Abstract
Iron (Fe) deficiency anaemia during pregnancy results in an increased risk of perinatal mortality and morbidity and is a significant factor for increased risk of disease in later life. Consequently we have developed a rat model to study the relationship between maternal Fe deficiency and postnatal growth and blood pressure in the offspring. Weanlings were fed a control or Fe-deficient diet prior to and throughout pregnancy. At term, all pups were cross-fostered to control fed dams and weaned onto control diet. At birth, pups from deficient dams had a greater mortality rate, were smaller and had reduced haematocrit and liver Fe levels. They also had larger hearts, smaller kidneys and spleens and unchanged livers (relative organ weight). The pups grew normally. At 6 weeks, male pups from deficient dams had a higher and females a lower blood pressure than their normal counterparts. At 10 and 16 weeks, blood pressure in the males from deficient dams was still raised and in the females was now greater than controls. The haematocrit was lower in males throughout the 16 weeks and in females until 10 weeks of age. There was no significant difference in the offsprings’ liver Fe stores at 6, 10 or 16 weeks. Duodenal Fe uptake in both the Fe-deficient mother and newborn offspring was significantly increased. By cross-fostering, we have eliminated confounding factors, such as maternal anaemia during lactation and show, unequivocally, that prenatal nutrition is critical for the development of normal postnatal function.
The impact of an inappropriate nutrition in utero on the growth and development of the fetus and its well-being in both the neonatal and adult life is now clear. Many studies have demonstrated a link between fetal nutrition, low birth weight and coronary heart disease, hypertension and impaired glucose tolerance in adults (Barker et al. 1993; Ravelli et al. 1998; Law et al. 2000). It has been proposed that adaptations made by the fetus to cope with inappropriate nutrition may lead to morphological and physiological changes that persist into postnatal life. These changes, though ensuring fetal survival, may have detrimental affects in later life (Barker, 1995).
The mechanism(s) by which these nutritional insults exert their effects remains unclear. To this end several animal models have been established using global caloric restriction (Woodall et al. 1996; Ozaki et al. 2001) or alteration in a specific dietary component. The specific dietary components thus far studied include protein (Langley-Evans & Jackson, 1994), fat (Koukkou et al. 1998) and minerals such as calcium (Bergel & Belizan, 2002) and iron (Fe) (Crowe et al. 1995; Lewis et al. 2001). While protein restriction is of great significance in underdeveloped countries, and increased fat intake is a westernised problem, it is the Fe deficiency models that are applicable to the aetiology of hypertension of both developed and developing countries.
Fe deficiency is the most common nutritional disorder in the world. The World Health Organisation estimates that 66-80 % of the population are suffering from Fe deficiency (WHO, 2003). Pregnant women are amongst the most susceptible group; in developing countries one in two pregnant women are thought to be suffering from Fe deficiency, while in western countries the situation is considered to be less serious, although a recent study carried out in the UK indicated that one-third of pregnant women attending the ante-natal clinic were Fe deficient (Fosset et al. 2003).
The rat models thus far established have indicated that maternal Fe deficiency, in common with the other rat models of fetal programming, can affect the cardiovascular system and alter both glucose and lipid metabolism in the offspring (Crowe et al. 1995; Lewis et al. 2001, 2002). However all of these effects can also be caused by Fe deficiency induced during postnatal life (Rao et al. 1983; Farrell et al. 1988; Olivetti et al. 1989; Borel et al. 1993; Tanne et al. 1994; Medeiros & Beard, 1998) and maternal Fe deficiency is known to have long-term effects on the Fe content of specific tissues (Felt & Lozoff, 1996). It has not yet been clearly established whether maternal Fe deficiency induces postnatal effects, by mechanisms such as have been described for other programming models or by simply causing a long-term lowering of the offspring's Fe status.
We have previously shown that although during maternal Fe deficiency placental Fe transport is upregulated, fetal growth retardation and fetal Fe deficiency occur (Gambling et al. 2001, 2002). Using this model the aim of this study is to establish whether any postnatal effects of maternal Fe deficiency are due to a continued reduction in the iron status of the offspring.
METHODS
Experimental animals
All experimental procedures were approved and conducted in accordance with the UK animals (Scientific Procedures) Act, 1986. Experiments were performed using weanling female rats of the Rowett hooded Lister strain. They were group housed in cages, under constant temperature and humidity. Controlled illumination with a 12 h light-dark cycle was maintained to assure regular oestrous cycles. All animals were fed ad libitum and provided with distilled water.
Twenty female weanling rats were fed control diet for 2 weeks, before being randomly placed on control or deficient diets. They were fed the diets for four weeks prior to mating. The rats were mated with males of the same strain. Mating was confirmed by detection of a vaginal plug and this day was denoted as day 0. The female rats were maintained on the same experimental diet throughout pregnancy. At birth, two pups from each litter were killed and samples taken for analyses as outlined below. Within 24 h of birth the litters were culled to eight, the remaining pups (from both control and Fe-deficient groups) were cross-fostered onto dams fed CRM, a standard laboratory chow diet (Special Diets Services, Witham, UK). The pups were weaned at 19 days onto the CRM diet and were group housed by litter and sex. All offspring were weighed three times weekly. At 6, 10 and 16 weeks of age a minimum of a further two pups from each litter were killed and samples taken. All animals were killed by stunning and cervical dislocation.
Experimental diets
The diets were based on a dried egg albumin diet (Williams & Mills, 1970) and conformed to American Institute of Nutrition guidelines for laboratory animals (AIN, 1980). FeSO4 was added to achieve levels of added Fe of 50 (control diet), or 7.5 mg kg−1 of diet. The diets were identical in all other respects. The experimental diets were as previously described (Gambling et al. 2001, 2002). Dietary ingredients were purchased from Mayjex Ltd (Chalfont-St Peter, UK), BDH Chemicals (Poole, UK) or Sigma (Poole, UK).
Haematological and atomic absorption spectrophotometric analysis
Maternal and neonatal blood and liver samples were analysed as previously described (Gambling et al. 2002). Briefly, haematocrits were measured by drawing blood into heparinised capillary tubes, centrifuged and read in a microhaematocrit reader. Tissue Fe levels were determined by graphite furnace atomic-spectrophotometry (Model 3100, Perkin-Elmer Corp, Norwalk, CT, USA).
59Fe uptake by the duodenum
59Fe uptake was measured using methods previously described (Smith et al. 2000). Briefly, 5 mm rings of proximal duodenal tissue were collected and incubated in balanced salt solution (BSS, in mM, Hepes 16, KCl 3.5, MgSO4 10, CaCl2 1, NaCl 125, glucose 10). The rings were allowed to evert prior to measuring uptake. Uptake was started by adding 59Fe-nitrilotriacetate (mother and neonates) or 59Fe-ascorbate (6 weeks). This latter condition was used as levels of uptake at 6 weeks were too low for reliable detection using Fe(III)NTA. Rings were incubated for 5 min and uptake terminated by washing in BSS containing a 10-fold concentration of non-radioactive Fe. Duodenal rings were fixed in glutaraldehyde (2 % v/v) and 59Fe uptake counted in a Cobra gamma counter (Packard). Data are expressed as μg (g wet weight)−1.
Blood pressure analysis
Blood pressure measurements were taken from the same group of animals at 6, 10 and 16 weeks, a total of 27 males and 19 females, from nine control and nine Fe-deficient litters. Systolic blood pressure was measured by recording arterial pulse in the tail (Model 229, Life Science Instruments, CA, USA). Prior to analysis, rats were maintained at 28 °C; during analysis they were restrained by hand. A 15 mm cuff was placed over the tail and inflated to 250 mmHg (males) or 200 mmHg (females). Five separate blood pressure readings were taken and the highest and lowest readings discarded, the average of the three remaining readings was recorded.
Glucose tolerance tests
Glucose tolerance tests were carried out on a subgroup of animals at 10 weeks of age. Following an overnight fast a blood sample was taken from the tail vein in order to assess fasting glucose levels. A glucose bolus (200 mg (100 g body weight)−1) was then administered by gavage. Further blood samples were taken from the tail vein at 15, 30, 60 and 90 min after glucose administration. Plasma glucose levels were measured by colorimetric analysis using a selective chemistry analyser (Labmedics, Manchester, UK). Insulin levels were measured using radio-immuno assay (MacRae et al., 1991).
Statistical analysis
For each dam, the litters were averaged and data recorded as a single point, rather than treating each offspring as a single point. This is the statistically more accurate option, although it may obscure trends that might be identified with high numbers of animals. Relative organ weights were calculated as percentage of total body weight (g (100 g)−1). All results are presented as mean ± S.E.M.
Where appropriate, data were analysed by unpaired t test. Where more complex variables were included, data were analysed in Genstat 6 release 6.1 (Lawes Agricultural Trust, Rothamstead, Berkshire, UK). As the data were unbalanced with respect to the sex distribution of offspring, residual maximum likelihood (REML) was used with age, maternal diet, sex and their interactions as fixed effects and litter as random effects (Patterson & Thompson, 1971).
RESULTS
Iron (Fe) deficiency reduced the maternal haematocrit, haemoglobin and liver Fe levels significantly (Table 1). Despite this, there were no differences in either the weight gain during gestation or the final weight between control and Fe-deficient dams (Table 2). Neither the pregnancy rate of the dams nor the number of pups in each litter was altered by Fe deficiency (Table 2). However the survival rate in the offspring born to Fe-deficient dams was significantly reduced with only 85 ± 7 % of them surviving the first 8 h compared to 99 ± 1 % of pups born to control dams (n = 18, P = 0.05). At this stage, we do not know what caused the increased mortality.
Table 1.
Indicators of maternal iron status on day of parturition
| Control dams | Fe-deficient dams | P | |
|---|---|---|---|
| Haematocrit(%) | 37 ± 1.5 | 26 ± 1.2 | 0.004 |
| Haemoglobin (μg l-1) | 201 ± 12 | 161 ± 10 | 0.03 |
| Liver Fe content (μg (g dry wt)-1) | 691 ± 155 | 408 ± 51 | 0.05 |
Table 2.
Maternal weight and pregnancy rate
| Control dams | Fe-deficient dams | P | |
|---|---|---|---|
| Weight at term (g) | 287 ± 4 | 301 ± 8 | n.s. |
| Weight gain (g) | 100 ± 4 | 109 ± 5 | n.s. |
| Pregnancy rate(%) | 90 | 90 | n.s. |
| Pups per litter | 11 ± 1 | 13 ± 1 | n.s. |
The male pups born to Fe-deficient dams were significantly smaller than those born to controls (P < 0.05, Fig. 1). In contrast, there were no significant differences between females (P = 0.055, Fig. 1). However, when the body weight data were combined, the pups born to Fe-deficient dams were smaller (P < 0.001, Fig. 1). The hearts of both male and female offspring were larger than controls (males P < 0.05, females P < 0.05, combined P < 0.01 Fig. 2A). In contrast, the kidneys of either male or female pups were not significantly smaller. When the data were combined, however, they were significantly decreased (P = 0.05 Fig. 2B). The spleens of Fe-deficient animals were also smaller than controls (males P < 0.05, females P < 0.05, combined P < 0.01 Fig. 2C). There were no significant changes in liver size in either sex or when the data were combined (Fig. 2D). No other organs were significantly affected. These differences had disappeared by the time the offspring were 6 weeks old (data not shown).
Figure 1. Maternal Fe deficiency induces a decrease in offspring birth weight.
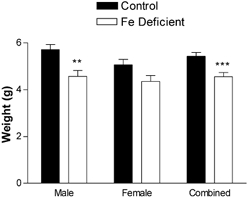
Offspring were weighed within 12 h of birth. Data are mean ± S.E.M. of pups taken from between five and nine litters in each experimental group. **P < 0.01, ***P < 0.001.
Figure 2. Maternal Fe deficiency leads to reduced organ weight in offspring at birth.
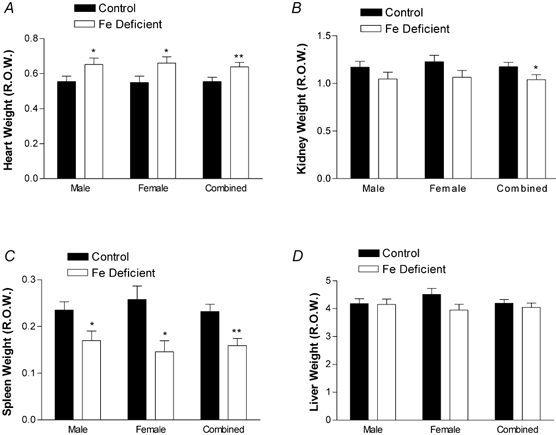
Heart (A), kidney (B), spleen (C) and liver (D) were dissected and weighed from a subset of offspring within 12 h of birth. Weights are expressed as relative organ weight (R.O.W.), and presented as a mean ± S.E.M. of pups taken from between five and nine litters in each experimental group. *P < 0.05 and **P < 0.01.
As would be expected, liver Fe levels were significantly lower at birth in offspring born to deficient mothers (P < 0.05). The differences had disappeared by the time they were 6 weeks old (Fig. 3A and B). The pups of anaemic mothers were themselves anaemic, as measured by haematocrit at birth (P < 0.05). However, in contrast to the Fe levels, the maternal diet continued to have a significant overall effect on the haematocrit throughout the 16 post-natal weeks (males P < 0.01, females P < 0.01, combined, P < 0.001). Considering each time point individually, the haematocrit in males remained significant (P < 0.05), but in the females was no longer significant by 10 weeks (Fig. 4).
Figure 3. Maternal Fe deficiency induces a decrease in neonatal liver Fe only at birth.
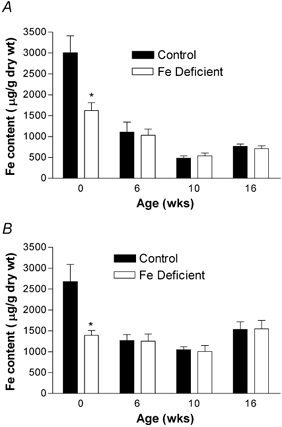
Samples were taken from male (A) and female (B) offspring livers at birth, 6, 10 and 16 weeks of age. Fe content was analysed by atomic absorption spectroscopy. Data are mean ± S.E.M. of pups taken from between five and nine litters in each experimental group. *P < 0.05. There was no significant effect of maternal diet on liver content after birth.
Figure 4. Effect of maternal Fe deficiency on offspring haematocrit.
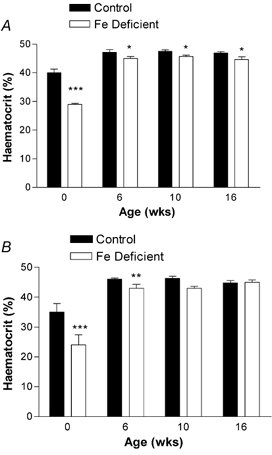
Blood samples were taken from subsets of male (A) and female (B) offspring at birth, 6, 10 and 16 weeks of age. Data are mean ± S.E.M. of pups taken from between five and nine litters. The data show a significant effect of maternal diet on haematocrit in males, but in females, the effect has lost significance by 10 weeks. *P < 0.05, **P < 0.01 and ***P < 0.001.
The Fe-deficient dams had increased duodenum Fe uptake (P < 0.01 Fig. 5). Pups born to deficient mothers also had increased iron uptake in their duodenums (P < 0.01 Fig. 5). However, these differences did not last and by 6 weeks of age duodenum Fe uptake was the same in offspring of both normal and Fe-deficient dams (Fig. 5).
Figure 5. Effect of maternal Fe deficiency on duodenal Fe uptake.
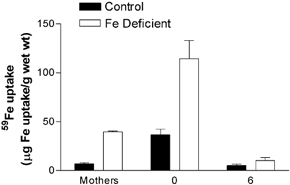
Duodenal 59Fe uptake was measured in both mothers and offspring within 12 h of birth and in a further subset of offspring at 6 weeks of age. Fe uptake is expressed as μg 59Fe uptake (g wet weight of duodenal tissue)−1. Data are mean ± S.E.M. of pups taken from between five and nine litters in each experimental group.
All pups born to Fe-deficient dams grew at the same rate as their control counterparts (Fig. 6A and B). The onset of puberty in females, as measured by weight fluctuations was not affected and neither was the fertility (as measured by pregnancy rate on mating) of female offspring of anaemic mothers altered (data not shown).
Figure 6. Postnatal growth of offspring was unaffected by maternal Fe deficiency.
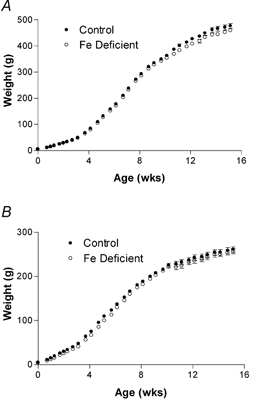
The weights of male (A) and female (B) offspring were recorded every three days. Data are mean ± S.E.M. of pups taken from between five and nine litters in each experimental group.
At 10 weeks of age, offspring glucose tolerance was determined by measuring the response to a glucose dose. There was no significant difference between control offspring and animals born to deficient dams in either plasma glucose (Fig. 7A and C) or insulin levels (Fig. 7B and D).
Figure 7. Maternal Fe deficiency has no effect on glucose tolerance or insulin levels in the offspring.
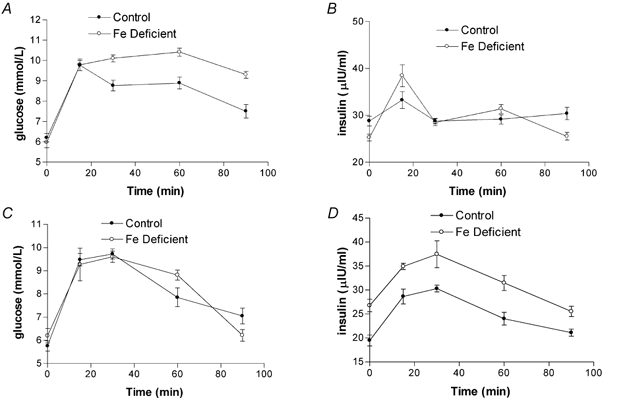
Fasting plasma glucose levels were measured in male (A and B) and female (C and D) offspring at 10 weeks of age. Following glucose administration, plasma glucose (A and C) and insulin (B and D) levels were then measured at varying time points. Data are mean ± S.E.M. of offspring taken from between five and nine litters in each experimental group.
In contrast, there was a significant difference in systolic blood pressure between those born to normal and those born to deficient dams. At 6 weeks, the females had lower blood pressure than those born to control dams, while male values were significantly higher (P < 0.01, Fig. 8). By 10 weeks of age, blood pressure in both females and males was higher (P < 0.05, Fig. 8). This difference was maintained into adulthood (P < 0.05, Fig. 8) Heart rates were not significantly affected (data not shown).
Figure 8. Maternal Fe deficiency leads to alterations in offspring blood pressure.

Blood pressure was measured by the tail cuff method in male (A) and female (B) offspring at 6, 10 and 16 weeks of age. Data are mean ± S.E.M. of offspring taken from between five and nine litters in each experimental group. The data show significant differences between offspring from anaemic and control males (P < 0.01). In the females, the blood pressure was significantly lower at 6 weeks (P < 0.01) and by 16 weeks had reversed to be higher than in controls (P < 0.05). *P < 0.05, **P < 0.01 and ***P < 0.001.
DISCUSSION
In this paper, we have shown that mild Fe deficiency in utero causes a significant increase in the blood pressures of both male and female offspring. The data presented in this paper both confirm and expand on data presented by Crowe et al. (1995) and Lewis et al. (2001), but also disagrees with some of their findings. As previously described, pups born to anaemic mothers have higher blood pressure as adults. The females show lower blood pressure at six weeks. This is similar to the data presented by Crowe et al. (1995), who showed at three weeks that combined males and females had lower blood pressure but that the trend was reversed by six weeks. As observed by Lewis et al. (2001), by three months both the male and female offspring of deficient dams show increased blood pressure. Why the blood pressure changes in the females is not clear. We suspect it may be related to the onset of puberty, but at this time the hypothesis remains to be tested.
As the Fe concentration of rat milk is related to the Fe status of the dam (Anaokar & Garry, 1981; O'Conner et al. 1988), this study employed a cross-fostering protocol for all offspring. This was considered particularly important since it has been established that alterations in maternal diet during lactation can impact on growth and development of the offspring (Desai et al. 1996) and the severity of the possible ‘programming’ effect seen (Dahri et al. 1991). Therefore the data presented here clearly establish that intra-uterine Fe deficiency alone is sufficient to generate an effect on the blood pressure of the resulting offspring.
The dietary regime used here is known to induce a mild but significant Fe deficiency in the mother (Gambling et al. 2002). We have chosen to induce deficiency before rather than after mating since this represents the human condition more closely, in that a significant proportion of women are already on the borderline of deficiency prior to conception (reviewed in Allen, 2000).
Consistent with our previous results, the level of Fe deficiency established in the offspring is less than that in the mother (Gambling et al. 2002) as a consequence of upregulation of placental Fe transport (Gambling et al. 2001). The pups are also born anaemic. The males remain anaemic, at least in terms of the haematocrit. The females also remain anaemic until 16 weeks of age. This effect is seen despite the fact that the Fe levels in the liver are restored to normal by six weeks. The observation is very surprising. We expected that if Fe stores were returned to normal, blood parameters would also be the same as in pups born to control animals. At present our hypothesis is that regulation of blood volume is altered, and this is also why blood pressure is increased. In support of this, kidney weight at birth is slightly reduced, which may suggest impaired function. However, the difference disappears in the adult offspring, so closer examination is required before conclusions can be drawn.
As with our data, previous groups have shown that pups born to anaemic mothers are smaller. However, in these earlier studies, the pups remained small and either did (Crowe et al. 1995) or did not (Lewis et al. 2001) show increased catch-up growth. Our animals grew at the same rate as controls, so that the difference remained arithmetically the same, but decreased as a fraction of total weight. This means that any size differences were insignificant. It is uncertain why the difference exists, but it may be related to the cross-fostering of the offspring, which would avoid any effects due to lack of iron in the maternal milk. Despite this, we still measured an increase in blood pressure. This argues against Crowe's hypothesis that postnatal growth, rather than prenatal exposure, is important in development of high blood pressure (Crowe et al. 1995).
Fe uptake across the neonatal gut is higher in deficient pups than controls. This is not surprising, but confirms that systemic regulation of Fe uptake across the gut is important. It is intriguing that maternal uptake is also stimulated, showing the gut has a considerable capacity to increase Fe transfer. By six weeks the uptake in the duodenums of the offspring has returned to normal, reflecting the normal liver status, and providing further indirect evidence that the reduced haematocrit is not a result of continued Fe deficiency anaemia.
Unlike Lewis et al. (2001), we did not observe differences in glucose tolerance between control and pups born to Fe-deficient dams. There are several possible explanations. Using other modalities, groups have shown that glucose responses do not always occur in parallel with blood pressure changes (W. D. Rees, unpublished data). Further there are often strain differences, and the diet protocol Lewis used was more severe but briefer than that used in this present study. Finally, it is also possible that stress induced by the treatment may have altered glucose responses and masked any effect.
The cause of the increased blood pressure remains a matter for further investigation. At this stage, we cannot exclude the possibility that there are changes in the mother that cause the alterations in the fetus, rather than simple Fe deficiency anaemia. For example, we have not measured maternal blood pressure or glucose tolerance.
In summary, the present paper has confirmed that the increased blood pressure is as a consequence of the intrauterine environment and excludes possible postnatal effects. The model has some advantages over others currently being used. It is a relatively mild treatment, generating small and controllable effects. The model is robust, since three different groups have now confirmed the effect of maternal Fe deficiency, despite using different strains of rat and dietary regimes (Crowe et al. 1995; Lewis et al. 2001; present study). It has also shown that the changes can occur in the absence of overt differences, such as size, between animals born to control or deficient mothers. It also has the advantage of being readily manipulated, since it will be possible to reverse the maternal deficiency easily and quickly by injecting Fe as Fe-transferrin, in order to define critical windows accurately.
There is one other important aspect of the model. In as much as 30 % of the population (Fosset et al. 2003), pregnant women suffer from anaemia over and above the normal anaemia of pregnancy. Our data imply that it is important to try and alleviate the condition as quickly as possible. Many studies have shown that maternal anaemia is not associated with decreased birth weight. However, it is becoming more apparent, as better indicators of Fe status are developed, that there are deleterious consequences. Our data suggest that these not only occur in the perinatal period but may also persist into adulthood.
Acknowledgments
This work was supported by SEERAD, European Union Framework V, The Wellcome Trust and the International Copper Association. We are grateful to Morven Cruikshank for technical assistance. We also thank Dr Ruth Danzeisen and Dr Cedric Fosset for their criticisms and discussions.
REFERENCES
- American Institute of Nutrition, (AIN) Second report of the ad hoc committee on standards for nutritional studies. J Nutr. 1980;110:741–744. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr. 2000;71:1280–1284. doi: 10.1093/ajcn/71.5.1280s. [DOI] [PubMed] [Google Scholar]
- Anaokar SG, Garry PJ. Effects of maternal iron nutrition during lactation on milk iron and rat neonatal iron status. Am J Clin Nutr. 1981;34:1505–1512. doi: 10.1093/ajcn/34.8.1505. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- Bergel E, Belizan J. A deficient maternal calcium intake during pregnancy increases blood pressure of the offspring in adult results. Br J Obst Gynaecol. 2002;109:540–545. [PubMed] [Google Scholar]
- Borel MJ, Beard JL, Farrell PA. Hepatic glucose production and insulin sensitivity and responsiveness in iron-deficient anemic rats. Am J Physiol. 1993;264:E380–390. doi: 10.1152/ajpendo.1993.264.3.E380. [DOI] [PubMed] [Google Scholar]
- Crowe C, Dandekar P, Fox M, Dhingra K, Bennet L, Hanson MA. The effects of anaemia on heart, placenta and body weight, and blood pressure in fetal and neonatal rats. J Physiol. 1995;488:515–519. doi: 10.1113/jphysiol.1995.sp020986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahri S, Snoeck A, Reusens-Billen B, Remacle C, Hoet JJ. Islet function in offspring of mothers on low-protein diet during gestation. Diabetes. 1991;40(suppl. 2):115–120. doi: 10.2337/diab.40.2.s115. [DOI] [PubMed] [Google Scholar]
- Desai M, Crowther NJ, Lucas A, Hales CN. Organ-selective growth in the offspring of protein-restricted mothers. Br J Nutr. 1996;76:591–603. doi: 10.1079/bjn19960065. [DOI] [PubMed] [Google Scholar]
- Farrell PA, Beard JL, Druckenmiller M. Increased insulin sensitivity in iron-deficient rats. J Nutr. 1988;118:1104–1109. doi: 10.1093/jn/118.9.1104. [DOI] [PubMed] [Google Scholar]
- Felt BT, Lozoff B. Brain iron and behavior of rats are not normalized by treatment of iron deficiency anemia during early development. J Nutr. 1996;126:693–701. doi: 10.1093/jn/126.3.693. [DOI] [PubMed] [Google Scholar]
- Fosset C, McGaw B, Abramovich D, McArdle H. Interactions between iron and copper metabolism during human pregnancy. Biol Trace Elem Res. 2003 doi: 10.1385/BTER:98:1:01. (in press) [DOI] [PubMed] [Google Scholar]
- Gambling L, Charania Z, Hannah L, Antipatis C, Lea RG, McArdle HJ. Effect of iron deficiency on placental cytokine expression and fetal growth in the pregnant rat. Biol Reprod. 2002;66:516–523. doi: 10.1095/biolreprod66.2.516. [DOI] [PubMed] [Google Scholar]
- Gambling L, Danzeisen R, Gair S, Lea RG, Charania Z, Solanky N, Joory KD, Srai SK, McArdle HJ. Effect of iron deficiency on placental transfer of iron and expression of iron transport proteins in vivo and in vitro. Biochem J. 2001;356:883–889. doi: 10.1042/0264-6021:3560883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukkou E, Ghosh P, Lowy C, Poston L. Offspring of normal and diabetic rats fed saturated fat in pregnancy demonstrate vascular dysfunction. Circulation. 1998;98:2899–2904. doi: 10.1161/01.cir.98.25.2899. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Jackson AA. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci. 1994;86:217–222. doi: 10.1042/cs0860217. [DOI] [PubMed] [Google Scholar]
- Law CM, Egger P, Dada O, Delgado H, Kylberg E, Lavin P, Tang GH, Von Hertzen H, Shiell AW, Barker DJ. Body size at birth and blood pressure among children in developing countries. Int J Epidemiol. 2000;29:52–57. doi: 10.1093/ije/30.1.52. [DOI] [PubMed] [Google Scholar]
- Lewis RM, Forhead AJ, Petry CJ, Ozanne SE, Hales CN. Long-term programming of blood pressure by maternal dietary iron restriction in the rat. Br J Nutr. 2002;88:283–290. doi: 10.1079/BJN2002656. [DOI] [PubMed] [Google Scholar]
- Lewis RM, Petry CJ, Ozanne SE, Hales CN. Effects of maternal iron restriction in the rat on blood pressure, glucose tolerance, and serum lipids in the 3-month-old offspring. Metabolism. 2001;50:562–567. doi: 10.1053/meta.2001.22516. [DOI] [PubMed] [Google Scholar]
- MacRae JC, Bruce LA, Hovell DFD, Hart IC, Inkster J, Atkinson T. Influence of protein nutrition on the response of growing lambs to exogenous bovine growth hormone. J Endocrinol. 1991;130:53–61. doi: 10.1677/joe.0.1300053. [DOI] [PubMed] [Google Scholar]
- Medeiros DM, Beard JL. Dietary iron deficiency results in cardiac eccentric hypertrophy in rats. Proc Soc Exp Biol Med. 1998;218:370–375. doi: 10.3181/00379727-218-44306. [DOI] [PubMed] [Google Scholar]
- O'Conner D, Picciano M, Sherman A. Impact of maternal iron deficiency on quality and quantity of milk ingested by neonatal rats. Br J Nutr. 1988;60:477–485. doi: 10.1079/bjn19880120. [DOI] [PubMed] [Google Scholar]
- Olivetti G, Lagrasta C, Quaini F, Ricci R, Moccia G, Capasso J, Anversa P. Capillary growth in anemia-induced ventricular wall remodelling in the rat heart. Circ Res. 1989;65:1182–1192. doi: 10.1161/01.res.65.5.1182. [DOI] [PubMed] [Google Scholar]
- Ozaki T, Nishina H, Hanson MA, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol. 2001;530:141–152. doi: 10.1111/j.1469-7793.2001.0141m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson HD, Thompson R. Recovery of inter-block information when block sizes are unequal. Biometrika. 1971;58:545–554. [Google Scholar]
- Rao GA, Crane RT, Larkin EC. Reduced plasma lecithin cholesterol acyl transferase activity in rats fed iron-deficient diets. Lipids. 1983;18:673–676. doi: 10.1007/BF02534532. [DOI] [PubMed] [Google Scholar]
- Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, Bleker OP. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351:173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- Smith MW, Debnam ES, Dashwood MR, Srai SKS. Structural and cellular adaptation of duodenal iron uptake in rats maintained on an iron-deficient diet. Pflugers Arch. 2000;439:449–454. doi: 10.1007/s004249900193. [DOI] [PubMed] [Google Scholar]
- Tanne Z, Coleman R, Nahir M, Shomrat D, Finberg JP, Youdim MB. Ultrastructural and cytochemical changes in the heart of iron-deficient rats. Biochem Pharmacol. 1994;47:1759–1766. doi: 10.1016/0006-2952(94)90303-4. [DOI] [PubMed] [Google Scholar]
- World Health Organisation. Battling Iron Deficiency Anaemia. 2003 www.who.int/nut/ida.htm.
- Williams RB, Mills CF. The experimental production of zinc deficiency in the rat. Br J Nutr. 1970;24:989–1003. doi: 10.1079/bjn19700102. [DOI] [PubMed] [Google Scholar]
- Woodall SM, Johnston BM, Breier BH, Gluckman PD. Chronic maternal undernutrition in the rat leads to delayed postnatal growth and elevated blood pressure of offspring. Pediatr Res. 1996;40:438–443. doi: 10.1203/00006450-199609000-00012. [DOI] [PubMed] [Google Scholar]


