Abstract
Cyclic nucleotide-gated (CNG) channels in rod photoreceptors transduce a decrease in cGMP into hyperpolarization during the light response. Insulin-like growth factor-1 (IGF-1) increases light responses by increasing the cGMP sensitivity of CNG channels, an event mediated by a protein tyrosine phosphatase. Native rod CNG channels are heteromultimers, composed of three CNGA1 subunits and one CNGB1 subunit. Previous studies on heterologously expressed rod CNG channels show that a specific tyrosine in the CNGA1 subunit (Y498) is required for modulation by protein tyrosine phosphatases, protein tyrosine kinases and IGF-1. Here we show that the CNGB1 subunit contains a specific tyrosine (Y1097) that is important for modulation of heteromeric channels by tyrosine phosphorylation. Direct biochemical measurements demonstrate 32P-labelling of CNGA1Y498 and CNGB1Y1097. Replacement of either Y498 of CNGA1 or Y1097 of CNGB1 with phenylalanine reduces modulation, and removal of both tyrosines eliminates modulation. Unlike CNGA1, CNGB1 does not exhibit activity dependence of modulation by tyrosine phosphorylation. Hence both CNGA1 and CNGB1 subunits contribute to phosphorylation-dependent modulation of rod CNG channels, but the phosphorylation states of the two subunits are regulated in different ways.
Cyclic nucleotide-gated (CNG) channels are fundamental for phototransduction in vertebrate rods and cones. Light initiates a G-protein-coupled cascade, decreasing the concentration of cGMP, leading to the closure of CNG channels. However, CNG channels are not invariant reporters of cGMP; rather, their sensitivity can be modulated by intracellular and extracellular signals (Kramer & Molokanova, 2001), which are potentially important for light or dark adaptation. Intracellular Ca2+ decreases the cGMP sensitivity of the rod CNG channel (Hsu & Molday, 1993; Chen et al. 1994; Gordon et al. 1995) via calmodulin and other Ca2+-binding proteins that bind to the channel. Phosphorylation, either on serine/threonine (Gordon et al. 1992) or on tyrosine (Molokanova et al. 1997, 1999) residues, also regulates cGMP sensitivity.
Rod CNG channels are heterotetramers, composed of two homologous subunits: CNGA1 (Kaupp et al. 1989), also known as the α-subunit, and CNGB1 (Koerschen et al. 1995), also known as the β-subunit (Bradley et al. 2001). CNGA1 subunits by themselves can form functional homomeric channels in various exogenous expression systems. However, CNGB1 can only form functional channels when co-expressed with CNGA1, or related olfactory CNG channel subunits (Finn et al. 1998). The expressed heteromeric (CNGA1/CNGB1) channel exhibits properties nearly identical to those of native channels, including single channel properties (conductance, gating behaviour), increased Ca2+ permeability, higher voltage dependence, sensitivity to cGMP and cAMP, and sensitivity to block by L-cis-diltiazem. Moreover, the presence of the CNGB1 subunit is necessary for certain forms of modulation. For example, CNGB1, but not CNGA1, contains Ca2+-calmodulin binding sites, and therefore heteromeric, but not homomeric, channels exhibit Ca2+-calmodulin regulation. Recent studies show that heteromeric CNG channels, both naturally occurring in rods and exogenously expressed in oocytes, are composed of three CNGA1 subunits and one CNGB1 subunit (Zhong et al. 2002; Weitz et al. 2002; Zheng et al. 2002); hence the single CNGB1 subunit accounts for all the special properties of heteromeric channels.
We have recently shown (Savchenko et al. 2001) that the sensitivity of rod CNG channels to cGMP is rapidly (< 30 s increased 2- to 3-fold by insulin-like growth factor-1 (IGF-1). In the intact eye, IGF-1 is released as a paracrine factor from the retinal pigment epithelium (Waldbillig et al. 1991), adjacent to rod outer segments. Application of IGF-1 on the isolated retina leads to an increase in the magnitude and speeding of rod light responses (Savchenko et al. 2001). Previous studies on homomeric CNGA1 channels have revealed that the molecular mechanism of IGF-1 modulation involves changes in tyrosine dephosphorylation catalysed by protein tyrosine kinases (PTKs) and phosphatases (PTPs), and have implicated a specific residue in the CNGA1 subunit (Y498) as the primary phosphorylation site involved in modulation (Molokanova et al. 1999). Replacement of the tyrosine with a phenylalanine at this position nearly eliminates modulation (Molokanova et al. 1999) and renders IGF-1 incapable of altering the cGMP sensitivity of homomeric channels (Savchenko et al. 2001).
Here we examine the role of CNGB1 in the modulation of heteromeric rod CNG channels. We find that the CNGB1 subunit also contains a tyrosine involved in modulation and that the CNGB1 subunit is even more important for IGF-1 modulation than is CNGA1. Finally, we present biochemical evidence directly demonstrating that both CNGA1Y498 and CNGB1Y1097 are the primary phosphorylation sites on the rod CNG channel.
METHODS
Electrophysiology
To generate homomeric channels, a cDNA clone encoding the CNGA1 subunit of the bovine rod photoreceptor CNG channel (Kaupp et al. 1989) was used for in vitro transcription, and the mRNA was then injected into Xenopus oocytes (50 nl per oocyte at 1 ng nl−1). Xenopus oocytes were collected from anaesthetized frogs (1 mg ml−1 2-amino benzoic acid), which were killed humanely after the final collection. The use and care of the animals in these experiments were approved by the UC Berkeley Animal Care and Use Committee. After 2-7 days, the vitelline membrane was removed from injected oocytes, and the oocytes were then placed in a chamber for patch clamp recording at 21-24 °C. Glass patch pipettes (2-3 MΩ) were filled with standard patch solution, containing (mM): 115 NaCl, 1 EDTA, 5 EGTA and 10 Hepes (pH 7.5), which also served as the standard bath solution and cGMP perfusion solution. After a gigaohm seal had been formed, inside-out patches were excised and the patch pipette was quickly (< 30 s) placed in the outlet of a 1 mm diameter tube for application of cGMP and other agents. We used a perfusion manifold containing eight different solutions and capable of solution changes within 50 ms.
Different concentrations of cGMP (50, 100, 250 and 2000 μM) were briefly (5 s each) applied on excised patches 1- 1.5 min after patch excision. The resulting CNG currents were recorded with an Axopatch 200A patch clamp amplifier (Axon Instruments, Union City, CA, USA), digitized, stored and later analysed. The membrane potential was held at -75 mV. Current responses were normalized to the maximal CNG current (Imax), elicited by saturating (2000 μM) cGMP. Normalized dose-response curves were fitted to the Hill equation: I/Imax = 1/(1 + (K1/2/A)nH), where A is the cGMP concentration and nH is the Hill coefficient, by using a non-linear least-squares fitting routine (Origin, Microcal Software, Northampton, MA, USA). Variability among measurements was expressed as mean ± S.E.M. Data sets were compared by using one-way ANOVA tests. The statistical significance reflects P < 0.05 unless otherwise stated.
To generate bovine rod photoreceptor heteromeric channels, mRNAs encoding CNGA1 (Kaupp et al. 1989) and CNGB1 (Koerschen et al. 1995) were co-injected into oocytes in a 1:10 ratio. The presence of heteromeric channels in excised patches was confirmed by application of saturating (2 mM) cGMP in conjunction with 10 μM L-cis-diltiazem, which selectively blocks channels containing CNGB1 subunits but is ineffective at blocking homomeric CNGA1 channels (Chen et al. 1993). Injection of CNGA1 and CNGB1 mRNA in a 1:8 (n = 6) or 1:10 (n = 14) ratio produced channels that were inhibited by 78 and 80 %, respectively, by 10 μM L-cis-diltiazem. In contrast, a 1:4 ratio generated channels inhibited by 60 %, suggesting that a CNGA1 to CNGB1 mRNA ratio of 1:10 is sufficient to saturate the CNGB1 subunit contribution to heteromeric channels, consistent with previous results (Shammat & Gordon, 1999).
For expression of hybrid CNGA2/CNGB1 channels, mRNAs encoding the CNGA2 subunit of the rat olfactory CNG channel (Dhallan et al. 1990) and CNGB1 (Koerschen et al. 1995) were co-injected into oocytes. The presence of CNGB1 in hybrid channels was confirmed by applying 10 μM L-cis-diltiazem (Finn et al. 1998). This inhibitor blocked channels resulting from CNGA2 to CNGB1 mRNA injection ratios of 1:1.5 and 1:3 by 36.8 ± 2.9 % (n = 3) and 32.8 ± 1.4 % (n = 6), respectively. These results suggest that a CNGA2 to CNGB1 injection ratio of 1:3 is sufficient to saturate the contribution of CNGB1 to hybrid channels.
Mutagenesis and construction of chimeric channels
cDNA for the CNGA1 subunit (Kaupp et al. 1989) and the full-length cDNA for the CNGB1 subunit (Korschen et al. 1995) were used as templates to generate mutant subunits with a phenylalanine in place of a tyrosine in position 498 of the CNGA1 subunit (CNGA1Y498F) and in position 1097 of the CNGB1 subunit (CNGB1Y1097F) using primer-mismatch PCR and Pfu DNA polymerase (Stratagene, La Jolla, CA, USA). To minimize the amount of PCR product in the final construct that would have to be sequenced, a small cassette containing the mutation was cut from the PCR product and inserted into the wild-type construct using existing restriction sites. All PCR-derived portions of the final constructs were confirmed by sequencing using Sequenase 2.0 (Sequenase 2.0 kits were from Amersham Life Sciences, Cleveland, OH, USA). The engineered mutant subunits were then sequenced on both strands to ensure polymerase fidelity and subcloned into the pGEM-HE vector (Liman et al. 1992) for use in the Xenopus expression system. mRNA was synthesized in vitro using a standard reverse transcription kit (mMessage mMachine, Ambion, Austin, TX, USA).
Biochemistry
CNGA1 and CNGB1 subunits were tagged with 6 ×His (His6) to allow for purification with Ni2+ beads. His6 tags were introduced at the COOH terminus of the CNGA1 and CNGB1 subunits using standard molecular biology techniques. DNA constructs encoding the tagged subunits were subcloned into pGEM-HE for in vitro synthesis of mRNA. Thirty to thirty-two oocytes were injected with 50 ng of mRNA encoding CNGA1 or a CNGA1/CNGB1 mixture (1:10 ratio) to produce either homomeric or heteromeric channels. 32P-Orthophosphate (at 0.2 mCi ml−1) was added to the oocyte incubation solution 24 h before harvesting. Before homogenization, the oocytes were pretreated with 500 μM pervanadate or 50 μM lavendustin A for 1 h. Oocytes were homogenized in 10 mM Tris pH 8.0, 100 mM NaCl, 1 mM β-mercaptoethanol, 10 mM sodium fluoride and 1 mM orthovanadate containing protease inhibitor cocktail (Sigma, St Louis, MO, USA). Homogenates were centrifuged at 700 g for 5 min to remove yolk granules and the supernatant was collected and subjected to ultracentrifugation at 100 000 g for 40 min. The pellet, containing the plasma membrane fraction, was solubilized in 400 μl of 8 M urea with 0.5 % Triton X-100 and 0.05 % SDS buffered with Tris-HCl, pH 8.0 (solubilization buffer). His6-tagged proteins were then purified using Ni2+-NTA agarose beads (Qiagen, Valencia, CA, USA). Beads, prewashed in solubilization buffer, were added to the samples and the mixture was left for 2 h with constant rotation. Beads were then washed extensively to remove unbound material, and proteins were eluted with 200 mM imidazole in solubilization buffer. The unbound and eluate fractions were analysed using SDS-PAGE with subsequent transfer onto nitrocellulose and probing with anti-His6 antibodies (His-probe H-15, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and autoradiography. To insure sufficiently high expression of CNG channels for biochemical experiments, groups of oocytes from individual frogs were selected on the basis of the magnitude of the cGMP-elicited current observed in excised patches. Groups of oocytes were used if patches exhibited ≥ 500 pA current in response to saturating (2000 μM) cGMP with a driving force of 75 mV.
RESULTS
Contribution of CNGB1 subunits to modulation of expressed CNG channels
To assess the role of the CNGB1 subunit in channel modulation, we first compared the behaviour of homomeric (CNGA1) and heteromeric (CNGA1/CNGB1) channels expressed in oocytes. Homomeric (CNGA1) channels exhibit a spontaneous increase in cGMP sensitivity after patch excision and a decrease in cGMP sensitivity after ATP application (Molokanova et al. 1997). The increase in sensitivity after excision results from dephosphorylation catalysed by spontaneously active PTPs in the excised patch. Likewise, the decrease in sensitivity elicited by ATP results from phosphorylation catalysed by PTKs in the patch. Figure 1A shows that heteromeric channels (CNGA1/CNGB1) exhibited changes in cGMP sensitivity that were similar in magnitude and time course to those observed in homomeric (CNGA1) channels. By 10 min after patch excision, the apparent affinity for cGMP (K1/2) reached steady state, decreasing from its initial value (1 min after patch excision) by 48.2 ± 2.4 % (n = 47) in homomeric and 42.7 ± 3.9 % (n = 12) in heteromeric channels (Fig. 1B).
Figure 1. Modulation of heteromeric wild-type rod CNG channels (CNGA1/CNGB1) resembles modulation of homomeric CNGA1 channels.
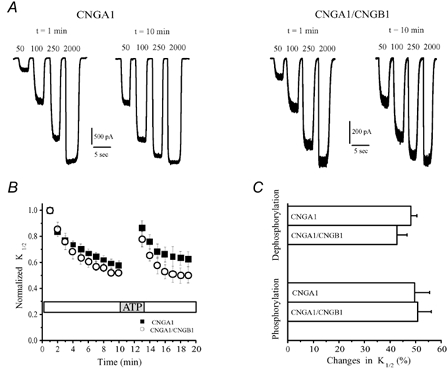
A, comparison of cGMP-elicited currents in homomeric and heteromeric channels at 1 and 10 min after patch excision. B, changes in the cGMP sensitivity in CNGA1 and CNGA1/CNGB1 channels after patch excision and ATP application. C, summary of changes in K1/2 elicited by phosphorylation/dephosphorylation in CNGA1 and CNGA1/CNGB1 channels. In this and other figures, changes in K1/2 during dephosphorylation were quantified by normalizing the K1/2 at 10 min after patch excision to the initial K1/2 (1 min after excision). Changes in K1/2 during phosphorylation were estimated by normalizing the change in K1/2 evoked by a 3 min application of 500 μM ATP to the K1/2 before ATP application.
It is possible that only the CNGA1 subunits are regulated by phosphorylation and are responsible for all of the modulation, with the CNGB1 subunits being neutral or uninvolved. To test whether CNGB1 subunits play an active role in supporting modulation, CNGB1 subunits were co-expressed with olfactory CNGA2 subunits. Homomeric expression of the CNGA2 subunit results in functional channels that exhibit no modulation by tyrosine phosphorylation, because these subunits lack a tyrosine at the position equivalent to Y498 in CNGA1 (F477 in CNGA2). Figure 2A shows that CNGA2/CNGB1 channels did indeed exhibit modulation, both after patch excision and as a result of ATP application. In fact, the modulation due to these processes in CNGA2/CNGB1 channels was at least half as large as that observed in homomeric or heteromeric rod channels (compare Fig. 2B with Fig. 1B). Hence, incorporation of CNGB1 is able to bestow ‘modulability’ on channels where it would otherwise be lacking.
Figure 2. The CNGB1 subunit of rod CNG channels introduces modulability when co-expressed with the olfactory CNGA2 subunit, which alone does not exhibit modulation.
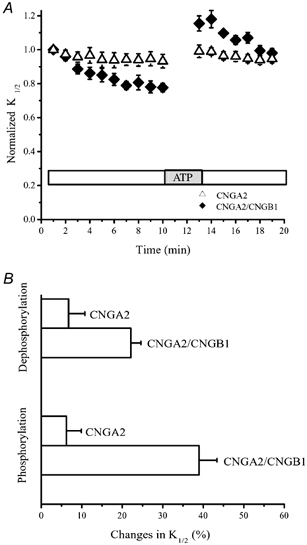
A, changes in the cGMP sensitivity after patch excision and following ATP application, for homomeric CNGA2 and hybrid CNGA2/CNGB1 channels. B, changes in the channel sensitivity as a result of phosphorylation and dephosphorylation.
To further confirm the active role of CNGB1 subunits in channel modulation, wild-type CNGB1 subunits were co-expressed with mutant rod CNGA1 subunits that are missing their crucial tyrosine phosphorylation site (CNGA1Y498F). The resulting channels did indeed exhibit changes in channel sensitivity after patch excision and ATP application that were much larger than the changes observed in homomeric CNGA1Y498F channels (Fig. 3A). The decrease in K1/2 after patch excision was 31.6 ± 4.5 % (n = 12) and recovery after ATP application was 25.4 ± 6.4 % (n = 10) (Fig. 3B). Hence, even when the CNGA1 subunits are mutated to prevent them from participating in modulation, the CNGB1 subunits continue to allow modulation to occur.
Figure 3. The CNGB1 subunit contributes to modulation of heteromeric rod CNG channels.
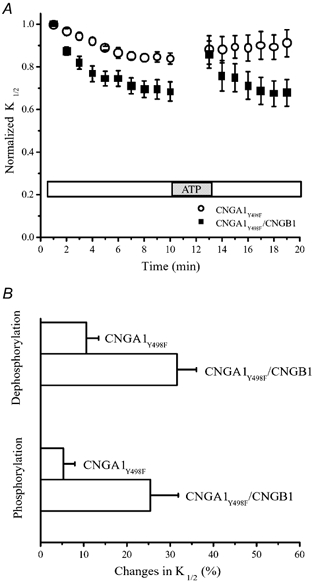
A, changes in cGMP sensitivity after patch excision and following ATP application for CNGA1Y498F and CNGA1Y498F/CNGB1 channels. B, changes in the channel sensitivity as a result of phosphorylation and dephosphorylation.
Does the modulation conferred by the CNGB1 subunit really result from changes in tyrosine phosphorylation, like the modulation mediated by the CNGA1 subunit? To address this question, we used inhibitors of PTKs and PTPs. To maintain channels in their phosphorylated state, immediately after patch excision CNGA1Y498F/CNGB1 channels were exposed to 100 μM vanadate, a PTP inhibitor. The increase in the cGMP sensitivity usually observed after patch excision was almost completely abolished under these conditions (n =10) (Fig. 4A), suggesting that this does indeed result from tyrosine dephosphorylation. To determine whether PTKs also regulate the cGMP sensitivity of these channels, excised patches were continuously exposed either to 500 μM ATP alone (n = 10), presumably maintaining the channels in a phosphorylated state, or to ATP plus 25 μM erbstatin, a PTK inhibitor (n = 8) (Fig. 4B). As expected, the cGMP sensitivity exhibited a gradual increase in the presence of erbstatin, because under these conditions PTPs can effectively dephosphorylate the channels while the opposing phosphorylation reaction is blocked. Hence, modulation mediated solely by the CNGB1 subunits appears to involve PTPs and PTKs, like modulation mediated by CNGA1 subunits.
Figure 4. The CNGB1 subunit-mediated changes in cGMP sensitivity are due to modulation by tyrosine phosphorylation/dephosphorylation.
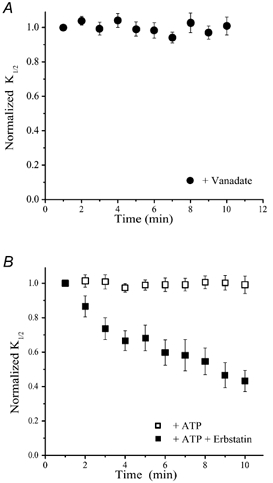
A, changes in the cGMP sensitivity after excision of membrane patches containing CNGA1Y498F/CNGB1 channels in the presence of a PTP inhibitor, vanadate (100 μM). B, changes in the cGMP sensitivity after excision of membrane patches containing CNGA1Y498F/CNGB1 channels in the presence of erbstatin (25 μM), a PTK inhibitor, and/or 500 μM ATP.
Identification of the crucial tyrosine phosphorylation site in the CNGB1 subunit
Amino acid sequence homology indicates that the CNGB1 subunit has a tyrosine at position 1097, the amino acid position equivalent to Y498F in the CNGA1 subunit. To test whether this tyrosine is important for modulation, site-directed mutagenesis was utilized to replace this tyrosine with phenylalanine (CNGB1Y1097F). The mutant CNGB1 subunit was then co-expressed with the corresponding mutant CNGA1 subunit (CNGA1Y498F). The resulting double mutant channels (CNGA1Y498F/ CNGB1Y1097F) exhibited a small change in cGMP sensitivity following patch excision and no change after ATP application (Fig. 5A), but these effects were indistinguishable from those exhibited by homomeric CNGA1Y498F channels. The change in sensitivity attributed to dephosphorylation was 10.6 ± 2.8 % (n = 10) for the double mutant heteromeric channel and 12.1 ± 2.6 % (n = 14) for the homomeric channel, and neither channel was significantly affected by ATP application (P < 0.05) (Fig. 5B). Hence, replacement of a single amino acid (Y1097) in the CNGB1 subunit eliminates its contribution to modulation, identifying Y1097 as a crucial site for regulating dynamic changes in channel sensitivity.
Figure 5. Identification of Y1097 as a site crucial for modulation by phosphorylation on the CNGB1 subunit.
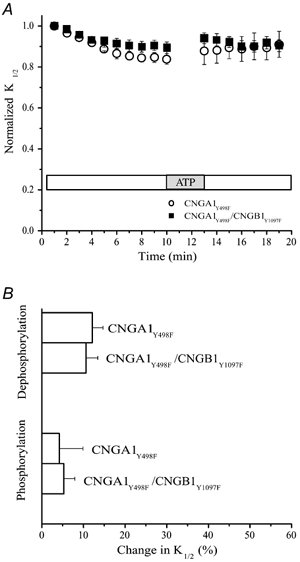
A, changes in the cGMP sensitivity after patch excision and following ATP application for CNGA1Y498F and CNGA1Y498F/CNGB1Y1097F channels. B, changes in the channel sensitivity as a result of phosphorylation and dephosphorylation.
Biochemical demonstration of CNG channel phosphorylation
Studies utilizing inhibitors of PTPs and PTKs, as well as site-directed mutagenesis to localize putative phosphorylation sites, provide strong evidence that both the CNGA1 and CNGB1 subunits of the rod CNG channel are regulated by tyrosine phosphorylation. However, until now, there has been no direct biochemical evidence for tyrosine phosphorylation, or indeed for any type of phosphorylation, of the rod channel. To detect phosphorylation of the channel, Xenopus oocytes expressing homomeric or heteromeric CNG channels were incubated for 24 h in a solution containing 32P-orthophosphate. Channel subunits were His6 tagged, allowing for purification of channel subunits with Ni2+-NTA agarose beads. Western blots, using anti-His6 antibodies, confirmed the expression of individual channel subunits, and autoradioagraphy was used to detect phosphorylation (Fig. 6).
Figure 6. Biochemical demonstration of tyrosine phosphorylation of CNGA1 and CNGB1 subunits.
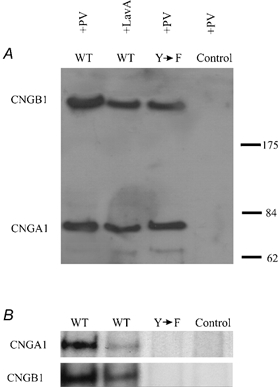
A, Western blot analysis (anti-His6) of wild-type (WT; CNGA1 and CNGB1) and mutant (Y→F; CNGA1Y498F and CNGB1Y1097F) channel subunits. Before homogenization, oocytes expressing heteromeric CNG channels and non-injected oocytes (Control) were pretreated with pervanadate (PV), a PTP inhibitor, or lavendustin A (LavA), a PTK inhibitor. Molecular mass markers (in kDa) are shown on the right. B, autoradiograms of wild-type (CNGA1 and CNGB1) and mutant (CNGA1Y498F and CNGB1Y1097F) subunits.
32P labelling of the CNGA1 subunit was increased by incubation of oocytes with 500 μM pervanadate for 1 h, to inhibit tyrosine dephosphorylation by PTPs, whereas incubation with lavendustin A, to block PTK activity, reduced labelling (n = 10). The mutant CNGA1Y498F subunit exhibited no detectable labelling even with pervanadate incubation (n = 3), consistent with Y498 being the major phosphorylation site. To examine phosphorylation of CNGB1 subunits, His6-tagged CNGB1 subunits were co-expressed with CNGA1 subunits to form heteromeric channels. CNGB1 subunits purified from the heteromeric channels did indeed display 32P labelling that was enhanced in pervanadate and blocked by lavendustin A (n = 3). Moreover, CNGB1Y1097F subunits did not exhibit labelling (n = 3). Hence Y498 in CNGA1 and Y1097 in CNGB1 are two major phosphorylation sites in the rod CNG channel.
Modulation by IGF-1
IGF-1 increases the amplitude of the rod light response by modulating the sensitivity of CNG channels to cGMP (Savchenko et al. 2001). Xenopus oocytes possess their own native IGF-1 receptors (Scavo et al. 1991; Zhu et al. 1998) and previous studies show that pretreatment of oocytes expressing homomeric CNGA1 channels leads to an increase in their cGMP sensitivity, measured immediately after patch excision (Savchenko et al. 2001). Figure 7A shows that incubation for 1 h with 1 μM IGF-1 resulted in a significant decrease (31.2 ± 3.2 %, n = 10) in the initial K1/2 for cGMP in wild-type CNGA1 channels (P < 0.05), but had no effect on mutant CNGA1Y498 channels, which are missing the crucial phosphorylation site. To determine the role of the CNGB1 subunit in IGF-1 modulation, we used oocytes expressing wild-type or mutant heteromeric channels with different numbers of putative phosphorylation sites. For wild-type heteromeric CNGA1/CNGB1 channels, which contain four phosphorylation sites, the K1/2 for cGMP activation was 117.4 ± 4.3 μM (n = 22) in control oocytes and 89.1 ± 3.3 μM (n = 8) in oocytes treated with IGF-1, a moderate increase in sensitivity (26.2 ± 3.7 %, n = 8). However, IGF-1 had a much larger effect on heteromeric CNGA1Y498F/CNGB1 channels (61.1 ± 4.2 %, n = 9), which have only one phosphorylation site, on the CNGB1 subunit. Without IGF-1, the initial K1/2 value for cGMP activation of these channels was 121.3 ± 5.5 μM (n = 24), but with IGF-1 pre-treatment the value decreased to 48.4 ± 2.5 μM (n = 9). In contrast, IGF-1 pre-treatment had no significant effect when neither CNGA1 nor CNGB1 subunits had phosphorylation sites (CNGA1Y498F/CNGB1Y1097F). These results show that the phosphorylation site on the CNGB1 subunit is important not only for modulation mediated by constitutively active PTPs and PTKs in oocytes but also for modulation triggered by the IGF-1 signalling cascade.
Figure 7. Insulin like growth factor-1 (IGF-1) triggers phosphorylation-dependent modulation of the CNGB1 subunit.
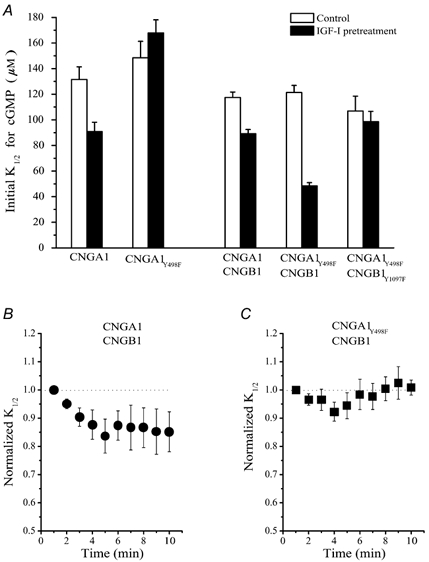
A, the apparent affinity for cGMP measured immediately after patch excision in control patches and patches pre-treated with 1 μM IGF-1 for 1 h. B, changes in the cGMP sensitivity in CNGA1/CNGB1 channels after excision of patches pre-treated with 1 μM IGF-1 for 1 h. C, changes in cGMP sensitivity of CNGA1Y498F/CNGB1 channels after excision of patches pre-treated with 1 μM IGF-1 for 1 h.
We were intrigued by the fact that the magnitude of the effect of IGF-1 in CNGA1Y498F/CNGB1 channels was higher than in wild-type CNGA1/CNGB1 channels. We found that IGF-1 did not completely dephosphorylate wild-type CNGA1/CNGB1 channels. The sensitivity of CNGA1/CNGB1 channels increased further by about 15 % after patch excision and removal of IGF-1, due to the action of constitutively active PTPs (Fig. 7B). In contrast, the sensitivity of CNGAY498F/CNGB1 channels did not change with time after patch excision, consistent with the idea that they were already fully dephosphorylated in response to IGF-1 (Fig. 7C).
Why does the effectiveness of IGF-1 in triggering dephosphorylation differ between the two channels? Previous work on homomeric CNGA1 channels showed that the rate of dephosphorylation by PTPs is dependent on the activation state of the channel, with channel opening favouring a faster dephosphorylation rate, and channel closing favouring a faster phosphorylation rate (Molokanova et al. 1999). In normal saline, CNG channels in intact oocytes have a low open probability (authors’ unpublished observations; also see Tibbs et al. 1997), so dephosphorylation triggered by pre-treating oocytes with IGF-1 should occur very slowly, and may be incomplete. If the activity dependence for CNGB1 subunits was different from that for CNGA1 subunits, the rate and extent of dephosphorylation in response to IGF-1 might also be different, possibly accounting for the difference in behaviour of CNGA1/CNGB1 versus CNGA1Y498F/CNGB1 channels.
To test this hypothesis, we compared the activity dependence of dephosphorylation of homomeric CNGA1 channels, in which all the phosphorylation sites are on the CNGA1 subunit, and heteromeric CNGA1Y498F/CNGB1 channels, in which the only phosphorylation site is on the CNGB1 subunit. Each type of channel was either kept open by applying saturating (2000 μM) cGMP or kept closed during a 10 min treatment period by using a solution devoid of cGMP. After a 10 min treatment period, the cGMP sensitivity of the channels in each group was compared to that obtained immediately after excising the patch (Fig. 8A). Homomeric CNGA1 channels behaved as described previously, with modulation resulting from dephosphorylation being greatest (2-fold increase in sensitivity) in open channels and least (about 10 % increase) in closed channels.
Figure 8. The activity dependence of modulation of CNGB1 subunits is different from that of CNGA1 subunits.
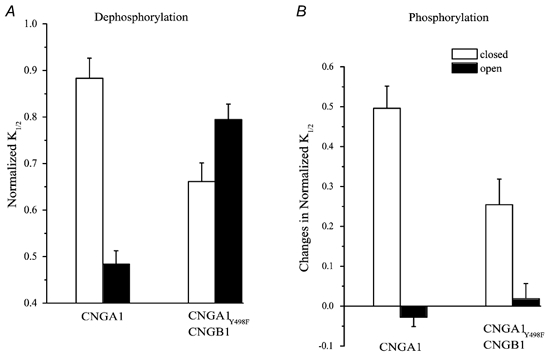
A, changes in cGMP sensitivity after patch excision elicited during a 10 min treatment period when channels were either kept open, by applying saturating cGMP, or kept closed, by using a cGMP-free solution. B, changes in cGMP sensitivity during phosphorylation were estimated by normalizing the change in K1/2 evoked by a 3 min application of 500 μM ATP to the K1/2 before ATP application in the presence or absence of saturating cGMP.
To test whether the CNGB1 subunit exhibits activity dependence, we examined mutant heteromeric channels containing wild-type CNGB1 subunits and CNGA1 subunits that are missing their phosphorylation site (CNGA1Y498F/CNGB1). Remarkably, the activity dependence of dephosphorylation of these channels, which contain phosphorylation sites only on CNGB1 subunits, was opposite to that seen with homomeric channels, which contain phosphorylation sites only on CNGA1 subunits. CNGA1Y498F/CNGB1 channels only exhibited spontaneous modulation reflecting tyrosine dephosphorylation in the absence of cGMP, when channels are closed.
In contrast to dephosphorylation, the activity dependence of phosphorylation of CNGA1Y498F/CNGB1 channels was similar to that observed in homomeric CNGA1 channels (Fig. 8B). Application of ATP on either type of channel in the closed state resulted in an increase in the K1/2 for cGMP. However, in both types of channel, keeping the channels open during ATP treatment, by applying saturating cGMP, eliminated changes in channel sensitivity. Hence, in both CNGA1 and CNGB1 subunits the PTK is apparently unable to catalyse spontaneous phosphorylation of subunits in their open state.
How can the difference in activity dependence of dephosphorylation of CNGA1 and CNGB1 subunits account for differences in IGF-1 modulation? For IGF-1 to be effective during pretreatment of intact oocytes, dephosphorylation must be possible from the closed state of the channel. Thus, CNGA1 subunits, which are dephosphorylated less efficiently when kept closed, are not affected by IGF-1 to the full extent, whereas CNGB1 subunits should be dephosphorylated efficiently from the closed state.
DISCUSSION
Native rod CNG channels contain CNGA1 and CNGB1 subunits with a 3:1 stoichiometry (Zhong et al. 2002; Weitz et al. 2002; Zheng et al. 2002). The basic architecture of CNGA1 and CNGB1 subunits is similar, but there are structural differences that have important functional implications. CNGB1, but not CNGA1, possesses a large, 572 amino acid cytoplasmic NH2 terminal glutamic acid-rich protein (GARP) region that may be important for channel localization (Chen et al. 1994). CNGB1 contains a Ca2+-calmodulin-binding site not present on the CNGA1 subunit (Grunwald et al. 1998). The CNGB1 subunit uniquely contains a region near the first membrane-spanning segment that regulates surface expression of heteromeric CNGA1/CNGB1 channels (Trudeau & Zagotta, 2002). The CNGB1 subunit cannot form functional homomeric channels but can contribute to heteromeric channels formed in conjunction with CNGA1. Heteromeric CNGA1/CNGB1 channels differ from homomeric CNGA1 channels in having a smaller single channel conductance, more ‘flickery’ single channel gating (Chen et al. 1993; Biel et al. 1996), increased sensitivity to activation by cAMP (Gordon et al. 1996; Pages et al. 2000) and higher sensitivity to block by L-cis-diltiazem. Moreover, heteromeric CNGA1/CNGB1 channels are inhibited by Ca2+-calmodulin binding, whereas homomeric CNGA1 channels are unaffected.
CNGA1 and CNGB1 subunits also have different protein partners in rod outer segments. Our previous studies with genistein, a PTK inhibitor, suggest that CNGA1 subunits, but not CNGB1 subunits, are ‘intimately’ associated with PTKs (Molokanova et al. 2000). Biochemical studies show that the CNGA1 subunit but not the CNGB1 subunit in native rod CNG channels binds to the Na+-Ca2+-K+ exchanger (Bauer & Drechsler, 1992; Schwarzer et al. 2000; Poetsch et al. 2001). The GARP region of the CNGB1 subunit organizes a protein complex including phosphodiesterase and guanylate cyclase, presumably controlling local cGMP turnover (Korschen et al. 1999). GARP also interacts with peripherin-2 in the rim region of outer segment disc membranes, interconnecting the plasma and disc membranes (Poetsch et al. 2001).
Our previous studies clearly implicated CNGA1 in the modulation by tyrosine phosphorylation of rod CNG channels (Molokanova et al. 1997, 1999). However, since the native channels are heteromeric, it is important to ask whether CNGB1 also plays a role. Modulation persists when CNGB1 subunits are co-expressed with other subunits incapable of modulation by tyrosine phosphorylation (CNGA2 or CNGA1Y498F), indicating that that the CNGB1 subunit must indeed contribute to the modulation. More specifically, a single tyrosine in the CNGB1 subunit (Y1097), in the position homologous to Y498 of the CNGA1 subunit, is crucial for modulation of heteromeric channels.
Despite extensive study by several laboratories, direct biochemical demonstration of CNG channel phosphorylation has been lacking. Rod outer segments contain high levels of protein kinases often implicated in ion channel modulation, including cAMP-dependent protein kinase and protein kinase C (Yarfitz & Hurley, 1994), but there has been only negative evidence for these particular enzymes being involved in modulating the activity of the rod CNG channel (Xiong et al. 1997; Jindrova & Detwiler, 1998). The evidence for modulation has relied exclusively on functional alterations in channel activity in response to the addition of more general protein kinase or phosphatase inhibitors (Gordon et al. 1992; Molokanova et al. 1997), or to exogenous application of protein phosphatases (Gordon et al. 1992; Savchenko et al. 2001). Here we provide direct biochemical evidence that both the CNGA1 and CNGB1 subunits, at least when they are expressed in oocytes, can be phosphorylated and that the same amino acids crucial for modulation are the primary phosphorylation sites. Preliminary results suggest that the CNGA1 subunit of native rod CNG channels can also be phosphorylated (D. Satpaev & R. H. Kramer, unpublished results). Previous difficulties in biochemically demonstrating phosphorylation of the rod CNG channel may have resulted from a high basal activity of PTPs and the small number of sites on each channel subunit (apparently only one).
The crucial tyrosine phosphorylation sites in CNGA1 and CNGB1 are conserved in many other channels containing cyclic nucleotide-binding sites, including CNGA3 from cones and all four of the known hyperpolarization-activated, cyclic nucleotide-gated (HCN) channels (for review see Richards & Gordon, 2000; Chen et al. 2002). All of the other cyclic nucleotide-binding channels (e.g. CNGA2 and CNGB2) contain a phenylalanine instead of a tyrosine at this position. The invariant presence of these very similar residues in every channel subunit suggests that this part of the sequence is crucial for channel function and that an aromatic amino acid must be present. Moreover, the presence of a tyrosine may be a useful indicator that a given subunit has the capacity to be modulated by extrinsic factors that signal through tyrosine kinase or phosphatase cascades, such as IGF-1. Hence, perhaps olfactory receptor neurons, thalamic pacemaker neurons and cardiac myocytes, all of which contain channels with the conserved tyrosine, can be acutely modulated by growth factors in a manner similar to that observed in rod photoreceptors.
The phosphorylation site of both the CNGA1 and CNGB1 subunits lies on the S6-side of the conserved cyclic nucleotide-binding domain. The structure of the cyclic nucleotide-binding site has been inferred from the crystal structure of the bacterial cAMP-binding protein catabolite gene-activating protein (Weber & Steitz, 1987). In this protein, the tyrosine equivalent to CNGA1Y498 and CNGB1Y1097 is located in the first segment of the eight-stranded β-roll structure, with the side group predicted to face away from the cyclic nucleotide contact binding pocket. While this position is not ideal for directly influencing cyclic nucleotide binding, it may be pivotal for regulating the coupling between ligand occupancy and movement of the channel gate, which is thought to involve the S6 segment. It is easy to imagine how phosphorylation of amino acids in protein regions that couple ligand binding to channel gating would influence the apparent affinity for cyclic nucleotides.
IGF-1 modulates the magnitude and kinetics of the rod light response, at least in part by triggering tyrosine dephosphorylation of the rod CNG channel (Savchenko et al. 2001). The effect of IGF-1 on native CNG channels in rods can be blocked by adding a PTP inhibitor and mimicked and occluded by applying exogenous PTPs. Application of IGF-1 on Xenopus oocytes expressing homomeric CNGA1 channels results in an increase in cGMP sensitivity, but if the crucial tyrosine is missing (CNGA1Y498), IGF-1 has no effect. We have now shown that CNGB1 subunits also participate in IGF-1 modulation, with a homologous tyrosine (Y1097) being crucial for IGF-1 action. Surprisingly, IGF-1 has a greater effect on cGMP sensitivity for CNGA1Y498F/CNGB1 channels than for homomeric CNGA1 channels, even though the latter channels should have twice as many tyrosine phosphorylation sites. In search of an explanation, we discovered an interesting difference in the activity-dependent behaviour of CNGA1 and CNGB1 subunits.
The modulation of rod CNG channels by PTPs and PTKs in Xenopus oocytes is strongly dependent on the activation state of the channel. Homomeric CNGA1 channels preferentially interact with PTPs and become dephosphorylated when they are opened by cGMP, and preferentially interact with PTKs and become phosphorylated when they are closed in the absence of cGMP. The activity dependence of phosphorylation and dephosphorylation of CNGB1 subunits cannot be examined in isolation from CNGA1 because CNGB1 by itself is not capable of forming functional channels. However, the activity dependence of the CNGB1 subunit can be assessed using mutant channels (CNGA1Y498F/ CNGB1), in which the one remaining tyrosine phosphorylation site is on the CNGB1 subunit. In these channels, both PTKs and PTPs are effective when cGMP is present and both are ineffective when cGMP is absent. Thus phosphorylation and dephosphorylation are affected in parallel by channel activation and the CNGB1 subunit has no preference for the PTK or PTP in either the open or closed states. The net result is that modulation of the CNGB1 subunit does not exhibit activity dependence. We propose that the reason that IGF-1 fails to fully modulate homomeric CNGA1 channels is that the channels are closed during IGF-1 pre-treatment of intact oocytes, thus the CNGA1 subunits are in an unfavourable state for IGF-1-triggered dephosphorylation. In contrast, IGF-1 completely modulates the CNGA1Y498F/CNGB1 channels, because the CNGB1 subunits do not have an unfavourable state for tyrosine dephosphorylation.
Even though there is only one CNGB1 subunit per native rod CNG channel, the lack of activity dependence of CNGB1 dephosphorylation potentially allows the channels to respond to IGF-1, independent of the activation state of the channel. In this scenario, exposure to IGF-1 will initiate tyrosine dephosphorylation of CNGB1, which increases the channel sensitivity to cGMP, leading to an increase in the probability of channel opening and, as a result, further dephosphorylation of the CNGA1 subunit and an increase in cGMP sensitivity. Removal of IGF-1 should reverse the process, turning off the PTP and allowing the CNGB1 subunit to become phosphorylated by PTKs. This should decrease the cGMP sensitivity of the channel, reducing the open probability and allowing for more complete phosphorylation of CNGA1 subunits. A test of this hypothesis awaits further physiological and biochemical experiments on native rod CNG channels.
Many questions remain about the assembly and mechanism of activation of heteromeric CNG channels, the influence of each of the subunits on heteromeric channel gating and ligand sensitivity, and the impact of proteins (e.g. PTKs and PTPs) that interact with either subunit on channel behaviour. It now seems clear that the rod CNG channel is an asymmetric channel composed of three CNGA1 subunits and one CNGB1 subunit (Zhong et al. 2002; Weitz et al. 2002; Zheng et al. 2002). However, three-dimensional cryo-electron microscopic reconstruction of CNG channels from bovine rods suggests a bilateral symmetry (Higgins et al. 2002), consistent with a ‘dimer of dimers’ model of gating (Liu et al. 1998). This implies that CNGA1 subunits can form a homophilic partnership with another CNGA1 subunit or a heterophilic partnership with a CNGB1 subunit. How do these different partnerships participate in gating? Is dephosphorylation of CNGA1 independent of or interdependent on the dephosphorylation of CNGB1 subunits? Does the dephosphorylation of the two subunits exhibit cooperativity as in the case of cGMP-dependent activation? Are CNGA1 and CNGB1 subunits modulated by phosphorylation or dephosphorylation in a consecutive or alternating manner? These are all interesting questions that await further study.
Numerous extracellular and intracellular signalling cascades converge on ion channels to fine tune their activity. Along with tyrosine phosphorylation, rod CNG channels are subject to modulation by serine/threonine phosphorylation, transition metals, phospholipid metabolites and Ca2+-calmodulin (reviewed in Kramer & Molokanova, 2001). It would be interesting to explore whether these forms of modulation are independent from each other or share a common molecular mechanism that could result in the interaction of several distinct modulation mechanisms at the level of CNG channels.
Acknowledgments
We thank Joseph Aslan and Floyd Maddox for excellent technical assistance. This work was supported by grants from the National Institutes of Health (EY12608 to R.H.K., and DA08102 to C.W.L.), and NRSA Postdoctoral Fellowship (5 F32 EY13506) to J.L.K.
REFERENCES
- Bauer PJ, Drechsler M. Association of cyclic GMP-gated channels and Na+-Ca2+-K+ exchangers in bovine retinal rod outer segment plasma membranes. J Physiol. 1992;451:109–131. doi: 10.1113/jphysiol.1992.sp019156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel M, Zong X, Ludwig A, Sautter A, Hofmann F. Molecular cloning and expression of the modulatory subunit of the cyclic nucleotide-gated cation channel. J Biol Chem. 1996;271:6349–6355. doi: 10.1074/jbc.271.11.6349. [DOI] [PubMed] [Google Scholar]
- Bradley J, Frings S, Yau KW, Reed R. Nomenclature for ion channel subunits. Science. 2001;294:2095–2096. doi: 10.1126/science.294.5549.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TY, Illing M, Molday LL, Hsu YT, Yau KW, Molday RS. Subunit 2 (or beta) of retinal rod cGMP-gated cation channel is a component of the 240-kDa channel-associated protein and mediates Ca2+-calmodulin modulation. Proc Natl Acad Sci U S A. 1994;91:11757–11761. doi: 10.1073/pnas.91.24.11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TY, Peng YW, Dhallan RS, Ahamed B, Reed RR, Yau KW. A new subunit of the cyclic nucleotide-gated cation channel in retinal rods. Nature. 1993;362:764–767. doi: 10.1038/362764a0. [DOI] [PubMed] [Google Scholar]
- Chen J, Piper DR, Sanguinetti MC. Voltage sensing and activation gating of HCN pacemaker channels. Trends Cardiovasc Med. 2002;12:42–45. doi: 10.1016/s1050-1738(01)00141-4. [DOI] [PubMed] [Google Scholar]
- Dhallan RS, Yau KW, Schrader KA, Reed RR. Primary structure and functional expression of a cyclic nucleotide-activated channel from olfactory neurons. Nature. 1990;347:184–187. doi: 10.1038/347184a0. [DOI] [PubMed] [Google Scholar]
- Finn JT, Krautwurst D, Schroeder JE, Chen TY, Reed RR, Yau KW. Functional co-assembly among subunits of cyclic-nucleotide-activated, nonselective cation channels, and across species from nematode to human. Biophys J. 1998;74:1333–1345. doi: 10.1016/S0006-3495(98)77846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SE, Brautigan DL, Zimmerman AL. Protein phosphatases modulate the apparent agonist affinity of the light-regulated ion channel in retinal rods. Neuron. 1992;9:739–748. doi: 10.1016/0896-6273(92)90036-d. [DOI] [PubMed] [Google Scholar]
- Gordon SE, Downing-Park J, Zimmerman AL. Modulation of the cGMP-gated ion channel in frog rods by calmodulin and an endogenous inhibitory factor. J Physiol. 1995;486:533–546. doi: 10.1113/jphysiol.1995.sp020832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SE, Oakley JC, Varnum MD, Zagotta WN. Altered ligand specificity by protonation in the ligand binding domain of cyclic nucleotide-gated channels. Biochemistry. 1996;35:3994–4001. doi: 10.1021/bi952607b. [DOI] [PubMed] [Google Scholar]
- Grunwald ME, Yu WP, Yu HH, Yau KW. Identification of a domain on the beta-subunit of the rod cGMP-gated cation channel that mediates inhibition by calcium-calmodulin. J Biol Chem. 1998;273:9148–9157. doi: 10.1074/jbc.273.15.9148. [DOI] [PubMed] [Google Scholar]
- Higgins MK, Weitz D, Warne T, Schertler GF, Kaupp UB. Molecular architecture of a retinal cGMP-gated channel: the arrangement of the cytoplasmic domains. EMBO J. 2002;21:2087–2094. doi: 10.1093/emboj/21.9.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YT, Molday RS. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature. 1993;361:76–79. doi: 10.1038/361076a0. [DOI] [PubMed] [Google Scholar]
- Jindrova H, Detwiler PB. Protein kinase C and IP3 in photoresponses of functionally intact rod outer segments: constraints about their role. Physiol Res. 1998;47:285–290. [PubMed] [Google Scholar]
- Kaupp UB, Niidome T, Tanabe T, Terada S, Bonigk W, Stuhmer W, Cook NJ, Kangawa K, Matsuo H, Hirode T, Miyata T, Numa S. Primary structure and functional expression from complementary DNA of the rod photoreceptor cGMP-gated channel. Nature. 1989;342:762–766. doi: 10.1038/342762a0. [DOI] [PubMed] [Google Scholar]
- Koerschen HG, Illing M, Seifert R, Sesti F, Williams A, Gotzes S, Colville C, Muller F, Dose A, Godde M, Molday L, Kaupp UB, Molday RS. A 240 kDa protein represents the complete beta subunit of the cyclic nucleotide-gated channel from rod photoreceptor. Neuron. 1995;15:627–636. doi: 10.1016/0896-6273(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Kramer RH, Molokanova E. Modulation of cyclic-nucleotide-gated channels and regulation of vertebrate phototransduction. J Exp Biol. 2001;204:2921–2931. doi: 10.1242/jeb.204.17.2921. [DOI] [PubMed] [Google Scholar]
- Liman ER, Tytgat J, Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- Liu DT, Tibbs GR, Paoletti P, Siegelbaum SA. Constraining ligand-binding site stoichiometry suggests that a cyclic nucleotide-gated channel is composed of two functional dimers. Neuron. 1998;21:235–248. doi: 10.1016/s0896-6273(00)80530-9. [DOI] [PubMed] [Google Scholar]
- Molokanova E, Maddox F, Luetje CW, Kramer RH. Activity-dependent modulation of rod photoreceptor cyclic nucleotide-gated channels mediated by phosphorylation of a specific tyrosine residue. J Neurosci. 1999;19:4786–4795. doi: 10.1523/JNEUROSCI.19-12-04786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molokanova E, Savchenko A, Kramer RH. Interactions of cyclic nucleotide-gated channel subunits and protein tyrosine kinase probed with genistein. J Gen Physiol. 2000;115:685–696. doi: 10.1085/jgp.115.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molokanova E, Trivedi B, Savchenko A, Kramer RH. Modulation of rod photoreceptor cyclic nucleotide-gated channels by tyrosine phosphorylation. J Neurosci. 1997;17:9068–9076. doi: 10.1523/JNEUROSCI.17-23-09068.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages F, Ildefonse M, Ragno M, Crouzy S, Bennett N. Coexpression of alpha and beta subunits of the rod cyclic GMP-gated channel restores native sensitivity to cyclic AMP: role of D604/N1201. Biophys J. 2000;78:1227–1239. doi: 10.1016/S0006-3495(00)76680-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poetsch A, Molday LL, Molday RS. The cGMP-gated channel and related glutamic acid-rich proteins interact with peripherin-2 at the rim region of rod photoreceptor disc membranes. J Biol Chem. 2001;276:48009–48016. doi: 10.1074/jbc.M108941200. [DOI] [PubMed] [Google Scholar]
- Richards MJ, Gordon SE. Cooperativity and cooperation in cyclic nucleotide-gated ion channels. Biochemistry. 2000;39:14003–14011. doi: 10.1021/bi001639i. [DOI] [PubMed] [Google Scholar]
- Savchenko A, Kraft TW, Molokanova E, Kramer RH. Growth factors regulate phototransduction in retinal rods by modulating cyclic nucleotide-gated channels through dephosphorylation of a specific tyrosine residue. Proc Natl Acad Sci U S A. 2001;98:5880–5885. doi: 10.1073/pnas.101524998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavo L, Shuldiner AR, Serrano J, Dashner R, Roth J, De Pablo F. Genes encoding receptors for insulin and insulin-like growth factor I are expressed in Xenopus oocytes and embryos. Proc Natl Acad Sci U S A. 1991;88:6214–6218. doi: 10.1073/pnas.88.14.6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer A, Schauf H, Bauer PJ. Binding of the cGMP-gated channel to the Na/Ca-K exchanger in rod photoreceptors. J Biol Chem. 2000;275:13448–13454. doi: 10.1074/jbc.275.18.13448. [DOI] [PubMed] [Google Scholar]
- Shammat IM, Gordon SE. Stoichiometry and arrangment of subunits in rod cyclic nucleotide-gated channels. Neuron. 1999;23:809–819. doi: 10.1016/s0896-6273(01)80038-6. [DOI] [PubMed] [Google Scholar]
- Tibbs GR, Goulding EH, Siegelbaum SA. Allosteric activation and tuning of ligand efficacy in cyclic-nucleotide-gated channels. Nature. 1997;386:612–615. doi: 10.1038/386612a0. [DOI] [PubMed] [Google Scholar]
- Trudeau MC, Zagotta WN. An intersubunit interaction regulates trafficking of rod cyclic nucleotide-gated channels and is disrupted in an inherited form of blindness. Neuron. 2002;34:197–207. doi: 10.1016/s0896-6273(02)00647-5. [DOI] [PubMed] [Google Scholar]
- Waldbillig RJ, Pfeffer BA, Schoen TJ, Adler AA, Shen-Orr Z, Scavo L, Le Roith D, Chader GJ. Evidence for an insulin-like growth factor autocrine-paracrine system in the retinal photoreceptor-pigment epithelial cell complex. J Neurochem. 1991;57:1522–1533. doi: 10.1111/j.1471-4159.1991.tb06347.x. [DOI] [PubMed] [Google Scholar]
- Weber IT, Steitz TA. Structure of a complex of catabolite gene activator protein and cyclic AMP refined at 2. 5 Å resolution. J Mol Biol. 1987;198:311–326. doi: 10.1016/0022-2836(87)90315-9. [DOI] [PubMed] [Google Scholar]
- Weitz D, Ficek N, Kremmer E, Bauer PJ, Kaupp UB. Subunit stoichiometry of the CNG channel of rod photoreceptors. Neuron. 2002;36:881–889. doi: 10.1016/s0896-6273(02)01098-x. [DOI] [PubMed] [Google Scholar]
- Xiong W, Nakatani K, Ye B, Yau KW. Protein kinase C activity and light sensitivity of single amphibian rods. J Gen Physiol. 1997;110:441–452. doi: 10.1085/jgp.110.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarfitz S, Hurley JB. Transduction mechanisms of vertebrate and invertebrate photoreceptors. J Biol Chem. 1994;269:14329–14332. [PubMed] [Google Scholar]
- Zheng J, Trudeau MC, Zagotta WN. Rod cyclic nucleotide-gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit. Neuron. 2002;36:891–896. doi: 10.1016/s0896-6273(02)01099-1. [DOI] [PubMed] [Google Scholar]
- Zhong H, Molday LL, Molday RS, Yau KW. The heteromeric cyclic nucleotide-gated channel adopts a 3A:1B stoichiometry. Nature. 2002;420:193–198. doi: 10.1038/nature01201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Ohan N, Agazie Y, Cummings C, Farah S, Liu XJ. Molecular cloning and characterization of Xenopus insulin-like growth factor-1 receptor: its role in mediating insulin-induced Xenopus oocyte maturation and expression during embryogenesis. Endocrinology. 1998;139:949–954. doi: 10.1210/endo.139.3.5824. [DOI] [PubMed] [Google Scholar]


