Abstract
This study examines three hypotheses that have been proposed to explain the effects of galvanic vestibular stimulation (GVS) in standing human subjects. The first assumes realignment to an altered representation of vertical. GVS-evoked body tilt produced under conditions of different stability was compared with perceptions of the vertical which subjects indicated by two means, a visual line and a manipulandum. GVS produced body tilt that increased in unstable conditions but there were no differences in the perceived vertical in any condition. The second hypothesis is that the altered vestibular signal is interpreted as a tilt of the support surface. The postural response evoked by tilting the support surface was compared with the GVS response under conditions of varying stability. These responses were different, particularly for the lower body where movements were oppositely directed. Standing on foam augmented GVS responses whereas standing with feet apart augmented platform-tilt responses. The third hypothesis is that GVS produces an illusion of movement, and this causes a reaction in the opposite direction. Perception of movement during GVS was determined in standing and immobilised subjects. Although immobilised subjects experienced illusions of movement in the direction opposite the sway response, this only happened after long periods of stimulation and never for standing where subjects accurately reported the true direction of sway. Thus, the results do not support any of these proposals. Instead, they and other observations support a simpler interpretation that the GVS signal is consistent with head movement and evokes an automated response to stabilise the head in space.
Galvanic vestibular stimulation (GVS) is usually applied to human subjects by passing a small direct current between the mastoid processes. It modulates the ongoing vestibular signal by increasing the firing rate of afferents on the cathodal side and decreasing the firing rate on the anodal side (Lowenstein, 1955; Goldberg et al. 1984; Courjon et al. 1987), causing standing subjects to sway towards the anodal side. It is uncertain how the central nervous system interprets and responds to this altered vestibular signal to produce the motor response. The response is complex, depends on task, posture and the availability of other sensory information. The size of the response increases when subjects stand with their feet close together (Day et al. 1997), stand on an unstable support surface (Fitzpatrick et al. 1994), or when proprioceptive, visual or tactile sources of sensory information are limited (Britton et al. 1993; Horak & Hlavacka, 2001; Day & Cole, 2002). The direction of the response is modified by the orientation of the head relative to the base of support (Lund & Broberg, 1983). Thus, the complexity of these responses made early suggestions of a simple direct effect on motor neurones via vestibulo-spinal or reticulo-spinal pathways (Coats, 1973) seem less probable, and different hypotheses to explain the sway reactions have come from recent experiments.
During constant GVS, subjects adopt a new final tilted position after the initial sway response. To explain this observation, it has been proposed that GVS alters an internal reference of the vertical that is used to align the body (Popov et al. 1986; Inglis et al. 1995; Hlavacka et al. 1996). Day el al. (1997) demonstrated that each body segment tilts in the direction of the anode by amounts that depend on the size of the base of support, and propose that the central nervous system (CNS) interprets the altered vestibular signal as a tilt of the support surface. A third suggestion is that GVS produces an illusion of movement towards the cathode and, in response to this, subjects move in the opposite direction to maintain balance (Fitzpatrick et al. 1994).
The present study examines these three different hypotheses concerning how GVS evokes a postural response. First, perceptions of the vertical were measured during GVS and compared with the evoked postural responses. Congruence of body alignment with perception of the vertical would support the theory that GVS alters an vertical reference that is used to align the body. Second, GVS-evoked postural responses are compared with responses evoked by tilts of the support surface. Similar alignments of the body segments relative to the support would argue for the proposal that the GVS signal is interpreted as a tilt of the support surface. Third, postural responses when subjects are standing and movement perceptions when subjects are immobilised are compared. The theory that the GVS response arises through a perception of movement in the opposite directions would be supported if the GVS tilt and perceptual responses have opposite directions and similar time courses.
METHODS
A total of 18 healthy adults (ages 24–57 yrs; 9 male) with no history of neurological disease or trauma participated in the experiments. The Institute's Human Ethics Committee approved the experiments and subjects provided informed consent in writing. All experiments conformed with the guidelines set out in the Declaration of Helsinki. Some findings have been presented in abstract form (Wardman et al. 2000).
Three experiments are described here. In the first experiment, subjects' perceptions of the vertical during GVS were determined while their actual body alignment was measured. The vertical was determined in two ways: (i) with a visual indicator and (ii) with a manipulandum. In the second experiment, the alignment of the body and segments after tilting the support surface was compared with responses to GVS. In the third experiment, subjects reported perceptions of movement during GVS when standing and when the body was immobilised.
Bipolar GVS was applied through electrodes (2 cm2 AgCl) attached over each mastoid process and stabilised with a headband. In all stimulus conditions, a constant-current source was used to pass 1 mA step impulses between the electrodes, with the anode on the right or left. Subjects could perceive the stimuli as bilateral cutaneous paraesthesia but were not told in advance whether a stimulus would be presented in the next trial.
Light-emitting diode (LED) markers were placed at the vertex of head, cervical vertebra (C7), sacral vertebra (S2), and back of left and right heels. Body movement was recorded by a digital video camera positioned 2.8 m behind the subject at a height of 1 m (Fig. 1A). For each trial the LEDs were digitised into X and Y co-ordinates with a resolution of 2 mm and converted into angles of body-segment tilt for the leg segment (S2-feet), body segment (C7 – S2) and head segment Head - C7). All angles were calculated relative to the segment orientation at the ‘go’ signal and stimulus onset.
Figure 1. Experimental setup.
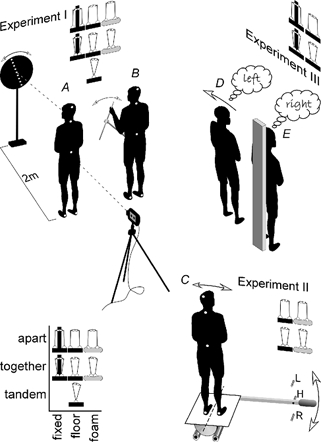
The setup for each experiment is illustrated along with a schematic table of the conditions of feet position and support tested in each. The table key is at the bottom left. In Experiment I, the subject stood in a darkroom and GVS was applied while postural responses were measured with a camera targeted at markers on the feet, pelvis, C7 vertebra and head. Simultaneously, the subject aligned a visual indicator to vertical using a hand-held control (A) or aligned a manual control to vertical (B). In Experiment II, the subject stood on a platform that was tilted about a sagittal axis between the feet at ground level. The platform was held at the tilted position to the left (L) or right (R) while postural responses were similarly measured with a camera (C). In Experiment III, the subject stood freely on the floor (D) or was immobilised by being strapped to an upright support (E). GVS was applied for different durations and the subject reported the direction of any perceived movement to the left or right.
To measure the size of the sway responses, it was necessary to identify the time at which subjects reliably attained a stable posture after GVS had commenced. Thus, in the first experiment conducted (i.e. vertical perception), data were digitised at 200 ms intervals and the traces were examined. Posture was shown to be stable 4 s after stimulus onset. Thus, in all experiments, body-segment tilt for statistical analysis was measured at 4 s.
Setup and protocol
Perception of the vertical
Vertical measured by visual indicator
Six subjects were studied in three one-hour sessions on different days, each testing a different body support. They stood in a darkroom that excluded all external light with a blindfold over the left eye. With the right eye they looked directly at the axis of a 50 cm black wheel placed 2 m away at eye level (Fig. 1A, left). The wheel had a line of LEDs that produced a retinal image of 14–16 deg of arc. Subjects wore dark sunglasses to mask object outlines from scattered LED light. The subject controlled the motorised wheel with a switch held in front of the body, turning it clockwise or anticlockwise at 20 deg s−1. Its position was measured from a potentiometer in the axis and zeroed to gravitational vertical.
Each trial began with the line of lights randomly tilted right or left by 20–40 deg. Subjects closed their eyes and waited 5–10 s for the countdown sequence ‘ready, set, go’. They were instructed to open the eyes on ‘go’ and align the lights to the vertical, indicating ‘OK’ when finished. GVS started with the ‘go’ command and stopped with the ‘OK’ response. Most trials lasted 5–6 s. Before the experiment started, subjects were given practice trials without GVS. The difference in the indicated vertical between the stimulus and no-stimulus conditions was calculated.
Three different surface supports, a hard floor, foam and an immovable body-support frame, were used to provide different levels of stability. In the first session, stimuli were delivered while subjects stood on the hard floor. They stood with the feet in three positions: (i) with one foot in front of the other, the ‘tandem Romberg’ position, (ii) with the feet side by side and together so that the medial surfaces touched, or (iii) with the feet side by side and parallel but 0.2 m apart. In the second session, subjects stood on a large 15 cm thick block of medium density foam with a compliance of 66 cm3 N−1. The feet were in two positions, (i) together and (ii) 20 cm apart. In the final session, subjects were strapped to an immoveable frame while they were in the standing position with feet also in two positions, together or 20 cm apart. A schematic table of these conditions is included alongside Fig. 1A. In the first session there were 54 randomised trials: three stimulus conditions (left anode, no stimulus, right anode), two wheel starting positions (left, right) and three stance conditions, all repeated three times. The second and third sessions each consisted of 36 randomised trials: three stimulus conditions, two wheel conditions and two stance conditions, all repeated three times.
Vertical measured by manual indicator
This experiment was carried out over two one-hour sessions on different days, each testing a different support surface. Subjects (n = 6) were blindfolded and stood in a darkroom. They indicated the gravitational vertical with a small rod that rotated in roll plane, and was attached to a bracket that was securely fitted to the pelvis (Fig. 1B). The rod could be turned clockwise or anticlockwise, felt secure and rigid to use and its position was measured from a servo-quality potentiometer in the axis. Position was measured with a resolution of 0.02 deg.
All trials started with the rod randomly tilted right or left by 20–40 deg. The subject stood with the hand close to the pointer and had been instructed how to manipulate it by using a small movement that would not destabilise posture. Subjects waited 5−10 s for the countdown sequence ‘ready, set, go’. On ‘go’ they aligned the rod with vertical, indicating ‘OK’ when finished. GVS started with the ‘go’ signal and stopped at ‘OK’. Most trials lasted 5–6 s. Before the experiments started, subjects were given practice trials without GVS.
In one session, trials were performed while subjects stood on a hard floor with feet together or with feet parallel but 20 cm apart. In the other session, subjects stood on the 15 cm thick foam, again with the feet together or apart. The presentation order of these trial blocks and the trials within each block were randomised.
Support surface tilt
In this experiment subjects (n = 12) stood on a platform which was tilted under them. The platform surface was either hard or foam and subjects stood with their feet together or 20 cm apart. The axis of rotation of the platform was at the level of the surface and passed antero-posterior along the line midway between the feet (Fig. 1C). The platform had a levered handle that could lock into one of three stops that rigidly held the platform. The middle stop held the platform level and the other two at 0.8 deg of inclination to the left or right. The platform was tilted by this 0.8 deg in 1 s and was maintained at this tilt for 5–6 s. This was achieved reliably by manual control because the level had a large mechanical advantage and frictional damping. The size of this disturbance was chosen because it was equivalent to the size of the head tilt produced by GVS in experiment I and in numerous previous studies in which 1 mA stimuli have been used. Subjects were not cued in advance about the onset of the tilt. This experiment consisted of 48 trials: two tilt conditions (left, right), two surface conditions (hard surface, foam), and two stance conditions (feet together, apart), all repeated six times. A schematic table of these conditions is included alongside Fig. 1B.
Perception of movement
Six subjects participated in this experiment that examined perceptions of movement during GVS of different durations for normal standing (Fig. 1D) and immobilised standing (Fig. 1E). For normal standing, subjects stood with the feet together or 20 cm apart. For immobilised standing, they were strapped to an immoveable frame at the pelvis, shoulders and forehead, but could position their feet together or apart. In both situations, they were blindfolded and stood quietly with their hands clasped in front of them. The subjects were instructed to attend to a period between audible ‘start’ and ‘stop’ signals. GVS at 1 mA and of 0.04, 0.4, 1 or 5 s duration commenced at the ‘start’ signal. The ‘stop’ signal was presented at 2 or 5 s for the 5 s stimulus. Subjects were then asked if they had felt any sideways movement of themselves, and if so, to report its direction as ‘left’ or ‘right’. They were instructed not to guess and only report a movement if they were certain of its direction. If they felt a reversal in the movement, they were to report the initial direction. The stance and feet conditions (normal, immobilised) × (together, apart) were block randomised between subjects. Within each block, four presentations of each combination of stimulus polarity and duration (anode left, right) × (0.04, 0.4, 1 and 5 s) were delivered in randomised order. Thus, there were 128 trials for each subject presented in four blocks of 32.
Statistical analysis
Tilt data for the GVS experiment (I) are given in the direction of the anodal electrode relative to the start position. The effects of the stimulus on segment tilt (leg, body and head) were determined by ANOVAs with test (visual or manual indicator), support (floor or foam) and stance (feet together or apart) as independent factors. For the support-surface tilt experiment (II), segment tilt data are given in the direction of the upward support-surface tilt. The tilt of each body segment was similarly determined by ANOVA (support × stance). The effects of GVS and support-surface tilt on individual body segments were compared by univariate ANOVAs with experiment (GVS, tilt), support (floor, foam) and stance (feet together, apart) as independent factor.
The effects of the stimulus on the two perceived verticals were determined by two univariate ANOVAs with support (floor, foam or immobilised) and stance (feet together or apart) as independent factors. Correlation analysis was used to compare the effect of GVS on head segment tilt with the effect of GVS on both visual and non-visual vertical tilt. A significance level of 5 % was used and data are expressed as the mean ± S.E.M.
Perceived movement directions from the perception of body movement experiment were coded as towards the anode or cathode. Data from all subjects were pooled (total = 128 trials × 6 subjects) and the net response in the direction of the anode was calculated as the fraction:
Differences between the net responses for different stimulus durations (0.04, 0.4, 1 and 5 s) were assessed by z-test for the feet-together and feet-apart data. Significance levels were Bonferroni corrected for multiple comparisons.
RESULTS
Perception of the vertical
Galvanic vestibular stimulation caused subjects to tilt towards the anodal side, more so when the feet were together and when standing on foam. GVS also caused subjects to report that the vertical was tilted towards the anode when they used the visual indicator, but not when they used the manual indicator. These results are described here in two sections; body tilt and subjective vertical.
Body tilt
The leg segment (feet-S2), body segment (S2–C7) and head segment (C7-vertex) all tilted in the direction of the anode. Sway commenced shortly after stimulus onset and continued for approximately 2 s after which tilt remained relatively stable. Figure 2 shows responses for one subject. Except for a small initial transient in the opposite direction, tilt of the leg segment was similar in timing but about half the size of the upper body. The body segment (not shown) was intermediate. Movements had similar time courses but differed in size for the different stance and support conditions. Tilt at 4 s after stimulus onset (arrow) was chosen as representative of the tilt during the later plateau period.
Figure 2. Typical postural response to GVS.
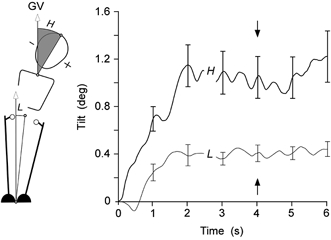
The alignment (group mean ± S.E.M.) of the leg (L) and head (H) body segments relative to the gravitational vertical (GV) are plotted for 6 s of continuous GVS for subjects standing with feet together on the floor. The tilt is in the direction of the anodal electrode. Following the initial tilt response that lasted 1–2 s, subjects attained a relatively constant posture. The alignments of the segments at 4 s (marked by arrows) were chosen to represent this steady-state posture.
The GVS-evoked sway responses during trials that used the visual pointer and the manual pointer were not significantly different. This applied for the tilts of the leg, body and head segments (ANOVA, F(1,52)= 0.39, 2.3 and 1.0, respectively). There were also no significant interactions between the indicator used and the test conditions. To simplify graphical presentation, segmental tilt data from the visual and non-visual test conditions are pooled in Fig. 3, but not in the statistical analysis below.
Figure 3. Alignment of body segments and perceptions of vertical during GVS.
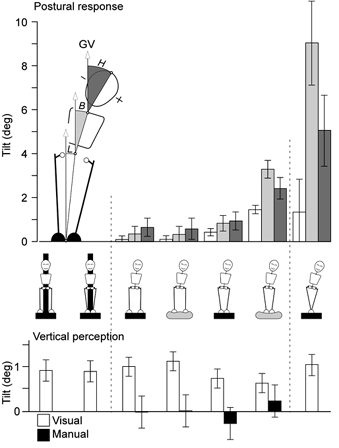
In the upper graph, the alignment of leg (L), body (B) and head (H) relative to the gravitational vertical (GV) are shown for the freestanding conditions indicated by the cartoons below. The two immobilised conditions (leftmost) are not shown. In the lower graph are the perceptions of the vertical that subjects reported simultaneously with these GVS responses. The subjective vertical was reported with the visual indicator during all conditions (open bars) and reported with the manual indicator in only the four freestanding conditions (filled bars).
There was approximately a fivefold range in the size of the tilt produced by GVS across the four stance and support conditions for feet together and apart, foam and firm support surface, (Fig. 3, groups between the broken lines). There were significant effects of stance on tilt of the leg (F(1,52)= 21.9, P < 0.001), body (F(1,52)= 19.7, P < 0.001) and head segments (F(1,52)= 5.2, P < 0.05), with greater tilts with the feet together. There were also significant effects of support surface on tilt of the leg (F(1,52)= 8.5, P < 0.01) and body segments (F(1,52)= 10.0, P < 0.01), with greater tilts on the foam. There was a significant interaction between stance and support on tilt of the leg (F(1,52)= 8.8, P < 0.01) and body segments (F(1,52)= 10.3, P < 0.01), evidenced by the disproportionately large tilts when standing on the foam with the feet together.
Vertical measured by visual pointer
Across all conditions subjects indicated that the visual vertical during GVS was tilted by 0.90 ± 0.09 deg towards the anodal side (0.89 ± 0.11 deg for standing and 0.92 ± 0.24 deg for immobilised trials). In the standing trials, there were no significant effects of stance (F(1,20)= 2.66) or support (F(1,20)= 0.005) on the visual vertical (Fig. 3) and furthermore, there were still no significant effects when data from the immobilised conditions were included in the analysis (F(5,30)= 0.636).
When standing with one foot in front of the other, subjects swayed very large amounts (rightmost in Fig. 3), and could not stand without stabilising themselves by other means in 58 of 72 stimulus trials. For these reasons, these data were not included in the statistical analysis. However, in the successful trials, the leg segment tilted 0.67 ± 2.24 deg, the body 9.46 ± 2.35 deg, and the head 7.10 ± 2.41 deg, whereas the tilt of the vertical indicated with the visual pointer was 1.18 ± 0.47 deg towards the anode.
Vertical measured by manual pointer
Subjects showed more variability when indicating the vertical with the manipulandum than with the visual pointer. On average, they indicated with the manual pointer that the vertical was tilted non-significantly by 0.02 ± 0.16 deg towards the anodal side during GVS. There were no significant effects of stance (F(1,20)= 0.03) or support (F(1,20)= 0.78) on the non-visual vertical.
The subjective verticals measured with visual and manual pointers during GVS were significantly different (F(1,30)= 22.3, P < 0.001). Correlation did not show an association between the GVS-evoked head tilt and either measure of the vertical (visual: r = 0.15 ± 0.06 deg; manual: r = 0.09 ± 0.16 deg). However, there was a significant difference between the intercepts of the two regression lines (visual 0.89 ± 0.11 deg; non-visual 0.02 ± 0.16 deg; P < 0.001)). Thus, the difference between the two measures is the constant offset (0.9 deg) when indicating with the visual pointer during GVS.
Support surface tilt
During these trials, the support surface was tilted over a period of approximately 1 s by an average of 0.82 ± 0.02 deg, slightly less than the lateral tilt produced by GVS (0.9 deg). In Fig. 4, the resulting tilts of the body segments are plotted relative to this final position of the support surface (shown as mean ± S.E.M.). Thus, the heights of the bars for each condition reflect the body shape or posture in the same way as those for the GVS responses in Fig. 3.
Figure 4. Alignments of the body and segments after tilting the support surface.
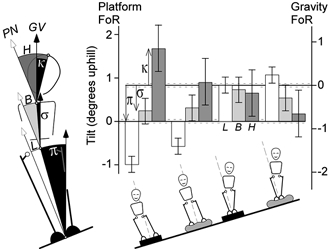
The alignment of the leg, body and head segments (L, B, H) is shown for each of the four freestanding conditions of stance and support indicated by the figures below. They are plotted in the platform frame of reference (FoR) on the left axis. They can also be read downward with the right axis (illustrated in the first group) to give the tilts in the opposite direction in the gravitational reference frame (κ, σ, π). The error bars on the Tilt and Vertical axes represent the S.E.M. associated with tilting the platform. Most obvious are the differences between the feet-apart and feet-together responses. Note the axis scale here spans just 3 deg whereas that of Fig. 3 spans 10 deg to illustrate the tandem stance data.
There was a significant effect of stance on tilt of the leg segment (F(1,44)= 72.9, P < 0.001). With the feet together, the leg segment tilted 0.94 ± 0.14 deg less than the support surface whereas, with the feet apart, it tilted 0.80 ± 0.14 deg more than the final support surface. Overall, body segment tilt was 0.48 ± 0.20 deg and head tilt was 0.86 ± 0.34 deg less than the final support surface tilt, and these were not affected by stance (body: F(1,44)= 0.9; head F(1,44)= 1.6). At no body segment was there significant effect of support or interaction between stance and support.
Body segment tilts relative to the floor produced by GVS were compared with body segment tilts relative to the platform produced by platform tilt. These showed significant differences for the leg (F(1,88)= 11.1, P < 0.001) and body segments (F(1,88)= 9.2, P < 0.01) but not the head segment (F(1,88)= 1.1). There were significant interactions between perturbation (GVS or platform) and stance (together or apart) on tilts of the leg, body and head segments (F(1,88)= 7.4, P < 0.05; F(1,88)= 6.5, P < 0.05; F(1,88)= 6.0, P < 0.05, respectively).
In Fig. 5, responses to GVS and platform tilt are compared for the most stable condition (feet apart on floor) and the most unstable condition (feet together on foam). The leftmost figures (A) show the alignment of the body relative to the gravitational vertical after the platform has been tilted. These figures are then tilted so that the platform is horizontal and the vertical is tilted (C). The alignment of the body and segments relative to the platform can then be compared with the GVS responses (D). Although there are complex interactions between segment, support and perturbation, it is apparent here that GVS and tilt of the support surface do not produce the same postures. With the feet apart, the leg segment is displaced in the direction opposite to that produced by GVS. With the feet apart (upper figures), there is greater arching of the body when the platform is tilted, whereas with the feet together (lower figures), the greater arching occurs with GVS. For all conditions, GVS causes the head to tilt more as balance becomes more unstable (Fig. 3) whereas platform tilt causes the head to tilt more as balance becomes more stable (Fig. 4).
Figure 5. Comparison of body segment alignments in response to tilt of the support surface and GVS.
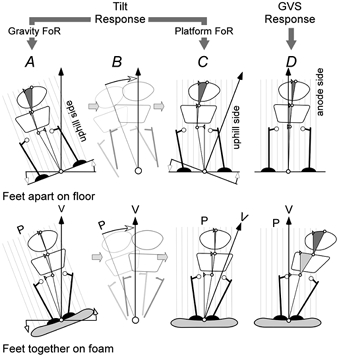
The figures show the angular alignment of the body and segments in response to tilt of the platform. The angular scale for each segment is magnified × 12.5. The upper figures are standing on a rigid platform with feet apart and the lower figures are standing on foam with feet together. The leftmost figures (A) are responses in the gravitational frame of reference (FoR). The black arrow (V) is the gravitational vertical and a grid (P) is drawn normal to the platform. These figures are tilted (B) so that the platform is level to yield the alignment in the platform surface FoR (C). If GVS produced a postural response based on an interpretation that the ground had tilted, then the measured GVS responses (D) should be similar to the tilt responses in platform co-ordinates (C).
Perception of movement
When standing freely, subjects reported movements towards the anode or not at all (Fig. 6, top plots). The opposite happened when subjects were immobilised. Apart from one occasion, they reported that they perceived movements towards the cathode or not at all (Fig. 6, bottom plots). However, the relationship between stimulus duration and frequency of perceived movements differed between the free standing and the immobilised situations. Subjects perceived no consistent movement regardless of whether they were standing or immobilised when the duration of the stimulus was short (40 ms). When the stimulus was very long (5 s) they nearly always perceived a movement towards the anode - the actual direction of sway - when standing, and nearly always towards the cathode when immobilised. For the two intermediate duration stimuli, very few movements were perceived when subjects were immobilised whereas a significant number more were perceived when standing for both 400 ms stimuli (feet together 44 %, apart 19 %; P < 0.01) and 1 s stimuli (feet together 60 %, apart 38 %; P < 0.01).
Figure 6. Frequency of reported directions of perceived movement during GVS.
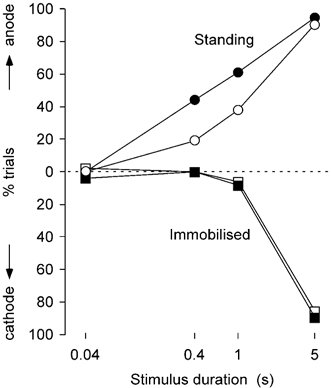
For all the data, the percentage of movements towards the anode (upper half of graph) or cathode (lower half) are plotted against the duration of the GVS stimulus. For 0.4 and 1 s stimuli, significantly more movements were detected during free standing than when immobilised.
The arrangement of the feet had no effect on the perception of movement when subjects were immobilised but had significant effects during free standing with 400 ms and 1 s stimuli, which produced approximately twice as many reports of movement when the feet were together than when they were apart (400 ms, 44 vs. 19 % P < 0.01; 1 s, 60 vs. 38 % P < 0.01).
DISCUSSION
Three hypotheses concerning the origin of the GVS-evoked sway response have been examined. The first hypothesis is that the response aligns the body to an altered internal model of the vertical and that GVS alters this model. The evidence here does not support this. The effects of GVS on body tilt and the perception of vertical were compared for different postural tasks. GVS caused the body to tilt differently depending on postural conditions but did not cause equivalent changes in the alignment of the vertical. The second hypothesis is that the altered vestibular signal evoked by GVS is interpreted as a tilt of the support surface and the sway is an organised response to it. Again, these experiments do not support this. Responses for GVS and tilt of the support were compared for different postural tasks. These responses were different; standing on foam caused the body to bend more with GVS whereas standing with feet apart caused it to bend more with platform tilt and for the lower body to move in the opposite direction to GVS. The third hypothesis is that the sway response is a reaction to a perception of movement in the opposite direction. This also appears to be incorrect because when subjects stood unrestrained, they perceived the actual direction of GVS-evoked body sway. However, in immobilised subjects, GVS produced illusions of movement in the opposite direction. It therefore appears that the vestibular signal produced by GVS is consistent with a movement of the head having been detected, and the postural correction is to maintain the position of the head in space.
Perception of the vertical
Subjects with unilateral peripheral vestibular loss indicate that the visual vertical is strongly deviated towards the side of the lesion. When they have to align the body to vertical in the absence of vision, on average they indicate the true vertical (Anastasopoulos et al. 1997) although the variance of this estimate is greater than that of normal subjects (Bisdorff et al. 1996). Normal subjects tested with GVS similarly showed no significant bias of this postural vertical (Bisdorff et al. 1996). Thus, subjects' estimates of the vertical using visual and non-visual indicators can differ, and it may be that subjects use visual and vestibular input to estimate the visual vertical but largely proprioceptive input to estimate the non-visual vertical. For this reason the present study measured the perception of the vertical during GVS using a visual indicator and a non-visual indicator. It is interesting that the results during GVS show a deviation of the visual vertical but not of the non-visual vertical, findings analogous to those in vestibular loss subjects.
When their posture is upright, normal subjects can, without vision, accurately (< 2 deg error) reproduce the orientation of a rod after it has been moved. After an imposed head tilt of 45 deg, they make systematic deviations of up to 10 deg in the opposite direction (Luyat et al. 2001). This suggests that perception of non-visual vertical can be affected by vestibular and neck proprioceptive inputs, at least for stimuli of this magnitude. In the present study, the vestibular perturbation produced by GVS at 1 mA did not affect the estimate of non-visual vertical.
Normal subjects can accurately align a visual indicator in a dark room to vertical to within 2 deg (Neal, 1926; Witkin & Asch, 1948; Mann et al. 1949) and tilting the head or body within 10 deg of vertical does not affect this (Graybiel, 1973). The perceived visual vertical can tilt beyond this with peripheral or central vestibular lesions (Friedmann 1970, 1971; Curthoys et al. 1991; Dieterich & Brandt, 1993; Brandt et al. 1994). Complete unilateral vestibular neurectomy causes a tilt of 10 –12 deg towards the affected side (Anastasopoulos et al. 1997; Bohmer & Mast, 1999) and also ocular torsion of similar magnitude (Curthoys et al. 1991). GVS appears to modulate the spontaneous firing of vestibular afferents, increasing firing frequency on the side of the cathode and decreasing it on the side of the anode (Lowenstein, 1955; Goldberg et al. 1984; Courjon et al. 1987). This imbalance can be considered analogous to a temporary vestibular lesion in that, as with pathological lesions, it caused subjects to perceive the visual vertical as tilted towards the side with hypofunction (Aarons & Goldenberg, 1964; Zink et al. 1997; Tardy-Gervet & Severac-Cauquil, 1998).
When perception of vertical is determined using a visual indicator, ocular torsion, mediated through vestibular-ocular reflexes, can influence the outcome. Curthoys et al. (1991) showed a high correlation between ocular torsion and perceived gravitational horizontal and suggested that in the absence of other visual information, subjects align the visual horizontal to the horizontal retinal meridian. The ocular torsion induced by GVS of 1 mA is between 0.5 and 1 deg (Zink et al. 1997, 1998; Severac-Cauquil et al. 1998; Watson et al. 1998). In the present study with the head and body immobilised, and in other studies with the head immobilised (Severac-Cauquil et al. 1998; Watson et al. 1998) the visual vertical also tilts by between 0.5 and 1 deg. Thus, the tilt of the visual vertical is consistent with ocular torsion. In the standing trials of the present study, alignment of the visual vertical during GVS remained the same - approximately 0.9 deg, irrespective of the head movement produced by GVS under the different postural conditions.
It therefore appears that an ocular torsion of 0.9 deg mediated through a GVS-evoked vestibulo-ocular reflex is superimposed on the normal ocular torsion produced by head tilt and results in the tilt of the visual vertical. Correcting for this gives the same result as the perception of the non-visual vertical. These results imply that there is no significant change in the internal representation of the vertical during GVS. Thus, we can conclude that the different postural sway responses evoked by GVS are not organized to maintain the alignment of the body with the vertical. To give an everyday analogy, it is as if you are standing and leaning into the wind. Standing requires balancing the sum of all the forces acting on the body; it does not require remaining aligned with the gravitational vector. With GVS, the vestibular system is detecting acceleration, or force, acting on the head.
Support surface tilt
The postural response evoked by GVS ultimately has the body segments tilted towards the anode. This new body position depends on the availability of peripheral somatosensory information (Horak & Hlavacka, 2001) and the stability determined by the width of the base of support (Day et al. 1997). Responses are small with the legs apart but can cause subjects to fall with tandem Romberg stance.
Based on different responses to GVS when sitting and when standing with different postures, Day et al. (1997) proposed that the nervous system interprets the GVS vestibular signal as a tilt of the support surface. The realignment of the body segments could be organised to keep the centre of mass of the body centred over the base of support. The idea is appealing because the vestibular system appears to have an important role in maintaining stability when the support moves. Sensory input from the legs is sufficient to control body sway when standing on a stable support surface but it does not allow labyrinthine defective subjects to control the position of the head and upper body during tilts or translations of the support surface (Martin, 1967; Allum et al. 1994; Horak et al. 2002). In contrast, normal subjects counter rotate or translate the head so that it maintains a fairly constant position in space and the body maintains its position over the base of support.
However, results from the present study indicate that, despite similarities at head level, the GVS-evoked postural response is different to the postural response produced by a tilt of the support surface. Standing on the foam had a large effect on the sway reaction to GVS but no effect when the surface support was tilted, and tilting the platform produced a relatively greater curvature of the body than GVS when the feet were apart. Thus, it appears as if the GVS response depends more on sensory information from the legs whereas the response to a platform tilt depends more on the adopted posture, which is whether the feet are apart or together in this situation.
There is little doubt that these differences arise because the afferent input and responses evoked by vestibular and peripheral sensory systems differ in the two situations. Although we have not measured the timing of movements at different joints, the support-surface tilt would be detected first in the muscles and joints of the legs, followed by upper body segments and finally the vestibular system, which could then modify the response. GVS evokes a postural response from a disturbance initially detected at the head, and produces a sequence of responses in postural muscles with shorter latencies in postural muscles more proximal to the vestibular system. Later responses, that appear to be influenced by non-vestibular inputs, have latencies that are shorter in distal postural muscles and cause greater tilts in more proximal body segments (Britton et al. 1993; Day et al. 1997; Ardic et al. 2000). The concept of ‘down- and up-channelling’ of sensory information described by Mergner & Rosemeier (1998) appears to apply here. Vestibular and proprioceptive information are internally combined in a ‘sensor fusion’, in which the relative weighting of inputs are changed according to the postural task.
Perception of movement
When GVS caused freely standing subjects to sway, they accurately perceived the direction of that movement. These were described for stimuli of 400 ms duration or greater, but not for 40 ms stimuli. In contrast, when subjects were immobilised GVS only evoked illusory movements for stimuli of 1 s or longer, and even at 1 s they were uncommon. Thus the illusory and the sway responses have time courses that prevent them being causally related. Furthermore, the sway response is affected by the subjects' posture, whereas the illusion is not.
However, long GVS stimuli produced illusions described as ‘tilt’ or ‘spin’ towards the cathodal side that commenced shortly after stimulus onset and lasted for the duration of the trial. Could a conscious reaction to an illusion explain some of the sway response in the opposite direction during very long stimuli? During free standing, there was never any doubt or confusion in the reports of the perception with the long stimuli; the movement was always clearly perceived in the direction of the actual movement evoked by GVS. Therefore, it appears that in free standing an illusory movement in the opposite direction to the eventual sway never develops.
Functional magnetic resonance imaging studies indicate that areas of the cortex associated with spatial orientation are activated during GVS (Lobel et al. 1998). GVS affects the pattern of irregularly firing vestibular afferents, and this could imitate the natural response to linear or angular head acceleration (Peterson, 1998; Goldberg, 2000). Subjects reported illusions of spin as well as tilt, and this could indicate that stimulation of semicircular canal and otolith afferents is perceived. Normally, the afferent firing of the utricular otolith increases with an ipsilateral-down lateral head tilt or medially directed linear acceleration, and decreases with ipsilateral-up head tilt or laterally directed linear acceleration. Afferent firing from the anterior and posterior semicircular canals also increases with ipsilateral-down head roll, and decreases with ipsilateral-up head roll (Highstein, 1996). If GVS increases the afferent firing from the utricle and the vertical canals on the cathodal side and decreases firing on the anodal side, such a change in afferent firing would indicate a naturally occurring linear acceleration or roll of the head in the direction of the cathode. If the CNS interprets GVS as a head movement, a compensatory postural reaction in the opposite direction would be expected. It is important to note that this response is likely to be automated and not based on perception.
Seated subjects, with the head immobilised, also perceive illusory movements towards the cathodal side shortly after GVS onset or changes in intensity (Watson et al. 1998). Thus, immobilising the subject seems necessary for the illusion of movement. During free standing, sensory input from non-vestibular sources, which is usually in the direction opposite the GVS illusion, may override the altered vestibular afferent discharge to provide a more accurate account of movement. Lower-limb sensory information is more sensitive at detecting body sway (Fitzpatrick & McCloskey, 1994), but subjects may prefer to rely on this information when making judgements about body movement regardless of sensitivity. An alternative explanation is that when the GVS sway reaction is prevented by the immobilising support, subjects perceive this as a ‘push’ in the opposite direction by the support, although it was not possible to ascertain this from subjective reports.
What is the basis of the GVS response?
The size of the GVS response is determined by the availability of other sensory information about posture and the stability of the posture. It diminishes when visual or tactile information is available (Britton et al. 1993) and increases when proprioceptive information is limited (Horak & Hlavacka, 2002). The response increases when subjects stand on foam, an unstable support (Fitzpatrick et al. 1994) or with the feet close together (Day et al. 1997) or in tandem stance. These observations seem inconsistent with the notion the response realigns to an altered representation of vertical or support surface because in each situation, GVS produces identical modulation of afferent discharge and therefore identical misalignments of the vertical and head. Rather than the pattern seen in Fig. 2, surely the head should realign to this same altered vertical and the segments below readjust to accommodate surface contact information. However, two recent studies show that much of the GVS response is a continuous movement rather than resetting the posture to a new static set point (Day & Cole, 2002; Wardman et al. 2003). It is easier to explain the variable head tilt if the GVS signal conveys movement rather than a static vertical as the head and upper body would continue moving until other sensory sources override the vestibular signal to arrest the movement.
The GVS response is highly automatic. Unlike the stimulus of a moving visual surround, which only causes sway when presented unexpectedly and habituates rapidly (Guerraz et al. 2001), the GVS response does not habituate even when self administered repeatedly (Guerraz & Day, 2001).
What is the ‘purpose’ of the vestibular response? This is obviously the big question of the vestibular role in standing balance generally rather than GVS specifically. However, the progressively greater bending of the body produced by GVS as balance becomes more unstable or other sensory input is lost suggests that the target of the control is the head and upper body. This coincides with observations that with rotation or translation of their support, normal subjects bend the body to keep the orientation and position of the head more stationary than other parts of the body whereas those with loss of vestibular function do not bend the body so that the head moves in line with the support (Martin, 1967; Horak et al. 2002).
This study tested three hypotheses that have been put forward to explain the postural response to GVS and the results do not support any of them. This is not what we expected at the outset and leaves us with some obligation to speculate on an alternative explanation for the GVS response. There may not be a simple explanation, but in line with the considerations above, we propose that the GVS signal conveys head movement and evokes an automatic response, modifiable by other sensory information about balance, that stabilises the head in gravito-inertial space.
Acknowledgments
This work was supported by the Clive and Vera Ramiacotti Foundation and the National Health and Medical Research Council of Australia. We thank Dr Brian Day for his assistance and ideas, and also one of the Journal referees for suggesting a fruitful experimental approach.
REFERENCES
- Aarons L, Goldenberg L. Galvanic stimulation of the vestibular system and perception of the vertical. Percept Mot Skills. 1964;19:59–66. doi: 10.2466/pms.1964.19.1.59. [DOI] [PubMed] [Google Scholar]
- Allum JH, Honegger F, Schicks H. The influence of a bilateral peripheral vestibular deficit on postural synergies. J Vestib Res. 1994;4:49–70. [PubMed] [Google Scholar]
- Anastasopoulos D, Haslwanter T, Bronstein A, Fetter M, Dichgans J. Dissociation between the perception of body verticality and the visual vertical in acute peripheral vestibular disorder in humans. Neurosci Lett. 1997;233:151–153. doi: 10.1016/s0304-3940(97)00639-3. [DOI] [PubMed] [Google Scholar]
- Ardic FN, Latt LD, Redfern MS. Paraspinal muscle response to electrical vestibular stimulation. Acta Otolaryngol. 2000;120:39–46. doi: 10.1080/000164800760370819. [DOI] [PubMed] [Google Scholar]
- Bisdorff AR, Wolsley CJ, Anastasopoulos D, Bronstein AM, Gresty MA. The perception of body verticality (subjective postural vertical) in peripheral and central vestibular disorders. Brain. 1996;119:1523–1534. doi: 10.1093/brain/119.5.1523. [DOI] [PubMed] [Google Scholar]
- Bohmer A, Mast F. Assessing otolith function by the subjective visual vertical. Ann N Y Acad Sci. 1999;871:221–311. doi: 10.1111/j.1749-6632.1999.tb09187.x. [DOI] [PubMed] [Google Scholar]
- Brandt T, Dieterich M, Danek A. Vestibular cortex lesions affect the perception of verticality. Ann Neurol. 1994;35:403–412. doi: 10.1002/ana.410350406. [DOI] [PubMed] [Google Scholar]
- Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD, Marsden CD. Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Exp Brain Res. 1993;94:143–151. doi: 10.1007/BF00230477. [DOI] [PubMed] [Google Scholar]
- Coats AC. Effect of varying stimulus parameters on the galvanic body-sway response. Ann Otol Rhinol Laryngol. 1973;82:96–102. doi: 10.1177/000348947308200119. [DOI] [PubMed] [Google Scholar]
- Courjon JH, Precht W, Sirkin DW. Vestibular nerve and nuclei unit responses and eye movement responses to repetitive galvanic stimulation of the labyrinth in the rat. Exp Brain Res. 1987;66:41–48. doi: 10.1007/BF00236200. [DOI] [PubMed] [Google Scholar]
- Curthoys IS, Dai MJ, Halmagyi GM. Human ocular torsional position before and after unilateral vestibular neurectomy. Exp Brain Res. 1991;85:218–225. doi: 10.1007/BF00230003. [DOI] [PubMed] [Google Scholar]
- Day BL, Cole J. Vestibular-evoked postural responses in the absence of somatosensory information. Brain. 2002;125:2081–2088. doi: 10.1093/brain/awf212. [DOI] [PubMed] [Google Scholar]
- Day BL, Severac-Cauquil A, Bartolomei L, Pastor MA, Lyon IN. Human body-segment tilts induced by galvanic stimulation: a vestibularly driven balance protection mechanism. J Physiol. 1997;500:661–672. doi: 10.1113/jphysiol.1997.sp022051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich M, Brandt T. Ocular torsion and tilt of subjective visual vertical are sensitive brainstem signs. Ann Neurol. 1993;33:292–299. doi: 10.1002/ana.410330311. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D, Gandevia SC. Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. J Physiol. 1994;478:363–372. doi: 10.1113/jphysiol.1994.sp020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick R, McCloskey DI. Proprioceptive, visual and vestibular thresholds for the perception of sway during standing in humans. J Physiol. 1994;478:173–186. doi: 10.1113/jphysiol.1994.sp020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann G. The judgement of the visual vertical and horizontal with peripheral and central vestibular lesions. Brain. 1970;93:313–328. doi: 10.1093/brain/93.2.313. [DOI] [PubMed] [Google Scholar]
- Friedmann G. The influence of unilateral labyrinthectomy on orientation in space. Acta Otolaryngol. 1971;71:289–298. doi: 10.3109/00016487109125366. [DOI] [PubMed] [Google Scholar]
- Goldberg JM. Afferent diversity and the organization of central vestibular pathways. Exp Brain Res. 2000;130:277–297. doi: 10.1007/s002210050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Smith CE, Fernandez C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol. 1984;51:1236–1256. doi: 10.1152/jn.1984.51.6.1236. [DOI] [PubMed] [Google Scholar]
- Graybiel A. Otolith function and human performance. Adv Otorhinolaryngol. 1973;20:485–519. doi: 10.1159/000393118. [DOI] [PubMed] [Google Scholar]
- Guerraz M, Thilo KV, Bronstein AM, Gresty MA. Influence of action and expectation on visual control of posture. Cogn Brain Res. 2001;11:259–266. doi: 10.1016/s0926-6410(00)00080-x. [DOI] [PubMed] [Google Scholar]
- Guerraz M, Day BL. Human body response to galvanic vestibular stimulation is not affected when the stimulus is self-triggered. J Physiol. 2001;531.P:142P. [Google Scholar]
- Highstein SM, Rabbitt RD, Boyle R. Determinants of semicircular canal afferent response dynamics in the toadfish, Opsanus tau. J Neurophysiol. 1996;75:575–596. doi: 10.1152/jn.1996.75.2.575. [DOI] [PubMed] [Google Scholar]
- Hlavacka F, Mergner T, Krizkova M. Control of body vertical by vestibular and proprioceptive inputs. Brain Res Bull. 1996;40:431–435. doi: 10.1016/0361-9230(96)00138-4. [DOI] [PubMed] [Google Scholar]
- Horak FB, Buchanan J, Creath R, Jeka J. Vestibulospinal control of posture. Adv Exp Med Biol. 2002;508:139–145. doi: 10.1007/978-1-4615-0713-0_17. [DOI] [PubMed] [Google Scholar]
- Horak FB, Hlavacka F. Somatosensory loss increases vestibulospinal sensitivity. J Neurophysiol. 2001;86:575–585. doi: 10.1152/jn.2001.86.2.575. [DOI] [PubMed] [Google Scholar]
- Inglis JT, Shupert CL, Hlavacka F, Horak FB. Effect of galvanic vestibular stimulation on human postural responses during support surface translations. J Neurophysiol. 1995;73:896–901. doi: 10.1152/jn.1995.73.2.896. [DOI] [PubMed] [Google Scholar]
- Lobel E, Kleine JF, Lebihan D, Leroywillig A, Berthoz A. Functional MRI of galvanic vestibular stimulation. J Neurophysiol. 1998;80:2699–2709. doi: 10.1152/jn.1998.80.5.2699. [DOI] [PubMed] [Google Scholar]
- Lowenstein O. The effect of galvanic polarization on the impulse discharge from sense endings in the isolated labyrinth of the thornback ray (Raja clavata) J Physiol. 1955;127:104–117. doi: 10.1113/jphysiol.1955.sp005241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund S, Broberg C. Effects of different head positions on postural sway in man induced by a reproducible vestibular error signal. Acta Physiol Scand. 1983;117:307–309. doi: 10.1111/j.1748-1716.1983.tb07212.x. [DOI] [PubMed] [Google Scholar]
- Luyat M, Gentaz E, Corte TR, Guerraz M. Reference frames and haptic perception of orientation: body and head tilt effects on the oblique effect. Percept Psychophys. 2001;63:541–554. doi: 10.3758/BF03194419. [DOI] [PubMed] [Google Scholar]
- Mann CW, Berthelot-Berry NH, Dauterive HJ., Jr The perception of the vertical: I. Visual and non-labyrinthine cues. J Exp Psychol. 1949;39:538–547. doi: 10.1037/h0063533. [DOI] [PubMed] [Google Scholar]
- Martin JP. The Basal Ganglia and Posture. London: Blackwell Science Inc; 1967. [Google Scholar]
- Mergner T, Rosemeier T. Interaction of vestibular, somatosensory and visual signals for postural control and motion perception under terrestrial and microgravity conditions - a conceptual model. Brain Res Brain Res Rev. 1998;28:118–135. doi: 10.1016/s0165-0173(98)00032-0. [DOI] [PubMed] [Google Scholar]
- Neal E. Visual localisation of the vertical. Am J Psychol. 1926;37:287–291. [Google Scholar]
- Peterson EH. Are there parallel channels in the vestibular nerve. News Physiol Sci. 1998;13:194–201. doi: 10.1152/physiologyonline.1998.13.4.194. [DOI] [PubMed] [Google Scholar]
- Popov KE, Smetanin BN, Gurfinkel VS, Kudinova MP, Shlykov VYu. Spatial perception and vestibulomotor responses in man. Neurophysiol. 1986;18:548–553. [PubMed] [Google Scholar]
- Severac-Cauquil A, Faldon M, Popov K, Bronstein A, Day BL. Torsional eye movements induced by galvanic vestibular stimulation in man. J Physiol. 1998;506.P:110P–111P. [Google Scholar]
- Tardy-Gervet MF, Severac-Cauquil A. Effect of galvanic vestibular stimulation on perception of subjective vertical in standing humans. Percep Mot Skill. 1998;86:1155–1161. doi: 10.2466/pms.1998.86.3c.1155. [DOI] [PubMed] [Google Scholar]
- Wardman DL, Day BL, Fitzpatrick RC. Position and velocity responses to galvanic vestibular stimulation during standing. J Physiol. 2003;547:293–299. doi: 10.1113/jphysiol.2002.030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardman DL, Taylor JL, Fitzpatrick RC. The effect of galvanic vestibular stimulation and standing on the perception of the visual vertical. Proc Aus Neurosci Soc. 2000;11:51. [Google Scholar]
- Watson SD, Brizuela AE, Curthoys IS, Colebatch JG, Macdougall HG, Halmagyi GM. Maintained ocular torsion produced by bilateral and unilateral galvanic (DC) vestibular stimulation in humans. Exp Brain Res. 1998;122:453–458. doi: 10.1007/s002210050533. [DOI] [PubMed] [Google Scholar]
- Witkin HA, Asch SE. Studies in space orientation. III. Perception of the upright in the absence of a visual field. J Exp Psychol. 1948;38:603–614. doi: 10.1037/h0055372. [DOI] [PubMed] [Google Scholar]
- Zink R, Bucher SF, Weiss A, Brandt T, Dieterich M. Effects of galvanic vestibular stimulation on otolithic and semicircular canal eye movements and perceived vertical. Electroencephalogr Clin Neurophysiol. 1998;107:200–205. doi: 10.1016/s0013-4694(98)00056-x. [DOI] [PubMed] [Google Scholar]
- Zink R, Steddin S, Weiss A, Brandt T, Dieterich M. Galvanic vestibular stimulation in humans-effects on otolith function in roll. Neurosci Lett. 1997;232:171–174. doi: 10.1016/s0304-3940(97)00610-1. [DOI] [PubMed] [Google Scholar]


