Abstract
To test the hypothesis that muscle protein synthesis (MPS) is regulated by the concentration of extracellular amino acids, we investigated the dose-response relationship between the rate of human MPS and the concentrations of blood and intramuscular amino acids. We increased blood mixed amino acid concentrations by up to 240 % above basal levels by infusion of mixed amino acids (Aminosyn 15, 44-261 mg kg−1 h−1) in 21 healthy subjects, (11 men 10 women, aged 29 ± 2 years) and measured the rate of incorporation of D5-phenylalanine or D3-leucine into muscle protein and blood and intramuscular amino acid concentrations. The relationship between the fold increase in MPS and blood essential amino acid concentration ([EAA], mM) was hyperbolic and fitted the equation MPS = (2.68 × [EAA])/(1.51 + [EAA]) (P < 0.01). The pattern of stimulation of myofibrillar, sarcoplasmic and mitochondrial protein was similar. There was no clear relationship between the rate of MPS and the concentration of intramuscular EAAs; indeed, when MPS was increasing most rapidly, the concentration of intramuscular EAAs was below basal levels. We conclude that the rates of synthesis of all classes of muscle proteins are acutely regulated by the blood [EAA] over their normal diurnal range, but become saturated at high concentrations. We propose that the stimulation of protein synthesis depends on the sensing of the concentration of extracellular, rather than intramuscular EAAs.
The regulation of the size of the lean body mass in human beings is not well understood. An attractive hypothesis is that the deposition of amino acids into tissues by protein synthesis and their release by protein breakdown is regulated in a linked fashion by the concentrations of amino acids, particularly the essential amino acids (EAAs; Millward et al. 1996; Wolfe & Miller, 1999). Thus, after a protein-containing meal, thus supplying amino acids to the blood, protein synthesis rises and protein breakdown falls. In skeletal muscle, which is the major contributor to the lean body mass in human beings, feeding appears to double the rate of deposition of muscle protein (Rennie et al. 1982) and much of this change can be attributed to the action of amino acids alone (Bennet et al. 1989, 1990), without much influence of insulin (Bennet et al. 1990; Svanberg et al. 1997). Until now it has been assumed that any nutrient-sensing mechanism associated with an increased supply of amino acids is intramuscular.
For diet-derived amino acids to occupy a pivotal role in the control of the size of the lean tissue mass in the human body, a number of conditions would have to be fulfilled. First, the amino acids, the concentrations of which need to be sensed, should not be capable of being made de novo (i.e. they should be truly essential). Indeed, there are good candidates, the most likely being the branched-chain amino acid leucine, the concentration of which has been suggested to be the main anabolic signal among the amino acids (Smith et al. 1992; Kimball et al. 1999); nevertheless, other EAAs such as valine seem to share this property inasmuch as large doses of them stimulate human muscle protein synthesis (MPS), whereas large doses of non-essential amino acids (NEAAs) have no effect (Smith et al. 1998).
Furthermore, the range of concentrations over which amino acids have their effect on protein synthesis and breakdown would have to be relatively narrow, since the diurnal variation in amino acid concentration in the blood is only ≈ ± 50 % of the daily mean (Bergstrom et al. 1990). Finally, the effects would have to be rapid, since the excursion in blood amino acid concentrations following a meal is complete within 3 h (Bergstrom et al. 1990) and we have recently demonstrated that when increased amino acid supply is sustained for up to 6 h, the protein synthetic machinery in muscle appears to become unresponsive after 2.5 h (Bohé et al. 2001). We predicted that human MPS would be stimulated acutely by increases in amino acid supply, most of the stimulation occurring in the 12 h fasted (post-absorptive)-to-fed range, with very little additional stimulation occurring at high concentrations of amino acids.
All 20 amino acids are required to make protein, so if protein anabolism is stimulated then the size of the intracellular pools of amino acids that show the biggest ratio between their contribution to protein and their presence in the free pool will decrease if they are not supplied fast enough (Waterlow et al. 1978). Such a phenomenon can be observed in the intramuscular free pool when a meal containing protein of a type unable to supply all of the amino acids required to make muscle protein is consumed. For example, when a meal containing 50 g of bovine serum albumin (which is deficient in isoleucine and methionine compared to muscle protein; Peters, 1985; Reeds et al. 1994) is given, the concentrations of isoleucine and methionine in the muscle fall, whereas other amino acids that are supplied in excess of their ability to contribute to the making of protein, rise (Bergstrom et al. 1990). A similar phenomenon occurs when haemoglobin-containing meals are taken or when the products of a gastric bleed are digested, because haemoglobin is deficient in isoleucine (Olde Damink et al. 1997). If only a single stimulatory amino acid is given (e.g. leucine), after the initial anabolic stimulation, all of the other amino acids show a fall in their concentration, and leucine oxidation rises, presumably because complete proteins can no longer be made (Alvestrand et al. 1990). We have confirmed this for a number of intramuscular EAAs after giving large doses of valine, leucine, phenylalanine and threonine, but not when giving NEAAs (Smith et al. 1992, 1998). A similar effect can be seen after the administration of large doses of insulin in muscle of type 1 diabetic patients (Bennet et al. 1991), when muscle protein breakdown is decreased beyond the capacity to maintain the muscle free pool, at a level able to supply protein synthesis and the concentration of rate-limiting amino acids, such as methionine and tyrosine, diminishes. If, for whatever reason, a rapid rise in demand for amino acid for MPS outstrips supply, then the intramuscular free pool size would diminish. This behaviour of the intramuscular amino acids would make them poorer indices of the existence of anabolic circumstances (e.g. in the post-prandial situation) than the extracellular amino acids because the hunting of the anabolic signal would militate against stability. Thus, we also predicted that MPS would respond to changes in extracellular amino acids in a simple, coherent, probably curvilinear fashion and that the response would be more consistent and steeper than to alterations of the intramuscular amino acids.
To test our predictions we decided to define the dose-response relationship between human MPS, as measured by incorporation of a stable isotope-labelled amino acid, and the availability, both intramuscularly and extracellularly, of amino acids supplied by an intravenous infusion of mixed amino acids. Achieving this was the major aim of the work. We also wished to determine whether the relationship is common for myofibrillar, sarcoplasmic and mitochondrial protein. To provide a metabolic context for the work we also measured the changes produced in blood glucose, urea and insulin.
METHODS
This study comprised two parts. First, we studied 15 normal post-absorptive volunteers (six men, nine women, mean ± s.e.m. age 25.3 ± 1.2 years and body mass 71.5 ± 3.3 kg). One subject was studied twice, the first time in the low-amino-acid-infusion group and the second time in the high-amino-acid-infusion group. The Institutional Review Board at the University of Texas Medical Branch (UTMB) approved the study, in line with the Declaration of Helsinki. Informed written consent to participate was obtained from all subjects. The subjects refrained from any but mild physical activity in the 2 days before admission to the study. The subjects were admitted to the Clinical Research Center of UTMB on the evening before the study and were given standard hospital food; they ate nothing after 12.00 a.m. until the end of the study, except a light breakfast at 6.00 a.m. containing 4 g protein, 18 g fat and 113 g carbohydrate. The rates of basal protein synthesis of each subject, measured 5.5-8 h later, showed values within the normal post-absorptive range observed in our laboratory (e.g. see Volpi et al. 2001). Forearm veins in different arms were cannulated for the infusion of tracer and mixed amino acids and for blood sampling. Tracer infusion started around 11.00 a.m. A primed constant infusion of D5-phenylalanine (D5-Phe) 99 atoms % (Cambridge Isotopes, Cambridge, MA, USA) was used to deliver tracer to the muscle (priming dose: 5.2 μmol kg−1, infusion rate: 0.1 μmol kg−1 min−1). After 3 h of tracer infusion we started a primed constant infusion of Aminosyn 15 (Abbot, Deerfield, IL, USA; 150 g l−1 of amino acids, the composition being as reported previously; Bohé et al. 2001) to which had been added 3 g l−1 of asparagine and 2.4 g l−1 of glycyltyrosine (a kind gift of Ortrud Brand, Kabi Fresenius, Uppsala, Sweden) to deliver amino acids missing or at low concentration. Subjects were randomly assigned to one of three groups (low, n = 6; medium, n = 4 and high, n = 6) to receive 43.5, 87 or 261 mg of mixed amino acids kg−1 h−1, respectively (with priming doses of 14.5, 29 or 87 mg amino acids kg−1, respectively).
After inspecting the preliminary results we realized that data from a set of previous studies, carried out under almost identical conditions, involving six subjects (five men and one woman aged 33 ± 1 years and weighing 80 ± 5 kg) would fit well with the pattern we were observing and we therefore incorporated them into the data set (those results have been reported previously; Bohé et al. 2001). In those subjects we had infused D3-α-ketoisocaproate (99 atoms %, D3-KIC, priming dose 13.2 μmol kg−1, 0.22 μmol kg−1 min−1, Cambridge Isotopes) and 162 mg kg−1 h−1 (priming dose 54 mg kg−1) of Aminosyn 15 without asparagine or the dipeptide. The doses used achieved increases in blood EAA concentrations of up to three times the basal, post-absorptive concentrations. The rationale was to alter the blood concentration of amino acids over a wide range covering the post-absorptive and post-prandial concentrations, plus concentrations likely to cause a near-saturation of MPS.
The delivery of the tracers was increased to match the increased supply of phenylalanine and leucine during the amino acid infusions. The infusion rate for D5-Phe was increased to 0.11, 0.13 and 0.18 μmol kg−1 min−1 (with priming doses of 0.26, 0.52 and 1.57 μmol kg−1, respectively) in the low, medium and high group, respectively. The infusion rate for D3-KIC was increased to 0.41 μmol kg−1 min−1, with a priming dose of 3.78 μmol kg−1, in the 162 mg kg−1 h−1 amino-acid-infusion-rate group. Blood samples were taken before the tracer infusion and at 30-60 min intervals up to 6.5 h.
Vastus lateralis biopsy samples were taken using Tilley-Henkel forceps (Dietrichson et al. 1987) at 30 and 180 min of the basal period before amino acid infusion and then at 60 and 210 min after the start of the amino acid infusion. Lidocaine (1 %) was used as a local anaesthetic. Two biopsy samples were taken from each leg, through the same incision but at 180 deg to each other and about 2-3 cm apart to minimize inflammatory interference. This was done to minimize scars from biopsy wounds for cosmetic reasons. One portion of the muscle biopsy (20-50 mg) was immediately frozen for measurement of mixed muscle protein synthetic rate and for measurement of intracellular amino acids (see later); a larger portion (100-120 mg) was immediately homogenized in a high-salt buffer containing protease and phosphatase inhibitors, as described previously (Bohé et al. 2001). The resulting homogenate was subjected to a scheme of differential centrifugation and further extraction in order to prepare fractions of myofibrillar, sarcoplasmic and mitochondrial proteins, as described previously (Bohé et al. 2001). Protein fractions were dissolved in 0.5 M NaOH then hydrolysed with 6 N HCl. Proteins were precipitated from serum using sulphosalicylic acid, and the amino acids from the HCl hydrolysate and in the sulphosalicylic supernatant were separated using strong cation-exchange chromatography derivatized using n-acetyl N-propyl reagents and subjected to selective ion monitoring gas chromatography mass spectrometry using a Hewlett Packard 5890 GCMS system, as described earlier (Wolfe, 1992). The rate of MPS was calculated by a comparison of the extent of labelling in the protein fractions with the labelling of the muscle free D5-Phe or D3-leucine (Bohé et al. 2001). The basal rate of MPS was calculated from data obtained from the first and second muscle biopsy samples (see above for timings), whereas the rate of MPS during amino acid infusion was calculated from the third and fourth biopsy samples, with appropriate averaging of the intracellular enrichment to calculate the precursor labelling. Individual rates for mixed muscle and all protein fractions were calculated as described previously and initially expressed as % h−1 (Rennie et al. 1982); they were then normalized to each individual's basal value to overcome the problem of baseline variation between subjects. Plasma and muscle amino acid concentrations were determined in the acetonitrile extracts using standard HPLC procedures. Free amino acid concentrations in blood were determined from deproteinized plasma samples. To 50 μl of plasma were added 100 μl of a norvaline internal standard and 100 μl of acetonitrile. The sample was mixed, stood on ice for 30 min and then diluted 1:15 with distilled, deionized water. Free amino acid concentrations in muscle were determined from 10-20 mg of muscle in 400 μl of 5 % perchloric acid, 50 μl of a norvaline internal standard. The tissue was ground on ice for 5 min and the mixture centrifuged at low speed (≈1000 g). The pH of the supernatant was adjusted to 5-7 with 5 N KOH and 1 N K2CO3 solutions. After centrifugation (≈1000 g), 100 μl of plasma fluid preparation and muscle supernatant were filtrated using hydrophilic membranes with a molecular weight cut-off of 5000 Da and centrifuged for 2 h at 5000 g. The resulting supernatants were analysed for free amino acid concentration by HPLC as described previously (Tipton et al. 1999). Intramuscular amino acid concentrations were calculated as described previously (Bergstrom et al. 1985). Muscle free amino acid concentrations were not measured in the subjects who received 162 mg kg−1 h−1 (Bohé et al. 2001), except for leucine, as part of the measurement of leucine free pool labelling for measurement of MPS. Blood glucose was determined using the glucose oxidase reaction, and urea was measured using a colorimetric method with urease and the Bertholet reaction, both using Sigma Kits (Sigma Diagnostics, St Louis, MO, USA). Serum insulin was determined using a radioimmunoassay (Coat-a-Count kit, Diagnostic Products Corporation, Los Angeles, CA, USA). Absolute values of insulin are not reported for the studies in which 162 mg kg−1 h−1 of amino acids were infused since we discovered subsequently to their assay that the values were incorrect, being elevated by between 1.5- and 2-fold through the inadvertent use of a standard curve made up with the wrong, but now unknown concentrations of standard insulin.
Statistics
The fit of the data to the rectangular hyperbola relationship between MPS and the amino acid concentrations was obtained using the CurveFit package from GraphPad Software (San Diego, CA, USA) and by PSI-Plot (Pearl River NY, USA). The goodness of fit was judged by the R2 metric (derived from the ratio of the absolute and normalized sum of the squares of the residuals) and the coefficient of determination. The P value was obtained from a χ2 test. The question of whether or not the intramuscular amino acid concentrations and serum insulin changed during the amino acid infusions was tested by two-way ANOVA for repeated measures. In all cases the null hypothesis was taken as being disproved when P < 0.05.
RESULTS
Plasma amino acids, tracer amino acid enrichments and urea
During the basal period, plasma total mixed amino acid concentrations were stable at 2.7 ± 0.1 mM, but rose in proportion to the rate of infusion (Fig. 1). For each infusion rate, a plateau in the amino acid concentration was achieved within 45 min of the start of infusion, whereupon there was no further significant change in any amino acid concentrations. Plasma tyrosine and asparagine concentrations, which had previously been found to fall progressively during infusion of Aminosyn 15 alone (i.e. without added glycyltyrosine and asparagine; Bohé et al. 2001), were steady throughout in subjects receiving amino acids at 43.5, 87 and 261 mg kg −1 h−1. The increases in the plasma concentrations of the EAAs were more pronounced than those of the NEAAs (Table 1), presumably because of the greater involvement of the latter in ureagenesis and gluconeogenesis, resulting in the observed increase in urea and glucose (see later).
Figure 1. Time course of changes in plasma total amino acid concentrations during infusion of mixed amino acids at four different rates.
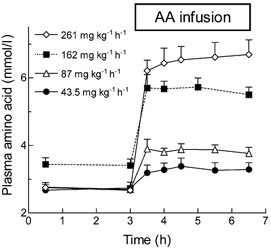
Values are means ± s.e.m. for 4-6 subjects per point.
Table 1.
Extracellular and intramuscular concentrations of EAAs and NEAAs before and during infusion of mixed amino acids at four different rates
| Amino acid infusion rate (mg kg−1 h−1) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 43.5 | 87 | 162 | 261 | |||||
| EC | IM | EC | IM | EC | IM | EC | IM | |
| EAA (mm) | ||||||||
| Before infusion | 0.92 ± 0.04 | 2.51 ± 0.23 | 0.95 ± 0.01 | 2.11 ± 0.05 | 1.25 ± 0.08 | n.d. | 0.92 ± 0.04 | 2.44 ± 0.28 |
| During infusion | 1.31 ± 0.05* | 2.25 ± 0.27 | 1.72 ± 0.03 | 1.94 ± 0.05* | 2.58 ± 0.17* | n.d. | 3.07 ± 0.13* | 3.58 ± 0.25* |
| % Change | +41 ± 3* | −8 ± 11* | +82 ± 5* | −8 ± 2* | +106 ± 8* | – | +235 ± 19* | +58 ± 25* |
| NEAA (mm) | ||||||||
| Before infusion | 1.78 ± 0.13 | 32.68 ± 3.18 | 1.78 ± 0.09 | 27.99 ± 1.96 | 2.17 ± 0.06 | n.d. | 1.83 ± 0.12 | 31.51 ± 4.24 |
| During infusion | 2.0 ± 0.17* | 27.17 ± 4.16* | 2.11 ± 0.15* | 25.11 ± 0.77* | 3.3 ± 0.25* | n.d. | 3.5 ± 0.29* | 32.95 ± 3.23 |
| % Change | +12 ± 3* | −17 ± 9* | +18 ± 5* | −10 ± 4* | +52 ± 9*– | — | +90 ± 1* | +16 ± 23 |
EC, extracellular; IM, intramuscular. Blood samples were taken 2.5 h apart before amino acid infusion and at 1, 1.5, 2.5 and 3.5 h during amino acid infusion (except in the 162 mg kg−1 h−1 amino acid infusion group where samples were taken at 1, 2 and 3.5 h during the infusion) Values are means ±s.e.m. for 4–6 subjects. No data are available for intramuscular ‘amino acids’ (i.c. EAAs + NEAAs) during infusion at 162 mg kg−1 h−1 (n.d. = not determined), but data for leucine suggest that the pattern is similar to that for the response seen in the group receiving amino acids at 261 mg kg−1 h−1. The values obtained during infusion were significantly different from those obtained in the basal state, as indicated by
P < 0.05 at least.
As expected from previous work, the enrichment of blood phenylalanine and leucine with D5-Phe and D3-leucine rapidly achieved plateau values of 12-13 % and 7-8 % (tracer/tracee ratios), respectively, within 30 min of their infusion and remained steady (i.e. varied by less than ± 10 % relative) throughout the remainder of the study, including the period of amino acid infusion (data not shown). Thus the addition of tracer to the amino acid infusion solutions was successful in achieving constant tracee amino acid labelling, despite varying blood concentrations.
Serum urea concentrations (initially 1.8 ± 0.3 mM) tended to drift downwards during the basal period by ≈12 % when the subjects had no exogenous supply of amino acids. This fall continued by another 10 % during 3.5 h of infusion at the lowest rate (43.5 mg kg−1 h−1), but at higher rates of infusion (87 mg kg−1 h−1) urea concentration stabilized for 30-60 min, then rose progressively. At the highest rate of amino acid infusion (261 mg kg−1 h−1), urea concentration rose progressively over 3.5 h to 2.9 ± 0.2 mM, suggesting that the capacity to make protein was overwhelmed.
Serum insulin and glucose
Insulin in the serum was unchanged from basal (11 ± 3 mIU l−1) during infusion of the lowest dose of amino acids (43.5 mg kg−1 h−1) when MPS rose by 30 % (Fig. 2). At the next highest rate of infusion (the medium dose, 87 mg kg−1 h−1) insulin rose transiently to 26 ± 8 mIU l−1, but fell rapidly within 30 min to values indistinguishable from the values achieved during the infusion of the low dose of amino acid. The plateau values attained for each were not significantly different (ANOVA) from basal. At the highest rate of amino acid infusion, serum insulin concentrations rose 4- to 5-fold within the first 15 min of infusion and then fell to a plateau value (36 ± 8 mIU l−1) at about three times the basal value. We discovered a technical problem in the measurement of insulin for the subjects studied during infusion of amino acids at 162 mg kg−1 h−1, which prevented the comparison of absolute values. Nevertheless, the pattern of response was very similar to that observed with the highest rate of infusion.
Figure 2. Changes in the concentrations of serum insulin and glucose during the infusion of mixed amino acids at four different rates.
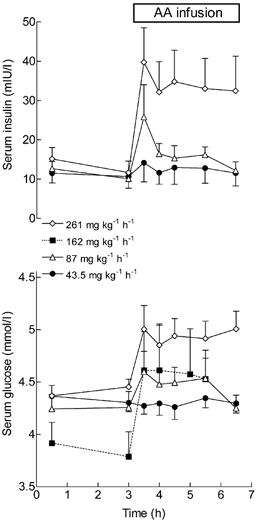
Serum insulin (top) and glucose (bottom) concentrations during infusion of mixed amino acids (AA infusion). Values are means ± s.e.m. for 4-6 subjects per point.
The basal serum glucose concentration, before amino acid infusion, was 4.2 ± 0.16 mM. Infusion of amino acids at the lowest rate (43.5 mg kg−1 h−1) had no effect on glucose concentrations, but the higher rates caused square-wave increases of serum glucose to plateau, the level of which was higher at each amino acid infusion rate (Fig. 2). At the highest rate (261 mg kg−1 h−1), blood glucose reached a mean value of 4.9 ± 0.25 mM.
Relationship between blood and intramuscular concentrations of amino acids
During the 1st hour of infusion time the extracellular concentrations of amino acids rose rapidly, but there were differential responses of the intramuscular amino acids. At the two lower doses (43.5 and 87 mg kg−1 h−1) the intramuscular EAA concentrations actually fell by about 8 %, despite rises of 41 and 82 %, respectively, in the extracellular concentrations (Table 1, Fig. 3). The fall was sustained at the lowest dose for the 3.5 h of the infusion, but at 87 mg kg−1 h−1 the concentration recovered to the basal value and did not rise above it (data not shown). At the highest dose, which raised the extracellular concentration by 235 %, the intracellular concentration rose rapidly to a mean value of 58 % above the basal value. We have data for all doses only for leucine. The pattern of change for leucine at the infusion rate of 162 mg kg−1 h−1 was almost identical to that for the rate of infusion at 261 mg kg−1 h−1, except that because we had a 30 min biopsy sample, we could observe a rise to 50 % of the final value within this time (data not shown). The relationship between the concentrations of extracellular and intracellular amino acids was not linear, as might have been expected, but was instead described by the J-shaped curve shown in Fig. 3.
Figure 3. Relationship between extracellular and intramuscular concentrations of EAAs during infusion of mixed amino acids at different rates.
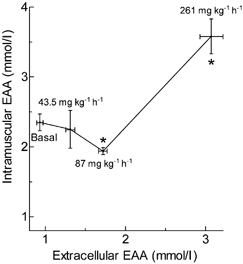
In the basal state, extracellular EAAs are the mean concentration of EAAs of two blood samples taken 2.5 h apart before the beginning of the mixed amino acid infusion, and intramuscular EAAs the mean concentration of EAAs from the first two muscle biopsy samples. During the infusion of mixed amino acid solution (at 43.5, 87 and 261 mg kg−1 h−1), extracellular EAAs represents the mean concentration of EAAs of blood samples taken at 1, 1.5, 2.5 and 3.5 h during the infusion, and intramuscular EAAs the mean concentration of EAAs from the two last muscle biopsy samples. Values are means ± s.e.m. for 4-6 subjects per point (with the exception of the 11 subjects at basal). Infusion rates of mixed amino acids are shown near to the points. Asterisks indicate the values of intramuscular amino acids that were significantly different from basal values (P < 0.05, repeated-measures ANOVA).
The intracellular NEAAs showed changes (Table 1) that were of the same general type as the EAAs, with falls at 43.5 mg kg−1 h−1 and 87 mg kg−1 h−1; the rise in intracellular NEAAs at the highest infusion rate was substantially blunted compared to that shown by the EAAs.
Relationship between MPS and blood and intramuscular amino acid concentrations
Elevations of +41 and +82 % in the concentrations of plasma EAAs above the basal values (corresponding to an elevation of +20 and +40 %, respectively, in the concentration of total amino acids) were associated with near-linear increases of 30 and 57 %, respectively, in the rates of mixed MPS measured between 1 and 3.5 h of amino acid infusion. The effect was seen in all of the main muscle protein fractions (myofibrillar, sarcoplasmic and mitochondrial), as well as in mixed muscle protein (Table 2 and Fig. 4). Further elevations in amino acid concentration caused little further increase in the rate of protein synthesis. We therefore attempted to fit the data to a Michaelis-Menten-type rectangular hyperbolic curve and discovered that the fit was best for the data from the studies carried out using the D5-Phe tracer for the mixed muscle protein data, which were the least variable. When we added the results reported previously (obtained with D3-leucine incorporation, and a mixed amino acid infusion rate of 162 mg kg−1 h−1; Bohé et al. 2001), the data conformed to an equation of the form: MPS = (2.68 × [EAA])/(1.51 + [EAA]), where MPS is the fold change in protein synthesis and the EAA concentration is in mM per blood water (upper panel of Fig. 4). The fit was very good (R2 = 0.62, P < 0.01) and better than for other possible shapes (e.g. linear or polynomial). The mean and standard deviation values for the maximal rate of protein synthesis and the concentration at which the rate was half-maximal were 2.68 ± 0.51 fold of basal and 1.51 ± 0.59 mM, respectively; 95 % confidence limits for these values were 1.65-3.7 and 0.3-2.71, respectively.
Table 2.
Rates of protein synthesis (% h−1) in mixed muscle protein and myofibrillar, mitchondrial and sarcoplasmic fractions of muscle before and during infusion of mixed amino acids at four different rates
| Amino acid infusion rate (mg kg−1 h−1) | ||||
|---|---|---|---|---|
| 43.5 | 87 | 162 | 261 | |
| Mixed muscle protein | ||||
| Before infusion | 0.057 ± 0.008 | 0.086 ± 0.009 | 0.076 ± 0.008 | 0.054 ± 0.002 |
| During infusion | 0.068 ± 0.007 | 0.134 ± 0.021 | 0.130 ± 0.029 | 0.1 ± 0.01 |
| % Change | +30 ± 25 | +57 ± 17 | +72 ± 38 | +88 ± 20 |
| Myofibrillar | ||||
| Before infusion | 0.059 ± 0.014 | 0.083 ± 0.023 | 0.085 ± 0.017 | 0.049 ± 0.005 |
| During infusion | 0.065 ± 0.014 | 0.101 ± 0.018 | 0.174 ± 0.036 | 0.095 ± 0.016 |
| % Change | +17 ± 27 | +40 ± 32 | +111 ± 43 | +93 ± 15 |
| Mitochondrial | ||||
| Before infusion | 0.053 ± 0.007 | 0.066 ± 0.015 | 0.119 ± 0.044 | 0.043 ± 0.006 |
| During infusion | 0.064 ± 0.006 | 0.113 ± 0.009 | 0.175 ± 0.046 | 0.086 ± 0.016 |
| % Change | +33 ± 27 | +90 ± 40 | +88 ± 109 | +112 ± 28 |
| Sarcoplasmic | ||||
| Before infusion | 0.067 ± 0.009 | 0.072 ± 0.015 | 0.108 ± 0.018 | 0.065 ± 0.009 |
| During infusion | 0.079 ± 0.008 | 0.127 ± 0.016 | 0.161 ± 0.017 | 0.134 ± 0.014 |
| % Change | +30 ± 24 | +80 ± 15 | +57 ± 16 | +140 ± 63 |
Values are means ± s.e.m. for 4–6 subjects (see Rennie et al. 1982 for method of fractional synthesis rate calculation). All of the rises in protein synthesis were significant at P < 0.05 compared to basal values.
Figure 4. Effect on mixed MPS of extracellular and intramuscular levels of EAAs.
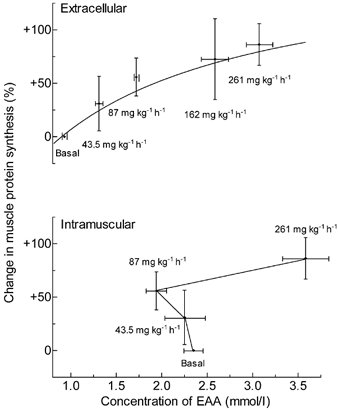
Upper panel, the effect on mixed MPS (measured as incorporation of D5-Phe or D3-leucine) in human skeletal muscle of extracellular EAA concentrations achieved by infusion of a mixed amino acid solution at different rates (shown near each point). Lower panel, the effect on mixed MPS (measured as incorporation of D5-Phe) in human skeletal muscle of intramuscular EAA concentrations achieved by infusion of a mixed amino acid solution at different rates (shown near each point). Values are means ± s.e.m. for 4-6 subjects per point (with the exception for the 11 subjects at basal), each point being the mean value of the individual changes in MPS and the mean EAA concentration over the period of measurement. The upper curve fits the equation: MPS = 2.68 ×[EAA]/(1.51 +[EAA]). No curve fit has been attempted for the lower graph.
A hyperbolic relationship between MPS and amino acid concentration was also seen for the extracellular NEAAs, but the curve was flatter and the error bars much greater (data not shown), presumably because of the extent of metabolism of the NEAAs.
When we plotted the alteration of the increase of MPS against the intramuscular amino acid concentrations, the resulting curve suggested that MPS was elevated as intramuscular amino acid concentrations fell (lower panel of Fig. 4). Thus, a simple curvilinear relationship such as that between the extracellular EAAs and MPS does not exist for intramuscular EAAs and MPS.
DISCUSSION
How generally applicable are our results? We contend that the most important variable having an effect upon MPS is the prevailing amino acid availability. Although the sensitivity and the capacity of the system may be altered by previous feeding of substantial protein meals (e.g. see Bohé et al. 2001), the small breakfast taken by our subjects 1 h before we made our measurements will have had little influence, since the amino acid and urea concentrations were near to those we commonly see in the overnight fasted state.
The results presented here have defined, we believe for the first time, the dose-response relationship between human MPS and EAA concentration in the blood. The results also confirm that the intramuscular concentration of EAAs fell as extracellular EAA concentrations rose in the physiological range, before rising as extracellular concentrations rise. The results support the hypothesis that there is a single curvilinear positive relationship between increase in MPS and extracellular EAAs, but that there is no such relationship between MPS and the intramuscular concentrations of EAAs. The results suggest that in the range occurring between the post-absorptive and mixed meal fed states, when blood EAAs rise by about 50-80 %, MPS rises almost linearly, but that as blood amino acids are elevated above this (e.g. by consumption of a high-protein meal or by use of high rates of amino acid infusion, clinically), MPS probably becomes saturated. This is supported by our observations that at high rates of amino acid infusion, serum urea concentration rose dramatically, presumably as a result of hepatic catabolism of amino acids delivered in excess of the capacity of the body to use them for protein synthesis.
The moderate changes in blood glucose observed were as expected from our previous work and are unlikely to be of any significance for the control of MPS.
In our studies, no attempt was made to manipulate the ambient insulin concentrations. The lowest rate of amino acid infusion had no effect on insulin concentration. At the next highest rate there was a slight very variable increase in insulin, but this was not significant and the insulin availability during the periods of measurement of MPS (calculated as the areas under the insulin-time curves; data not shown) was not different during infusion at the two lowest rates, whereas the rate of MPS increased by ≈30 and ≈60 %, respectively. This suggests that serum insulin, apart from having a likely permissive effect at low concentrations, had little or no part in the increase of MPS (from 30 to 60 %) seen with the elevation of blood amino acid concentration by ≈40-80 % above basal. This suggestion is strengthened by the observation that when insulin availability was markedly stimulated as a result of the infusion of the highest dose of amino acids, there was no additional effect on the rate of MPS, further suggesting that above ≈15-20 mIU l−1, insulin has no stimulatory effect on MPS. These observations fit well with those of the results of Svanberg et al. (1997), who showed that the stimulation of MPS in re-fed rats was dependant on dietary protein but not insulin, and the results of Yoshizawa et al. (1997, 1998), who showed that stimulation of protein translation was dependent upon amino acids for activation of the signalling elements involved in its control.
Our results also show that with small rises of blood amino acid concentrations achieved by infusion of low doses of amino acids, MPS rises at a time when the concentration of intramuscular amino acids is falling. The signalling mechanism involved in linking the rise in amino acid availability and MPS over the steepest part of the curve cannot depend upon the size of the intramuscular amino acid pool because this falls when MPS is stimulated (i.e. the sensed quantity would have to provide a signal of a sign opposite to that required for MPS stimulation). Thus, we suggest that it is the extracellular EAA concentration that is sensed and signalled. If this is so there must be a membrane-associated EAA sensor, which is unlikely to be an amino acid transporter, but which detects changes in extracellular amino acid concentrations and signals to the protein synthetic machinery in a way either independent of the concentrations of intramuscular EAAs, or with a much greater sensitivity.
The question arises as to whether or not our data implicate the importance of any particular amino acid, the obvious candidate being leucine, which has been suggested many times to be able by itself to stimulate MPS in animal and human muscle. Our results are consistent with such a role for leucine; however, its behaviour was indistinguishable from that of the other branched-chain amino acids, and indeed of the EAAs as a whole. So, if leucine does have a particular signalling role we would predict that it includes stimulation of a leucine membrane detector.
We propose that the fall in intramuscular EAAs observed when blood amino acid concentrations rise modestly might be explained as follows, and by reference to Fig. 5. As a result of stimulation of MPS via increased exogenous amino acids (which we suggest is signalled via the sensor on the muscle membrane) there is a markedly increased rate of deposition of amino acids into muscle protein. With only modest increases in blood amino acid concentrations, this occurs faster than can be accommodated by a stimulation of membrane transport (Taylor et al. 1999) and thus intramuscular amino acid concentrations fall, even though the flux through the intracellular compartment increases. This could explain how previous observations that linked the rate of inward amino acid transport and MPS failed to find a relationship between transport and corresponding changes in intramuscular concentrations (Wolfe & Miller, 1999).
Figure 5. Hypothetical scheme to explain the effect of increased extracellular EAAs on MPS.
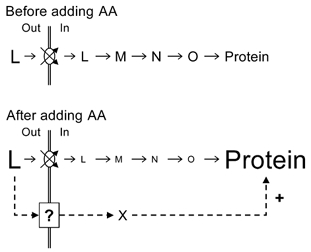
Hypothetical scheme showing how increased extracellular EAAs (L) may modulate MPS (the last stage in the sequence L-Protein) and cause diminution in intramuscular EAAs. Stages M-O simply represent biochemical entities involved in the initiation and elongation of protein synthesis. The increased availability of the extracellular amino acids is signalled to the protein synthetic apparatus via a membrane-bound sensor labelled ‘?’, which is independent of the amino acid transporters. The sensor must transmit a signal through another unknown mechanism (X).
We see no merit in a scheme of loss of amino acids from muscle by a membrane-transport-mediated effect because it is difficult to identify a mechanism that would have the observed effect in a situation in which all of the EAAs in the external pool were rising (Taylor et al. 1999). However, a decrease in the concentration of substrate during increased flux through a pathway resulting in increased synthesis of product is a common feature of metabolic regulation; the behaviour is described by the crossover theorem (Heinrich & Rapoport, 1973). In the present case, this occurs at a low to moderate supply of extracellular amino acids; when the supply becomes overwhelming (e.g. at the higher rates of amino acid infusion), the intramuscular concentration rises, but without any further increase in MPS (Table 2 and Fig. 4), which appears to be saturated.
The biological advantage of a system in which amino-acid-mediated stimulation of protein synthesis is signalled via a sensor outside the cell is not immediately obvious. However, it would have major advantages in allowing control of anabolism from a compartment that is not acutely affected by intermediary metabolism or by, as observed here, diminution of substrate supply, as the intramuscular compartment is where protein synthesis is stimulated (Fig. 2 and Fig. 4). The concentration of amino acids in the blood is the result of a number of processes (e.g. delivery from the gut and proteolysis, removal by hepatic and muscular catabolism, and protein synthesis) and is effectively the integral of these, changing with a time constant of change longer than those of the individual components. It would therefore be advantageous to sample this pool and regulate protein synthesis with respect to it. The possibility that, as hypothesized elsewhere, the control mechanisms of MPS and breakdown are linked (Millward et al. 1996; Wolfe & Miller, 1999) and possibly coordinated via amino acid concentration, would also have great biological utility, enabling the acutely fine regulation of the lean body mass in response to dietary intake from day to day over a lifetime.
Mortimore and colleagues have indeed produced evidence that proteolysis in the liver is controlled not by the intracellular concentration of amino acids, but rather by their extracellular availability (Mortimore et al. 1991, 1994). Further evidence of the importance of an extracellular amino acid sensor/signaller is our observation that the activity of P70S6 kinase, a protein intimately involved in the activation of the translational stage of protein synthesis (Kimball et al. 1998), and the phosphorylation of eIF4BP1, which is involved in control of initiation (Kimball et al. 1998), are both increased in human muscle during raised extracellular amino acid supply (Rennie, 2001a, 2001b), presumably at a time when intramuscular amino acid concentration falls.
Our results have some relevance to the question of the requirement of the human body for protein. They suggest that quite modest increases in amino acid supply, within the diurnal concentration range, result in substantial changes in the rates of synthesis, and thus probably the deposition of protein in muscle. Together with our previous results showing that stimulation of MPS is time limited (Bohé et al. 2001), it appears that only modest amounts of dietary amino acids would be needed to achieve maximal stimulation of the muscle anabolic processes (i.e. for adults of average weight, 55-75 kg × 0.260 mg kg−1 h−1 × 2 h, or of the order of 30-40 g of protein). This is probably somewhat lower than the current FAO/WHO/UNU recommendation of 0.8 g kg−1 day−1 and much lower than that of 1.2 g kg −1day−1 proposed by some workers for the elderly (Campbell et al. 2001). The current results could have important implications for deciding upon protein requirements in circumstances in which the availability of protein is limited.
Acknowledgments
These studies were conducted at the General Clinical Research Center (GCRC), University of Texas Medical Branch at Galveston, funded by a grant (M01 RR-00073) from the National Center for Research Resources, NIH grants G15780 and AG17231, grants from the UK MRC and The Welcome Trust. M.J.R. is grateful to the University of Dundee for leave of absence during a sabbatical to UTMB Galveston, and to the Shriners Burns Hospital, grant 8490, for support. J.B. thanks the Hospices Civils de Lyon and the Ville de Lyon (Prix Innovalyon 1998) for financial support. We thank Elena Volpi and Dennis Gore for clinical cover, and Elisabeth Boersheim and David W. Hart for technical assistance.
REFERENCES
- Alvestrand A, Hagenfeldt L, Merli M, Oureshi A, Eriksson LS. Influence of leucine infusion on intracellular amino acids in humans. Eur J Clin Invest. 1990;20:293–298. doi: 10.1111/j.1365-2362.1990.tb01858.x. [DOI] [PubMed] [Google Scholar]
- Bennet WM, Connacher AA, Jung RT, Stehle P, Rennie MJ. Effects of insulin and amino acids on leg protein turnover in IDDM patients. Diabetes. 1991;40:499–508. doi: 10.2337/diab.40.4.499. [DOI] [PubMed] [Google Scholar]
- Bennet WM, Connacher AA, Scrimgeour CM, Rennie MJ. The effect of amino acid infusion on leg protein turnover assessed by L-[15N]phenylalanine and L-[13C]leucine exchange. Eur J Clin Invest. 1990;20:37–46. doi: 10.1111/j.1365-2362.1990.tb01789.x. [DOI] [PubMed] [Google Scholar]
- Bennet WM, Connacher AA, Scrimgeour CM, Smith K, Rennie MJ. Increase in anterior tibialis muscle protein synthesis in healthy man during mixed amino acid infusion: studies of incorporation of [1–13C]leucine. Clin Sci. 1989;76:447–454. doi: 10.1042/cs0760447. [DOI] [PubMed] [Google Scholar]
- Bergstrom J, Fürst P, Hültman E. Free amino acids in muscle tissue and plasma during exercise in man. Clin Physiol. 1985;5:155–160. doi: 10.1111/j.1475-097x.1985.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Bergstrom J, Fürst P, Vinnars E. Effect of a test meal, without and with protein, on muscle and plasma free amino acids. Clin Sci. 1990;79:331–337. doi: 10.1042/cs0790331. [DOI] [PubMed] [Google Scholar]
- Bohé J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532:575–579. doi: 10.1111/j.1469-7793.2001.0575f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WW, Trappe TA, Wolfe RR, Evans WJ. The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci. 2001;56:M373–380. doi: 10.1093/gerona/56.6.m373. [DOI] [PubMed] [Google Scholar]
- Dietrichson P, Coakley J, Smith PEM, Griffiths RD, Helliwell TR, Edwards RHT. Conchotome and needle percutaneous biopsy of skeletal muscle. J Neurol Neurosurg Psychiatry. 1987;50:1461–1467. doi: 10.1136/jnnp.50.11.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich R, Rapoport TA. Linear theory of enzymatic chains; its application for the analysis of the crossover theorem and of the glycolysis of human erythrocytes. Acta Biol Med Ger. 1973;31:479–494. [PubMed] [Google Scholar]
- Kimball SR, Horetsky RL, Jefferson LS. Signal transduction pathways involved in the regulation of protein synthesis by insulin in L6 myoblasts. Am J Physiol. 1998;274:C221–228. doi: 10.1152/ajpcell.1998.274.1.C221. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Shantz LM, Horetsky RL, Jefferson LS. Leucine regulates translation of specific mRNAs in L6 myoblasts through mTOR-mediated changes in availability of eIF4E and phosphorylation of ribosomal protein S6. J Biol Chem. 1999;274:11647–11652. doi: 10.1074/jbc.274.17.11647. [DOI] [PubMed] [Google Scholar]
- Millward DJ, Fereday A, Gibson NR, Pacy PJ. Post-prandial protein metabolism. Baillieres Clin Endocrinol Metab. 1996;10:533–549. doi: 10.1016/s0950-351x(96)80696-3. [DOI] [PubMed] [Google Scholar]
- Mortimore GE, Khurana KK, Miotto G. Amino acid control of proteolysis in perfused livers of synchronously fed rats. Mechanism and specificity of alanine co-regulation. J Biol Chem. 1991;266:1021–1028. [PubMed] [Google Scholar]
- Mortimore GE, Wert JJ, Jr, Miotto G, Venerando R, Kadowaki M. Leucine-specific binding of photoreactive Leu7-MAP to a high molecular weight protein on the plasma membrane of the isolated rat hepatocyte. Biochem Biophys Res Commun. 1994;203:200–208. doi: 10.1006/bbrc.1994.2168. [DOI] [PubMed] [Google Scholar]
- Olde Damink SW, Dejong CH, Deutz NE, Soeters PB. Decreased plasma and tissue isoleucine levels after simulated gastrointestinal bleeding by blood gavages in chronic portacaval shunted rats. Gut. 1997;40:418–424. doi: 10.1136/gut.40.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters T., Jr Serum albumin. Adv Protein Chem. 1985;37:161–245. doi: 10.1016/s0065-3233(08)60065-0. [DOI] [PubMed] [Google Scholar]
- Reeds PJ, Fjeld CR, Jahoor F. Do the differences between the amino acid compositions of acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states. J Nutr. 1994;124:906–910. doi: 10.1093/jn/124.6.906. [DOI] [PubMed] [Google Scholar]
- Rennie MJ. Myofibrillar protein synthesis (MPS) and the activity of p70s6 kinase in human skeletal muscle: the effects of contractile activity and essential amino acids (EAA) J Physiol. 2001b;531.P:39P. [Google Scholar]
- Rennie MJ, Edwards RH, Halliday D, Matthews DE, Wolman SL, Millward DJ. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci. 1982;63:519–523. doi: 10.1042/cs0630519. [DOI] [PubMed] [Google Scholar]
- Smith K, Barua JM, Watt PW, Scrimgeour CM, Rennie MJ. Flooding with L-[1–13C]leucine stimulates human muscle protein incorporation of continuously infused L-[1–13C]valine. Am J Physiol. 1992;262:E372–376. doi: 10.1152/ajpendo.1992.262.3.E372. [DOI] [PubMed] [Google Scholar]
- Smith K, Reynolds N, Downie S, Patel A, Rennie MJ. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol. 1998;275:E73–78. doi: 10.1152/ajpendo.1998.275.1.E73. [DOI] [PubMed] [Google Scholar]
- Svanberg E, Jefferson LS, Lundholm K, Kimball SR. Postprandial stimulation of muscle protein synthesis is independent of changes in insulin. Am J Physiol. 1997;272:E841–847. doi: 10.1152/ajpendo.1997.272.5.E841. [DOI] [PubMed] [Google Scholar]
- Taylor PM, Rennie MJ, Low SY. Biomembrane transport and interorgan flows: the amino acids. In: Van Winkle LJ, editor. Biomebrane Transport. New York: Academic; 1999. pp. 295–325. [Google Scholar]
- Tipton KD, Gurkin BE, Matin S, Wolfe RR. Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. J Nutr Biochem. 1999;10:89–95. doi: 10.1016/s0955-2863(98)00087-4. [DOI] [PubMed] [Google Scholar]
- Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001;286:1206–1212. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterlow JC, Garlick PJ, Millward DJ. Protein Turnover in Mammalian Tissues and in the Whole Body. Amsterdam: Elsevier-North Holland; 1978. [Google Scholar]
- Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. New York: Wiley-Liss; 1992. [Google Scholar]
- Wolfe RR, Miller SL. Amino acid availability controls muscle protein metabolism. Diabetes Nutr Metab. 1999;12:322–328. [PubMed] [Google Scholar]
- Yoshizawa F, Kimball SR, Jefferson LS. Modulation of translation initiation in rat skeletal muscle and liver in response to food intake. Biochem Biophys Res Commun. 1997;240:825–831. doi: 10.1006/bbrc.1997.7652. [DOI] [PubMed] [Google Scholar]
- Yoshizawa F, Kimball SR, Vary TC, Jefferson LS. Effect of dietary protein on translation initiation in rat skeletal muscle and liver. Am J Physiol. 1998;275:E814–820. doi: 10.1152/ajpendo.1998.275.5.E814. [DOI] [PubMed] [Google Scholar]


