Abstract
The recently discovered apical calcium channels CaT1 (TRPV6) and ECaC (TRPV5) belong to a family of six members called the ‘TRPV family’. Unlike the other four members which are nonselective cation channels functioning as heat or osmolarity sensors in the body, CaT1 and ECaC are remarkably calcium-selective channels which serve as apical calcium entry mechanisms in absorptive and secretory tissues. CaT1 is highly expressed in the proximal intestine, placenta and exocrine tissues, whereas ECaC expression is most prominent in the distal convoluted and connecting tubules of the kidney. CaT1 in the intestine is highly responsive to 1,25-dihydroxyvitamin D3 and shows both fast and slow calcium-dependent feedback inhibition to prevent calcium overload. In contrast, ECaC only shows slow inactivation kinetics and appears to be mostly regulated by the calcium load in the kidney. Outside the calcium-transporting epithelia, CaT1 is highly expressed in exocrine tissues such as pancreas, prostate and salivary gland. In these tissues it probably mediates re-uptake of calcium following its release by secretory vesicles. CaT1 also contributes to store-operated calcium entry in Jurkat T-lymphocytes and prostate cancer LNCaP cells, possibly in conjunction with other cellular components which link CaT1 activity to the filling state of the calcium stores. Finally, CaT1 expression is upregulated in prostate cancer and other cancers of epithelial origin, highlighting its potential as a target for cancer therapy.
As the most abundant cation in the human body, calcium repesents a key component of the mineral phase of teeth and bones and is maintained nearly constant in the blood and extracellular fluids (Brown, 1991). In its ionized form, calcium (Ca2+) serves as an intracellular messenger that participates in muscle contraction, neurotransmission, enzyme and hormone secretion, as well as many other biological processes, e.g. cell cycle regulation and programmed cell death (Clapham, 1995; Berridge et al. 2000). In spite of the fact that all of the calcium in our bodies is derived from our diet, over the past two decades, the detailed molecular mechanism of Ca2+ entry into the body has received little attention compared with that directed at understanding Ca2+ signalling. Nevertheless, our understanding of Ca2+ absorption has been advanced by the identification of the apical Ca2+ entry channels in Ca2+-transporting epithelia, CaT1 from the intestine (Peng et al. 1999) and ECaC from the kidney (Hoenderop et al. 1999). In addition to mediating luminal calcium uptake into epithelial cells, CaT1 also exhibits properties similar to those of the Ca2+ release-activated Ca2+ channels (Yue et al. 2001), and contributes to the store-operated calcium entry in the human Jurkat T-lymphocytes (Cui et al. 2002) and LNCaP prostate cancer cells (Vanden Abeele et al. 2003). Thus, CaT1 is a link between Ca2+ transport and Ca2+ signalling. CaT1 expression is up-regulated in prostate carcinoma (Peng et al. 2001b; Wissenbach et al. 2001) and other cancers of epithelial origin (Zhuang et al. 2002), suggesting an important pathological role for CaT1. In this review, we will summarize the biology of the recently identified Ca2+ entry channels CaT1 and ECaC.
The cation channels of the TRPV family function as sensors as well as Ca2+ transporters
CaT1 and ECaC are TRP-related, six-membrane-spanning channels, belonging to a gene subfamily comprising six members (Peng et al. 2001a). The first TRP protein was identified in Drosophila photoreceptor cells and the name TRP was based on the gene locus trp, which, when mutated, causes a transient receptor potential (light response) rather than a sustained response, thereby disrupting visual excitation downstream of IP3 production (Montell & Rubin, 1989). This gene subfamily was recently termed TRPV, with the V denoting ‘vanilloid’ because the first mammalian member of the family identified was the vanilloid receptor VR1 (Montell et al. 2002). The TRPV ion channels are much more similar to the Caenorhabditis elegans OSM-9 channel, which is required for olfaction, mechanosensation, and olfactory adaptation (Colbert et al. 1997). VR1 (TRPV1) (Caterina et al. 1997), as well as CaT1 (TRPV6) (Peng et al. 1999) and ECaC (TRPV5) (Hoenderop et al. 1999), were identified using an expression cloning approach based on their capacity to mediate an increase in intracellular Ca2+ in transfected cells (VR1) or Ca2+ influx into Xenopus laevis oocytes (CaT1, ECaC). The other members of the family, VRL-1/GRC (TRPV2) (Caterina et al. 1999; Kanzaki et al. 1999), OTRPC4/VR-OAC/VRL-2/Trp12 (TRPV4) (Strotmann et al. 2000; Liedtke et al. 2000; Wissenbach et al. 2000; Delany et al. 2001) and TRPV3/VRL3 (Smith et al. 2002; Xu et al. 2002; Peier et al. 2002), were identified based on sequence homology. All of the six human genes in the family have now been identified (Peng et al. 2001a). The TRPV family has two branches (Fig. 1): CaT1 and ECaC with 75.6 % amino acid identity and the other four channels with ˜30 % identity to CaT1 and ECaC. CaT1 and ECaC are Ca2+-selective channels and serve as apical Ca2+ entry mechanisms in Ca2+-transporting epithelia; the other four members are nonselective channels and serve as sensors. VR1, VRL-1 and TRPV3 are heat sensors and are activated over different temperature ranges (Clapham, 2002a). VR1 is also ligand gated and can be activated by capsaicin (Caterina et al. 1997). OTRPC4 is activated under hypotonic conditions and serves as an osmoreceptor (Strotmann et al. 2000; Liedtke et al. 2000). The CaT1 and ECaC genes, TRPV6 and TRPV5, respectively, are localized on chromosome 7q33–35; the genes for the three heat sensors (TRPV1–3) are localized in 17p11.2–13.3, while the OTRPC4 gene (TRPV4) is on a different chromosome (12q24.1). Thus there is a good relationship between the chromosomal localization and the functions of the genes in this family (Table 1), suggesting that gene duplication events occurred during the development of the gene family.
Figure 1. Phylogenetic tree of the TRP supper family and related channels.

The tree was generated with the TREEVIEW program (Page, 1996) using sequence alignment produced by ClustalW at http://www.ebi.ac.uk/clustalw/#. The bar indicates 0.1 amino-acid substitutions per site. Human protein sequences were used for the alignment. Each sequence was obtained by input individual gene name in the UniGene web site: http://www.ncbi.nlm.nih.gov/UniGene/
Table 1.
A family of Ca2+-permeable channles encoded by TRPV genes
| Gene | Locus# | Protein | Function | Distribution* | PCa/PNa | Activation | Inhibition |
|---|---|---|---|---|---|---|---|
| TRPV1 | 17p13.3 | VR1 | Heat sensor | Trieminal and dorsal root ganglia | 9.6 (capsaicin-activated current) 3.8 (heat-activated current) | Heat (threshold 42° C), capsaicin, resiniferatoxin, anandamide, acidic pH | Ruthenium red (IC50∼0.15 μM), capsazepine, iodo-resiniferatoxin, Sb-366791 |
| TRVP2 | 17p11.2 | VRL-1GRC | Heat sensor | Brain, spinal cord, lung, spleem, intestine, kidney | 2.9 (heat-activated current) | Heat (threshold 53° C) | Ruthenium red (IC50 < 1 μM), La3+, SKF 96365 |
| TRPV3 | 17p13.3 | VRL3, TRPV3 | Heat sensor | Central nervous system, spinal cord, skin, testis | 12.1 (heat-activated current) | Heat (threshold 37 °C) | Ruthenium red (IC50 < 10 μM) |
| TRPV4 | 12q24.1 | OTRPC4 (VR-OAC, VRL,TRP12) | Osmoreceptor | Kidney, trachea, salivary gland, fat, testis | 6 | Hypotonic osmolarity, 4α-phorbol didecanoate, | Ruthenium red (IC50 < 1 μ M), La3+, Gd3+ |
| TRPV5 | 7q35 | ECaC (ECaCl, CaT2) | Apical Ca2+ transporter | Kidney | 107 | Constitutive active, low [Ca2+]I, alkaline pH | Ruthenium red (IC50∼0.1 μM), Gd3+, La3+, Cd2+, econazole, miconazole |
| TRPV6 | 7q33–34 | CATI (ECAC2, CaT-L) | Apical Ca2+ transporter | Intestine, placenta, pancreas, prostate, salivary gland, testis, kidney | 130 | Constitutive active, low [Ca2+]i Ca2+ store depletion alkaline pH | Ruthenium red, (IC50∼9μM), Gd3+, La3+, Cd2+, Pb2+, econazole, miconazole |
Only tissues with high expression levels are llisted.
Gene mapping data are based on that provided in the Uni Gene web site at http://www.ncbi.nlm.nih.gov/UniGene/
This group of ion channels and the other channels in the TRP supperfamily, the TRPC (canonical) and TRPM (melastatin) products (Montell et al. 2002), together with polycystin 2 and related proteins (products of PKD2, PKD2L and PKD2L2 genes), the mucolipins (products of MCOLN1–3), and sperm-associated cation channels (products of CATSPER1–2), share a common six-membrane-spanning molecular architecture with hyperpolarization-activated and cyclic nucleotide-gated K+ channels (HCN and CNG products) as well as voltage-gated and Ca2+-activated K+ channels (some KCN products). The TRPV and TRPC family members also have three to four ankyrin repeats in their N-terminal region. However, very little sequence identity is shared between the TRPV and the ‘canonical’TRPC channels. When CaT1 is aligned with the TRPC family members using the BLAST engine for local alignment with the default setting, there is no significant homology between CaT1 and TRPC1 or TRPC6, and very low homology (18–24 % identities) between CaT1 and TRPC3, TRPC4, TRPC5, and TRPC7 over a stretch of 136–147 amino acids covering the putative pore region and transmembrane domain 6. Using the same approach, CaT1 shows 23 % identity to polycystin 2 over a stretch of 227 amino acids, 24 % to melastatin 1 (TRPM1) over a stretch of 143 amino acids and no significant similarity to the recently identified mucolipins (MCOLN1–3). The phylogenetic tree (Fig. 1) for the human TRP, PKD2 and MCOLN families shows the sequence diversity of this group of functional channels.
Functional and pharmacological properties
When expressed in X. laevis oocytes, CaT1 and ECaC show constitutive activity and saturation kinetics with apparent K0.5 values ranging from 0.2 to 0.66 mM (Peng et al. 1999, 2000a; 2000b Hoenderop et al. 1999). Ca2+ influx is not coupled to Na+, Cl− or H+ gradients, though transport activities are sensitive to pH, with increased activities at alkaline pH. Like most electrogenic transport processes, the current-voltage relationships of both channels suggest that a hyperpolarizing potential favours Ca2+ influx. Both CaT1 and ECaC are permeable to Ba2+ and Sr2+ but not to Mg2+. The macroscopic properties of the channels indicate that they function as facilitative transporters, mediating cellular uptake of calcium down its electrochemical gradient, with saturation kinetics but no obvious gating mechanisms (e.g. by ligand or voltage).
As members of the family of six-membrane-spanning channels, both CaT1 and ECaC exhibit characteristic pore and single channel properties (Nilius et al. 2000; Yue et al. 2001; Vassilev et al. 2001). In the nominal absence of extracellular divalent cations, when using Na+ as charge carrier, single channel conductances of 42 and 78 pS were obtained for rat CaT1 (Yue et al. 2001) and rabbit ECaC, respectively (Nilius et al. 2000). Single channel activities for these channels using divalent cations as charge carriers have not yet been reported. Both channels show high selectivity for Ca2+, with Ca2+-to-Na+ permeability ratios (PCa/PNa) of over 100. In the presence of monovalent cations and Ca2+ these channels exhibit the so-called anomalous mole fraction behaviour - currents decrease initially as extracellular Ca2+ levels are reduced, but at very low extracellular Ca2+ levels, currents then increase beyond the amplitude seen in the presence of high Ca2+ levels, due to the increasing permeability to monovalent cations. This behaviour is thought to be related to the affinity difference between monovalent and divalent cations in the channel pore. The presence of extracellular Mg2+ was shown to block the monovalent cation currents of CaT1 (Yue et al. 2001) and ECaC (Nilius et al. 2000). Mg2+ blockage may contribute to the inward rectification of the monovalent cation currents, possibly preventing the exit of Ca2+ under depolarizing conditions. However, CaT1 also has intrinsic inward rectification, independent of Mg2+ blockage (Voets et al. 2003).
CaT1 and ECaC can be blocked by trivalent and divalent cations. La3+, Gd3+, Pb2+, Cd2+ and Cu2+ are among the most effective blockers of CaT1 and ECaC (Peng et al. 1999, 2000a; 2000b; Nilius et al. 2001a). The difference in the IC50 for inhibition by Cd2+ (˜5 μM) and the K0.5 for the Cd2+ current (1.3 mM) suggest that two binding sites are present in the ECaC (CaT2) channel (Peng et al. 2000a). The CaT1 and ECaC channels are relatively insensitive to L-type voltage-gated channels blockers (Peng et al. 1999, 2000 a; Hoenderop et al. 1999). The classical L-type channel blockers nifedipine, diltiazem and verapamil (100 μM) inhibited CaT1-mediated Ca2+ uptake by 10–15 % (Peng et al. 1999) and ECaC-mediated Ca2+ currents by less than 10 % (Peng et al. 2000a). Preincubation with verapamil increased the inhibition of ECaC-mediated Ca2+ current to ˜ 50 % (Peng et al. 2000a). The imidazole derivatives econazole and miconazole have been found to be among the most effective organic blockers of ECaC with IC50 values of 1.27 μM and 1.77 μM for the Na+ current, respectively (Nilius et al. 2001a). Another imidazole derivative, SKF96365, is rather ineffective in blocking ECaC (Nilius et al. 2001a). Econazole, miconazole and SKF96365 have also been shown to inhibit Ca2+ uptake in oocytes expressing CaT1 and ECaC, with econazole being the most effective inhibitor (˜50 % inhibition at 50 μM). SKF96365 was almost ineffective at this concentration (J.-B. Peng & M. A. Hediger, unpublished observations). Ruthenium red is currently the most effective blocker of ECaC (IC50= 111 nM) (Nilius et al. 2001a), but it is a much less effective blocker of CaT1 (IC50= 9 μM) (Hoenderop et al. 2001b). None of the above blockers are specific enough to discriminate these channels from others, and the mechanisms of inhibition are unclear.
A single negatively charged residue, Asp542, was found to determine Ca2+ permeation and Mg2+ blockade in ECaC (Nilius et al. 2001b). However, another study indicated that Asp542 plays a critical role in Ca2+ and Mg2+ affinity but that it is not a major determinant of Ca2+ permeation (Jean et al. 2002). The corresponding residue in CaT1 is also Asp542 and it is critical for Ca2+ transport mediated by CaT1 (Fig. 2; J.-B. Peng & M. A. Hediger, unpublished observation). The corresponding aspartate residue in VR1 was reported to determine the pore properties of VR1, including the permeability to Mg2+ and sensitivity to ruthenium red (Garcia-Martinez et al. 2000). The residue was suggested to form a ring of negative charges that produces a high affinity binding site for cationic molecules at the extracellular entryway of VR1 (Garcia-Martinez et al. 2000). As ECaC, CaT1 and VR1 share significant sequence similarity, and yet VR1 is a nonselective cation channel, it therefore appears that additional residues in CaT1 and ECaC are required for the unique high selectivity of these channels for Ca2+ over monovalent cations and Mg2+.
Figure 2. Molecular architecture and feedback inactivation of CaT1.
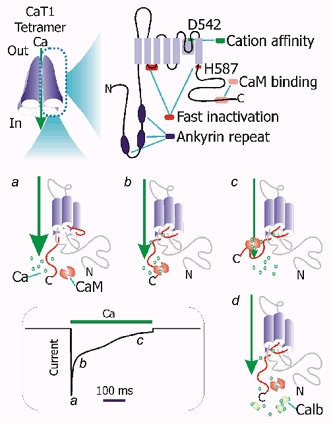
Upper panel: key domains and residues in CaT1. A CaT1 tetramer, the functional unit of the channel, is shown on the left. Ankyrin repeats may mediate protein-protein interaction; D542 is a key residue cation affinity or permeation. The first intracellular loop and H587 are involved in the fast phase of inactivation. The calmodulin (CaM) binding site close to the carboxyl terminus is involved in the slow phase of Ca2+-dependent inactivation. Lower panel: fast and slow phases of inactivation and involved residues and domains in CaT1. A typical Ca2+-dependent inactivation process of Ca2+ current of CaT1 is shown in the box based on that described by Niemeyer et al. (2001) and Nilius et al.(2002). The currents and the presumed corresponding CaT1 states are labelled a, b, and c; d shows the putative role of calbindin D9k (Calb) on Ca2+ influx: buffering the Ca2+ underneath the channel thereby releases the channel from Ca2+ feedback inhibition.
Auto-regulatory mechanism by intracellular Ca2+
As sustained increases in intracellular Ca2+ may cause cell death, Ca2+-transporting cells express calbindins to buffer the increases in intracellular Ca2+ resulting from calcium entry through these channels. To avoid elevations in intracellular Ca2+ to toxic levels that are beyond the buffering capacity of the calbindins, both CaT1 and ECaC exhibit Ca2+-dependent inactivation (Niemeyer et al. 2001; Nilius et al. 2002). CaT1 shows both a fast phase (within 50 ms) and a slow phase of inactivation (over a period of ˜1 s). The slow phase involves direct binding of calmodulin to the CaT1 C-terminal region (Niemeyer et al. 2001) (Fig. 2). CaT1′s calmodulin binding site is conserved among different species, but is not present in the same region of ECaC (Niemeyer et al. 2001). Ca2+-dependent binding of calmodulin to CaT1 inactivates the CaT1 channel. A protein kinase C site is present within the calmodulin binding site in human CaT1 but not in the other species studied so far. The phosphorylation of this protein kinase C site prevents calmodulin binding, thereby maintaining the activity of CaT1 so as to allow more Ca2+ to enter the cell (Niemeyer et al. 2001). ECaC shows essentially no fast phase of inactivation compared with CaT1. The first intracellular loop of CaT1 determines the fast phase of inactivation (Nilius et al. 2002) and its sequence is not conserved between CaT1 and ECaC (Fig. 2). Of note, the first intracellular loop is entirely encoded by one exon - an example of a single exon endowing a distinct functional feature. In addition to the first intracellular loop, H587, a positively charged amino acid residue downstream of the last transmembrane domain of CaT1 has also been identified as being involved in fast inactivation of CaT1 (Suzuki et al. 2002).
The feedback inhibition is a two-edged sword: it protects the cell from Ca2+ overload but it also limits the flow of Ca2+ into the cell. CaT1 activity is inversely related to the intracellular Ca2+ level on its cytoplasmic side (Bodding et al. 2002). Thus, the availability of calbindin D9k to buffer the local increase in Ca2+ will reduce the Ca2+ feedback inhibition and in turn, increase the flow of Ca2+ into the cell. Therefore, a coordinated increase in the expression of both the apical channel and intracellular calbindin is necessary to achieve maximal Ca2+ influx at the apical side (Fig. 2d).
Roles in Ca2+-transporting epithelia
The intestine, kidney and placenta are three major organs involved in Ca2+ transport that participate in Ca2+ absorption from the diet, renal tubular Ca2+ reabsorption, and Ca2+ transport from the maternal to the fetal circulation, respectively. The transcellular pathway which allows uphill Ca2+ transport against an electrochemical gradient occurs mostly in the proximal intestine, the distal tubule of the nephron and the placental syncytium. In this pathway, Ca2+ crosses the polarized epithelium by entering the epithelial cells across the apical membrane and exiting across the basolateral membrane. The extracellular Ca2+ concentration is in the millimolar range and the intracellular Ca2+ concentration is around 100 nM; thus an energy-consuming process, mediated by the Ca2+ pump and/or the Na+-Ca2+ exchanger, is used to overcome the ˜10 000 times Ca2+ concentration difference and the ˜60 mV membrane potential difference, allowing Ca2+ to exit the cell. In contrast to the basolateral side, there is a favourable electrochemical gradient for Ca2+ entry at the apical membrane. The proteins that mediate this step should work like facilitative transporters with saturation kinetics in the millimolar range but without any ion coupling or gating mechanisms. Both CaT1 and ECaC possess these properties and are well suited as apical Ca2+ entry mechanisms. As the Ca2+ concentration in the intestinal lumen can vary greatly, the sudden appearance of a high level of Ca2+ in the intestinal lumen could be disastrous. Therefore, a fast inactivation mechanism is needed for the intestinal channel. In contrast, a fast inactivation mechanism is not necessary in the renal distal tubule, where the Ca2+ concentration is relatively constant. Thus, the fast inactivation property of CaT1 makes it more suitable for the intestinal environment.
Indeed, CaT1 is the major apical Ca2+ entry channel in the mouse (Weber et al. 2001; Van Cromphaut et al. 2001), rat (Peng et al. 2000a) and human intestine (Barley et al. 2001; Peng 2000a; 2000b). CaT1 protein localizes in the apical membrane of the small and large intestines of both mouse and human (Zhuang et al. 2002). In the kidney, however, ECaC is the major apical channel in the mouse (Van Cromphaut et al. 2001) and rat (Peng et al. 2000a); in human kidney, in contrast, we found that the abundance of CaT1 mRNA is about 10 times greater than that of ECaC (Peng et al. 2001a). An apical localization of ECaC has been observed in rabbit (Hoenderop et al. 2000), rat (Hoenderop et al. 2001a) and mouse kidney (Loffing et al. 2001) in the distal segment of the nephron. In all of the animal models examined to date, ECaC is expressed in the distal segment (DCT-2) of the distal convoluted tubule and connecting tubule (CNT), in the same cells that contain additional machinery for cytosolic Ca2+ transport, including calbindin D28k and the basolateral Na+-Ca2+ exchanger 1 (NCX1). The localization of CaT1 in the kidney is less clear. It has been reported to be expressed in the thick ascending limb (Suzuki et al. 2000), and more recently, to be co-expressed with ECaC in the distal tubules, where it has been suggested to form a heterotetramer with the latter (Hoenderop et al. 2003). Using mRNA samples from human kidney cortex and outer medulla, we found that the level of ECaC mRNA in the cortex is 3 times that in the outer medulla. In contrast, CaT1 mRNA appears to be higher in the outer medulla than in the cortex (J.-B. Peng & M. A. Hediger, unpublished observation). Given the great differences in the localization and levels of expression of the two channels reported to date, the likelihood and significance of heterotetramerization of the two proteins in vivo requires further evaluation.
In the human placenta, the level of CaT1 mRNA is about 1000 times that of ECaC (Peng et al. 2001a). In situ hybridization demonstrated the presence of CaT1 transcript in trophoblasts and syncytiotrophoblasts (Wissenbach et al. 2001). In cultured cytotrophoblast cells isolated from human term placenta, Ca2+ uptake activity was correlated with the secretion of human chorionic gonadotrophin and the mRNA levels of CaT1 and ECaC (Moreau et al. 2002b). The uptake of Ca2+ by trophoblast cells was insensitive to L-type Ca2+ channel modulators but was inhibited by ruthenium red with an IC50 of 9 μM, indicating that CaT1 is the major channel. This is consistent with the report that CaT1 but not ECaC was detected by Northern blot in these cells (Moreau et al. 2002a).
Calcium absorption is regulated by 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), the active form of vitamin D. Studies on the action of 1,25(OH)2D3 on Ca2+ transport have focused on the calbindins (Wasserman & Fullmer, 1995; Christakos et al. 1992). The possible significance of the effects of 1,25(OH)2D3 on CaT1 expression was first explored in a human intestinal cell line, Caco-2, which has been used for studies of Ca2+ transport (Wood et al. 2001). The induction of CaT1 by 1,25(OH)2D3 in Caco-2 cells was more robust than that of calbindin D9k and preceded the latter by several hours (Wood et al. 2001). Regulation of CaT1 by vitamin D was soon confirmed in a study using vitamin D receptor-null mice (Van Cromphaut et al. 2001). Duodenal CaT1 mRNA was reduced more than 90 % with a 3-fold decrease in calcium absorption in two VDR-KO strains on a normal calcium diet (Van Cromphaut et al. 2001). Calbindin D9k was decreased only in one strain and plasma membrane Ca2+-ATPase PMCA1b expression was normal in both VDR-null strains, indicating that the decrease in CaT1 expression is responsible for the decrease in calcium absorption (Van Cromphaut et al. 2001). These two studies both show that among the proteins involved in transcellular Ca2+ transport the apical entry channel CaT1 is most robustly regulated by vitamin D (Wood et al. 2001; Van Cromphaut et al. 2001). CaT1 mRNA expression level, as well as its induction by 1,25(OH)2D3 correlate better to transcellular calcium transport in Caco-2 cell lines than calbindin D9k and PMCA1 (Fleet et al. 2002). These studies suggest that instead of the intracellular diffusion step mediated by calbindin D9k, the CaT1-mediated apical entry step is the rate-limiting step for vitamin D-regulated Ca2+ transport.
In a study using human duodenal biopsies from 20 normal subjects, CaT1 mRNA levels were not significantly correlated with vitamin D metabolites, but were moderately correlated with calbindin D9k and more strongly with the PMCA1 (Barley et al. 2001). However, the results of this study may have been affected considerably by the uncontrolled calcium intake of the subjects, a factor that was later found to have a significant impact on CaT1 expression (Van Cromphaut et al. 2001). CaT1 expression was greatly reduced on a high calcium diet and greatly increased on a low calcium diet (Van Cromphaut et al. 2001), and the extent of the reduction was independent of the vitamin D receptor (Song et al. 2003).
Vitamin D regulation of ECaC in the kidney is controversial. It has been reported that vitamin D depletion reduces the expression of ECaC in rat kidney and that repletion of vitamin D restores its expression (Hoenderop et al. 2001a); however, in the VDR-null mice, ECaC expression was not decreased in two strains of VDR-null mice on a normal diet (Van Cromphaut et al. 2001). In mice lacking 25-hydroxyvitamin D3-1α-hydroxylase (1α-OHase), a key enzyme for 1,25(OH)2D3 production, substantial decreases in the levels of expression of ECaC, calbindins and the Na+-Ca2+ exchanger 1 (NCX1) were found, along with a decrease in serum calcium level (Hoenderop et al. 2002). High calcium intake restored the levels of ECaC, calbindin D28k (but not the calbindin D9k) and NCX1, as well as the serum calcium level (Hoenderop et al. 2002). It appears that the level of expression of ECaC is positively regulated by the calcium load indicated by the serum calcium level (Hoenderop et al. 2002). The apparent increase in ECaC induced by 1,25(OH)2D3 may reflect the increase in the calcium load in the kidney as a result of increased expression of intestinal CaT1. In contrast to the observation that only duodenal CaT1 in the VDR-null mouse was severely reduced compared to calbindin D9k and PMCA1b, in the 1α-OHase null mice on a normal diet, coordinated changes of ECaC, calbindins D9k and D28k and NCX1 were observed. This indicates that transcellular Ca2+ transport in the distal tubules is a coordinated process involving all of the participating proteins.
CaT1 and the Ca2+ release-activated Ca2+ (CRAC) channel
Ca2+ entry in Ca2+-transporting cells does not require a specific signal; in other nonexcitable cells, however, Ca2+ enters in response to the release of Ca2+ from intracellular calcium stores - a process termed ‘capacitative Ca2+ entry’ or ‘store-operated Ca2+ entry’ (Putney, 1997; Parekh & Penner, 1997). In this process, plasma membrane Ca2+ channels are activated in response to calcium store depletion, often upon stimulation by a G protein-coupled receptor. The Ca2+ release-activated Ca2+ (CRAC) channel refers to the store-operated channel in the rat basophilic leukemia (RBL) and Jurkat T-lymphocyte cell lines. Its biophysical properties have been extensively studied compared to other store-operated channels described in a variety of cell types. The molecular identity of the CRAC channel is still unknown and its identification is of significant importance.
When using Na+ as a charge carrier, in the absence of divalent cations, single channel recordings in Jurkat T-lymphocytes uncovered a channel activity that was attributed to CRAC channels (Kerschbaum & Cahalan, 1999). Interestingly, CaT1, when expressed in Chinese hamster ovary cells, exhibited pore properties indistinguishable in many respects from those of CRAC (Yue et al. 2001). These include high selectivity for Ca2+ (PCa/PNa > 100), an order of selectivity for divalent cations (Ca2+ >> Ba2+ > Sr2+ > Mn2+), loss of selectivity in the absence of divalent cations, block by La3+, the anomalous mole fraction effect, whole-cell current kinetics and single channel conductance to Na+ in divalent ion-free conditions (Yue et al. 2001). In addition, when CaT1 is expressed at low levels, it can be activated by depletion of calcium stores. Thus CaT1 was proposed to constitute part or all of the CRAC channel pore, with the assumption that other cellular components are necessary for controlling CaT1 via calcium stores (Yue et al. 2001).
Despite the similarity of the CaT1 and CRAC channel currents, a comparison of CaT1 expressed in human embryonic kidney (HEK 293) cells and CRAC in rat basophilic leukaemia (RBL) cells revealed certain differences between the CaT1 and CRAC channel properties (Voets et al. 2001). These include the following: (1) CRAC (but not CaT1) can be activated by ionomycin-mediated store depletion and can be blocked by 2-aminoethoxydiphenyl borate (2-APB); (2) CaT1 (but not CRAC) can be blocked in a voltage-dependent manner by an increase in intracellular Mg2+; and (3) the channels display a difference in relative permeability to Na+ and Cs+ (Voets et al. 2001). Further comparison of the properties of CaT1 exogenously expressed in HEK 293 cells and RBL cells revealed that CaT1 current was constitutively active in HEK 293 cells, whereas in RBL cells it was either constitutively active at a high current density or was activated by calcium store depletion - a matter which depends on the expression levels of CaT1 (and perhaps also on the matched up-regulation of other cellular components) (Schindl et al. 2002). Both constitutively active and store depletion-activated CaT1 currents were distinguishable from endogenous ICRAC in RBL cells by their current-voltage relationship in divalent cation-free conditions. The store depletion-activated CaT1 current was blocked by 2-APB, as was the endogenous ICRAC, indicating that a common 2-APB-sensitive regulatory component controls the activation of both CRAC and CaT1 in the RBL (Schindl et al. 2002). Another study showed that exogenously expressed human CaT1 in RBL and HEK 293 cells was not activated by store depletion; rather, the activity of CaT1 is affected by the local Ca2+ concentration close to the pore (Bodding et al. 2002). The authors suggested that CaT1 activity is inversely related to the intracellular Ca2+ levels and that it serves as an intracellular Ca2+ sensor in these cells (Bodding et al. 2002).
In spite of the studies showing that the CaT1 current may not be ICRAC, studies in Jurkat T-lymphocytes (Cui et al. 2002) and in the prostate cancer epithelial cell line LNCaP (Vanden Abeele et al. 2003) suggest that CaT1 contributes significantly to the endogenous store-operated Ca2+ entry in both cells. The CaT1 mRNA level in Jurkat T-lymphocytes is 2.5 % of that detected in placenta (Cui et al. 2002) - a level that is comparable to CaT1 expression in epithelial cells of the small intestine. CaT1 contributed to the store-operated currents when expressed in Jurkat T-lymphocytes, and over-expression of a dominant negative CaT1 mutant in the same cells resulted in suppression of endogenous ICRAC (Cui et al. 2002). In LNCaP cells, in which CaT1 is endogenously expressed at a considerable level and is negatively regulated by androgen (Peng et al. 2001b), anti-sense hybrid depletion of CaT1 decreased store-operated current by approximately 50 %, whereas up-regulation of CaT1 mRNA by 60 % through anti-androgen treatment enhanced the current by 30 % (Vanden Abeele et al. 2003). The CaT1 contribution may have been underestimated, given that the anti-sense approach generally cannot completely eliminate CaT1 expression. Thus, CaT1 appears to represent a major component of the store-operated Ca2+ entry in LNCaP cells.
Recently, the biophysical properties of the CRAC channel have been redefined since recent studies using Jurkat T-lymphocytes revealed that the monovalent cation current previously attributed to ICRAC (Kerschbaum & Cahalan, 1999) corresponds to the Mg2+-inhibited cation current (IMIC) (Prakriya & Lewis, 2002) (also known as the Mg-nucleotide-regulated metal ion currents (MagNuM); Hermosura et al. 2002). A similar conclusion was reached based on studies using RBL cells (Kozak et al. 2002); Bakowski & Parekh, 2002a). The IMIC is likely to be mediated by TRPM7 (also known as ChaK1; Ryazanov et al. 1999), LTRPC7 (Nadler et al. 2001) and TRP-PLIK (Runnels et al. 2001)). The following properties of ICRAC differ from IMIC: activation by store depletion, resistance to suppression by 8 mM intracellular Mg2+, inhibition by SKF 96365 and low Cs+ permeability (Prakriya & Lewis, 2002). The single channel openings of the CRAC channel in Jurkat T-lymphocyte were not detectable, even under divalent cation-free conditions, when using a monovalent cation as a charge carrier, and a 0.2 pS conductance was estimated from noise analysis (Prakriya & Lewis, 2002), much lower than that estimated for CaT1 (42 pS). In contrast, IMIC (TRPM7?) has a ˜44 pS single channel conductance (Prakriya & Lewis, 2002). Thus, the newly described biophysical properties of the CRAC channel differ significantly from those of CaT1, calling into question whether and/or to what extent CaT1 contributes to the CRAC channel (Clapham, 2002b) (for a detailed discussion see Bakowski & Parekh, 2002b).
The CRAC channel is one of the store-operated Ca2+ channels whose properties have been well defined in RBL cells and Jurkat T-lymphocytes using biophysical approaches (see Clapham, 2002b). Store-operated Ca2+ entry or capacitative Ca2+ entry has been described in many cell types (Parekh & Penner, 1997). CRAC and/or other Ca2+-permeable channels contribute to Ca2+ entry in a variety of cells such as prostate cancer LNCaP cells (Skryma et al. 2000) and pancreatic acinar cells (Raraty et al. 2000). Whether or not CaT1 is a part of the CRAC pore, it probably plays an important role in store-operated Ca2+ entry in cells where it is highly expressed such as in Jurkat T-lymphocytes (Cui et al. 2002), LNCaP cells (Vanden Abeele et al. 2003) and probably also pancreatic acinar and other secretory cells. Given that CaT1 is a constitutively active calcium entry channel, one question that arises is how CaT1 can play a role as a store-operated channel. CaT1 is constitutively active on its own, consistent with its role in Ca2+ transporting epithelia (Fig. 3; see constitutively active mode). We propose that, in other cells, including those not involved in Ca2+ transport, CaT1 is inactive until it is stimulated. As previously suggested (Yue et al. 2001) and further supported by another study (Schindl et al. 2002), other cellular components may govern the store-operated property of CaT1. These components may function as a molecular switch, turning off CaT1 when it is bound to the channel (Fig. 3, molecular switch mode). The component may be present in cells with significant store-operated Ca2+ entry pathways but absent in Ca2+ transporting epithelia. It is likely that 2-APB acts on this component rather than on the channel itself (Schindl et al. 2002).
Figure 3. Modes of CaT1 activation.
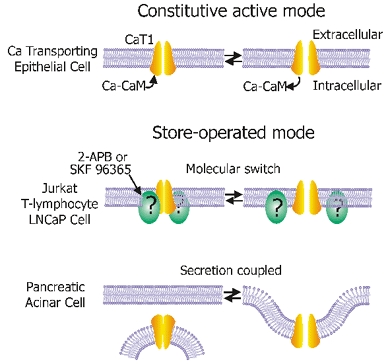
Constitutive active mode: CaT1 alone is constitutively active and works in this mode in Ca2+-transporting epithelia such as the brush-border membrane of small intestine. The channels are subject to Ca2+-dependent feedback regulation and this process is partially mediated by calmodulin (CaM). Store-operated mode: CaT1 is not active in unstimulated cells and is activated by calcium store depletion. Two putative mechanisms are shown. Molecular switch mode: another cellular component serves as a molecular switch to shut CaT1 off when it is associated with CaT1 or to activate CaT1 upon its dissociation. This component might be the target of 2-APB or SKF 96365, which may promote the association state, as suggested by studies in Jurkat T-lymphocytes or LNCaP cells. Secretion-coupled mode: inactivated CaT1 may localize in the membrane of intracellular vesicles. Upon exocytosis, CaT1 is incorporated into plasma membrane. The plasma membrane-associated CaT1 takes up Ca2+ released from the vesicle to refill the calcium store or to trigger further exocytosis.
CaT1 function in exocrine tissues
The tissues that express CaT1 at the highest levels, as assessed by Northern analysis or in situ hybridization, are those with exocrine functions, including pancreas, salivary gland and prostate (Peng et al. 2000b; Wissenbach et al. 2001). The use of immunocytochemistry confirmed CaT1 expression in these tissues and also revealed the expression of CaT1 in stomach, mammary gland and sweat gland (Zhuang et al. 2002).
The roles of CaT1 in these glandular tissues have not yet been studied, but it is possible that it serves some common function. If CaT1 is present in the membrane of zymogen granules, it would have to be in an inactive state to prevent release of the calcium from the zymogen granules. Once it is incorporated into the plasma membrane, it would then become active to take up Ca2+ released from the granule. In the pancreas, CaT1 staining was mostly restricted to the apical secretory pole, with both intracellular and apical membrane staining (Zhuang et al. 2002). This pattern of immunostaining suggests the possibility that CaT1 is localized in zymogen granules, although further studies are needed for verification. The proteins for cellular signalling, including the IP3 receptors (Nathanson et al. 1994; Yule et al. 1997) and G protein-coupled receptors such as the muscarinic type 3 receptor and cholecystokinin receptors (Shin et al. 2001), are localized in the apical region. The localization of CaT1 in the granular area overlaps with the small trigger zone where Ca2+ waves and oscillations originate in a pancreatic acinar cell (Kasai et al. 1993). As CaT1 is a Ca2+-selective Ca2+ channel, its involvement in Ca2+ signalling in pancreatic acinar cells is likely. However, the role of CaT1 in the process has not been shown. CaT1 may play a role in the clearance of Ca2+ released from the zymogen granules by re-uptake of Ca2+ into the cells, thereby replenishing the Ca2+ pool - equivalent to store-operated Ca2+ entry (Fig. 3; secretion-coupled mode). Hyperstimulation of pancreatic acinar cells by cholecystokinin evokes a sustained increase of intracellular Ca2+ due to store-operated Ca2+ entry which in turn causes trypsin activation and vacuole formation in the apical granular pole similar to what happens in acute pancreatitis (Raraty et al. 2000). The possible role of CaT1 in this process has yet to be investigated.
The reuptake mechanism is necessary for exocrine function as secretory vesicles have high calcium content (e.g. 10–100 mM). The coincidence of the apical localization of the Ca2+ selective channel CaT1 in exocrine glandular cells in the pancreas, prostate, stomach and mammary gland (Zhuang et al. 2002) and the high calcium content in the secretory vesicles suggest a common reuptake mechanism in these cells. While CaT1 is highly expressed in pancreatic acinar cells, ECaC has been reported to be present in the pancreatic islets (Janssen et al. 2002), suggesting distinct roles of these two channels in exocrine and endocrine function, respectively.
Up-regulation of CaT1 expression in cancers
Up-regulation of CaT1 in prostate cancer was reported at the mRNA level (Peng et al. 2001b; Wissenbach et al. 2001) and later confirmed at the protein level (Zhuang et al. 2002). We found that CaT1 was expressed in both benign prostatic hyperplastic (BPH) tissues and prostate cancers in the epithelial cells. However, the CaT1 transcript was significantly increased in cancerous prostate tissues. The expression level of CaT1 mRNA increased with the degree of malignancy of prostate cancer (Peng et al. 2001b). Wissenbach et al. (2001) did not detect CaT1 expression in BPH samples or in the organ-confined primary Gleason grade 3 tumour. In contrast, the highest levels of CaT1 mRNA were detected in high-grade tumours with extraprostatic extension. Even higher levels of CaT1 expression were detected in prostate cancer with lymph node metastases and in recurrent lesions (Wissenbach et al. 2001).
In the androgen-sensitive prostatic cancer cell line LNCaP, which expresses CaT1 endogenously, the CaT1 transcript level is negatively regulated by androgen (Peng et al. 2001b). CaT1 expression levels in the LNCaP cells is positively related to the store-operated Ca2+ entry in the cells (Vanden Abeele et al. 2003). If this is also true in vivo, then androgen withdrawal therapy could increase CaT1 expression, which might be a signal for proliferation of the tumour cells. Conversely, strategies that reduced CaT1 expression could be beneficial in the treatment of prostate cancer.
Pathological up-regulation of CaT1 does not appear to be restricted to prostate cancer but seems to be a general phenomenon of cancers of epithelial origin. Zhuang et al. (2002) found CaT1 protein to be present at elevated levels in comparison with normal tissues in a series of prostate, breast, thyroid, colon and ovarian carcinomas, consistent with previous reports of up-regulation of CaT1 mRNA in prostate cancer tissues. Increased CaT1 expression might mean an increase in the level of intracellular Ca2+ (if CaT1 is in the constitutively active mode), or increased Ca2+ influx in response to stimuli (if CaT1 is in store-operated mode) or both. Ca2+ is involved in cell differentiation and proliferation, as well as apoptosis (Berridge et al. 2000). If CaT1 expression is linked to cell proliferation and/or apoptosis, altered CaT1 function might have significant implications for tumour growth. Thus, inhibition of CaT1 might be a therapeutic strategy to prevent uncontrolled growth of cancers of epithelial origin.
Concluding remarks
Recent studies of the Ca2+ entry channels CaT1 and ECaC have provided us with a better understanding of the transcellular pathway of Ca2+ transport and its regulation. Although rapid progress has been made in this new field, there are still many issues to be addressed. Neither of the channels has been directly shown to mediate apical calcium entry in the transcellular pathway of calcium transport. The channels have been proposed to serve as gatekeepers of transcellular calcium transport, but the roles of all of the participating proteins have not yet been carefully evaluated in a functional setting, although this will now become possible given the identification of the major players in calcium transport. The roles of CaT1 beyond Ca2+ transport are emerging but are yet to be investigated in further detail. CaT1′s possible role in exocrine function is uncharacterized to date. The cause and the consequence of CaT1 overexpression in cancers may help identify CaT1 as a target for cancer therapy.
REFERENCES
- Bakowski D, Parekh AB. Monovalent cation permeability and Ca2+ block of the store-operated Ca2+ current ICRAC in rat basophilic leukemia cells. Pflugers Arch. 2002a;443:892–902. doi: 10.1007/s00424-001-0775-8. [DOI] [PubMed] [Google Scholar]
- Bakowski D, Parekh AB. Permeation through store-operated CRAC channels in divalent-free solution: potential problems and implications for putative CRAC channel genes. Cell Calcium. 2002b;32:379–391. doi: 10.1016/s0143416002001914. [DOI] [PubMed] [Google Scholar]
- Barley NF, Howard A, O'Callaghan D, Legon S, Walters JR. Epithelial calcium transporter expression in human duodenum. Am J Physiol Gastrointest Liver Physiol. 2001;280:G285–290. doi: 10.1152/ajpgi.2001.280.2.G285. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Bodding M, Wissenbach U, Flockerzi V. The recombinant human TRPV6 channel functions as Ca2+ sensor in human embryonic kidney and rat basophilic leukemia cells. J Biol Chem. 2002;277:36656–36664. doi: 10.1074/jbc.M202822200. [DOI] [PubMed] [Google Scholar]
- Brown EM. Extracellular Ca2+ sensing, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol Rev. 1991;71:371–411. doi: 10.1152/physrev.1991.71.2.371. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Christakos S, Gill R, Lee S, Li H. Molecular aspects of the calbindins. J Nutr. 1992;122:678–682. doi: 10.1093/jn/122.suppl_3.678. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Signal transduction. Hot and cold TRP ion channels. Science. 2002a;295:2228–2229. doi: 10.1126/science.1070766. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Sorting out MIC, TRP, and CRAC ion channels. J Gen Physiol. 2002b;120:217–220. doi: 10.1085/jgp.20028618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Bian JS, Kagan A, McDonald TV. CaT1 contributes to the stores-operated calcium current in Jurkat T-lymphocytes. J Biol Chem. 2002;277:47175–47183. doi: 10.1074/jbc.M205870200. [DOI] [PubMed] [Google Scholar]
- Delany NS, Hurle M, Facer P, Alnadaf T, Plumpton C, Kinghorn I, See CG, Costigan M, Anand P, Woolf CJ, Crowther D, Sanseau P, Tate SN. Identification and characterization of a novel human vanilloid receptor-like protein, VRL-2. Physiol Genomics. 2001;4:165–174. doi: 10.1152/physiolgenomics.2001.4.3.165. [DOI] [PubMed] [Google Scholar]
- Fleet JC, Eksir F, Hance KW, Wood RJ. Vitamin D-inducible calcium transport and gene expression in three Caco-2 cell lines. Am J Physiol Gastrointest Liver Physiol. 2002;283:G618–625. doi: 10.1152/ajpgi.00269.2001. [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez C, Morenilla-Palao C, Planells-Cases R, Merino JM, Ferrer-Montiel A. Identification of an aspartic residue in the P-loop of the vanilloid receptor that modulates pore properties. J Biol Chem. 2000;275:32552–32558. doi: 10.1074/jbc.M002391200. [DOI] [PubMed] [Google Scholar]
- Gerasimenko OV, Gerasimenko JV, Belan PV, Petersen OH. Inositol trisphosphate and cyclic ADP-ribose-mediated release of Ca2+ from single isolated pancreatic zymogen granules. Cell. 1996;84:473–480. doi: 10.1016/s0092-8674(00)81292-1. [DOI] [PubMed] [Google Scholar]
- Hermosura MC, Monteilh-Zoller MK, Scharenberg AM, Penner R, Fleig A. Dissociation of the store-operated calcium current I(CRAC) and the Mg-nucleotide-regulated metal ion current MagNuM. J Physiol. 2002;539:445–458. doi: 10.1113/jphysiol.2001.013361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenderop JG, Dardenne O, Van Abel M, Van Der Kemp AW, Van Os CH, Arnaud R, Bindels RJ. Modulation of renal Ca2+ transport protein genes by dietary Ca2+ and 1,25-dihydroxyvitamin D3 in 25-hydroxyvitamin D3–1 alpha-hydroxylase knockout mice. FASEB J. 2002;16:1398–1406. doi: 10.1096/fj.02-0225com. [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Hartog A, Stuiver M, Doucet A, Willems PH, Bindels RJ. Localization of the epithelial Ca(2+) channel in rabbit kidney and intestine. J Am Soc Nephrol. 2000;11:1171–1178. doi: 10.1681/ASN.V1171171. [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Muller D, Kemp AW, Hartog A, Suzuki M, Ishibashi K, Imai M, Sweep F, Willems PH, Os CH, Bindels RJ. Calcitriol controls the epithelial calcium channel in kidney. J Am Soc Nephrol. 2001a;12:1342–1349. doi: 10.1681/ASN.V1271342. [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Van Der Kemp AW, Hartog A, Van De Graaf SF, Van Os CH, Willems PH, Bindels RJ. Molecular identification of the apical Ca2+ channel in 1,25-dihydroxyvitamin D3-responsive epithelia. J Biol Chem. 1999;274:8375–8378. doi: 10.1074/jbc.274.13.8375. [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Vennekens R, Muller D, Prenen J, Droogmans G, Bindels RJ, Nilius B. Function and expression of the epithelial Ca(2+) channel family: comparison of mammalian ECaC1 and 2. J Physiol. 2001b;537:747–761. doi: 10.1111/j.1469-7793.2001.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenderop JG, Voets T, Hoefs S, Weidema F, Prenen J, Nilius B, Bindels RJ. Homo- and heterotetrameric architecture of the epithelial Ca(2+) channels TRPV5 and TRPV6. EMBO J. 2003;22:776–785. doi: 10.1093/emboj/cdg080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen SW, Hoenderop JG, Hermus AR, Sweep FC, Martens GJ, Bindels RJ. Expression of the novel epithelial Ca2+ channel ECaC1 in rat pancreatic islets. J Histochem Cytochem. 2002;50:789–798. doi: 10.1177/002215540205000605. [DOI] [PubMed] [Google Scholar]
- Jean K, Bernatchez G, Klein H, Garneau L, Sauve R, Parent L. Role of aspartate residues in Ca(2+) affinity and permeation of the distal ECaC1. Am J Physiol Cell Physiol. 2002;282:C665–672. doi: 10.1152/ajpcell.00443.2001. [DOI] [PubMed] [Google Scholar]
- Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biolog. 1999;1:165–170. doi: 10.1038/11086. [DOI] [PubMed] [Google Scholar]
- Kasai H, Li YX, Miyashita Y. Subcellular distribution of Ca2+ release channels underlying Ca2+ waves and oscillations in exocrine pancreas. Cell. 1993;74:669–677. doi: 10.1016/0092-8674(93)90514-q. [DOI] [PubMed] [Google Scholar]
- Kerschbaum HH, Cahalan MD. Single-channel recording of a store-operated Ca2+ channel in Jurkat T lymphocytes. Science. 1999;283:836–839. doi: 10.1126/science.283.5403.836. [DOI] [PubMed] [Google Scholar]
- Kozak JA, Kerschbaum HH, Cahalan MD. Distinct properties of CRAC and MIC channels in RBL cells. J Gen Physiol. 2002;120:221–235. doi: 10.1085/jgp.20028601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffing J, Loffing-Cueni D, Valderrabano V, Klausli L, Hebert SC, Rossier BC, Hoenderop JG, Bindels RJ, Kaissling B. Distribution of transcellular calcium and sodium transport pathways along mouse distal nephron. Am J Physiol Renal Physiol. 2001;281:F1021–1027. doi: 10.1152/ajprenal.0085.2001. [DOI] [PubMed] [Google Scholar]
- Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, Clapham DE, Harteneck C, Heller S, Julius D, Kojima I, Mori Y, Penner R, Prawitt D, Scharenberg AM, Schultz G, Shimizu N, Zhu MX. A unified nomenclature for the superfamily of TRP cation channels. Mol Cell. 2002;9:229–231. doi: 10.1016/s1097-2765(02)00448-3. [DOI] [PubMed] [Google Scholar]
- Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- Moreau R, Daoud G, Bernatchez R, Simoneau L, Masse A, Lafond J. Calcium uptake and calcium transporter expression by trophoblast cells from human term placenta. Biochim Biophys Acta. 2002a;1564:325–332. doi: 10.1016/s0005-2736(02)00466-2. [DOI] [PubMed] [Google Scholar]
- Moreau R, Hamel A, Daoud G, Simoneau L, Lafond J. Expression of calcium channels along the differentiation of cultured trophoblast cells from human term placenta. Biol Reprod. 2002b;67:1473–1479. doi: 10.1095/biolreprod.102.005397. [DOI] [PubMed] [Google Scholar]
- Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a Mg. ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- Nathanson MH, Fallon MB, Padfield PJ, Maranto AR. Localization of the type 3 inositol 1, 4, 5-trisphosphate receptor in the Ca2+ wave trigger zone of pancreatic acinar cells. J Biol Chem. 1994;269:4693–4696. [PubMed] [Google Scholar]
- Niemeyer BA, Bergs C, Wissenbach U, Flockerzi V, Trost C. Competitive regulation of CaT-like-mediated Ca2+ entry by protein kinase C and calmodulin. Proc Natl Acad Sci U S A. 2001;98:3600–3605. doi: 10.1073/pnas.051511398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Hoenderop JG, Vennekens R, Hoefs S, Weidema AF, Droogmans G, Bindels RJ. Fast and slow inactivation kinetics of the Ca2+ channels ECaC1 and ECaC2 (TRPV5 and TRPV6). Role of the intracellular loop located between transmembrane segments 2 and 3. J Biol Chem. 2002;277:30852–30858. doi: 10.1074/jbc.M202418200. [DOI] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Vennekens R, Hoenderop JG, Bindels RJ, Droogmans G. Pharmacological modulation of monovalent cation currents through the epithelial Ca2+ channel ECaC1. Br J Pharmacol. 2001a;134:453–462. doi: 10.1038/sj.bjp.0704272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Vennekens R, Prenen J, Hoenderop JG, Bindels RJ, Droogmans G. Whole-cell and single channel monovalent cation currents through the novel rabbit epithelial Ca2+ channel ECaC. J Physiol. 2000;527:239–248. doi: 10.1111/j.1469-7793.2000.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Vennekens R, Prenen J, Hoenderop JG, Droogmans G, Bindels RJ. The single pore residue Asp542 determines Ca2+ permeation and Mg2+ block of the epithelial Ca2+ channel. J Biol Chem. 2001b;276:1020–1025. doi: 10.1074/jbc.M006184200. [DOI] [PubMed] [Google Scholar]
- Page RDM. TREEVIEW: An application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S, Patapoutian A. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- Peng JB, Brown EM, Hediger MA. Structural conservation of the genes encoding CaT1, CaT2, and related cation channels. Genomics. 2001a;76:99–109. doi: 10.1006/geno.2001.6606. [DOI] [PubMed] [Google Scholar]
- Peng JB, Chen XZ, Berger UV, Vassilev PM, Brown EM, Hediger MA. A rat kidney-specific calcium transporter in the distal nephron. J Biol Chem. 2000a;275:28186–28194. doi: 10.1074/jbc.M909686199. [DOI] [PubMed] [Google Scholar]
- Peng J-B, Chen X-Z, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, Hediger MA. Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J Biol Chem. 1999;274:22739–22746. doi: 10.1074/jbc.274.32.22739. [DOI] [PubMed] [Google Scholar]
- Peng J-B, Chen X-Z, Berger UV, Weremowicz S, Morton CC, Vassilev PM, Brown EM, Hediger MA. Human calcium transport protein CaT1. Biochem Biophys Res Commun. 2000b;278:326–332. doi: 10.1006/bbrc.2000.3716. [DOI] [PubMed] [Google Scholar]
- Peng J-B, Zhuang L, Berger UV, Adam RM, Williams BJ, Brown EM, Hediger MA, Freeman MR. CaT1 expression correlates with tumor grade in prostate cancer. Biochem Biophys Res Commun. 2001b;282:729–734. doi: 10.1006/bbrc.2001.4638. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. Separation and characterization of currents through store-operated CRAC channels and Mg(2+)-inhibited cation (MIC). channels. J Gen Physiol. 2002;119:487–507. doi: 10.1085/jgp.20028551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JWJ. Capacitative Calcium Entry. Austin, TX, USA: Blackwell Science Inc; 1997. [Google Scholar]
- Raraty M, Ward J, Erdemli G, Vaillant C, Neoptolemos JP, Sutton R, Petersen OH. Calcium-dependent enzyme activation and vacuole formation in the apical granular region of pancreatic acinar cells. Proc Natl Acad Sci U S A. 2000;97:13126–13131. doi: 10.1073/pnas.97.24.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–1047. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- Ryazanov AG, Pavur KS, Dorovkov MV. Alpha-kinases: a new class of protein kinases with a novel catalytic domain. Curr Biol. 1999;9:R43–R45. doi: 10.1016/s0960-9822(99)80006-2. [DOI] [PubMed] [Google Scholar]
- Schindl R, Kahr H, Graz I, Groschner K, Romanin C. Store depletion-activated CaT1 currents in rat basophilic leukemia mast cells are inhibited by 2-aminoethoxydiphenyl borate. Evidence for a regulatory component that controls activation of both CaT1 and CRAC (Ca(2+) release-activated Ca(2+) channel). channels. J Biol Chem. 2002;277:26950–26958. doi: 10.1074/jbc.M203700200. [DOI] [PubMed] [Google Scholar]
- Shin DM, Luo X, Wilkie TM, Miller LJ, Peck AB, Humphreys-Beher MG, Muallem S. Polarized expression of G protein-coupled receptors and an all-or-none discharge of Ca2+ pools at initiation sites of [Ca2+]i waves in polarized exocrine cells. J Biol Chem. 2001;276:44146–44156. doi: 10.1074/jbc.M105203200. [DOI] [PubMed] [Google Scholar]
- Skryma R, Mariot P, Bourhis XL, Coppenolle FV, Shuba Y, Abeele FV, Legrand G, Humez S, Boilly B, Prevarskaya N. Store depletion and store-operated Ca2+ current in human prostate cancer LNCaP cells: involvement in apoptosis. J Physiol. 2000;527:71–83. doi: 10.1111/j.1469-7793.2000.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- Song Y, Kato S, Fleet JC. Vitamin D receptor (VDR) knockout mice reveal VDR-independent regulation of intestinal calcium absorption and ECaC2 and Calbindin D(9k) mRNA. J Nutr. 2003;133:374–380. doi: 10.1093/jn/133.2.374. [DOI] [PubMed] [Google Scholar]
- Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Ishibashi K, Ooki G, Tsuruoka S, Imai M. Electrophysiologic characteristics of the Ca-permeable channels, ECaC and CaT, in the kidney. Biochem Biophys Res Commun. 2000;274:344–349. doi: 10.1006/bbrc.2000.3135. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Ohki G, Ishibashi K, Imai M. A single amino acid mutation results in a rapid inactivation of epithelial calcium channels. Biochem Biophys Res Commun. 2002;291:278–285. doi: 10.1006/bbrc.2002.6416. [DOI] [PubMed] [Google Scholar]
- Van Cromphaut SJ, Dewerchin M, Hoenderop JG, Stockmans I, Van Herck E, Kato S, Bindels RJ, Collen D, Carmeliet P, Bouillon R, Carmeliet G. Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci U S A. 2001;98:13324–13329. doi: 10.1073/pnas.231474698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Abeele F, Roudbaraki M, Shuba Y, Skryma R, Prevarskaya N. Store-operated Ca2+ current in prostate cancer epithelial cells: Role of endogenous Ca2+ transporter type 1. J Biol Chem. 2003;278:15381–15389. doi: 10.1074/jbc.M212106200. [DOI] [PubMed] [Google Scholar]
- Vassilev PM, Peng JB, Hediger MA, Brown EM. Single-channel activities of the human epithelial Ca2+ transport proteins CaT1 and CaT2. J Membr Biol. 2001;184:113–120. doi: 10.1007/s00232-001-0085-2. [DOI] [PubMed] [Google Scholar]
- Voets T, Janssens A, Prenen J, Droogmans G, Nilius B. Mg(2+)-dependent gating and strong inward rectification of the cation channel TRPV6. J Gen Physiol. 2003;121:245–260. doi: 10.1085/jgp.20028752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Prenen J, Fleig A, Vennekens R, Watanabe H, Hoenderop JG, Bindels RJ, Droogmans G, Penner R, Nilius B. CaT1 and the calcium release-activated calcium channel manifest distinct pore properties. J Biol Chem. 2001;276:47767–47770. doi: 10.1074/jbc.C100607200. [DOI] [PubMed] [Google Scholar]
- Wasserman RH, Fullmer CS. Vitamin D and intestinal calcium transport: facts, speculations and hypotheses. J Nutr. 1995;125:1971S–1979S. doi: 10.1093/jn/125.suppl_7.1971S. [DOI] [PubMed] [Google Scholar]
- Weber K, Erben RG, Rump A, Adamski J. Gene structure and regulation of the murine epithelial calcium channels ECaC1 and 2. Biochem Biophys Res Commun. 2001;289:1287–1294. doi: 10.1006/bbrc.2001.6121. [DOI] [PubMed] [Google Scholar]
- Wissenbach U, Bodding M, Freichel M, Flockerzi V. Trp12, a novel Trp related protein from kidney. FEBS Lett. 2000;485:127–134. doi: 10.1016/s0014-5793(00)02212-2. [DOI] [PubMed] [Google Scholar]
- Wissenbach U, Niemeyer BA, Fixemer T, Schneidewind A, Trost C, Cavalie A, Reus K, Meese E, Bonkhoff H, Flockerzi V. Expression of CaT-like, a novel calcium-selective channel, correlates with the malignancy of prostate cancer. J Biol Chem. 2001;276:19461–19468. doi: 10.1074/jbc.M009895200. [DOI] [PubMed] [Google Scholar]
- Wood RJ, Tchack L, Taparia S. 1,25-Dihydroxyvitamin D3 increases the expression of the CaT1 epithelial calcium channel in the Caco-2 human intestinal cell line. B M C Physiol. 2001;1:11. doi: 10.1186/1472-6793-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, Distefano PS, Curtis R, Clapham DE. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- Yue L, Peng JB, Hediger MA, Clapham DE. CaT1 manifests the pore properties of the calcium-release-activated calcium channel. Nature. 2001;410:705–709. doi: 10.1038/35070596. [DOI] [PubMed] [Google Scholar]
- Yule DI, Ernst SA, Ohnishi H, Wojcikiewicz RJ. Evidence that zymogen granules are not a physiologically relevant calcium pool. Defining the distribution of inositol 1, 4, 5-trisphosphate receptors in pancreatic acinar cells. J Biol Chem. 1997;272:9093–9098. doi: 10.1074/jbc.272.14.9093. [DOI] [PubMed] [Google Scholar]
- Zhuang L, Peng JB, Tou L, Takanaga H, Adam RM, Hediger MA, Freeman MR. Calcium-selective ion channel, CaT1, is apically localized in gastrointestinal tract epithelia and is aberrantly expressed in human malignancies. Lab Invest. 2002;82:1755–1764. doi: 10.1097/01.lab.0000043910.41414.e7. [DOI] [PubMed] [Google Scholar]


