Abstract
Neurons were obtained from the CA1 region of the hippocampus of newborn rats and maintained in culture. Channels were activated by pentobarbitone in cell-attached, inside-out or outside-out patches, normally by applying pentobarbitone in flowing bath solution. Currents were outwardly rectifying and blocked by bicuculline, properties of GABAA channels in these cells. Maximum channel conductance increased as pentobarbitone concentration was increased to 500 μm but conductance then decreased as pentobarbitone concentration was raised further. The best fit of a Hill-type equation to the relationship between maximum channel conductance and pentobarbitone concentration (up to 500 μm) gave an EC50 of 41 μm, a maximum conductance of 36 pS and a Hill coefficient of 1.6. Bicuculline decreased the maximum conductance of the channels activated by pentobarbitone, with an IC50 of 224 μm. Diazepam increased channel conductance, with a maximum effect being obtained with 1 μm diazepam. Diazepam (1 μm) decreased the EC50 of the pentobarbitone effect on channel conductance from 41 μm to 7.2 μm and increased maximum conductance to 72 pS. We conclude that GABAA channel conductance is related to the concentration of the allosteric agonist pentobarbitone.
In neurons obtained from the CA1 region of hippocampal slices from neonatal rats and maintained in culture, chloride channels activated by GABA (GABAA channels) are outwardly rectifying and can have conductances much greater than 30 pS at positive potentials (Curmi et al. 1993; Gage & Chung, 1994). Drugs such as diazepam and pentobarbitone increase the conductance of low-conductance channels (< 30 pS) activated by GABA in these cells (Eghbali et al. 1997, 2000). In outside-out patches, maximum channel conductance increases with GABA concentration (Birnir et al. 2001). The effect of GABA concentration on channel conductance was not tested in cell-attached or inside-out patches because of the technical difficulty of changing the GABA concentration in the tip of the patch pipette when studying channels in these patch configurations.
The main aim of this investigation was to investigate the influence of agonist concentration on channel conductance. Although it was previously shown that GABA concentration could influence the conductance of GABAA channels that appeared after a delay in outside-out patches from the same preparation as used here (Birnir et al. 2001), we wished to study the effect of agonist concentration in cell-attached and inside-out patches as well as outside-out patches. We therefore chose an allosteric agonist, pentobarbitone, that readily crosses cell membranes. An advantage with pentobarbitone compared with GABA as an agonist is that in its undissociated form it is lipid soluble and can equilibrate with the cell membrane and eventually with the solution in the tip of a patch pipette when applied at varying concentrations in the bath solution. This allowed us to test the effect of pentobarbitone concentration on channel conductance in cell-attached and inside-out patches. There is good evidence that GABAA channels can be activated directly by barbiturates (Mathers & Barker, 1980; Nicoll & Wojtowicz, 1980; Schwartz et al. 1986; Yang & Olsen, 1987; Robertson, 1989; Rho et al. 1996; Thompson et al. 1996) but the binding site is different from the binding site for GABA (Amin & Weiss, 1993), i.e. pentobarbitone is an allosteric agonist. In spite of this, activation by pentobarbitone can be inhibited by bicuculline (Nicoll & Wojtowicz, 1980; Rho et al. 1996), which competes with GABA for its binding site.
In this paper, we describe the effects of pentobarbitone concentration on the conductance of GABAA channels. The kinetics of channels activated by pentobarbitone have been extensively studied by others (Rho et al. 1996; Akk & Steinbach, 2000; Serafini et al. 2000; Krampfl et al. 2002) and they are not described here because it would add little new information.
We found that channels activated by pentobarbitone were outwardly rectifying and could have conductances higher than 30 pS at positive potentials (though generally less than the conductance of channels activated by GABA), and maximum channel conductance varied with pentobarbitone concentration.
METHODS
Cell culture
Primary cultures of hippocampal neurons from neonatal rats were prepared as described previously (Curmi et al. 1993). Briefly, newborn rats were killed rapidly by decapitation using protocols approved by the Australian National University Animal Ethics Committee (JBM5100). Their hippocampi were then removed and cells obtained by trituration were grown on glass coverslips coated with poly-L-lysine. The culture medium was 87 % minimum essential medium (MEM Gibco) and glucose (360 mg (100 ml)−1), supplemented with 10 % fetal bovine serum (Gibco), 1 % penicillin-streptomycin and 0.001 % serum extender. The cultures were incubated at 37 °C in a controlled atmosphere of 5 % CO2-95 % air for 5-24 days. Individual coverslips were transferred to the recording bath for experiments.
Solutions
The bath solution contained (mM): NaCl 135, KCl 3, MgCl2 2, CaCl2 2 and Tes 10, adjusted to a pH of 7.3 with NaOH (1 M), and had an osmolarity of 280-300 mosmol l−1. In experiments on inside-out and cell-attached patches, the patch pipette contained (mM): NaCl 140, MgCl2 2, CaCl2 0.5 and Tes 10 and for outside-out patches the patch pipette contained (mM): NaCl 140, MgCl2 2, CaCl2 0.5, EGTA 5 and Tes 10. Experiments were carried out at room temperature (20-22 °C). Drugs used were pentobarbitone (Sigma), bicuculline methiodide (Sigma) and diazepam (Hoffman-La Roche). Diazepam was dissolved in dimethylsulphoxide (DMSO), then in bath solution. Aliquots of the stock solutions were added to the bath solutions to give the required concentrations. The maximum final concentration of DMSO in the experiments was 190 μm, a concentration that does not activate or modulate GABAA receptors in the cultured neurons (n = 6). Drugs were dissolved in bath solution and applied to a patch either in the flowing bath solution or occasionally through a fine perfusion tube positioned close to the patch. The flow rate into the bath was 2-3 ml min−1 and the bath volume was 0.5-1 ml so that drugs applied through the bath would reach the concentration in the inflowing solution only gradually. Apart from time of onset of effects, these methods produced no significant difference in the final channel conductance.
Recording currents
Patch pipettes were made from borosilicate glass (GC150f-15, Clarke Electromedical Instruments, UK) on a two-stage vertical puller (L/M-3P-A, List Medical). Pipettes were coated within 50 μm of their tip with Sylgard (184 Silicon elastone, Dow-Corning, Midland, MI, USA). Their tips were fire-polished to create a smooth and clean tip. Pipettes had a resistance of 10-15 MΩ when filled with pipette solution. Single-channel currents were recorded in cell-attached, inside-out or outside-out patches with an Axopatch 1C amplifier (Axon Instruments), filtered at 5 kHz, digitized at 44 kHz (Sony PCM) and stored on video tape. For analysis, currents were digitized using an IBM-compatible PC and digital to analog interface. The maximum amplitude of single-channel currents was measured from records of channel activity filtered at 5 kHz and digitized at 10 kHz. A channel opening was defined as a direct (< 200 μs) transition from, or to, the baseline current level. Intermediate levels occurring during opening of a channel that closed without delay to the baseline level (or vice versa) were considered subconductance states. Channels that opened to and closed from a lower current level were considered subconductance states of the largest channels or perhaps a second kind of channel in the patch. If smaller openings never obviously superimposed on larger openings, it was most likely that they were subconductance states. Normally, 300-1000 such channel openings were measured. Channel conductance was calculated by dividing current amplitude by the difference between the reversal potential and the pipette potential.
Analysis of channel conductance-concentration data
The average conductance of channels at different pentobarbitone concentrations was fitted with the Hill-type equation:
| (1) |
where γ is average single-channel conductance, γmax the maximum single-channel conductance, [PB] the pentobarbitone concentration, EC50 the pentobarbitone concentration where γ = γmax/2 and n the Hill coefficient.
In the presence of varying concentrations of bicuculline, the relationship between average channel conductance and bicuculline concentration was fitted with the equation:
| (2) |
where γ0 is the conductance of channels when the bicuculline concentration ([BIC]) is zero and IC50 is the bicuculline concentration that halves the conductance of the channels.
RESULTS
Channels activated by pentobarbitone
Pentobarbitone applied in the bath solution activated channels in outside-out, inside-out, and cell-attached patches as illustrated in Fig. 1. Before application of pentobarbitone, there was no channel activity (see all-points histograms to the right of control traces) and application of 100 μm pentobarbitone in the bath elicited channel activity. The reversibility of this effect of pentobarbitone in a cell-attached patch is shown in Fig. 1C. The channels shown had a conductance of about 25 pS. Some patches showed frequent subconductance states, others did not. Subconductance activity occurred in several forms. In one form, there were occasional bursts of low amplitude (< 10 pS) ‘spikey’ currents. In another form, current amplitude could increase or decrease in a series of ill-defined steps. This kind of activity could produce inter-peak non-zero probabilities in all-points histograms. In none of the patches was there a clearly defined, consistent subconductance level. Invariably, the maximum conductance level occurred most frequently and was best defined.
Figure 1. Single-channel currents activated directly by 100 μm pentobarbitone.
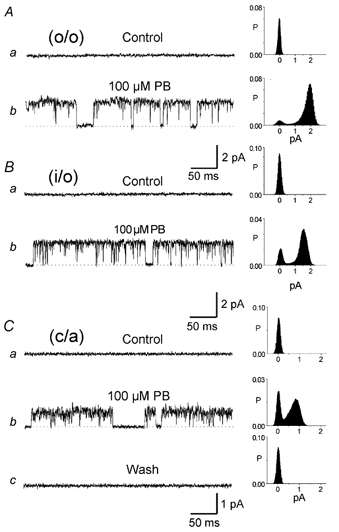
Openings are upward in all traces and the dotted lines show the closed level. All-points histograms shown alongside traces were obtained from 20 s segments of data sampled at 10 kHz. A, currents recorded in an outside-out (o/o) patch (pipette potential, +80 mV) before (a) and after (b) exposing the patch to 100 μm pentobarbitone in the bath solution. B, currents recorded in an inside-out (i/o) patch (Vp =−60 mV) before (a) and after (b) exposing the patch to 100 μm pentobarbitone in the bath solution. C, currents recorded in a cell-attached (c/a) patch (Vp =−40 mV) before (a) and after (b) exposing the cell to 100 μm pentobarbitone in the bath solution. Channel activity disappeared when pentobarbitone was washed out of the bath (c).
Characteristics of channels activated by pentobarbitone
The relationship between membrane potential and the amplitude of single-channel currents activated by pentobarbitone is illustrated for an inside-out patch in Fig. 2. The currents (activated by 100 μm pentobarbitone) reversed at about 0 mV. With 143 mM Cl− on both sides of the patch (circles), the reversal potential was close to 0 mV and there was outward rectification similar to that reported previously for channels activated by GABA in these cells (Curmi et al. 1993). Similar outward rectification and a reversal potential at 0 mV were seen in channels activated by pentobarbitone in other inside-out and outside-out patches exposed to symmetrical Cl− concentrations (not shown). When the bath solution was changed to one containing 30 mM Cl− (by replacing 113 mM NaCl with 113 mM sodium gluconate), the reversal potential changed to −40 mV, close to the calculated chloride equilibrium potential of −39 mV, and there was still outward rectification (Fig. 2, squares). It was concluded that the currents activated by pentobarbitone were chloride currents.
Figure 2. Current-voltage characteristics of channels activated by pentobarbitone.
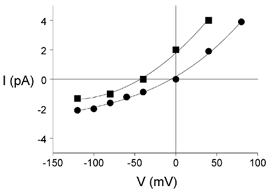
Relationship between amplitude of single-channel currents (I (pA)) activated by 100 μm pentobarbitone and membrane potential (V (mV) =−Vp) recorded from an inside-out patch. In symmetrical chloride solutions (143 mM, •) the reversal potential was close to 0 mV. When the chloride concentration in the bath solution was changed to 30 mM (▪), the reversal potential shifted from 0 mV to −40 mV.
The relationship between pentobarbitone concentration and maximum single-channel conductance
In order to study the relationship between pentobarbitone concentration and maximum single-channel conductance in the same patch, a series of different concentrations of pentobarbitone was applied to a patch through the flowing bath solution. Single-channel currents recorded in an outside-out patch exposed to concentrations of pentobarbitone ranging from 20 μm to 10 mM are shown in Fig. 3A-F. Before exposure of the patch to pentobarbitone, no channel activity was evident (Fig. 3A). Raising the concentration of pentobarbitone from 20 to 50 μm increased the maximum single-channel current amplitude while the reversal potential remained at 0 mV (not shown): the average maximum single-channel conductance increased from 10 to 35 pS. As the concentration of pentobarbitone was increased up to 500 μm, channel conductance increased. However, at pentobarbitone concentrations above 500 μm, channel conductance decreased as can be seen in Fig. 3E and F.
Figure 3. The effect of pentobarbitone concentration on single-channel currents.
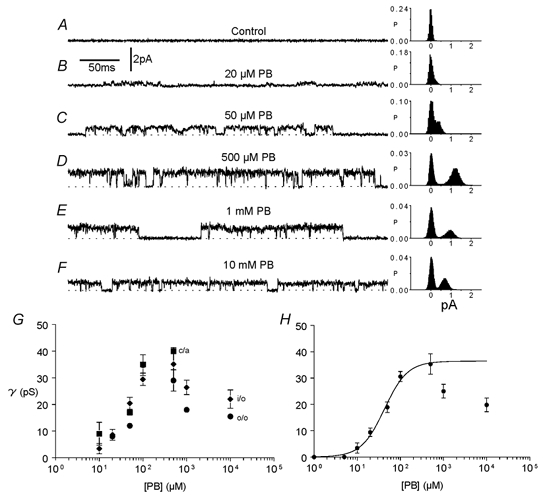
Records were all from the same outside-out patch at a pipette potential of +60 mV before (A) and during exposure to a range of pentobarbitone (PB) concentrations (B-F). All-points histograms shown alongside traces were constructed from 10 s segments of data sampled at 10 kHz. G, relationship between pentobarbitone concentration and channel conductance (γ). Average results were obtained from four cell-attached patches (▪), 39 inside-out patches (♦) and 15 outside-out patches (•). Data points represent the mean conductance ± 1 s.e.m. if larger than the symbol. H, all data from all patches were averaged and fitted using the Hill equation (eqn (1), Methods) over the range of 1−500 μm pentobarbitone. The maximum channel conductance was estimated to be 36 pS. The EC50 and the Hill coefficient were 41 μm and 1.6 respectively.
In Fig. 3G, similar results obtained in four cell-attached patches (squares), 39 inside-out patches (diamonds) and 15 outside-out patches (circles) exposed to a range of pentobarbitone concentrations and average maximum conductances (± 1 s.e.m.) are shown. Channel conductances were measured directly from current records as described in the Methods. Channel conductance at first increased with pentobarbitone concentration and then decreased at higher concentrations (above 500 μm). The fit of a Hill-type equation (eqn (1), Methods) to average conductance obtained from all types of patches vs. pentobarbitone concentrations from 1 to 500 μm (Fig. 3H) gave an EC50 (pentobarbitone concentration for half-maximum conductance) of 41 μm, a Hill coefficient of 1.6 and a maximum single-channel conductance of 36 pS.
Effects of bicuculline on currents activated by pentobarbitone
GABA and bicuculline are thought to bind to the same site on GABAA receptors. Bicuculline also antagonizes the direct agonist effects of barbiturates (Robertson, 1989; Uchida et al. 1996; Ueno et al. 1997). We found that bicuculline modulated both the open probability and conductance of channels activated by pentobarbitone. Results obtained in an experiment on an outside-out patch are shown in Fig. 4A-D. This patch showed very little substate activity and this is reflected in the all-points histograms. Single-channel currents activated directly by 100 μm pentobarbitone had a maximum amplitude of 3.2 pA (40 pS, Vp = +80 mV, Fig. 4A). Little change in either the amplitude or the open probability of single-channel currents was caused by 20 μm bicuculline (trace and histogram, Fig. 4B). Following exposure of the patch to 100 μm pentobarbitone + 100 μm bicuculline, the amplitude of single-channel currents decreased to 1.6 pA (20 pS, Fig. 4C) and the relative areas of the open and closed channel components of the histogram in Fig. 4C also show that the bicuculline caused a decrease in channel open probability. It can be seen that openings at 1.6 pA were not prominent before exposure to bicuculline so that it is unlikely that the bicuculline was preferentially affecting higher-conductance channels. Raising the concentration of bicuculline to 500 μm decreased the amplitude of single-channel currents even further to 1.2 pA (15 pS, Fig. 4D). The histogram in Fig. 4D also shows a decrease in open probability but there were still some channel openings even at this high bicuculline concentration. Again channel openings to 1.2 pA were very infrequent at lower bicuculline concentrations. There were still small-amplitude channels with a conductance of about 5 pS in the presence of 100 μm pentobarbitone + 2 mM bicuculline (not shown). Conductances of channels activated by 100 μm pentobarbitone and measured directly from current records (see Methods) in another outside-out patch before and after exposure to 500 μm bicuculline are shown in Fig. 4E and F. Before the bicuculline, maximum single-channel current was about 3 pA and there were no lower amplitude currents (Fig. 4E). After exposure to 500 μm bicuculline (Fig. 4F), there were no single-channel currents with an amplitude above 1.5 pA but single-channel currents now had a maximum amplitude of 1.2 pA on average, a level not seen before exposure to the bicuculline.
Figure 4. Effect of bicuculline concentration on channels activated by pentobarbitone.
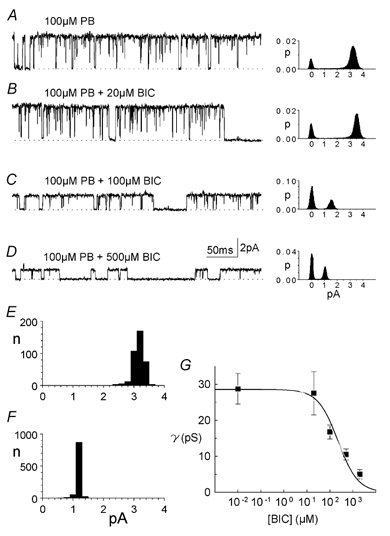
Currents were activated in an outside-out patch by 100 μm pentobarbitone (Vp =+80 mV) and the patch was then exposed to varying concentrations of bicuculline (A, no bicuculline; B, C and D, 20 μm, 100 μm and 500 μm, respectively). The corresponding all-points histograms are from 10 s current records sampled at 10 kHz. E, frequency histogram of channel amplitude measured in another outside-out patch exposed to 100 μm pentobarbitone. Ordinate in E and F, number of observations. F, frequency histogram of channel amplitude measured in the same outside-out patch as in E exposed to 100 μm pentobarbitone plus 500 μm bicuculline. G, relationship between bicuculline concentration and average maximum conductance of channels activated by pentobarbitone. Currents were activated with 100 μm pentobarbitone in outside-out patches. Averaged data (n = 6) fitted with a simple binding equation (eqn (2), Methods) gave a bicuculline IC50 of 224 μm. Vertical bars show ± 1 s.e.m.
Similar results were obtained in six outside-out patches: single-channel conductance and open probability were both reduced as bicuculline concentration was increased. The average conductance of channels in the six outside-out patches activated by 100 μm pentobarbitone, measured directly from current records, is plotted against bicuculline concentration in Fig. 4G. Fitting the data with a simple equation (eqn (2), Methods) gave an IC50 of 224 μm.
Effects of diazepam on currents activated by pentobarbitone
The benzodiazepine diazepam has been shown to potentiate the effectiveness of GABAA receptors by increasing the probability of channel opening (Study & Barker, 1981; Rogers et al. 1994) and, more recently, the conductance (Eghbali et al. 1997; Guyon et al. 1999) of channels activated by GABA. The binding site and molecular changes underlying the effects of benzodiazepines on GABAA receptors have not been established.
We found that diazepam increases both the maximum conductance and open probability of channels directly activated by pentobarbitone. This is illustrated in records from an inside-out patch (Vp −40 mV) in Fig. 5. Small channels (18 pS) were activated by 10 μm pentobarbitone (Fig. 5A). At the arrow near the beginning of the second trace (Fig. 5B), a solution containing 10 μm pentobarbitone + 1 μm diazepam started to flow into the bath. The maximum single-channel conductance increased gradually to 75 pS at the end of the trace in Fig. 5C. The gradual increase in conductance may have been caused by a gradual increase in diazepam concentration in the bath. Forty seconds later (Fig. 5D), currents from two channels were superimposing, each with a maximum conductance of 75 pS, indicating that channel open probability had increased. The mean current of 0.3 pA before exposure to 1 μm diazepam increased by over 10-fold to 3.2 pA in the presence of the diazepam. Similar increases in both the maximum conductance and open probability of channels activated by pentobarbitone were observed in 17 inside-out and two outside-out patches in the presence of 1 μm diazepam. The effectiveness of diazepam was dependent on its concentration as illustrated in Fig. 6A-C. Small channels (12 pS) were first activated in an outside-out patch (Vp = +60 mV) with 20 μm pentobarbitone (Fig. 6A) and then the patch was exposed to 20 μm pentobarbitone + 1 μm diazepam. Maximum single-channel current amplitude increased to 5 pA (83 pS, Fig. 6B). The histograms in Fig. 6A and B reveal that channel open probability also increased. Increasing the diazepam concentration to 10 μm decreased maximum single-channel current amplitude to 3.8 pA (63 pS, Fig. 6C). Similar results for the effect of diazepam on maximum channel conductance were obtained in another seven inside-out and two outside-out patches and these results are summarized in Fig. 6D in which relative channel conductance (γ(DZ/CON)) is plotted against diazepam concentration. The maximal effect on maximum single-channel conductance was observed at 1 μm diazepam. At higher concentrations, the enhancement by diazepam was less.
Figure 5. Effect of diazepam on channels activated by pentobarbitone.
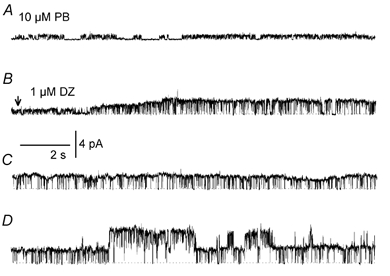
Single-channel currents were recorded in an inside-out patch activated by 10 μm pentobarbitone (PB) (Vp =−40 mV) before (A) and after (arrow in B) exposure to 1 μm diazepam (DZ). There was a gradual increase in the single-channel conductance (traces in B and C are continuous). Thirty seconds later (D), two channels were opening in the patch.
Figure 6. Effect of diazepam concentration on maximum conductance of channels activated by pentobarbitone.
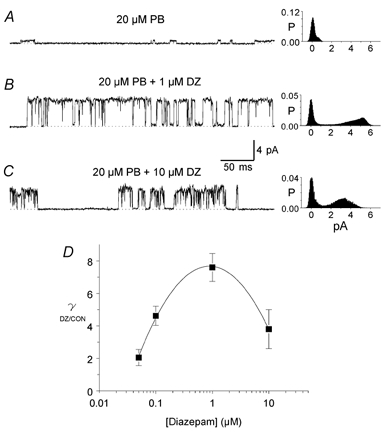
A, low-conductance channels activated in an outside-out patch (Vp =+60 mV) with 20 μm pentobarbitone (PB). B, single-channel currents recorded in the same patch following exposure to 20 μm pentobarbitone plus 1 μm diazepam (DZ). C, currents recorded following exposure of the patch to 20 μm pentobarbitone + 10 μm diazepam. The corresponding all-points histograms on the right of each trace were obtained from 10 s current records sampled at 10 kHz. D, relationship between diazepam concentration and average maximum conductance of channels activated by 20 μm pentobarbitone measured in 10 patches (7 inside-out and 3 outside-out) in which channels were activated with 20 μm pentobarbitone and then exposed to a range of diazepam concentrations. Average maximum conductance in any patch was normalized to channel conductance measured before exposure to diazepam (γ (DZ/CON)). The line is the best fit of a third order polynomial. Vertical bars show ± 1 s.e.m.
Effect of diazepam on pentobarbitone EC50
It has been suggested that diazepam increases the affinity of GABAA receptors for GABA. We therefore examined whether diazepam affects the concentration dependence of the agonist effect of pentobarbitone. Since the maximum potentiation of currents activated by pentobarbitone occurred at 1 μm diazepam, we used this concentration to test whether diazepam influences the effectiveness of pentobarbitone as an agonist. The effect of 1 μm diazepam on the relationship between maximum single-channel conductance and pentobarbitone concentration (n = 19) is illustrated in Fig. 7. The line through the data points in Fig. 7A is the best fit of a Hill-type equation to the data. In the presence of 1 μm diazepam, maximum channel conductance was increased to 71.6 pS. The shift in the pentobarbitone EC50 caused by the diazepam is shown more clearly in Fig. 7B in which conductance is normalized to the maximum conductance before (dashed line) and after (continuous line) exposure to the diazepam. Addition of the diazepam decreased the pentobarbitone EC50 from 41 to 7.2 μm.
Figure 7. Effect of diazepam on the relationship between pentobarbitone concentration and maximum channel conductance.
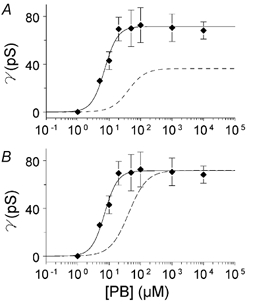
A, data points show average maximum channel conductance (± 1 s.e.m.) for channels activated by pentobarbitone + 1 μm diazepam (19 patches; 17 inside-out and 2 outside-out). The continuous line is the best fit of eqn (1) to the points. The dashed line shows for comparison the line fitted to the data in the absence of diazepam from Fig. 3H. B, the dashed line has been scaled up to the same maximum as the continuous line to illustrate the change in pentobarbitone EC50.
DISCUSSION
Our results show that maximum GABAA channel conductance varies with agonist (pentobarbitone) concentration. Although we had shown previously that pentobarbitone could influence the conductance of channels activated by GABA (Eghbali et al. 2000), in those experiments pentobarbitone was not the agonist. There is evidence that the modulatory and agonist effects of pentobarbitone occur at different sites: for example a single site mutation in the β1 subunit abolishes the modulating effect of pentobarbitone, leaving its direct agonist action intact (Dalziel et al. 1999).
Single-channel currents that were activated by pentobarbitone in cell-attached, inside-out and outside-out patches had characteristics of GABAA channels. They were chloride channels because currents reversed at a potential close to that predicted for a chloride-selective channel and no other ion had an equilibrium potential close to the reversal potential. They showed outward rectification, were depressed by bicuculline and were potentiated by diazepam, all characteristics of channels activated by GABA in these cells (Fatima-Shad & Barry, 1992; Curmi et al. 1993; Eghbali et al. 1997, 2000). However, there were some differences between channels activated by GABA and pentobarbitone. The maximum conductance of channels activated by pentobarbitone was about 40 pS, significantly less than observed when GABA was the agonist (Birnir et al. 2001), although maximum conductance was increased by diazepam (Fig. 7).
Influence of pentobarbitone concentration
Why channel conductance is influenced by agonist concentration and drugs in these receptors remains unexplained. It has not been reported in many other studies on GABAA receptors in cultured neurons and expression systems and may be due to the presence of unusual receptors in cultured hippocampal neurons from neonatal rats, a preparation that is not widely used. On the other hand, an influence of benzodiazepines (Guyon et al. 1999; Birnir et al. 2000a), bicuculline and pentobarbitone (Birnir et al. 2000a) on single-channel conductance has also been reported in neurons in situ. A dependence of channel conductance on ligand concentration has been observed in homomeric glutamate channels (Rosenmund et al. 1998) and cyclic nucleotide-gated channels (Ruiz & Karpen, 1997).
The maximum conductance of channels increased with pentobarbitone concentration up to a concentration of 500 μm. The increase in maximum channel conductance caused by increasing pentobarbitone concentration could be due to the conversion of lower conductance (subconductance) states of a channel into higher conductance states, or to the recruitment of another channel whose maximum conductance increased with an increase in pentobarbitone concentration. Our results favour the former explanation. Lower conductance levels became less frequent as higher conductance levels appeared. For example, this can be seen in the all-points histograms in Fig. 3C and D. The peak at about 0.4 pA (Fig. 3C) is not there when the main peak has shifted to about 1.3 pA (Fig. 3D).
A similar dependence of channel conductance on agonist concentration has been reported for channels activated by GABA in outside-out patches from cultured hippocampal neurons obtained from neonatal rats (Birnir et al. 2001). We now report a similar phenomenon in cell-attached and inside-out patches with pentobarbitone, an allosteric agonist that can cross cell membranes in its undissociated form. The average conductance increased from less than 8 pS (10 μm pentobarbitone) to almost 40 pS in the presence of 500 μm pentobarbitone. GABAA channels directly activated by pentobarbitone in outside-out patches from cultured rat hippocampal neurons have been previously described (Rho et al. 1996). The unitary conductance was 30 pS for channels directly activated by pentobarbitone (30 and 300 μm) but no correlation was reported between pentobarbitone concentration and single-channel conductance. From the concentration-conductance curve we obtained (Fig. 3H), the average conductances of channels activated by 30 μm and 300 μm pentobarbitone in our cells were about 15 pS and 30 pS.
An inhibitory effect of higher concentrations of pentobarbitone (1 mM or greater) described here for single-channel conductance has been reported previously for currents activated by barbiturates (Akaike et al. 1985; Parker et al. 1986; Yakushiji et al. 1989; Cestari et al. 1996; Rho et al. 1996; Akk & Steinbach, 2000). The mechanisms underlying the inhibitory effect of pentobarbitone are not clear. At high concentrations, pentobarbitone may block channels or bind at another binding site that inhibits the response.
The maximum conductance of channels activated by pentobarbitone was less than the conductance of channels activated by GABA in the same cells (Birnir et al. 2001) or in adult hippocampal neurons in slices (Gray & Johnston, 1985; Birnir et al. 2000b). It is known that different combinations of GABAA receptor subunits show different sensitivities to pentobarbitone. The degree of affinity and efficacy for the direct activation by pentobarbitone, depends on the type of α subunit present (Thompson et al. 1996; Fisher et al. 1997). In receptors containing α6 subunits, pentobarbitone is more effective than GABA, producing a larger maximum whole-cell current than that obtainable with a maximal concentration of GABA (154 %). With the other α subunits, pentobarbitone is less effective than GABA, the maximum response ranging between 45 % of maximal GABA response for α5 to 83 % for α2. Our results are consistent with the absence of α6 subunits in the receptors.
Effects of bicuculline
Bicuculline binds to GABAA receptors at the same site as GABA and competitively inhibits GABA binding and GABAA currents (Zukin et al. 1974; Ueno et al. 1997). Although pentobarbitone does not bind at the same site as GABA (Amin & Weiss, 1993), it has been reported that bicuculline inhibits currents activated by pentobarbitone but with a higher IC50 than that required for block of currents activated by GABA (Rho et al. 1996; Thompson et al. 1996; Ueno et al. 1997). In experiments on cultured rat hippocampal neurons, 100 μm bicuculline reduced the amplitude of whole-cell currents activated directly by 300 μm pentobarbitone, but even at higher concentrations of bicuculline the inhibition was not complete (Rho et al. 1996). In another study on α1β2γ2s receptors expressed in oocytes, currents activated by GABA were completely blocked by 100 μm bicuculline, whereas this concentration of bicuculline had little effect on currents directly activated by 100 μm pentobarbitone (Thompson et al. 1996).
The observations that bicuculline could inhibit the effects of pentobarbitone but that higher concentrations were required than to block receptors activated by GABA were not unexpected, therefore. We have reported that the bicuculline IC50 for channels activated by GABA in the same preparation was 19.2 ± 2 μm (Birnir et al. 2001). In contrast, 20 μm bicuculline had no effect on the conductance of channels activated by 100 μm pentobarbitone and block was not complete even with 2 mM bicuculline. It appears that high concentrations of bicuculline can partially inhibit channels activated by agonists acting at sites other than the GABA binding site. However, the depression of channel conductance by bicuculline is unusual but not without precedent (Birnir et al. 2000a, 2001). The depression of channel conductance by bicuculline is not due to selective removal of a different population of high-conductance channels, leaving already existing, low-conductance channels. This is illustrated in the all-points histograms in Fig. 4. In Fig. 4B, there is a single prominent peak at about 3.5 pA but no peak at 1.5 pA, whereas in Fig. 4C, in which the peak at 3.5 pA has disappeared, there is now a prominent peak at 1.5 pA. Direct measurement of single-channel openings (Fig. 4E and F) confirms this. Before exposure to the bicuculline, there were no channel openings to 1.2 pA, the main single-channel current amplitude after exposure to 500 μm bicuculline. The bicuculline is reducing the conductance of channels in some way, perhaps by making high-conductance states less favourable.
Effects of diazepam
Diazepam (1 μm) made pentobarbitone a more effective agonist, shifting the EC50 from 41 μm to 7.2 μm (Fig. 7). In addition, diazepam increased the maximum conductance of channels activated by maximal concentrations of pentobarbitone to 70-80 pS (Figs 5–7). It is also interesting to note that diazepam was able to increase the amplitude of currents depressed by high concentrations of pentobarbitone (compare Fig. 3 and Fig. 7). Hence diazepam, an allosteric modulator of GABAA receptors, can relieve the depression of single-channel conductance caused by higher concentrations of pentobarbitone.
The EC50 of pentobarbitone for general anaesthesia is about 50 μm, and pentobarbitone at concentrations only 20 % higher than the EC50 should be able to anaesthetize almost all animals (Franks & Lieb, 1994). The EC50 for pentobarbitone in this study is comparable to the clinical concentration that induces general anaesthesia. Therefore, direct activation of GABAA receptors by pentobarbitone may contribute to its depressant effects.
REFERENCES
- Akaike N, Hattori K, Inomata N, Oomura Y. Gamma-aminobutyric-acid- and pentobarbitone-gated chloride currents in internally perfused frog sensory neurones. J Physiol. 1985;360:367–386. doi: 10.1113/jphysiol.1985.sp015622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Steinbach JH. Activation and block of recombinant GABAA receptors by pentobarbitone: a single-channel study. Br J Pharmacol. 2000;130:249–258. doi: 10.1038/sj.bjp.0703335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin J, Weiss DS. GABAA receptor needs two homologous domains of the beta-subunit for activation by GABA but not by pentobarbital. Nature. 1993;366:565–569. doi: 10.1038/366565a0. [DOI] [PubMed] [Google Scholar]
- Birnir B, Eghbali M, Cox GB, Gage PW. GABA concentration sets the conductance of delayed GABAA channels in outside-out patches from rat hippocampal neurons. J Membr Biol. 2001;181:171–183. doi: 10.1007/s00232-001-0021-5. [DOI] [PubMed] [Google Scholar]
- Birnir B, Eghbali M, Everitt AB, Gage PW. Bicuculline, pentobarbital and diazepam modulate spontaneous GABAA channels in rat hippocampal neurons. Br J Pharmacol. 2000a;131:695–704. doi: 10.1038/sj.bjp.0703621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnir B, Everitt AB, Lim MS, Gage PW. Spontaneously opening GABAA channels in CA1 pyramidal neurones of rat hippocampus. J Membr Biol. 2000b;174:21–29. doi: 10.1007/s002320001028. [DOI] [PubMed] [Google Scholar]
- Cestari IN, Uchida I, Li L, Burt D, Yang J. The agonistic action of pentobarbital on GABAA beta-subunit homomeric receptors. Neuroreport. 1996;7:943–947. doi: 10.1097/00001756-199603220-00023. [DOI] [PubMed] [Google Scholar]
- Curmi JP, Premkumar LS, Birnir B, Gage PW. The influence of membrane potential on chloride channels activated by GABA in rat cultured hippocampal neurones. J Membr Biol. 1993;136:273–280. doi: 10.1007/BF00233666. [DOI] [PubMed] [Google Scholar]
- Dalziel JE, Cox GB, Gage PW, Birnir B. Mutant human alpha(1)beta(1)(T262Q) GABAA receptors are directly activated but not modulated by pentobarbital. Eur J Pharmacol. 1999;385:283–286. doi: 10.1016/s0014-2999(99)00710-4. [DOI] [PubMed] [Google Scholar]
- Eghbali M, Curmi JP, Birnir B, Gage PW. Hippocampal GABAA channel conductance increased by diazepam. Nature. 1997;388:71–75. doi: 10.1038/40404. [DOI] [PubMed] [Google Scholar]
- Eghbali M, Gage PW, Birnir B. Pentobarbital modulates gamma-aminobutyric acid-activated single-channel conductance in rat cultured hippocampal neurons. Mol Pharmacol. 2000;58:463–469. [PubMed] [Google Scholar]
- Fatima-Shad K, Barry PH. A patch-clamp study of GABA- and strychnine-sensitive glycine-activated currents in post-natal tissue-cultured hippocampal neurons. Proc Roy Soc B. 1992;250:99–105. doi: 10.1098/rspb.1992.0136. [DOI] [PubMed] [Google Scholar]
- Fisher JL, Zhang J, MacDonald RL. The role of alpha1 and alpha6 subtype amino-terminal domains in allosteric regulation of gamma-aminobutyric acida receptors. Mol Pharmacol. 1997;52:714–724. doi: 10.1124/mol.52.4.714. [DOI] [PubMed] [Google Scholar]
- Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- Gage PW, Chung S-H. Influence of membrane potential on conductance sublevels of chloride channels activated by GABA. Proc Roy Soc B. 1994;255:167–172. doi: 10.1098/rspb.1994.0024. [DOI] [PubMed] [Google Scholar]
- Gray R, Johnston D. Rectification of single GABA-gated chloride channels in adult hippocampal neurons. J Neurophysiol. 1985;54:134–142. doi: 10.1152/jn.1985.54.1.134. [DOI] [PubMed] [Google Scholar]
- Guyon A, Laurent S, Paupardin-Tritsch D, Rossier J, Eugene D. Incremental conductance levels of GABAA receptors in dopaminergic neurones of the rat substantia nigra pars compacta. J Physiol. 1999;516:719–737. doi: 10.1111/j.1469-7793.1999.0719u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krampfl K, Wolfes H, Dengler R, Bufler J. Kinetic analysis of the agonistic and blocking properties of pentobarbital on recombinant rat alpha(1)beta(2)gamma(2s) GABAA receptor channels. Eur J Pharmacol. 2002;435:1–8. doi: 10.1016/s0014-2999(01)01558-8. [DOI] [PubMed] [Google Scholar]
- Mathers DA, Barker JL. (−)Pentobarbital opens ion channels of long duration in cultured mouse spinal neurons. Science. 1980;209:507–509. doi: 10.1126/science.6248961. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Wojtowicz JM. The effects of pentobarbital and related compounds on frog motoneurons. Brain Res. 1980;191:225–237. doi: 10.1016/0006-8993(80)90325-x. [DOI] [PubMed] [Google Scholar]
- Parker I, Gundersen CB, Miledi R. Actions of pentobarbital on rat brain receptors expressed in xenopus oocytes. J Neurosci. 1986;6:2290–2297. doi: 10.1523/JNEUROSCI.06-08-02290.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho JM, Donevan SD, Rogawski MA. Direct activation of GABAA receptors by barbiturates in cultured rat hippocampal neurons. J Physiol. 1996;497:509–522. doi: 10.1113/jphysiol.1996.sp021784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B. Actions of anaesthetics and avermectin on GABAA chloride channels in mammalian dorsal root ganglion neurones. Br J Pharmacol. 1989;98:167–176. doi: 10.1111/j.1476-5381.1989.tb16878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CJ, Twyman RE, Macdonald RL. Benzodiazepine and beta-carboline regulation of single GABAA receptor channels of mouse spinal neurones in culture. J Physiol. 1994;475:69–82. doi: 10.1113/jphysiol.1994.sp020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Stern-Bach Y, Stevens CF. The tetrameric structure of a glutamate receptor channel. Science. 1998;280:1596–1599. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- Ruiz ML, Karpen JW. Single cyclic nucleotide-gated channels locked in different ligand-bound states. Nature. 1997;389:389–392. doi: 10.1038/38744. [DOI] [PubMed] [Google Scholar]
- Schwartz RD, Suzdak PD, Paul SM. Gamma-aminobutyric acid (GABA)- and barbiturate-mediated 36 Cl− uptake in rat brain synaptoneurosomes: evidence for rapid desensitization of the GABA receptor-coupled chloride ion channel. Mol Pharmacol. 1986;30:419–426. [PubMed] [Google Scholar]
- Serafini R, Bracamontes J, Steinbach JH. Structural domains of the human GABAA receptor 3 subunit involved in the actions of pentobarbital. J Physiol. 2000;524:649–676. doi: 10.1111/j.1469-7793.2000.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Study RE, Barker JL. Diazepam and (−)-pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neurons. Proc Nat Acad Sci U S A. 1981;78:7180–7184. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SA, Whiting PJ, Wafford KA. Barbiturate interactions at the human GABAA receptor: dependence on receptor subunit combination. Br J Pharmacol. 1996;117:521–527. doi: 10.1111/j.1476-5381.1996.tb15221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida I, Cestari IN, Yang J. The differential antagonism by bicuculline and SR95531 of pentobarbitone-induced currents in cultured hippocampal neurons. Eur J Pharmacol. 1996;307:89–96. doi: 10.1016/0014-2999(96)00156-2. [DOI] [PubMed] [Google Scholar]
- Ueno S, Bracamontes J, Zorumski C, Weiss DS, Steinbach JH. Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor. J Neurosci. 1997;17:625–634. doi: 10.1523/JNEUROSCI.17-02-00625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakushiji T, Oyama Y, Akaike N. Comparative study on barbiturates using isolated single neurons: GABA-mimetic action and augmentatory action on GABA response. Brain Res. 1989;488:357–360. doi: 10.1016/0006-8993(89)90730-0. [DOI] [PubMed] [Google Scholar]
- Yang JS, Olsen RW. Gamma-aminobutyric acid receptor-regulated 36 Cl− flux in mouse cortical slices. J Pharmacol Exp Ther. 1987;241:677–685. [PubMed] [Google Scholar]
- Zukin SR, Young AB, Snyder SH. Gamma-aminobutyric acid binding to receptor sites in the rat central nervous system. Proc Natl Acad Sci U S A. 1974;71:4802–4807. doi: 10.1073/pnas.71.12.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]


