Abstract
Neuro-anatomical studies in the mammalian cochlea have previously identified a subpopulation of approximately 5% of primary auditory neurones, designated type II spiral ganglion neurones (sgnII). These neurones project to outer hair cells and their supporting cells, within the ‘cochlear amplifier’ region. Physiological characterization of sgnII has proven elusive. Whole-cell patch clamp of spiral ganglion neurones in P7-P10 rat cochlear slices provided functional characterization of sgnII, identified by biocytin or Lucifer yellow labelling of their peripheral neurite projections (outer spiral fibres) subsequent to electrophysiological characterisation. SgnII terminal fields comprised multiple outer hair cells and supporting cells, located up to 370 μm basal to their soma. SgnII firing properties were defined by rapidly inactivating A-type-like potassium currents that suppress burst firing of action potentials. Type I spiral ganglion neurones (sgnI), had shorter radial projections to single inner hair cells and exhibited larger potassium currents with faster activation and slower inactivation kinetics, compatible with the high temporal firing fidelity seen in auditory nerve coding. Based on these findings, sgnII may be identified in future by the A-type current. Glutamate-gated somatic currents in sgnII were more potentiated by cyclothiazide than those in sgnI, suggesting differential AMPA receptor expression. ATP-activated desensitising inward currents were comparable in sgn II and sgnI. These data support a role for sgnII in providing integrated afferent feedback from the cochlear amplifier.
Type II spiral ganglion neurones (sgnII) in the mammalian cochlea have remained an enigma. These neurones compriseonly around 5 % of the primary auditory neurone population, but provide exclusive afferent innervation to the majority of sensory hair cells, the outer hair cells (ohc) (Berglund & Ryugo, 1987; Brown, 1987; Echteler, 1992), and supporting cells (Fechner et al. 2001). The central fibres of sgnII project in a frequency-specific distribution to the cochlear nucleus (Berglund & Brown, 1994). SgnII peripheral neurites (outer spiral fibres, osf) travel in the outer spiral bundle (osb) between Deiters’ cells for several hundred microns (Berglund & Ryugo, 1987; Brown, 1987; Echteler, 1992). In the basal (high frequency) region, sgnII innervate mainly outer row (row 3) ohc and supporting cells, and in the apex (lower frequency region) sgnII innervate all three rows (Echteler, 1992; Fechner et al. 2001).
The electromotility of ohc provides the cellular basis of the ‘cochlear amplifier’, the electromechanical process that counteracts viscous damping, and is responsible for the exquisite sensitivity of basilar membrane responses (Ashmore & Kolston, 1994). The ‘amplifier-enhanced’ displacement of the basilar membrane is transduced by inner hair cells (ihc), and encoded by type I spiral ganglion neurones (sgnI; around 95 % of primary auditory neurones). The physiological properties of sgnI have been determined, including their membrane conductances and firing characteristics (Robertson, 1984; Brown, 1994; Lin, 1997; Mo & Davis, 1997; Glowatzki & Fuchs, 2002; Jagger & Housley, 2002; Salih et al. 2002). In contrast, little is known about sgnII beyond their innervation pattern. The lack of information on the function of sgnII reflects their relative scarcity, but also the difficulties of recording in vivo (Robertson, 1984; Brown, 1994; Robertson et al. 1999), and the ambiguities of their identification in vitro (Mo & Davis, 1997). Using a cochlear slice preparation (Jagger et al. 2000), we have now determined the distinct electrophysiological phenotype of unequivocally identified sgnII innervating the cochlear amplifier region.
METHODS
Cochlear slice preparation
Slices were made from cochleae of 7-10 day old (P7-P10) Wistar rats, as described previously (Jagger et al. 2000). Rats were killed by intraperitoneal injection of sodium pentobarbitone (90 mg kg−1). The University of Auckland Animal Ethics Committee approved all procedures. Temporal bones were removed and the cochleae dissected and mounted. Slices were cut on a vibratome (Leica, Heerbrugg, Switzerland) at 400 μm thickness, in order to conserve outer spiral fibres in the outer spiral bundle.
Electrophysiology
Voltage-clamp and current-clamp recordings were obtained from spiral ganglion neurones in neonatal cochlear slices as described previously (Jagger & Housley, 2002; Salih et al. 2002). Cells were imaged using a × 40 water-immersion objective and computer-enhanced infrared differential interference contrast (DIC) optics on an upright microscope (Axioskop, Zeiss, Oberkochem, Germany). Slices were bathed in artificial perilymph solution containing (mM): NaCl 140, KCl 4; MgCl2 2; CaCl2 1.5; Hepes 10; and glucose 10. Patch pipettes were filled with the following solution (mM): KCl 140; NaCl 10; MgCl2 2; Hepes 5; EGTA 5; and glucose 10. In most experiments biocytin (0.5 %) or Lucifer yellow (0.2 %) were added for post hoc confirmation of cell type, by identification of neurite projections to outer hair cells (sgnII) or inner hair cells (sgnI). Currents were recorded with pCLAMP6 software and an Axopatch 200B patch amplifier, lowpass filtered at 1-5 kHz and digitized at 5-20 kHz with a Digidata 1200 series interface (Axon Instruments, Foster City, CA, USA). Voltage errors due to series resistance were compensated at 70-90 % online, and residual errors corrected during analysis. Junction potentials were compensated during analysis. For comparisons between inactivation kinetics of sgnII and sgnI currents, voltage-clamp protocols incorporated a negative pre-pulse (-100 mV, 1000 ms) to maximally enable A-type currents. To construct activation curves the A-type component was isolated from non-inactivating currents by a subtractive voltage protocol. The currents activated by test potentials (200 ms, -80 mV to +60 mV) from a -40 mV holding potential were subtracted from those activated by test potentials (as before) from a -100 mV holding potential. Subtracted currents were normalized between maximum and minimum values. Inactivation curves were constructed by plotting the peak current activated during test potentials (+40 mV, 200 ms), against the potential of pre-conditioning pulses (-120 mV to +60 mV, 1 s). The current was normalized between the maximum outward current activated (following the -120 mV conditioning), and the minimum (non-inactivating) current activated following depolarized conditioning (above 0 mV). Thus, the non-inactivating current components were not included in these analyses. In current-clamp mode, depolarising current was injected at the resting membrane potential (300 ms square-wave pulses, 50 pA increments). The latency to first action potential was measured from the onset of the stimulus waveform to the peak of the first action potential above firing threshold. Analysis used pCLAMP6 (Axon Instruments), and Origin software (Microcal Software Inc., Northampton, MA, USA). Single modified Boltzmann functions were estimated for plots of potassium conductance activation and potassium current, using a least-squares fitting procedure (Origin). Values are given throughout as means ± standard deviation (S.D.). Comparisons were carried out using Student's unpaired or paired t test (Origin), with a probability level less than 0.05 considered significant.
4-Aminopyridine (4AP) was made as a stock solution in dimethyl sulfoxide (DMSO), and diluted such that the final DMSO concentration was ≤ 0.1 %. Ligand-gated channel activators were dissolved directly in artificial perilymph, and applied from a pressure pipette held within 50 μm of the soma (ATP), or by bath application (glutamate and cyclothiazide; 2-3 ml min−1). Reagents were obtained from Sigma unless stated. Videomicrographs of all cells were taken before recordings, stored on sVHS videotape and the cross-sectional soma area was computed (0.05 μm2 resolution) with Image Pro Plus software (Media Cybernetics, Silver Spring, MD, USA). All experiments were carried out at room temperature (20-25 °C).
Detection of whole-cell labelling
Cochlear slices containing cells filled with biocytin were fixed in 4 % paraformaldehyde (PFA) for 40 min. Endogenous peroxidase activity was quenched (50 % methanol + 1 % H2O2 in distilled water for 40 min), and slices permeabilized (1 % Triton-X in PBS for 40 min). Slices were incubated in fresh avidin-biotin complex (Vector Laboratories) for 40 min, and washed in PBS. The biocytin labelling was then resolved using nickel-intensified diaminobenzidine (DAB) solution with H2O2 (Vector Laboratories). Slices were dehydrated in an ethanol series, cleared and mounted in methyl-salicylate, or cleared in xylene and mounted in Histomount. Digital microscopy, including extended focus of low-magnification images and outer spiral fibre length measurements, was carried out using Axiovision (Zeiss) and Image Pro Plus software. Cochlear slices containing cells filled with Lucifer yellow were fixed in 4 % PFA for 40 min. Slices were washed in PBS and then examined under epifluorescence (excitation 450-490 nm, emission measured > 520 nm) on the Axioskop microscope (Zeiss), or with confocal fluorescence (TCS SP2, Leica; excitation 458/476 nm, emission 490-600 nm).
RESULTS
Using 400 μm thick cochlear slices that presented the circumferential ohc region, whole-cell voltage clamp and current clamp recordings were made in situ from spiral ganglion neurones. Prior to commencing this study we had confirmed that this slice configuration retained sgnII with intact peripheral neurites. This was achieved using peripherin immunolabelling (D. Jagger & G. Housley, unpublished data). Peripherin is a neuronal intermediate filament protein which demarcates sgnII from sgnI from around P4 in the rat cochlea (Hafidi et al. 1993). In this study, sgnII identification was achieved by imaging the label (biocytin or Lucifer yellow) included in the pipette solution during the electrophysiological characterisation. Neurones were confirmed as sgnII on the determination of their traced outer spiral fibres (Fig. 1A and B), within the osb in the ohc region (Fig. 1C). SgnI were identified by their short radial fibres projecting to a single ihc (Fig. 2). Most of the cells could not be securely classified, as their peripheral neurites could not be located.
Figure 1. Biocytin and Lucifer yellow labelling confirmed the identity of type II spiral ganglion neurones by resolving their outer spiral fibres.
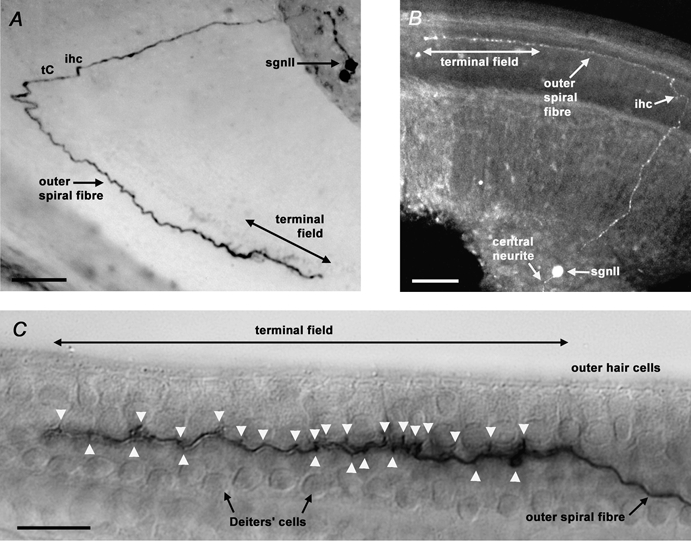
A, extended-focus view of a biocytin-filled type II spiral ganglion neurone (sgnII) in the mid-apical region of a P8 slice, revealing an outer spiral fibre travelling 340 μm in the basal direction from the inner hair cell (ihc) region and the tunnel of Corti (tC). Ten outer hair cells (ohc) in rows 1 and 2 were innervated within the terminal field. The other cell labelled in the ganglion had type I neurone-like (sgnI) properties, but had no neurites evident. Scale bar = 50 μm. B, confocal optical section reconstruction of a Lucifer yellow-filled sgnII in the apical region of a P7 slice. Outer spiral fibre length was 310 μm, with 13 ohc contacted in row 1. Scale bar = 50 μm. C, lateral view of the neurite terminal field of a biocytin-filled P9 apical sgnII. Terminals (arrowheads) appear to be onto ohc and Deiters’ cells. 16 ohc in row 1 were innervated by this neurone. Scale bar = 20 μm
Figure 2. Biocytin labelling confirmed the identity of type I spiral ganglion neurones by resolving their radial fibres.

Extended-focus view of a biocytin-filled type I spiral ganglion neurone (sgnI) in the basal region of a P8 slice, revealing a radial fibre innervating a single inner hair cell (ihc). Scale bar = 50 μm. Inset, detail of sgnI dendrite, showing terminal contacts on the modiolar face and the basal pole of the ihc. Scale bar = 20 μm.
A total of eight sgnII (seven mid-apical/apical, one basal) were securely identified. These sgnII had a range of cross-sectional soma areas (94-164 μm2, measured from videomicrographs taken before electrophysiological recordings). The cells could be divided into two groups, those of small area (94-109 μm2, mean 101 ± 6 μm2, n = 4), and those of noticeably larger area (130-164 μm2, mean 148 ± 15 μm2, n = 4). SgnII from the apical region innervated between six and sixteen ohc in the inner row (row 1) and middle row (row 2). The basally directed osf length ranged 120-340 μm for the apical region sgnII, and 370 μm for the single basal region sgnII. An example of a biocytin-filled mid-apical turn sgnII is shown in Fig. 1A, and a more apically located Lucifer yellow-filled sgnII in Fig. 1B. Where a lateral aspect was presented, the outer spiral fibre could be seen travelling underneath the ohc and between the Deiters’ cells, before terminating onto both these cell types via en passant terminals (Fig. 1C, arrowheads). We did not observe collateral connections to Hensen's cells, as seen in the apical turn of the adult guinea-pig cochlea (Fechner et al. 2001). The peripheral neurite of the basal turn sgnII travelled in all three rows of the osb, before innervating nine ohc in the outer row (row 3) only. These observations of peripheral neurite morphology were essential for our secure identification of sgnII.
The whole-cell recordings also identified 17 sgnI, with radial projections innervating single ihc (Fig. 2). There was often branching within the dendritic endings (see Fig. 2 inset), particularly in apical region sgnI at P7-P8. The identified sgnI had measured cross-sectional soma areas ranging from 92-163 μm2 (mean 130 ± 25 μm2), spanning the observed range of sgnII areas. The peripheral and central neurites of identified sgnII were generally thinner than those of sgnI (typically < 0.5 μm, and approximately 1 μm, respectively). We did not undertake an extensive quantitative analysis due to the limited data available. Detailed comparisons of sgnII and sgnI processes have been presented previously (e.g. Berglund & Ryugo, 1987; Brown, 1987; Brown et al. 1988). An additional 19 cells in the present study had an electrophysiological phenotype matching the labelled sgnII (see below), but did not have neurites that could be fully traced. These cells had soma areas ranging 74-168 μm2. We also recorded from 114 untraceable cells with sgnI-like responses (area 83-166 μm2).
Voltage-clamp and current-clamp recordings revealed a consistent electrophysiological phenotype within identified sgnII, which was distinct from the phenotype of identified sgnI. Under voltage clamp the mean zero-current potential (Vz) of sgnII (-59 ± 6 mV, n = 8) was significantly less negative than that of sgnI (-73 ± 7 mV, n = 17; P < 0.05, Student's unpaired t test). While both sgnII and sgnI exhibited inactivating inward sodium and outward potassium currents during depolarising voltage steps (Fig. 3A), the rate of inactivation of the sgnII outward current was noticeably faster (see below). Both cell types exhibited variable amounts of slowly activating inward currents during hyperpolarising voltage steps. The characteristics of the inward sodium currents and the hyperpolarisation-activated inward currents in sgnII and sgnI were not studied in detail. The sgnII had higher input resistance (510 ± 70 MΩ, n = 8) compared with that of sgnI (360 ± 120 MΩ, n = 17; P < 0.05, Student's unpaired t test).
Figure 3. Type II spiral ganglion neurones displayed dominant inactivating outward currents.

A, rapidly inactivating outward currents in type II spiral ganglion neurone (sgnII; left panel, marked *), and outward currents dominated by a slower inactivation time constant in type I spiral ganglion neurone (sgnI; right panel). Transient inward sodium currents are evident at the start of the depolarizing voltage steps, and slowly activating inward currents were evident during hyperpolarizing voltage steps in both cells. B, voltage-dependent activation kinetics of the outward currents in identified sgnII (▪, n = 8) and identified sgnI (○, n = 17). In both cell types the time to peak current decreased with increasing depolarisation. The curves are single exponential fits to the mean data, with 32 mV per efold increase in activation time for sgnII and 80 mV for sgnI.C, relative amplitudes of peak and steady-state (s-s) currents in identified sgnII and sgnI. Currents measured at beginning (peak) and end (s-s) of 200 ms test potentials to +40 mV (holding potential -100 mV). D, outward currents of identified sgnII had shorter primary inactivation time constants (▪) than those of identified sgnI (•). Non-traced neurones with sgnII-like electrical phenotype (□), and sgnI-like electrical phenotype (○) are included for comparison. E, primary inactivation time constants of sgnII and sgnI plotted as a percentage of total inactivation. SgnII currents were dominated by a short primary time constant ( < 55 ms, 64-80 % contribution), whereas sgnI current inactivation showed longer and less dominant primary time constants (>60 ms, 22-69 % contribution). The data in D and E were determined during 2000 ms depolarising steps to +40 mV, following -100 mV conditioning (1000 ms). F, voltage dependence of activation (▪) and steady-state inactivation (□) for A-type currents in the same sgnII shown in A. A-type currents derived by subtraction (see Methods). Activation threshold was close to -50 mV. Activation and inactivation voltage dependence were described by single modified Boltzmann relationships (continuous lines), with half-maximal activation (V0.5) estimated at -12.2 mV (slope factor, k = 12.0 mV per efold change in conductance), and half-maximal inactivation (V0.5) estimated at -76.4 mV (k = 20.0). G, bath-applied 5 mM 4-aminopyridine (4AP) selectively blocked the A-type potassium current in sgnII (2000 ms voltage step to +40 mV, from 1000 ms conditioning potential at -100 mV). In the presence of 4AP, only a ‘residual’ fast-activating sustained outward current remained.
SgnII outward current activation kinetics demonstrated a pronounced voltage dependence (Fig. 3A, left panel). The time-to-peak outward current decreased from a mean of 17.0 ms at -30 mV to 3.0 ms at +50 mV (Fig. 3B). In comparison, sgnI currents had a significantly faster activation rate (mean time-to-peak of 9.8 ms at -30 mV), but less voltage dependency. We have also studied the relative contributions of inactivating and non-inactivating currents in identified sgnII and sgnI (Fig. 3C). A procedure used previously to categorise unidentified neonatal sgn (Jagger & Housley, 2002) allowed calculation of an ‘inactivation index’ for each cell. During maximal outward currents activation (+40 mV test potentials, 200 ms, -100 mV holding potential) the peak current was divided by the pseudo steady-state sustained current at the end of the test potential. Identified sgnII displayed peak currents of 4.5 ± 1.4 nA (n = 8) and sustained currents of 1.6 ± 0.7 nA (n = 8). The identified sgnI displayed significantly larger peak (9.4 ± 2.7 nA, n = 17; P < 0.05, Student's unpaired t test) and sustained (7.0 ± 1.9 nA, n = 17; P < 0.05, Student's unpaired t test) currents. The inactivation index of identified sgnII (3.2 ± 1.1, n = 8) was significantly larger than that of identified sgnI (1.3 ± 0.2, n = 17; P < 0.05, Student's unpaired t test). The highest index for a single sgnI was 1.7, whereas the lowest index for a single sgnII was 2.1. This analysis further demonstrated the distinction between the inactivation characteristics of identified sgnII and sgnI.
The inactivation kinetics of the outward currents in both sgnII and sgnI were best fitted by double exponential functions (measured during +40 mV test potentials). During prolonged voltage steps (2000 ms at +40 mV from -100 mV conditioning potential, not shown) in eight identified sgnII, the primary (short) exponential time constant of 43 ± 11 ms contributed 71 ± 6 % of the total inactivation. In contrast, the primary component of outward current inactivation in 17 identified sgnI was twice as slow (primary time constant 89 ± 21 ms; P < 0.05, Student's unpaired t test), and contributed significantly less to the overall inactivation (39 ± 13 %; P < 0.05, Student's unpaired t test). Data for the populations of identified sgnII and sgnI, and the populations of non-identified neurones, are displayed in Fig. 3D and E. These data show that the outward current inactivation kinetics are a distinct marker for rat sgnII at these ages.
The voltage dependence of activation and steady-state inactivation of the outward current in a single sgnII are shown in Fig. 3F. For all identified sgnII tested, the current activation was half maximal at -10.9 ± 5.2 mV (n = 6). The steady-state inactivation of the outward current was relieved by hyperpolarising conditioning (1000 ms), with half-maximal inactivation at -72.5 ± 15 mV (n = 6). In identified sgnI the steady-state inactivation curve could not be fitted by a single Boltzmann function. Although inactivating currents in sgnI showed a decrease in amplitude following depolarising conditioning, the more dominant sustained (non-inactivating) current also showed a decreased amplitude following depolarized conditioning (not shown). Recovery of the sgnII outward current from inactivation (‘reactivation’, using -100 mV conditioning) followed a bi-exponential time-course, with time constants of 48 ms (contributing 35 % to total recovery) and 450 ms (65 %) in one sgnII, 65 ms (54 %) and 530 ms (46 %) in another (data not shown). In the two identified sgnII tested, the inactivating outward current was blocked 73 % and 69 % by 1 mM 4-aminopyridine (4AP), and was blocked completely in one cell by 5 mM 4AP (Fig. 3G). The isolated 4AP-sensitive A-type current had a primary inactivation time constant of 43 ms (contributing 81 %), and a secondary time constant of 320 ms (19 %; not shown), equivalent to the whole-cell current data described above.
In identified sgnII under voltage-clamp conditions, bath-applied glutamate (100 μM, 25 s, not shown) activated small sustained inward currents at -70 mV (-45 ± 13 pA, n = 4). When glutamate was co-applied with cyclothiazide (CTZ, 100 μM), an inhibitor of AMPA receptor desensitisation (Patneau et al. 1993), these currents were greatly potentiated (-1277 ± 197 pA, n = 4; P < 0.05, Student's paired t test). Slices were pre-incubated with CTZ for 60 s prior to co-application of glutamate (25 s). Similar results were seen in five unconfirmed sgnII. Glutamate-activated inward currents in identified sgnI (-24 ± 10 pA, n = 7) were also potentiated by cyclothiazide (to -253 ± 55 pA, n = 7; P < 0.05, Student's paired t test), but to a lesser degree than for sgnII (P < 0.05; Student's unpaired t test). ATP (100 μM, not shown) applied directly to sgnII activated desensitising inward currents (peak = -278 ± 99 pA, n = 3), which were comparable to those elicited in sgnI (-206 ± 90 pA, n = 9; P > 0.05, Student's unpaired t test). The desensitisation time constant of the sgnII response to ATP was approximately 2 s, as reported previously for sgnI (Jagger et al. 2000; Salih et al. 2002). Inward current responses to both glutamate and ATP were recorded in three identified sgnII and seven unconfirmed sgnII, using sequential drug applications.
In current clamp experiments the resting membrane potential of identified sgnII (-64 ± 3 mV, n = 5) was less negative than that of identified sgnI (-77 ± 5 mV, n =9; P < 0.05, Student's unpaired t test). There was no evidence of spontaneous firing activity at the resting potential in the identified sgnII studied (0/5), but spontaneous firing was observed in some identified sgnI (3/9). The basis of this spontaneous firing was not investigated. In response to depolarising current injections (+50 to +700 pA) at the resting potential, sgnII fired a single, short-latency action potential at the start of the current injection (Fig. 4A, left panel). This was followed by a characteristic slowly depolarising response, which increased in amplitude with increased current injection. In comparison, sgnI fired one or two action potentials at the start of current injections. The slowly depolarising response was absent in all sgnI (Fig. 4A, right panel). The latency to first action potential in identified sgnII (4.8 ± 0.5 ms, n = 5) was not significantly different to that in identified sgnI (4.2 ± 0.8 ms, n = 9; P > 0.05, Student's unpaired t test). Steady-state current- voltage relationships (Fig. 4B) of identified sgnII (n = 4) and identified sgnI (n = 8) reflected the higher input resistance of sgnII.
Figure 4. Outward current inactivation shaped the type II spiral ganglion neurone voltage response.

A, under current clamp conditions, comparison of voltage responses of the type II spiral ganglion neurone (sgnII; left panel) and type I spiral ganglion neurone (sgnI; right panel) demonstrated that sgnII more rapidly recruit input resistance during square wave current injections (100-700 pA) at the resting potential. This was consistent with the inactivation of the dominant outward currents in sgnII. Resting potentials of -64 mV (sgnII) and -77 mV (sgnI) are offset for clarity. B, steady-state current-voltage relationships for groups of sgnII (▪; n = 4 cells) and sgnI (•; n = 8 cells). SgnII showed a steeper slope around the resting potential reflecting their higher input resistance despite lower resting potentials. SgnI showed a pronounced rectification attributable to their greater steady-state voltage-dependent conductance.
DISCUSSION
The properties of type II spiral ganglion neurones (sgnII), and the role they play in mammalian hearing have defied description. Experimental difficulties arise from their relatively low abundance within the spiral ganglion, which has stacked the odds against successful microelectrode recordings (Robertson, 1984; Brown, 1994; Robertson et al. 1999). In addition, sgnII may be selected against by the conditions used in isolated cell preparations (Mo & Davis, 1997). The cochlear slice preparation presents the sgnII somata within the spiral ganglion, with a proportion retaining their peripheral neurites. Although whole-cell recordings undertaken in the ganglion do not give an adequate description of distant mechanisms such as axonal action potentials, or synaptic currents (Glowatzki & Fuchs, 2002), a comparison of membrane properties of the somata of sgnII and type I spiral ganglion neurones (sgnI) has provided significant new data. The characteristics of sgnII identified here contrast significantly with those of sgnI, likely reflecting a difference in the functions of these sub-populations of primary auditory neurones. These experiments revealed that all sgnII maintained a substantial membrane potential, which has not been evident in microelectrode studies (Robertson, 1984; Brown, 1994; Robertson et al. 1999).
We note that studies using animals with an immature auditory system should be interpreted with caution. The functional onset of hearing has been placed between P11 and P14 in rats. Auditory nerve-brainstem (ABR) evoked responses can be first recorded at P11 in response to air-conducted stimuli (Geal-Dor et al. 1993). The period P7-P10 was chosen in the present study as sgnII and sgnI peripheral arborizations are sufficiently differentiated to allow their discrimination (Echteler, 1992; Hafidi et al. 1993). Also, myelination (Romand & Romand, 1985) does not yet preclude whole-cell patch clamp recordings, or hinder visualization of cells within the ganglion. The comparable somatic areas of sgnII and sgnI were unexpected, as sgnII were generally smaller than sgnI in fixed adult rat cochlear tissue (Hafidi, 1998). However, noticeably large sgnII innervating ohc have been observed in the adult mouse cochlea (Berglund & Ryugo, 1987). These anomalies notwithstanding, the significant differences in sgnII and sgnI properties identified in this study (including potassium current inactivation kinetics, resting membrane potential, input resistance, and the cyclothiazide-sensitivity of glutamate-gated currents) mean that there should no longer be ambiguities in identification of rat sgn at these ages. The ability to identify sgnII in mixed neuronal populations, such as in the cochlear slice, should encourage further study.
The glutamate responses of sgnII and sgnI were modulated strongly by cyclothiazide, suggesting an involvement of AMPA-type glutamate receptors (Patneau et al. 1993). AMPA receptors have been shown as the dominant post-synaptic glutamate receptor type in sgnI (Niedzielski & Wenthold, 1995; Matsubara et al. 1996; Glowatzki & Fuchs, 2002). In excitotoxicity studies, AMPA receptor agonists perfused into the cochlea cause osmotic swelling and membrane disruption in sgnI afferent dendrites below ihc (Pujol et al. 1985). Such swelling was absent in the dendrites of adult rat sgnII (Pujol et al. 1985), suggesting a differential expression of AMPA receptors between ihc and ohc afferent synapses, and arguing against glutamate as the ohc/sgnII afferent transmitter. In trial experiments glutamate applied directly to unidentified sgn somata gave similar results to those shown here in response to bath application. We assume that the recorded responses here, are due to the activation of receptors on or close to the soma. The role of somatic AMPA receptors in neonatal sgnII and sgnI is not clear. Somatic AMPA receptors have been demonstrated in adult guinea-pig sgnI, where the somatic receptor characteristics appeared to match those at the inner hair cell synapse (Ruel et al. 1999). The responses of sgnII and sgnI to ATP are best explained by the activation of ATP-gated ion channels incorporating P2X2 subunits in a heteromultimeric assembly (Salih et al. 2002). P2X2 subunits have been localized to the post-synaptic thickening of the adult guinea-pig ohc-sgnII synapse by immunogold labelling (Housley et al. 1999).
Our results suggest that the dominant inactivating outward current in sgnII is an A-type current, due to its fast kinetics of inactivation and recovery from inactivation, the voltage-dependence of its activation and inactivation, and its block by 4-aminopyridine (Rogawski, 1985). Voltage-dependent potassium channel subunits (Kv) that assemble into A-type channels (including Kv1.4, Kv3.4, Kv4.1, Kv4.2, and Kv4.3) have been detected by RT-PCR of whole neonatal and adult rat spiral ganglion (P. Laslo, D. Jagger & G. Housley, unpublished observations). Furthermore, expression of the fast inactivating potassium channel subunit Kv4.2 has been demonstrated in the adult mouse spiral ganglion (Adamson et al. 2002), with densest antibody labelling occurring in the apical region. The identified sgnII and sgnI in the present study displayed a differential distribution of inactivating and non-inactivating outward currents. The non-inactivating currents had greater magnitude in sgnI than in sgnII. Non-inactivating potassium currents have been implicated in rapid adaptation by postnatal gerbil sgnI (Lin, 1997), and mouse sgnI (Adamson et al. 2002). Some of this current is probably carried by the delayed rectifier potassium channel subunit Kv3.1 (Adamson et al. 2002). The non-inactivating component of IK in postnatal gerbil sgnI (Lin, 1997) showed a strong dependence on holding potential, as seen in the present study. In a study of unidentified sgn from the neonatal rat (P2-P6) the outward currents showed a wide range of inactivation profiles (Jagger & Housley, 2002). It is possible that sgn with prominent A-type currents in that study may have been sgnII, particularly those with currents activated from depolarized holding potentials and with the highest inactivation indices.
The properties of A-type currents enable neurones to fire repetitively at low frequencies, by regulating the first spike latency and the inter-spike interval (Rogawski, 1985). It was therefore surprising that the differential distribution of this current between sgnII and sgnI did not result in contrasting firing patterns in our preparation. In particular, there was no significant difference in the first spike latency in identified sgnII and sgnI. Similar short latencies were also observed in neonatal neurones (P2-P6) displaying prominent A-type currents in the same preparation (Jagger & Housley, 2002). The inconsistencies of the firing patterns of sgnII in cochlear slices require further study. However, in a recent in vitro study (Reid & Davis, 2003), putative murine neonatal sgnII (P4-P7; identified by peripherin expression) displayed firing characteristics that were consistent with the expression of A-type potassium channel subunits. Neurones fired action potentials with longer latency and slower accommodation compared to their peripherin-negative counterparts.
SgnI provide a continuous and rapid coding of the activity of single ihc. The observation of spontaneous firing in some of the identified sgnI in the present study may have corresponded to spontaneous glutamate release from their innervated ihc (Glowatzki & Fuchs, 2002). In vivo, auditory stimuli activate coincident action potentials in numerous sgnI, allowing phase-locking of signals up to 2 kHz in the auditory nerve (Trussell, 1999). Study of somatically initiated firing suggest that sgnI preserve the timing of high frequency stimuli by the generation of single, short-latency action potentials (Lin, 1997; Mo & Davis, 1997; Adamson et al. 2002). This is achieved by substantial outward rectification near the resting potential, due to the somatic expression of specific potassium channel subunits (Adamson et al. 2002). Further work is required to determine the relevance of somatic potassium channel expression, and whether fidelity is first achieved by the presence of such mechanisms at the sgnI postsynaptic membrane.
The anatomical and electrophysiological properties of sgnII preclude them from coding such high frequency signals. It seems more likely that sgnII may signal afferent information from the cochlear amplifier region, which has lower spatial and temporal resolution, such as in the hypothesized mechanism of feedback gain control for the active and non-linear biomechanics of the organ of Corti (Kim, 1984). It is estimated that the cochlear amplifier is located around 0.5 octaves basal to the peak displacement of the basilar membrane for a given frequency (Ashmore & Kolston, 1994). The sgnII peripheral dendritic arborization (Robertson, 1984; Berglund & Ryugo, 1987; Brown, 1987; Echteler, 1992; Brown, 1994; Fechner et al. 2001) is therefore ideally organized to code the spatial integration of cochlear amplifier activity. While the physiological stimulus to the sgnII receptive field remains undetermined (Robertson, 1984; Brown, 1994; Robertson et al. 1999), based on our voltage clamp data and the responses of putative sgnII in vitro (Reid & Davis, 2003), we suggest that sgnII would generate a non-bursting spike train in vivo, matched to integrating inputs from the distributed terminal field. We predict that the A-type current contributes to the temporal integration of cochlear amplifier activity by coding the summation of multiple inputs into an intensity-graded firing rate. As such, the distinct characteristics of the A-type current define it as a marker for the identification of type II spiral ganglion neurones.
Acknowledgments
This work was supported by the Marsden Fund (Royal Society of New Zealand), and the Health Research Council of New Zealand. We thank A. Wolff (Faculté de Médecine Pitié-Salpêtriére, UMR CNRS 7000, Paris, France) for providing SE411 peripherin antiserum and S. Vlajkovic for assistance during initial immunocytochemistry experiments. The University of Auckland Biomedical Imaging Research Unit is thanked for imaging support.
REFERENCES
- Adamson CL, Reid MA, Mo ZL, Bowne-English, Davis RL. Firing features and potassium channel content of murine spiral ganglion neurons vary with cochlear location. J Comp Neurol. 2002;447:331–350. doi: 10.1002/cne.10244. [DOI] [PubMed] [Google Scholar]
- Ashmore JF, Kolston PJ. Hair cell based amplification in the cochlea. Curr Op Neurobiol. 1994;4:503–508. doi: 10.1016/0959-4388(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Berglund AM, Brown MC. Central trajectories of type II spiral ganglion cells from various cochlear regions in mice. Hear Res. 1994;75:121–130. doi: 10.1016/0378-5955(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Berglund AM, Ryugo DK. Hair cell innervation by spiral ganglion neurons in the mouse. J Comp Neurol. 1987;255:560–570. doi: 10.1002/cne.902550408. [DOI] [PubMed] [Google Scholar]
- Brown MC. Morphology of labeled afferent fibers in the guinea pig cochlea. J Comp Neurol. 1987;260:591–604. doi: 10.1002/cne.902600411. [DOI] [PubMed] [Google Scholar]
- Brown MC. Antidromic responses of single units from the spiral ganglion. J Neurophysiol. 1994;71:1835–1847. doi: 10.1152/jn.1994.71.5.1835. [DOI] [PubMed] [Google Scholar]
- Brown MC, Berglund AM, Kiang NY, Ryugo DK. Central trajectories of type II spiral ganglion neurons. J Comp Neurol. 1988;278:581–590. doi: 10.1002/cne.902780409. [DOI] [PubMed] [Google Scholar]
- Echteler SM. Developmental segregation in the afferent projections to mammalian auditory hair cells. Proc Natl Acad Sci U S A. 1992;89:6324–6327. doi: 10.1073/pnas.89.14.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fechner FP, Nadol JB, Burgess BJ, Brown MC. Innervation of supporting cells in the apical turns of the guinea pig cochlea is from type II afferent fibers. J Comp Neurol. 2001;429:289–298. doi: 10.1002/1096-9861(20000108)429:2<289::aid-cne9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Geal-Dor M, Freeman S, Li G, Sohmer H. Development of hearing in neonatal rats: air and bone conducted ABR thresholds. Hear Res. 1993;69:236–42. doi: 10.1016/0378-5955(93)90113-f. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nature Neurosci. 2002;5:147–154. doi: 10.1038/nn796. [DOI] [PubMed] [Google Scholar]
- Hafidi A, Despres G, Romand R. Ontogenesis of type II spiral ganglion neurons during development: peripherin immunohistochemistry. Int J Dev Neurosci. 1993;11:507–512. doi: 10.1016/0736-5748(93)90024-8. [DOI] [PubMed] [Google Scholar]
- Hafidi A. Peripherin-like immunoreactivity in type II spiral ganglion cell body and projections. Brain Res. 1998;805:181–190. doi: 10.1016/s0006-8993(98)00448-x. [DOI] [PubMed] [Google Scholar]
- Housley GD, Kanjhan R, Raybould NP, Greenwood D, Salih SG, Järlebark L, Burton LD, Setz VC, Cannell MB, Soeller C, Christie DL, Usami S, Matsubara A, Yoshie H, Ryan AF, Thorne PR. Expression of the P2X2 receptor subunit of the ATP-gated ion channel in the cochlea: implications for sound transduction and auditory neurotransmission. J Neurosci. 1999;19:8377–8388. doi: 10.1523/JNEUROSCI.19-19-08377.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger DJ, Housley GD. A-type potassium currents dominate repolarisation of neonatal rat primary auditory neurones in situ. Neuroscience. 2002;109:169–82. doi: 10.1016/s0306-4522(01)00454-7. [DOI] [PubMed] [Google Scholar]
- Jagger DJ, Robertson D, Housley GD. A technique for slicing the rat cochlea around the onset of hearing. J Neurosci Meth. 2000;104:77–86. doi: 10.1016/s0165-0270(00)00322-8. [DOI] [PubMed] [Google Scholar]
- Kim DO. Functional roles of the inner- and outer-hair-cell subsystems in the cochlea and brainstem. In: Berlin CI, editor. Hearing Science: Recent Advances. San Diego: College-Hill Press; 1984. pp. 241–262. [Google Scholar]
- Lin X. Action potentials and underlying voltage-dependent currents studied in cultured spiral ganglion neurons of the postnatal gerbil. Hear Res. 1997;108:157–79. doi: 10.1016/s0378-5955(97)00050-6. [DOI] [PubMed] [Google Scholar]
- Matsubara A, Laake JH, Davanger S, Usami S, Ottersen OP. Organization of AMPA receptor subunits at a glutamate synapse: a quantitative immunogold analysis of hair cell synapses in the rat organ of Corti. J Neurosci. 1996;16:4457–4467. doi: 10.1523/JNEUROSCI.16-14-04457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo ZL, Davis RL. Endogenous firing patterns of murine spiral ganglion neurons. J Neurophysiol. 1997;77:1294–1305. doi: 10.1152/jn.1997.77.3.1294. [DOI] [PubMed] [Google Scholar]
- Niedzielski AS, Wenthold RJ. Expression of AMPA, kainate, and NMDA receptor subunits in cochlear and vestibular ganglia. J Neurosci. 1995;15:2338–2353. doi: 10.1523/JNEUROSCI.15-03-02338.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patneau DK, Vyklicky L, Mayer ML. Hippocampal neurons exhibit cyclothiazide-sensitive rapidly desensitizing responses to kainate. J Neurosci. 1993;13:3496–3509. doi: 10.1523/JNEUROSCI.13-08-03496.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol R, Lenoir M, Robertson D, Eybalin M, Johnstone BM. Kainic acid selectively alters auditory dendrites connected with cochlear inner hair cells. Hear Res. 1985;18:145–151. doi: 10.1016/0378-5955(85)90006-1. [DOI] [PubMed] [Google Scholar]
- Reid MA, Davis RL. Firing properties of putative type II spiral ganglion neurons in vitro. Abstr Assoc Res Otolaryngol. 2003;26:p248. [Google Scholar]
- Robertson D. Horseradish peroxidase injection of physiologically characterized afferent and efferent neurones in the guinea pig spiral ganglion. Hear Res. 1984;15:113–121. doi: 10.1016/0378-5955(84)90042-x. [DOI] [PubMed] [Google Scholar]
- Robertson D, Sellick PM, Patuzzi R. The continuing search for outer hair cell afferents in the guinea pig spiral ganglion. Hear Res. 1999;136:151–158. doi: 10.1016/s0378-5955(99)00120-3. [DOI] [PubMed] [Google Scholar]
- Rogawski MA. The A-current: how ubiquitous a feature of excitable cells is it? Trends Neurosci. 1985;8:214–219. [Google Scholar]
- Romand R, Romand MR. Qualitative and quantitative observations of spiral ganglion development in the rat. Hear Res. 1985;18:111–120. doi: 10.1016/0378-5955(85)90002-4. [DOI] [PubMed] [Google Scholar]
- Ruel J, Chen C, Pujol R, Bobbin RP, Puel JL. AMPA-preferring glutamate receptors in cochlear physiology of adult guinea-pig. J Physiol. 1999;518:667–680. doi: 10.1111/j.1469-7793.1999.0667p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salih SG, Jagger DJ, Housley GD. ATP-gated currents in rat primary auditory neurones in situ arise from a heteromultimeric P2X receptor subunit assembly. Neuropharmacology. 2002;42:386–395. doi: 10.1016/s0028-3908(01)00184-8. [DOI] [PubMed] [Google Scholar]
- Trussell LO. Synaptic mechanisms for coding timing in auditory neurons. Annu Rev Physiol. 1999;61:477–496. doi: 10.1146/annurev.physiol.61.1.477. [DOI] [PubMed] [Google Scholar]


