Abstract
It has been suggested that magnesium deficiency is correlated with many diseases. 31P NMR experiments were carried out in order to investigate the effects of Na+ substitution on Mg2+ depletion in smooth muscle under divalent cation-free conditions. In the taenia of guinea-pig caeci, the intracellular free Mg2+ concentration ([Mg2+]i) was estimated from the chemical shifts of (1) the β-ATP peak alone and (2) β- and γ-ATP peaks. Both estimations indicated that [Mg2+]i decreased only very slowly in Mg2+-free, Ca2+-free solutions in which Na+ was substituted with large cations such as NMDG (N-methyl-D-glucamine) and choline. Furthermore, the measurements of tension development supported the suggestion of preservation of intracellular Mg2+ with NMDG substitution. Substituting extracellular Na+ with the small cation, Li+, also shifted the β-ATP peak towards a lower frequency, but the frequency shift was significantly less than that seen upon Na+ substitution with K+. The estimated [Mg2+]i depletion was, however, comparable with that seen after Na+ substitution with K+ using the titration curves of metal-free and Mg2+-bound ATP obtained in Li+-based model solutions. It was concluded that Mg2+ rapidly decreases only when small cations were the major electrolyte of the extracellular medium. Na+ substitutions with NMDG, choline or Li+ had little effect on intracellular ATP concentration after 100 min treatment.
Epidemiological studies suggest that magnesium is involved in many important diseases, e.g. diabetes mellitus, hypertension, cardiovascular and cerebrovascular diseases (Ebel & Günther, 1983; Shattock et al. 1987; Paolisso & Barbagallo 1997; Yang, 1998; Kao et al. 1999; McGuigan et al. 2002). Magnesium deficiency is normally considered as a risk factor for these diseases. One of the important roles of Mg2+ is presumably the antagonistic effect on Ca2+ movement. For example, intracellular Mg2+ is known to suppress Ca2+-induced Ca2+ release through ryanodine receptors (Ogawa et al. 2000, 2002), store-operated Ca2+ influx (or current: ICRAC) (Kerschbaum & Cahalan, 1998; Braun et al. 2000), etc. The role for Mg2+ as an intracellular signalling molecule, especially as a chronic regulator, is becoming established.
The guinea-pig taenia caeci is a smooth muscle-rich tissue (Gabella, 1981), and can be a good model for [Mg2+]i measurements using 31P NMR to assess the role of Mg2+, a signalling molecule affecting intracellular homeostasis. We have previously described several properties of Mg2+ depletion in this smooth muscle by changing the extracellular divalent cation concentrations. The intracellular free-Mg2+ concentration ([Mg2+]i) was rapidly decreased only when extracellular Mg2+ and Ca2+ were simultaneously removed from the extracellular solution and this [Mg2+]i depletion was not prevented by removal of Na+ (K+ substitution; Nakayama & Tomita, 1990, 1991). These observations imply the presence of a passive Mg2+ efflux pathway that is blocked by extracellular Ca2+, and is not coupled with Na+ flux. Similar passive Mg2+ flux pathways have also been reported in cardiac myocytes (Handy et al. 1996). It is thought that the passive Mg2+ pathways, along with Na+-Mg2+ exchange, which can pump Mg2+ out of the cell using energy from the Na+ gradient across the plasma membrane (Nakayama & Tomita, 1991; Nakayama et al. 1994), regulate [Mg2+]i together, and thereby can alter the smooth muscle cell function.
In the present study, using 31P NMR, we investigated properties of Mg2+ depletion in terms of extracellular monovalent cations. Extracellular Na+ was substituted with Li+, NMDG (N-methyl-D-glucamine) or choline. Tension development was also measured to reinforce 31P NMR measurements. The intracellular free-Mg2+ concentration ([Mg2+]i) was estimated from the chemical shifts of (1) the β-ATP peak alone and (2) β- and γ-ATP peaks. In the former, pHi obtained from the PME (phosphomonoester)-1 peak was used to correct the [Mg2+]i value, while in the latter, [Mg2+]i and pHi were concomitantly estimated by solving simultaneous equations. In the present [Mg2+]i estimation we mainly used the pH dependence of the apparent dissociation constant of MgATP described by Zhang et al. (1997), and occasionally used that described by Bock et al. (1985) to compare our previous estimation of [Mg2+]i. The results suggested that entry of certain cations was required to rapidly decrease [Mg2+]i even under Ca2+-free, Mg2+-free conditions. Further, it was shown that care should be taken with [Mg2+]i estimation in Li+-rich solutions, because this monovalent cation significantly alters the chemical shifts of metal-free and Mg2+-bound β-ATP.
Recently, molecular cloning and functional characterization of a Mg2+- and Ca2+-permeable divalent cation channel, LTRPC7, has been reported (Nadler et al. 2001; Hermosura et al. 2002).
METHODS
Animals
Guinea-pigs (300–400 g) of either sex were killed by cervical dislocation and exsanguination following stunning, and the taeniae were dissected from the caecum. All procedures conformed with the Guiding Principles for the Care and Use of Animals in the Field of Physiological Sciences published by The Physiological Society of Japan, Japanese Government Animal Protection and Managing Law (No. 105) and Japanese Government Notification on Methods on Sacrificing Animals (No. 40).
31P nuclear magnetic resonance
The taeniae weighing 0.4–0.6 g were isometrically mounted in a sample tube of 10 mm diameter, and were superfused with physiological saline solutions at a constant flow rate of 12 ml min−1. The temperature in the sample tube was kept at 32 °C.
Changes in phosphorus compounds were measured using 31P NMR. A NMR spectrometer (JEOL GSX270W, Tokyo, Japan) was operated at 109.4 MHz. Radiofrequency pulses corresponding to a flip angle of 30 deg were repeated at 0.6 s intervals. 31P NMR spectra were normally obtained by accumulation of 2500 free induction decays (FIDs) over 25 min, and a line broadening of 15 Hz was applied. Spectral peak resonances were measured relative to that of PCr (phosphocreatine) in parts per million (p.p.m.). Digital resolution was set to be approximately 0.005 p.p.m.
Measurements were started after equilibrating preparations in ‘normal’ solution for at least 100 min. Under exposure to the ‘normal’ solution, six major peaks were observed in the guinea-pig taenia caeci as described previously (Nakayama & Tomita, 1990, 1991): phosphomonoesters (PME), inorganic phosphate (Pi), PCr and the γ-, α- and β-peaks of ATP. The PME consisted of two peaks resonating at around 6.8 and 6.3 p.p.m., and they were assigned as PME-1 and −2. Concentrations of the phosphorous compounds were estimated by integrating the spectral peak areas (Nakayama et al. 1988, 1995).
The composition of the normal solution was as follows (mM): NaCl, 137.9; KHCO3, 5.9; CaCl2, 2.4; MgCl2, 1.2; glucose, 11.8; Hepes (N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid), 5; pH adjusted to 7.4–7.5 at 32 °C. The ionic composition was modified iso-osmotically. Mg2+- and Ca2+-free solutions contained 1 mM EDTA. Ca2+-free solutions contained 0.1 mM EGTA (ethyleneglycol-bis-(β-aminoethylether)N,N,N′,N′-tetraacetic acid). The solutions used in the NMR experiments were aerated with oxygen gas.
Estimation of [Mg2+]i
The intracellular free-Mg2+ concentration ([Mg2+]i) was estimated from the ratio of metal-free to total ATP (Φ), assuming the dissociation constant of MgATP (KD,MgATP; Dillon, 1996):
| (1) |
The metal free to total ATP ratio is expressed by the chemical shift of the observed ATP peak (δo):
| (2) |
where δf and δb are the chemical shifts of metal-free and Mg2+-bound forms of ATP, respectively. Since among the three ATP peaks chemical shift of β-ATP (δoβ) is mostly shifted by changes in Mg2+ binding, this ATP peak was mainly used to estimate [Mg2+]i in the present study:
| (3) |
KD,MgATP is actually a pH-dependent function: KD,MgATP(pH). Recently, using Mg2+-sensitive electrodes, McGuigan's group (Zhang et al. 1997) has re-evaluated KD,MgATP values at 25 and 37 °C. Both KD,MgATP at 25 °C (KD,MgATP,25 (pH)) and 37 °C (KD,MgATP,37 (pH)) are expressed as quadratic functions of pH:
| (4a) |
| (4b) |
In the present [Mg2+]i estimation, we mainly used a KD,MgATP(pH) function at 32 °C (KD,MgATP,32 (pH)) derived from these new pH functions of KD,MgATP at 25 and 37 °C using the van't Hoff isochore (Nakayama et al. 2002).
| (4c) |
where ψ= (1/TC−1/TB)/(1/TA−1/TB), and TA, TB and TC are absolute temperatures of 25, 37 and 32 °C, respectively.
The new estimation procedures provide values that are approximately twice those of KD,MgATP,32 in the pH range of 6.5–8 compared with those used in our previous study. As a result, the estimated [Mg2+]i values using the new KD,MgATP,32 (pH) are proportionally increased. It may be noteworthy that the resting [Mg2+]i calculated by use of the new KD,MgATP,32 (pH) is similar to that measured with a Mg2+-sensitive fluorescent dye (Tashiro & Konishi, 1997).
KD,MgATP(pH) can be also calculated from a measured apparent dissociation constant KDa,MgATP at a specific pH (pHa) (Bock et al. 1985):
| (5) |
where pK is minus the log of dissociation constant between H+ and ATP. In some [Mg2+]i estimations, we also applied a KD,MgATP,32 of 41 μM (pH 7.2; Nakayama & Tomita, 1990) in order to compare our previous studies.
As described previously (Nakayama et al. 1994), the chemical shifts of metal-free and Mg2+-bound β-ATP are expressed as sigmoid functions of pH (δfβ(pH); δbβ(pH)). Thus, the estimation of [Mg2+]i was corrected by intracellular pH (pHi):
| (6) |
where Fβ represents a pH function for β-ATP. The pHi can be estimated from the chemical shifts of the inorganic phosphate (Pi) (pKa 6.70; chemical shifts of H2PO4− and HPO42−= 3.15 and 5.72 p.p.m., respectively; Nakayama & Tomita, 1990) and PME-1 peaks (phosphorylethanolamine: pKa 5.70; chemical shifts of protonated and deprotonated form = 3.27 and 6.95 p.p.m., respectively: Nakayama & Tomita, 1991; Nakayama et al. 1995). Since the Pi peak was often hard to resolve under exposure to Mg2+-free, Ca2+-free solutions, the chemical shift of the PME-1 peak was normally used to estimate pHi.
Analogous with the eqn (6), [Mg2+]i is expressed as a function of the chemical shift of the observed γ-ATP peak (δoγ) and pHi:
| (7) |
where Fγ represents a pH function for γ-ATP. In the present study, in order to estimate [Mg2+]i, we primarily used eqn (6) (the chemical shift of the β-ATP peak alone) with the pHi obtained from the PME-1 peak. However, we also solved simultaneous eqns of (6) and (7) in order to determine whether the changes in the chemical shift of the ATP peaks systematically account for the intracellular ionic environment. (Thus, we could suggest intracellular K+ was nearly completely replaced with Li+, when extracellular Na+ was substituted with Li+ under divalent cation-free conditions.)
As described above, the chemical shift of metal-free ATP (δf) is expressed as a sigmoidal function of pH:
| (8) |
where δf,p and δf,d are the chemical shifts of protonated and deprotonated forms of metal-free ATP, respectively, nH is the Hill coefficient and pK is minus the log of the dissociation constant between H+ and ATP. A similar sigmoid function is derived for the pH dependency of Mg2+-bound ATP (δb(pH)) using the parameters. In Li+-based model solutions, the an-isotropies of δf(pH) and δb(pH) significantly differed from those previously obtained in K+-based solutions (Fig. 1B). Thus, when extracellular Na+ was replaced with Li+ in Mg2+-free, Ca2+-free solutions, the sigmoid curves obtained in the Li+-based model solutions were used for [Mg2+]i estimation. On the other hand, the Na+-rich condition had little effect on the pH dependency of δfβ(pH) and δbβ(pH) compared with those in K+-based solution (Fig. 1A). The four parameters used in curve fitting are listed in Table 1. The composition of the Na+-based model solutions was as follows (mM): NaCl, 150; Hepes, 10; ATP, 5; PCr, 1. EDTA (1 mM) and MgCl2 (10 mM) were added in metal-free ATP and Mg2+-bound ATP solutions, respectively. For Li+-based model solutions 150 mM LiCl was used instead of NaCl.
Figure 1. Titration data for metal free- (filled square) and Mg2+-bound ATP (filled circle).
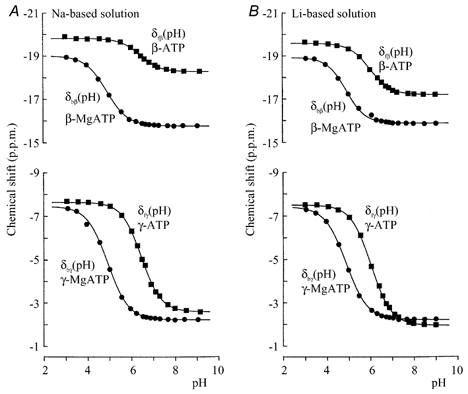
The data points are fitted by sigmoid curves (eqn (8)). The fitting parameters are shown in Table 1. A Na+-based solution was used in A. In B, Li+ was used instead of Na+.
Table 1.
Fitting parameters for pH dependency of the chemical shifts of ATP shown in Fig. 1
| δf,d | δf,p | ηH | pK | ||
|---|---|---|---|---|---|
| A. Na+-based solution | |||||
| β-peak | Free-ATP (δf) | –18.27 | –19.80 | –0.09 | 6.46 |
| MgATP (δb) | –15.76 | –19.01 | –0.90 | 4.91 | |
| γ-peak | Free-ATP(δf) | –2.59 | –7.63 | –0.98 | 6.46 |
| MgATP(δb) | –2.21 | –7.45 | –0.92 | 4.93 | |
| B. Li+-based solution | |||||
| β-peak | Free-ATP(δf) | –17.20 | –19.59 | –0.93 | 6.01 |
| MgATP(δb) | –15.86 | –18.95 | –0.88 | 4.89 | |
| γ-peak | Free-ATP(δf) | –1.96 | –7.49 | –0.94 | 6.02 |
| Mg-ATP(δb) | –2.22 | –7.42 | –0.89 | 4.87 | |
| C. K+-based solution | |||||
| β-peak | Free-ATP(δf) | –18.59 | –19.79 | –1.00 | 6.48 |
| MgATP(δb) | –15.79 | –19.12 | –0.90 | 4.84 | |
| γ-peak | Free-ATP(δf) | –2.78 | –7.61 | –1.01 | 6.48 |
| MgATP(δf) | –2.26 | –7.57 | –0.92 | 4.86 | |
Cis reproduced from Table 1 in Nakayama et al (1994), nH Hill coefficient; pK,–log of the dissociation constant between H+ and ATP; δf,d and δf, p are the chemical shifts of protonated and deprotonated forms of metal-free forms of ATP, respectively.
Measurements of mechanical activity
Mechanical activity was measured in separate experiments. The taenia caeci (1.5–2 mm in width, 15 mm in length) were mounted in an organ bath, and continuously superfused with extracellular solutions at a constant flow rate of 2 ml min−1 (Nakayama & Tomita, 1990).
Statistics
Numerical data are expressed as means ± standard deviation. Differences between means were evaluated by Student's paired or unpaired t test, and a P value of less than 0.05 was taken as a statistically significant difference.
RESULTS
Tension development after exposure to Ca2+- and Mg2+-free solutions
Spontaneous tension development was measured in smooth muscle strips of the guinea-pig taenia caeci. Figure 2 shows typical responses to removal of intracellular divalent cations in the absence of Na+. In Fig. 2A, after observing control spontaneous mechanical activity, both extracellular Mg2+ and Ca2+ were removed, and Na+ was substituted with K+. This treatment completely terminated spontaneous activity after producing a transient rise in the tension development. After 100 min exposure to the divalent cation-free solution, reapplication of 2.4 mM Ca2+ (its normal concentration) to the extracellular medium produced only a small increase in the resting tension, but no spontaneous activity was observed. Subsequent addition of 1.2 mM Mg2+, in the presence of 2.4 mM Ca2+, also produced only a gradual, small increase in the resting tension development over 100 min. Returning the extracellular Na+ concentration to that of the control (superfusing normal solution) fully restored the tension development (n = 6) with some delay.
Figure 2. Effects of removal and reapplication of extracellular cations on spontaneous mechanical activity.
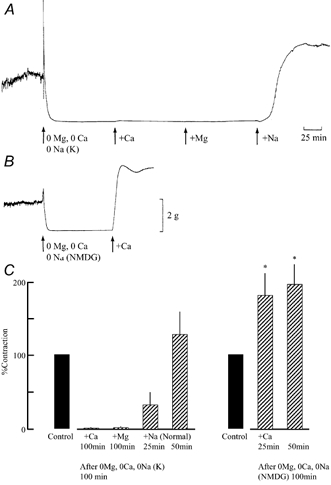
The extracellular divalent cations and Na+ were iso-osmotically replaced with K+ in (A) or with NMDG in (B). After 100 min exposure to divalent cation-free, Na+-free solutions, 2.4 m M Ca2+ was added (in both A and B). A, Mg2+ (1.2 mM) was added 100 min after the reapplication of Ca2+. Subsequently, after 100 min the Na+ concentration was returned to the initial concentration. The histogram in C shows averaged magnitude of mechanical response to the reapplication of extracellular cations (n = 6 for each experiment). The magnitude was normalized by taking the control mechanical activity (in ‘normal’ solution) as 100 %. * Significant difference (unpaired t-test, P < 0.05) compared with the mechanical response to Ca2+ reapplication in Mg2+-free, Ca2+-free solution (K+ substitution) at 100 min.
The divalent cation-free solution used in Fig. 2B also did not contain any Na+, but in this solution Na+ was substituted with NMDG. The treatment with this solution also abolished spontaneous mechanical activity after a transient rise in the tension development (Fig. 2B). In contrast to the substitution with K+, the subsequent reapplication of 2.4 mM Ca2+ alone after 100 min removal of extracellular divalent cations, restored tension development. Qualitatively the same responses to the reapplication of Ca2+ were observed in five other muscle strips. The histogram in Fig. 2C summarises the mechanical responses to reapplication of cations after treatment with a divalent cation-free, Na+-free solution. The magnitude of mechanical response to Ca2+ reapplication was statistically significantly larger for NMDG substitution.
Changes in [Mg2+]i under Ca2+- and Mg2+-free conditions
The differences that we observed in the contractile activity with Na+, K+ and NMDG substitutions suggest distinct changes in the intracellular environment including, but perhaps not limited to, changes in the high-energy phosphates and Mg2+. We thus applied 31P NMR in the smooth muscle preparation to study intracellular phosphorus compounds and relating ions better.
During exposure to the K+-rich, divalent cation-free solution, the β-ATP peak, as observed with 31P NMR, was significantly shifted towards lower frequency (to the right), and also became broad such that the chemical shift was difficult to determine with precision (Fig. 3A). The β-ATP peak in Fig. 3Ab is around −18.2 p.p.m. (100 min exposure). Due to a relatively slow chemical exchange rate between MgATP and free ATP, the width of the observed ATP peak increased as the observed peak got closer to the middle of the free and Mg2+-bound ATP peak positions (Nageswara Rao, 1984). Also, treatments which can induce large changes in [Mg2+]i, would enlarge the distribution of [Mg2+]i in multi-cellular preparations. Therefore, the observation of the broadening ATP peak was considered to be supporting evidence for a fall in [Mg2+]i. The γ- and α-ATP peaks also had clearly detectable leftward shifts. Further, the half-width of the PCr peak was unchanged, indicating that heterogeneity of the magnetic field could not account for the changes in ATP. We previously showed similar changes in ATP peaks when [Mg2+]i was decreased by removal of divalent cations in the presence of extracellular Na+ (Nakayama & Tomita, 1990; Nakayama & Nomura, 1995).
Figure 3. 31p NMR spectra obtained in divalent cation-free, Na+- free solutions.
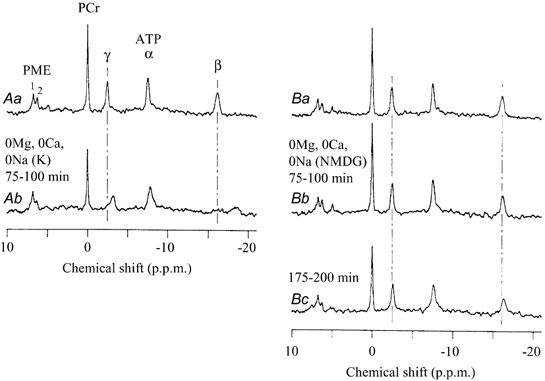
After observing control 31P NMR spectra (Aa and Ba), extracellular divalent cations and Na+ were iso-osmotically substituted with K+ for 100 min (A) or with NMDG for 200 min (B). Vertical dashed lines represent the initial chemical shifts of γ- and β-ATP. Each spectrum was obtained from the sum of 2500 NMR signals over 25 min. The 31P NMR spectra (Ab, Bb and Bc) were obtained during exposures to divalent cation-free, Na+-free solutions for 75–100 and 175–200 min, respectively.
The [Mg2+]i estimated from the chemical shift of β-ATP decreased from 0.87 ± 0.07 to 0.013 ± 0.006 mM (n = 4) after 100 min (filled squares in Fig. 4A). This estimation was performed by correcting the apparent dissociation constant of MgATP (KD,MgATP), and the chemical shifts of metal-free and Mg2+-bound β-ATP (δfβ(pH); δbβ(pH)) using pHi (7.06 ± 0.09 in control; 7.25 ± 0.09 after 100 min, n = 4) obtained from the chemical shift of PME-1 (Nakayama & Nomura, 1995). Simultaneous estimation of [Mg2+]i and pHi from the chemical shifts of the β- and γ-ATP peaks also revealed a similar reduction of [Mg2+]i (Table 2). Zhang et al. (1997) have reported a new equation to describe the pH dependence of KD,MgATP. In this study we mainly used a pH function for KD,MgATP (KD,MgATP(pH); eqn (4)) derived from that described by Zhang et al. (1997; see Methods). In order to compare our previous studies, another KD,MgATP(pH) described by Bock et al. (1985; eqn (5)) was used in Fig. 4B. [Mg2+]i in the control becomes approximately half, however, the time course of depletion under the Mg2+-free, Ca2+-free condition is the same (filled squares in Fig. 4B).
Figure 4. The time course of changes in [Mg2+]i during exposure to divalent cation-free, Na+-free solutions.
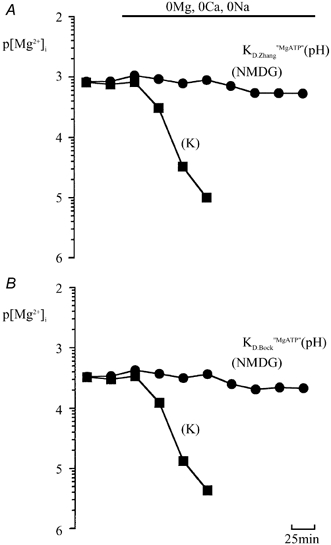
Changes in [Mg2+]i induced by substituting extracellular divalent cations and Na+ with K+ (filled square)and NMDG(filled circle). Each point represents the negative logarithm of the mean [Mg2+]i value (p[Mg2+]i). [Mg2+]i was estimated from the chemical shifts of the β-ATP and PME-1 peaks using eqn (6). The pH dependence of KD,MgATP described by Zhang et al. (1997) (KD,Zhang,MgATP(pH)) was used in A, while that described by Bock et al. (1984) (KD,Bock,MgATP(pH)) was used in B.
Table 2.
Changes in [Mg2+]i during exposure to a Mg2+-free, Ca2+-free solution in which Na+was substituted with K+
| Control (mm) | Mg2+-free, Ca2+-free (K+) 50 min (mM) | 100 min (mm) | |
|---|---|---|---|
| A. [Mg2+]i from β-ATP and PME—1 | 0.87 ± 0.07 | 0.29 ± 0.15* | 0.013 ± 0.006* |
| B. [Mg2+]i from β and γ-ATP | 0.778 ± 0.09 | 0.31 ± 0.20* | 0.010 ± 0.005* |
| C. [Mg2+]i using KD.mgATP described by Bock et al (1985) (eqn (5)) | 0.35 ± 0.03 | 0.12 ± 0.06* | 0.005 ± 0.003* |
[Mg2+]i was estimated with three methods (n = 4) A, [Mg2+]i was estimated from the chemical shift of β-ATP. KD,mgATP was corrected by pHi from the chemical shift of PME-1 using the KD,mgATP (pH) of Zhang et al. (1997) modified for 32 °C (Nakayama et al. 2002) (eqn (4c)). B, [Mg2+]i was estimated from the chemical shifts of β-and gamma-ATP peaks (Nakayama et al. 1994) using the same KD,mgATP(pH) as in A. C, [Mg2+]i was estimated bybthe same method as in A (from the chemical shifts of γ-ATP and PME-1), eqn (4c).
Significant difference when evaluated with paired t-test (P < 0.05).
NMDG is considered to be plasma membrane-impermeable (Flatman & Smith, 1990). In contrast to K+ substitution, when extracellular Na+ was replaced with equimolar NMDG, removal of Mg2+ and Ca2+ shifted the β-ATP peak towards lower frequency only very slowly (from −16.16 ± 0.01 to −16.20 ± 0.06 p.p.m. after 100 min, and to −16.39 ± 0.12 p.p.m. after 200 min, n = 3; Fig. 3B). This indicates a slow decrease in [Mg2+]i: 0.88 ± 0.01 mM in control; 0.86 ± 0.15 mM after 100 min; 0.51 ± 0.14 mM after 200 min, n = 3 (by using the chemical shifts of β-ATP and PME-1, and KD,MgATP(pH) of eqn (4); filled squares in Fig. 4A). During exposure to the divalent cation-free solution for 200 min, pHi changed from 7.04 ± 0.01 to 6.96 ± 0.09, while the concentrations of ATP and PCr decreased to approximately 74 and 81 % of the control, respectively (n = 3). The [Mg2+]i values estimated simultaneously from the chemical shifts of β- and γ-ATP, and using KD,MgATP of Bock et al (1985) (eqn (5)) are listed in Table 3. All methods of estimation indicate essentially the same tendency for a slow decrease in [Mg2+]i with NMDG substitution, although control [Mg2+]i is smaller in the case of KD,MgATP described by Bock et al. (1985; filled circles in Fig. 4B). In one experiment, instead of NMDG, we used choline chloride (with 10 μM atropine), which is also considered to be impermeable across the plasma membrane. This substitution of extracellular Na+ also only slowly decreased [Mg2+]i under a divalent cation-free condition: 0.78 mM in control; 0.60 mM after 100 min; 0.33 mM after 200 min.
Table 3.
Slow decrease in [Mg2+]i in a [Mg2+]i -free, Ca2+-free solution in which Na+ was substituted with NMDG
| Control (mM) | Mg2+-free, Ca2+-free (NMDG) 100 min (mM) | 200 min (mM) | |
|---|---|---|---|
| A. [Mg2+]i from β-ATP and PME—1 | 0.88 ± 0.01 | 0.86 ± 0.15* | 0.51 ± 0.14* |
| B. [Mg2+]i from β and γ-ATP | 0.84 ± 0.01 | 0.89 ± 0.21* | 0.53 ± 0.14* |
| C. [Mg2+]i using KD,mgATP described by Bock et al (1985) (eqn (5)) | 0.36 ± 0.01 | 0.35 ± 0.06* | 0.21 ± 0.06* |
The three methods used to estimate [Mg2+]i in A–C are the same as those used in Table 2.
Significant difference (P <0.05) from the control (n = 3).
As mentioned above, we have previously used K+ to substitute extracellular Na+ (Nakayama & Tomita, 1990). In the present study, we also substituted extracellular Na+ with Li+, which is often used to suppress Na+-Ca2+ exchange (e.g. Kimura et al. 1987), and is also known to be permeable to ion channels in the plasma membrane (e.g. Yang & Sachs, 1990). Figure 5 shows typical spectra obtained by exposures to a Mg2+-free, Ca2+-free solution in which Na+ was substituted with equimolar Li+. After 100 min the β-ATP peak shifted towards a lower frequency by only 0.24 p.p.m. (from −16.17 ± 0.03 to −16.43 ± 0.04 p.p.m., n = 3). According to the chemical shifts of metal-free and Mg2+-bound forms of ATP measured in Li+-based model solutions (Fig. 1), the observed chemical shift of β-ATP corresponds to a large decrease in [Mg2+]i from 0.79 ± 0.07 to 0.16 ± 0.03 mM after 100 min (n = 3, Table 4). It is noteworthy that [Mg2+]i depletion would be underestimated by applying the calibration curves obtained in K+- or Na+-based model solutions (Fig. 1C in Nakayama et al. 1994; Fig. 1 in the present study), due to the lower frequency shift of the deprotonated form of metal-free β-ATP in Li+-rich solutions (δf,d in Table 1B). Furthermore, after 100 min exposure to the (Li+-based) Mg2+-free, Ca2+-free medium, simultaneous estimation of [Mg2+]i and pHi from the chemical shifts of β- and γ-ATP (solving simultaneously eqns (6) and (7)) provided solutions, only when the calibration curves for Li+-based model solutions were applied. This suggested that intracellular K+ was almost fully replaced with Li+ after 100 min.
Figure 5. 31p NMR spectra obtained in a divalent cation-free, Na+-free solution which was made by iso-osmotically substituting with Li+.
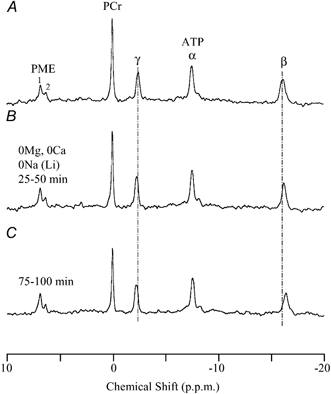
Vertical dashed lines represent the initial chemical shifts of γ- and β-ATP. The spectrum (a) shows control; (b) and (c) are 31P NMR spectra obtained during exposures to divalent cation-free, Na+-free solutions for 25–50 and 75–100 min, respectively.
Table 4.
Changes in [Mg2+]i in a Mg2+-free, Ca2+ free solution in which Na+ was substitued with Li+
| Control (mM) | Mg2+-free, Ca2+ free (Li+) 50 min (mM) | 100 min (mM) | |
|---|---|---|---|
| A. [Mg2+]i from β-ATP and PME—1 | 0.79 ± 0.07 | 0.30 ± 0.04* | 0.16 ± 0.03* |
| B. [Mg2+]i from β and γ-ATP | 0.75 ± 0.13 | 0.33 ± 0.05* | 0.17 ± 0.03* |
| C. [Mg2+]i using KD,mgATP described by Bock et al (1985) (eqn (5)) | 0.32 ± 0.03 | 0.12 ± 0.01* | 0.064 ± 0.010* |
The three methods used to estimate [Mg2+]i in A–C are the same as those used in Table 2. Calibration curves obtained in K+-based model solutions were used for control, while those in Li+ -based solutions were used for Mg+-free, Ca+-free conditions (for bothe and min).
Significant difference (P <0.05) from the control (n = 3).
Changes in intracellular ATP and pH
ATP is an important Mg2+ buffer and chelator, and is also reported to affect Mg2+ permeability across the plasma membrane (Nadler et al. 2001). On the other hand, pHi is likely to modulate the intracellular Mg2+ buffering capacity through competition between H+ and Mg2+ for intracellular cation-binding sites, including ATP-binding of Mg2+. The dissociation constant of MgATP used in the [Mg2+]i estimation involves a term of pHi (see eqn (4)). Table 5 compares intracellular ATP concentration ([ATP]=[MgATP + metal-free ATP]) and pHi 100 min after exposure to divalent cation-free, Na+-free solutions. Substitution of extracellular Na+ with a large cation, NMDG or choline, had little effect on [ATP] after 100 min: 99 ± 6 (n = 3) or 106 % (n = 1), respectively. When extracellular Na+ was replaced with K+, [ATP] decreased to 52 ± 8 % after 100 min (vs. control, n = 4). In contrast, when Li+, another small cation substitution was used, the reduction of [ATP] was much smaller (to 90 ± 2 %, n = 3).
Table 5.
[ATP] and ΔpHi after 100 min exposure to divalent cation-free Na+-free solutions
| Na+substitution | [ATP]% | ΔpHi | n |
|---|---|---|---|
| K+ | 52 ± 8 | 0.19 ± 0.05* | 4 |
| Li+ | 90 ± 2* | 0.33 ± 0.06* | 3 |
| NMDG | 99 ± 6* | –0.11 ± 0.02+ | 3 |
| Choline | 106 | –0.06 | 1 |
[ATP] is expressed as percentage of the control.
Significant difference (P < 0.05) compared with the change to K+ substitution; n, number of experiments.
The concentration of ATP was estimated by integrating the β-ATP peak area, because the γ- and α-ATP peaks involve a contribution from both ADP and nicotinamide adenine dinucleotide (NAD/NADH). The γ- and α-ATP peak areas were also reduced upon K+ substitution, suggesting a reduction of the adenosine pool.
When small monovalent cations were used as a substitute for Na+, pHi (estimated from the PME-1 peak) became alkaline during divalent cation-free, Na+-free treatments (Table 5; 0.19 ± 0.05 for K+ (n = 4) and 0.33 ± 0.06 for Li+ (n = 3) after 100 min). On the other hand, with large divalent cations pHi was relatively more stable and decreased slightly (−0.06 for choline (n = 1) and −0.11 ± 0.02 for NMDG (n = 3)).
DISCUSSION
When intracellular Mg2+ is depleted, Mg-bound ATP decreases and free ATP (and ADP) concentrations increase. These are all intracellular signalling molecules, which are known to control a variety of cellular functions, ranging from oxidative phosphorylation to contraction. During Mg2+ depletion, for example, respiration is impaired and Ca2+ movement is affected (Nakayama & Tomita, 1990). It is therefore suggested that Mg2+ homeostasis is an important metabolic component for intracellular metabolism.
31P NMR measurements demonstrated that simultaneous removal of extracellular Mg2+ and Ca2+ significantly decreased intracellular Mg2+, even though Na+ was replaced with K+ (Nakayama & Tomita, 1990, 1991; re-estimation in the present study). These results suggested that the passive Mg2+ pathway blocked by extracellular Ca2+, not Na+-Mg2+ exchange, played a major role in Mg2+ depletion under Mg2+-free, Ca2+-free conditions. In the present study, we have further examined effects of several cations as Na+ replacements on Mg2+ depletion. The experiments suggested that substitution of extracellular Na+ with NMDG or choline prevented intracellular Mg2+ depletion under divalent cation-free conditions, and that [Mg2+]i was significantly decreased when Li+ was used as a replacement.
In one of our previous studies (Nakayama & Tomita, 1991) we showed reapplication of Na+ was required to restore [Mg2+]i. This phenomenon is explained by Mg2+ influx through reverse mode Na+-Mg2+ exchange when the passive Mg2+ flux pathway is inhibited by Ca2+. (Na+-Mg2+ exchange is the main Mg2+ pathway in the presence of extracellular Ca2+). In the present study, the changes in the mechanical activity observed are considered to reflect the changes in [Mg2+]i. Hence after treatment with a divalent cation-free, Na+-free solution (K+ substitution) the mechanical activity was still impaired even when Mg2+ was reapplied in the presence of Ca2+, but was restored following reapplication of Na+ (Fig. 2B). Furthermore, when NMDG was used as a Na+ substitute, [Mg2+]i was not depleted following removal of divalent cations in the absence of Na+ (Fig. 3B), and reapplication of Ca2+ alone could recover mechanical activity. These results are consistent with the notion that [Mg2+]i is an important factor for the mechanical activity in smooth muscle.
Large cations, such as NMDG and choline, are known to be largely excluded from entering the cell (e.g. Flatman & Smith, 1990). On the other hand, Na+ and K+ ions, physiological cations, are permeable across the plasma membrane, and are also actively transported. Li+ is a small cation, and is known to permeate through several ion channels in the plasma membrane. Furthermore, after 50 min or longer exposure to a Mg2+-free, Ca2+-free solution in which Na+ was substituted with Li+, only the calibration curves obtained from Li+-based model solutions allowed us to estimate [Mg2+]i and pHi simultaneously from the chemical shifts of the β- and γ-ATP peaks. This suggests that intracellular cations were largely replaced with Li+ after 50 min. (Using 87Rb NMR, we previously measured changes in the intracellular Rb+ concentration, which could be used as an index of exchangeable intracellular K+ (Nakayama & Nomura, 1995). In the absence of extracellular Ca2+, application of ouabain decreased preloaded Rb+ to less than 20 % after 50 min. In the absence of both Ca2+ and Mg2+, intracellular K+ would be depleted more quickly by replacing extracellular Na+ with Li+. This result supported the deduction from the simultaneous estimation of [Mg2+]i and pHi.
In red blood cells, it has been reported that Mg2+ efflux is stimulated by a long exposure (several hours) to Mg2+- and Ca2+-free solution, despite substituting extracellular Na+ with NMDG (Flatman & Smith, 1990, 1996). The discrepancy between studies in red blood cells and smooth muscle (present study) may be explained by the fact that sizable amounts of ATP still remained 200 min after exposure to divalent cation-free solutions in smooth muscle, i.e. the reduction of intracellular Mg2+ capacity in red blood cells, due to ATP depletion, may increase [Mg2+]i, and consequently increase Mg2+ efflux (Flatman & Smith, 1996).
Recently, a Mg2+- and Ca2+ permeable channel has been described. This channel is regulated by changes in physiological concentrations of intracellular MgATP: MagNuM (Mg-nucleotide-regulated metal ion current = LTRPC7; Hermosura et al. 2002). This channel is ubiquitously expressed, therefore it is possibly involved in the Mg2+ depletion seen in our experiments. Since Mg2+ and Ca2+ are competitive in this channel, simultaneous removal of both cations would facilitate Mg2+ efflux. When extracellular Na+ was replaced with K+, removal of divalent cations from the extracellular medium also significantly decreased [Mg2+]i and was accompanied by a significant loss of [ATP]. On the other hand, when Na+ was substituted with Li+, NMDG or choline, the reduction of [ATP] was not significant, although only Li+ substitution caused a significant reduction in [Mg2+]i. The fact that [ATP] loss and changes in [Mg2+]i did not correlate with each other, suggested that the MgATP-dependent regulation of the LTRPC7 channel is not a primary contributor, even though this channel could be involved in the depletion of Mg2+. (Competition between Mg2+ and Ca2+ in this channel is considered to be a primary mechanism contributing to our observation.) Furthermore, [ATP] might be underestimated in the broader ATP peaks seen upon K+ substitution. Nevertheless, the [Mg-bound ATP] decreases in parallel with [Mg2+]i. It is probable that LTRPC7 would be further activated by reduction of MgATP (through recovery from MgATP-dependent blockage: Nadler et al. 2001), when [Mg2+]i is decreased to some extent, especially upon K+ or Li+ substitution.
It has also been shown that the reversal potential of the LTRPC7 current is close to zero (in the absence of extracellular divalent cations). If similar Mg2+-permeable channels are responsible for the Mg2+ depletion, Mg2+ efflux without accompanying influx of cations, i.e. upon substitution of Na+ with NMDG or choline, would hyperpolarize the cell membrane, and then consequently prevent Mg2+ efflux. (With a simple charge balance calculation, Mg2+ efflux of only 2.5 pC cell−1 (8–25 × 10−6) of [Mg2+]i alters the membrane potential by 50 mV, assuming a smooth muscle membrane capacitance of 50 pF.)
In addition to the maintenance of electric charge balance across the plasma membrane as described above, entry of some cations into the cell could be important to replace Mg2+ at anionic sites (Sparrow, 1969). Indeed, independent groups have reported competition between Mg2+ and Li+ as used in the present study, at intracellular anionic sites including ATP (Mota de Freitas et al. 1994; Gow et al. 1999). Another explanation is that large cations, such as NMDG and choline, may directly block the passive Mg2+ pathway. The contribution of this mechanism is, however, less possible, because [Mg2+]i significantly increased, even when Na+-Mg2+ exchange is blocked by replacing extracellular Na+ with NMDG in Ca2+-free, Mg2+-containing solutions (Nakayama & Tomita, 1991; Nakayama et al. 1994). Furthermore, it has been shown that substitution of extracellular Na+ with choline has little effect on the membrane current through LTRPC7 (Nadler et al. 2001), a candidate of passive Mg2+ pathway. (Only intracellular choline blocks outward LTRPC7 current.)
Zhang et al. (1997) have published a new pH-dependent dissociation constant of MgATP (KD,MgATP(pH)) that was directly measured with Mg2+-selective electrodes. We have mainly used this KD,MgATP(pH) in the present study. For some of the [Mg2+]i estimation, we have also used KD,MgATP(pH) of Bock et al. (1985) in order to compare [Mg2+]i values with those in our previous studies. This KD,MgATP(pH) provided about twofold smaller [Mg2+]i values in all experimental conditions; however the tendency of [Mg2+]i change in each substitution was essentially the same: removal of extracellular Mg2+ and Ca2+ depleted [Mg2+]i, when Na+ was substituted with K+ or Li+, but not when NMDG and choline were used (Tables 2–4). In ‘normal’ solution, the [Mg2+]i estimated using KD,MgATP(pH) of Zhang et al. (1997) and Bock et al. (1985) were ˜0.81 and ˜0.33 mM, respectively (Nakayama & Clark, 2003). In light of the numerous cellular functions in smooth muscle, e.g. mitochondrial respiration (Pyne et al. 2001), the former estimation method seems to be preferable (Nakayama & Clark, 2003), but the real value may be between the two estimations. Further evaluation is required in this respect.
Comparison between [Mg2+]i (Tables 2–4) and pHi (Table 5) suggests a tendency toward depletion of [Mg2+]i that is accompanied by intracellular alkalosis. Both [Mg2+]i and [H+] (=10-pH) significantly decreased with either K+ or Li+ substitution. On the other hand, [Mg2+]i only slowly decreases with NMDG or choline substitution, while pHi was stable or only decreased slightly. However, the intracellular alkalosis is not considered to be a primary (requisite) mechanism to induce the discrepancy of [Mg2+]i depletion between large and small cations used as a substitute for Na+, for the following reasons. (1) The rise of pHi to 7.4 seen with small cation substitutions was occasionally observed under conditions which do not significantly reduce [Mg2+]i, e.g. when Ca2+ alone was removed from the extracellular solution for an extended period of time (Nakayama & Nomura, 1995). (2) The degree of intracellular alkalosis was smaller for K+ (Table 5), while [Mg2+]i decreased by a greater degree (Tables 2 and 4). (3) Due to the pH dependence of the apparent dissociation constant of MgATP (KD,MgATP(pH); eqn (4)), the intracellular alkalosis would enhance the fall in [Mg2+]i, i.e. alkalosis decreases the value of the KD,MgATP, and consequently the [Mg2+]i estimate is less upon intracellular alkalosis, even though the metal-free ATP ratio is the same. The metal-free ATP ratios themselves were, however, significantly different between small and large monovalent cation substitutions, e.g. ˜87 % for K+ and ˜12 % for NMDG after 100 min.
In conclusion, using 31P NMR we have demonstrated that [Mg2+]i depletion caused by removal of extracellular Mg2+ and Ca2+ was prevented by substituting Na+ with large membrane-impermeable cations. It is suggested that rapid depletion of [Mg2+]i requires some cations to enter the cell. Measurements of spontaneous tension development supported this phenomenon on Mg2+ transport, although care should be taken in estimating [Mg2+]i, when Li+-rich solution is used.
Acknowledgments
The authors are grateful to Dr H. Fukatsu and Professors T. Ishigaki and T. Tomita, Nagoya University for pertinent help and advice. This work was supported by research grants from Monbusho International Scientific Research Program (Japan), and for Cardiovascular Diseases (11C-1) from the Ministry of Health and Welfare (Japan). J.F.C. is supported by the NIH HL67186 and NS42697.
REFERENCES
- Bock JL, Wenz B, Gupta RK. Changes in intracellular Mg adenosine triphosphate and ionized Mg2+ during blood storage: detection by 31P nuclear magnetic resonance. Blood. 1985;65:1526–1530. [PubMed] [Google Scholar]
- Braun FJ, Broad LM, Armstrong DL, Putney JW., Jr Stable activation of single CRAC-channels in divalent cation-free solutions. J Biol Chem. 2000;276:1063–1070. doi: 10.1074/jbc.M008348200. [DOI] [PubMed] [Google Scholar]
- Dillon PF. 31P Nuclear magnetic resonance spectroscopy. In: Bárány M, editor. Biochemistry of Smooth Muscle Contraction. San Diego USA: Blackwell Science Inc; 1996. pp. 393–404. [Google Scholar]
- Ebel H, Günther T. Role of magnesium in cardiac disease. J Clin Chem Clin Biochem. 1983;21:249–265. doi: 10.1515/cclm.1983.21.5.249. [DOI] [PubMed] [Google Scholar]
- Flatman PW, Smith LM. Magnesium transport in ferret red cells. J Physiol. 1990;431:11–25. doi: 10.1113/jphysiol.1990.sp018318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman PW, Smith LM. Magnesium transport in magnesium-loaded ferret red blood cells. Pflugers Arch. 1996;432:995–1002. doi: 10.1007/s004240050227. [DOI] [PubMed] [Google Scholar]
- Gabella G. Structure of smooth muscle. In: Bülbring E, Brading AF, Jones AW, Tomita T, editors. Smooth Muscle: An Assessment of Current Knowledge. London: Blackwell Science Inc; 1981. pp. 127–156. [Google Scholar]
- Gow IF, Flatman PW, Ellis D. Lithium induced changes in intracellular free magnesium concentration in isolated rat ventricular myocytes. Mol Cell Biochem. 1999;198:129–133. doi: 10.1023/a:1006973109874. [DOI] [PubMed] [Google Scholar]
- Handy RD, Gow IF, Ellis D, Flatman PW. Na-dependent regulation of intracellular free magnesium concentration in isolated rat ventricular myocytes. J Mol Cell Cardiol. 1996;28:1641–1651. doi: 10.1006/jmcc.1996.0154. [DOI] [PubMed] [Google Scholar]
- Hermosura MC, Monteilh-Zoller MK, Scharenberg AM, Penner R, Fleig A. Dissociation of the store-operated calcium current I(CRAC) and the Mg-nucleotide-regulated metal ion current MagNuM. J Physiol. 2002;539:445–458. doi: 10.1113/jphysiol.2001.013361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao WhL, Folsom AR, Neito FJ, Mo J-P, Watson RL, Brancati FL. Serum and dietary magnesium and the risk for type 2 diabetes mellitus. Arch Intern Med. 1999;159:2151–2159. doi: 10.1001/archinte.159.18.2151. [DOI] [PubMed] [Google Scholar]
- Kerschbaum HH, Cahalan MD. Monovalent permeability, rectification, and ionic block of store-operated calcium channels in Jurkat T lymphocytes. J Gen Physiol. 1998;111:521–537. doi: 10.1085/jgp.111.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J, Miyamae S, Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol. 1987;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuigan JAS, Elder HY, Günzel D, Schlue W-R. Magnesium homeostasis in heart: a critical reappraisal. J Clin Basic Cardiol. 2002;5:5–22. [Google Scholar]
- Mota de Freitas D, Amari L, Srinivasan C, Rong Q, Ramasamy R, Abraha A, Geraldes CFGC, Boyd MK. Competition between Li+ and Mg2+ for the phosphate groups in the human erythrocyte membrane and ATP: An NMR and fluorescence study. Biochemistry. 1994;33:4101–4110. doi: 10.1021/bi00180a002. [DOI] [PubMed] [Google Scholar]
- Nadler MJS, Hermosura MC, Inabe K, Perraud A-L, Zhu Q, Strokes AJ, Kurosaki T, Kinet J-P, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a MgATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- Nageswara Rao BD. Phosphorus-31 NMR of enzyme complexes. In: Gorenstein GD, editor. Phosphorus-31 NMR. London: Blackwell Science Inc; 1984. pp. 57–103. [Google Scholar]
- Nakayama S, Clark JF. Smooth muscle and NMR review: An overview of smooth muscle metabolism. Mol Cell Biochem. 2003;244:17–30. [PubMed] [Google Scholar]
- Nakayama S, Hachisuka T, Itoh K, Matsumoto T, Tomita T. Phosphomonoesters in the guinea-pig taenia caeci: pH-dependency of the phosphomonoester peaks in 31P-NMR. Jpn J Physiol. 1995;45:411–422. doi: 10.2170/jjphysiol.45.411. [DOI] [PubMed] [Google Scholar]
- Nakayama S, Nomura H. Mechanisms of intracellular Mg2+ regulation affected by amiloride and ouabain in the guinea-pig taenia caeci. J Physiol. 1995;488:1–12. doi: 10.1113/jphysiol.1995.sp020941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama S, Nomura H, Smith LM, Clark JF. Simultaneous estimation of intracellular free Mg2+ and pH using a new pH-dependent dissociation constant of MgATP. Jpn J Physiol. 2002;52:323–326. doi: 10.2170/jjphysiol.52.323. [DOI] [PubMed] [Google Scholar]
- Nakayama S, Nomura H, Tomita T. Intracellular-free magnesium in the smooth muscle of guinea pig taenia caeci: A concomitant analysis for magnesium and pH upon sodium removal. J Gen Physiol. 1994;103:833–851. doi: 10.1085/jgp.103.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama S, Seo Y, Takai A, Tomita T, Watari H. Phosphorous compounds studied by 31P nuclear magnetic resonance spectroscopy in the taenia of guinea-pig caecum. J Physiol. 1988;402:565–578. doi: 10.1113/jphysiol.1988.sp017222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama S, Tomita T. Depletion of intracellular free Mg2+ in Mg2+- and Ca2+-free solution in the taenia isolated from guinea-pig caecum. J Physiol. 1990;421:363–378. doi: 10.1113/jphysiol.1990.sp017949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama S, Tomita T. Regulation of intracellular free magnesium concentration in the taenia of guinea-pig caecum. J Physiol. 1991;435:559–572. doi: 10.1113/jphysiol.1991.sp018525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Kurebayashi N, Murayama T. Putative roles of type 3 ryanodine receptor isoforms (RyR3) Trends Cardiovasc Med. 2000;10:65–70. doi: 10.1016/s1050-1738(00)00050-5. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Murayama T, Kurebayashi N. Ryanodine receptor isoforms of non-mammalian skeletal muscle. Front Biosci. 2002;7:d1187–1194. doi: 10.2741/A832. [DOI] [PubMed] [Google Scholar]
- Paolisso G, Barbagallo M. Hypertension, diabetes mellitus, and insulin resistance: the role of intracellular magnesium. Am J Hypertens. 1997;10:368–370. doi: 10.1016/s0895-7061(96)00342-1. [DOI] [PubMed] [Google Scholar]
- Pyne GJ, Cadoux-Hudson TA, Clark JF. Cerebrospinal fluid from subarachnoid haemorrhage patients causes excessive oxidative metabolism compared to vascular smooth muscle force generation. Acta Neurochir (Wien) 2001;143:59–63. doi: 10.1007/s007010170139. [DOI] [PubMed] [Google Scholar]
- Shattock MJ, Hearse DJ, Fry CH. The ionic basis of anti-ischemic and anti-arrhythmic properties of magnesium in the heart. J Am Coll Nutr. 1987;6:27–33. doi: 10.1080/07315724.1987.10720162. [DOI] [PubMed] [Google Scholar]
- Sparrow MP. Interaction of 28Mg with Ca and K in the smooth muscle of guinea-pig taenia coli. J Physiol. 1969;205:19–38. doi: 10.1113/jphysiol.1969.sp008948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro M, Konishi M. Basal intracellular free Mg2+ concentration in smooth muscle cells of guinea-pig tenia cecum: Intracellular calibration of the fluorescent indicator fraptra. Biophys J. 1997;73:3358–3370. doi: 10.1016/S0006-3495(97)78360-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C-Y. Calcium and magnesium in drinking water and risk of death from cerebrovascular disease. Stroke. 1998;29:411–414. doi: 10.1161/01.str.29.2.411. [DOI] [PubMed] [Google Scholar]
- Yang X-C, Sachs F. Characterization of stretch-activated ion channels in Xenopus oocytes. J Physiol. 1990;431:103–122. doi: 10.1113/jphysiol.1990.sp018322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Truttmann AC, Lüthi D, McGuigan JAS. Apparent Mg2+-adenosine 5′-triphosphate dissociation constant measured with Mg2+ macroelectrodes under conditions pertinent 31P-NMR ionized magnesium determinations. Anal Biochem. 1997;251:246–250. doi: 10.1006/abio.1997.2238. [DOI] [PubMed] [Google Scholar]


