Abstract
Large conductance calcium- and voltage-activated potassium (BK) channels are widely expressed in the mammalian central nervous system. Although the activity of BK channels in endocrine and vascular cells is regulated by protein kinases and phosphatases associated with the channel complex, direct evidence for such modulation in neurons is largely lacking. Single-channel analysis from inside-out patches isolated from the soma of dissociated rat cerebellar Purkinje neurons demonstrated that the activity of BK channels is regulated by multiple endogenous protein kinases and protein phosphatases in the membrane patch. The majority of BK channels were non-inactivating and displayed a ‘low’ activity phenotype determined at +40 mV and 1 μM intracellular free calcium. These channels were activated by cAMP-dependent protein kinase (PKA) associated with the patch and the extent of PKA activation was limited by an opposing endogenous type 2A-like protein phosphatase (PP2A). Importantly, PKA activation was dependent upon the prior phosphorylation status of the BK channel complex dynamically controlled by protein kinase C (PKC) and protein phosphatase 1 (PP1). In contrast, Purkinje cells also displayed a low proportion of non-inactivating BK channels with a ‘high’ activity under the same recording conditions and these channels were inhibited by endogenous PKA. Our data suggest that: (1) multiple endogenous protein kinases and phosphatases functionally couple to the BK channel complex to allow conditional modulation of BK channel activity in neurons, and (2) native, phenotypically distinct, neuronal BK channels are differentially sensitive to PKA-dependent phosphorylation.
Large conductance calcium- and voltage-activated potassium (BK) channels are widely expressed in the mammalian nervous system where their unique regulation by both transmembrane voltage and intracellular free calcium levels allows them to exert a powerful influence on neuronal excitability (Vergara et al. 1998; Gribkoff et al. 2001; Hu et al. 2001; Sah & Faber, 2002). BK channel sensitivity to voltage and calcium is potently modified by reversible protein phosphorylation of serine/threonine or tyrosine residues within the channel, or closely associated regulatory proteins (Levitan, 1999). Thus the dynamic interactions between competing protein kinases and protein phosphatases at the channel complex provide an important mechanism to tune BK channel function and behaviour.
Increasing electrophysiological and biochemical evidence suggests that BK channel proteins are associated with a variety of protein kinases and protein phosphatases within a regulatory complex at the plasma membrane of excitable cells (White et al. 1991; Bielefeldt & Jackson, 1994; Reinhart & Levitan, 1995; Levitan, 1999; Wang et al. 1999; Hall & Armstrong, 2000; Shipston, 2001; Tian et al. 2001). However native BK channels show considerable diversity in their modulation by distinct kinase/phosphatase signalling pathways depending on the cell type under investigation (Levitan, 1999; Shipston, 2001). Such phenotypic diversity is likely to arise as a result of alternative pre-mRNA splicing from the single gene (KCNMA1) encoding the pore forming α-subunits (Tseng-Crank et al. 1994; Xie & McCobb, 1998; Shipston, 2001; Tian et al. 2001; Zhou et al. 2001), association of α-subunits with regulatory β-subunits (McManus et al. 1995; Dworetzky et al. 1996; Xia et al. 1999; Weiger et al. 2000; Jin et al. 2002; Wang et al. 2002) and/or associated proteins (Schopperle et al. 1998; Xia et al. 1998; Wang et al. 1999; Zhou et al. 1999) as well as through differential assembly of BK channels with protein kinase/protein phosphatase signalling complexes.
Although BK channels have been demonstrated to be regulated by endogenous protein kinases and protein phosphatases in a number of systems (Levitan, 1999; Schubert & Nelson, 2001; Shipston, 2001) such evidence is, surprisingly, limited from defined cells of the nervous system (Bielefeldt & Jackson, 1994; Lee et al. 1995; Smith & Ashford, 2000). However, reconstitution of rat brain channels into artificial lipid bilayers results in a diversity of BK channel phenotypes with distinct modulation by serine/ threonine protein kinases and protein phosphatases that remain intimately associated with the channels in the bilayer (Reinhart et al. 1991; Reinhart & Levitan, 1995). Moreover native neuronal BK channels have been reported to be regulated by distinct phosphorylation-dependent pathways (Bielefeldt & Jackson, 1994; Lee et al. 1995; Smith & Ashford, 2000).
To further examine the functional diversity of native BK channel regulation by endogenous protein kinases and protein phosphatases in a defined neuronal population we examined the regulation of native BK channels in rat cerebellar Purkinje neurons. Cerebellar Purkinje neurons display some of the highest levels of BK channel expression in the adult mammalian central nervous system (Knaus et al. 1996). Furthermore, considerable diversity in the sensitivity to calcium and voltage as well as single-channel conductance has been reported in mammalian Purkinje neurons (Gruol, 1984; Gruol et al. 1991; Jacquin & Gruol, 1999; Womack & Khodakhah, 2002). BK channels have also been reported to modify the physiology of cerebellar Purkinje neurons (Cingolani et al. 2002; Womack & Khodakhah, 2002). Although the precise functional role of BK channels in Purkinje neurons has not been clearly defined (Llinas & Sugimori, 1980; Gruol et al. 1992; Deschutter & Bower, 1994; Raman & Bean, 1999; Muller et al. 2000; Womack & Khodakhah, 2001) we have exploited this model system to address whether (1) native BK channels of distinct phenotype are differentially sensitive to the same kinase signalling pathway and (2) multiple endogenous protein kinases and phosphatases functionally couple to the BK channel complex to allow fine tuning of BK channel activity in neurons.
METHODS
Preparation of freshly dissociated Purkinje neuron cell bodies
Rats aging from 10 to 17 postnatal days (mean age: 13.9 ± 0.2 days) were killed by decapitation following cervical dislocation according to UK Home Office guidelines. After removal of the cerebellum, the vermis was isolated and the two cerebellar hemispheres discarded. Neurons were dissociated following the method described by Raman & Bean (1999). Briefly, the tissue piece was first incubated in the following solution (mM): 82 NaSO4, 30 KSO4, 5 MgCl2, 10 Hepes, 10 glucose, pH 7.4. containing 3 mg ml−1 protease XXIII (Sigma) for 8 to 14 min at 37 °C under constant oxygenation. Tissue was rinsed for 5 min in the above solution containing trypsin inhibitor (1 mg ml−1, Sigma) and bovine serum albumin (1 mg ml−1, Sigma), before being transferred to Locke solution at room temperature that contained (mM): 130 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 10 glucose and 10 Hepes, pH 7.4), and mechanically dissociated using a fire-polished pipette. Cells were then plated in a 500 μl dish for electrophysiological recording and used within 3 h of dissociation. The bottom of the recording dish consisted of a microscope slide pre-coated with poly-lysine (BDH Laboratory Supplies). Osmolarity of all solutions was adjusted to 295-300 mosmol l−1.
Single-channel recording
All experiments were performed in the isolated inside-out configuration of the patch clamp technique at room temperature (20-24 °C) using an equimolar potassium gradient. The intracellular (bath) solution contained (mM): 140 potassium gluconate, 2 MgCl2, 5 BAPTA, 15 Hepes, 1 ATP, pH 7.2 with free calcium buffered to 1 μM. The extracellular (patch pipette) solution contained (mM): 130 potassium gluconate, 10 KCl, 10 Hepes, 1 MgCl2, 16 sucrose, pH 7.4. The osmolarity of both solutions was adjusted to 295-300 mosmol l−1.
Patch pipettes were pulled from borosilicate glass with filament (GC150F-7.5; Harvard Apparatus) on a horizontal puller (Flaming-Brown, P-97, Sutter Instruments). To minimise the number of channels within a single patch, the patch pipette resistance, under the potassium gluconate recording conditions used, was typically 20-25 MΩ. In preliminary experiments, drug addition was performed by continuous gravity driven perfusion at a flow rate of 1 ml min−1 using a bath volume of 0.5 ml. However, to maintain patch stability over tens of minutes of recording and minimise flow-induced changes in activity observed at perfusion rates > 1 ml min−1, the majority of experiments were performed by direct drug application to the bath. As such, the time of drug application in all figures is given with no correction for perfusion or diffusion time. In all experiments BK channel activity was allowed to stabilise for at least 7-10 min following inside-out patch excision before drug application.
Data acquisition and voltage protocols were controlled by an Axopatch 200B amplifier and pCLAMP6 software (Axon Instruments Inc., Union City, USA). All recordings were sampled at 10 kHz, filtered at 2 Hz and analysed without further post-acquisition filtering. Note that for figure presentation, single-channel traces are displayed at 0.4 kHz for printing resolution. Single-channel open probability (Po) was derived either from single-channel analysis using pSTAT for patches with more than four channels, or in the case of patches with more than four channels, by an integration-over-baseline algorithm using Igor Pro 4.1 (WaveMetrics, Lake Oswego, OR, USA). NPo (number of functional channels × open probability of channel) values were determined as follows: all-point histograms were plotted to obtain the ‘offset’, i.e. leak current, as well as the single-channel current amplitude from the peak intervals and number of channels in the patch. After subtraction of the offset from the traces these were integrated and the integral divided by integration time and single-channel current amplitude gives NPo.
The activity of non-inactivating channels was determined at a holding potential of +40 mV. The mean percentage (%) change in channel activity after a treatment, in patches with low to moderate levels of channel expression, mean Po or NPo was determined by averaging the channel activity immediately prior to the treatment and after treatment. For analysis of sustained activation, or inhibition, the mean percentage change was determined by the ratio of the mean channel activity determined over 5 min starting 8-10 min after drug application to a 5 min period immediately prior to drug application. For analysis of transient activation, an area under the curve algorithm was applied during channel activation and expressed as a ratio of the mean activity determined over 5 min immediately prior to drug application. For inactivating channels, a voltage pulse protocol using alternating 3 min steps at -60 mV and +40 mV was used. The inactivation time course was obtained by calculating the NPo in each 5 s period during the first 2 min after the start of the pulse to +40 mV. Inactivation was mostly complete after 1 min at +40 mV (see Results).
Mean change in activity was expressed as a percentage (%) of the pre-treatment control ± S.E.M. Data were analysed by ANOVA with significance between different treatments at P < 0.01.
Chemicals
Unless otherwise stated, reagents were purchased from Sigma Chemical company (Poole, Dorset, UK) and were of the highest analytical grade. Okadaic acid and phorbol myristate acetate (PMA) were from Alexis Biochemicals (Nottingham, UK). Protein phosphatase inhibitor 2 (PPI2), the protein kinase A inhibitor (PKI15-24) peptide and purified rat brain protein kinase C catalytic subunit (PKC) were from Calbiochem (Nottingham, UK).
RESULTS
Phenotypic variation of non-inactivating BK channels in rat cerebellar neurones
Analysis of non-inactivating large conductance calcium-activated potassium (BK) channels in 101 independent isolated inside-out patches from acutely isolated cerebellar Purkinje cell bodies of 10- to 17-day-old (13.9 ± 0.2 days) rats (Fig. 1) revealed a heterogeneity in single-channel open probability (Po). In an equimolar (140 mM) potassium gradient with 1 μM intracellular free calcium and 1 mM MgATP at a fixed membrane potential of +40 mV, the majority (73/101, 73 %) of channels had a starting Po of less than 0.25. In contrast, a few patches (9/101) had a starting Po of 0.8 or greater, while a further 19 patches contained channels whose activity fell between these two populations or contained mixed populations of high- and low-activity channels. The single-channel conductance of the low (< 0.25) and high (> 0.8) Po groups was not significantly different (201 ± 3.9 pS (n = 11) vs. 194 ± 2.9 pS (n = 9)). The heterogeneity in single-channel open probability observed between patches probably arose from both intra- and intercellular variation in BK channel phenotype.
Figure 1. Characterisation of cerebellar Purkinje BK channels as a function of ‘basal’ activity.
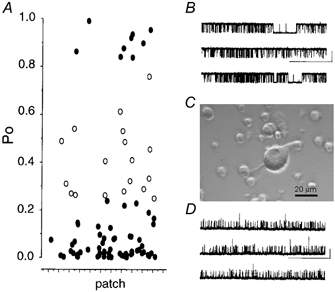
A, mean channel open probabilities (Po) plotted for each isolated inside-out patch in which the maximal number of channels was determined. All recordings were performed in the absence of any treatment in equimolar K+ gradient with 1 μM free calcium and 1 mM ATP with the membrane depolarised to +40 mV (n = 91). Channels with mean Po below or equal to 0.25, as well as Po above 0.8, are represented as filled circles. Channels with intermediate Po values (i.e. between 0.25 and 0.8) are represented as open circles. B and D, representative traces from ‘high’ activity (B, Po = 0.92) and ‘low’ activity (D, Po = 0.02) channel under identical recording conditions as above. (Scale bars: 10 pA, 20 s.) C, representative photograph of a freshly dissociated cerebellar Purkinje cell body from a 12-day-old rat. Note the large size of the Purkinje cell body, with characteristic ‘pear’ shape with dendritic stump and some axonal extension retained during cell isolation, with respect to the smaller cerebellar granule cells isolated in parallel.
Opposite PKA regulation of phenotypically distinct non-inactivating BK channels
Functional differences between the ‘low’ and ‘high’ activity populations of non-inactivating channels with respect to endogenous protein kinase A (PKA) associated with the patch was examined by analysing changes in open probability in response to activation of PKA with cAMP under the recording conditions used above.
Application of maximal concentrations (1 mM) of cAMP, to the intracellular face of patches containing ‘high’ activity non-inactivating BK channels resulted in a robust, sustained inhibition of BK channel Po by 53 ± 16 % in 4/4 patches (Fig. 2). The inhibitory action of cAMP was prevented by prior application of the PKA inhibitor peptide (PKI, 0.45 μM) before cAMP application (in 2/2 patches).
Figure 2. Sustained inhibition of ‘high’ activity BK channels by cAMP.
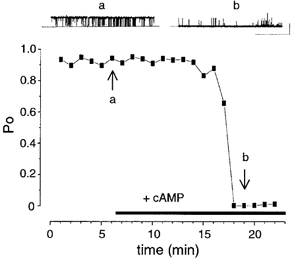
Representative traces and corresponding time course plot from a patch containing ‘high’ (stable starting Po > 0.80) activity BK channels. The two arrows labelled a and b, indicate the respective mean Po for the corresponding traces in the upper panels. (Scale bars: 10 pA, 10 s.)
In direct contrast, non-inactivating channels with a stable, low (< 0.25) starting Po were transiently activated (24 ± 12-fold (range 9- to 40-fold) in 7/7 patches) upon application of cAMP to the intracellular face of the patch. The duration of the transient activation was typically less than 3 min (Fig. 3) in the continued presence of cAMP. In three patches tested, the transient effect of cAMP was repeatable in the same patch. In these experiments, cAMP was applied resulting in a transient activation followed by a slow washout of cAMP. A subsequent application of cAMP resulted in a second transient activation similar in time course and magnitude to the initial response.
Figure 3. ‘Low’ activity BK channels are transiently activated by cAMP.
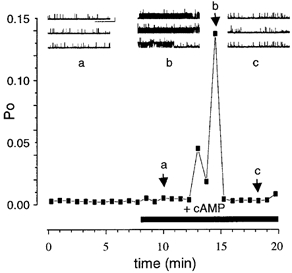
Representative traces and corresponding time course plot from a patch containing ‘low’ (stable starting Po < 0.25) activity non-inactivating BK channels. The transient increase in channel Po occurs in the continued presence of cAMP, arising from a very low and steady resting Po and fully recovers. Note that the patch contained three non-inactivating channels and no inactivating BK channels. The three arrows labelled a, b and c indicate the Po for the corresponding traces in the upper panels. (Scale bars: 10 pA, 5 s.)
Elevation of cAMP to 5 mM did not result in a sustained response in these channels. cAMP alone had no effect on channel activity in the presence of the PKA inhibitor peptide (PKI, 0.45 μM) in 6/6 patches or in the absence of ATP (4/4 patches).
The transient phosphorylation-dependent activation of ‘low’ activity, non-inactivating BK channels in the continued presence of saturating concentrations of cAMP could potentially result from at least two distinct mechanisms: (1) ‘low’ activity non-inactivating channels are tightly regulated by associated protein phosphatases that antagonise the effect of cAMP-dependent protein phosphorylation or (2) cAMP-dependent phosphorylation results in transient removal of inactivation from inactivating BK channels that may be present in the same patch.
In several systems PKA regulation of BK channels has been reported to be antagonised by PP2A (Reinhart et al. 1991; Tian et al. 1998). To test whether the ‘low’ activity’ BK channels are regulated by endogenous PP2A-like protein phosphatases we exposed patches to 3 nM okadaic acid, which blocks PP2A, but not PP1, prior to application of cAMP to the intracellular face of the channels. Pretreatment of patches with 3 nM okadaic acid resulted in a sustained activation of channel activity upon subsequent application of cAMP in 6/6 patches (Fig. 4 and Fig. 7) supporting a role for dephosphorylation in the rapid reversal of cAMP action. The mean activation was 26 ± 20-fold (ranging from 2.1- to 127.9-fold), determined between 5 and10 min after application (P < 0.01, Fig. 4 and Fig. 7). Okadaic acid alone (3 nM) had no significant effect on channel activity over the same time course (94 ± 21 % of control, n = 5, Fig. 7). The sustained effect of cAMP in the presence of 3 nM okadaic acid was completely blocked by pretreatment of patches with the PKA inhibitor peptide, PKI (118 ± 69 % of control, n = 3, Fig. 5 and Fig. 7). Taken together these data suggest that PP2A serves as a brake to limit the action of cAMP-dependent phosphorylation on ‘low’ activity phenotype BK channels.
Figure 4. Sustained activation of ‘low’ activity BK channels by cAMP in the presence of 3 nM okadaic acid.
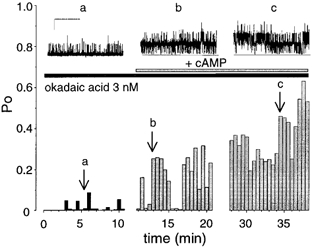
Representative traces and corresponding diary plot of channel open probability against time from a patch expressing ‘low’ activity (stable starting Po < 0.25) BK channels pretreated with 3 nM okadaic acid and exposed to cAMP. The arrows labelled a, b, and c, indicate the channel activity for the different traces illustrated in the upper panels. (Scale bars: 10 pA, 10 s.)
Figure 7. Summary of effects of protein phosphatase inhibitors on sustained cAMP-dependent activation of ‘low’ activity BK channels.
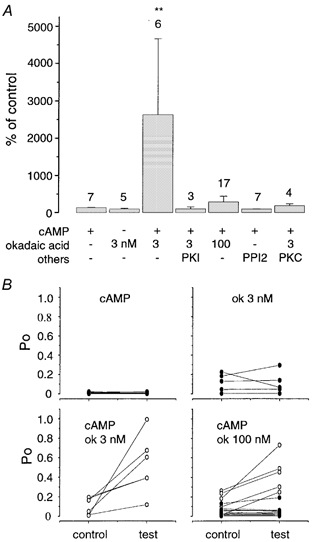
A, bar graph summary of the different treatments. Each column represents the mean change in Po in the respective treatment, measured 10 min after application of cAMP compared to the corresponding period before cAMP application. The number of patches tested is given above each bar. Note that application of cAMP in the absence of protein phosphatase inhibitors results in transient activation (Fig. 3) and thus activation is negligible over the period used to determine sustained activity. Patches were pretreated (at least 10 min) in the absence (-) or presence (+) of the respective phosphatase inhibitor (okadaic acid (ok) at 3 or 100 nM, protein kinase A inhibitor peptide (PKI) at 0.45 μM, the specific protein phosphatase I inhibitor peptide (PPI2) at 20 nM or 0.8 μM protein kinase C together with 100 nM PMA as indicated in the figure. All patches were then exposed to cAMP. Data are given as means ± S.E.M. Statistical difference between groups using an ANOVA is indicated by ** (P < 0.01). B, individual data obtained under the different treatments summarised in A. Each panel represents a different treatment. The resting Po (control) and the Po measured 10 min after cAMP (or okadaic acid alone in upper right panel) application (test) are linked by a straight line. In each panel, the open circles represent mean sustained increases greater than 2-fold whereas channels with no, or less than 2-fold, changes are shown by filled circles. Note that all resting Po values were below 0.25.
Figure 5. The sustained stimulatory effect of cAMP is mediated by PKA-dependent protein phosphorylation.
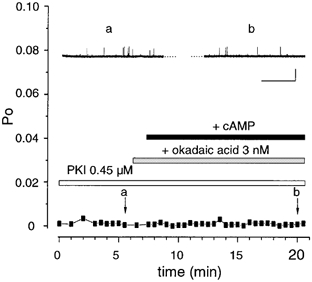
Representative traces and corresponding diary plot from a patch containing ‘low’ activity (stable starting Po < 0.25) BK channels pretreated with 3 nM okadaic acid and in the presence of the PKA inhibitor peptide PKI5-24. The two arrows labelled a and b, indicate the respective mean Po for the corresponding traces in the upper panels. (Scale bars: 10 pA, 10 s).
Inactivating BK channels do not play a significant role in the transient or sustained activation of ‘low’ activity BK channels
Although dephosphorylation by PP2A clearly plays an important role in limiting cAMP-dependent protein phosphorylation of the ‘low’ activity BK channels, both the transient effect of cAMP alone and the sustained activation in the presence of 3 nM okadaic acid could potentially result from removal of inactivation from inactivating BK channels present in the patch. Inactivating channels were observed in 44 % of patches and displayed characteristics of slowly inactivating BK channels previously reported in rat hippocampal (Hicks & Marrion, 1998) and cortical neurones (Smith & Ashford, 2000). Rat Purkinje neuron inactivating BK channels displayed a wide range of inactivation rates (16.9 ± 5.4 s ranging from 6.3 s to 22.7 s (Fig. 6A) and relief of inactivation upon hyperpolarisation. In preliminary experiments, the activity of inactivating channels was highly variable (as previously reported using similar calcium and voltage protocols (Smith & Ashford, 2000)) over the time course (> 15 min) of experiments when voltage step protocols were used to analyse inactivating BK channels. Furthermore, patches in which inactivating BK channels were exclusively present were extremely rare, thus further precluding direct analysis.
Figure 6. Inactivating BK channels do not contribute significantly to activation by cAMP.
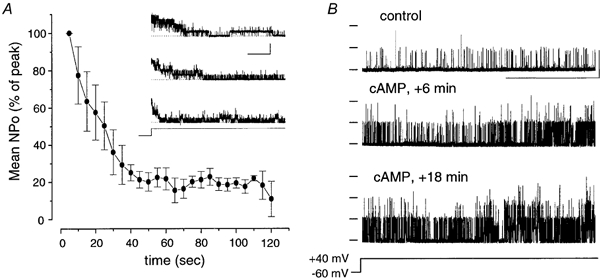
A, time course plot of normalised NPo measured over 5 s periods averaged from three patches from independent neurons expressing inactivating BK channels. Representative traces of inactivating channels from these neurons are shown in the inset, illustrating the variability in the inactivation time course between patches. Note that in all three cases, the inactivation was complete within a minute. The data were measured under the ionic conditions as in Fig. 1 with the patch potential stepped from -60 mV to +40 mV for 3 min as indicated in voltage protocol. (Scale bars: 20 pA, 20 s.) B, sustained activation of BK channel activity by cAMP in the presence of 3 nM okadaic acid in patches lacking functional inactivating channels. Representative single-channel traces from a patch recorded as in A using the voltage step protocol before (control) or after (+ cAMP) exposure to cAMP. (Scale bars: 10 pA, 20 s.)
However, transient activation of ‘low’ activity non-inactivating BK channels by cAMP was observed in 4/4 patches that had previously been characterised as not containing inactivating BK channels (Fig. 3). Furthermore, using a voltage step protocol that allows monitoring of inactivating BK channels over the full time course of the assay (Fig. 6), cAMP in the presence of 3 nM okadaic acid also resulted in a sustained activation of BK channel activity in 5/5 patches that did not contain inactivating BK channels (Fig. 6B). Thus although we cannot exclude inactivating BK channels as having a minor contribution to the cAMP-stimulated activation it is unlikely they are a significant source.
PKA regulation of ‘low’ activity BK channels is conditional on prior channel phosphorylation status controlled by protein kinase C and protein phosphatase 1
Inhibition of endogenous PP2A, using 3 nM okadaic acid, resulted in a sustained activation of ‘low’ activity BK channels by cAMP, suggesting that PP2A limits the extent of PKA-dependent activation. Paradoxically, increasing the concentration of okadaic acid to 100 nM, which blocks both PP2A and protein phosphatase 1 (PP1), resulted in one of two distinct responses: either sustained activation or no response to cAMP (Fig. 7B). In the majority (12/17) of patches, no significant effect of cAMP, either transient or sustained, was observed. As both PP2A and PP1 are inhibited in these experiments our data would suggest that in these 12 patches, endogenous PP1 activity is required for cAMP activation of ‘low’ activity BK channels i.e. endogenous PP1 acts as a functional ‘switch’ determining channel regulation by PKA. As simultaneous inhibition of PP1 and PP2A results in a complete loss of cAMP-mediated stimulation in these 12 patches, the data would suggest that PP1 acts antagonistically to PP2A. More importantly, an endogenous protein kinase must be active under our recording conditions, in the presence of MgATP, to phosphorylate sites sensitive to dephosphorylation by PP1. However, in 5/17 patches, cAMP in the presence of 100 nM okadaic acid resulted in a robust (> 2-fold) sustained stimulation of channel activity (Fig. 7B) not significantly different from the effect of cAMP when PP2A alone is inhibited using 3 nM okadaic acid (mean activation for the channels in five patches that were activated by cAMP in the presence of 100 nM okadaic acid was 758 ± 285 %). This small proportion (in 5/17 patches) of channels that underwent sustained activation in response to cAMP when both PP1 and PP2A were inhibited by 100 nM okadaic acid may represent a distinct channel population. Alternatively, they probably represent channels in which the endogenous protein kinase that phosphorylates the PP1-sensitive site is not functionally associated with the patch. Thus although the mean cAMP-dependent activation when PP1 and PP2A were inhibited by 100 nM okadaic acid for all 17 patches was only 289 ± 152 % (Fig. 7A) this was composed of two distinct populations of response: either no effect of cAMP (70 % of patches) or a sustained activation (30 %) (Fig. 7B).
To further test the hypothesis that endogenous PP1 activity is required for the functional effect of cAMP-dependent phosphorylation, and thus PP1 acts as a conditional switch of ‘low’ activity BK channel regulation by PKA, we inhibited endogenous PP1 alone using the specific peptide inhibitor PPI2. If PP1 does indeed act as a ‘switch’, our model would predict that cAMP would have no effect (either sustained or transient) in patches in which PP1 is inhibited but the endogenous kinase that phosphorylates the PP1-sensitive site is active. Indeed, in contrast to 7/7 patches containing ‘low’ activity BK channels that responded with a transient activation in the presence of cAMP alone (Fig. 3), only in 1/7 patches were channels transiently activated when PP1 alone was inhibited using PPI2 (Fig. 7). Sustained increase in channel activity upon cAMP application was never observed in any patch (7/7 patches) in the presence of PPI2. It is likely that in the one patch in which channels were transiently activated by cAMP, the endogenous protein kinase that phosphorylates the PP1-sensitive site was not functionally associated with the patch.
Taken together these data suggest that PP1 acts as a conditional switch of cAMP-dependent phosphorylation of BK channels in rat Purkinje cells. Thus under normal conditions PP1 would control the phosphorylation status of the channel complex, and inhibition of PP1 (using either 100 nM okadaic acid or the peptide inhibitor PPI2) would be predicted to allow sites normally kept dephosphorylated by PP1 to be phosphorylated by an endogenous protein kinase. Under our recording conditions, the presence of MgATP would support endogenous protein kinase activity in the isolated inside-out patch. In such a model we would predict that inhibition of this endogenous protein kinase would allow cAMP activation of the channels when PP1 is inhibited. Conversely, activation of endogenous protein kinase activity (or exogenous kinase application) would be predicted to completely occlude cAMP-dependent activation of ‘low’ activity BK channels in Purkinje neurons.
Previous studies of recombinant bovine BK channels expressed in HEK 293 cells demonstrated that protein kinase C (PKC) phosphorylation of PKC sites within the intracellular C-terminus of the channel occludes channel regulation by exogenous PKA (Zhou et al. 2001). To address whether the endogenous protein kinase that phosphorylates the PP1-sensitive site in ‘low’ activity BK channels is a member of the PKC family we took two approaches.
Firstly, under conditions in which both PP1 and PP2A are simultaneously inhibited (using 100 nM okadaic acid) we addressed whether inhibition of endogenous PKC activity increased the efficacy of cAMP activation of ‘low’ activity BK channels. For these assays we used the specific PKC inhibitor bisindolylmaleimide I (BIS), at concentrations (100 nM) that block PKC but not PKA activity. Under these conditions as PP1, PKC and PP2A are all inhibited we would predict a sustained activation by cAMP. Indeed, in 5/6 (83 %) of patches tested in the presence of 100 nM okadaic acid and 100 nM BIS, a robust sustained activation of BK channel activity was observed upon application of cAMP (Fig. 8). This is in contrast to channels in only 5/17 (29 %) of patches activated by cAMP in the presence of 100 nM okadaic acid alone (Fig. 7B). Thus inhibition of endogenous PKC, simultaneously with inhibition of PP1 and PP2A, increases the proportion of ‘low’ activity BK channels that are responsive to cAMP. The mean cAMP activation in the presence of 100 nM okadaic acid and 100 nM BIS in the five patches that responded to cAMP was 771 ± 286 %, n = 5 compared to 758 ± 285 % for channels in the five patches that were activated in the presence of 100 nM okadaic acid alone (Figs 7A and B and 8). These data support a model in which the endogenous protein kinase controlling the PP1-sensitive site is indeed a member of the PKC family.
Figure 8. Inhibiting PKC with BIS increases efficiency of cAMP activation in the presence of 100 nM okadaic acid.
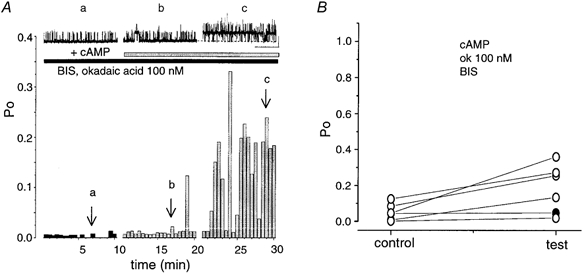
A, representative channel traces and diary plots of channel activity in patches containing ‘low’ activity BK channels pretreated with 100 nM okadaic acid (ok) and 100 nM BIS before application of cAMP. The arrows labelled a, b, and c, correspond to the recordings above, illustrating the increase in the activity of BK channels (scale bars: 10 pA, 10 s). B, data from individual patches showing mean Po before (control) and 10 min after cAMP application (test) are linked by a straight line. Note: under these conditions, 5/6 patches (83 %) patches responded with a sustained increase greater than 2-fold (open circles). In one patch the increase was less than 2-fold (1.83-fold, filled circle).
Secondly, we maximally elevated PKC activity at the intracellular face of ‘low’ activity channels by exposing isolated patches to a combination of the PKC activator phorbol myristate acetate (PMA) and purified rat brain PKC catalytic subunit prior to cAMP application in the presence of 3 nM okadaic acid. Thus PMA should activate any endogenous PMA-sensitive PKC in the patch whereas exogenous constitutively active PKC would allow channel phosphorylation in patches in which endogenous PKC was not functionally associated with the patch. Under these conditions, cAMP had no significant effect on channel activity in 4/4 patches (Fig. 7A and Fig. 9). The mean percentage activation in response to cAMP under these conditions was 118 ± 43 %, n = 4. This is in contrast to the sustained activation by cAMP in the presence of 3 nM okadaic acid alone in 6/6 patches (Fig. 4 and Fig. 7A and B). Furthermore, in one experiment, PMA and PKC were first applied prior to a cAMP application in the presence of 3 nM okadiac acid, resulting in no significant cAMP activation. PMA and PKC were then washed from the patch and a subsequent application of cAMP (under conditions in which PP2A, but not PP1, is inhibited by 3 nM okadaic acid) resulted in a sustained activation of BK channel activity in the same patch. These data support the hypothesis that PKC phosphorylation occludes cAMP stimulation of ‘low’ activity BK channels in Purkinje neurons (Fig. 9).
Figure 9. Activation of PKC prevents cAMP-stimulated activation of BK channels.
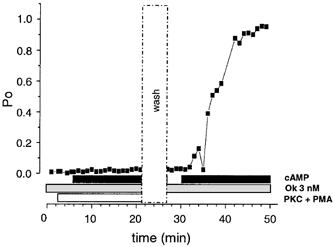
Representative time-course plot of ‘low’ activity BK channel open probability in patches pretreated with 0.8 μM PKC catalytic subunit and 100 nM PMA (open horizontal bar), before application of cAMP (filled horizontal bar) in the presence of 3 nM okadaic acid (ok, shaded bar). Under these conditions cAMP has no effect on channel open probability. In this patch after washout (vertical open dashed bar, wash) a subsequent cAMP application in the presence of 3 nM okadaic acid resulted in a sustained increase in channel open probability.
Taken together, these data support a model in which PP1 and PKC act at a common site(s) acting as a switch to determine the response of ‘low’ activity BK channels to cAMP-dependent phosphorylation.
DISCUSSION
The dynamic interplay of protein kinases and protein phosphatases is essential for coordinated regulation of ion channel behaviour by reversible protein phosphorylation (Armstrong & White, 1992; Levitan, 1999). In several native and recombinant expression systems, distinct protein kinases and protein phosphatases have been reported to interact to determine BK channel activity. Furthermore, several studies have examined the functional impact of reversible phosphorylation on neuronal BK channels (Bielefeldt & Jackson, 1994; Lee et al. 1995; Smith & Ashford, 2000). However, few studies have directly addressed the interplay between endogenous protein kinases and phosphatases on BK channels in defined neurons.
Here we demonstrate, through analysis of non-inactivating BK channels from the soma of rat cerebellar Purkinje neurones, that the interplay between multiple, endogenous serine/threonine protein kinases and protein phosphatases closely associated with the BK channel complex provides a mechanism for the coordinated regulation of BK channel activity in Purkinje neurons. Furthermore, phenotypically distinct BK channels expressed in a defined neuronal cell system may be oppositely regulated by protein kinase A-dependent protein phosphorylation.
Phenotypic variation of BK channels in rat Purkinje neuron cell bodies
Characterisation of the intrinsic properties of BK channels expressed in the cell bodies of acutely dissociated rat cerebellar Purkinje cells under defined conditions of membrane depolarisation and intracellular free calcium (+40 mV and 1 μM respectively) revealed at least three phenotypically distinct BK channels: ‘high’ and ‘low’ activity non-inactivating BK channels and inactivating BK channels. Similar phenotypic diversity has been previously reported in several mammalian Purkinje neuron models (Gruol, 1984; Gruol et al. 1991; Jacquin & Gruol, 1999; Womack & Khodakhah, 2002). The majority of channels observed in isolated inside out patches were non-inactivating and displayed resting mean open probabilities of < 0.2 determined at +40 mV and 1 μM free calcium in the presence of MgATP. A small (≈10 %) proportion of patches displayed non-inactivating BK channels with a ‘high’ resting (> 0.8) mean open probability with a single-channel conductance (≈200 pS in equimolar 140 mM potassium) not significantly different from the low activity BK channels. In cortical neurons, mode switching of channels between high and low activity states has been reported (Smith & Ashford, 2000), but under our assay conditions similar spontaneous mode switching between ‘high’ and ‘low’ activity states was never observed in the same channel. A significant proportion (44 %) of patches also contained inactivating BK channels with characteristics similar to those previously reported in rat hippocampal (Hicks & Marrion, 1998) and cortical (Smith & Ashford, 2000) neurons. These channels displayed variable but slow (of the order of tens of seconds) inactivation that could be relieved upon hyperpolarisation.
‘High’ and ‘low’ activity BK channels are differentially regulated by endogenous PKA
To examine whether phenotypically distinct BK channels may be differentially regulated by endogenous cAMP-dependent protein kinase activity, we assayed the regulation of non-inactivating BK channels with ‘low’ and ‘high’ resting mean open probabilities in isolated inside-out patches. Direct analysis of endogenous PKA regulation of inactivating BK channels was not undertaken under the recording conditions used, due to several factors including the low proportion of patches containing exclusively inactivating BK channels and the variability in activity of inactivating channels with time.
Activation of endogenous PKA resulted in a sustained inhibition of ‘high’ activity non-inactivating BK channels whereas ‘low’ activity non-inactivating BK channels were transiently activated by endogenous PKA. The rapid reversal (transient) of PKA-dependent activation of ‘low’ activity BK channels, even in the continued presence of cAMP, resulted from the activity of a low nanomolar okadaic acid-sensitive PP2A-like phosphatase present in the patch as sustained PKA-dependent activation could be achieved by inhibiting the phosphatase activity with 3 nM okadaic acid. ‘Low’ activity BK channels were transiently activated by endogenous PKA while ‘high’ activity channels undergo sustained inhibition, under identical recording conditions in which phosphatases would be predicted to be active. This suggests that phosphatase activity associated with either channel phenotype is likely to be distinct, i.e. ‘basal’ phosphatase activity associated with ‘high’ activity channels is not sufficient to overcome the PKA-mediated inhibition. The molecular basis for the distinct phenotype and differential sensitivity of ‘high’ and ‘low’ activity BK channels to PKA in Purkinje neurons is at present unknown. Such functional diversity is likely to result either from BK channel assembly from distinct alternatively spliced variants of the pore-forming α-subunits (Tian et al. 2001), or through differential phosphorylation-dependent modulation of α-subunits by regulatory β-subunits (Dworetzky et al. 1996; Jin et al. 2002).
As inactivating BK channels were present in 44 % of patches, a contribution to the transient or sustained cAMP-activation of ‘low’ activity BK channels could potentially result from PKA-dependent relief from inactivation of inactivating BK channels present in the same patch (Hicks & Marrion, 1998; Smith & Ashford, 1998; Smith & Ashford, 2000). However, in patches that did not contain inactivating BK channels, identified using voltage protocols to relieve inactivation upon hyperpolarisation (Hicks & Marrion, 1998; Smith & Ashford, 2000), transient or sustained cAMP-dependent activation of ‘low’ activity BK channels was observed in the absence or presence of 3 nM okadaic acid, respectively. Furthermore, although dephosphorylation has been suggested to remove inactivation from slowly inactivating BK channels in rat cortical neurons, application of exogenous PKA in this system had no effect on channel inactivation (Smith & Ashford, 2000). Thus, inactivating BK channels are unlikely to play a significant role in the PKA-activation of ‘low’ activity BK channels reported here.
PP2A acts as a brake on PKA-activation of ‘low’ activity BK channels
‘Low’ activity BK channels demonstrated complex modulation by reversible protein phosphorylation mediated by distinct endogenous kinases and phosphatases associated with the patch. A working model of ‘low’ activity BK channel regulation is illustrated in Fig. 10. As previously reported for other neuronal BK channels (Reinhart et al. 1991; Lee et al. 1995), PKA activity associated with the patch stimulated channel activity in isolated inside-out patches. However, in cerebellar Purkinje neurons, PKA activation was transient resulting in return of BK channel activity within a few minutes, even in the continued presence of cAMP to activate PKA. The transient activation was sensitive to low concentrations (3 nM) of okadaic acid, resulting in a sustained activation, suggesting that a protein phosphatase 2A (PP2A)-like enzyme is responsible for limiting PKA activation of BK channels in Purkinje neurons. Indeed, PP2A is expressed at high levels in rat Purkinje neuron cell bodies at the age used in this study (Hashikawa et al. 1995). Okadaic acid (3 nM) on its own had no effect on BK channel activity suggesting that PP2A specifically antagonises the stimulatory action of PKA. Thus intrinsic PP2A activity at the patch is sufficiently high to allow rapid reversal of PKA-dependent phosphorylation but does not regulate the BK channel complex in the absence of PKA-dependent phosphorylation. Similar antagonism of PKA-mediated activation of BK channel activity by PP2A has been reported in rat brain BK channels reconstituted into bilayers (Reinhart et al. 1991). Furthermore, in endocrine cells, PP2A also blocks PKA inhibition of BK channel activity (Tian et al. 1998). Together these data suggest that PKA and PP2A may act as a functional kinase/phosphatase ‘module’ to control the phosphorylation status of BK channels and hence determine the ‘dynamic range’ of PKA activation irrespective of whether PKA activates or inhibits the native channel.
Figure 10. Schematic model of antagonistic action of protein kinases and phosphatases regulating ‘low’ activity BK channels in rat Purkinje neurones.
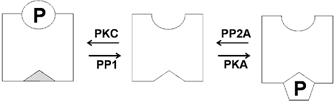
‘Low’ activity BK channels in Purkinje cell bodies are activated by protein kinase A-dependent phosphorylation (PKA) at site(s) (indicated by hexagon) independent of protein kinase C-dependent phosphorylation (indicated by circle). PKA activation is conditional on dephosphorylation of the PKC site(s) by protein phosphatase 1 (PP1) and the effect of PKA is antagonised by a protein phosphatase 2A-like (PP2A) protein phosphatase. Thus activation of PKA alone results in transient activation of channel activity and this activation may be sustained upon blockade of PP2A with 3 nM okadaic acid. Inhibition of PP1 (using 100 nM okadaic acid or the peptide inhibitor PPI2) allows PKC-mediated phosphorylation of the channel, rendering the channel functionally insensitive to subsequent PKA-dependent phosphorylation (indicated by filled triangle).
PKA activation of ‘low’ activity BK channels is conditional on a PKC/PP1 site
Intriguingly PKA activation of ‘low’ activity Purkinje BK channels was conditional on the prior phosphorylation state of the channel controlled by the dynamic interplay between endogenous PKC and PP1 activities. Inhibition of PP1 activity closely associated with the channel in isolated patches, using 100 nM okadaic acid or the peptide inhibitor PPI2, had no effect on channel activity per se but largely prevented subsequent channel activation by PKA. Thus, under conditions in which PP1 activity is inhibited, but phosphorylation can be supported by MgATP, an endogenous protein kinase must be active and lead to phosphorylation of the channel or associated proteins that makes the channel insensitive to regulation by PKA. In patches in which PPI was inhibited, PKA activation of ‘low’ activity BK channels was observed by co-applying a specific PKC inhibitor, bisindolylemaleimaide I (BIS-I), at concentrations (100 nM) that inhibit PKC but not PKA activity, suggesting that the endogenous kinase was a member of the PKC family. Furthermore, increasing PKC activity (through co-application of PMA to activate endogenous PKC along with exogenous application of catalytic PKC subunits) at the intracellular face of the channel prevented subsequent PKA stimulation of the channel. Although we have not defined the endogenous PKC isoform(s) that mediate this effect, Purkinje neurons express both typical and atypical PKC isoforms at high levels as well as PP1 (Hashikawa et al. 1995; Barmack et al. 2000).
In many neurons (Doerner et al. 1988) and endocrine cells (Shipston & Armstrong, 1996; Tian et al. 1999; Hall & Armstrong, 2000), PKC is a potent inhibitor of BK channel activity. In pituitary GH4C1 cells, PKC has been proposed to limit the maximal activity of BK channels, irrespective of the prevailing voltage and calcium conditions (Hall & Armstrong, 2000). Furthermore, the sensitivity of cloned bovine BK channels to modulation by PKA in HEK 293 cells has been reported to be dependent upon the prior PKC-dependent phosphorylation of the channel (Zhou et al. 2001). Cloned bovine BK channels in which the tandem BK channel C-terminal PKC consensus motifs are deleted are activated by PKA, whereas channel variants expressing functional PKC phosphorylation motifs are insensitive to PKA (Zhou et al. 2001). A similar conditional action of PKA, dependent upon prior PKC-dependent phosphorylation, is also observed in other channels such as voltage-activated sodium channels (Li et al. 1993). Whether PKC phosphorylation of the ‘low’ activity channels in our studies prevents subsequent PKA-mediated phosphorylation of the channel per se or modifies the functional impact of PKA phosphorylation remains to be determined. Our data demonstrate that PKC limits the availability of ‘low’ activity BK channels to subsequent modulation by PKA, and that the PKC phosphorylation sites are sensitive to PP1 but not PP2A.
A model for phosphorylation regulation of ‘low’ activity BK channels in rat Purkinje neurons
Taken together our data would support a model such as that outlined in Fig. 10 for the dynamic regulation of non-inactivating BK channels with the ‘low’ activity phenotype. The phosphorylation status of the channel complex dynamically controlled by PKC/PP1 acts as a molecular switch to determine the sensitivity of BK channels to subsequent regulation by PKA. In turn, PP2A acts as a rheostat to modify the ‘gain’ of PKA activation of BK channel activity. Such a model is analogous to that proposed for regulation of BK channels in pituitary GH4C1 cells in which PKC/PP1 limits the maximal activity of BK channels, whereas PKA/PP2A acts to shift the calcium sensitivity of the channel (Hall & Armstrong, 2000). As PKA inhibits BK channel activity in GH4C1 cells, taken together this would support a model in which the site(s) regulated by PKC/PP1 and PKA/PP2A are independent. It remains to be determined whether ‘high’ activity channels in Purkinje neurons are also dynamically regulated by such competing sites. Increasing evidence from mutagenesis studies reveals that the pore-forming α-subunit may be a direct target for both PKA- and PKC-mediated regulation of mammalian BK channels (Nara et al. 1998; Tian et al. 2001; Zhou et al. 2001). We cannot exclude that some of the effects observed here result from changes in the phosphorylation state of closely associated regulatory (McManus et al. 1995; Dworetzky et al. 1996; Xia et al. 1999; Weiger et al. 2000; Jin et al. 2002; Wang et al. 2002) or accessory subunits (Schopperle et al. 1998; Xia et al. 1998; Wang et al. 1999; Zhou et al. 1999). Irrespective of the molecular target(s), differential modulation of these protein kinase and phosphatase signalling pathways would provide a powerful, dynamic mechanism to modify BK channel activity and regulation in neurons.
The expression level of BK channels in cerebellar Purkinje neurons is amongst the highest within neurons of the mammalian central nervous system (Knaus et al. 1996). However, although BK channels are reported to contribute to the afterhyperpolarisation and modify action potential duration, frequency and calcium signalling, their functional role in Purkinje neurons is largely unknown (Llinas & Sugimori, 1980; Gruol et al. 1992; Deschutter & Bower, 1994; Raman & Bean, 1999; Muller et al. 2000; Womack & Khodakhah, 2001; Cingolani et al. 2002; Womack & Khodakhah, 2002). Previous studies have reported a wide variety of BK channel phenotypes in cerebellar Purkinje neuron isolated cell bodies as well as intact neurons in culture and cerebellar slices (Gruol, 1984; Gruol et al. 1991; Jacquin & Gruol, 1999; Womack & Khodakhah, 2002). For example in cultured rat Purkinje neurons, BK channels with high calcium sensitivity predominate (Jacquin & Gruol, 1999), whereas in acutely dissociated mouse Purkinje neurons, BK channels with lower calcium sensitivity predominate (Womack & Khodakhah, 2002). Our data suggest that an additional level of BK channel functional variation in Purkinje neurons is through the opposite and multi-level regulation of phenotypically distinct BK channels by reversible protein phosphorylation. Importantly, the dynamic interplay between distinct protein kinase and protein phosphatase signalling pathways on Purkinje BK channels most likely provides a powerful mechanism to modify BK channel activity in response to activation/inhibition of these diverse signalling pathways. The ability to switch and modify the dynamic range of BK channels at physiological voltages, and intracellular free calcium in Purkinje neurons, may provide a powerful cellular computational tool essential for the integration of Purkinje cell function.
Acknowledgments
We thank members of the Membrane Biology Group for useful discussions. This work was funded by The Wellcome Trust.
REFERENCES
- Armstrong DL, White RE. An enzymatic mechanism for potassium channel stimulation through pertussis-toxin-sensitive G-proteins. Trends Neurosci. 1992;15:403–408. doi: 10.1016/0166-2236(92)90192-b. [DOI] [PubMed] [Google Scholar]
- Barmack NH, Qian ZY, Yoshimura J. Regional and cellular distribution of protein kinase C in rat cerebellar Purkinje cells. J Comp Neurol. 2000;427:235–254. doi: 10.1002/1096-9861(20001113)427:2<235::aid-cne6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Jackson MB. Phosphorylation and dephosphorylation modulate a Ca2+-activated K+ channel in rat peptidergic nerve terminals. J Physiol. 1994;475:241–254. doi: 10.1113/jphysiol.1994.sp020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani LA, Gymnopoulos M, Boccaccio A, Stocker M, Pedarzani P. Developmental regulation of small-conductance Ca2+-activated K+ channel expression and function in rat Purkinje neurons. J Neurosci. 2002;22:4456–4467. doi: 10.1523/JNEUROSCI.22-11-04456.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschutter E, Bower JM. An active membrane model of the cerebellar Purkinje-cell. 1. simulation of current clamps in slice. J Neurophysiol. 1994;71:375–400. doi: 10.1152/jn.1994.71.1.375. [DOI] [PubMed] [Google Scholar]
- Doerner D, Pitler DA, Alger BE. Protein kinase C activators block specific calcium and potassium current components in isolated hippocampal neurones. J Neurosci. 1988;8:4069–4078. doi: 10.1523/JNEUROSCI.08-11-04069.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworetzky S, Boissard C, Lum-Ragan J, Mckay M, Post-Munson D, Trojnacki J, Chang C, Gribkoff V. Phenotypic alteration of a human BK (hslo) channel by hslo beta subunit co-expression: changes in blocker sensitivity, activation/relaxation kinetics, and protein kinase A modulation. J Neurosci. 1996;16:4543–4550. doi: 10.1523/JNEUROSCI.16-15-04543.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribkoff VK, Starrett JE, Dworetzky SI. Maxi-K potassium channels: form, function, and modulation of a class of endogenous regulators of intracellular calcium. Neuroscientist. 2001;7:166–177. doi: 10.1177/107385840100700211. [DOI] [PubMed] [Google Scholar]
- Gruol DL. Single channel analysis of voltage-sensitive K+ channels in cultured Purkinje neurons. Biophys J. 1984;45:53–55. doi: 10.1016/S0006-3495(84)84105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol DL, Deal CR, Yool AJ. Developmental-changes in calcium conductances contribute to the physiological maturation of cerebellar Purkinje neurons in culture. J Neurosci. 1992;12:2838–2848. doi: 10.1523/JNEUROSCI.12-07-02838.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol DL, Jacquin T, Yool AJ. Single-channel K+ currents recorded from the somatic and dendritic regions of cerebellar Purkinje neurons in culture. J Neurosci. 1991;11:1002–1015. doi: 10.1523/JNEUROSCI.11-04-01002.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SK, Armstrong DL. Conditional and unconditional inhibition of calcium-activated potassium channels by reversible protein phosphorylation. J Biol Chem. 2000;275:3749–3754. doi: 10.1074/jbc.275.6.3749. [DOI] [PubMed] [Google Scholar]
- Hashikawa T, Nakazawa K, Mikawa S, Shima H, Nagao M. Immunohistochemical localization of protein phosphatase isoforms in the rat cerebellum. Neurosci Res. 1995;22:133–136. doi: 10.1016/0168-0102(95)00886-x. [DOI] [PubMed] [Google Scholar]
- Hicks G, Marrion N. Ca2+-dependent inactivation of large conductance Ca2+-activated K+ (BK) channels in rat hippocampal neurones produced by pore block from an associated particle. J Physiol. 1998;508:721–734. doi: 10.1111/j.1469-7793.1998.721bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Shao L-R, Chavoshy S, Gu N, Trieb M, Behrens R, Laake P, Pongs O, Knaus H-G, Ottersen OP, Storm JF. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci. 2001;21:9585–9597. doi: 10.1523/JNEUROSCI.21-24-09585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquin TD, Gruol DL. Ca2+ regulation of a large conductance K+ channel in cultured rat cerebellar Purkinje neurons. Eur J Neurosci. 1999;11:735–739. doi: 10.1046/j.1460-9568.1999.00478.x. [DOI] [PubMed] [Google Scholar]
- Jin P, Weiger T, Wu Y, Levitan I. Phosphorylation-dependent functional coupling of hSlo calcium-dependent potassium channel and its hβ4 subunit. J Biol Chem. 2002;277:10014–10020. doi: 10.1074/jbc.M107682200. [DOI] [PubMed] [Google Scholar]
- Knaus H-G, Schwarzer C, Koch R, Eberhart A, Kaczorowski G, Glossman H, Wunder F, Pongs O, Garcia M, Sperk G. Distribution of high-conductance Ca2+-activated K+ channels in rat brain: targeting to axons and nerve terminals. J Neurosci. 1996;16:955–963. doi: 10.1523/JNEUROSCI.16-03-00955.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Rowe ICM, Ashford MJ. Characterization of an ATP-modulated large-conductance Ca2+-activated K+ channel present in rat cortical-neurons. J Physiol. 1995;488:319–337. doi: 10.1113/jphysiol.1995.sp020969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan IB. Modulation of ion channels by protein phosphorylation - how the brain works. Adv Sec Mess Phosphoprotein Res. 1999;33:3–22. doi: 10.1016/s1040-7952(99)80003-2. [DOI] [PubMed] [Google Scholar]
- Li M, West JW, Numann R, Murphy BJ, Scheuer T, Catterall WA. Convergent regulation of sodium channels by protein kinase C and cAMP-dependent protein kinase. Science. 1993;261:1439–1442. doi: 10.1126/science.8396273. [DOI] [PubMed] [Google Scholar]
- Llinas R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O, Helms L, Pallanck L, Ganetzky B, Swanson R, Leonard R. Functional role of the beta-subunit of high-conductance calcium-activated potassium channels. Neuron. 1995;14:645–650. doi: 10.1016/0896-6273(95)90321-6. [DOI] [PubMed] [Google Scholar]
- Muller YL, Reitstetter R, Yool AJ. Antisense knockdown of calcium-dependent K+ channels in developing cerebellar Purkinje neurons. Dev Brain Res. 2000;120:135–140. doi: 10.1016/s0165-3806(00)00004-3. [DOI] [PubMed] [Google Scholar]
- Nara M, Dhulipala PD, Wang YX, Kotlikoff MI. Reconstitution of beta-adrenergic modulation of large conductance, calcium-activated potassium (maxi-K) channels in Xenopus oocytes. Identification of the cAMP-dependent protein kinase phosphorylation site. J Biol Chem. 1998;273:14920–14924. doi: 10.1074/jbc.273.24.14920. [DOI] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J Neurosci. 1999;19:1663–1674. doi: 10.1523/JNEUROSCI.19-05-01663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart PH, Chung SK, Martin BL, Brautigan DL, Levitan IB. Modulation of calcium-activated potassium channels from rat brain by protein kinase A and phosphatase 2A. J Neurosci. 1991;11:1627–1635. doi: 10.1523/JNEUROSCI.11-06-01627.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart PH, Levitan IB. Kinase and phosphatase-activities intimately associated with a reconstituted calcium-dependent potassium channel. J Neurosci. 1995;15:4572–4579. doi: 10.1523/JNEUROSCI.15-06-04572.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ESL. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol. 2002;66:345–353. doi: 10.1016/s0301-0082(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Schopperle W, Holmqvist M, Zhou Y, Wang J, Wang Z, Griffith L, Keselman I, Kusinitz F, Dagan D, Levitan I. Slob, a novel protein that interacts withthe slowpoke calcium-dependent potassium channel. Neuron. 1998;20:565–573. doi: 10.1016/s0896-6273(00)80995-2. [DOI] [PubMed] [Google Scholar]
- Schubert R, Nelson MT. Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci. 2001;22:505–512. doi: 10.1016/s0165-6147(00)01775-2. [DOI] [PubMed] [Google Scholar]
- Shipston MJ. Alternative splicing of potassium channels: a dynamic switch of cellular excitability. Trends Cell Biol. 2001;11:353–358. doi: 10.1016/s0962-8924(01)02068-2. [DOI] [PubMed] [Google Scholar]
- Shipston M, Armstrong D. Activation of protein kinase C inhibits calcium-activated potassium channels in rat pituitary tumour cells. J Physiol. 1996;493:665–672. doi: 10.1113/jphysiol.1996.sp021413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M, Ashford M. Mode switching characterises the activity of large-conductance potassium channels recorded from rat cortical fused nerve terminals. J Physiol. 1998;513:733–747. doi: 10.1111/j.1469-7793.1998.733ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Ashford MLJ. Inactivation of large-conductance, calcium-activated potassium channels in rat cortical neurons. Neuroscience. 2000;95:33–50. doi: 10.1016/s0306-4522(99)00425-x. [DOI] [PubMed] [Google Scholar]
- Tian L, Duncan RR, Hammond SL, Coghill LC, Wen H, Rusinova R, Clark AG, Levitan IB, Shipston MJ. Alternative splicing switches potassium channel sensitivity to protein phosphorylation. J Biol Chem. 2001;276:1717–1720. doi: 10.1074/jbc.C000741200. [DOI] [PubMed] [Google Scholar]
- Tian L, Knaus H-G, Shipston MJ. Glucocorticoid regulation of calcium-activated potassium channels mediated by serine/threonine protein phosphatase. J Biol Chem. 1998;273:13531–13536. doi: 10.1074/jbc.273.22.13531. [DOI] [PubMed] [Google Scholar]
- Tian L, Philp JAC, Shipston MJ. Glucocorticoid block of protein kinase C signalling in mouse pituitary corticotroph AtT20 D16 : 16 cells. J Physiol. 1999;516:757–768. doi: 10.1111/j.1469-7793.1999.0757u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng-Crank J, Foster C, Krause J, Mertz R, Godinot N, Dichiara T, Reinhart P. Cloning, expression, and distribution of functionally distinct Ca2+-activated K+ channel isoforms from human brain. Neuron. 1994;13:1315–1330. doi: 10.1016/0896-6273(94)90418-9. [DOI] [PubMed] [Google Scholar]
- Vergara C, Latorre R, Marrion N, Adelman J. Calcium-activated potassium channels. Curr Opin Neurobiol. 1998;8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- Wang YW, Ding JP, Xia XM, Lingle CJ. Consequences of the stoichiometry of Slo1 alpha and auxiliary beta subunits on functional properties of large-conductance Ca2+-activated K+ channels. J Neurosci. 2002;22:1550–1561. doi: 10.1523/JNEUROSCI.22-05-01550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhou Y, Wen H, Levitan I. Simultaneous binding of two protein kinases to a calcium-dependent potassium channel. J Neurosci. 1999;19:1–7. doi: 10.1523/JNEUROSCI.19-10-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiger TM, Holmqvist MH, Levitan IB, Clark FT, Sprague S, Huang WJ, Ge P, Wang CC, Lawson D, Jurman ME, Glucksmann MA, Silos-Santiago I, Distefano PS, Curtis R. A novel nervous system beta subunit that downregulates human large conductance calcium-dependent potassium channels. J Neurosci. 2000;20:3563–3570. doi: 10.1523/JNEUROSCI.20-10-03563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RE, Schonbrunn A, Armstrong DL. Somatostatin stimulates Ca2+-activated K+ channels through protein dephosphorylation. Nature. 1991;351:570–573. doi: 10.1038/351570a0. [DOI] [PubMed] [Google Scholar]
- Womack M, Khodakhah K. Both BK and SK channels contribute to the spontaneous firing pattern of cerebellar Purkinje neurons. Biophys J. 2001;80:994. [Google Scholar]
- Womack MD, Khodakhah K. Characterization of large conductance Ca2+-activated K+ channels in cerebellar Purkinje neurons. Eur J Neurosci. 2002;16:1214–1222. doi: 10.1046/j.1460-9568.2002.02171.x. [DOI] [PubMed] [Google Scholar]
- Xia XM, Ding JP, Lingle CJ. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J Neurosci. 1999;19:5255–5264. doi: 10.1523/JNEUROSCI.19-13-05255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Hirschberg B, Smolik S, Forte M, Adelman J. dslo interacting protein 1, a novel protein that interacts with large-conductance calcium-activated potassium channels. J Neurosci. 1998;18:2360–2369. doi: 10.1523/JNEUROSCI.18-07-02360.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, McCobb DP. Control of alternative splicing of potassium channels by stress hormones. Science. 1998;280:443–446. doi: 10.1126/science.280.5362.443. [DOI] [PubMed] [Google Scholar]
- Zhou XB, Arntz C, Kamm S, Motejlek K, Sausbier U, Wang GX, Ruth P, Korth M. A molecular switch for specific stimulation of the BKCa channel by cGMP and cAMP kinase. J Biol Chem. 2001;276:43239–43245. doi: 10.1074/jbc.M104202200. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Schopperle WM, Murrey H, Jaramillo A, Dagan D, Griffith LC, Levitan IB. A dynamically regulated 14–3-3, slob, and slowpoke potassium channel complex in Drosophila presynaptic nerve terminals. Neuron. 1999;22:809–818. doi: 10.1016/s0896-6273(00)80739-4. [DOI] [PubMed] [Google Scholar]


