Abstract
The relationship between the electrophysiological properties of motoneurones and their muscle units has been established in animal models. A functionally significant relationship exists whereby motoneurones with long post-spike afterhyperpolarizations (AHPs) innervate slow contracting muscle units. The purpose of this study was to determine whether the time course of the AHP as measured by its time constant is associated with the contractile properties of its muscle unit in humans. Using an intramuscular fine wire electrode, 46 motor units were recorded in eight subjects as they held a low force contraction of the first dorsal interosseus muscle for approximately 10 min. By applying a recently validated transform to the interspike interval histogram, the mean voltage versus time trajectory of the motoneurone AHP was determined. Spike-triggered averaging was used to extract the muscle unit twitch from the whole muscle force with strict control over force variability and motor unit discharge rate (interspike intervals between 120 and 200 ms). The AHP time constant was positively correlated to the time to half-force decay (ρ = 0.36, P < 0.05) and twitch duration (ρ = 0.57, P < 0.001); however, time to peak force failed to reach significance (ρ = 0.27, P < 0.07). These results suggest that a similar functional relationship exists in humans between the motoneurone AHP and the muscle unit contractile properties.
The modulation of motoneurone discharge rate is a fundamental process by which muscle force is controlled. The interaction between motoneurone discharge characteristics and the muscle unit contractile response is central to force gradation and maintenance. For example, the minimum discharge frequency corresponds to the rate at which consecutive muscle unit twitches start to summate (Kernell, 1983; Kernell et al. 1999). Within the primary range of discharge, both the minimum frequency and range of motoneurone discharge have been shown to correlate with the motoneurone post-spike afterhyperpolarization (AHP) time course (Kernell, 1965).
The observation that type-S motor units have longer mean AHP durations than type-FF and type-FR motor units is well established across mammalian muscle groups (Eccles et al. 1958; Hammarberg & Kellerth, 1975; Dum & Kennedy, 1980; Zengel et al. 1985; Gardiner & Kernell, 1990; Gardiner, 1993; Bakels & Kernell, 1993a,b). A positive correlation between the AHP duration and twitch contraction time within a motor unit pool has also been shown to exist (Zengel et al. 1985; Cope et al. 1986; Gardiner, 1993; Bakels & Kernell, 1993a,b).
Since the contraction times of human motor units in many muscles of the arm and hand are organized along a continuum (Young & Mayer, 1981; Romaiguere et al. 1989; Thomas et al. 1991; Fuglevand et al. 1999), it is reasonable to expect that a similar organization exists in these motor units; such an association has yet to be established. The purpose of this study was to examine whether the motoneurone AHP time course is related to time-dependent contractile properties of its muscle unit. We utilized a novel transform of the interspike interval (ISI) histogram (Matthews, 1996, 1999b, 2002a) that estimates the temporal properties of the AHP. The muscle unit contractile properties were derived using spike-triggered averaging of the whole muscle force. Consequently, the motoneurone AHP-muscle unit relationship could be examined using a minimally invasive, purely volitional protocol. Preliminary results have been reported previously in abstract form (Gossen et al. 2002).
METHODS
Experiments were performed on the first dorsal interosseus (FDI) muscle of the right hand from eight healthy adults (6 males and 2 females) aged 29-49 years. Subjects had no history of neuromuscular disorders and volunteered with informed, written consent. The study was approved by the University Ethics Committee. All experiments conformed to the Declaration of Helsinki.
Apparatus
The apparatus used has been described previously (Gossen et al. 2003). Briefly, subjects sat with the right shoulder abducted to roughly 45 deg and the elbow flexed at 90 deg. The hand was placed in a pronated position in a custom-made Sansplint cast that secured the hand at the wrist. The thumb was abducted and the third to fifth fingers were fixed in a flexed position. A custom-made finger splint (mean mass 11.4 ± 1.8 g) was made for each subject that maintained the index finger in full extension along the horizontal plane while allowing abduction. Two 10 cm stainless steel wires were anchored to the splint over the midline of the middle phalanx of the index finger. The first wire was perpendicular to the index finger, parallel to the horizontal plane to transmit abduction force, while the second wire was perpendicular to the horizontal plane to transmit flexion force. Hooks at the end of each wire were connected to separate force transducers (Grass FT-10, range 0-20 N, minimum reliable resolution 1 mN; Grass-Telefactor, RI, USA) that recorded abduction and flexion forces. Force was sampled at 2.5 kHz using a 16-bit data acquisition system (Power 1401, Cambridge Electronic Design, UK). Following the completion of all motor unit data collection, subjects were brought into the laboratory to perform a maximal voluntary contraction (MVC) using the aforementioned transducers reconfigured to accommodate higher forces (range 0-100 N).
Single motor unit potentials were recorded intramuscularly using 50 μm stainless steel fine wire (California Fine Wire, Grover Beach, CA, USA) bipolar electrodes and sampled at 25 kHz. A dual window discriminator (Model DDIS-1, Bak Electronics Inc., MD, USA) identified individual action potentials for the purpose of auditory and visual feedback. The electrode was threaded through a disposable 3 cm long, 25 gauge hypodermic needle (Becton Dickinson and Company, NJ, USA), with a hook of approximately 1-2 mm in length formed at the recording end of the electrode. The needle was used to insert the electrode into the FDI to a depth of approximately 1 cm and then withdrawn, leaving the wire embedded within the muscle. All wire electrode- hypodermic needle assemblies were sealed and autoclaved (AMSCO Autoclave, USA) for 45 min prior to use.
Off-line motor unit discrimination was accomplished using a template-matching algorithm (Spike2, version 4.15, Cambridge Electronic Design) that classified motor units according to their amplitude and shape. Care was taken to ensure that identified motor unit action potentials were associated with the correct motor unit. Any sections of data where motor unit action potentials could not be classified with 100 % certainty were excluded from subsequent analysis.
Experimental protocol
Subjects were required to perform a 10 min sustained isometric contraction. The first half of the contraction provided a discharge rate suitable for spike-triggered averaging (STA). Assessment of motor unit contractile properties using STA of whole muscle force requires slow tonic discharge of the isolated motor unit (Stein et al. 1972; Kossev et al. 1994; Thomas, 1995). Visual feedback of motor unit discharge rate, displayed as a 1 s running mean, was given through a 17 in computer monitor positioned approximately 1.5 m from the subject. A frequency marker of 7 Hz was displayed on the computer monitor and subjects were instructed to maintain a motor unit discharge rate as close as possible to the 7 Hz target frequency for a 5 min period.
Subjects were instructed to produce abduction forces and to try to minimize flexion. STA was performed on both abduction and flexion force and a vector sum STA twitch was derived. Subjects were successful in minimizing flexion force since the resultant twitch did not differ appreciably from the abduction twitch.
The second half of the isometric contraction (i.e. the final 5 min) provided additional ISIs necessary to characterize the time course of the motoneurone AHP (see below). Subjects were asked to maintain a steady low discharge rate (below 12 Hz) but, unlike the first half of the isometric contraction, they were free to perform small gradual changes in the mean discharge rate of the motor unit; large and/or abrupt changes in discharge rate were discouraged. Once the subject achieved a small change in the mean discharge rate, they were instructed to maintain that frequency for at least 30 s before changing the discharge rate.
Motor unit contractile properties
Motor unit contractile properties were assessed using STA of whole muscle force (Stein et al. 1972). Identified motor unit action potentials were used as triggers to average whole muscle abduction and flexion force within the first 5 min of the isometric contraction. For the purpose of averaging, a 220 ms window of force was used (200 ms following and 20 ms preceding the trigger). To control the effects that the mean motor unit discharge rate and its variability can have on the resulting STA twitch (Nordstrom et al. 1989; Gossen et al. 2003), acceptable pre- and post-trigger ISIs used in the averaging process were restricted to a range of 120-200 ms. We previously found that mean whole muscle force and its variability can affect the STA twitch amplitude and time course (Gossen et al. 2003). Thus, STA was performed in a region where whole muscle force was stable and its variability, as measured by its coefficient of variation, was minimal.
Peak twitch force (PF, mN) was measured from a baseline defined by the onset of the rise in force. Time to peak force (TTP, ms) was defined as the time between the onset of the rise in force and the peak force. Time to half-force decay (HFD, ms) was the time taken for twitch force to decay from PF to half that value. Twitch duration (DUR, ms) was calculated as the time from the onset of the rise in force to the return to baseline. If force did not fully return to baseline, a linear extrapolation of the descending phase of the STA twitch to baseline was performed.
Motoneurone AHP properties
Estimation of the motoneurone AHP trajectory was performed using the interval death rate transform of the motor unit discharge ISI histogram (Matthews, 1996). The interval death rate represents the probability of an ISI being terminated (i.e. spike occurring) per unit time following a spike (Moore et al. 1966; Matthews, 1996). The interval death rate plot is then converted to a voltage (expressed in units of noise variance or ‘noise units’) versus time trajectory that can be used to determine the time course of the motoneurone AHP. The transform estimates the decay of the last portion of the AHP's exponential tail (Matthews, 1996, 2002b). The details underlying the methodology and validity of the transform have previously been discussed (Matthews, 1996, 1999a, 2002a,b;Powers & Binder, 2000).
For the determination of the AHP trajectory, a 10 min period of tonic discharge was analysed. From identified motor unit action potentials, a 1 s running mean of the discharge rate (i.e. 500 ms preceding and 500 ms following an action potential) was calculated using a raised cosine bell function (Spike2). Only intervals between 50 and 300 ms were used in the calculation of the running mean. The instantaneous value of the running mean centred on each ISI was used to classify (or ‘slice’) the ISIs from the spike train into sub-populations for the purpose of grouping intervals with similar mean discharge rates (Matthews, 1996). Each sub-population of ISIs was then used to construct a statistically stationary histogram (bin size 5 ms) that represented the distribution of ISIs about a given mean discharge rate. Thus for each motor unit, several histograms were constructed that separated the ‘moment-to-moment’ variability with a steady mean input from ‘longer term’ variability due to changes in the mean level of synaptic drive.
Each of these histograms was subsequently converted into a death rate plot and a transform then applied (corresponding to a 4 ms membrane time constant, see Fig. 13 in Matthews, 1996) which converted these plots into a trajectory that represented a segment of the final portion of the motoneurone's AHP. These trajectory segments were overlapped to form a compound trajectory that was fitted with a simple first order exponential curve, the time constant (τ, ms) of which served as an index of the AHP time course (Fig. 3; Matthews, 1996).
Figure 1. Frequency histograms summarizing contractile properties of the 46 STA twitches.
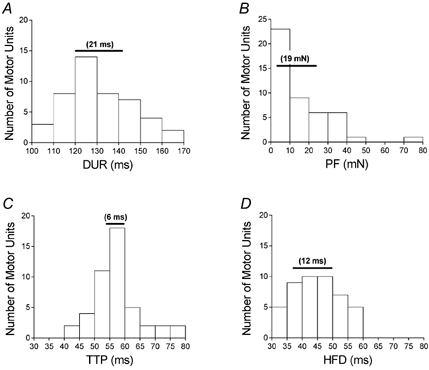
A, duration (DUR); B, peak force (PF); C, time to peak force (TTP); D, time to half-force decay (HFD). Horizontal bars depict the data contained between the 25th and 75th percentiles. The range is indicated in parentheses above each bar. Note the narrow dispersion of the TTP data in comparison to the HFD data.
Figure 3. Plots of AHP compound trajectories (A-C) and corresponding STA twitches (D-F) for 3 different motor units.
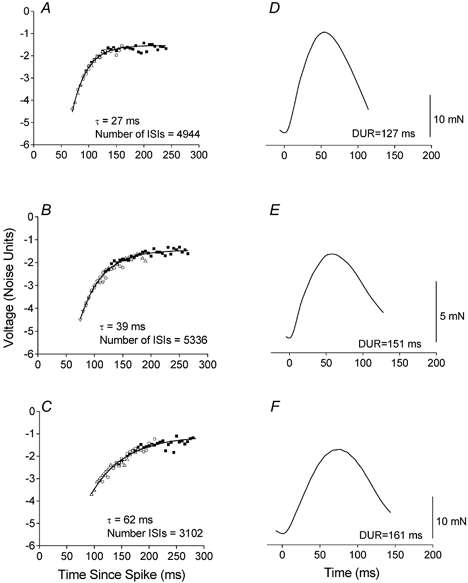
Note a longer time constant (τ) was associated with longer twitch durations. The compound trajectory in A was derived from the sliced histograms in Fig. 2. The different symbols correspond to transforms performed on separate sliced histograms (i.e. 3, 4 and 3 ISI histograms were transformed to create the compound trajectories in A, B and C, respectively).
Central to the accuracy of the compound trajectory is the spike train slicing process and the shape of the resulting ISI histograms. If a histogram was generated with mixed means (i.e. statistically non-stationary) the resulting parameters of the exponential fit applied to the final compound trajectory would be an inaccurate representation of the true AHP trajectory. Although numerous distortions are discussed (see Fig. 6 in Matthews, 1996), we primarily used the presence of a sharp bend in the compound trajectory referred to as a ‘kink’. Specifically, the early portion of the trajectory abruptly curves and approaches a final plateau much earlier than if a series of truly stationary histograms were used to generate the compound trajectory. A trajectory kink is particularly noticeable when a simple exponential fit is attempted through the compound trajectory. With this in mind, each population of intervals recorded from a motor unit was initially sliced such that three or more histograms, each containing roughly 1000-2000 intervals, were generated as a first approximation. A compound trajectory was created and fitted with a simple exponential. If the resulting trajectory exhibited signs of a kink or failed to come to a stable plateau, the slicing process was repeated by altering the number of histograms, the number of intervals per histogram, or both, until the resulting compound trajectory could be smoothly fitted with a first order exponential function.
STA twitch properties were plotted as function of the time constant derived from the exponential fit. To determine whether a parametric Pearson r or non-parametric Spearman rho (ρ) correlation coefficient would be used to test the relationship between the AHP time constant and STA twitch properties, a Shapiro-Wilk normality test was performed on all data. Statistical significance for all analyses was set at P ≤ 0.05. All values are expressed as means ± S.D.
RESULTS
STA analysis
Forty-six motor units were analysed. The Shapiro-Wilk test indicated that the AHP time constant, PF and TTP were not normally distributed. Thus, the Spearman ρ was used as a test of correlation. The mean MVC peak force for the eight subjects was 32.8 ± 13.8 N. The mean abduction force held during STA was 5.7 ± 3.8 % MVC (range 1.4-16.7 % MVC). ISIs used for STA had a consistent mean pre-trigger (144 ± 5 ms) and post-trigger (145 ± 5 ms) value. Mean ISI variability during STA was also similar across motor units (S.D. pre-trigger ISI, 19 ± 2 ms; S.D. post-trigger ISI, 19 ± 2 ms). The mean number of averages per twitch was 293 ± 148 over a mean time of 182 ± 66 s. Within the averaging windows, the mean coefficient of variation of whole muscle abduction force was 8.8 ± 4.5 % while the mean motor unit discharge rate that the subjects maintained was 7.8 ± 1.0 Hz.
Mean measurements of motor unit contractile properties were consistent with previous studies that examined the FDI using STA (PF, 15 ± 15 mN, range 1-75 mN; TTP, 57 ± 8 ms, range 42-76 ms; HFD, 45 ± 8 ms, range 33-59 ms). Mean DUR was 131 ± 15 ms (range 101-164 ms). Figure 1 summarizes the distribution of the contractile properties of the motor units.
PF did not significantly correlate with TTP (ρ = 0.29, n.s.) or HFD (ρ = 0.37, n.s.). Mean abduction force as a percentage of MVC correlated positively with PF (ρ = 0.57, P < 0.001). That is, within our sample, motor units with a large PF were recorded when the abduction force was higher.
AHP analysis
Each AHP compound trajectory involved transforming a minimum of three sliced histograms per motor unit. A mean of 4840 ± 1334 ISIs were used per analysis with a minimum of 1000 ISIs per histogram for most trajectories. Although the minimum number of ISIs required for a good estimate of the AHP has not been investigated in detail, the number of ISIs used was, on average, greater than the number used in the validation of the transform (2340 ± 1690; Powers & Binder, 2000). The mean firing rate of motor units across all trials was usually kept below 10 Hz (range 6.3-11.0 Hz). The resulting mean time constant of compound AHP trajectories was 37 ± 8 ms.
An example of an ISI histogram and the resulting sliced histograms is illustrated in Fig. 2. The sliced histograms (Fig. 2B-D) exhibited the typical trend of increasing positive skew with decreasing mean discharge rate (Warren et al. 1993; Matthews, 1996). These sliced histograms gave rise to the compound trajectory in Fig. 3A. Figure 3 illustrates three motoneurone AHP trajectories and their corresponding STA twitches. Increases in STA twitch duration from Fig. 3D-F were accompanied by increases in the AHP time constants.
Figure 2. Representative example of a series of ISI histograms derived from a single motor unit.
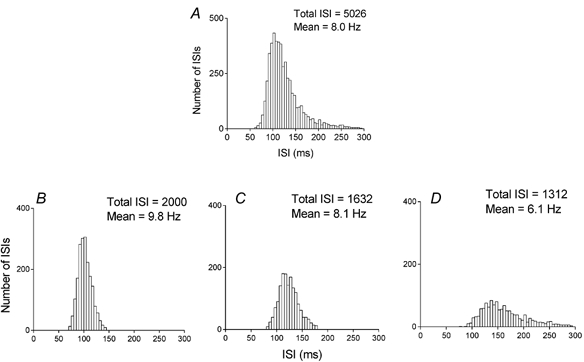
A, raw ISI histogram containing all intervals from the trial. B-D, sliced histograms extracted from A illustrating the ISI characteristics during the fastest (B), middle (C) and slowest (D) periods of motor unit discharge. The histogram in D gives rise to the plateau of the final compound trajectory (see Fig. 3A). Due to the comparatively smaller number of ISIs contained in the histogram in D (i.e. particularly above ≈200 ms), caution needs to be exercised when fitting the final compound trajectory. See text for further details.
There was a tendency for TTP to increase with the AHP time constant, but the correlation failed to reach significance (ρ = 0.27, P < 0.07, Fig. 4A). A positive correlation was observed between the AHP time constant and HFD (ρ = 0.36, Fig. 4B). The correlation became considerably larger and more significant when DUR was plotted as a function of the AHP time constant (ρ = 0.57, Fig. 4C).
Figure 4. Relationship between AHP time constant and time-dependent STA twitch measurements.
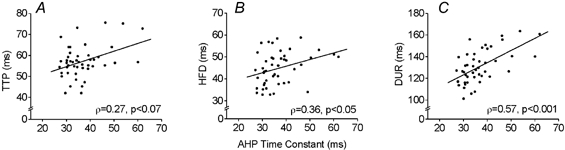
A, TTP; B, HFD; C, DUR. HFD and DUR were significantly correlated to AHP time constant. Despite the positive trend of TTP to increase with the AHP time constant, the relationship was not significant.
Sixteen of forty-six motor units required extrapolation of force to baseline to determine DUR. Due to possible errors associated with extrapolation, TTP + HFD was used as an alternative measure of twitch duration (as used in Cope et al. 1986 and Gardiner, 1993), and was plotted as a function of the AHP time constant. This resulted in a slight drop in the correlation but it still remained statistically significant (ρ = 0.45, P < 0.01). Thus, regardless of the definition of DUR used, its correlation with the AHP time constant remained greater than the correlation of the AHP time constant with TTP or HFD. No relationship was found between the PF and AHP time constant (ρ = 0.25, n.s.) or between the mean abduction force during STA and the AHP time constant (ρ = 0.10, n.s.).
AHP trajectory reliability
The time constant of the compound trajectory's exponential fit can be influenced by the plateau formed by the lowest frequency trajectory (Matthews, 1996). In fitting an exponential curve the longest intervals were often discarded, otherwise they could have a quite unreasonably large effect, especially in relation to their numbers (Fig. 2). To monitor the effects that long ISIs included in the analysis could have on the time constant of the exponential fit, the upper cut-off ISI was often varied, resulting in a greater weighting of the exponential fit to the earlier portion of the trajectory.
Long ISIs could be of particular concern with compound trajectories exhibiting long plateaus as seen in Fig. 3A and B. The long ISIs had small effects on the shape of the final exponential fit. For example, in Fig. 3A excluding all ISIs above 200 ms had a negligible effect on the final time constant (τ = 28 ms) while excluding all ISIs above 150 ms still resulted in a comparable curve (τ = 30 ms). In general, time constants were robust despite differences in duration of the plateaus across motor units.
DISCUSSION
The purpose of this study was to determine whether the time course of the motoneurone AHP was correlated with the contractile properties of its muscle unit. The association between the AHP time course and motor unit contractile properties is functionally important since the AHP limits the steady-state firing of a motoneurone (Kernell, 1965; Gustafsson, 1974; Zengel et al. 1985). A positive correlation between the AHP time course and the contractile time of its muscle unit would optimize the amount of force summation. In other words, a slower contracting muscle unit would require a lower discharge rate for force summation, and would be innervated by a motoneurone with a longer AHP duration; conversely, a fast contracting muscle unit that is innervated by a motoneurone with a short AHP duration would have a relatively fast discharge rate.
The presence of a positive correlation between the AHP time course and muscle unit contractile time found in the present study (Fig. 4B and C) is not unexpected, as there exists evidence suggesting that motoneurones have a considerable effect on the muscle unit speed of shortening and relaxation (Buller et al. 1960; Vrbová et al. 1995). Although such a relationship, termed continuous match (Bakels & Kernell, 1993b), has been observed in the cat soleus (Cope et al. 1986) and rat gastrocnemius muscles (Gardiner, 1993; Bakels & Kernell, 1993b), Bakels & Kernell (1993a) did not find evidence for this relationship in the rat tibialis anterior muscle. They suggested that the absence of a continuous match resulted from the relatively homogeneous motor unit distribution within the muscle. Interestingly, although motor units with a relatively narrow range of STA twitch contractile properties were examined in the current study, this did not preclude a positive relationship between the AHP time constant and the STA twitch duration.
There were consistencies between our findings and those of previous work characterizing FDI motor units, such as a unimodal distribution of TTPs (Milner-Brown et al. 1973; Young & Mayer, 1981; Elek et al. 1992; Kossev et al. 1994), a lack of correlation between the time course of the STA twitch and PF (Young & Mayer, 1981; Elek et al. 1992; Kossev et al. 1994), and larger PF values at higher voluntary abduction forces (Milner-Brown et al. 1973; Stephens & Usherwood, 1977). However, there was a smaller range of contractile properties in comparison with other studies characterizing FDI using STA. In other studies, for example, lower limits of TTP ranged from 25-33 ms and PF values were found in excess of 100 mN (Milner-Brown et al. 1973; Stephens & Usherwood, 1977; Kossev et al. 1994; Carpentier et al. 2001). In our study, the shortest TTP was 42 ms and the largest PF was 75 mN suggesting a bias towards weaker and slower contracting motor units. The small dispersion of TTP values (Fig. 1C) probably contributed to the lack of correlation between TTP and the AHP time constant (Fig. 4A).
Despite the absence of large and fast contracting motor units, we found that HFD and DUR were positively correlated to the AHP time course, with DUR displaying the highest correlation coefficient. This finding supports the concept advanced by previous studies (Young & Mayer, 1981; Thomas et al. 1986; Kossev et al. 1994) that the contractile properties of human FDI motor units are distributed along a continuum as opposed to a bimodal grouping. Interestingly, the lack of correlation between TTP and the AHP time constant coexisting with a significant correlation between HFD and the AHP time constant parallels the finding of Gardiner (1993). As suggested by Gardiner, the finding of a stronger relationship (Fig. 4) and greater range of HFD (Fig. 1) than TTP to the AHP time constant (or the AHP half-decay time used by Gardiner) may be indicative of the greater importance of the former in determining the degree of summation at subtetanic frequencies (Gardiner, 1993). Our finding of no correlation between the AHP time constant and PF is also consistent with those of Gardiner (1993), who found no relationship between twitch PF and AHP time to peak or half-decay time; significant correlations between motoneurone properties and twitch PF were limited to AHP amplitude and axonal conduction velocity (Gardiner, 1993). Because the AHP trajectories of the present study do not accurately predict AHP amplitude (Matthews, 1996), we cannot comment as to whether a similar relationship exists in the human FDI. Also, it is not known to what degree the twitch peak force was distorted by motor unit synchronization during STA (Taylor et al. 2002). Future studies using intramuscular microstimulation (Taylor & Stephens, 1976; Elek et al. 1992) may be a useful alternative for characterizing motor unit contractile properties.
The results of the present study expand on the physiological relevance of the transform developed by Matthews (1996). The time constants found generally agreed with typical trajectories obtained from several human muscles including the FDI, brachioradialis, biceps brachii, flexor carpi radialis, tibialis anterior and soleus (Matthews, 1996). We have established that a relationship exists between the AHP time constant and the temporal characteristics of the motor unit twitch. This is particularly impressive given the inferential nature of the derivation of both the STA contractile properties and the AHP time constant.
The compound AHP trajectories are distance to threshold estimates of the true AHP trajectory. In making this estimate, it is assumed that the threshold for spike initiation is constant throughout the ISI (Matthews, 1996; Powers & Binder, 2000). Variation in the voltage threshold for spike initiation occurs during the ISI (Powers & Binder, 2001) and can result in a reduction in the distance to threshold at given points within the ISI (Powers & Binder, 2000). This can lead to differences between the estimated and true AHP trajectory. In about 30 % of cases (9/31), Powers & Binder (2000) found the predicted AHP trajectory differed from the true trajectory in magnitude and time course. The error amounted to a mean difference of roughly 25 % between the estimated and true trajectory, at a time point corresponding to the 25th percentile of the cumulative interval histogram (see their Fig. 7). Thus, the estimation process may have contributed to the scatter of data we observed. In general, however, the AHP estimate is quite close to the actual AHP trajectory (Powers et al. 2002).
One major confounding factor in STA is the effect that the motor unit discharge rate has on the resulting STA twitch (Monster & Chan, 1977; Calancie & Bawa, 1986; Kossev et al. 1994). We have shown that the reliability of the STA is improved when the pre- and post-trigger ISIs used in the averaging are restricted to 120-200 ms (Gossen et al. 2003). This created a sampling limitation in the present study because motoneurones with shorter AHP durations would have had a greater number of short ISIs (Kernell, 1965, 1983). This made it difficult for us to sample from faster contracting motor units because the subject would have had considerable difficulty maintaining a slow steady discharge rate of 7 Hz.
In conclusion, despite the sampling limitations associated with STA of whole muscle force, a significant positive relationship exists between the time course of the AHP and the speed of the motor unit contraction in the human FDI muscle, consistent with that seen in small mammals. The AHP histogram transform is a rather new analytical tool. The functional relevance of the transform has been supported in the present study by the demonstration of an AHP-muscle unit speed relationship.
Acknowledgments
We wish to thank Dr P. B. C. Matthews for his valuable comments regarding the derivation of the AHP trajectories. The study was supported by the Natural Sciences and Engineering Research Council of Canada.
REFERENCES
- Bakels R, Kernell D. Average but not continuous speed match between motoneurons and muscle units of rat tibialis anterior. J Neurophysiol. 1993a;70:1300–1306. doi: 10.1152/jn.1993.70.4.1300. [DOI] [PubMed] [Google Scholar]
- Bakels R, Kernell D. Matching between motoneurone and muscle unit properties in rat medial gastrocnemius. J Physiol. 1993b;463:307–324. doi: 10.1113/jphysiol.1993.sp019596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller AJ, Eccles JC, Eccles RM. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol. 1960;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calancie B, Bawa P. Limitations of the spike-triggered averaging technique. Muscle Nerve. 1986;9:78–83. doi: 10.1002/mus.880090113. [DOI] [PubMed] [Google Scholar]
- Carpentier A, Duchateau J, Hainaut K. Motor unit behaviour and contractile changes during fatigue in the human first dorsal interosseus. J Physiol. 2001;534:903–912. doi: 10.1111/j.1469-7793.2001.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope TC, Bodine SC, Fournier M, Edgerton VR. Soleus motor units in chronic spinal transected cats: physiological and morphological alterations. J Neurophysiol. 1986;55:1202–1220. doi: 10.1152/jn.1986.55.6.1202. [DOI] [PubMed] [Google Scholar]
- Dum RP, Kennedy TT. Physiological and histochemical characteristics of motor units in cat tibialis anterior and extensor digitorum longus muscles. J Neurophysiol. 1980;43:1615–1630. doi: 10.1152/jn.1980.43.6.1615. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. The action potentials of the alpha motoneurones supplying fast and slow muscles. J Physiol. 1958;142:275–291. doi: 10.1113/jphysiol.1958.sp006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elek JM, Kossev A, Dengler R, Schubert M, Wohlfahrt K, Wolf W. Parameters of human motor unit twitches obtained by intramuscular microstimulation. Neuromuscul Disord. 1992;2:261–267. doi: 10.1016/0960-8966(92)90058-e. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Macefield VG, Bigland-Ritchie B. Force-frequency and fatigue properties of motor units in muscles that control digits of the human hand. J Neurophysiol. 1999;81:1718–1729. doi: 10.1152/jn.1999.81.4.1718. [DOI] [PubMed] [Google Scholar]
- Gardiner PF. Physiological properties of motoneurons innervating different muscle unit types in rat gastrocnemius. J Neurophysiol. 1993;69:1160–1170. doi: 10.1152/jn.1993.69.4.1160. [DOI] [PubMed] [Google Scholar]
- Gardiner PF, Kernell D. The “fastness” of rat motoneurones: time-course of afterhyperpolarization in relation to axonal conduction velocity and muscle unit contractile speed. Pflugers Arch. 1990;415:762–766. doi: 10.1007/BF02584018. [DOI] [PubMed] [Google Scholar]
- Gossen ER, Ivanova T, Garland SJ. The time course of the motoneuron post-spike afterhyperpolarization is matched to the temporal characteristics of the muscle unit twitch in the human first dorsal interosseus muscle. Soc Neurosci Abst Online. 2002 [Google Scholar]
- Gossen ER, Ivanova T, Garland SJ. Factors affecting the stability of the spike-triggered averaged force in the human firstdorsal interosseus muscle. J Neurosci Methods. 2003;126:155–164. doi: 10.1016/s0165-0270(03)00077-3. [DOI] [PubMed] [Google Scholar]
- Gustafsson B. Afterhyperpolarization and the control of repetitive firing in spinal neurones of the cat. Acta Physiol Scand. 1974;(suppl. 416):1–47. [PubMed] [Google Scholar]
- Hammarberg C, Kellerth JO. Studies of some twitch and fatigue properties of different motor unit types in the ankle muscles of the adult cat. Acta Physiol Scand. 1975;95:231–242. doi: 10.1111/j.1748-1716.1975.tb10047.x. [DOI] [PubMed] [Google Scholar]
- Kernell D. The limits of firing frequency in cat lumbosacral motoneurones possessing different time course of afterhyperpolarization. Acta Physiol Scand. 1965;65:87–100. [Google Scholar]
- Kernell D. Functional properties of spinal motoneurons and gradation of muscle force. Adv Neurol. 1983;39:213–226. [PubMed] [Google Scholar]
- Kernell D, Bakels R, Copray JC. Discharge properties of motoneurones: how are they matched to the properties and use of their muscle units. J Physiol (Paris) 1999;93:87–96. doi: 10.1016/s0928-4257(99)80139-9. [DOI] [PubMed] [Google Scholar]
- Matthews PB. Relationship of firing intervals of human motor units to the trajectory of post-spike after-hyperpolarization and synaptic noise. J Physiol. 1996;492:597–628. doi: 10.1113/jphysiol.1996.sp021332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PB. Properties of human motoneurones and their synaptic noise deduced from motor unit recordings with the aid of computer modelling. J Physiol (Paris) 1999a;93:135–145. doi: 10.1016/s0928-4257(99)80144-2. [DOI] [PubMed] [Google Scholar]
- Matthews PB. The effect of firing on the excitability of a model motoneurone and its implications for cortical stimulation. J Physiol. 1999b;518:867–882. doi: 10.1111/j.1469-7793.1999.0867p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PB. A new way of using modelling to estimate the size of a motoneurone's EPSP. Adv Exp Med Biol. 2002a;508:193–197. doi: 10.1007/978-1-4615-0713-0_23. [DOI] [PubMed] [Google Scholar]
- Matthews PB. Measurement of excitability of tonically firing neurones tested in a variable-threshold model motoneurone. J Physiol. 2002b;544:315–332. doi: 10.1113/jphysiol.2002.024984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB, Yemm R. The orderly recruitment of human motor units during voluntary isometric contractions. J Physiol. 1973;230:359–370. doi: 10.1113/jphysiol.1973.sp010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monster AW, Chan H. Isometric force production by motor units of extensor digitorum communis muscle in man. J Neurophysiol. 1977;40:1432–1443. doi: 10.1152/jn.1977.40.6.1432. [DOI] [PubMed] [Google Scholar]
- Moore GP, Perkel DH, Segundo JP. Statistical analysis and functional interpretation of neuronal spike data. Annu Rev Physiol. 1966;28:493–522. doi: 10.1146/annurev.ph.28.030166.002425. [DOI] [PubMed] [Google Scholar]
- Nordstrom MA, Miles TS, Veale JL. Effect of motor unit firing pattern on twitches obtained by spike-triggered averaging. Muscle Nerve. 1989;12:556–567. doi: 10.1002/mus.880120706. [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Relationship between the time course of the afterhyperpolarization and discharge variability in cat spinal motoneurones. J Physiol. 2000;528:131–150. doi: 10.1111/j.1469-7793.2000.t01-1-00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol. 2001;143:137–263. doi: 10.1007/BFb0115594. [DOI] [PubMed] [Google Scholar]
- Powers RK, Turker KS, Binder MD. What can be learned about motoneurone properties from studying firing patterns. Adv Exp Med Biol. 2002;508:199–205. doi: 10.1007/978-1-4615-0713-0_24. [DOI] [PubMed] [Google Scholar]
- Stein RB, French AS, Mannard A, Yemm R. New methods for analysing motor function in man and animals. Brain Res. 1972;40:187–192. doi: 10.1016/0006-8993(72)90126-6. [DOI] [PubMed] [Google Scholar]
- Stephens JA, Usherwood TP. The mechanical properties of human motor units with special reference to their fatiguability and recruitment threshold. Brain Res. 1977;125:91–97. doi: 10.1016/0006-8993(77)90361-4. [DOI] [PubMed] [Google Scholar]
- Taylor A, Stephens JA. Study of human motor unit contractions by controlled intramuscular microstimulation. Brain Res. 1976;117:331–335. doi: 10.1016/0006-8993(76)90742-3. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Steege JW, Enoka RM. Motor-unit synchronization alters spike-triggered average force in simulated contractions. J Neurophysiol. 2002;88:265–276. doi: 10.1152/jn.2002.88.1.265. [DOI] [PubMed] [Google Scholar]
- Thomas CK. Human motor units studied by spike-triggered averaging and intraneural motor axon stimulation. Adv Exp Med Biol. 1995;384:147–160. doi: 10.1007/978-1-4899-1016-5_12. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Johansson RS, Bigland-Ritchie B. Attempts to physiologically classify human thenar motor units. J Neurophysiol. 1991;65:1501–1508. doi: 10.1152/jn.1991.65.6.1501. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Ross BH, Stein RB. Motor-unit recruitment in human first dorsal interosseous muscle for static contractions in three different directions. J Neurophysiol. 1986;55:1017–1029. doi: 10.1152/jn.1986.55.5.1017. [DOI] [PubMed] [Google Scholar]
- Vrbová G, Gordon T, Jones R. Nerve-Muscle Interaction. 2. London: Chapman & Hall; 1995. Plasticity of muscles and their motor units; pp. 109–136. [Google Scholar]
- Warren JD, Miles TS, Turker KS. Properties of synaptic noise in tonically active human motoneurons. J Electromyogr Kinesiol. 1993;2:189–202. doi: 10.1016/1050-6411(92)90023-C. [DOI] [PubMed] [Google Scholar]
- Young JL, Mayer RF. Physiological properties and classification of single motor units activated by intramuscular microstimulation in the first dorsal interosseus muscle in man. In: Desmedt JE, editor. Motor Unit Types Recruitment and Plasticity in Health and Disease. Basel: Karger; 1981. pp. 17–25. [Google Scholar]
- Zengel JE, Reid SA, Sypert GW, Munson JB. Membrane electrical properties and prediction of motor-unit type of medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1985;53:1323–1344. doi: 10.1152/jn.1985.53.5.1323. [DOI] [PubMed] [Google Scholar]


