Abstract
5-HT1A receptor agonists lower body temperature. We have investigated whether activation of 5-HT1A receptors inhibits cutaneous sympathetic discharge so that dilatation of the cutaneous vascular bed lowers body temperature by increasing heat transfer to the environment. We measured ear pinna blood flow in conscious rabbits (with chronically implanted Doppler ultrasound flow probes), and postganglionic sympathetic vasomotor nerve activity in anaesthetized rabbits. Recordings from conscious rabbits were made in a cage at 26 °C and the rabbit was then transferred to a cage at 10 °C. The ear pinna Doppler signal fell from 56 ± 4 cm s−1 in the 26 °C cage to 4 ± 1 cm s−1 (P < 0.0001, n = 24) after 30 min in the 10 °C cage, and body temperature increased from 38.8 ± 0.2 to 39.0 ± 0.2 °C (P < 0.01, n = 24). The 5-HT1A agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT; 0.1 mg kg−1 I.V.) reversed the cold-induced fall in ear pinna blood flow (Doppler signal increased from 5 ± 1 to 55 ± 8 cm s−1, P < 0.001, n = 7) within 5 min when administered 30 min after transfer to the 10 °C cage, and prevented the fall in ear pinna blood flow when administered before the rabbit was transferred to the 10 °C cage. Body temperature decreased after administration of 8-OH-DPAT. These changes were abolished by the specific 5-HT1A antagonist WAY-100635 (0.1 mg kg−1 I.V.). In anaesthetized rabbits, 8-OH-DPAT (0.1 mg kg−1 I.V.) reduced resting postganglionic cutaneous sympathetic vasomotor discharge, and prevented the increase normally elicited by cooling the trunk. Our experiments constitute the first demonstration that activation of 5-HT1A receptors powerfully inhibits cold-induced increases in cutaneous sympathetic vasomotor discharge, thereby dilating the cutaneous vascular bed and increasing transfer of heat to the environment.
Identification of the different 5-hydroxytryptamine (5-HT) receptor subtypes has facilitated our understanding of the contribution of 5-HT to the regulation of body temperature. Activation of 5-HT1A receptors decreases body temperature (Hjorth, 1985; Gudelsky et al. 1986; Cryan et al. 1999). Activation of 5-HT2A receptors increases body temperature (Gudelsky et al. 1986; Löscher et al. 1990; Mazzola-Pomietto et al. 1995). However, the relevant neuroanatomical pathways and underlying neurotransmitter mechanisms mediating these effects remain to be elucidated. The task is especially complicated because 5-HT alters so many psychological, behavioural and physiological variables (Barnes & Sharp, 1999). Pharmacological and physiological studies of the mechanisms underlying the temperature effects of agents acting at 5-HT1A receptors often focus on body temperature per se, without determining the relative contributions of heat production and/or heat loss to the temperature equation.
Similar considerations apply to neuroanatomical studies. Interest in the role of 5-HT in temperature control has focused on upper brainstem and forebrain 5-HT-innervated regions. Much less attention has been paid to possible contributions of the thermoregulatory role of 5-HT via regulation of heat exchange with the environment through the cutaneous circulation, i.e. on heat dissipation rather than heat production. Even when temperature studies have focused on 5-HT neurons in the medullary raphe region, interpretation of the results has emphasized possible ascending projections of the cells (Dickenson, 1977; Berner et al. 1999), rather than descending projections to cutaneous sympathetic preganglionic neurons in the spinal cord. Central neuroanatomical organization of the descending central control of the cutaneous circulation includes a brainstem relay in raphe magnus/pallidus and the parapyramidal region of the medulla oblongata (Blessing & Nalivaiko, 2000; Nalivaiko & Blessing, 2001; Tanaka et al. 2002). Neurons in this medullary region include the B1-B3 bulbospinal cells that synthesize 5-HT (Loewy, 1981; Steinbusch, 1981; Skagerberg & Bjorklund, 1985; Nicholas et al. 1992). Transneuronal intra-axonal tracing experiments in rats show that 5-HT neurons are amongst the early wave of virus-containing cells after injection of virus into the tail (Smith et al. 1998), which is the principal heat-exchanging cutaneous vascular bed in this species. 5-HT1A receptors have been demonstrated on raphe/ parapyramidal spinally projecting neurons present in the medulla oblongata (Helke et al. 1997). Thus 5-HT1A agonists could lower body temperature by inhibiting the action of cutaneous premotor sympathetic neurons, including 5-HT neurons located in this region.
We have now determined whether activation of 5-HT1A receptors by the specific agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) (Arvidsson et al. 1987) can reduce body temperature by increasing cutaneous blood flow, thereby facilitating transfer of heat from the body. In conscious rabbits we first induced vasoconstriction of the ear pinna vascular bed by exposing the animals to a cold environment. We then determined whether 5-HT1A receptor activation reverses this physiologically induced, sympathetically mediated cutaneous vasoconstriction. In anaesthetized rabbits we then directly measured postganglionic cutaneous sympathetic nerve activity, determining whether stimulation of 5-HT1A receptors inhibits ongoing cutaneous sympathetic discharge activity and reduces cold-induced cutaneous sympathetic discharge. We determined whether WAY-100635, a specific 5-HT1A antagonist (Forster et al. 1995), reverses and/or prevents the changes induced by 8-OH-DPAT.
METHODS
Ear pinna blood flow in conscious unrestrained rabbits
Experiments were performed on 31 conscious unrestrained New Zealand White rabbits (2.5-4.5 kg) purchased from Nanowie Rabbit Farm, Torquet, Australia. Rabbits were frequently handled and transferred between cages in the animal house. Each rabbit was used in up to five of the experimental conditions, with at least 3 days elapsing between each experiment. Experimental procedures were approved by the Flinders University Animal Welfare Committee. For implantation of probes, rabbits were anaesthetized with midaxolam and hypnorm (0.4 mg kg−1 and 0.3 mg kg−1 I.M. respectively), a chronically implanted Doppler ultrasonic flow probe (Iowa Doppler Products, IA, USA) was positioned around the central ear pinna artery, and a telemetric temperature probe (Data Sciences International, St Paul, MN, USA) was implanted intraperitoneally (Pedersen & Blessing, 2001). At the conclusion of the surgical procedures each rabbit was given carprofen (4 mg kg−1 S.C.; Pfizer Pty Ltd, West Ryde, NSW, Australia) as an analgesic agent. Rabbits were given supplemental vegetables in the diet for 1 week after surgery. All animals ate, drank and moved freely on the first post-operative day.
Animals were studied in temperature-controlled cages equipped with a swivel device and flexible cable that attached to a socket fixed to the animal's skull, so that blood flow recordings could be made while the conscious animal moved freely within the cage. Food and water were continuously available. At the end of the experiments rabbits were killed by intravenous injection of 2 ml of pentobarbitone sodium (325 mg ml−1).
In initial experiments, the set-point of the temperature-controlled cage was reduced from 26 to 15 °C after a 30 min control recording period. Because it took 20-30 min to reduce the cage temperature from 26 to 15 °C, subsequent experiments were carried out by transferring the animal from the 26 °C cage to a second cage already maintained at 10 °C, with the lower temperature chosen so that cold-induced cutaneous vasoconstriction was more marked. Ear pinna blood flow and body temperature were assessed throughout the experiment, except for a brief period during transfer from the 26 °C cage to the 10 °C cage. Temperature and Doppler signals were processed (Triton Technology, San Diego, CA, USA) and digitized (40 and 2 Hz for flow and temperature signals, respectively) using PowerLab and Chart software (ADInstruments, Sydney, Australia) and a Macintosh computer.
Postganglionic cutaneous vasculature sympathetic discharge in anaesthetized rabbits
Experiments were performed on six male New Zealand White rabbits (2.5-3.5 kg). Animals were given a single dose of methylscopolamine bromide (50 μg I.V.) to reduce airway secretions, and then anaesthetized with urethane (Sigma Chemical Co., Castle Hill, Australia; 1.5 g kg−1 I.V., infused via the right ear marginal vein over 20 min). Fur was shaved from the trunk and neck. An endotracheal tube was inserted via a tracheostomy. The left femoral artery and vein were cannulated for measurement of systemic arterial pressure and for intravenous drug infusion, respectively.
The animal was mounted prone in a Kopf stereotaxic frame, with a water jacket positioned around the trunk. Warm water (36-48 °C) was circulated (1-2 l min−1) through the water jacket to maintain body temperature between 38 and 39 °C. A thermocouple was attached to the abdominal skin under the water jacket to monitor skin temperature. Another thermocouple was inserted 6 cm into the rectum to measure core body temperature. Circulating cold water (10-20 °C) through the jacket for 5-12 min lowered skin temperature and this was followed by a delayed fall in body (rectal) temperature. Recirculation of warm water reversed these changes.
The left cervical sympathetic trunk was exposed from the dorsolateral aspect and the intact nerve was placed across a pair of silver-wire electrodes. A small (approximately 0.1 mm diameter) nerve fascicle was dissected from the central ear branch of the posterior auricular artery approximately 3 cm from the base of the ear. The distal end of the nerve was cut and the nerve was placed over bipolar silver-silver chloride wire electrodes. The nerves were covered with a mixture of paraffin oil and Vaseline to prevent drying. Multiunit nerve action potential recordings were made using a Neurolog NL100 preamplifier and Neurolog NL104 amplifier (NL125 filters 100-1000 Hz) (Digitimer Ltd, Hertfordshire, UK). The noise level was determined from inspection of the signal between bursts of discharge and confirmed at the end of the experiment by abolishing all postganglionic sympathetic discharge with hexamethonium (see below). All recorded signals, including the raw nerve signal, were recorded on videotape for offline analysis. A Grass 7P10B signal conditioning unit (Grass Telefactor, West Warwick, RI, USA), was used to full wave rectify the raw nerve signal bursts that exceeded the noise level, and the supra-threshold signal was integrated with a Neurolog NL705 (root mean square, time constant 500 ms).
On completion of the surgery animals were neuromuscularly blocked with vecuronium bromide (1-1.5 mg kg−1 I.V.) and mechanically ventilated with 100 % oxygen. End tidal CO2 (Normocap CO2 monitor, Datex, Helsinki, Finland) was kept at 30-40 mmHg. After neuromuscular block, adequate anaesthesia was determined by the absence of any increase in arterial pressure in response to possibly painful procedures and by ensuring the absence of a withdrawal reflex to paw squeeze during periods when the return of active respiratory effort indicated that neuromuscular block was no longer present. If anaesthesia was inadequate, supplemental urethane (150 mg kg−1 I.V.) was administered over 5 min. When anaesthesia was adequate, supplemental vecuronium bromide (0.5 mg kg−1 I.V.) was administered to maintain neuromuscular block.
The cervical sympathetic trunk was electrically stimulated with a single rectangular pulse of 0.5 ms with current strength (50-500 μA) at twice the threshold level required to produce an evoked potential in the ear pinna cutaneous sympathetic nerve. A peristimulus time histogram (16 sweeps) was constructed to confirm the sympathetic nature of the ear pinna nerve from which recordings were being made, and to confirm that the nerve was in place on the electrodes during periods of low or absent spontaneous activity. We then measured the increase in nerve discharge elicited by perfusing cold water through the jacket. After recovery following reperfusion of warm water, when nerve discharge was stable, we administered 8-OH-DPAT (0.1 mg kg−1 I.V.). The effect on resting nerve discharge was assessed 5 min after the injection. Responses to electrical stimulation of the cervical sympathetic trunk and to trunkal cooling were again assessed. In three of six animals we then administered WAY-100635 (0.1 mg kg−1 I.V.) and determined the effect on ear pinna sympathetic discharge. In all animals, at the end of the experiments, the ear pinna sympathetic nerve response to stimulation of the left cervical sympathetic trunk was confirmed after injection of the ganglionic blocking agent hexamethonium bromide (50 mg kg−1 I.V.). At the end of the experiments rabbits were killed by intravenous injection of 2 ml of pentobarbitone sodium (325 mg ml−1).
Pharmacological agents
All drugs were administered intravenously; into the marginal ear vein contralateral to the implanted ear pinna Doppler probe in conscious rabbits and into the femoral vein in anaesthetized rabbits. WAY-100635, 8-OH-DPAT and hexamethonium bromide were purchased from Sigma Chemical Company (Castle Hill, Australia) and dissolved in Ringer solution.
Statistical analysis
Data were analysed with Chart (ADInstruments, Sydney, Australia), IgorPro (WaveMetrics, Lake Oswega, OR, USA) and Statview (SAS Institute, Cary, NC, USA) software. Conscious unrestrained rabbits have variable ‘baseline’ pulsatile ear pinna blood flow signals, with episodic sudden falls from high levels to near-zero levels, and gradual return to the previous high level within approximately 1 min (Yu & Blessing, 1997). For each animal in a particular condition, we measured mean ear pinna blood flow and body temperature averaged over a 2 min period when the baseline flow was not affected by these alerting responses. Examples of the time period selected for measurement during the control period are indicated as a bar in the graph of ear pinna blood flow for each of the four flow traces shown in Fig. 1 and Fig. 2. Details of other measurement times are given in the appropriate Results section. For analysis of data from anaesthetized rabbits, integrated nerve activity signal, and arterial pressure, core body temperature, skin temperature and end-tidal CO2 were digitized with PowerLab (100 Hz) and displayed on a Macintosh computer.
Figure 1. 8-Hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) reverses and prevents cold-induced ear pinna vasoconstriction.
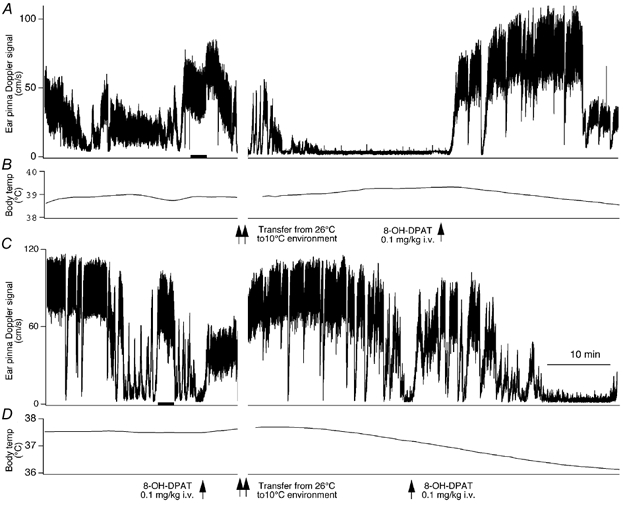
Records of ultrasonic Doppler signal measuring phasic ear pinna blood flow (A and C) and body temperature (B and D) in conscious freely moving rabbits. The initial 30 min recording was obtained with the rabbit in a 26 °C cage. At the time point indicated by the double vertical arrows the animal was transferred to a 10 °C cage. 8-OH-DPAT (0.1 mg kg−1 I.V.) was administered at the time indicated by the single vertical arrows. A and B, 8-OH-DPAT was administered 30 min after transfer to the 10 °C cage. C and D, 8-OH-DPAT was administered before transfer to the 10 °C cage, and then again 30 min after transfer. Records in A and C, both before and after 8-OH-DPAT, exhibit sudden alerting-related falls in ear pinna blood flow, with return to the pre-fall level in approximately 1 min. The 10 min time bar in C applies to all panels. The 2 min bar in the control periods of A and C indicates the when the mean flow was measured in these records.
Figure 2. WAY-100635 prevents and reverses the vasodilating action of 8-OH-DPAT.

Records of ultrasonic Doppler signal measuring phasic ear pinna blood flow (A and C) and body temperature (B and D) in conscious freely moving rabbits. The initial 20 min recording was obtained with the rabbit in a 26 °C cage. At the time point indicated by the first pair of double vertical arrows the animal was transferred to a 10 °C cage. At the time point indicated by the second pair of double vertical arrows the animal was transferred back to 26 °C cage. 8-OH-DPAT (0.1 mg kg−1) or WAY-100635 (0.1 mg kg−1 I.V.) was administered at the times indicated by the single vertical arrow. Alerting-related falls also occurred during the brief handling period required for intravenous injection. This explains why the vasoconstricting effect of WAY-100635 seems so abrupt in A. The 10 min time bar applies to all panels. The 2 min bar in the control periods of A and C indicates the when the mean flow was measured in these records.
Group data were analysed by repeated measures analysis of variance, with comparison of particular post-injection values with each other and with the corresponding control values. Factorial analysis of variance was used to compare values from corresponding time points in vehicle and drug-treated animals. Fisher's protected t test was used to determine significant differences, with the significance threshold set at the 0.05 level.
RESULTS
Ear pinna blood flow and body temperature in conscious rabbits
In control experiments to determine whether the process of transfer from one cage to another causes stress-related changes in ear pinna blood flow, rabbits were transferred from the 26 °C cage to a second similar cage, also maintained at 26 °C. The mean Doppler ear pinna blood flow signal was 65 ± 9 cm s−1 before transfer and 65 ± 10 cm s−1 30 min after transfer (P > 0.05, n = 5). Corresponding body temperature values were 37.8 ± 0.5 and 38.1 ± 0.5 °C (P > 0.05, n = 5). Thus the process of cage transfer did not, of itself, cause any significant change in ear pinna blood flow or body temperature.
In all rabbits, after transfer from the 26 °C cage to the second cage maintained at 10 °C, mean ear pinna blood flow fell and stabilized at a very low level within 15 min (Fig. 1A and Fig. 2A). Effects on ear pinna blood flow and body temperature of a 30 min exposure to 10 °C after abrupt transfer from the 26 °C cage were statistically assessed by combining the data from the 24 rabbits in the different conditions (Table 1) that received no treatment before transfer to the 10 °C cage. Ear pinna blood flow fell from 56 ± 4 cm s−1 in the 26 °C cage to 4 ± 1 cm s−1 (P < 0.0001, n = 24) after 30 min in the 10 °C cage. Body temperature slightly increased (Fig. 1B), from 38.77 ± 0.16 to 39.04 ± 0.18 °C, during the first 30 min after transfer from the 26 °C cage to the 10 °C cage (+0.27 ± 0.08 °C, n = 24, P < 0.01).
Table 1.
Modification of cold-induced changes in cutaneous blood flow and body temperature by 5-HT1A receptors
| A. Cage temperature gradually from 26 to 15°C, then 8-OH-DPAT | ||||||
| Cage temperature 26°C | After 20 min at cage temperature 15°C | 5 min after 8-OH-DPAT (0.01 mg kg−1) | 5 min after 8-OH-DPAT (0.1 mg kg−1) | |||
| Ear pinna flow signal (cm s−1) | 44 ± 6(7) | 6 ± 2(7)* | 10 ± 4(6)*† | 55 ± 8(7)‡§ | ||
| B. Rabbit transferred from 26°C cage to 10°C cage, then 8-OH-DPAT or vehicle | ||||||
| 26°C cage | 30 min after transfer to 10°C cage | 5 min after 8-OH-DPAT (0.1 mg kg−1) or vehicle | 30 min after 8-OH-DPAT (0.1 mg kg−1) or vehicle | |||
| 8-OH-DPAT | ||||||
| Ear pinna blood flow (cm s−1) | 56 ± 7(7) | 5 ± 1 (7)* | 55 ± 8 (7)†‡ | 5 ± 1 (6)* | ||
| Body temperature(°C) | 38.8 ± 0.3(7) | 9.2 ± 0.4(7)* | 39.2 ± 0.4(7) | 38.7 ± 0.4(7)*‡ | ||
| Vehicle | ||||||
| Ear pinna blood flow (cm s−1) | 47 ± 10(5) | 5 ± 2(5)* | 3 ± 1(5)*§ | 2 ± 1(5)* | ||
| Body temperature(°C) | 39.1 ± 0.2(6) | 39.4 ± 0.2(6)‡ | 39.2 ± 0.4(6)‡ | 39.2 ± 0.1(6)‡ | ||
| C. 8-OH-DPAT in 26°C cage, transfer to 10°C cage, second 8-OH-DPAT injection after 30 min | ||||||
| 26°C cage, before 8-OH-DPAT | 26°C cage 5 min after 8-OH- DPAT (0.1 mg kg−1) | 5-30 min after transfer to 10°C cage | 60 min after transfer to 10°C cage | |||
| Ear pinna flow signal (cm s−1) | 78 ± 7(7) | 52.0 ± 5(7)* | 35 ± 7(7)‡ | 2 ± 1(7)† | ||
| Body temperature (°C) | 37.9 ± 0.4(7) | 38.0 ± 0.4(7) | 37.6 ± 0.3(7)‡ | 37.0 ± 0.3(7)† | ||
| D. Transfer from 26°C cage to 10°C cage, then 8-OH-DPAT, then WAY-100635 | ||||||
| 26°C cage | 30 min after transfer to 10°C cage | 5 min after 8-OH-DPAT (0.1 mg kg−1) | 5 min after WAY-100635 (0.1 mg kg−1) | 30 min after WAY-100635 | 15 min after transfer back to 26°C cage | |
| Ear pinna flow signal (cm s−1) | 51 ± 8(6) | 3 ± 1(6)* | 51 ± 10(6)†‡ | 4 ± 1(6)* | 4 ± 1(6)*§ | 41 ± 8(6)‡ |
| Body temperature(°C) | 38.4 ± 0.4(5) | 38.4 ± 0.4(5)‡ | 38.3 ± 0.4(5)‡ | 38.2 ± 0.4(5)‡ | 38.7 ± 0.5(5)‡ | 39.5 ± 0.6(5)* |
| E. Transfer from the 26°C cage to the 10°C cage, then WAY-100635 and then 8-OH-DPAT | ||||||
| 26°C cage | 30 min after transfer to 10°C cage | 5 min after WAY-100635 (0.1 mg kg−1) | 5 min after 8-OTH-DPAT (0.1 mg kg−1) | 30 min after 8-OTH-DPAT (0.1 mg kg−1) | 26°C cage | |
| Ear pina flow signal (cm s−1) | 69 ± 8(6) | 5 ± 1(6)* | 5 ± 1(6)*† | 4 ± 1(6)*† | 5 ± 1(6)*† | 44 ± 11(6)§ |
| Body temperatur(°C) | 38.7 ± 0.4(6) | 39.1 ± 0.4(6)‡ | 39.3 ± 0.4(6)‡ | 39.3 ± 0.4(6)‡ | 39.5 ± 0.4(6)‡ | 39.5 ± 0.6(6)‡ |
| F. WAY-100635 given in 26°C cage, then rabbit transferreed to 10°C cage, then 8-OH-DPAT | ||||||
| 26°C cage before WAY-100635 | 26°C cage 5 min after WAY-100635 (0.1 mg kg−1) | 30 min after transfer to 10°C cage | 5 min after 8-OH-DPAT (0.1 mg kg−1) | 30 min after 8-OH-DPAT (0.1 mg kg−1) | 26°C cage | |
| Ear pinna flow signal (cm s−1) | 51 ± 5(5) | 45 ± 5(5)§ | 6 ± 1(5)* | 7 ± 1(5)*‡ | 6 ± 1(5)*‡† | 25 ± 5(5)*† |
| Body temperature(°C) | 39.1 ± 0.6(5) | 39.2 ± 0.5(5)§ | 39.3 ± 0.5(5)§ | 39.3 ± 0.5(5)§ | 39.4 ± 0.4(5)§ | 39.5 ± 0.6(5)§ |
Effect on ear pinna blood flow and body temperature (mean ± s.e.m.) of intravenous administration of 8-hydroxy-2-(di-n-propy1amino)tetralin (8-OH-DPAT) or WAY-100635, either in a 26°C cage with gradual reduction of cage temperature to 15°C (A) or with transfer of the rabbit from the 26°C cage to a second cage maintained at 10°C (B–F). The heading of each subsection describes the experimental condition for that subsection. Explanation of symbols: A, *significantly different from 26°C control value, P < 0.01; †not significantly different from 20 min post transfer value, P > 0.05; ‡significantly different from 20 min post transfer value, P < 0.01; and §not significantly different from 26°C control value, P > 0.05. B, *significantly different from 26°C control value, P < 0.01; †significantly different from 30 min post transfer value, P < 0.01; ‡not significantly different from 26°C control value, P > 0.05; and §not significantly different from 30 min post transfer value, P > 0.05. C, *significantly different from 26°C control value before 8-OH-DPAT, P < 0.05; †significantly different from 26°C control value after 8-OH-DPAT, P < 0.01; and ‡not significantly different from 26°C control value after 8-OH-DPAT, P > 0.05. D, *significantly different from 26°C control value, P < 0.01; †significantly different from 30 min post transfer value, P < 0.01; ‡not significantly different from 26°C control value, P > 0.05; and §not significantly different from value 5 min after WAY-100635, P > 0.05. E, *significantly different from 26°C control value, P < 0.01; †not significantly different from 30 min post transfer value, P > 0.05; and ‡not significantly different from 26°C control value, P > 0.05. F, *significantly different from 26°C control value after WAY-100635, P < 0.01; †significantly different from 30 min post transfer value, P > 0.01; ‡not significantly different from 30 min post transfer value, P > 0.05; and §not significantly different from 26°C control value after WAY-100635, P > 0.05.
Effect of drug treatment on ear pinna blood flow and body temperature in rabbits exposed to a cold environment
Treatment with 8-OH-DPAT after gradual reduction in cage-temperature from 26 to 15 °C
Administration of 0.01 mg kg−1 8-OH-DPAT, 20 min after cage temperature was reduced to 15 °C and ear pinna blood flow had fallen to a very low level, did not significantly change ear pinna blood flow (Table 1A). Subsequent administration of 0.1 mg kg−1 8-OH-DPAT increased ear pinna blood flow within a few minutes of administration, restoring flow to the levels initially observed in the warm environment (Table 1A).
Treatment with 8-OH-DPAT or vehicle after transfer from the 26 °C cage to the 10 °C cage
Within 3 min of injection of 8-OH-DPAT (0.1 mg kg−1), administered 30 min after transfer from the 26 °C cage to the 10 °C cage, ear pinna blood flow rapidly and substantially increased from the very low cold-induced level to a level similar to that observed when the animal was in the 26 °C chamber (Fig. 1A and Table 1B). The 8-OH-DPAT-induced increase in flow lasted approximately 20 min after which time flow once again decreased to low levels (Fig. 1A and Table 1B). When a higher dose of 8-OH-DPAT (0.5 mg kg−1) was administered 30 min after the previous 0.1 mg kg−1 dose, ear pinna blood flow increased again and remained at a high level for the duration of an additional 30 min observation period, so that at the end of this time ear pinna flow was 53 ± 5 cm s−1, significantly greater than the flow value after 30 min cold exposure (P < 0.01) and not significantly different from the flow value recorded at 26 °C before transfer to the cold (P > 0.05, n = 6).
There was a small rise in body temperature during the first 30 min after transfer from the 26 °C cage to the 10 °C cage (Fig. 1A and Table 1B). In the 30 min period after administration of 8-OH-DPAT (0.1 mg kg−1), body temperature decreased by approximately 0.5 °C (Fig. 1A and Table 1B).
In a separate group of rabbits, Ringer vehicle (2 ml) administered 30 min after transfer from the 26 °C cage to the 10 °C cage did not change ear pinna blood flow (Table 1B). In these animals there was no significant change in body temperature during the first 30 min after transfer to the 10 °C cage (Table 1B).
Treatment with 8-OH-DPAT before transfer from the 26 °C cage to the 10 °C cage
Administration of 8-OH-DPAT (0.1 mg kg−1) at 26 °C caused a small fall in ear pinna blood flow which lasted for the duration of the 5 min period before the rabbit was transferred to the 10 °C cage. (Fig. 1C and Table 1C). In these rabbits the mean ear pinna blood flow for the period 5-30 min after transfer to the 10 °C cage was 35 ± 7 cm s−1 (n = 7), significantly greater (P < 0.01) than 7 ± 2 cm s−1 (n = 12), the corresponding value in rabbits receiving 8-OH-DPAT or vehicle 30 min after transfer to the 10 °C cage, but with no treatment before transfer to the cold. In rabbits treated in the 26 °C cage with 8-OH-DPAT, body temperature decreased during the first 30 min after transfer to the 10 °C cage (−0.42 ± 0.15 °C, P < 0.01, n = 7). In contrast, in rabbits receiving no treatment before transfer to the cold (combined groups), body temperature slightly increased after transfer (+0.27 ± 0.08 °C, n = 24, P < 0.01). Therefore treatment with 8-OH-DPAT before transfer to the cold substantially prevented cold-induced ear pinna vasoconstriction, and increased the amount of heat transferred from the body to the cold environment.
Treatment with 8-OH-DPAT after transfer from the 26 °C cage to the 10 °C cage, and then treatment with WAY-100635
In rabbits transferred from the 26 °C cage to the 10 °C cage, administration of 8-OH-DPAT (0.1 mg kg−1) 30 min after transfer reversed cold-induced ear pinna vasoconstriction (Fig. 2A and Table 1D) in a manner very similar to that observed in a previous experiment (Fig. 1A and Table 1B). When WAY-100635 (0.1 mg kg−1) was administered 10 min after 8-OH-DPAT, ear pinna blood flow fell promptly to a very low level and remained at a very low level for the 30 min period during which the rabbit was kept in the 10 °C cage. The initial rapid fall in ear pinna blood flow after WAY-100635 (Fig. 2A) reflects the alerting-related effect associated with the injection procedure. After the rabbit was transferred back to the 26 °C cage, within 15 min ear pinna blood flow returned to the levels originally observed in this cage (Fig. 2A and B, and Table 1D). In this subgroup of rabbits, there was no significant change in body temperature during the 30 min in the 10 °C cage, nor did temperature change after administration of 8-OH-DPAT (Table 1D).
Treatment with WAY-100635 after transfer from the 26 °C cage to the 10 °C cage, and then treatment with 8-OH-DPAT
In rabbits transferred from the 26 °C cage to the 10 °C cage, ear pinna blood flow fell to very low levels in the usual manner. Administration of WAY-100635 (0.1 mg kg−1) 30 min after transfer did not alter ear pinna blood flow (Table 1E), nor did subsequent administration of 8-OH-DPAT (0.1 mg kg−1). When rabbits were returned to the 26 °C cage, ear pinna blood flow increased to a level not significantly different from the level previously observed in this cage (Table 1E). In this subgroup of rabbits body temperature did not significantly change during the 30 min exposure to cold, nor did it change significantly during the 30 min period after administration of WAY-100635 and then 8-OH-DPAT (Table 1E).
Treatment with WAY-100635 before transfer from the 26 °C cage to the 10 °C cage, and then treatment with 8-OH-DPAT
Ear pinna blood flow did not change when WAY-100635 (0.1 mg kg−1) was administered 5 min before the end of the 30 min control period in the 26 °C cage. After transfer to the 10 °C cage, ear pinna blood flow fell to very low levels in the usual manner (Fig. 2B and Table 1F). Subsequent administration of 8-OH-DPAT did not alter ear pinna blood flow at either 5 or 30 min after injection (Fig. 2B and Table 1F). When the rabbit was returned to the warm 26 °C cage, ear pinna blood flow increased, although the level reached in the group data was not as high as observed during the initial control period (Table 1F). Body temperature did not change significantly from the control value recorded in the 26 °C cage at any stage (Table 1F).
Cutaneous sympathetic nerve activity in anaesthetized rabbits
When stable nerve recordings were obtained, rabbits were maintained with warm water circulating through the water jacket so that skin temperature was 39.4 ± 0.4 °C and body (rectal) temperature was 38.6 ± 0.2 °C. The identity of the nerve fibres as sympathetic was verified by testing the response to electrical stimulation of the intact cervical sympathetic trunk. Single pulse stimulation (50-500 μA, 0.5 ms) of the cervical sympathetic trunk in six rabbits increased the discharge of the ear pinna sympathetic nerve with a response latency of 95 ± 5 ms and response duration of 72 ± 10 ms. The conduction velocity from stimulation site to recording site was 1.1 ± 0.1 ms. The maximum amplitude of the response was 25 ± 5 μV.
The trunk skin of the rabbit was then cooled by circulating cold water (10 °C) through the water jacket. This procedure rapidly lowered skin temperature to 23.5 ± 2.3 °C and caused a delayed fall in core temperature to 37.9 ± 0.3 °C. The cooling procedure increased ear sympathetic nerve discharge to 172 ± 19 % of pre-cooling level (P < 0.01, n = 5). Records from one rabbit are shown in Fig. 3. When the animal was rewarmed by re-introducing the warm water into the jacket, nerve activity gradually declined towards the pre-cooling baseline level. When nerve discharge was again reasonably stable, we injected 8-OH-DPAT (0.1 mg kg−1 I.V.). Arterial pressure was unchanged by this procedure (95 ± 6 mmHg before and 93 ± 5 mmHg 5 min after 8-OH-DPAT, P > 0.05, n = 6). Administration of 8-OH-DPAT caused nerve activity to fall, within a minute or so of the injection (Fig. 3) so that 5 min after the injection nerve activity was 5 ± 2 % (n = 6, P < 0.01) of pre-injection discharge level. When the rabbit was cooled 5-10 min after administration of 8-OH-DPAT, the increase in sympathetic nerve discharge elicited by the cooling procedure was reduced to 14 ± 8 % (n = 6, P < 0.01) of the pre-8-OH-DPAT response to cooling. The animal was then warmed. Nerve activity recovered to the pre-injection level 52 ± 12 min after the injection in four to six animals (data not shown). In the other two animals, nerve activity did not recover during the 1 h observation period. WAY-100635 (0.1 mg kg−1) was administered approximately 20 min after readministration of 8-OH-DPAT (0.1 mg kg−1) in three rabbits. In each case, WAY-100635 restored ear pinna sympathetic nerve discharge to the level recorded before 8-OH-DPAT (data not shown). Five min after administration of WAY-100635 the amplitude of the discharge was 159 ± 65 % of the amplitude before 8-OH-DPAT (n = 3).
Figure 3. 8-OH-DPAT inhibits cutaneous sympathetic nerve discharge.

Recording of ear pinna sympathetic nerve discharge (A), 30 s bins (B), skin temperature (C), body temperature (D) and arterial pressure (E) from an anaesthetized rabbit. The circled numbers (1-3) in A correspond to the circled numbers on the X axis in E, indicating the time period during which the nerve recording shown in A was made. The trunk skin was cooled during the time indicated by the single horizontal arrows and warmed during the period indicated by the double horizontal arrow. 8-OH-DPAT (0.1 mg kg−1 I.V.) was administered at the time shown by the vertical arrow. The 10 min time base bar in E also applies to B, C and D.
Administration of 8-OH-DPAT did not change (P > 0.05, n = 4) the latency, the amplitude or the duration of sympathetic nerve discharge elicited by single pulse stimulation of the cervical sympathetic trunk (Fig. 4A and B). Administration of hexamethonium abolished the evoked response (Fig. 4C and D).
Figure 4. Ear pinna sympathetic nerve discharge evoked by stimulation of cervical sympathetic trunk.
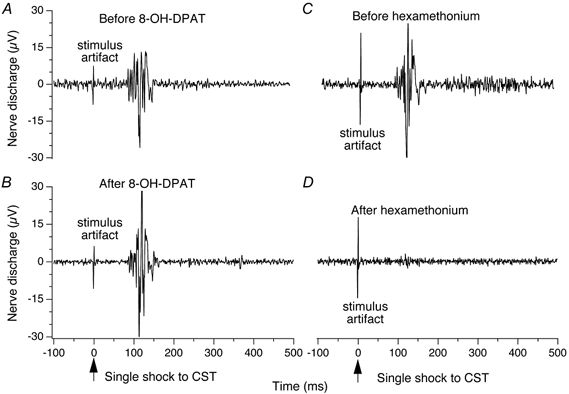
Peristimulus histograms (average of 16 sweeps) of ear pinna sympathetic nerve discharge evoked by single shock electrical stimulation of the ipsilateral cervical sympathetic trunk (CST), administered at time zero. A and B demonstrate that the evoked response is unaffected 5 min after 8-OH-DPAT (0.1 mg kg−1 I.V.). C and D demonstrate that the evoked response is abolished 5 min after hexamethonium bromide (50 mg kg−1 I.V.).
DISCUSSION
Our experiments provide the first demonstration, in any species, that 5-HT1A receptors have a powerful inhibitory effect on activity in the central neural pathway mediating temperature-related activation of sympathetic outflow to the cutaneous vascular bed. In conscious unrestrained rabbits, stimulating 5-HT1A receptors with 8-OH-DPAT delayed constriction of the ear pinna vascular bed elicited by subsequently exposing the rabbit to a cold environment, and reversed cold-induced cutaneous vasoconstriction when administered after the cold exposure. The specific 5-HT1A receptor antagonist WAY-100635 prevented and reversed the vasodilating action of 8-OH-DPAT, and prevented the fall in body temperature.
The dose of 8-OH-DPAT that entirely reversed cold-induced cutaneous vasoconstriction (0.1 mg kg−1) is at the low end of the range of doses found to decrease body temperature in rats (Hjorth, 1985; Gudelsky et al. 1986; Cryan et al. 1999). Since the ear pinna is a major vascular bed for heat-exchange in the rabbit (Grant et al. 1932) and since body temperature fell, it is likely that loss of heat from the body via dilated cutaneous vessels contributes to the fall in body temperature occurring in association with 8-OH-DPAT. The smaller temperature fall in rabbits compared with rats may be related to the difference in body size. Since body temperature is a complex variable, depending on the relationship between heat production and heat loss, our findings explain why the hypothermic effect of 8-OH-DPAT might be greater in a colder environment (Nicholas & Seiden, 2003).
Our electrophysiological recordings from postganglionic sympathetic axons accompanying the ear pinna cutaneous vessels confirm that nerve discharge is responsive to changes in skin and core body temperature in the rabbit (Riedel et al. 1972), in the manner also described for rat tail sympathetic nerve discharge (Owens et al. 2002). Activation of 5-HT1A receptors with 8-OH-DPAT substantially reduced ongoing cutaneous sympathetic nerve activity, and substantially prevented the increase in cutaneous sympathetic nerve activity normally elicited by reducing the temperature of the water in the jacket surrounding the animal. In contrast, 8-OH-DPAT did not affect nerve discharge evoked by electrical stimulation of preganglionic sympathetic axons in the cervical sympathetic trunk. Thus the cutaneous sympathoinhibitory action of the drug is substantially within the central nervous system, in the brain and/or spinal cord, but not in the periphery.
5-HT1A receptors occur in the CNS pathway regulating cold-induced cutaneous sympathetic vasomotor activity
Because 8-OH-DPAT inhibits a naturally induced normothermic response, it is likely that the neurons with 5-HT1A receptors normally participate in the central neural regulation of this response. Our findings thus suggest the presence of inhibitory 5-HT1A receptors in the central sympathetic pathway that normally regulates cutaneous vasoconstriction in response to exposure to a cold environment. However, although the 5-HT1A antagonist WAY-100635 prevented and reversed the cutaneous vasodilating activity of 8-OH-DPAT, when administered at 26 °C the antagonist did not change baseline ear pinna flow; nor did it alter physiologically elicited cutaneous vasoconstriction when administered before the rabbit was transferred to the 10 °C environment, or prevent the physiological cutaneous vasodilatation normally elicited by transferring the rabbit from cold to warm. Thus, although 5-HT1A receptors are linked in to the central pathway normally regulating the sympathetic vasoconstrictor response to cold, in the physiological situation they are not essential links in this pathway.
Understanding of the cellular physiology of 5-HT1A receptors derives largely from studies of neurons in the dorsal and median raphe nuclei in the pons and midbrain (Sprouse & Aghajanian, 1987). These 5-HT1A receptors are considered to be inhibitory somatodendritic autoreceptors present principally on neurons that synthesize 5-HT (De Vry et al. 1998; Barnes & Sharp, 1999). The electrophysiological studies that have focused on raphe magnus/ pallidus neurons (Pan et al. 1993; Bayliss et al. 1997; Mason, 1997) support the autoreceptor view. However the assumption that 5-HT1A receptors are exclusively or even principally present on 5-HT perikarya has recently been brought into question for the dorsal raphe nucleus (Kirby et al. 2003). The receptors seem also to be present on non-5-HT neurons in this nucleus.
Because a major subclass of 5-HT1A receptors are thought to be autoreceptors and not to receive synaptic inputs, it has been difficult to determine how they are physiologically integrated into neural circuitry regulating physiological functions. The 5-HT1A antagonist WAY-100635 potently and selectively antagonizes the actions of specific 5-HT1A pharmacological agonists, but so far the antagonist, given by itself, has not been shown to have major effects on physiological processes. This is also consistent with the idea that the 5-HT1A receptors relevant to our results are non-innervated somatodendritic receptors, not direct links in neural pathways mediating physiological processes.
Medullary raphe region and 5-HT1A inhibition of cutaneous sympathetic vasomotor activity
Regulation of cutaneous blood flow is coordinated, at the lower brainstem level, by bulbospinal sympathetic premotor neurons located in the rostral midline medulla oblongata, in raphe magnus/pallidus and the parapyramidal region (Blessing & Nalivaiko, 2000; Nalivaiko & Blessing, 2001; Tanaka et al. 2002). In conscious rats, focal inhibition of neuronal activity in a similar raphe region causes a fall in body temperature (Zaretsky et al. 2003), presumably at least partially resulting from heat loss from the dilated cutaneous bed. 5-HT1A receptors have been demonstrated to occur on raphe-spinal neurons present in the medulla oblongata (Helke et al. 1997). Our preliminary evidence indicates that local microinjection of 8-OH-DPAT into raphe magnus/pallidus in rabbits substantially inhibits resting postganglionic sympathetic nerve discharge (Y. Ootsuka and W. W. Blessing, unpublished observations). This is consistent with our hypothesis that a subpopulation of the cutaneous sympathoinhibitory 5-HT1A receptors activated in our study is present on bulbospinal neurons in raphe magnus/pallidus and the parapyramidal region. Clearly there may be relevant 5-HT1A receptors, either autoreceptors or post-synaptic receptors, in other regions of the nervous system.
Possible involvement of rostral medullary bulbospinal 5-HT neurons in the thermoregulatory process is suggested by the transneuronal tracing study of Smith and colleagues (1998), demonstrating that cutaneous sympathetic premotor neurons in this region include 5-HT-synthesizing neurons as well as non-5-HT-synthesizing cells. The lowest axonal conduction velocity for thermosensitive raphe-spinal neurons in the study by Rathner and colleagues (2001) was 3.4 m s−1, suggesting that the particular neurons studied were not small 5-HT-synthesizing cells. However many of the 5-HT neurons, especially the subependymal cells, are small, with soma diameters in the order of 15 μm (Skagerberg & Bjorklund, 1985). Their descending axons are thus presumably thin and unmyelinated, making them difficult to activate in antidromic stimulation studies.
5-HT1A receptors, anxiety, stress-induced hyperthermia and cutaneous blood flow
Body temperature can increase in anxiety-provoking situations (Zethof et al. 1995; Oka et al. 2001). 5-HT1A receptor agonists reduce this stress-induced hyperthermia (Groenink et al. 1996; van der Heyden et al. 1997; Olivier et al. 1998; Mendoza et al. 1999; Pattij et al. 2002), and heat loss via cutaneous vasodilatation could contribute to this reduction. 5-HT1A agonist drugs are in clinical use as anxiolytic agents (De Vry et al. 1998). Buspirone, an anxiolytic with marked 5-HT1A agonist properties, causes a fall in body temperature both in experimental animals and in humans, and buspirone decreases stress-induced hyperthermia as well as the increase in skin conductance elicited by exposing humans to a sudden aversive white noise stimulus (Lecci et al. 1990; Young et al. 1993; Zethof et al. 1995; Bond et al. 2003). Our present study suggests that cutaneous vasodilatation as a result of 5-HT1A receptor activation is likely to contribute to the hypothermic effect of buspirone-like anxiolytic agents.
Clozapine and olanzapine, atypical antipsychotic agents with anxiolytic properties, reverse cutaneous vasoconstriction and hyperthermia elicited by 3,4-methylenedioxymethamphetamine (MDMA, ecstasy), at least partially via increased heat loss secondary to the marked cutaneous sympathoinhibitory actions of these drugs (Pedersen & Blessing, 2001; Blessing et al. 2003). Clozapine's complex pharmacological profile includes 5-HT1A agonist and 5-HT2A antagonist properties (Mason & Reynolds, 1992; Arnt & Skarsfeldt, 1998; Barnes & Sharp, 1999). The present study demonstrates that 5-HT1A agonist effects could contribute to cutaneous sympathoinhibition and vasodilatation induced by clozapine and olanzapine. A recent study suggests that 5-HT2A antagonism could also contribute (Blessing & Seaman, 2003).
Conclusion
Our findings suggest that inhibitory 5-HT1A receptors are present in the CNS pathway normally activating cutaneous sympathetic vasomotor nerve activity in response to cold. Neuronal localization of these receptors may include the perikarya and dendrites of bulbospinal premotor sympathoexcitory neurons in the rostral medullary raphe region, including a population of neurons that also synthesize 5-HT.
Acknowledgments
This study was supported by the National Health and Medical Research Council. We thank Kate Barber, Melissa Blair and Robyn Flook for technical assistance.
REFERENCES
- Arnt J, Skarsfeldt T. Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology. 1998;18:63–101. doi: 10.1016/S0893-133X(97)00112-7. [DOI] [PubMed] [Google Scholar]
- Arvidsson LE, Johansson AM, Hacksell U, Nilsson JL, Svensson K, Hjorth S, Magnusson T, Carlsson A, Andersson B, Wikstrom H. (+)-cis-8-Hydroxy-1-methyl-2-(di-n-propylamino)tetralin: a potent and highly stereoselective 5-hydroxytryptamine receptor agonist. J Med Chem. 1987;30:2105–2109. doi: 10.1021/jm00394a029. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Li YW, Talley EM. Effects of serotonin on caudal raphe neurons: activation of an inwardly rectifying potassium conductance. J Neurophysiol. 1997;77:1349–1361. doi: 10.1152/jn.1997.77.3.1349. [DOI] [PubMed] [Google Scholar]
- Berner NJ, Grahn DA, Heller HC. 8-OH-DPAT-sensitive neurons in the nucleus raphe magnus modulate thermoregulatory output in rats. Brain Res. 1999;831:155–164. doi: 10.1016/s0006-8993(99)01426-2. [DOI] [PubMed] [Google Scholar]
- Blessing WW, Nalivaiko E. Regional blood flow and nociceptive stimuli in rabbits: patterning by medullary raphe, not ventrolateral medulla. J Physiol. 2000;524:279–292. doi: 10.1111/j.1469-7793.2000.t01-2-00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing WW, Seaman B. 5-hydroxytryptamine(2A) receptors regulate sympathetic nerves constricting the cutaneous vascular bed in rabbits and rats. Neuroscience. 2003;117:939–948. doi: 10.1016/s0306-4522(02)00810-2. [DOI] [PubMed] [Google Scholar]
- Blessing WW, Seaman B, Pedersen NP, Ootsuka Y. Clozapine reverses hyperthermia and sympathetically mediated cutaneous vasoconstriction induced by MDMA (Ecstasy) in rabbits and rats. J Neurosci. 2003;23:6385–6391. doi: 10.1523/JNEUROSCI.23-15-06385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond AJ, Wingrove J, Baylis M, Dalton J. Buspirone decreases physiological reactivity to unconditioned and conditioned aversive stimuli. Psychopharmacology. 2003;165:291–295. doi: 10.1007/s00213-002-1295-8. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Kelliher P, Kelly JP, Leonard BE. Comparative effects of serotonergic agonists with varying efficacy at the 5-HT(1A) receptor on core body temperature: modification by the selective 5-HT(1A) receptor antagonist WAY 100635. J Psychopharmacol. 1999;13:278–283. doi: 10.1177/026988119901300310. [DOI] [PubMed] [Google Scholar]
- De Vry J, Schohe-Loop R, Heine HG, Greuel JM, Mauler F, Schmidt B, Sommermeyer H, Glaser T. Characterization of the aminomethylchroman derivative BAY x 3702 as a highly potent 5-hydroxytryptamine1A receptor agonist. J Pharmacol Exp Ther. 1998;284:1082–1094. [PubMed] [Google Scholar]
- Dickenson AH. Specific responses of rat raphe neurones to skin temperature. J Physiol. 1977;273:277–293. doi: 10.1113/jphysiol.1977.sp012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster EA, Cliffe IA, Bill DJ, Dover GM, Jones D, Reilly Y, Fletcher A. A pharmacological profile of the selective silent 5-HT1A receptor antagonist, WAY-100635. Eur J Pharmacol. 1995;281:81–88. doi: 10.1016/0014-2999(95)00234-c. [DOI] [PubMed] [Google Scholar]
- Grant RT, Bland EF, Camp PD. Observations on the vessels and nerves of the rabbit's ear with special reference to the reaction to cold. Heart. 1932;16:69–101. [Google Scholar]
- Groenink L, Van Der Gugten J, Zethof TJ, van der Heyden JA, Olivier B. Neuroendocrine effects of diazepam and flesinoxan in the stress-induced hyperthermia test in mice. Pharmacol Biochem Behav. 1996;54:249–254. doi: 10.1016/0091-3057(95)02177-9. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Koenig JI, Meltzer HY. Thermoregulatory responses to serotonin (5-HT) receptor stimulation in the rat. Evidence for opposing roles of 5-HT2 and 5-HT1A receptors. Neuropharmacology. 1986;25:1307–1313. doi: 10.1016/0028-3908(86)90101-2. [DOI] [PubMed] [Google Scholar]
- Helke CJ, Capuano S, Tran N, Zhuo H. Immunocytochemical studies of the 5-HT(1A) receptor in ventral medullary neurons that project to the intermediolateral cell column and contain serotonin or tyrosine hydroxylase immunoreactivity. J Comp Neurol. 1997;379:261–270. [PubMed] [Google Scholar]
- Hjorth S. Hypothermia in the rat induced by the potent serotoninergic agent 8-OH-DPAT. J Neural Transm. 1985;61:131–135. doi: 10.1007/BF01253058. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Pernar L, Valentino RJ, Beck SG. Distinguishing characteristics of serotonin and non-serotonin-containing cells in the dorsal raphe nucleus: electrophysiological and immunohistochemical studies. Neuroscience. 2003;116:669–683. doi: 10.1016/s0306-4522(02)00584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecci A, Borsini F, Mancinelli A, D'Aranno V, Stasi MA, Volterra G, Meli A. Effect of serotoninergic drugs on stress-induced hyperthermia (SIH) in mice. J Neural Transm Gen Sect. 1990;82:219–230. doi: 10.1007/BF01272765. [DOI] [PubMed] [Google Scholar]
- Loewy AD. Raphe pallidus and raphe obscurus projections to the intermediolateral cell column in the rat. Brain Res. 1981;222:129–133. doi: 10.1016/0006-8993(81)90946-x. [DOI] [PubMed] [Google Scholar]
- Löscher W, Witte U, Fredow G, Ganter M, Bickhardt K. Pharmacodynamic effects of serotonin (5-HT) receptor ligands in pigs: stimulation of 5-HT2 receptors induces malignant hyperthermia. Naunyn Schmiedebergs Arch Pharmacol. 1990;341:483–493. doi: 10.1007/BF00171727. [DOI] [PubMed] [Google Scholar]
- Mason P. Physiological identification of pontomedullary serotonergic neurons in the rat. J Neurophysiol. 1997;77:1087–1098. doi: 10.1152/jn.1997.77.3.1087. [DOI] [PubMed] [Google Scholar]
- Mason SL, Reynolds GP. Clozapine has sub-micromolar affinity for 5-HT1A receptors in human brain tissue. Eur J Pharmacol. 1992;221:397–398. doi: 10.1016/0014-2999(92)90731-i. [DOI] [PubMed] [Google Scholar]
- Mazzola-Pomietto P, Aulakh CS, Wozniak KM, Hill JL, Murphy DL. Evidence that 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI)-induced hyperthermia in rats is mediated by stimulation of 5-HT2A receptors. Psychopharmacology. 1995;117:193–219. doi: 10.1007/BF02245187. [DOI] [PubMed] [Google Scholar]
- Mendoza DL, Bravo HA, Swanson HH. Antiaggresive and anxiolytic effects of gepirone in mice, and their attenuation by WAY 100635. Pharmacol Biochem Behav. 1999;62:499–509. doi: 10.1016/s0091-3057(98)00171-3. [DOI] [PubMed] [Google Scholar]
- Nalivaiko E, Blessing WW. Raphe region mediates changes in cutaneous vascular tone elicited by stimulation of amygdala and hypothalamus in rabbits. Brain Res. 2001;891:130–137. doi: 10.1016/s0006-8993(00)03210-8. [DOI] [PubMed] [Google Scholar]
- Nicholas AC, Seiden LS. Ambient temperature influences core body temperature response in rat lines bred for differences in sensitivity to 8-hydroxy-dipropylaminotetralin. J Pharmacol Exp Ther. 2003;305:368–374. doi: 10.1124/jpet.102.045088. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Pieribone VA, Arvidsson U, Hökfelt T. Serotonin-, substance P- and glutamate/aspartate-like immunoreactivities in medullo-spinal pathways of rat and primate. Neuroscience. 1992;48:545–559. doi: 10.1016/0306-4522(92)90401-m. [DOI] [PubMed] [Google Scholar]
- Oka T, Oka K, Hori T. Mechanisms and mediators of psychological stress-induced rise in core temperature. Psychosom Med. 2001;63:476–486. doi: 10.1097/00006842-200105000-00018. [DOI] [PubMed] [Google Scholar]
- Olivier B, Zethof TJ, Ronken E, van de JA. Anxiolytic effects of flesinoxan in the stress-induced hyperthermia paradigm in singly-housed mice are 5-HT1A receptor mediated. Eur J Pharmacol. 1998;342:177–182. doi: 10.1016/s0014-2999(97)01482-9. [DOI] [PubMed] [Google Scholar]
- Owens NC, Ootsuka Y, Kanosue K, McAllen RM. Thermoregulatory control of sympathetic fibres supplying the rat's tail. J Physiol. 2002;543:849–858. doi: 10.1113/jphysiol.2002.023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ZZ, Wessendorf MW, Williams JT. Modulation by serotonin of the neurons in rat nucleus raphe magnus in vitro. Neuroscience. 1993;54:421–429. doi: 10.1016/0306-4522(93)90263-f. [DOI] [PubMed] [Google Scholar]
- Pattij T, Groenink L, Hijzen TH, Oosting RS, Maes RA, van de Gugten J, Olivier B. Autonomic changes associated with enhanced anxiety in 5-HT(1A) receptor knockout mice. Neuropsychopharmacology. 2002;27:380–390. doi: 10.1016/S0893-133X(02)00317-2. [DOI] [PubMed] [Google Scholar]
- Pedersen NP, Blessing WW. Cutaneous vasoconstriction contributes to hyperthermia induced by 3,4-methylenedioxymethamphetamine (ecstasy) in conscious rabbits. J Neurosci. 2001;21:8648–8654. doi: 10.1523/JNEUROSCI.21-21-08648.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathner JA, Owens NC, McAllen RM. Cold-activated raphe-spinal neurons in rats. J Physiol. 2001;535:841–854. doi: 10.1111/j.1469-7793.2001.t01-1-00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel W, Iriki M, Simon E. Regional differentiation of sympathetic activity during peripheral heating and cooling in anesthetized rabbits. Pflugers Arch. 1972;332:239–247. [PubMed] [Google Scholar]
- Skagerberg G, Bjorklund A. Topographic principles in the spinal projections of serotonergic brainstem neurons in the rat. Neuroscience. 1985;15:445–480. doi: 10.1016/0306-4522(85)90225-8. [DOI] [PubMed] [Google Scholar]
- Smith JE, Jansen AS, Gilbey MP, Loewy AD. CNS cell groups projecting to sympathetic outflow of tail artery: neural circuits involved in heat loss in the rat. Brain Res. 1998;786:153–164. doi: 10.1016/s0006-8993(97)01437-6. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse. 1987;1:3–9. doi: 10.1002/syn.890010103. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Nagashima K, McAllen RM, Kanosue K. Role of the medullary raphe in thermoregulatory vasomotor control in rats. J Physiol. 2002;540:657–664. doi: 10.1113/jphysiol.2001.012989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heyden JA, Zethof TJ, Olivier B. Stress-induced hyperthermia in singly housed mice. Physiol Behav. 1997;62:463–470. doi: 10.1016/s0031-9384(97)00157-1. [DOI] [PubMed] [Google Scholar]
- Young AH, McShane R, Park SB, Cowen PJ. Buspirone-induced hypothermia in normal male volunteers. Biol Psychiatry. 1993;34:665–666. doi: 10.1016/0006-3223(93)90161-6. [DOI] [PubMed] [Google Scholar]
- Yu YH, Blessing WW. Cutaneous vasoconstriction in conscious rabbits during alerting responses detected by hippocampal theta-rhythm. Am J Physiol. 1997;272:R208–216. doi: 10.1152/ajpregu.1997.272.1.R208. [DOI] [PubMed] [Google Scholar]
- Zaretsky DV, Zaretskaia MV, Di Micco JA. Stimulation and blockade of GABAA receptors in the raphe pallidus: Effects on body temperature, heart rate and blood pressure in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R110–116. doi: 10.1152/ajpregu.00016.2003. [DOI] [PubMed] [Google Scholar]
- Zethof TJ, van de JA, Tolboom JT, Olivier B. Stress-induced hyperthermia as a putative anxiety model. Eur J Pharmacol. 1995;294:125–135. doi: 10.1016/0014-2999(95)00520-x. [DOI] [PubMed] [Google Scholar]


