Abstract
There is evidence in experimental animals that, in addition to receiving fusimotor drive, muscle spindles are subject to modulation by the sympathetic nervous system. We examined the validity of this idea in human subjects by recording from muscle spindles in the relaxed ankle and toe extensor muscles during a strong and sustained physiological activation of muscle sympathetic outflow. Unitary recordings were made from 20 primary and 17 secondary muscle spindle afferents via a tungsten microelectrode inserted percutaneously into the peroneal nerve in 10 awake, healthy subjects seated with the legs supported in the extended position. ECG, blood pressure, respiration and calf circumference were also recorded. The majority of the muscle spindles were spontaneously active at rest; a background discharge was induced in four silent spindles by vibrating the tendon. A sustained increase in muscle vasoconstrictor activity, an increase in calf volume and a fall in pulse pressure were produced by subjects performing a 30–40 s maximal inspiratory breath-hold. Despite this strong increase in muscle sympathetic outflow no significant changes occurred in the discharge of either primary or secondary muscle spindle afferents, measured as a change in mean frequency and variability over sequential 5 s epochs and compared with the preceding period of rest. Strong chemoreceptor-driven sympathetic bursts during sustained expiratory breath-holds also failed to modulate the firing of 14 spindle endings. We conclude that a sustained, physiological increase in muscle sympathetic activity causes no detectable change in muscle spindle firing, lending no support to the concept that the sympathetic nervous system can influence the sensitivity of human muscle spindles directly.
The muscle spindle is a complex, highly sensitive stretch receptor that is unique in the somatosensory system in having its own efferent innervation - the fusimotor (γ) neurones - which can modulate the static and dynamic sensitivity of the spindle ending. While it is known that the fusimotor system can operate independently of the skeletomotor (α-motor) system in experimental animals, in human subjects very few examples of independent control have been demonstrated (Gandevia et al. 1985, 1997; Hagbarth, 1993; Kakuda et al. 1996). However, it has been proposed that there is another means of modulating spindle sensitivity - via the sympathetic nervous system (Passatore & Filipi, 1982; Passatore et al. 1985, 1996; Grassi et al. 1987, 1991, 1993a 1993b; Roatta et al. 2002). According to this idea, an increase in sympathetic outflow would serve to change spindle sensitivity to stretch and hence play a role in motor control, for example in states of stress as well as in certain pathological conditions such as sympathetically maintaned pain (Roatta et al. 2002). All of the evidence to support this idea has come from studies in experimental animals, in which changes in spindle sensitivity to controlled stretch stimuli have been assessed during electrical stimulation of the cervical or lumbar sympathetic chain.
Some features of the study by Roatta et al. (2002) cast doubt, however, on the validity of the extrapolation of their results to humans. First, the study showed that stimulation of the sympathetic chain could sometimes lead to increases and sometimes to decreases in the resting discharge of muscle spindles. Second, continuous (45–90 s) stimulation at a frequency of 10 Hz was used, which is unphysiological. Unlike the synchronous firing induced by electrical stimulation, sympathetic neurones fire asynchronously, and irregular stimulation has been shown to be more effective than regular stimulation (e.g. Kunimoto et al. 1992). More importantly, sympathetic neurones do not fire at such high rates. For instance, the median firing rate of single sympathetic neurones in the renal, splenic and mesenteric nerves in the anaesthetized cat is 0.9 Hz (Meckler & Weaver, 1988; Stein & Weaver, 1988), and in awake humans, individual cutaneous vasoconstrictor and sudomotor neurones discharge very irregularly with an average frequency of 0.5–0.6 Hz during the specific increases in sympathetic drive associated with cold-induced vasoconstriction (Macefield & Wallin, 1999b) or heat-induced sweating (Macefield & Wallin, 1996). Moreover, human muscle vasoconstrictor neurones fire at only ˜1 Hz during the intense sympathetic activation associated with a maximal inspiratory breath-hold (Macefield & Wallin, 1999a), congestive heart failure (Macefield et al. 1999) or obstructive sleep apnoea (Elam et al. 2002).
Against this background, the purpose of the present study was to assess whether changes in the resting activity of muscle spindles in relaxed muscles occur in awake healthy human subjects during a sustained physiological increase in sympathetic drive to muscle blood vessels and, presumably, also to the muscle spindles. To this end, afferent activity from muscle spindles was recorded while the subjects made inspiratory or expiratory apnoeas, maneouvres known to cause marked increases in human muscle sympathetic activity.
METHODS
Data were obtained from 10 healthy subjects, six male and four female, ranging in age from 18 to 28 years. Each subject provided informed written consent to the procedures, which were approved by the human ethics committee of the University of New South Wales and conformed with the Declaration of Helsinki. Subjects were semirecumbent in a chair with their backs at 45 deg and their legs supported horizontally. Electrocardiographic activity (ECG) was recorded over the chest with standard Ag-AgCl electrodes, respiratory movements with a strain-gauge transducer around the chest (Pneumotrace, UFI, Morro Bay, CA, USA) and continuous blood pressure using radial arterial tonometry (NIBP 7000, Colin Corp., Japan). In most subjects changes in blood volume of the leg contralateral to the nerve recording were assessed by changes in leg circumference, measured by an elastic strain gauge (Pneumotrace, UFI) around the calf. In some experiments, surface EMG was recorded via Ag-AgCl electrodes over the receptor-bearing muscle to confirm that the muscles were relaxed. The common peroneal nerve was located at the fibular head by palpation and electrical stimulation via a surface probe. A tungsten microelectrode (type 25–10–1, Frederick Haer Co., Brunswick, ME, USA or type TM33B20, World Precision Instruments, Sarasota, FL, USA) was inserted percutaneously into a motor fascicle of the nerve, and unitary recordings were made from 37 muscle spindle afferents in the tibialis anterior, extensor digitorum longus, extensor hallucis longus or peroneus longus muscles. All were activated by taps over the parent muscle belly and responded to passive stretch of the muscle. Primary muscle spindle endings were classified as such if they had an irregular background discharge (Nordh et al. 1983) and a high dynamic sensitivity to passive muscle stretch (Edin & Vallbo, 1990a), and generated an off-discharge at the end of a slow voluntary contraction of the receptor-bearing muscle (Edin & Vallbo, 1990b). Secondary endings had a regular background discharge (Nordh et al. 1983), a weak dynamic response to passive stretch (Edin & Vallbo, 1990a) and no off-discharge in the relaxation phase of a voluntary contraction (Edin & Vallbo, 1990b). For some muscle spindles located in the ankle- or toe-extensors, vibration (1.5 mm, 10–150 Hz) was applied over the tendon of the relevant muscle using a servo-controlled motor.
A sustained increase in muscle sympathetic activity was evoked by asking subjects to perform an inspiratory-capacity apnoea, in which subjects hold their breath at maximal lung volume against a closed glottis for 30–40 s (Macefield & Wallin, 1995b, 1999a; Macefield, 1998). In addition, some subjects performed an end-expiratory apnoea, holding their breath in expiration for as long as they could. On reaching the asphyxic breaking point, subjects were asked to take a single breath (in then out) and then to continue with the apnoea, resuming normal breathing after three such cycles. By this means a few series of large, chemoreceptor-driven muscle sympathetic bursts were generated close to the asphyxic breaking point (Macefield & Wallin, 1995a).
Neural activity was amplified (1 × 104), filtered (0.3–5.0 kHz), digitized at 12.8 kHz (12 bits) and stored on computer with ECG (digitized at 3.2 kHz), blood pressure (at 400 Hz), respiration (at 200 Hz) and calf volume (at 200 Hz) via the SC/ZOOM data acquisition and analysis system (Department of Physiology, University of Umeå, Sweden). Spindle-afferent spikes were discriminated using the inbuilt spike recognition facility. During off-line analysis the mean frequency was calculated over 5 s epochs from the computed instantaneous frequency of the unit. Discharge variability was calculated as the standard deviation over the 5 s period divided by the mean frequency (coefficient of variation) and presented as a percentage. Means and standard deviations were calculated over the 5 s period immediately preceding the inspiratory-capacity apnoea and compared to consecutive 5 s periods measured from the plateau phase of the apnoea. A copy of the nerve signal was root mean square (RMS) processed to emulate a leaky integrator (time constant 100 ms) in order to illustrate the underlying sympathetic bursts (mean voltage neurogram). For the end-expiratory apnoeas, the large sympathetic bursts were used to average the instantaneous-frequency record (burst-triggered averaging). All statistical evaluation of the data was performed using STATISTICA for Windows v6.0 (StatSoft Inc., Tulsa, OK, USA). Repeated-measures ANOVA was used to assess whether there was any change in either mean discharge frequency or discharge variability across each 5 s measurement period. Values are expressed as means and S.E.M., and differences were considered statistically significant at P < 0.05.
RESULTS
Unitary recordings were made from 37 muscle spindle afferents in relaxed muscles of the leg. With the exception of four afferents all had a background discharge. Twenty were classified as primary endings, with a mean discharge variability at rest of 15.0 ± 2.2 %. Seventeen afferents with a very regular discharge were classified as secondary endings (variability 3.0 ± 0.2 %). One primary ending was driven by the arterial pulse, firing once per heart beat, presumably owing to its location near a blood vessel.
Figure 1 shows experimental records from a secondary muscle spindle ending in the tibialis anterior. The afferent was spontaneously active at rest, presumably because the weight of the foot caused a passive plantarflexion, thereby stretching the receptor-bearing muscle. As shown in Fig. 1A, its frequency could be decreased by passive dorsiflexion of the foot (which unloaded the spindle). Records from a primary ending are shown in Fig. 2. This unit, located in the extensor digitorum longus, was also spontaneously active at rest, responding dynamically to passive plantarflexion of the toes.
Figure 1. Effects of sympathetic activation on a spindle secondary ending.
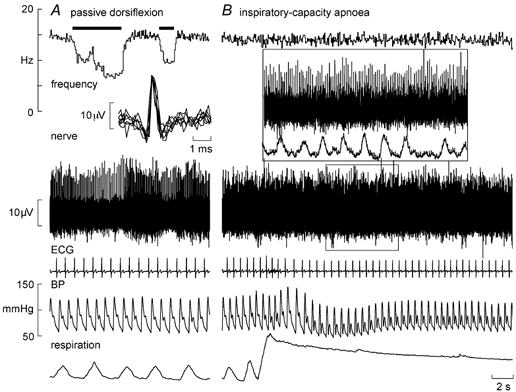
Recording from a spontaneously active secondary muscle spindle ending in tibialis anterior during passive dorsiflexion of the ankle, which unloads the spindle (A), and a sustained maximal inspiratory breath-hold, which increases muscle sympathetic drive but does not change the spindle firing (B). Superimposed spikes are shown in the inset of A. The inset of B shows an expanded section and illustrates the far-field muscle sympathetic activity detected from the same microelectrode (RMS-processed nerve signal). BP, blood pressure.
Figure 2. Effects of sympathetic activation on a spindle primary ending.
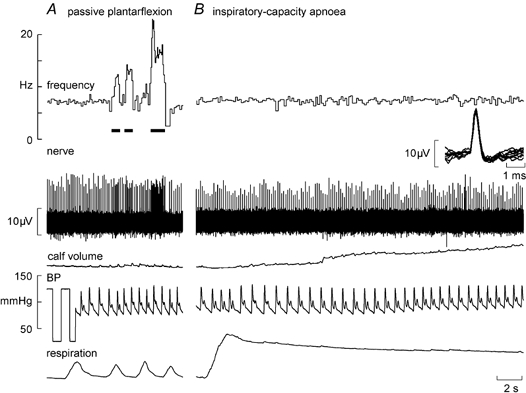
Recording from a spontaneously active primary muscle spindle ending in extensor digitorum longus during passive plantarflexion of the toes, which stretches the spindle (A), and a sustained maximal inspiratory breath-hold, which increases muscle sympathetic drive but does not change the spindle firing (B). Calf volume, calf circumference measured contralaterally.
Inspiratory-capacity apnoeas
In most subjects, a maximal inspiratory breath-hold (inspiratory-capacity apnoea) caused an initial fall in blood pressure and a sustained fall in pulse pressure. When measured, calf volume (recorded from the contralateral leg) increased during the apnoea (see Fig. 2), presumably reflecting the high intrathoracic pressure and consequent venous congestion peripherally. During 11 spindle afferent recordings multi-unit muscle sympathetic activity was also discernable; this far-field activity was highlighted in the RMS-processed version of the nerve signal (see inset of Fig. 1B). As demonstrated previously (Macefield & Wallin, 1995b, 1999; Macefield, 1998), the apnoea led to a sustained increase in muscle sympathetic activity.
Regardless of their identity as primary or secondary muscle spindle endings, none of the afferents changed their background discharge during the manouevre. This was quantified for 37 spindle afferents by measuring mean frequency and its variability every 5 s during the apnoea. The total number of apnoeas analysed was 55: for 21 units only one apnoea was performed, whereas duplicate runs were analysed for 14 units and triplicate runs for two units. There were no significant changes in either mean frequency or variability for either type of spontaneously active spindle ending (Table 1). The four afferents that were silent at rest (one primary, three secondaries) were given a tonic discharge by vibrating the tendons at 20, 30 or 50 Hz. The primary ending fired with every cycle (1:1) whereas each of the secondary endings fired with every second or third cycle of the vibration (1:2 or 1:3). An example of a secondary ending (in extensor digitorum longus) that fired once every second cycle of a 20 Hz sinusoid is shown in Fig. 3A. There were no changes in firing of any of these endings during the inspiratory-capacity apnoea, i.e. there was no shift towards 1:1 entrainment for those endings firing at a subharmonic of the vibration cycle and no shift towards 2:1 or 3:1 entrainment for those firing 1:1. The pulse-synchronous firing of one primary ending did not change during the apnoea - it continued to fire only once per heart beat, its mean frequency simply reflecting the changes in heart rate during the course of the manouevre.
Table 1.
Mean discharge frequency and variability of primary and secondary endings
| Time during apnoea | Rest | 0–5 s | 5–10 s | 10–15 s | 15–20 s |
|---|---|---|---|---|---|
| Mean frequency (Hz) | |||||
| Primaries (n=18) | 9.6 ± 1.0 | 9.7 ± 1.0 | 9.4 ± 1.0 | 9.4 ± 1.0 | 9.4 ± 1.0 |
| Secondaries (n=15) | 10.5 ± 0.8 | 10.5 ± 0.8 | 10.5 ± 0.8 | 10.5 ± 0.8 | 10.3 ± 0.8 |
| Mean variability (%) | |||||
| Primaries | 20.9 ± 3.8 | 22.0 ± 3.2 | 22.9 ± 3.7 | 25.9 ± 5.1 | 22.6 ± 4.5 |
| Secondaries | 3.9 ± 0.4 | 4.1 ± 0.4 | 4.3 ± 0.4 | 4.4 ± 0.6 | 3.9 ± 0.4 |
Mean (± S.E.M.) discharge frequency and variability of primary and secondary muscle spindle endings at rest and during consecutive 5 s periods during inspiratory-capacity apnoea. Data were obtained for 48 runs in 33 afferents (data from the vibrated and pulse-driven units were excluded). There were no significant changes in either parameter during the manouevre.
Figure 3. Effects of sympathetic activation on a spindle secondary ending.
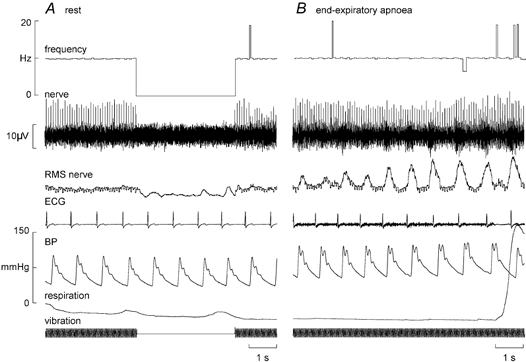
Recording from a silent secondary muscle spindle ending in extensor digitorum longus. A, the ending was activated by vibrating the tendon at 20 Hz, firing with every second cycle. B, the the unit's entrainment pattern did not change during an end-expiratory apnoea. Multi-unit sympathetic activity was also recorded concurrently from the same microelectrode, illustrated in the RMS-processed nerve signal.
End-expiratory apnoeas
Fourteen spindles (nine primaries, five secondaries) were studied during end-expiratory apnoeas, in which series of large chemoreceptor-driven sympathetic bursts were generated towards the asphyxic breaking-point. An example of a silent spindle that was entrained by vibration (1:2) is shown in Fig. 3B. As with the inspiratory apnoeas there was no shift towards 1:1 firing during the bursts, other than the erratic 1:1 firing also observed at rest (Fig. 3A). A spontaneously active ending is shown in Fig. 4A. None of these 14 endings changed their firing during the apnoea. For four spindle recordings clear sympathetic bursts could be discerned in the nerve signal. These bursts were then used to trigger averaging of the instantaneous frequency profile of the spindle ending and thereby assess whether there was any change in frequency that was time-locked to the sympathetic bursts. Figure 4B shows an example from the secondary ending shown in Fig. 4A. Averaging of 55 large sympathetic bursts in this recording failed to reveal any short-term change in spindle firing. The remaining three endings likewise failed to show a short-term change in mean frequency during the averaged sympathetic burst.
Figure 4. Effects of sympathetic activation on a spindle secondary ending.
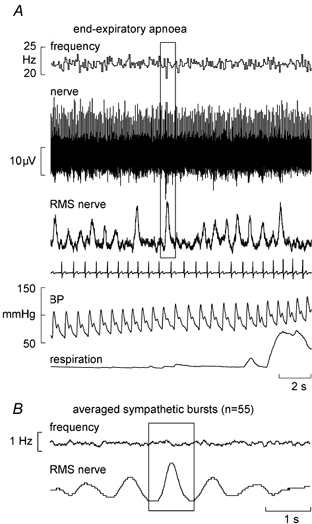
A, recording from a spontaneously active secondary muscle spindle ending in tibialis anterior (same unit as in Fig. 1) during one series of an end-expiratory apnoea. The box centred over a sympathetic burst illustrates the source of synchronization for the averaging. B, burst-triggered average discharge frequency of the spindle shown in A, calculated from 55 large sympathetic bursts generated during the end-expiratory apnoea.
DISCUSSION
This study is the first to examine whether activation of the sympathetic nervous system affects the firing of muscle spindles in human subjects. The motivation for this investigation was to search for evidence of a direct sympathetic innervation of muscle spindles in awake human subjects, as has been reported in several studies in anaesthetized (or decerebrate) experimental animals (Passatore & Filipi, 1982; Passatore et al. 1985, 1996; Grassi et al. 1987, 1991, 1993a 1993b; Roatta et al. 2002). Despite the use of a strong and sustained increase in muscle sympathetic nerve activity we failed to observe any changes in spontaneous or evoked discharge in primary or secondary muscle spindles in relaxed human leg muscles.
Methodological considerations
We used a physiological method of increasing muscle sympathetic nerve activity, asking subjects to perform inspiratory or expiratory breath-holds. These maneouvres are known to cause marked increases in muscle sympathetic outflow (Macefield & Wallin, 1995a 1995b, 1999; Macefield, 1998) and in the present study the increase was confirmed in all electrode sites in which sympathetic activity could be monitored in parallel with the spindle-afferent recording. The observed decrease in arterial pulse pressure (Macefield & Wallin, 1995b) and increase in calf volume (as measured by changes in calf circumference in the present study) both reflect a decrease in venous return. This is due to the high intrathoracic pressure, which reduces the transmural pressure across the great vessels in the chest, unloading the low-pressure baroreceptors and thereby increasing muscle vasoconstrictor drive (Macefield, 1998).
All of the spindles sampled in the present study were recorded in relaxed muscles (confirmed in some experiments by surface EMG), which avoids any unintentional changes in spindle firing consequent to a change in muscle activation (either unloading of the spindle or a fusimotor-driven increase). The majority of the spindles were spontaneously active, in which the ongoing discharge reflects the prevailing length of the relaxed receptor-bearing muscle (Vallbo, 1974). However, there are limitations of the study. It may be that sympathetic effects on spindle sensitivity are only observed when the fusimotor system is activated during a volitional contraction, though this has not been tested in experimental animals. However, it is known that sympathetically mediated changes in spindle firing occur in curarized animals, in which the fusimotor system is paralysed (Passatore et al. 1985; Roatta et al. 2002), so this would suggest that any sympathetic modulation of spindle sensitivity is not mediated via γ-motoneurones. In the present study, we did not test the dynamic and static sensitivity of the spindle endings to controlled stretch - only changes in resting discharge (static sensitivity) were examined. Nevertheless, the fact that there were no changes in firing of those afferents stimulated by vibration (or by the arterial pulse) suggests that no change in dynamic sensitivity occurred. Moreover, the animal data indicate that changes in both resting and evoked spindle firing can be induced by electrical stimulation of sympathetic nerves, although the most consistent finding is of a reduced sensitivity to stretch (e.g. Roatta et al. 2002). The firing rates of human muscle spindles are low at rest (˜–15 Hz) but they are very sensitive to small changes in length, so any increase or decrease in discharge should have been detectable. Calculated from the spontaneously active endings presented in Table 1, the mean resting discharge of both types of spindle ending was 10.0 ± 0.6 Hz (S.D. = 4.5 Hz, n = 48). Power analysis revealed that a difference of 2 Hz could be detected with a power of 70 %, and a difference of 4 Hz with a power of 100 %. The mean difference in firing rate during the inspiratory-capacity apnoea for the pooled data of Table 1 was 0.1 Hz. Repeated-measures ANOVA showed no significant difference in discharge frequency for different time epochs.
Implications
In the studies in experimental animals, a non-specific activation of the sympathetic nervous system - involving many target tissues - was produced by electrical stimulation of the cervical or lumbar sympathetic chain (Passatore & Filipi, 1982; Passatore et al. 1985, 1996; Grassi et al. 1987, 1991, 1993a 1993b; Roatta et al. 2002). Not withstanding our concerns about the stimulation frequencies employed in these studies (see Introduction), raising the issue as to whether the responses observed are truly physiological, we are concerned that the use of the terms ‘sympathetic activation’ or ‘stress’ can be misleading, given that the sympathetic nervous system exhibits a high degree of specificity in its actions (Jänig & McLachlan, 1992). Our observations promote caution in extrapolating data from anaesthetized animals to awake human subjects.
Conclusions
Our study has failed to demonstrate any direct or indirect effects of muscle sympathetic nerve activity on the firing of muscle spindles in relaxed human leg muscles. Thus, the evidence for a direct sympathetic modulation of muscle spindle function obtained in experimental animals cannot be extrapolated to humans.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (program grant 002306) and the Swedish Medical Research Council (grant 12170).
REFERENCES
- Edin BB, Vallbo ÅB. Dynamic response of human muscle spindle afferents to stretch. J Neurophysiol. 1990a;63:1297–1306. doi: 10.1152/jn.1990.63.6.1297. [DOI] [PubMed] [Google Scholar]
- Edin BB, Vallbo ÅB. Muscle afferent responses to isometric contractions and relaxations in humans. J Neurophysiol. 1990b;63:1307–1313. doi: 10.1152/jn.1990.63.6.1307. [DOI] [PubMed] [Google Scholar]
- Elam M, McKenzie D, Macefield V. Mechanisms of sympatho-excitation: single-unit analysis of muscle vasoconstrictor neurons in awake OSAS subjects. J Appl Physiol. 2002;93:297–303. doi: 10.1152/japplphysiol.00899.2001. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Burke D. Effect of training on voluntary activation of human fusimotor neurons. J Neurophysiol. 1985;54:1422–1429. doi: 10.1152/jn.1985.54.6.1422. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Wilson LR, Inglis JT, Burke D. Mental rehearsal of motor tasks recruits α-motoneurones but fails to recruit human fusimotor neurones selectively. J Physiol. 1997;505:259–266. doi: 10.1111/j.1469-7793.1997.259bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi C, Filipi GM, Passatore M. Tension development in lumbrical muscles and concomitant increase of activity in A alpha and A beta afferents during sympathetic stimulation in the cat. Brain Res. 1987;437:15–23. doi: 10.1016/0006-8993(87)91581-2. [DOI] [PubMed] [Google Scholar]
- Grassi C, Passatore M. Anti-fatigue action exerted by the sympathetic nervous system in the rabbit digastric muscle. Funct Neurol. 1991;6:255–258. [PubMed] [Google Scholar]
- Grassi C, Deriu F, Artusio E, Passatore M. Modulation of the jaw jerk reflex by the sympathetic nervous system. Archiv Ital Biol. 1993a;131:213–226. [PubMed] [Google Scholar]
- Grassi C, Deriu F, Passatore M. Effect of sympathetic nervous system activation on the tonic vibration reflex in rabbit jaw closing muscles. J Physiol. 1993b;469:601–613. doi: 10.1113/jphysiol.1993.sp019832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth K-E. Microneurography and applications to issues of motor control: Fifth Annual Stuart Reiner Memorial Lecture. Muscle Nerve. 1993;16:693–705. doi: 10.1002/mus.880160702. [DOI] [PubMed] [Google Scholar]
- Jänig W, McLachlan EM. Specialized functional pathways are the building blocks of the autonomic nervous system. J Autonom Nerv Syst. 1992;41:3–14. doi: 10.1016/0165-1838(92)90121-v. [DOI] [PubMed] [Google Scholar]
- Kakuda N, Vallbo ÅB, Wessberg J. Fusimotor and skeletomotor activities are increased with precision finger movement in man. J Physiol. 1996;492:921–929. doi: 10.1113/jphysiol.1996.sp021358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimoto M, Kirno K, Elam M, Karlsson T, Wallin BG. Neuro-effector characteristics of sweat glands in the human hand activated by irregular stimuli. Acta Physiol Scand. 1992;146:261–269. doi: 10.1111/j.1748-1716.1992.tb09415.x. [DOI] [PubMed] [Google Scholar]
- Macefield VG. Sustained activation of muscle sympathetic outflow during lung inflation depends on a high intrathoracic pressure. J Autonom Nerv Syst. 1998;68:135–139. doi: 10.1016/s0165-1838(97)00129-x. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Rundqvist B, Sverrisdottir YB, Wallin BG, Elam M. Firing properties of single muscle vasoconstrictor neurons in the sympathoexcitation associated with congestive heart failure. Circulation. 1999;100:1708–1713. doi: 10.1161/01.cir.100.16.1708. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG. Modulation of muscle sympathetic activity during spontaneous and artificial ventilation and apnoea in humans. J Autonom Nerv Syst. 1995a;53:137–147. doi: 10.1016/0165-1838(94)00173-h. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG. Effects of static lung inflation on sympathetic activity in human muscle nerves at rest and during asphyxia. J Autonom Nerv Syst. 1995b;53:148–158. doi: 10.1016/0165-1838(94)00174-i. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG. Discharge behaviour of single sympathetic neurones innervating human sweat glands. J Autonom Nerv Syst. 1996;61:277–286. doi: 10.1016/s0165-1838(96)00095-1. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG. Firing properties of single vasoconstrictor motoneurones in subjects with high levels of muscle sympathetic activity. J Physiol. 1999a;516:293–301. doi: 10.1111/j.1469-7793.1999.293aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG. Respiratory and cardiac modulation of single vasoconstrictor and sudomotor neurones to human skin. J Physiol. 1999b;516:303–314. doi: 10.1111/j.1469-7793.1999.303aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG, Vallbo ÅB. The discharge behaviour of single vasoconstrictor motoneurones in human muscle nerves. J Physiol. 1994;481:799–809. doi: 10.1113/jphysiol.1994.sp020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckler RL, Weaver LC. Characteristics of ongoing and reflex discharge of single splenic and renal sympathetic postganglionic fibres in cats. J Physiol. 1988;396:163–175. doi: 10.1113/jphysiol.1988.sp016955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordh E, Hulliger M, Vallbo ÅB. The variability of inter-spike intervals of human spindle afferents in relaxed muscles. Brain Res. 1983;271:89–99. doi: 10.1016/0006-8993(83)91367-7. [DOI] [PubMed] [Google Scholar]
- Passatore M, Deriu F, Grassi C, Roatta S. A comparative study of changes operated by sympathetic nervous system activation on spindle afferent discharge and on tonic vibration reflex in rabbit jaw muscles. J Autonom Nerv Syst. 1996;57:163–167. doi: 10.1016/0165-1838(95)00074-7. [DOI] [PubMed] [Google Scholar]
- Passatore M, Filipi GM. A dual effect of sympathetic nerve stimulation on jaw muscle spindles. J Autonom Nerv Syst. 1982;6:347–361. doi: 10.1016/0165-1838(82)90006-6. [DOI] [PubMed] [Google Scholar]
- Passatore M, Grassi C, Filipi GM. Sympathetically-induced development of tension in jaw muscles: the possible contraction of intrafusal muscle fibres. Pflugers Arch. 1985;405:297–304. doi: 10.1007/BF00595681. [DOI] [PubMed] [Google Scholar]
- Roatta S, Windhorst U, Ljubisavljevic M, Johansson H, Passatore M. Sympathetic modulation of muscle spindle afferent sensitivity to stretch in rabbit jaw closing muscles. J Physiol. 2002;540:237–248. doi: 10.1113/jphysiol.2001.014316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RD, Weaver LC. Multi- and single-fibre mesenteric and renal sympathetic responses to chemical stimulation of intestinal receptors in cats. J Physiol. 1988;396:155–172. doi: 10.1113/jphysiol.1988.sp016956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo ÅB. Afferent discharge from human muscle spindles in non-contracting muscles. Steady state impulse frequency as a function of joint angle. Acta Physiol Scand. 1974;90:303–318. doi: 10.1111/j.1748-1716.1974.tb05593.x. [DOI] [PubMed] [Google Scholar]


