Abstract
Arterial interstitial cells of Cajal (ICC)-like cells (AIL cells) with a multipolar, irregular, elongated shape and with numerous thin (often less than 1 μm), sometimes branching, processes with lengths up to ≈60 μm were isolated enzymatically from 1st to 7th order branches of guinea-pig mesenteric artery. Some of the processes of AIL cells were growing (average speed ≈0.15 μm min−1) and their growth was blocked by 10 μm latrunculin B, an inhibitor of actin polymerisation. Staining with BODIPY phalloidin, a fluorescent dye selective for F-actin, showed the presence of F-actin in the processes of AIL cells. Voltage clamp of single AIL cells revealed an inward current that was four times more dense than in myocytes and was abolished by 10 μm nicardipine, and an outward current carried exclusively by potassium ions that was reduced by 1 mm 4-aminopyridine and/or 100 nm iberiotoxin but unaffected by 10 nm dendrotoxin-K. Imaging of intracellular ionised calcium with fluo-4 using a laser scanning confocal microscope showed local or global calcium transients lasting several seconds in ≈28 % of AIL cells. When membrane current was recorded simultaneously, the calcium transients were found to correspond to long-lasting transient outward currents, which occurred at potentials positive to −40 mV. Unlike myocytes, AIL cells did not contract in response to 1 mm caffeine or 5 μm noradrenaline, although they responded with a [Ca2+]i increase. The segments of intact arteries did not stain for c-kit, a marker of ICCs. Single AIL cells stained positive for vimentin, desmin and smooth muscle myosin. The presence of ICC-like cells is demonstrated for the first time in the media of resistance arteries.
A number of cell types cooperate in the walls of resistance vessels to provide an appropriate flow of blood through a tissue. The functions of endothelial and smooth muscle cells have been investigated in numerous studies; the roles of nerves and nerve endings are similarly well recognised. Longer-term changes in the regulation of blood flow may require remodelling of the blood vessel wall and division of smooth muscle cells or their progenitors. Healthy blood vessels contain a few inflammatory cells (macrophages, monocytes and lymphocytes), perhaps fibroblasts, and possibly contractile pericytes (Diaz-Flores et al. 1991; Hirschi & D'Amore, 1996). In addition to the above-mentioned cell types, we have recently found multipolar cells with numerous, long, fine processes in enzymatic dispersions of small mesenteric arteries (Pucovsky & Bolton, 2002) which do not fit into any of the above cell categories. Similar cells were described by Meyling 50 years ago (Meyling, 1953). Such cells located in the adventitia of cerebral blood vessels have been noticed previously in sections of whole vessels studied by transmission electron microscopy (Dahl & Nelson, 1964), but this observation has received little attention and studies on single cells have not been performed.
Multipolar cells with processes, first described in the intestine by Ramón y Cajal (1911) and others, were later named the interstitial cells of Cajal (ICCs; Thuneberg, 1982). First thought to be primitive neurones, ICCs show a morphology distinct from the surrounding smooth muscle cells (myocytes), their main feature being the presence of numerous thin cytoplasmic processes. Similar cells were also found throughout the gastrointestinal tract (e.g. colon: Faussone-Pellegrini & Cortesini, 1984; oesophagus: Faussone-Pellegrini & Cortesini, 1985; stomach: Faussone-Pellegrini et al. 1989) and in the urinary tract (urethra: Sergeant et al. 2000; urinary bladder: McCloskey & Gurney, 2002). All of these organs perform complex patterns of muscular activity and ICCs or ICC-like cells in their walls have been suggested to play an important role in pacemaking and/or in the mediation of neural impulses to smooth muscle (Barajas-Lopez et al. 1989; Burns et al. 1996; Sergeant et al. 2000; Ward et al. 2000a). Similar cells in resistance vessels may have some, as yet unsuspected, roles.
This paper provides initial data on the structure and function of cells from the resistance arteries of guinea-pig with similar morphology to ICCs, and which will be termed ‘arterial ICC-like’ (AIL) cells. It was found that AIL cells are located in the vessel wall and are functionally different from myocytes, but also from the ICCs found in the gastrointestinal and urinary tract.
METHODS
Cell preparation
Experiments were done on single cells isolated from 1st to 7th order branches (see Table 1 for diameters) of guinea-pig mesenteric artery and on segments of these arteries. The procedure is similar to the one already described (Pucovsky et al. 2002). Briefly, male Dunkin-Hartley guinea-pigs weighing 250-450 g were humanely killed by cervical dislocation followed by exsanguination, which was in accordance with the UK Animals (Scientific Procedures) Act 1986 (Schedule 1). The mesenterium was then taken out and mesenteric arteries were cleaned of surrounding tissue, cut out and incubated at 37 °C for 30 min in a Ca2+- and Mg2+-free physiological saline solution (PSS; for composition see below) containing collagenase, soybean trypsin inhibitor and bovine serum albumin (BSA; all at 1 mg ml−1) and protease (0.5 mg ml−1). The pieces of tissue were then taken out, rinsed twice with Ca2+- and Mg2+-free PSS and gently triturated with a wide-bore glass pipette to yield single cells. Aliquots of the suspension of single cells were then placed in experimental chambers and cells were left at 4 °C for 45-60 min to attach to glass coverslips forming the bottom of the experimental chamber. The cells were stored at 4 °C in PSS (mM: NaCl 120, KCl 6, glucose 12, Hepes 10, MgCl2 1.2, CaCl2 2.5, pH set to 7.4 with NaOH) and used within 12 h of isolation. Test substances were applied by changing the bathing solution. All experiments were done at room temperature (20-23 °C).
Table 1.
External diameters of mesenteric articles
| Order | Diameter (μm) | n (vessels) | n (animals) |
|---|---|---|---|
| 1 | 505 ± 14 | 15 | 8 |
| 2 | 409 ± 19 | 15 | 8 |
| 3 | 327 ± 18 | 16 | 8 |
| 4 | 212 ± 15 | 20 | 8 |
| 5 | 178 ± 10 | 25 | 8 |
| 6 | 146 ± 9 | 30 | 8 |
| 7 | 128 ± 10 | 22 | 8 |
Diameters represent means ± s.e.m.
Confocal microscopy
The cells were imaged using a Zeiss LSM 510 laser scanning confocal microscope (Carl Zeiss, Jena, Germany). Experimental chambers containing cells were placed on the stage of an Axiovert 100M inverted microscope. The excitation beam was produced by an argon (488 nm) or helium/neon laser (543 and 633 nm), and delivered to the specimen via a Zeiss Apochromat × 63 oil immersion objective (numerical aperture 1.4). The pinhole size was adjusted to 0.98-1.04 Airy units, setting the optical section thickness to less than 0.7-0.9 μm. Lateral resolution was established by scanning 0.2 μm fluorescent beads and was found to be 0.4 μm. Emitted fluorescence was captured using LSM 510 software (release 2.5, Carl Zeiss, Jena) running on a Windows NT work station. During a time series protocol, x-y images (typically 90-200 images per series; image depth of 8 bits), were taken at specified intervals. In some cases, to improve temporal resolution, a single line of 256 pixels, usually positioned over a subplasmalemmal region of the cell parallel to the cell membrane or along the longitudinal axis of the cell, was repeatedly scanned every 50 ms. Successive images of the scanned line were aligned parallel to each other from left to right to form a line-scan image in which the horizontal dimension reflects time and the vertical dimension shows position along the scan line. When the cells were scanned in three dimensions, z-slices were 0.1-0.2 μm apart.
Electron microscopy
Cells adherent to plastic coverslips or tissues were fixed in 4 % glutaraldehyde in sodium cacodylate buffer (pH 7.2) for 2 h or overnight. They were then washed in cacodylate buffer (2 × 15 min) and post-fixed with 1 % osmium tetroxide in cacodylate buffer (2 h). After another wash in cacodylate buffer (2 × 15 min) they were dehydrated through ascending grades of ethanol (35, 70, 90 and 2 × 100 %, 30 min each).
For transmission electron microscopy, the cells or tissues were transferred through propylene oxide (30 min) into a 1:1 mixture of propylene oxide and Spur's resin and left overnight. They were then transferred through two changes of Spur's resin (2 × 2 h), placed in embedding moulds and cured in an oven at 65 °C. Tissue sections were cut using a Reichert OMU4 ultramicrotome with a diamond knife. Sections were picked up on 200 mesh copper grids and stained with 2 % uranyl acetate in 30 % ethanol followed by staining with Sato's lead stain. Grids with sections on them were viewed with a Zeiss EM 900 transmission electron microscope.
For scanning electron microscopy, the cells on coverslips were transferred from the 100 % ethanol into a Poloron critical point drying apparatus, substituted with liquid CO2 and passed through the (critical point) drying cycle. The coverslips were removed and fixed to aluminium stubs, sputter-coated with gold and viewed with a Zeiss EM 940 scanning electron miscroscope.
Electrophysiology
Membrane current in single cells was recorded by whole-cell or amphotericin-perforated patch (Rae et al. 1991) configuration of the voltage clamp technique, using an Axopatch 200A (Axon Instruments Inc., Union City, CA, USA) voltage clamp amplifier. Borosilicate glass pipettes had resistances of 2.5-6 MΩ. They were filled with K+-based pipette solution (mM: KCl 120, NaCl 4, MgCl2 5, Na2ATP 1, creatine 5, glucose 10, Hepes 10, EGTA 0.05, pH adjusted to 7.4 with KOH). In the case of the perforated-patch technique (during simultaneous calcium imaging and voltage clamping) 240 μg ml−1 of amphotericin B was included in the pipette solution. In experiments where the nature of outward current was studied, either low [Cl−] (mM: NaCl 20, sodium glutamate 100, KCl 6, glucose 12, Hepes 10, MgCl2 1.2, CaCl2 2.5, pH set to 7.4 with NaOH) or high [K+] external solution (mM: NaCl 6, KCl 120, glucose 12, Hepes 10, MgCl2 1.2, CaCl2 2.5, pH set to 7.4 with KOH) were used. The junction potentials of these solutions were found to be < 1 mV when using a KCl bridge to reference electrode. In experiments where Ca2+-free external solution was required, CaCl2 was omitted and 0.1 mM EGTA was added to the PSS. In experiments where calcium current was studied, the pipette solution contained 124 mM CsCl instead of KCl and NaCl and 10 mM EGTA to bind the intracellular calcium, and the external solution contained 120 mM CsCl instead of NaCl and KCl and 5 mM BaCl2 instead of CaCl2. Voltage clamp pulses were generated and data were captured on-line using a Digidata 1200 interface run under the pCLAMP program (Axon Instruments Inc.). Signals were sampled at 0.2-5 kHz, filtered and then analysed, corrected for leakage, and plotted using MicroCal Origin software (MicroCal Software Inc., Northampton, MA, USA).
Immunohistochemistry
The single cells or tissue segments were fixed by either 4 % paraformaldehyde solution in PSS (for staining of vimentin, smooth muscle myosin, F-actin, or desmin) or 100 % acetone (for staining of c-kit) at 4 °C for 10 min (single cells) or 30 min (artery segments and pieces of small intestine), and washed 4 × 10 min with PSS. They were then incubated with PSS containing 1 % BSA and 0.3 % Triton X-100 for 1 h at room temperature, which was followed by incubation with primary antibodies in PSS containing 1 % BSA and 0.3 % Triton X-100 either overnight at 4 °C or for 1-2 h at room temperature. After this step, the cells or tissues were washed 4 × 10 min with PSS containing 1 % BSA and 0.3 % Triton X-100 and incubated with secondary antibodies (conjugated with fluorescent probes) in PSS with BSA and Triton X-100 for 1-2 h at room temperature. After removing the unbound secondary antibodies by washing the preparations 4 × 10 min with PSS containing BSA and Triton X-100, the preparations were imaged using the laser scanning confocal microscope. Antibodies used were: vimentin: mouse monoclonal antibodies (clone V9; dilution 1 : 250) and Alexa Fluor 633-conjugated goat anti-mouse antibodies (1 : 500); smooth muscle myosin: mouse monoclonal antibodies (clone hSM-V; 1 : 500) and Alexa Fluor 633-conjugated goat anti-mouse antibodies (1 : 400); c-kit: rat monoclonal antibodies (clone ACK45; 1 : 200) and Alexa Fluor 488 chicken anti-rat antibodies (1 : 500); desmin: mouse monoclonal antibodies (clone DE-U-10; 1:200) and Alexa Fluor 633-conjugated goat anti-mouse antibodies (1 : 500). F-actin was stained with BODIPY 558/568 phalloidin (5 u ml−1). PSS contained penicillin (20 u ml−1) and streptomycin (20 μg ml−1) at all times during immunohistochemical experiments.
Chemicals
Collagenase (type 1A), trypsin inhibitor (type II-S), bovine serum albumin, protease (type X), amphotericin B, (-)-noradrenaline bitartarate salt, caffeine, nicardipine, iberiotoxin, 4-aminopyridine, dimethyl sulphoxide (DMSO), mouse monoclonal antibodies against smooth muscle myosin, vimentin and desmin were purchased from Sigma-Aldrich. Dendrotoxin-K and latrunculin B were purchased from Calbiochem. The rat monoclonal antibodies against c-kit were from Research Diagnostics. Fluo-4 AM, Pluronic F-127, BODIPY 558/568 phalloidin and all the secondary antibodies conjugated with fluorescent dyes were bought from Molecular Probes.
Fluo-4 AM, the membrane-permeant ester of the Ca2+-sensitive fluorescent dye (absorption maximum: 494 nm, emission maximum: 516 nm), was solubilised immediately before use in DMSO containing 0.025 % (w/v) Pluronic F-127 by ultrasonication for 5 min to yield a stock solution of 4 mM and this solution was then added into PSS so that the final concentration of fluo-4 AM was 5 μM. The final concentration of DMSO was 0.125 % (v/v). After the cells had settled on the coverslips, they were incubated in PSS containing fluo-4 AM and left to load the dye for 30 min at room temperature. They were then washed with PSS and left to allow de-esterification of the dye for another 30 min.
Stock solutions of amphotericin B, latrunculin B and nicardipine were also prepared in DMSO. All the other substances were dissolved in deionised water.
Analysis of data
Raw confocal imaging data were processed and analysed using Zeiss LSM510 software or custom-written routines in IDL 5.4 programming language (Research Systems, Inc., Boulder, CO, USA). The patch clamp data were analysed using the pCLAMP 6 software suite (Axon Instruments Inc.). Statistical evaluation was done using MicroCal Origin software (MicroCal Software, Inc.) and final images were produced using CorelDraw 10 software (Corel Corporation, Ottawa, Ontario, Canada).
To reduce the scatter of points and partly eliminate the effect of photomultiplier noise, the raw image data were first smoothed using 3-pixel-wide boxcar averaging and fluorescence values less than 5 intensity units (I.U.) were cut off. The intensity of the fluorescence in the images was then normalised (F/F0) using the self-ratio approach, as described elsewhere (Cheng et al. 1993).
Statistics
Data are expressed as mean values ± standard error of the mean (s.e.m.) for the number of cells (n) analysed. Statistical significance was calculated using Student's t test for unpaired observations unless otherwise indicated and the differences where P < 0.05 were considered significant.
RESULTS
Morphology
Enzymatic dispersion of segments of mesenteric artery branches yielded a heterogeneous suspension of cells (Fig. 1A). The majority of cells in the suspension were myocytes, spindle-shaped, ≈40-50 μm long and ≈4-7 μm wide cells with a rugged membrane surface and no processes (Fig. 1D). However, 6.4 ± 1.3 % of cells in the suspension (n = 8 digestions, each one from a different animal, total of 6940 cells counted) had a multipolar elongated irregular shape, a smooth cellular membrane and numerous processes up to ≈60 μm long and often less than 1 μm in diameter (Fig. 1B and C).
Figure 1. Morphology of arterial ICC-like cells (AIL cells) and myocytes.
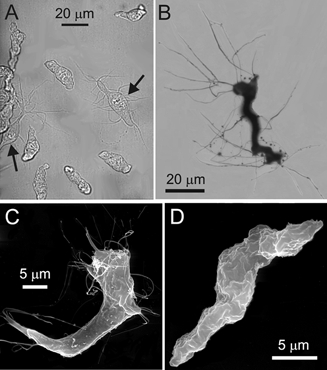
A, transmitted light image of single freshly isolated myocytes and AIL cells (arrows). B, light microscopic image of an AIL cell stained with 1 % osmium tetroxide. C, scanning electron micrograph of an AIL cell. Note the fine processes. D, scanning electron micrograph of a myocyte.
The transmission electron micrographs of these arterial ICC-like (AIL) cells revealed a multilobar nucleus (not all lobes shown) surrounded by well-developed smooth endoplasmic reticulum, and Golgi apparatus and mitochondria in the central part of cytoplasm (Fig. 2A). More to the periphery, filaments and dense bodies were found (Fig. 2A and B), and caveolae were located just under the plasmalemma (Fig. 2A, inset). The distinctive feature of the AIL cells, thin processes, contained the cytoplasmatic matrix sandwiched between two parallel membranes (Fig. 2A and C), and sometimes filaments could be seen running along the longitudinal axis of the process (Fig. 2C).
Figure 2. Ultrastructure of AIL cells and myocytes.
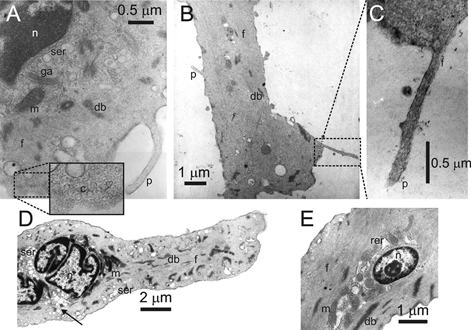
Transmission electron micrographs of AIL cells (A, B and C) and of myocytes (D and E). n, nucleus; ser, smooth endoplasmic reticulum; rer, rough endoplasmic reticulum; ga, golgi apparatus; m, mitochondrion; db, dense body; f, filaments; c, caveolae (inset in A); p, thin process.
The myocytes also had a nucleus consisting of several lobes (Fig. 2D) encircled with perinuclear smooth and rough endoplasmic reticulum with vesicles (Fig. 2D and E) and mitochondria located mainly in the apical regions of the perinuclear space (Fig. 2D), intertwined with the sarcoplasmic reticulum (Fig. 2E). Encapsulating the region around the nucleus were the filaments running along the longitudinal axis of the myocyte, and dense bodies scattered among them (Fig. 2D and E). In the subplasmalemmal space, the vesicles of sarcoplasmic reticulum could be found around the circumference of the cell (Fig. 2D). Sometimes the continuity between the subplasmalemmal and perinuclear sarcoplasmic reticulum could be seen (Fig. 2D, arrow).
Enzymatic digestion of tissue surrounding the mesenteric artery yielded only droplets of fat and virtually no cells, which suggests that AIL cells are closely associated with or contained within the arteries and do not originate from the surrounding connective or fatty tissue.
AIL cells were detected in the suspension of single cells 80 min after cutting out the mesenterium or 30 min after the integrity of arteries was damaged by enzymatic digestion lasting 10 min. These AIL cells had 7 ± 1.5 processes (n = 10) with an average length of 10.4 ± 0.6 μm (n = 70), the longest one measuring 30.0 μm.
Growth of processes and active change of shape
Some processes of AIL cells were found to be growing in length (Fig. 3A, also available as a video clip ‘Growth’ in supplementary material online). Out of 23.3 ± 1.8 processes per cell (n = 32), 13.8 ± 1.0 or 63.9 ± 3.8 % were growing, at an average speed of 0.149 ± 0.005 μm min−1 (n = 443 processes, 32 cells, 8 guinea-pigs), as calculated from a series of 90 transmitted-light images taken every 20 s. The speed of growth of processes was variable, with the maximum speed observed being 0.776 μm min−1 and the minimum speed being 0.012 μm min−1 (Fig. 3D). The speed of growth of individual processes was non-uniform as well –in some processes it declined with time (Fig. 3C, leftmost pair of bars), and in the others it came to a full stop. The direction of growth seemed random, as some processes grew past other cells or between pairs of cells without touching them. Also, the bodies of 9 out of 32 AIL cells actively changed their shape (Fig. 3A, rightmost image, open arrows; Fig. 4A, also available as a video clip ‘Shape change’ in supplementary material online).
Figure 3. Growth of processes in AIL cells is latrunculin B-sensitive.
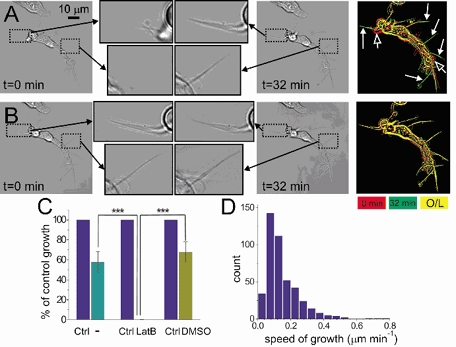
Transmitted light images of an AIL cell at the beginning (t = 0 min) and at the end (t = 32 min) of either a control series of images (A), or a series taken in the presence of 10 μm latrunculin B, an actin polymerisation inhibitor (B). Two frames from the same position of each of four images are shown enlarged (indicated with dotted line frames and black arrows). The frames in A show growth of processes in the control series, while the frames in B show that the same processes stopped growing in the presence of latrunculin B. The rightmost images in A and B show the overlap (O/L) of cell contours (yellow) from the beginning (red) and the end of image series (green). Note green-coloured processes (filled white arrows) indicative of growth in A, but not in B. Also, open arrows in A show changes in shape of the AIL cell, which were also blocked by latrunculin B (B). C, summary data on growth of processes in AIL cells observed over two consecutive periods of 32 min. Growth during the first period was expressed as 100 % (Ctrl). The right-hand bars in the three pairs of bars indicate nothing added (−), 10 μm latrunculin B (LatB), and 0.1 % DMSO (vehicle for latrunculin B) added in the second 32 min-long period. *** P < 0.0001. D, frequency histogram of average speeds of growth of processes (μm min−1).
Figure 4. Change of body shape of AIL cells and staining of F-actin in myocytes and AIL cells.
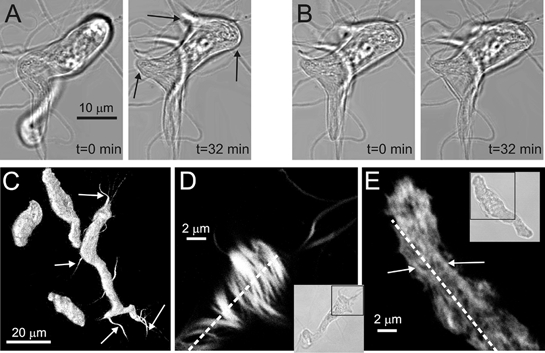
A, transmitted light images of an AIL cell under resting conditions showing the change of cell's body shape (arrows) over a first period of 32 min. B, 10 μm latrunculin B blocked further changes in body shape over a second period of 32 min. C, BODIPY 558/568 phalloidin fluorescence indicating the presence of F-actin in the bodies of three myocytes and in the body and processes of an AIL cell. To visualise the cells and especially the whole length of processes, none of which lies in only one confocal plane, an infinite-focus projection of a series of z-sections is shown. White arrows indicate some of the processes of the AIL cell. D, a single confocal plane of a portion of an AIL cell (shown in the inset as a transmitted-light image) stained with BODIPY 558/568 phalloidin is shown. Actin stress fibres (white and shades of grey) are arranged in various directions, many of them roughly at right angles, to the long axis of the cell (broken line). E, similar to D, but a portion of a myocyte (shown in the inset as a transmitted-light image) is shown. Actin filaments are more densely packed and do not seem organised into thick strands. The few distinguishable strands (arrows) run roughly parallel to the long axis of the cell (broken line).
The growth of processes was completely inhibited and the active change of shape greatly reduced by 10 μM latrunculin B, an actin polymerisation inhibitor (Spector et al. 1983; Fig. 3B and 4B, also available as video clips ‘Latrunculin B’ and ‘Shape change’, respectively, in supplementary material online). The speed of growth was observed over two 32 min periods, the growth during the first period when nothing was added being expressed as 100 %. During the second 32 min period when nothing was added (−), the speed of growth was 57.8 ± 10.4 % (n = 43 processes, 3 cells, 1 guinea-pig) of that during the first 32 min period. In the presence of 10 μM latrunculin B, growth during the second period was 0.1 ± 0.1 % of that during the first period (n = 42 processes, 3 cells, 1 guinea-pig; P < 0.0001; Fig. 3C). In the presence of 0.1 % DMSO (the vehicle for latrunculin B), growth during the second period was 67.6 ± 10.3 % (n = 43 processes, 3 cells, 1 guinea-pig) of control.
Staining of the AIL cells with BODIPY 558/568 phalloidin, a fluorescent derivative of phalloidin used as a marker for polymerised (F (fibrillar)-actin, showed the presence of F-actin in the processes of AIL cells (Fig. 4C, arrows and 4D) as well as in the bodies of myocytes (Fig. 4C and E) and AIL cells (Fig. 4C and D). Actin in the AIL cells was organised into thick strands running in various directions, often roughly perpendicular to the long axis of the cell (Fig. 4D), with spaces between the individual strands. In contrast to AIL cells, actin in the myocytes was more densely packed and did not seem organised into thick strands. The few distinguishable superficial strands were parallel to the long axis of the cell (Fig. 4E).
Electrophysiology
The capacitance of AIL cells was significantly higher than that of myocytes (16.03 ± 0.33 pF, n = 105 AIL cells vs. 13.31 ± 0.37 pF, n = 78 myocytes, P < 0.001) which suggests that on average, the surface area of an AIL cell was larger than that of a myocyte.
The average resting membrane potential of AIL cells was −32.60 ± 1.88 mV (n = 16 cells) and, although lower on average, was not significantly different from the resting membrane potential of myocytes which was −37.84 ± 2.32 mV (n = 28 cells, P > 0.05). Also, the input resistance of AIL cells (9.98 ± 2.94 GΩ, n = 104) measured at −60 mV did not differ significantly from that of myocytes (9.88 ± 1.24 GΩ, n = 78, P > 0.05).
The inward current of AIL cells (Fig. 5A and C) was recorded using either the whole-cell or perforated-patch voltage-clamp technique with Cs+ ions replacing Na+ and K+ ions in both external and pipette solution and with Ba2+ ions as charge carriers. The [Ca2+]i was kept low with 10 mM EGTA in the pipette solution. The inward current had relatively low density (approx. −1.5 pA pF−1) at 0 mV which was the maximum of the current density-voltage (I-V) curve (Fig. 5C); it peaked within ≈10-20 ms and then inactivated (n = 10). The activation curve of this current (Fig. 5C, inset) was constructed from tail current amplitudes (obtained by extrapolating to the zero-time value after fitting an exponential curve). The voltage of half-maximal activation (V0.5) was −13.7 mV and the slope factor k was −15.3 mV when fitted to a Boltzmann curve. The inward current in myocytes had a roughly four times lower density and its I−V curve (Fig. 5E, n = 8) had a similar shape to that of AIL cells. Nicardipine (10 μM) abolished the inward current in AIL cells (n = 7, Fig. 5A and C), suggesting that it was through voltage-dependent Ca2+ channel(s). In myocytes, the same concentration of nicardipine suppressed but did not abolish the inward current (n = 8, Fig. 5E) indicating the presence of a dihydropyridine-insensitive component, as suggested by Morita et al. (1999). No fast inward Na+ current was observed when Na+-containing solutions setting quasi-physiological ionic gradients were used (n = 12, not shown), suggesting that AIL cells are not a subpopulation of neurones.
Figure 5. Inward and outward current in voltage-clamped cells.
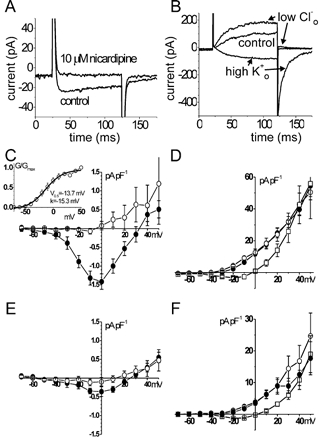
A, original traces of inward current in AIL cells obtained by stepping from the holding potential of −60 mV to 0 mV for 100 ms. I−V relationship for the inward current in AIL cells (C) and myocytes (E). •, control; ○, with nicardipine (10 μm). Inset in C, activation curve of inward current in AIL cells. G/Gmax, relative conductance; V0.5, voltage of half-maximal activation; k, slope factor. The inward current of AIL cells was ≈4 times more dense than in myocytes. Nicardipine abolished the inward current in AIL cells and inhibited it in myocytes. B, original traces of outward current (100 ms steps from −60 mV to −10 mV) in AIL cells. I−V relationship for the outward current in AIL cells (D) and myocytes (F). •, control; ○, in low [Cl−]o physiological salt solution (PSS); □, in high [K+]o PSS. The outward current of AIL cells was ≈twice as dense as in myocytes. In both the AIL cells and myocytes, increasing the [K+]o to 120 mm shifted the curve to the right and lowering the [Cl−]o to 33.4 mm did not change the outward current significantly.
In order to determine the carrier ion(s) of the outward current, the [Cl−]o or [K+]o was changed to 33.4 or 120 mM, respectively, shifting the equilibrium potential for Cl− ions from +0.1 mV to +35.0 mV or for K+ ions from −76.8 mV to −1.3 mV. The density of the outward current of AIL cells was, at 0 mV, roughly ten times higher than that of the inward current (Fig. 5B and D vs. 5A and C) and twice as high as the outward current in myocytes (Fig. 5D vs. 5F). The I−V curve of outward current in both types of cell was, on average, unchanged when extracellular solution with low [Cl−] was used (AIL cells: n = 6 vs. n = 7 in control; myocytes: n = 9). However, when extracellular solution with high [K+] was used, the I−V curve of outward current shifted to the right and its equilibrium potential was −2.3 mV (AIL cells: n = 4, Fig. 5B and D) and +1.5 mV (myocytes: n = 9, Fig. 5F), very close to the calculated equilibrium potential for K+ ions of −1.3 mV, suggesting that the outward current was carried exclusively by K+ ions.
Iberiotoxin (IbTX; 10−8 M), a specific blocker of calcium-activated potassium (BKCa) channels (Galvez et al. 1990), inhibited outward current of AIL cells substantially only at potentials more positive than +30 mV (n = 5 vs. n = 12-15 in control). In contrast to IbTX, the inhibitory effect of 4-aminopyridine (4-AP; 1 mM), a blocker of the delayed rectifier type of voltage-dependent K+ channels (Beech & Bolton, 1989), was less dependent on membrane potential and reduced the outward current by ≈30-40 % (n = 7-8). Combination of IbTX and 4-AP had an additive effect and inhibited the outward current by ≈60 % (n = 10, Fig. 6A and B).
Figure 6. Pharmacology of the outward current in AIL cells.
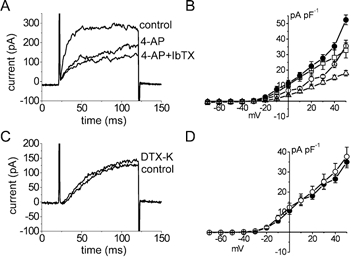
A and C, original traces of outward current (100 ms steps from −60 mV to +20 mV in A or to 0 mV in C) under control conditions and in the presence of (A) 4-aminopyridine (4-AP, 1 mm), or both iberiotoxin (IbTX, 10 nm) and 4-AP or (C) dendrotoxin K (DTX-K, 10 nm). B and D, I−V relationships for the outward current. •, control; ○ in B, with 4-AP; □, with IbTX; ▵, with 4-AP and IbTX; ○ in D, with DTX-K. The inhibitory effect of 4-AP was voltage independent. IbTX exerted its inhibitory effect at potentials positive to +30 mV and DTX-K was ineffective.
It has been shown that dendrotoxin-K (DTX-K) is a specific inhibitor of K+ channels containing at least one Kv1.1 subunit and that it blocks a component of the outward current in murine fundus ICCs, but not in the myocytes from the same tissue (Hatton et al. 2001). However, DTX-K (10−8 M) was ineffective in AIL cells from the guinea-pig small mesenteric arteries (n = 7-8 vs. n = 6-8 in controls, Fig. 6C and D), suggesting the absence of K+ channels containing the Kv1.1 subunit in these cells.
Intracellular calcium events
In order to study the changes in intracellular calcium concentration ([Ca2+]i) the cells were loaded with a calcium-sensitive dye, fluo-4, and imaged using a laser scanning confocal microscope. In some experiments, designed to study simultaneously the effect of intracellular calcium events on membrane current, the cells were patched with a micropipette and held in a voltage-clamp mode.
In contrast to myocytes, where the majority of cells showed spontaneous activity in the form of brief localised transient calcium releases taking place just under the plasmalemma (Ca2+ sparks, Fig. 7B and D), only 27.8 % (27 out of 97) of AIL cells showed changes in [Ca2+]i, and these were larger in spatial spread, longer in duration and of higher intensity than the ones in myocytes (Table 2). The AIL cells mainly produced repetitive calcium transients (24/27 cells, 88.9 % of cases, Fig. 7A) –elevations of [Ca2+]i lasting several seconds (average full duration at half maximum (FDHM) was 2.8 ± 0.3 s, n = 28, compared to 50.6 ± 1.6 ms in myocytes, n = 145) and including a major part of the confocal plane of the cell (19/24) or only a part of it (5/24, Fig. 7C, indicated by arrows). These discharges had an average full width at half maximum (FWHM) of 15.7 ± 1.2 μm (n = 28) compared to 2.5 ± 0.1 μm (n = 145) in myocytes, were irregular in rhythm and their frequency varied from 0.005 to 0.070 s−1. In 8 out of 27 cells, brief localised transient calcium events, similar to Ca2+ sparks in myocytes, were observed in the central cytoplasm, away from the plasmalemma (6/8) or in the processes (2/8, Fig. 7E). When AIL cells were patched, depolarisation to potentials more positive than −40 mV elicited long-lasting transient outward currents (more than 1 s duration), which corresponded with the calcium events in the cell (n = 5, Fig. 7C). The cells without changes in the [Ca2+]i did not produce transient outward currents when patched (not shown). In contrast to AIL cells, myocytes produced brief (less than 100 ms duration) ‘spontaneous’ transient outward currents (STOCs) at potentials positive to −60 mV (Fig. 7D).
Figure 7. Intracellular calcium events and membrane current in AIL cells and myocytes.
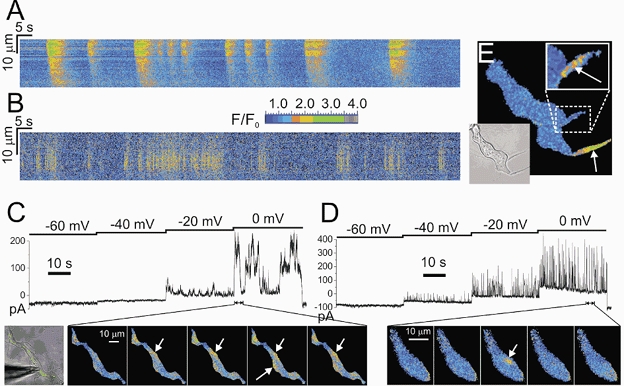
A, line-scan self-ratioed (F/F0) image obtained by scanning approximately parallel to the long axis of an AIL cell for ≈200 s. Long-lasting calcium transients with a spatial spread often > 10 μm are visible in red and yellow. B, line-scan image obtained by scanning a line positioned over a frequent-discharge site, roughly parallel to the cell membrane of a myocyte, for ≈200 s. Ca2+ sparks (which appear as yellow vertical lines on this timescale) are markedly briefer and have spatial spreads less than ≈10 μm. C, membrane current and calcium transients measured simultaneously in an AIL cell. The cell was loaded with a calcium-sensitive dye, fluo-4, and patched in a whole-cell voltage-clamp mode. The voltage protocol (shown above the membrane current record) evoked relatively long-lasting bursts of outward current at potentials positive to −40 mV, which coincided with the long-lasting transient calcium events (shown in red and yellow, indicated by white arrows), imaged using a laser scanning confocal microscope (bottom row in C). D, brief membrane current and calcium transients (white arrow indicating a Ca2+ spark) in a myocyte. Experimental conditions were the same as in C. ‘Spontaneous’ transient outward currents (STOCs) appeared at potentials positive to −60 mV and (some of them) coincided with the Ca2+ sparks. Both STOCs and Ca2+ sparks were markedly briefer than equivalent events in the AIL cell. E, Ca2+ sparks in the processes of an AIL cell (indicated by white arrows, also in the upper inset). Lower inset, transmitted light image of the AIL cell. All the images except the leftmost one in C and the lower inset in E show self-ratioed relative fluorescence (F/F0) colour-coded as shown by the colour scale between panels A and B.
Table 2.
Comparison of the intracellular calcium events in AIL cells and myocytes
| AIL cells | Myocytes | Statistical significance | |
|---|---|---|---|
| Max. (F/F0) | 3.19 ± 0.13 | 2.48 ± 0.05 | P < 10−7 |
| FWHM (μm) | 15.67 ± 1.22* | 2.50 ± 0.07 | P < 10−7 |
| FDHM (ms) | 2766.3 ± 284.4 | 50.6 ± 1.6 | P < 10−7 |
| n (events) | 28 | 145 | |
| n (cells) | 5 | 5 | |
| n (animals) | 2 | 4 |
Max, maximum intensity of a calcium event in relative fluorescence units; FWHM, full width at half maximum; FDHM, full duration at half maximum.
The FWHM of calcium events in AIL cells was dependent on cell length.
Responses to contractile agents
Caffeine, a substance known to release calcium from the intracellular stores through its effect on ryanodine receptors (RyRs; Schmid et al. 1990) and noradrenaline, a Ca2+-mobilising and contractile agent in smooth muscle cells, were used to compare the responses of myocytes and AIL cells to contractile agents.
Administration of caffeine (1 mM) elicited a generalised transient [Ca2+]i increase (measured as fluorescence intensity) in all the examined myocytes (n = 16) and AIL cells (n = 10; Fig. 8A, panel ii vs. i). Moreover, caffeine contracted all the myocytes studied (16 out of 16) by 15.7 ± 2.9 % of their original length (Fig. 8Aiii and the rightmost panel, lower cell; P < 0.0001) but did not produce any shortening of the AIL cells (by 0.3 ± 0.5 % of their original length, n = 10, P > 0.5; Fig. 8A, upper cell).
Figure 8. Response to caffeine and noradrenaline: AIL cells do not contract, myocytes do.
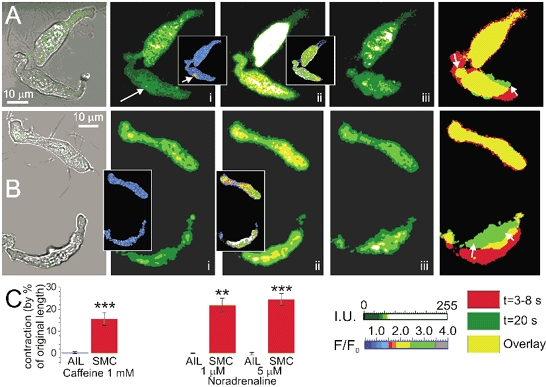
A, combined transmitted light (grey) and fluo-4 fluorescence (green) image of an AIL cell (upper) and a myocyte. Raw fluorescence images of these cells before the application (Ai), at the beginning of response to 1 mm caffeine (within 3−8 s after application, Aii) and 20 s after the application, at the peak of the mechanical response of a myocyte (Aiii). Insets in Ai and Aii show normalised fluorescence images, where Ca2+ spark in the myocyte (indicated by white arrow) can be seen more clearly. [Ca2+]i rose in both cells in response to caffeine. The rightmost image shows the overlap of cell contours (yellow) from Aii (red) and Aiii (green). Note the excess of red colour on the myocyte contour, indicative of its shortening. The AIL cell did not contract. B, similar to A, but showing reaction to 5 μm noradrenaline. The [Ca2+]i rose in both the AIL cell and the myocyte in response to noradrenaline, but only the myocyte contracted. C, summary data on mechanical responses of AIL cells and smooth muscle cells (SMC) expressed as the percentage by which the cell contracted (cell length before the application of the agonist was taken as 100 %). ** P < 0.001; *** P < 0.0001. The upper colour scale (green to white) applies to raw fluorescence images, the lower one to self-ratioed (F/F0) fluorescence images in the insets.
Noradrenaline (1-5 μM), a neuromediator of sympathetic nerves, which are known to innervate the arteries, elevated [Ca2+]i in all the AIL cells studied (n = 15; Fig. 8Bii, upper cell) and in 96 % of myocytes (same figure, lower cell; only one cell out of 24 failed to respond to 1 μM noradrenaline by increasing [Ca2+]i). However, noradrenaline did not produce mechanical response in AIL cells (1 μM noradrenaline: by 0.0 ± 0.2 % of original length, n = 5, P > 0.05; 5 μM noradrenaline: by −0.1 ± 0.5 % of original length, n = 9, P > 0.05; Fig. 8Biii and the rightmost panel, upper cell), except in one case, where 5 μM noradrenaline caused the AIL cell to contract by 11.6 % of its original length. In contrast to AIL cells, all the myocytes which responded to noradrenaline by increasing [Ca2+]i also contracted (1 μM noradrenaline: by 21.8 ± 3.2 % of original length, n = 7, P < 0.01; 5 μM noradrenaline: by 24.4 ± 2.7 % of original length, n = 17, P < 0.0001; Fig. 8B, lower cell).
Immunohistochemistry
The myocytes and AIL cells were labelled with primary antibodies raised against several cellular markers reported by other laboratories to be present or absent in myocytes or ICCs (or interstitial cells) and thus useful in distinguishing cell types (Gabbiani et al. 1981; Epperson et al. 2000; Sergeant et al. 2000; Hatton et al. 2001; McCloskey & Gurney, 2002) –c-kit, vimentin, smooth muscle myosin and desmin, followed by incubation with fluorescent secondary antibodies.
Segments of small mesenteric arteries of guinea-pig were labelled for c-kit, a tyrosine kinase receptor for stem cell factor, which is a selective marker of the ICCs in the gastrointestinal tract (Ward et al. 1994). However, acetone-fixed segments of mesenteric arteries did not stain for this marker (Fig. 9A, left), although the same procedure revealed a network of interconnected cells with multiple processes in the wall of small intestine taken from the same animal (Fig. 9A, right). The live segments of mesenteric arteries, paraformaldehyde-fixed artery segments and pieces of the wall of small intestine, and single live or fixed AIL cells or myocytes did not stain for c-kit (not shown).
Figure 9. Immunohistochemistry of AIL cells.
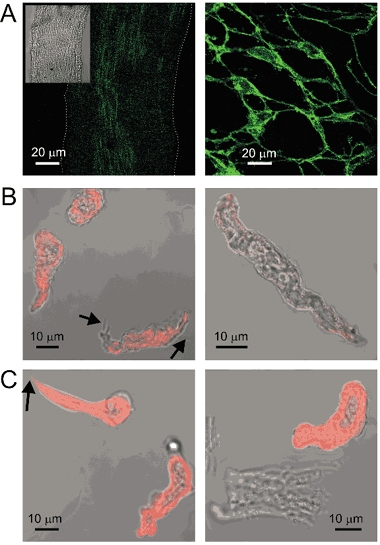
A, c-kit staining of fixed guinea-pig small mesenteric artery (left) and small intestine (right) at the same magnification. The staining of the artery for c-kit was weak (white dotted line indicates the contour of the vessel; transmitted light image in the inset) and not different from the control where primary antibodies were omitted (not shown), but the identical protocol in the small intestine of the same guinea-pig revealed a network of c-kit-positive interstitial cells of Cajal. B, both myocytes and the AIL cells (processes indicated by black arrows) stained positive for vimentin (left). The identical protocol on myocytes isolated from the longitudinal layer of small intestine produced only weak fluorescence (right). C, both myocytes and the AIL cells stained positive for smooth muscle myosin (left). The identical protocol did not stain ventricular cardiomyocytes isolated from the same guinea-pig, although it stained the myocytes that were isolated together with them, presumably from the cardiac vessels (right). The fluorescence images (shown in green or red pseudocolours) were obtained by confocal laser scanning microscopy and were combined with transmitted light images in A (inset), B and C.
Staining for vimentin, an intermediate filament used to distinguish ICCs from myocytes (Sergeant et al. 2000; Hatton et al. 2001), produced staining of both arterial myocytes and AIL cells (Fig. 9B, left). The non-specificity of antibodies against vimentin was excluded in an experiment where an identical procedure produced only weak staining of myocytes isolated from the longitudinal layer of small intestine taken from the same animal (Fig. 9B, right).
Labelling of the smooth muscle myosin also stained both myocytes and, somewhat surprisingly, AIL cells (Fig. 9C, left). The selectivity of antibodies against smooth muscle myosin was confirmed on the suspension of cells isolated from the ventricle of the same animal, where an identical procedure stained myocytes from local blood vessels, but not the cardiomyocytes (Fig. 9C, right).
In a manner similar to staining of vimentin and smooth muscle myosin, both the AIL cells and myocytes stained positive for another intermediate filament, desmin (not shown). The fixing and incubation procedure used in the immunohistochemical studies, lasting over 18 h and involving several changes of solution, damaged the processes on the AIL cells, which resulted in their number being lower in these images. However, the AIL cells could still be recognised by their irregular shape and some remaining processes. Moreover, no unstained cells in the field of view were observed in any of these experiments, which would be expected if there were differences in the expression of vimentin, smooth muscle myosin and desmin between myocytes and AIL cells. In none of the above cases did the primary or secondary antibodies alone produce fluorescence.
Methylene blue, used for staining the ICCs before c-kit (Barajas-Lopez et al. 1989), did not stain the AIL cells and myocytes selectively, either in tissue or as single cells (not shown).
Localisation of AIL cells within the vessels
In order to find out where exactly the AIL cells are localised in mesenteric arteries, the transverse sections of whole vessels were imaged using transmission electron microscopy. Cells with thin processes were found in the media, both in the layer of cells immediately under the basal lamina (Fig. 10a and b) and in the deeper layers (Fig. 10c), scattered among the myocytes. The sections through the processes were 4-5 μm long, presumably because longer processes extend out of the plane of a single section.
Figure 10. Localisation of AIL cells in the arteries.
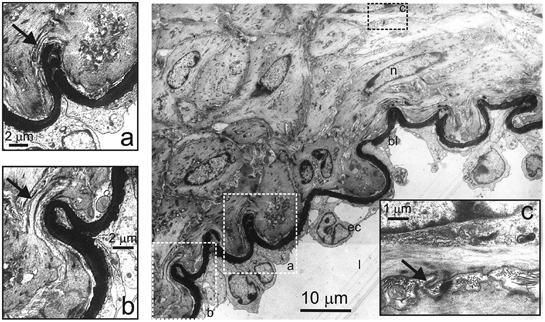
Transmission electron micrograph of a transverse section of mesenteric artery. Rectangles delineated by the broken line show areas which are shown enlarged in a, b and c. l, vessel lumen; ec, endothelial cell; bl:,basal lamina; n, nucleus. Arrows indicate processes. The cells with thin processes can be seen in the media, both in the layer immediately under the basal lamina (a and b) and deeper in the media (c).
DISCUSSION
The view that ICCs are present only in the gastrointestinal tract gives way to a broader hypothesis in the light of recent data that ICCs or ICC-like cells accompany smooth muscle cells throughout the body. These types of cells were recently found in the urethra (Sergeant et al. 2000), bladder (McCloskey & Gurney, 2002), lymphatic vessels (McCloskey et al. 2002) and portal vein (Povstyan et al. 2003), the last being the first observation of ICCs in the vasculature. While previously ICCs were found in low-pressure vessels, this work studies for the first time the ICC-like cells found in resistance arteries.
Differences from and similarities to other cell types
The AIL cells described in this paper can be distinguished from myocytes both morphologically and functionally. The most obvious feature of AIL cells is the presence and growth of thin processes and active and slow change of shape of their bodies. The basis of these phenomena seems to be the reorganisation of the actin stress fibres network and actin fibre elongation. Unlike myocytes, AIL cells did not contract despite the presence of smooth muscle myosin in them. The AIL cells seldom produced Ca2+ sparks, which are a feature of myocytes in this artery (Gordienko et al. 1999; Pucovsky et al. 2002). In rare cases when AIL cells did produce Ca2+ sparks, these originated in the central cytoplasm or in the processes rather than in the subplasmalemmal space. Instead, AIL cells mainly produced calcium transients lasting several seconds and involving a major portion of the AIL cell, which elicited long-lasting transient outward currents, instead of typical STOCs characteristic of myocytes. This suggests easier recruitment of additional calcium release sites in AIL cells than in myocytes and could reflect different spatial arrangement of the intracellular calcium release channels or a lower threshold necessary for their activation. Also, both the voltage-dependent inward and outward currents had higher density in AIL cells than in myocytes.
There are also a number of similarities between the AIL cells and the myocytes, suggesting their common origin. They have similar ultrastructure, including the presence of smooth muscle myosin, vimentin and desmin filaments. They also have qualitatively similar membrane currents, including a voltage-dependent calcium current (also described in mesenteric myocytes by Morita et al. 1999) and outward current carried by potassium ions, including delayed rectifier and Ca2+-activated K+ current (described in mesenteric myocytes by Benham et al. 1986; Cole et al. 1996; Lu et al. 2001; McDaniel et al. 2001). The AIL cells stained positive for smooth muscle myosin (SMM), although they were shown to be non-contractile. Although SMM was generally accepted to be a specific marker of the contractile type of differentiated myocytes, its presence has been demonstrated in the non-contractile precursors of myocytes such as myofibroblasts (Buoro et al. 1993; Plateroti et al. 1998), or human fibroblast line WI38 (Painter et al. 1975).
The AIL cells were also functionally different from the ICCs or ICC-like cells described in other tissues, including the portal vein. The main difference lies in the fact that AIL cells did not stain positive for c-kit, which is generally regarded as a marker for ICCs. Although the majority of ICCs or ICC-like cells stain positive for this marker (Ward et al. 1994; McCloskey & Gurney, 2002; McCloskey et al. 2002; Povstyan et al. 2003) this is not the first observation of c-kit-negative ICC-like cells, as Sergeant et al. (2000) were the first to describe them in the rabbit urethra. Clearly, the relation between the presence of c-kit antigen and ICC-like phenotype is not simple and seems to be dependent on tissue (and its integrity) and source species. Unlike the ICCs of the dog, guinea-pig and mouse (Hatton et al. 2001) and like ICCs of the rabbit portal vein (Povstyan et al. 2003) and the myocytes of all the above species, the K+ channels of AIL cells did not contain a Kv1.1 subunit, which is dendrotoxin K-sensitive.
It is unlikely that the AIL cells are pericytes, fibroblasts, or neurones. Pericytes are normally found as a single layer around capillaries or postcapillary venules but are contractile (Diaz-Flores et al. 1991; Hirschi & D'Amore, 1996). The AIL cells were obtained from vessels which were > 120 μm in external diameter and had more than three layers of myocytes in the vessel wall (Fig. 10). Fibroblasts are generally regarded as precursors of a variety of cell types, including myocytes (e.g. following injury), but do not have processes. They normally have a well-developed rough endoplasmic reticulum and Golgi apparatus reflecting their secretory function (Olsen et al. 1975) but these organelles were not found to be prominent in AIL cells, and their nuclei were multilobar, not oval as in fibroblasts. Nor did AIL cells display a voltage-dependent sodium current, which argues against their being a type of nerve cell.
It is also unlikely that AIL cells are damaged myocytes, because they had even more dense membrane currents than myocytes and a similar input resistance (about 10 GΩ), indicating no measurable increase in membrane conductance (or non-specific electrical ‘leak’) which might be expected if the membrane was unusually damaged. Although the membrane potential was less negative than that of myocytes, it was not significantly so. The processes were true extensions of the cell, containing cytoplasm, and this continuity was confirmed by electron microscopy. Indeed, it is difficult to imagine why the dispersal procedure should cause long fine processes to be extruded, as ‘blebbing’ of the cell membrane is a more usual response of myocytes to cell damage (Fay & Delise, 1973; Maruyama et al. 1987).
Localisation of AIL cells
None of the markers used in this study could discriminate the AIL cells from the myocytes. Therefore the most obvious feature of AIL cells, their processes, was used to establish the location of AIL cells in arteries. The results suggest that the AIL cells can be found in the layer of media immediately under the basal lamina, but also scattered among the myocytes in the deeper layers of media.
Another two facts support this observation by suggesting that the cells with processes were already present in the intact tissue: (1) the speed of elongation of processes was not high enough to account for the length of processes found on the AIL cells immediately after their isolation; (2) if the processes were a result of tissue injury, the percentage of AIL cells in suspension would have to increase over time, with the majority of myocytes gradually transforming into AIL cells, which was not observed.
Possible physiological role(s) of AIL cells
The ICCs in the gastrointestinal tract (Langton et al. 1989; Ördög et al. 1999) and interstitial cells in the urinary tract (Sergeant et al. 2000) and rabbit portal vein (Povstyan et al. 2003) were shown to produce spontaneous depolarisations. The ICCs in the intestines were shown to contain numerous mitochondria, considered important for their pacemaking function (e.g. Thuneberg, 1982; Komuro & Zhou, 1996; Ward et al. 2000b). Also, in rabbit urethra interstitial cells it was shown that rhythmic depolarisations were caused by opening of calcium-dependent chloride channels (ClCa; Sergeant et al. 2000, 2002). The number of mitochondria in AIL cells was similar to that in myocytes, judging from electron micrographs and from staining the cells with Mitotracker Orange CMTMRos (V. Pucovsky and T. B. Bolton, unpublished observations). Moreover, the AIL cells did not have voltage-dependent Cl− current and, at the potentials where spontaneous transient inward currents (STICs, through ClCa channels) were routinely observed in urethral interstitial cells, no STICs or inward currents were observed in AIL cells, suggesting an absence of ClCa channels. Also, the rare calcium transients in AIL cells were irregular in rhythm. Thus, the role of AIL cells as pacemakers is unlikely.
The localisation of AIL cells throughout the media and especially in the layer of cells just under the basal lamina makes unlikely the possibility that they might act as intermediaries in neural transmission, as suggested for ICCs in the intestine (Ward et al. 2000a).
The processes of AIL cells lack varicosities. It is conceivable that they might receive rather than send signals or help the cell during migration. The low-pressure vessels like veins or lymphatic vessels require rhythmic mechanical activity to help with the flow of blood or lymph, with a clear role for pacemaking cells in such a tissue. This is not necessary in arteries where there is a sufficient pressure gradient to move the blood towards capillaries. This higher pressure might cause AIL cells to develop only to an intermediate stage between the myocytes and ICCs, having some characteristics of both these cell types, and act as a pool of cells ready to differentiate either way. Indeed, the application of cyclic strain was shown to increase the expression of smooth muscle myosin in neonatal rat vascular myocytes (Reusch et al. 1996). The capability of the ICCs to interconvert with myocytes under certain conditions, such as long-term treatment with c-kit antibody, and to revert to their ICC form upon cessation of treatment (Torihashi et al. 1999) together with many similarities between the myocytes and AIL cells suggests that myocytes, AIL cells and ICCs are all members of a single cell spectrum.
Acknowledgments
This work was supported by a British Heart Foundation Programme grant and by the Wellcome Trust grant 042293.
Supplementary material
The online version of this paper can be found at:
DOI: 10.1113/jphysiol.2003.046243
and contains three video clips entitled:
Growth, Shape change and Latrunculin B.
REFERENCES
- Barajas-Lopez C, Berezin I, Daniel EE, Huizinga JD. Pacemaker activity recorded in interstitial cells of Cajal of the gastrointestinal tract. Am J Physiol. 1989;257:C830–835. doi: 10.1152/ajpcell.1989.257.4.C830. [DOI] [PubMed] [Google Scholar]
- Beech DJ, Bolton TB. Two components of potassium current activated by depolarization of single smooth muscle cells from the rabbit portal vein. J Physiol. 1989;418:293–309. doi: 10.1113/jphysiol.1989.sp017841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham CD, Bolton TB, Lang RJ, Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabit jejunum and guinea-pig mesenteric artery. J Physiol. 1986;371:45–67. doi: 10.1113/jphysiol.1986.sp015961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buoro S, Ferrarese P, Chiavegato A, Roelofs M, Scatena M, Pauletto P, Passerini-Glazel G, Pagano F, Sartore S. Myofibroblast-derived smooth muscle cells during remodelling of rabbit urinary bladder wall induced by partial outflow obstruction. Lab Invest. 1993;69:589–602. [PubMed] [Google Scholar]
- Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci U S A. 1996;93:12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Cole WC, Clement-Chomienne O, Aiello EA. Regulation of 4-aminopyridine-sensitive, delayed rectifier K+ channels in vacsular smooth muscle by phosphorylation. Biochem Cell Biol. 1996;74:439–447. doi: 10.1139/o96-048. [DOI] [PubMed] [Google Scholar]
- Dahl E, Nelson E. Electron microscopic observations on human intracranial arteries. Neurology. 1964;10:158–164. doi: 10.1001/archneur.1964.00460140044007. [DOI] [PubMed] [Google Scholar]
- Diaz-Flores L, Gutierrez R, Varela H, Rancel N, Valladares F. Microvascular pericytes: a review of their morphological and functional characteristics. Histol Histopathol. 1991;6:269–286. [PubMed] [Google Scholar]
- Epperson A, Hatton WJ, Callaghan B, Doherty P, Walker RL, Sanders KM, Ward SM, Horowitz B. Molecular markers expressed in cultured and freshly isolated interstitial cells of Cajal. Am J Physiol. 2000;279:C529–539. doi: 10.1152/ajpcell.2000.279.2.C529. [DOI] [PubMed] [Google Scholar]
- Faussone-Pellegrini MS, Cortesini C. Ultrastructural peculiarities of the inner portion of the circular layer of colon. I. Research in the human. Acta Anat (Basel) 1984;120:185–189. doi: 10.1159/000145918. [DOI] [PubMed] [Google Scholar]
- Faussone-Pellegrini MS, Cortesini C. Ultrastructural features and localization of the interstitial cells of Cajal in the smooth muscle coat of human esophagus. J Submicrosc Cytol. 1985;17:187–197. [PubMed] [Google Scholar]
- Faussone-Pellegrini MS, Pantalone D, Cortesini C. An ultrastructural study of the interstitial cells of Cajal of the human stomach. J Submicrosc Cytol Pathol. 1989;21:439–460. [PubMed] [Google Scholar]
- Fay FS, Delise CM. Contraction of isolated smooth-muscle cells –structural changes. Proc Natl Acad Sci U S A. 1973;70:641–645. doi: 10.1073/pnas.70.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G, Schmid E, Winter S, Chaponnier C, De Chastonay C, Vandekerckhove J, Weber K, Franke WW. Vascular smooth muscle cells differ from other smooth muscle cells: predominance of vimentin filaments and a specific alpha-type actin. Proc Natl Acad Sci U S A. 1981;78:298–302. doi: 10.1073/pnas.78.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, Kaczorowski GJ, Garcia ML. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990;265:11083–11090. [PubMed] [Google Scholar]
- Gordienko DV, Zholos AV, Bolton TB. Membrane ion channels as physiological targets for local Ca2+ signalling. J Microsc. 1999;196:305–316. doi: 10.1046/j.1365-2818.1999.00599.x. [DOI] [PubMed] [Google Scholar]
- Hatton WJ, Mason HS, Carl A, Doherty P, Latten MJ, Kenyon JL, Sanders KM, Horowitz B. Functional and molecular expression of a voltage-dependent K+ channel (Kv1. 1) in interstitial cells of Cajal. J Physiol. 2001;533:315–327. doi: 10.1111/j.1469-7793.2001.0315a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KK, D'Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–698. [PubMed] [Google Scholar]
- Komuro T, Zhou DS. Anti-c-kit protein immunoreactive cells corresponding to the interstitial cells of Cajal in the guinea-pig small intestine. J Auton Nerv Syst. 1996;61:169–174. doi: 10.1016/s0165-1838(96)00078-1. [DOI] [PubMed] [Google Scholar]
- Langton PD, Ward SM, Carl A, Norell MA, Sanders KM. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc Natl Acad Sci U S A. 1989;86:7280–7284. doi: 10.1073/pnas.86.18.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhang J, Tang G, Wang R. Modulation of voltage-dependent K+ channel current in vascular smooth muscle cells from rat mesenteric arteries. J Membr Biol. 2001;180:163–175. doi: 10.1007/s002320010067. [DOI] [PubMed] [Google Scholar]
- McCloskey KD, Gurney AM. Kit-positive cells in the guinea pig bladder. J Urol. 2002;168:832–836. [PubMed] [Google Scholar]
- McCloskey KD, Hollywood MA, Thornbury KD, Ward SM, McHale NG. Kit-like immunopositive cells in sheep mesenteric lymphatic vessels. Cell Tissue Res. 2002;310:77–84. doi: 10.1007/s00441-002-0623-y. [DOI] [PubMed] [Google Scholar]
- McDaniel SS, Platoshyn O, Yu Y, Sweeney M, Miriel VA, Golovina VA, Krick S, Lapp BR, Wang JY, Yuan JX. Anorexic effect of K+ channel blockade in mesenteric arterial smooth muscle and intestinal epithelial cells. J Appl Physiol. 2001;91:2322–2333. doi: 10.1152/jappl.2001.91.5.2322. [DOI] [PubMed] [Google Scholar]
- Maruyama I, Kobayashi M, Yoshida C, Momose K. Ultrastructure of single smooth muscle cells contracted by carbachol and calcium ion. J Pharmacobiodyn. 1987;10:396–403. doi: 10.1248/bpb1978.10.396. [DOI] [PubMed] [Google Scholar]
- Meyling HA. Structure and significance of the peripheral extension of the autonomic nervous system. J Comp Neurol. 1953;99:495–543. doi: 10.1002/cne.900990304. [DOI] [PubMed] [Google Scholar]
- Morita H, Cousins H, Onoue H, Ito Y, Inoue R. Predominant distribution of nifedipine-insensitive, high voltage-activated Ca2+ channels in the terminal mesenteric artery of guinea pig. Circ Res. 1999;85:596–605. doi: 10.1161/01.res.85.7.596. [DOI] [PubMed] [Google Scholar]
- Olsen BR, Berg RA, Kishida Y, Prockop DJ. Further characterization of embryonic tendon fibroblasts and the use of immunoferritin techniques to study collagen biosynthesis. J Cell Biol. 1975;64:340–355. doi: 10.1083/jcb.64.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ördög T, Ward SM, Sanders KM. Interstitial cells of Cajal generate electrical slow waves in the murine stomach. J Physiol. 1999;518:257–269. doi: 10.1111/j.1469-7793.1999.0257r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter RG, Sheetz M, Singer SJ. Detection and ultrastructural localization of human smooth muscle myosin-like molecules in human non-muscle cells by specific antibodies. Proc Natl Acad Sci U S A. 1975;72:1359–1363. doi: 10.1073/pnas.72.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plateroti M, Rubin DC, Duluc I, Singh R, Foltzer-Jourdainne C, Freund JN, Kedinger M. Subepithelial fibroblast cell lines from different levels of gut axis display regional characteristics. Am J Physiol. 1998;274:G945–954. doi: 10.1152/ajpgi.1998.274.5.G945. [DOI] [PubMed] [Google Scholar]
- Povstyan OV, Gordienko DV, Harhun MI, Bolton TB. Identification of interstitial cells of Cajal in the rabbit portal vein. Cell Calcium. 2003;33:223–239. doi: 10.1016/s0143-4160(02)00197-5. [DOI] [PubMed] [Google Scholar]
- Pucovský V, Bolton TB. Cells with non-myocyte phenotype in the media of guinea pig mesenteric arteries. Biophys J. 2002;82:416a. [Google Scholar]
- Pucovský V, Gordienko DV, Bolton TB. Effect of nitric oxide donors and noradrenaline on Ca2+ release sites and global intracellular Ca2+ in myocytes from guinea pig small mesenteric arteries. J Physiol. 2002;539:25–39. doi: 10.1113/jphysiol.2001.012978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Histologie du système nerveux de l' homme et des vertébrés. Paris, Maloine. 1911;2:891–942. [Google Scholar]
- Reusch P, Wagdy H, Reusch R, Wilson E, Ives HE. Mechanical strain increases smooth muscle and decreases nonmuscle myosin expression in rat vascular smooth muscle cells. Circ Res. 1996;79:1046–1053. doi: 10.1161/01.res.79.5.1046. [DOI] [PubMed] [Google Scholar]
- Schmid A, Dehlinger-Kremer M, Schulz I, Gogelein H. Voltage-dependent InsP3-insensitive calcium channels in membranes of pancreatic endoplasmic reticulum vesicles. Nature. 1990;346:374–376. doi: 10.1038/346374a0. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, Thornbury KD, McHale NG. Specialised pacemaking cells in the rabbit urethra. J Physiol. 2000;526:359–366. doi: 10.1111/j.1469-7793.2000.t01-2-00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant GP, Thornbury KD, McHale NG, Hollywood MA. Characterization of norepinephrine-evoked inward currents in interstitial cells isolated from the rabbit urethra. Am J Physiol. 2002;283:C885–894. doi: 10.1152/ajpcell.00085.2002. [DOI] [PubMed] [Google Scholar]
- Spector I, Shochet NR, Kashman Y, Groweiss A. Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science. 1983;219:493–495. doi: 10.1126/science.6681676. [DOI] [PubMed] [Google Scholar]
- Thuneberg L. Interstitial cells of Cajal: intestinal pacemaker cells. Adv Anat Embryol Cell Biol. 1982;71:1–130. [PubMed] [Google Scholar]
- Ward SM, Beckett EAH, Wang XY, Baker F, Khoyi M, Sanders KM. Interstitial Cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000a;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Ördög T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000b;525:355–361. doi: 10.1111/j.1469-7793.2000.t01-1-00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


