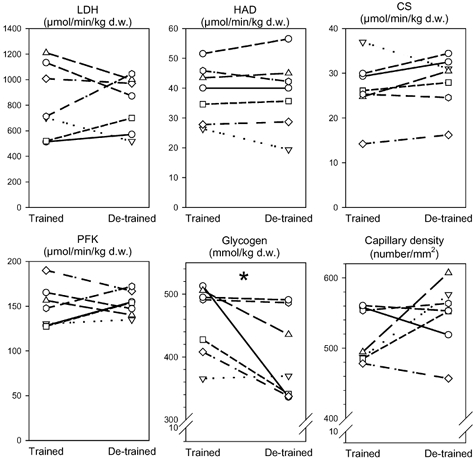
Seven young, healthy men carried out a heavy resistance training programme for 90 days (trained state) and thereafter abstained from training for a further 90 days (de-trained state). Skeletal muscle biopsies from vastus lateralis were taken in the trained and de-trained states and were analysed for lactate dehydrogenase (LDH), short chain β-hydroxyacyl-CoA dehydrogenase (HAD), citrate synthase (CS), phosphofructokinase (PFK) enzyme activities, and glycogen content and capillary density. Individual values are shown. * Significant difference between trained and de-trained state (P < 0.05).
Enzyme activities of LDH, HAD, CS and PFK in skeletal muscle did not change from the trained to the de-trained state (Fig. 2).
Glucose metabolism
Fasting glucose, insulin and C-peptide concentrations in plasma were unaltered by de-training (Table 1). Glycogen content in the skeletal muscle decreased 12 ± 5 % with de-training (from 458 ± 22 mmol (kg dry weight muscle)−1) in the trained state to 400 ± 26 mmol (kg dry weight muscle)−1) in the de-trained state; P = 0.047; Fig. 2).
Similar plasma insulin concentrations were achieved during the clamp in the trained state (step 1: 353 ± 12 pM; step 2: 11 742 ± 693 pM) and in the de-trained state (step 1: 384 ± 15 pM; step 2: 12 151 ± 666 pM).
Fuel metabolism and fate of glucose
Whole body
Insulin-stimulated glucose uptake rates during the entire 180 min clamp are shown in Fig. 3. No difference was seen at clamp step 1, while an 11 ± 4 % decrease (P < 0.05) from the trained to the de-trained state was seen at clamp step 2 (final 30 min of the clamp step).
Figure 3. Glucose uptake in legs and whole body.
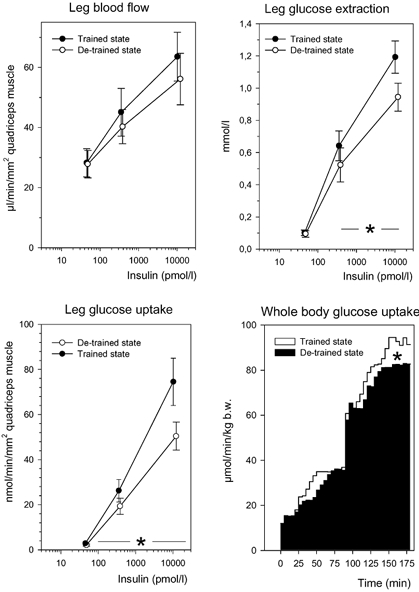
Seven young, healthy men carried out a heavy resistance training programme for 90 days (trained state) and thereafter abstained from training for a further 90 days (de-trained state). Euglycaemic, hyperinsulinaemic clamps combined with the leg balance technique were performed in the trained and de-trained states. Basal and insulin-stimulated values of leg blood flow, leg glucose extraction and leg glucose uptake are shown (means ± S.E.M.). Whole body glucose uptake rates (= glucose infusion rates) are shown (lower, right) as the mean of averaged 5 min intervals. * Significant difference between the trained and de-trained states (P < 0.05).
Skeletal muscle
Glucose uptake rates per leg before and during the two insulin infusion rates were always greater in the trained state (0.02 ± 0.01, 0.22 ± 0.04 and 0.62 ± 0.09 mmol min−1, respectively) compared with the de-trained state (0.02 ± 0.005, 0.15 ± 0.03, and 0.40 ± 0.04 mmol min−1, respectively) (P = 0.041, main effect). However, during de-training the muscle mass of the legs had diminished (Table 1), and therefore data on glucose uptake rates are given relative to cross-sectional area of the thigh muscles, measured by MRI-scans (Fig. 3). The thigh is the site of the leg where one would anticipate the largest change in muscle mass (i.e. decrease) with de-training. Correcting for differences in thigh muscle mass therefore - if anything - underestimates the differences in glucose uptake rates. Even so, the difference in leg glucose uptake between the training states was significant (P = 0.042, main effect) (Fig. 3). There was a significant interaction (P = 0.004) between training states and time points during the clamps. The diminished glucose uptake in the leg with de-training was due to a diminished glucose extraction during insulin stimulation (P < 0.05), while the blood flow per unit of muscle was not influenced by de-training (Fig. 3).
The difference in glucose uptake rates primarily reflected a difference (P < 0.05) in non-oxidative glucose disposal, but a slight, but significant (P < 0.05) difference also existed in oxidation of glycosyl units at the highest insulin concentration (Fig. 4).
Figure 4. Glucose metabolism.
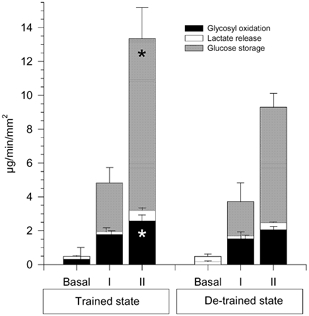
Seven young, healthy men carried out a heavy resistance training programme for 90 days (trained state) and thereafter abstained from training for a further 90 days (de-trained state). Oxidative and non-oxidative leg glucose metabolism was calculated by means of indirect calorimetry before (basal) and during steps I and II in euglycaemic, hyperinsulinaemic clamps in the trained and de-trained states. Data are means ± S.E.M. * Significant difference between the trained and de-trained states (P < 0.05).
DISCUSSION
The aim of this study was not to demonstrate that resistance training, like endurance training, increases whole body insulin action. Many studies have shown this previously (Yki-Järvinen & Koivisto, 1983; Miller et al. 1994; Zachwieja et al. 1996; Eriksson et al. 1998; Ishii et al. 1998; Takala et al. 1999) and the present study confirms these findings. The aim was rather to establish if skeletal muscle specifically also adapts qualitatively to a de-training resistance programme, or if the changes in insulin action are merely due to increases in muscle mass.
There are currently two opposing views. On the one hand, it is claimed that insulin-stimulated whole body glucose uptake increases with resistance training, even when differences in fat mass are corrected for or where no differences in body composition between the groups exist (Miller et al. 1994; Zachwieja et al. 1996; Eriksson et al. 1998; Ishii et al. 1998). On the other hand, two cross-sectional studies using sedentary people and weight lifters report opposite findings (Yki-Järvinen & Koivisto, 1983; Takala et al. 1999). In one (Takala et al. 1999), the glucose uptake rate in skeletal muscle was indirectly estimated by a glucose tracer and positron emission tomography.
In the present study we have used a different approach. Glucose uptake rates were estimated by the glucose clamp technique, but in addition leg glucose uptake was measured by the leg balance technique. Although the leg contains non-muscular tissue, skeletal muscle is predominant, and - metabolically speaking - the leg consists almost entirely of muscle. It is difficult to measure precisely (in vivo) the amount of skeletal muscle in one leg, and instead of estimating the volume of the leg by circumference measures or by water displacement we chose to obtain one MRI scan of the mid-thigh and by visual inspection and planimetry we aimed to accurately determine the skeletal muscle area and subsequently relate the glucose uptake data to this area. Due to the costs we were not in a position to obtain complete MRI scans of the whole leg. However, since we used MRI scans from a portion of the leg where the largest changes in muscle mass would be expected (mid-thigh), we have in fact made an over-correction of the data, and thus we have underestimated the difference in insulin action between the two states of training.
We are therefore now able to conclude that insulin-stimulated glucose uptake rates per unit of muscle mass decrease significantly with de-training (Fig. 3). It therefore seems likely that resistance training increases skeletal muscle insulin action - in line with the well-known effects of endurance training. We did not find any significant correlation between changes in leg glucose uptake rates and changes in muscle mass (data not shown), which supports the conclusion that the effect of resistance training cannot be ascribed to a mere increase in fat-free mass.
Thus, resistance training may also be considered in the treatment of insulin resistance, and not only with a view to increasing the muscle mass. Resistance exercise is probably very attractive to the typically overweight type 2 diabetic patient, who may be reluctant to take endurance exercise. The intensity of the resistance exercise does not necessarily need to be as heavy as in the present study. An increase in insulin action of 23–48 % has been found to occur with light-to-moderate resistance exercise (2–3 sets of 8–20 repetitions at 40–60 % of RM, 3–5 times a week) (Eriksson et al. 1998; Ishii et al. 1998), but interestingly, moderate-to-heavy resistance exercise (1–4 sets of 4–15 repetitions at 75–90 % RM, 3–4 times a week) has not been shown to be more efficient in terms of increasing insulin action (increases of 22–24 %) (Miller et al. 1994; Zachwieja et al. 1996).
Data for whole-body glucose uptake rates during the two steps of the clamp showed that a significant difference between the trained and de-trained states was only obtained at clamp step 2, with the highest insulin concentration (Fig. 3). At clamp step 1, almost identical glucose infusion rates were achieved in the final part, but clearly the onset of insulin action was slower in the de-trained state (Fig. 3). The implications for patients with type 2 diabetes may at first glance seem trivial, since whole body glucose uptake rates were only significantly different at the high (supra-physiological) insulin concentration, and were not significantly different at the lower insulin concentration. However, the insulin concentration at clamp step 1 was not at the high end of the physiological insulin concentration among patients with type 2 diabetes, in whom insulin concentrations more than twice as high (800–900 pM) are frequently seen in the post-prandial phase (Larsen et al. 1997).
Insulin-stimulated blood flow per unit of muscle was not different in the trained compared with the de-trained state. This is in contrast to findings where endurance training has been used (Dela et al. 1992). This difference in blood flow response between endurance- and resistance-trained muscle, may be partly explained by the fact that endurance training is more likely to induce capillary growth. It is furthermore consistent with our finding of unchanged capillary density in the present study (Fig. 2). The difference in leg glucose uptake therefore reflected a difference in glucose extraction (main effect P < 0.05) (Fig. 3). One might then expect that the amount of GLUT4 protein changed in parallel, but although this was not measured in the present study due to lack of biopsy material, the data showing unchanged GLUT4 mRNA levels do not indicate that this occurred (Fig. 1). Whether or not GLUT4 protein levels change in response to resistance training is at present unclear. In one study, 14 days of resistance de-training did not change GLUT4 protein levels, even though indices of insulin sensitivity diminished (Houmard et al. 1993). In contrast, Tabata et al. (1999) showed that even mild resistance training every day during 19 days of bedrest (which itself decreases GLUT4 protein) resulted in a 30 % increase in GLUT4 protein levels. The precise mechanism behind the resistance training-induced qualitative improvement in insulin action is therefore not clear, but may be related to differences in GLUT4 protein localisation in trained vs. untrained skeletal muscle. The effect of resistance training and/or de-training may also be downstream in the insulin signalling pathway. Thus, in healthy people and in patients with type 2 diabetes undergoing a resistance training programme, we have recently found significant increases in protein kinase B and glycogen synthase content in the skeletal muscle (F. Dela, unpublished data).
Indirect calorimetric calculations revealed that the major difference in leg glucose handling in the two states of training was a decrease in non-oxidative glucose disposal with de-training (Fig. 4). With endurance training the major effect is also in non-oxidative glucose disposal (Dela et al. 1992), and is usually explained by a training-induced increase in glycogen synthase (GS) enzyme activity (Vestergaard et al. 1994) and in GS mRNA (Dela et al. 1994). In the present study, maximal glucose storage capacity decreased with de-training, as indicated by lower levels of non-oxidative glucose disposal at maximal insulin concentrations. This finding, and also the lower whole body glucose uptake rates at clamp step 2, must reflect post-insulin receptor adaptations to de-training. Such adaptations will also be operating at submaximal insulin levels. Correspondingly, at submaximal insulin concentrations glucose storage was also lower, although not significantly so. Furthermore, glycogen content in the skeletal muscle, which reflects postprandial glucose storage at submaximal insulin concentrations, was significantly lower in the de-trained state. In the present study we could not detect changes in GS mRNA content, and since the muscle biopsies were obtained in the basal, non-insulin-stimulated state, an increase in GS enzyme activity would probably not have been seen. Biochemical support for the calorimetric data is therefore not available in the present study, and to our knowledge is not available in the literature.
The muscle biopsies were further analysed for CS, HAD, LDH and PFK enzyme activity. Changes in these enzyme activities are considered a hallmark of muscle adaptation to endurance training, and therefore the lack of such changes indicates that endurance training was not taken up by the subjects, as compensation for the termination of the strength training programme.
Due to the design of the study we can only draw conclusions about the effects of de-training. The training-induced adaptations in glucose metabolism may not be exactly the opposite of those seen with de-training. Furthermore, since we did not perform pre-training experiments, we cannot be sure that residual effects of the training were not present after 90 days of de-training. However, from endurance training studies it is known that the training effect on glucose metabolism is short lived, lasting about 1 week (Dela et al. 1992), making it unlikely that any residual effects were still present after de-training. Another possibility could be that the training effect was not due to a long-term adaptation, but rather was the effect of the last resistance exercise bout (2 days prior). In contrast to endurance exercise studies, where an acute effect of exercise has been shown to last 48 h, but not 5 days (Mikines et al. 1988b), with resistance exercise in the present study this possibility is less likely.
In conclusion, we have demonstrated that abstaining from resistance training for 90 days results in attenuation of insulin-stimulated glucose uptake in the whole body, and more importantly, an attenuation of insulin-stimulated glucose uptake per unit of skeletal muscle. Thus, in response to the cessation of resistance training, skeletal muscle adapts qualitatively with decreased insulin sensitivity.
Acknowledgments
The financial support of the Danish National Research Foundation (J. nr. 504–14), the Danish Diabetes Association, the Novo-Nordisk Foundation, the Direktør Jacob Madsen & hustru Olga Madsens Fond and The Foundation of 1870 is gratefully acknowledged. Regitze Kraunsøe, Jeppe Bach, Vigdis Hoel Christie and Ann-Christina Henriksen are thanked for excellent technical assistance.
REFERENCES
- Andersen JL, Aagaard P. Myosin heavy chain IIX overshoot in human skeletal muscle. Muscle Nerve. 2000;23:1095–1104. doi: 10.1002/1097-4598(200007)23:7<1095::aid-mus13>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Craig BW, Everhart J, Brown R. The influence of high-resistance training on glucose tolerance in young and elderly subjects. Mech Ageing Develop. 1989;49:147–157. doi: 10.1016/0047-6374(89)90098-5. [DOI] [PubMed] [Google Scholar]
- Dela F. On the influence of physical training on glucose homeostasis. Acta Physiol Scand. 1996;158:5–41. [PubMed] [Google Scholar]
- Dela F, Larsen JJ, Mikines KJ, Galbo H. Normal effect of insulin to stimulate leg blood flow in NIDDM. Diabetes. 1995;44:221–226. doi: 10.2337/diab.44.2.221. [DOI] [PubMed] [Google Scholar]
- Dela F, Mikines KJ, Galbo H. Physical activity and insulin resistance in man. In: Reaven GM, Laws A, editors. Insulin Resistance. Totowa, NJ, USA: Blackwell Science Inc; 1998. pp. 97–120. [Google Scholar]
- Dela F, Mikines KJ, Linstow VM, Secher NH, Galbo H. Effect of training on insulin mediated glucose uptake in human skeletal muscle. Am J Physiol. 1992;263:E1134–1143. doi: 10.1152/ajpendo.2006.263.6.E1134. [DOI] [PubMed] [Google Scholar]
- Dela F, Ploug T, Handberg A, Petersen LN, Larsen JJ, Mikines KJ, Galbo H. Physical training increases muscle GLUT-4 protein and mRNA in patients with NIDDM. Diabetes. 1994;43:862–865. doi: 10.2337/diab.43.7.862. [DOI] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson J, Tuominen J, Valle T, Sundberg S, Sovijarvi, Lindholm H, Tuomilehto J, Koivisto V. Aerobic endurance exercise or circuit-type resistance training for individuals with impaired glucose tolerance. Horm Metab Res. 1998;30:37–41. doi: 10.1055/s-2007-978828. [DOI] [PubMed] [Google Scholar]
- Heled Y, Shapiro Y, Shani Y, Moran DS, Langzam L, Braiman L, Sampson SR, Meyerovitch J. Physical exercise prevents the development of type 2 diabetes mellitus in Psammomys obesus. Am J Physiol Endocrinol Metab. 2002;282:E370–375. doi: 10.1152/ajpendo.00296.2001. [DOI] [PubMed] [Google Scholar]
- Higginson J, Wackerhage H, Woods N, Schjerling P, Ratkevicius A, Grunnet N, Quistorff B. Blockades of mitogen-activated protein kinase and calcineurin both change fibre-type markers in skeletal muscle culture. Pflugers Arch. 2002;445:437–443. doi: 10.1007/s00424-002-0939-1. [DOI] [PubMed] [Google Scholar]
- Houmard JA, Hortobagyi T, Neufer PD, Johns RA, Fraser DD, Israel RG, Dohm GL. Training cessation does not alter GLUT-4 protein levels in human skeletal muscle. J Appl Physiol. 1993;74:776–781. doi: 10.1152/jappl.1993.74.2.776. [DOI] [PubMed] [Google Scholar]
- Ingelbrecht IL, Mandelbaum CI, Mirkov TE. Highly sensitive northern hybridization using a rapid protocol for downward alkaline blotting of RNA. Biotechniques. 1998;25:420–426. doi: 10.2144/98253st03. [DOI] [PubMed] [Google Scholar]
- Ishii T, Yamakita T, Sato T, Tanaka S, Fujii S. Resistance training improves insulin sensitivity in NIDDM subjects without altering maximal oxygen uptake. Diabetes Care. 1998;21:1353–1355. doi: 10.2337/diacare.21.8.1353. [DOI] [PubMed] [Google Scholar]
- Ivy JL, Zderich TW, Fogt DL. Prevention and treatment of non-insulin-dependent diabetes mellitus. In: Holloszy JO, editor. Exercise and Sport Sciences Reviews. New York: Blackwell Science Inc; 1999. pp. 1–35. [PubMed] [Google Scholar]
- Jonsdottir IH, Schjerling P, Ostrowski K, Asp S, Richter EA, Pedersen BK. Muscle contractions induce interleukin-6 mRNA production in rat skeletal muscles. J Physiol. 2000;528:157–163. doi: 10.1111/j.1469-7793.2000.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen JJ, Dela F, Kjær M, Galbo H. The effect of moderate exercise on postprandial glucose homeostasis in NIDDM patients. Diabetologia. 1997;40:447–453. doi: 10.1007/s001250050699. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. New York: Blackwell Science Inc; 1972. pp. 1–291. [Google Scholar]
- Mikines KJ, Farrell PA, Sonne B, Tronier B, Galbo H. Postexercise dose-response relationship between plasma glucose and insulin secretion. J Appl Physiol. 1988a;64:988. doi: 10.1152/jappl.1988.64.3.988. [DOI] [PubMed] [Google Scholar]
- Mikines KJ, Sonne B, Farrell PA, Tronier B, Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol. 1988b;254:E248–259. doi: 10.1152/ajpendo.1988.254.3.E248. [DOI] [PubMed] [Google Scholar]
- Miller JP, Pratley RE, Goldberg AP, Gordon P, Rubin M, Treuth MS, Ryan AS, Hurley BF. Strength training increases insulin action in healthy 50- to 65-yr-old men. J Appl Physiol. 1994;77:1122–1127. doi: 10.1152/jappl.1994.77.3.1122. [DOI] [PubMed] [Google Scholar]
- Miller WJ, Sherman WM, Ivy JL. Effect of strength training on glucose tolerance and post-glucose insulin response. Med Sci Sports Exerc. 1984;16:539–543. [PubMed] [Google Scholar]
- Narici MV, Hoppeler H, Kayser B, Landoni L, Claassen H, Gavardi C, Conti M, Cerretelli P. Human quadriceps cross-sectional area, torque and neural activation during 6 months strength training. Acta Physiol Scand. 1996;157:175–186. doi: 10.1046/j.1365-201X.1996.483230000.x. [DOI] [PubMed] [Google Scholar]
- Qu Z, Andersen JL, Zhou S. Visualisation of capillaries in human skeletal muscle. Histochem Cell Biol. 1997;107:169–174. doi: 10.1007/s004180050101. [DOI] [PubMed] [Google Scholar]
- Schjerling P. The importance of internal controls in mRNA quantification. J Appl Physiol. 2001;90:401–402. doi: 10.1152/jappl.2001.90.1.401. [DOI] [PubMed] [Google Scholar]
- Smutok MA, Reece C, Kokkinos PF, Farmer CM, Dawson PK, Devane J, Patterson J, Goldberg AP, Hurley BF. Effects of exercise training modality on glucose tolerance in men with abnormal glucose regulation. Int J Sport Med. 1994;15:283–289. doi: 10.1055/s-2007-1021061. [DOI] [PubMed] [Google Scholar]
- Smutok MA, Reece C, Kokkinos PF, Farmer C, Dawson P, Shulman R, Devane-Bell J, Patterson J, Charabogos C, Goldberg AP. Aerobic versus strength training for risk factor intervention in middle- aged men at high risk for coronary heart disease. Metabolism. 1993;42:177–184. doi: 10.1016/0026-0495(93)90032-j. [DOI] [PubMed] [Google Scholar]
- Szczypaczewska M, Nazar K, Kaciuba-Uscilko H. Glucose tolerance and insulin response to glucose load in body builders. Int J Sports Med. 1989;10:34–37. doi: 10.1055/s-2007-1024870. [DOI] [PubMed] [Google Scholar]
- Tabata I, Suzuki Y, Fukunaga T, Yokozeki T, Akima H, Funato K. Resistance training affects GLUT-4 content in skeletal muscle of humans after 19 days of head-down bed rest. J Appl Physiol. 1999;86:909–914. doi: 10.1152/jappl.1999.86.3.909. [DOI] [PubMed] [Google Scholar]
- Takala TO, Nuutila P, Knuuti J, Luotolahti M, Yki-Kärvinen H. Insulin action on heart and skeletal muscle glucose uptake in weight lifters and endurance athletes. Am J Physiol. 1999;276:E706–711. doi: 10.1152/ajpendo.1999.276.4.E706. [DOI] [PubMed] [Google Scholar]
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- Vestergaard H, Andersen PH, Lund S, Schmitz O, Junker S, Pedersen O. Pre- and posttranslational upregulation of muscle-specific glycogen synthase in athletes. Am J Physiol. 1994;266:E92–101. doi: 10.1152/ajpendo.1994.266.1.E92. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H, Koivisto VA. Effects of body composition on insulin sensitivity. Diabetes. 1983;32:965–969. doi: 10.2337/diab.32.10.965. [DOI] [PubMed] [Google Scholar]
- Zachwieja JJ, Toffolo G, Cobelli C, Bier DM, Yarasheski KE. Resistance exercise and growth hormone administration in older men: effects on insulin sensitivity and secretion during a stable-label intravenous glucose tolerance test. Metabolism. 1996;45:254–260. doi: 10.1016/s0026-0495(96)90063-3. [DOI] [PubMed] [Google Scholar]


