Abstract
The intrinsic properties of thalamic neurons are influenced by synaptic activities in ascending pathways and corticofugal projections, as well as by the actions of neurotransmitters released by generalised modulatory systems. We focused on the effects of corticothalamic projections on the hyperpolarisation-activated cation current Ih. Intracellular recordings of thalamocortical neurons in the dorsal lateral geniculate (dLG) nucleus were performed in cats under ketamine-xylazine anaesthesia. At variance with the conventional way of recording intracellularly from thalamic neurons after partial or total ablation of the grey and white matter overlying the dLG, we preserved intact corticothalamic neuronal loops. Stimulating electrodes inserted into the optic tract and light-emitting-diodes as photic stimulation were used to identify the dLG neurons. The expression of the depolarising sag due to Ih depended on the state of cortical networks. Thalamic dLG Ih, induced by hyperpolarising current steps, was detected during the periods of cortical disfacilitation that occur during the cortical slow (< 1 Hz) oscillation, whereas Ih was absent during the active (depolarised) periods. The possibility that the excitatory corticothalamic projections could preclude the generation of the Ih was tested by applying a concentrated K+ solution (3 M) to the primary visual cortex. The same dLG neurons that did not display Ih before application of K+ were able to produce hyperpolarisation-activated depolarising sags during K+-induced cortical depression. Our data suggest that the thalamic clock-like delta oscillation, which results from an interplay between Ih and the low-threshold calcium current (IT), as described in preparations without cerebral cortex, is prevented in dLG neurons when corticothalamic loops are intact.
The rich innervation of thalamic dorsal lateral geniculate (dLG) neurons, which are targets of incessant inputs from retina, visual cortex and brainstem core modulatory systems, may affect their intrinsic properties. The hyperpolarisation-activated cation current (Ih) was described in slices from dLG nucleus, and its role in the generation of rhythmic low-threshold Ca2+ spikes within the frequency range of 1-4 Hz was assessed both in vitro and by computer simulations (McCormick & Pape, 1990; Leresche et al. 1991; Soltesz et al. 1991; Toth & Crunelli, 1992; McCormick & Huguenard, 1992). In vivo intracellular recordings, with partial or total ablation of neocortex to expose the thalamus, showed the presence of Ih (Steriade et al. 1991; Curró Dossi et al. 1992; Nuñez et al. 1992). Despite thalamic synchronisation by synchronous corticothalamic volleys (Steriade et al. 1991), the clock-like delta rhythm rarely occurs at the cortical level in intact-brain preparations, thus raising the possibility that this oscillation is not operational when cortex is preserved. This does not preclude the expression of a cortical component of delta sleep activity that survives thalamectomy (Villablanca, 1974; Steriade et al. 1993b).
It is therefore reasonable to investigate whether the dynamics of the currents described in dLG neurons of cortically deafferented preparations are different from those in intact-cortex animals. This is why we adopted a technique for reaching the dLG nucleus without removing the overlying cortex and without damaging the corticothalamic and thalamocortical projections crossing through the white matter. We challenged the intrasomatic compartment of neurons with hyperpolarising pulses, which have been shown to evoke Ih currents in previous deafferented preparations. Our results show that the expression of the Ih is limited to the epochs during which cortical networks are silent. These results call for a re-evaluation of the functional role of Ih during normal sleep oscillations.
METHODS
Forty-five adult cats of both sexes were deeply anaesthetised with ketamine and xylazine (10-15 mg kg−1 and 2-3 mg kg−1, respectively, I.M.). EEG and heart rate were monitored continuously during the experiments to ascertain that deep level of anaesthesia was maintained. Additional doses of anaesthetic were given at the first sign of a change in EEG pattern or an accelerated pulse rate (>110 beats min−1). All pressure points to be incised were infiltrated with lidocaine (lignocaine). The surgical procedure started with intubation, injection with gallamine triethiodide to induce neuromuscular blockade, and artificial ventilation (20-30 cycles min−1). The end-tidal CO2 concentration was maintained at ≈3.7 ± 0.4 % by adjusting the O2 concentration in the airflow of the ventilation. Craniotomy holes exposed the cerebral cortex and allowed the stereotaxical insertion of the stimulating electrodes and recording micropipettes. Cisternal drainage, hip suspension, pneumothorax, and filling of the hole in the calvarium with a 4 % solution of agar were used to enhance the stability of the intracellular recordings. Body temperature was maintained at 37-39 °C with a heating pad. Glucose (5 % solution, 10 ml I.V.) was given every 3- 4 h during experiments.
Intracellular recordings from dLG nucleus were obtained with glass micropipettes (tip diameter < 0.5 μm) filled with potassium acetate (3 M, impedance 35-50 MΩ). These microelectrodes crossed the ectosylvian gyrus and were lowered into the dLG under stereotaxical control. Low impedance (< 3 MΩ) glass micropipettes inserted into the cortical depth (≈1 mm) were used to record the electrocorticogram (ECoG). The signals recorded with glass pipettes were passed through a high-impedance amplifier with active bridge circuitry (Neurodata, bandpass DC to 9 kHz). Macroelectrodes were used for the stimulation of the ipsilateral optic tract (stimuli lasting for 0.1-0.2 ms, intensity of 0.05-0.8 mA). Steady light stimulation was performed with light emitting diodes (LEDs, luminous intensity up to 20 mcd) placed at 50 mm in front of cat's eyes. The ambient light was switched off during LED stimulation. All signals were digitally converted (20 kHz sampling rate) and recorded for off-line analysis.
At the end of the experiments the animals received a lethal dose of intravenous pentobarbital sodium. All experimental procedures were performed according to the NIH guiding principles and were also approved by the committee for animal care of Laval University.
RESULTS
Database and cellular identification
From a total of over 200 impaled cells in the 45 cats, we retained for the present study only the intraneuronal recordings made in the dLG nucleus (72 cells). Criteria for good quality intracellular recordings were: resting membrane potential (Vm) more negative than −60 mV, overshooting action potentials and stable recordings for at least 20-30 min. The average (± S.D.) cell input resistance, tested with hyperpolarising pulses of 1 nA, was 20.2 ± 0.3 MΩ.
Besides the stereotaxical procedures, the identification criteria of recording from dLG neurons were based on the following response features: (a) monosynaptic EPSPs, occasionally leading to action potentials, evoked by electric stimulation of the optic tract (Fig. 1A); (b) modulation of intracellular dLG activities by the ambient light (Fig. 1B); and (c) the ability of the recorded dLG neurons to trigger low-threshold Ca2+ spikes at adequate levels of hyperpolarisation (Fig. 1C). The average latency of the optic tract-induced responses was 1.8 ± 0.23 ms (range 1.52-2.21 ms; n = 72).
Figure 1. Neuronal identification.
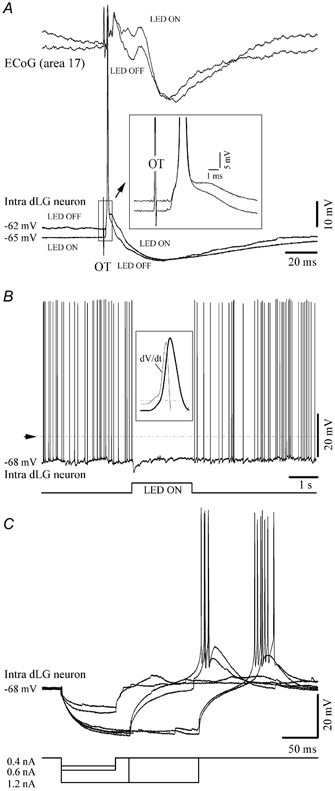
A, cortical depth-EEG (ECoG) and intracellular recording of a dLG neuron. Optic tract (OT) stimulus elicited a neuronal depolarisation with 1.2 ms latency that triggered an action potential followed by an IPSP. The resting Vm and the synaptic response were modulated by the background illumination (LED ON or LED OFF). B, light modulation of the membrane potential and discharge pattern of a dLG neuron. The dotted line indicates the firing threshold for action potentials (around −57 mV). One such action potential is enlarged in the inset in order to show that it is triggered by a subthreshold fast pre-potential event (the break is detected with the dV/dt of the intracellular trace). The LED ON period is devoid of such synaptic events, and thus of action potentials. In this and the following figures the resting membrane potential is indicated. C, intrasomatic hyperpolarising current pulses in a dLG neuron elicited a low-threshold Ca2+ spike at the end of the pulse. All polarities are with positivity upward.
Absence of Ih during hyperpolarising pulses
Since in previous in vitro and in vivo studies (see Introduction) the Ih was elicited with hyperpolarising pulses, we applied similar hyperpolarising pulses of 0.4-1.2 nA. This intensity was chosen so as to produce, depending on the input resistance of recorded neurons (see section above), hyperpolarisations of about 10-15 mV, close to values achieved during natural slow-wave sleep (Hirsch et al. 1983). We additionally applied hyperpolarising pulses of higher intensity (up to 1.75 nA) in order to reach very hyperpolarised Vm values, around −100 mV (Fig. 2 and Fig. 3; n = 5), similar to those attained in in vitro preparations (e.g. in Luthi & McCormick, 1998). The hyperpolarising pulses were applied at various Vm values. With the exception of the episodes of cortical disfacilitation (either due to the slow cortical oscillation or to the functional depression; see below) no hyperpolarisation-activated depolarising sag was elicited in all recorded dLG neurons (n = 72) from our intact brain preparation (Fig. 1C and Fig. 2). At least 20 pulses were applied in each of the recorded cells.
Figure 2. Absence of Ih during long lasting hyperpolarising pulses of different intensities.
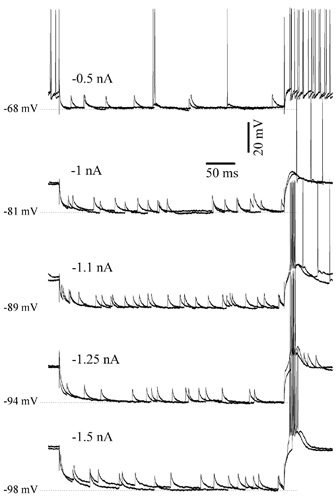
Intracellular hyperpolarising pulses of various intensities in a dLG neuron failed to evoke a hyperpolarisation-activated depolarising sag. All pulses were delivered during active phases of the slow oscillation.
Figure 3. Relationship between cortical slow oscillation and the expression of the Ih in dLG neurons.
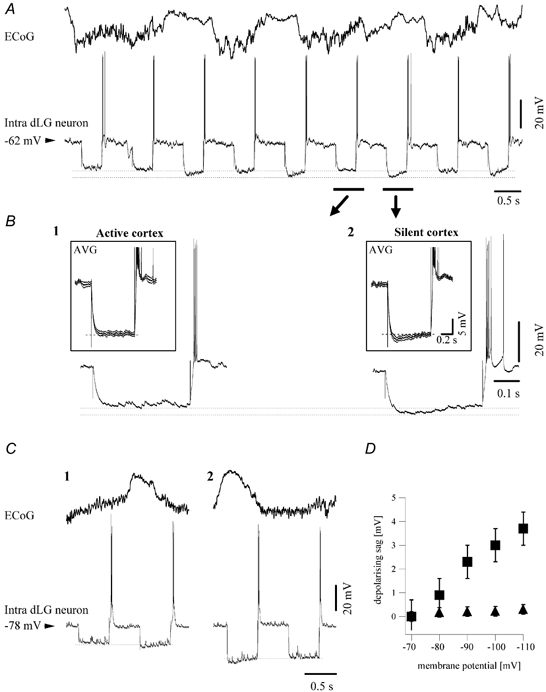
A, ECoG and intracellular recording of a dLG neuron. The Ih was elicited in the dLG neuron by hyperpolarising current pulses only when the cortex was in a period of disfacilitation (positive waves). B, individual sweeps of hyperpolarising current pulses expanded from the trace above. They correspond to a pulse delivered during active EEG (1) and another pulse delivered during disfacilitation (2). For each situation, the average pulse responses (n = 20) are depicted in the insets. These pulse responses are surrounded by the inferior and superior envelopes (upper and lower traces) of the standard deviation of the averages. Each of the chosen sweeps occurred entirely during either active, negative ECoG (1) or silent, positive ECoG (2) epochs of the slow oscillation. The expression of Ih was associated with an increased input resistance. C, pulses of different intensities (0.75 nA in 1, and 1.5 nA in 2) applied to the same cell, inducing more negative membrane potentials (−92 mV in C1 and −103 mV in C2) showed a similar dependency of Ih expression on the network activity. D, the amplitude of the depolarising sag as a function of the membrane potential reached during the hyperpolarising pulse. ▪, amplitude of the depolarising sag during silent phases of the cortical slow oscillation, ▴, amplitude of the depolarising sag during active state of the cortical network. Data are results from the average of 30 pulses delivered at each current intensity in the cell depicted in panel C.
Cortical modulation of the Ih
The major difference between the present preparation and those in previous studies (McCormick & Pape, 1990; Soltesz et al. 1991; Nuñez et al. 1992) was that the corticothalamic projections were intact, and we therefore hypothesised that cortical synaptic impingement on thalamic neurons might preclude the expression of this current. We tested this hypothesis in two ways: (a) by analysing, during the slow (< 1 Hz) oscillation, the impact of periodic hyperpolarisations associated with disfacilitation in cortical networks (Fig. 3); and (b) by reversibly depressing cortical activity (Fig. 4). In both cases, Ih appeared to be concealed by the synaptic activity arising in the cortex.
Figure 4. Intracellular recording in dLG nucleus before, during and after cortical depression.
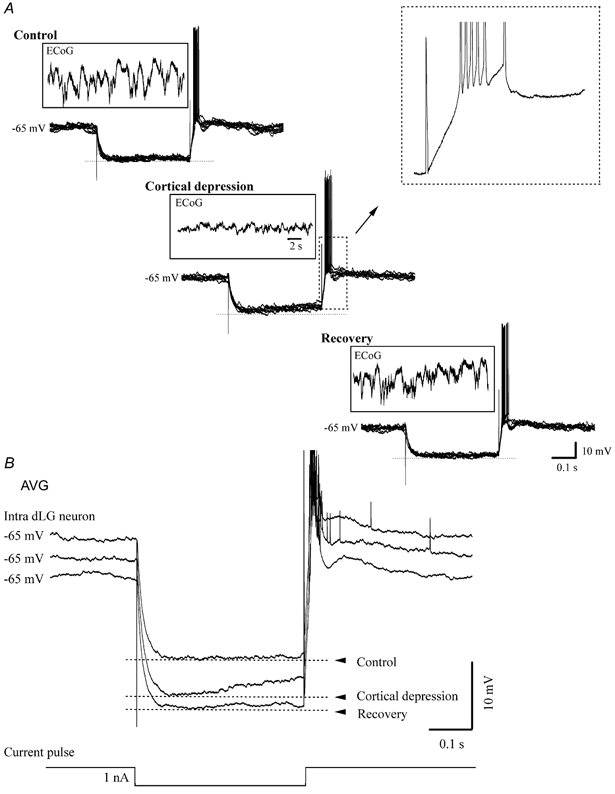
A, superimposition of individual sweeps during hyperpolarising current pulses and ECoG recording before, during and after cortical depression induced by topic application of a concentrated K+ solution on the primary area 17 of the visual cortex. Only those sweeps occurring at comparable Vm values (−65 mV) were retained for analysis. B, averaged sweeps (n = 20) before, during and after cortical depression. Ih was observed only during cortical depression.
We took advantage of the fact that the anaesthesia (ketamine and xylazine) used in this study induces a sleep-like pattern, with a slow (0.6-0.9 Hz) oscillation, which is similar to that recorded during natural sleep in cats and humans (Contreras & Steriade, 1995; Amzica & Steriade, 1997; Achermann & Borbély, 1997; Simon et al. 2000). The slow oscillation comprises two different activity levels in cortical networks: an active (‘up’) state during which cortical neurons are depolarised and fire action potentials, and a silent (‘down’) state in which cortical neurons are hyperpolarised by a disfacilitation process (Contreras et al. 1996; Massimini & Amzica, 2001).
Hyperpolarising pulses injected through the recording pipette were superimposed over the ongoing activity (Fig. 3A). Some of them occurred during the ECoG negative waves (Fig. 3B1) associated with neuronal depolarisation, while others fell during the ECoG positive waves (Fig. 3B2) associated with neuronal hyperpolarisation. The responses of dLG neurons to current steps applied during active states were devoid of depolarising sags, while such sags were expressed when the current pulse was applied during the silent phases of the slow oscillation (n = 65; 90.3 % of cases). The amplitude of the depolarising sag was in all cases higher than the standard deviation of the averaged responses (Fig. 3B, averages in insets). This result suggests that the absence of an active cortical network constitutes a necessary condition for the expression of hyperpolarisation-activated depolarising sags. Moreover, as indicated by the voltage deflection at the onset of the hyperpolarising pulse (dotted lines in Fig. 3B and C), the apparent input resistance of dLG neurons was initially higher during disfacilitated periods than during active ones (15.8 % increase, n = 64 cells).
It is possible that the more negative Vm attained during the disfacilitated epoch, due to the increased input resistance mentioned above, is responsible for the triggering of a depolarising sag. Application of current pulses of higher intensity that displaced the Vm to a more negative value than that displayed during the disfacilitated period produced, however, a similar type of response as a function of the state of the cortical network (Fig. 3C; n = 5). This suggests that the expression of Ih during silent cortical epochs is not exclusively related to the increase in input resistance or Vm (Fig. 3D).
Persistent depression of cortical synaptic activity was produced by spreading depression induced by topical application of a concentrated K+ solution (3 M) on the primary visual area 17 by soaking a small (≈10 mm2) filter paper previously laid on the cortical surface (Fig. 4). Care was taken to avoid any spillage onto the neighbouring dura mater (as a consequence of cisternal drainage, the brain had reached a lower position 2-3 mm below the dura mater), and no heart rate variation was noticed during and after this manoeuvre. The cortical depression was confirmed by a drastic reduction in the amplitude of the field potential in the background activity. Frequently, the spreading depression produced unstable recording conditions in the thalamus and loss of the intracellular recording. However, in some cases (n = 12) we were able to keep the thalamic recording going beyond the duration of the cortical spreading depression. In these cases, the following cellular behaviour was noted: (a) as in all other intra-dLG recordings, intracellular hyperpolarising pulses did not elicit any Ih before cortical depression during periods when the cortical network was active (Fig. 4, control); (b) after the induction of cortical spreading depression, the initial negative voltage deflection induced by current steps increased by ≈15 % and a depolarising sag was present (Fig. 4, cortical depression); and (c) recovery from the spreading depression was associated with restoration of normal amplitudes and patterns of cortical field potentials and input resistance, as well as with the absence of Ih in dLG neurons (Fig. 4, recovery). The ability of thalamic neurons to produce low-threshold Ca2+ spikes at the end of the hyperpolarising pulse was preserved during spreading depression.
DISCUSSION
These experiments show that the expression of the hyperpolarisation-activated depolarising sag in dLG neurons is prevented during the active (‘up’) phases of sleep-like slow oscillations by synaptic activity arising in the cortex and that the expression of Ih is unveiled during cortical disfacilitation periods or K+-induced depression of cortical activity.
The average somatic input resistance of the neurons in our database (20.2 MΩ) was not significantly different from that previously recorded in an in vivo dLG study in decorticated preparations (Nuñez et al. 1992). As the cortical input to the dLG neurons reaches distant dendrites (Robson, 1983; Wilson & Forestner, 1995), it is unlikely that the resistance of the somatic membrane would be affected by the presence or absence of cortical inputs. Besides, with greatly reduced brainstem-thalamic cholinergic inputs during slow-wave sleep (Steriade et al. 1990), the cortical input to dLG neurons constitutes the majority of synapses (Van Horn et al. 2000). Corticothalamic linkages contact thalamocortical neurons through direct glutamatergic synapses and indirectly affect these neurons through local-circuit and/or perigeniculate inhibitory neurons.
In contrast to retinal inputs, which act exclusively on fast ionotropic (AMPA and NMDA) glutamatergic receptors (Crunelli et al. 1987; Hartveit & Heggelund, 1990), glutamatergic corticogeniculate axons also act on metabotropic receptors, producing large depolarisations and increased input resistance of dLG neurons (McCormick & von Krosigk, 1992; Turner & Salt, 2000; Hughes et al. 2002). The removal of the latter effect might withdraw a prolonged depolarising pressure and a source of increased dendritic conductance. On the other hand, the cortical excitation of thalamic interneurons results in perisomatic inhibition of relay neurons (Wilson & Forestner, 1995) associated with shunting of currents travelling from dendrites to soma. Thus, the presence or absence of visual cortex appears to be a modulating factor of transmembrane currents that might interfere with the expression of Ih.
The extent of the cortical ablation would therefore be expected to modify the strength of Ih. Anatomical studies have shown that the corticogeniculate axons pass through the white matter underneath the supra- and ectosylvian gyri (Nelson & LeVay, 1985; Senoh & Naito, 1991), which were removed in our previous in vivo studies (Nuñez et al. 1992). Ih was present in decorticated animals and to a large degree in some slice preparations (see Introduction). In the present preparation with intact corticothalamic projections, hyperpolarising currents comparable to those used in previous studies elicited Ih only during the short time periods of cortical disfacilitation, which corresponded to the rhythmic and synchronous hyperpolarisations of cortical neurons. The cortical neuronal membrane during the slow (< 1 Hz) oscillation alternates between a depolarised and a hyperpolarised level. The former level is made up of excitatory as well as inhibitory synaptic potentials (Steriade et al. 1993a), while the latter betrays network disfacilitation (Contreras et al. 1996) due to a progressive depletion of extracellular Ca2+ ions that reduce the efficacy of synapses (Massimini & Amzica, 2001). Therefore, the hyperpolarising phase of the slow oscillation has similar effects on dLG relay neurons as a functional deafferentation.
The reduced expression of Ih in intact preparations has important consequences for the incidence and generation mechanisms of clock-like delta oscillations in the visual thalamus. Beyond the interference of retinal inputs with clock-like delta oscillations in dLG neurons (see Fig. 7 in Nuñez et al. 1992), the periodic activation of the cortical network during the depolarising phase of the slow oscillation would disrupt any possible sequences of the clock-like delta cycles in dLG neurons. The duration of the hyperpolarising phase of the cortical slow oscillation ranges from 0.3 to 0.7 s and is too short to allow more than 1-2 cycles of the intrinsic clock-like delta activity, at its fastest 4 Hz limit (Amzica & Steriade, 1998). Moreover, the onset of the cortical hyperpolarisation is associated with progressive disfacilitation in the corticothalamic network, and thus with a reduced ability to express coherent activity, which is an essential requirement for these oscillations to be apparent at the EEG level. This would explain why such oscillations were not seen in any of the cells of the present study and are not apparent in the EEG of intact animals.
In spite of this, Ih could still promote delta rhythmicity during conditions associated with prolonged loss of consciousness such as coma or burst suppression, during which the cortical activity is depressed. Burst suppression is accompanied by a steady hyperpolarisation, of about 10 mV, of cortical neurons and long periods of reduced responsiveness to synaptic volleys, while thalamocortical neurons display clock-like delta oscillations (see Fig. 9 and Fig. 10 in Steriade et al. 1994). The utility of Ih under such conditions could consist of its ability to maintain a minimal neuronal activity in the thalamus with negligible energetic impact on target structures.
Acknowledgments
We are grateful to Denis Paré for helpful discussion and to Pierre Giguère and Denis Drolet for technical assistance. The work was supported by grants from the Canadian Institutes of Health Research, Human Frontier Science Program, and National Institute of Health (USA). D.A.N. is a doctoral student and F.A. is a scholar of Fonds de la Recherche en Santé du Québec.
REFERENCES
- Achermann P, Borbély AA. Low-frequency (< 1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience. 1997;81:213–222. doi: 10.1016/s0306-4522(97)00186-3. [DOI] [PubMed] [Google Scholar]
- Amzica F, Steriade M. The K-complex: its slow (< 1 Hz) rhythmicity and relation to delta waves. Neurology. 1997;49:952–959. doi: 10.1212/wnl.49.4.952. [DOI] [PubMed] [Google Scholar]
- Amzica F, Steriade M. Electrophysiological correlates of sleep delta waves. Electroencephalogr Clin Neurophysiol. 1998;107:69–83. doi: 10.1016/s0013-4694(98)00051-0. [DOI] [PubMed] [Google Scholar]
- Contreras D, Steriade M. Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci. 1995;15:604–622. doi: 10.1523/JNEUROSCI.15-01-00604.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D, Timofeev I, Steriade M. Mechanisms of long-lasting hyperpolarizations underlying slow sleep oscillations in cat corticothalamic networks. J Physiol. 1996;494:251–264. doi: 10.1113/jphysiol.1996.sp021488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V, Kelly JS, Leresche N, Pirchio M. On the excitatory post-synaptic potential evoked by stimulation of the optic tract in the rat lateral geniculate nucleus. J Physiol. 1987;384:603–618. doi: 10.1113/jphysiol.1987.sp016472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curró Dossi R, Nuñez A, Steriade M. Electrophysiology of a slow (0.5–4 Hz) intrinsic oscillation of cat thalamocortical neurones in vivo. J Physiol. 1992;447:215–234. doi: 10.1113/jphysiol.1992.sp018999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartveit E, Heggelund P. Neurotransmitter receptors mediating excitatory input to cells in the cat lateral geniculate nucleus. II. Nonlagged cells. J Neurophysiol. 1990;63:1361–1372. doi: 10.1152/jn.1990.63.6.1361. [DOI] [PubMed] [Google Scholar]
- Hirsch JC, Fourment A, Marc ME. Sleep-related variations of membrane potential in the lateral geniculate body relay neurons of the cat. Brain Res. 1983;259:308–312. doi: 10.1016/0006-8993(83)91264-7. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Cope DW, Blethyn KL, Crunelli V. Cellular mechanisms of the slow (< 1 Hz) oscillation in thalamocortical neurons in vitro. Neuron. 2002;33:947–958. doi: 10.1016/s0896-6273(02)00623-2. [DOI] [PubMed] [Google Scholar]
- Leresche N, Lightowler S, Soltesz I, Jassik-Gerschenfeld D, Crunelli V. Low-frequency oscillatory activities intrinsic to rat and cat thalamocortical cells. J Physiol. 1991;441:155–174. doi: 10.1113/jphysiol.1991.sp018744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi A, McCormick DA. H-current: properties of a neuronal and network pacemaker. Neuron. 1998;21:9–12. doi: 10.1016/s0896-6273(00)80509-7. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Huguenard JR. A model of the electrophysiological properties of thalamocortical relay neurons. J Neurophysiol. 1992;68:1384–1400. doi: 10.1152/jn.1992.68.4.1384. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Von Krosigk M. Corticothalamic activation modulates thalamic firing through glutamate ‘metabotropic’ receptors. Proc Natl Acad Sci U S A. 1992;89:2774–2778. doi: 10.1073/pnas.89.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M, Amzica F. Extracellular calcium fluctuations and intracellular potentials in the cortex during the slow sleep oscillation. J Neurophysiol. 2001;85:1346–1350. doi: 10.1152/jn.2001.85.3.1346. [DOI] [PubMed] [Google Scholar]
- Nelson SB, Levay S. Topographic organization of the optic radiation of the cat. J Comp Neurol. 1985;240:322–330. doi: 10.1002/cne.902400308. [DOI] [PubMed] [Google Scholar]
- Nuñez A, Amzica F, Steriade M. Intrinsic and synaptically generated delta (1–4 Hz) rhythms in dorsal lateral geniculate neurons and their modulation by light-induced fast (30–70 Hz) events. Neuroscience. 1992;51:269–284. doi: 10.1016/0306-4522(92)90314-r. [DOI] [PubMed] [Google Scholar]
- Robson JA. The morphology of corticofugal axons to the dorsal lateral geniculate nucleus in the cat. J Comp Neurol. 1983;216:89–103. doi: 10.1002/cne.902160108. [DOI] [PubMed] [Google Scholar]
- Senoh K, Naito J. A WGA-HRP study of the fiber arrangement in the cat optic radiation: a demonstration via three-dimensional reconstruction. Exp Brain Res. 1991;87:473–483. doi: 10.1007/BF00227073. [DOI] [PubMed] [Google Scholar]
- Simon NR, Manshanden I, Lopes Da, Silva FH. A MEG study of sleep. Brain Res. 2000;860:64–76. doi: 10.1016/s0006-8993(00)01974-0. [DOI] [PubMed] [Google Scholar]
- Soltesz I, Lightowler S, Leresche N, Jassik-Gerschenfeld D, Pollard CE, Crunelli V. Two inward currents and the transformation of low-frequency oscillations of rat and cat thalamocortical cells. J Physiol. 1991;441:175–197. doi: 10.1113/jphysiol.1991.sp018745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Amzica F, Contreras D. Cortical and thalamic cellular correlates of electroencephalographic burst-suppression. Electroencephalogr Clin Neurophysiol. 1994;90:1–16. doi: 10.1016/0013-4694(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Steriade M, Curró Dossi R, Nuñez A. Network modulation of a slow intrinsic oscillation of cat thalamocortical neurons implicated in sleep delta waves: cortically induced synchronization and brainstem cholinergic suppression. J Neurosci. 1991;11:3200–3217. doi: 10.1523/JNEUROSCI.11-10-03200.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Datta S, Paré D, Oakson G, Curró Dossi R. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990;10:2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nuñez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993a;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nuñez A, Amzica F. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993b;13:3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth T, Crunelli V. Computer simulation of the pacemaker oscillations of thalamocortical cells. Neuroreport. 1992;3:65–68. doi: 10.1097/00001756-199201000-00017. [DOI] [PubMed] [Google Scholar]
- Turner JP, Salt TE. Synaptic activation of the group I metabotropic glutamate receptor mGlu1 on the thalamocortical neurons of the rat dorsal lateral geniculate nucleus in vitro. Neuroscience. 2000;100:493–505. doi: 10.1016/s0306-4522(00)00280-3. [DOI] [PubMed] [Google Scholar]
- Van Horn SC, Erisir A, Sherman SM. Relative distribution of synapses in the A-laminae of the lateral geniculate nucleus of the cat. J Comp Neurol. 2000;416:509–520. [PubMed] [Google Scholar]
- Villablanca J. Role of the thalamus in sleep control: Sleep-wakefulness studies in chronic diencephalic and athalamic cats. In: Petre-Quadens O, Schlag J, editors. Basic Sleep Mechanisms. New York: Academic; 1974. pp. 51–81. [Google Scholar]
- Wilson JR, Forestner DM. Synaptic inputs to single neurons in the lateral geniculate nuclei of normal and monocularly deprived squirrel monkeys. J Comp Neurol. 1995;362:468–488. doi: 10.1002/cne.903620404. [DOI] [PubMed] [Google Scholar]


