Abstract
Creatine kinase (CK) has a central role in skeletal muscle, acting as a fast energy buffer and shuttle between sites of energy production (mitochondria) and consumption (cross-bridges and ion pumps). Unexpectedly, isolated fast-twitch skeletal muscle cells of mice deficient in both cytosolic and mitochondrial CK (CK-/-) are highly fatigue resistant during stimulation protocols that stress aerobic metabolism. We have now studied different aspects of mitochondrial function in CK-/- skeletal muscle. Intact, single fibres of flexor digitorum brevis (FDB) muscles were fatigued by repeated tetanic stimulation (70 Hz, 350 ms duration, duty cycle 0.14). Under control conditions, CK-/- FDB fibres were more fatigue resistant than wild-type fibres. However, after mitochondrial inhibition with cyanide, force declined markedly faster in CK-/- fibres than in wild-type fibres. The rapid force decline in CK-/- fibres was not due to decreased myoplasmic [Ca2+] during tetani (measured with indo-1), which in these fibres remained virtually constant during fatigue in the presence of cyanide. Intact, single fibres of highly oxidative soleus muscles were fatigued by repeated tetani (50 Hz, 500 ms duration, duty cycle 0.5). All CK-/- soleus fibres tested (n = 9) produced > 40% force at the end of the fatiguing stimulation period (500 tetani), whereas force fell to < 40% before 500 tetani in two of three wild-type fibres. Mitochondrial [Ca2+] (measured with rhod-2 and confocal microscopy) increased during repeated tetanic stimulation in CK-/- but not in wild-type FDB fibres. In conclusion, mitochondria and energy shuttling operate effectively in CK-/- fibres and this is associated with an increase in mitochondrial [Ca2+].
Creatine kinase (CK) catalyses the reaction: phosphocreatine (PCr) + ADP + H+ ⇌ creatine (Cr) + ATP. This reaction is presumed to play a key role in energy metabolism, acting both as a temporal and a spatial energy buffer (Wallimann et al. 1992; Wyss & Kaddurah-Daouk, 2000). There are two isoforms of CK in skeletal muscle cells: (1) cytosolic CK (M-CK) concentrated at the myofibrillar M-line and in association with sarcoplasmic reticulum (SR) Ca2+ pumps, and (2) mitochondrial CK (ScCKmit or MtCK) situated in the intermembrane space of mitochondria. Genetically modified mice lacking both these isoforms of CK have been generated (CK-/- mice) (Steeghs et al. 1997). Unexpectedly, CK-/- mice are fully viable and display only mild alterations of basal muscle physiology. Skeletal muscle cells of these mice have a normal total creatine concentration and the PCr/Cr ratio in resting muscles is only moderately decreased (Steeghs et al. 1997; Dahlstedt et al. 2000). Nevertheless, during a period of repeated contractions PCr is not hydrolysed in CK-/- muscle, whereas wild-type muscle displays a marked reduction of the PCr/Cr ratio (Steeghs et al. 1997; Dahlstedt et al. 2000; Gorselink et al. 2001). Tetanic force and free myoplasmic [Ca2+] ([Ca2+]i) decrease more in CK-/- fibres than in wild-type fibres during a period of high-intensity exercise (Steeghs et al. 1997; Dahlstedt et al. 2000). A recent study from our laboratory showed that this decreased performance is a direct consequence of CK deficiency since injection of CK into CK-/- cells almost fully restored the wild-type phenotype (Dahlstedt et al. 2003).
CK-/- muscle cells display major adaptations in energy metabolism systems. These changes are particularly clear in fast-twitch skeletal muscle cells, which show a major increase in the number of mitochondria and increased oxidative capacity (Steeghs et al. 1998; De Groof et al. 2001; Kaasik et al. 2003). Shuttling of Cr from sites of fast energy consumption (cross-bridges and ion pumps) to the mitochondria and transport of PCr in the opposite direction is supposed to be important for an effective mitochondrial energy production. Since CK-/- muscle fibres lack both myoplasmic and mitochondrial CK, this shuttling process cannot occur. Therefore, it seems likely that the full potential of the increased mitochondrial content cannot be utilized. We recently exposed intact fast-twitch CK-/- fibres to a period of low-intensity fatiguing stimulation (350 ms tetani initially given at 2.5 s interval) (Dahlstedt et al. 2000). The unexpected outcome of this study was that CK-/- fibres were markedly more fatigue resistant than wild-type fibres. This might be a consequence of the increased mitochondrial concentration in CK-/- fibres and mitochondrial respiration and energy shuttling would then work effectively despite the lack of CK. In addition, the absence of PCr breakdown during fatigue in CK-/- fibres minimizes the increase in inorganic phosphate ions (Pi), which is considered to be a major cause of fatigue (Stackhouse et al. 2001; Allen & Westerblad, 2001; Westerblad et al. 2002).
In the present study the following three hypotheses were tested. (1) Inhibition of mitochondrial respiration has a larger effect on fatigue development in fast-twitch CK-/- fibres than wild-type fibres. This hypothesis was tested by producing fatigue in the presence of a saturating concentration of cyanide. (2) The lack of CK leads to a faster fatigue development in oxidative, fatigue resistant muscle fibres. The rationale behind this hypothesis is that oxidative fibres contain a markedly higher proportion of MtCK than glycolytic fibres and their respiration is stimulated by Cr (Yamashita & Yoshioka, 1991; Veksler et al. 1995; Kuznetsov et al. 1996). This hypothesis was tested by producing fatigue in oxidative, fatigue resistant fibres isolated from soleus muscles (Marechal & Beckers-Bleukx, 1993; Steeghs et al. 1998). (3) The increased mitochondrial energy production in CK-/- fibres is associated with altered mitochondrial Ca2+ homeostasis. This hypothesis was tested by measuring mitochondrial [Ca2+], which remains constant during fatiguing stimulation of wild-type FDB fibres (Lännergren et al. 2001).
METHODS
Animals
Animals were housed at room temperature with a 12 h light-12 h dark cycle. Food and water were provided ad libitum. CK-/- mice were generated as described previously (Steeghs et al. 1997) and C57Bl/6 mice served as wild-type controls. Adult female and male mice were used and these were killed by rapid neck disarticulation. Thereafter the hindlimb flexor digitorum brevis (FDB) and soleus muscles were isolated. All procedures were approved by the Stockholm North local ethical committee.
Solutions
Muscle fibres were superfused with a Tyrode solution of the following composition (mM): NaCl, 121; KCl, 5.0; CaCl2, 1.8; MgCl2, 0.5; NaH2PO4, 0.4; NaHCO3, 24.0; EDTA, 0.1, glucose, 5.5; 0.2 % fetal calf serum was added to the solution to improve muscle fibre survival. The solution was bubbled with 5 % CO2- 95 % O2, which gives a pH of 7.4. Sodium cyanide (NaCN, 2 mM) was used to block oxidative phosphorylation in mitochondria. Experiments were performed at room temperature (24 °C).
Single fibre dissection, mounting and stimulation
Intact, single fibres from FDB and soleus muscles were dissected following procedures described elsewhere (Lännergren & Westerblad, 1987; Bruton et al. 2003). The isolated fibre was mounted between an Akers 801 force transducer (SensoNor, Horten, Norway) and an adjustable holder in a chamber that was continuously superfused with Tyrode solution. The fibre length was adjusted to that giving maximum tetanic force and the fibre diameter was measured. Tetanic stimulation was achieved by supramaximum current pulses (duration 0.5 ms) delivered via platinum plate electrodes lying parallel to the fibre.
Stimulation protocols
FDB fibres
Tetani (70 Hz, 350 ms duration) were given at an interval of 2.5 s, i.e. with a duty cycle (tetanic duration divided by tetanic interval) of 0.14. Fibres were fatigued by 100 tetanic stimulations or until force was reduced to 40 % of the force in the first tetanus. The fibre was allowed to rest for 90 min after fatiguing stimulation and a test tetanus was then produced. The fibre was then exposed to 2 mM cyanide for 15 min before starting a second fatigue run. Fatigue was produced with the same tetanic duration and interval and 50 tetani were produced.
Soleus fibres
Initially the force-frequency relationship was studied by producing one 500 ms contraction every 1 min and the stimulation frequency was varied between 1 and 100 Hz. The force was measured as the mean over 100 ms where force was maximum and is expressed relative to the force produced in 100 Hz tetani. For comparison, the force-frequency relationship was also studied in isolated FDB fibres.
Isolated soleus fibres were fatigued by 50 Hz, 500 ms tetani at 1 s intervals (i.e. with a duty cycle of 0.5). Fatiguing stimulation was stopped after 500 tetani or when force had fallen to 40 % of the initial value.
[Ca2+]i measurements
[Ca2+]i was measured in some FDB fibres. These fibres were pressure injected with the fluorescent Ca2+ indicator indo-1 (Molecular Probes Europe B.V., Leiden, The Netherlands). Indo-1 was mixed in a buffer (150 mM KCl, 10 mM Hepes, pH 7.1) to a final concentration of 10 mM and microinjected into the fibre. The fluorescence of indo-1 was measured with a system consisting of a xenon lamp, a monochromator, and two photomultiplier tubes (PTI, Photo Med GmbH, Wedel, Germany). The excitation light was set to 360 ± 5 nm and the light emitted at 405 ± 5 and 495 ± 5 nm was measured. The ratio of the light emitted at 405 nm to that at 495 nm was converted to [Ca2+]i as described elsewhere (Andrade et al. 1998).
Fibres were allowed to rest for at least 60 min after being injected with indo-1. Some control contractions were then performed to assure that force was stable and > 90 % of that before injection. During contractions, fluorescence and force signals were sampled on-line and stored in a desktop computer for subsequent data analysis. The mean fluorescence ratio during tetanic stimulation was measured and translated into [Ca2+]i.
Measurement of mitochondrial [Ca2+] and the fractional area of the fibre occupied by mitochondria
FDB and soleus fibres were incubated in 5 μM rhod-2-AM (Molecular Probes) for 90-120 min at room temperature. Rhod-2 is a positively charged molecule that preferentially loads into mitochondria with relatively little left in the myoplasm (Babcock et al. 1997; Bruton et al. 2003). The fibre was washed for at least 30 min following loading. The fluorescence of rhod-2 was detected with confocal microscopy as described previously (Lännergren et al. 2001; Bruton et al. 2003). Briefly, sections of ≈30-50 μm (90-150 pixels) × 200 μm (600 pixels) close to the surface of fibres were scanned with a BioRad MRC 1024 confocal unit (BioRad Microscopy Division, Hertfordshire, England) attached to a Nikon Diaphot 200 inverted microscope. A Nikon Plan Apo × 40 oil immersion objective (N.A. 1.3) was used. Rhod-2 was excited with 568 nm light and the emitted light collected through a 585 nm long-pass filter. A single scan of the fibre with laser power set to ≤ 12 % of the maximum 15 mW was used to obtain each image. No filtering, smoothing or averaging procedures were used during the acquisition of images. Confocal images were analysed offline with ImageJ (NIH, USA, http://rsb.info.nih.gov/ij/). To demonstrate mitochondrial Ca2+ uptake in wild-type FDB fibres, localized sarcolemmal damage was deliberately induced by scanning a narrow band of the fibre (< 1 μm; 4 000 sweeps in the line scan mode) with maximum laser power.
Confocal images of rhod-2 fluorescence in rested CK-/- and wild-type FDB fibres and CK-/- soleus fibres were used to estimate the fractional area occupied by mitochondria. The distribution of light intensity was measured and 2 S.D. was subtracted from each image, which equalised the differences in basal fluorescence associated with the intrinsic fluorescence of the regularly repeating arrays of muscle proteins. Images were then automatically thresholded and converted into binary objects. The total area occupied by positive pixels was considered to represent mitochondria. The fractional area of mitochondria in the muscle fibres was obtained by dividing the number of positive pixels by the total number of pixels, ignoring regions smaller than 5 pixels that were considered too small to be mitochondria (increasing the limit to 10 pixels had no obvious effect on the measurements).
Mitochondrial [Ca2+] was measured in some FDB fibres. The average pixel intensity in areas containing mitochondria (defined as described above) was considered to be the mitochondrial signal, while measurements of the mean pixel intensity from nearby areas devoid of distinctly visible mitochondria served as background (such areas showed little change (< 10 %) in fluorescence during experiments). In each image, the mitochondrial rhod-2 intensity (F) was obtained by subtracting the background signal from the mitochondrial signal. Relative changes in F during repeated tetanic stimulation were quantified by dividing F measured at each time point by that measured in the rested fibre (F0).
A fixed protocol with 50 repeated contractions was used in the experiments where mitochondrial [Ca2+] was measured. The stimulation frequency (70 Hz), tetanic duration (350 ms) and tetanic interval (2.5 s) were identical to those used for the other experiments on FDB fibres. Stimulation was briefly stopped to record a confocal image after 10 and 25 contractions. Images were also obtained before and immediately after fatiguing stimulation, and after 1, 2.5, 5 and 10 min of recovery.
Statistics
Values are presented as means ± S.E.M. Student's paired and unpaired t tests were used as appropriate. Statistical significance was set at P < 0.05.
RESULTS
Force measurements in FDB fibres before and during cyanide exposure
Tetanic force production of unfatigued FDB CK-/- muscle fibres was significantly (P < 0.05) lower (231 ± 20 kN m−2; n = 9) than that of wild-type fibres (327 ± 28 kN m−2; n = 7), which agrees with previous results (Dahlstedt et al. 2000). CK-/- fibres were more fatigue resistant than wild-type fibres during a bout of repeated tetanic contractions produced under control conditions (Fig. 1A). All CK-/- fibres endured the full fatiguing stimulation period (i.e. 100 tetani) and the mean force in the 100th tetanus was about 80 % of the original force. On the other hand, force decreased to < 70 % of the original in all wild-type fibres. After 90 min of recovery, tetanic force had almost fully recovered in both CK-/- and wild-type fibres (Fig. 1B). Fibres were then exposed to 2 mM cyanide for 15 min. This had little effect on the force produced in a single tetanus in CK-/- fibres (increased from 90 to 93 % of the original), whereas it caused a significant (P < 0.05) decrease from 98 to 90 % in wild-type fibres (Fig. 1C).
Figure 1. CK-/- FDB fibres are more fatigue resistant than wild-type fibres under normal conditions but fatigue more rapidly when mitochondrial respiration is inhibited by cyanide.
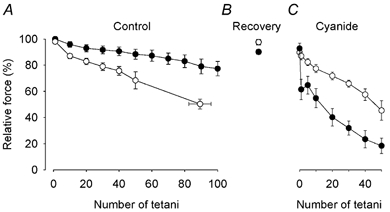
Mean data (± S.E.M.) of tetanic force production in CK-/- (•; n = 9) and wild-type (○; n = 7) fibres. Fibres were fatigued by a maximum of 100 repeated tetanic contractions under control conditions (A), allowed to recover for 90 min (B), and then fatigued again by 50 tetani in the presence of cyanide (2 mM) (C).
Figure 2 shows original force records from fatigue runs in cyanide produced in a CK-/- fibre (A) and in a wild-type fibre (B). Fatigue developed very rapidly in the CK-/- fibre, whereas the wild-type fibre was more fatigue resistant. Thus, the results were opposite to those under control conditions (cf. Fig. 1A and C). After 50 tetani in cyanide, the force developed in CK-/- fibres was reduced to 20 ± 6 % of that at the start of the fatigue run, which compares to 88 ± 4 % under control conditions; corresponding values for wild-type fibres were 48 ± 9 % in cyanide and 71 ± 5 % under control conditions.
Figure 2. Mitochondrial inhibition with cyanide results in a fast force decline during repeated tetanic stimulation in CK-/- FDB fibres.
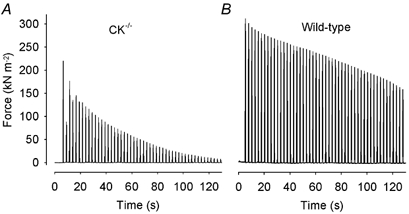
Representative force records obtained in a CK-/- (A) and a wild-type (B) fibre during fatigue in the presence of cyanide. Note the marked force decrease in the second tetanus in the CK-/- fibre.
One conspicuous finding in CK-/- fibres during fatigue in cyanide was a major decrease in tetanic force in the second tetanus by 35 ± 6 % (P < 0.001), which was partially reversed in subsequent tetani (see Fig. 1C and Fig. 2A). In CK-/- fibres, a significant (P < 0.01) reduction of tetanic force was also observed in the second fatiguing tetanus under control conditions, but the decrease was markedly smaller (11 ± 3 %) than during cyanide exposure and fully reversed in the third to fifth fatiguing tetanus.
In contrast to wild-type fibres, the shape of tetanic force records was markedly changed in CK-/- fibres during fatigue in the presence of cyanide; in fatigue the initial rate of force rise was substantially decreased and force was still increasing at the end of the tetanus. To quantify this, we measured the relative rate of force change (i.e. rate of force change divided by peak force) during the last 100 ms of tetanic stimulation. During fatigue in cyanide, this rate increased by 1.17 ± 0.21 s−1 (P < 0.001) in CK-/- fibres, whereas it was not significantly changed in wild-type fibres (0.40 ± 0.21 s−1; P = 0.1).
[Ca2+]i measurements in FDB fibres before and during cyanide exposure
[Ca2+]i was measured in a subset of the muscle fibres described above, i.e. five CK-/- fibres and four wild-type fibres. Mean data of tetanic [Ca2+]i during fatigue under control conditions (Fig. 3A) were similar to those reported previously (Dahlstedt et al. 2000). That is, tetanic [Ca2+]i remained almost constant in CK-/- fibres, whereas it displayed an early increase followed by a decrease in wild-type fibres. Resting [Ca2+]i showed a minor increase during fatigue, from 71.6 ± 9.1 to 112 ± 14.9 nM, in CK-/- fibres (measured immediately before the 100th fatiguing tetanus). The increase in resting [Ca2+]i tended to be larger in wild-type fibres (from 78.4 ± 13.4 to 199 ± 39.4 nM) but the difference was not significant.
Figure 3. Changes in tetanic [Ca2+]i show a similar pattern during fatigue produced under control conditions and with mitochondrial inhibition.
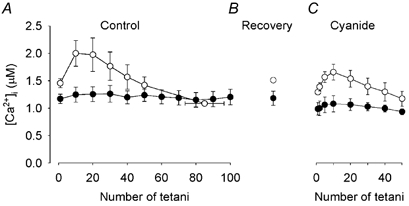
Mean data (± S.E.M.) of tetanic [Ca2+]i of CK-/- (•; n = 5) and wild-type (○; n = 4) FDB fibres. Fibres were fatigued by repeated tetanic stimulation under control conditions (A), allowed to recover for 90 min (B), and finally fatigued again in the presence of cyanide (C).
After 90 min of recovery, tetanic [Ca2+]i was similar to the starting value both in CK-/- and wild-type fibres (105 ± 3 % and 101 ± 9 % of the original, respectively) (Fig. 3B). Resting [Ca2+]i was also similar to the pre-fatigue value: 102 ± 7 % in CK-/- fibres and 112 ± 10 % in wild-type fibres. Exposure to cyanide resulted in a significant (P < 0.05) decrease in tetanic [Ca2+]i by 16 ± 3 % in CK-/- fibres and by 12 ± 2 % in wild-type fibres (Fig. 3C). Cyanide exposure also resulted in a significant (P < 0.05) increase in resting [Ca2+]i by 42 ± 17 % in CK-/- fibres and 28 ± 5 % in wild-type fibres.
Figure 4A shows original [Ca2+]i and force records of selected tetani during fatigue in cyanide obtained in a CK-/- fibre. Intriguingly, tetanic [Ca2+]i showed little change during fatigue in this CK-/- fibre despite the marked decline in force. Corresponding records from a wild-type fibre are shown in Fig. 4B. In this fibre, [Ca2+]i showed a pattern similar to that observed under control conditions, i.e. an early increase followed by a decrease. Thus, mean data of tetanic [Ca2+]i during fatigue in cyanide show a picture similar to that during fatigue induced under control conditions (cf. Fig. 3A and C): little change in CK-/- fibres but an initial increase followed by a gradual decline in wild-type fibres. It is worth noting that the marked decrease in tetanic force in CK-/- fibres in the second fatiguing tetanus was not accompanied by any reduction in tetanic [Ca2+]i either during cyanide exposure (see Fig. 3C) or under control conditions (data not shown). Resting [Ca2+]i increased to a similar extent during fatigue in cyanide in CK-/- fibres (from 97.6 ± 5.8 to 247.1 ± 49.6 nM) and wild-type fibres (from 113.4 ± 16.0 to 266.3 ± 44.9 nM).
Figure 4. Mitochondrial inhibition results in a rapid force decline during fatigue in CK-/- FDB fibres.
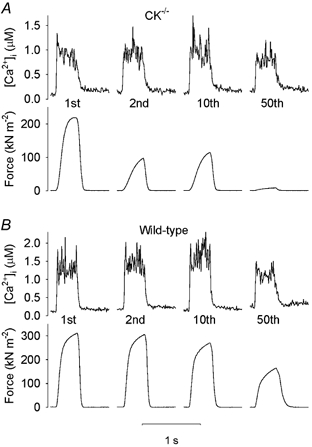
Representative records of [Ca2+]i (upper part) and force (lower part) from selected tetani during fatigue in the presence of cyanide obtained in a CK-/- fibre (A) and in a wild-type fibre (B). The tetanus number is indicated below each [Ca2+]i record.
Force measurements and fatigue in soleus fibres
The force-frequency relationship of CK-/- soleus fibres was slightly shifted to the left as compared to wild-type fibres (Fig. 5). However, the difference was not significant at any frequency studied and the frequency giving 50 % force was 23.6 ± 2.8 Hz (n = 9) and 29.1 ± 4.2 Hz (n = 3) for CK-/- and wild-type fibres, respectively (P = 0.33). Unexpectedly, the frequency giving 50 % force in these soleus fibres was similar to that in FDB fibres (26.1 ± 1.4 Hz for CK-/- (n = 7) and 30.4 ± 2.0 Hz for wild-type (n = 6)).
Figure 5. The force-frequency relationship of CK-/- soleus fibres is slightly shifted to the left as compared to wild-type fibres but the difference is not significant.
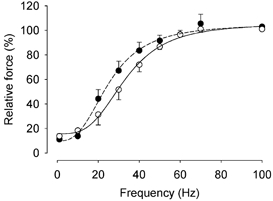
Mean data (± S.E.M.) from CK-/- (•, n = 9) and wild-type (○, n = 3) soleus fibres. The force at 100 Hz is set to 100 % in each fibre.
Isolated soleus fibres were fatigued by repeated 500 ms 50 Hz tetani given at 1 s intervals. At the start of fatiguing stimulation, tetanic force was significantly lower (P < 0.05) in the CK-/- fibres (264 ± 20 kN m−2) than in the wild-type fibres (330 ± 25 kN m−2). During the initial part of fatiguing stimulation, tetanic force decreased by ≈15 % in CK-/- fibres while it was little affected in wild-type fibres (Fig. 6). Thereafter tetanic force remained stable in CK-/- fibres for the rest of the fatiguing stimulation period. Conversely, after ≈50 fatiguing contractions, tetanic force showed a gradual decline in wild-type fibres and force fell to 40 % of the initial value (and stimulation was stopped) before the end of the 500 tetani in two of the three fibres.
Figure 6. CK-/- soleus fibres are at least as fatigue resistant as wild-type fibres.
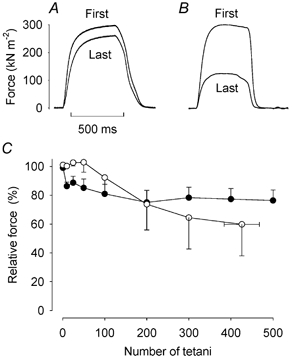
Representative records of force in the first and last fatiguing tetanus of a CK-/- (A) and a wild-type (B) soleus fibre. Time bar applies to both fibres. C shows mean data (± S.E.M.) from CK-/- (•, n = 9) and wild-type (○, n = 3) soleus fibres. The force of the first tetanus is set to 100 % in each fibre.
Slowing of relaxation is generally a prominent feature in fatigue (Westerblad et al. 1997). Intriguingly, neither CK-/- nor wild-type soleus fibres showed such slowing (see Fig. 6A and B). At the start of fatiguing stimulation, the half-relaxation time was markedly longer (P < 0.01) in CK-/- fibres (101.3 ± 9.5 ms) than in wild-type fibres (38.3 ± 11.5 ms). In the last fatiguing tetanus, the half-relaxation time had actually decreased in CK-/- fibres (78.7 ± 7.3 ms, P < 0.01), whereas it showed little change in wild-type fibres (34.0 ± 10.0 ms).
Estimates of the mitochondrial content in FDB and soleus muscles
The results above strongly indicate that increased mitochondrial capacity is a key factor behind the increased fatigue resistance of CK-/- fibres. We used confocal images of rhod-2 fluorescence to get an estimate of the fractional area of fibres occupied by mitochondria (see Methods). These estimates showed a significantly larger (P < 0.05) mitochondrial content in CK-/- (24.7 ± 4.9 %, n = 5) as compared to wild-type (9.9 ± 2.7 %, n = 5) FDB fibres. This is in reasonable agreement with previous reports showing ≈twofold difference in the concentration of mitochondria and mitochondrial proteins between CK-/- and wild-type fast-twitch muscle fibres (Steeghs et al. 1998; De Groof et al. 2001; Kaasik et al. 2003). The mitochondrial content was also estimated in CK-/- soleus fibres and amounted to 33.1 ± 2.9 % (n = 3).
Measurements of mitochondrial [Ca2+] in FDB fibres during fatigue under control conditions
Mitochondrial [Ca2+] was measured with rhod-2 in CK-/- and wild-type FDB muscle fibres during fatigue produced under control conditions. Confocal microscopy images from a CK-/- fibre (Fig. 7A) show a marked increase in the rhod-2 fluorescence (i.e. increased mitochondrial [Ca2+]) close to the cell surface during fatiguing stimulation. This increase reached its maximum after 25 contractions and then declined. Five minutes after the end of fatiguing stimulation, the rhod-2 fluorescence was back to the initial level. Mean data show that in CK-/- fibres (n = 5), the mitochondrial [Ca2+] signal increased during fatigue reaching a peak with a threefold increase in F/F0 a fter 25 fatiguing contractions (Fig. 7B). Conversely, wild-type fibres (n = 5) did not show any significant change in the rhod-2 fluorescence during fatigue. However, mitochondria of wild-type FDB fibres were able to accumulate detectable amounts of Ca2+ after localized damage to the sarcolemma was induced by repeated scanning of a small area with maximum laser power (Bruton et al. 2003). Figure 7C shows images from one such experiment. In this fibre, the rhod-2 fluorescence was increased ≈threefold 2 min after induction of sarcolemmal damage. Similar results were obtained in one more fibre. These results indicate that mitochondria of wild-type FDB fibres can take up Ca2+ under certain conditions.
Figure 7. Mitochondrial [Ca2+] increases during repeated tetanic stimulation in CK-/- FDB fibres.
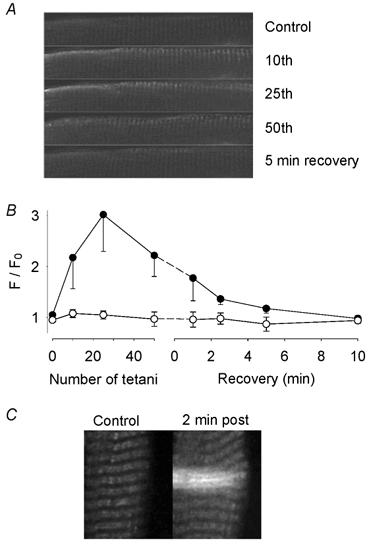
A, confocal images obtained from a CK-/- fibre loaded with rhod-2 to measure mitochondrial [Ca2+]. Images obtained under control conditions, after 10, 25 and 50 repeated contractions, and after 5 min of recovery. The height of each image is 30 μM. The rhod-2 fluorescence signal increased (brighter image) during the stimulation period, especially at the cell surface. B, mean data (± S.E.M.) of the relative change in rhod-2 fluorescence in CK-/- (•; n = 5) and wild-type (○; n = 5) FDB fibres. The fluorescence at each time point (F) is expressed relative to the fluorescence under control conditions (F0). C, confocal images from a wild-type FDB fibre before (Control) and 2 min after (2 min post) localized damage to the sarcolemma was induced by excessive laser light. This resulted in a local increase in the rhod-2 fluorescence in two horizontal rows of mitochondria. Note that this would reflect an increase in mitochondrial [Ca2+] since it was not accompanied by any major contraction, which would be the consequence of a major increase in myoplasmic [Ca2+]. The total width of the image is 30 μm.
DISCUSSION
The main novel results of the present study are the following. (1) Inhibition of mitochondrial respiration in CK-/- FDB fibres results in a very rapid force decrease during fatiguing stimulation, which is not accompanied by any reduction of tetanic [Ca2+]i. (2) CK-/- soleus fibres are at least as fatigue resistant as their wild-type counterparts. (3) In contrast to the situation in wild-type FDB fibres, mitochondrial [Ca2+] increases during repeated contractions in CK-/- FDB fibres.
Force and [Ca2+]i changes during fatigue in FDB fibres following cyanide administration
Although tetanic force declined more rapidly after mitochondrial inhibition with cyanide in both CK-/- and wild-type FDB fibres, the effect of cyanide was markedly larger in CK-/- fibres (see Fig. 1), which agrees with recent results obtained in CK-/- and wild-type diaphragm muscles (Dzeja et al. 2003). Thus, the increased fatigue resistance seen under control conditions in CK-/- FDB fibres (present results; Dahlstedt et al. 2000) would be related to an increased oxidative capacity, which is in agreement with an about twofold increase in the concentration of mitochondria and mitochondrial proteins in fast-twitch CK-/- fibres (present results) (Steeghs et al. 1998; De Groof et al. 2001; Kaasik et al. 2003). Furthermore, ATP generated by mitochondria is effectively channelled to myofibrils and SR in fast-twitch CK-/- muscle cells, which appears to differ from the situation in wild-type cells (Kaasik et al. 2003).
In wild-type fibres, there is a characteristic pattern of tetanic [Ca2+]i changes during fatigue that remains during cyanide exposure, i.e. an early increase followed by a gradual decline (see Fig. 3). This did not occur in CK-/- fibres, neither under control conditions nor during mitochondrial inhibition, which indicates that such changes in tetanic [Ca2+]i are related to CK activity and PCr breakdown (Balog et al. 2000; Allen & Westerblad, 2001; Duke & Steele, 2001). An alternative explanation would be that mitochondrial Ca2+ uptake in the mitochondria-rich CK-/- fibres buffers the early increase in tetanic [Ca2+]i. However, this seems unlikely because an early increase in tetanic [Ca2+]i followed by a decline is also observed during fatiguing stimulation in highly oxidative mouse soleus fibres (Bruton et al. 2003).
Both CK-/- and wild-type FDB fibres displayed a decrease in tetanic [Ca2+]i after a 15 min rest period in cyanide. When mitochondrial respiration is inhibited, fibres will depend on anaerobic glycolysis and in wild-type fibres also on PCr breakdown for a supply of ATP. This will affect the intracellular energy balance and changes in the ATP/ADP ratio can be expected. Thus, a likely mechanism behind the decrease in tetanic [Ca2+]i is inhibition of SR Ca2+ release channels due to reduced [ATP] coupled to increased [Mg2+] (Lamb, 2002). A reduced ATP/ADP ratio would also decrease the ability of SR to accumulate Ca2+ (Macdonald & Stephenson, 2001), which may explain the observed increase in resting [Ca2+]i upon cyanide exposure. In CK-/- fibres, tetanic [Ca2+]i remained relatively constant during fatiguing stimulation in the presence of cyanide. Thus, it seems that cyanide exposure resulted in a robust change in the ATP/ADP ratio in the vicinity of SR Ca2+ release channels (i.e. the triadic region), which was little affected by the increased energy demand associated with repeated contractions. This would then suggest a microenvironment in the triadic region with a high capacity to generate ATP via anaerobic glycolysis. In support of this, the triadic region has been shown to contain a high concentration of glycolytic enzymes (Han et al. 1992).
One unexpected finding of the present study is the discrepancy between the rapid force decline and the stable tetanic [Ca2+]i during fatigue of CK-/- fibres in the presence of cyanide. This is particularly clear in the second tetanus where force is reduced by ≈35 % while [Ca2+]i remains unchanged. This uncoupling between tetanic [Ca2+]i and force obviously reflects a marked decrease in myofibrillar Ca2+ sensitivity and/or reduced ability of cross-bridges to generate force (Westerblad & Allen, 1996). Two possible mechanisms can be advanced to account for these changes. First, reduced intracellular pH decreases both myofibrillar Ca2+ sensitivity and cross-bridge force production at the temperature of this study, whereas it has little effect on voltage-activated SR Ca2+ release (Westerblad & Allen, 1993; Lamb & Stephenson, 1994). During fatiguing stimulation in the presence of cyanide, anaerobic glycolysis would be the dominant source of energy production in CK-/- fibres and hence acidification can be expected. Furthermore, hydrogen ions are normally consumed during intensive activity due to PCr hydrolysis but this cannot occur in CK-/- fibres. Second, the rapid decrease in force in CK-/- fibres may result from a major reduction in the ATP/ADP ratio at the myofibrils, which is known to reduce the rate of cross-bridge cycling (Cooke & Pate, 1985; Westerblad et al. 1998). This slowing would increase the time taken for force to reach its maximum during a tetanic contraction. Consequently, the tetanic duration (350 ms) used in the present study might have been too short for maximum force to be attained. In line with this, we observed a high relative rate of force rise towards the end of the 350 ms tetanic contractions in CK-/- fibres fatigued in the presence of cyanide (see Fig. 4).
Force measurements and mitochondrial function in isolated soleus fibres
Soleus muscles of CK-/- mice and their wild-type controls both contain about 45 % fast-twitch type IIa fibres and 55 % slow-twitch type I fibres (Steeghs et al. 1998). FDB muscles of wild-type C57 mice contain mainly fast-twitch type IIa (≈60 %) and IIx (≈35 %) fibres (Raymackers et al. 2000). The force-frequency relationships of our isolated soleus and FDB fibres were similar, which indicates that our isolated soleus fibres were mainly fast-twitch type IIa fibres. At any rate, these soleus fibres were markedly more fatigue resistant than FDB fibres. All CK-/- soleus fibres sustained 500 tetanic contractions at a duty cycle of 0.5, whereas even the most fatigue resistant CK-/- FDB fibres cannot sustain 100 tetanic contractions at a duty cycle of 0.35 (Dahlstedt et al. 2000); the difference between the fatigue resistance of soleus and FDB fibres was even larger in wild-type mice. Thus, despite similar kinetics of isometric contractions, soleus fibres are more fatigue resistant than FDB fibres. This can be due to a higher mitochondrial content in soleus fibres, as indicated by the present estimates of mitochondrial concentration. However, the difference in mitochondrial concentration between CK-/- FDB and soleus fibres was rather modest, which suggests that additional factors also contribute.
Isolated soleus fibres of CK-/- mice were at least as fatigue resistant as wild-type fibres (see Fig. 6). This shows that under the present experimental conditions, the flux of ATP/ADP between mitochondria and sites of energy consumption (i.e. cross-bridges and ion pumps) is sufficient despite the absence of CK-mediated energy shuttling. Moreover, mitochondria can work effectively despite the lack of MtCK even in these highly oxidative fibres.
Effects of CK deficiency on contractile performance
The present results and several previous studies show that the contractile performance of CK-/- skeletal muscle cells is surprisingly normal and in some situations even superior to wild-type cells (e.g. increased fatigue resistance). This raises the question why skeletal muscle cells have CK. In other words, what are the direct negative effects on contractile function of CK deficiency? One obvious disadvantage of CK deficiency is that force cannot be sustained during periods of very high-intensity contractions (duty cycle ≈0.7) (Steeghs et al. 1997; Dahlstedt et al. 2003). However, this intensity is well above that required during any normal activity (Hennig & Lømo, 1985) and hence the functional consequences for the intact animal may be limited. Another disadvantage is that under control conditions, CK-/- fibres produce lower force per cross-sectional area than wild-type fibres. Structural adaptations in CK-/- cells, including a marked increase in mitochondria, will reduce the space available for myofibrils. This, together with an increased myoplasmic Pi in resting muscles, reduces the maximum force generating capacity (Dahlstedt et al. 2001). Finally, Cr has been shown to be a powerful stimulator of mitochondrial respiration in oxidative muscle cells (Veksler et al. 1995; Kuznetsov et al. 1996). One functionally important aspect of this is that mitochondrial respiration can be activated during periods of high energy demand without major changes in ADP (Kay et al. 2000; Walsh et al. 2001). In the absence of CK, ADP has to increase substantially in order to fully activate mitochondrial respiration. Moreover, a higher ADP level is required to activate mitochondrial respiration in fast-twitch CK-/- muscles as compared to wild-types (Kay et al. 2000; Kaasik et al. 2003). While increased ADP has limited effect on isometric force production, it decreases the rate of cross-bridge cycling and hence reduces the shortening speed of skeletal muscle cells (Cooke & Pate, 1985; Westerblad et al. 1998). Thus, a major effect of MtCK might be to minimize the ADP increase required to activate mitochondrial respiration. The effect of CK deficiency on contractile performance may then be more pronounced during a series of dynamic contractions with active shortening than during the present experiments with repeated isometric contractions.
Mitochondrial Ca2+ accumulation in CK-/- FDB fibres
Results from our laboratory recently showed that FDB fibres of NMRI mice do not accumulate Ca2+ in the mitochondria during a series of repeated contractions (Lännergren et al. 2001), which is consistent with the present results of wild-type fibres (see Fig. 7). Conversely, FDB fibres of CK-/- mice showed a significant increase in mitochondrial [Ca2+]. Uptake of Ca2+ from the cytosol into the mitochondria is considered to occur via a uniporter that is driven by the large membrane potential over the inner mitochondrial membrane (Loew et al. 1994; Duchen et al. 1998). The function of this uniporter is considered to be modulated in a complex way by adenine nucleotides and Pi (Litsky & Pfeiffer, 1997), the concentrations of which are known to differ between wild-type and CK-/- muscles (Steeghs et al. 1997; Dahlstedt et al. 2000). In addition, structural alterations in mitochondria lacking MtCK may result in an increased Ca2+ influx. Nevertheless, the increase in mitochondrial Ca2+ would be beneficial since mitochondria contain Ca2+-activated dehydrogenases and Ca2+ stimulates mitochondrial respiration (Jouaville et al. 1999; Kavanagh et al. 2000; Cortassa et al. 2003). Thus, the increase in mitochondrial [Ca2+] may limit the increase in ADP required to fully activate the mitochondria lacking MtCK.
Conclusions
Compared to wild-type fibres, CK-/- FDB fibres have a higher mitochondrial content, are more fatigue resistant and their myofibrillar function is more sensitive to inhibition of mitochondrial respiration. Thus, mitochondrial respiration as well as the energy shuttling between mitochondria and sites of energy consumption can function effectively in the absence of CK. This is achieved by major adaptations both in mitochondrial function and in intracellular energy channelling (Kaasik et al. 2003). One sign of mitochondrial adaptation is the increase in mitochondrial [Ca2+] observed during repeated tetani in CK-/- but not in wild-type FDB fibres.
Acknowledgments
We thank Professor Be Wieringa (Department of Cell Biology and Histology, University of Nijmegen) for donation of the CK-/- mice and helpful discussions. This work was supported by grants from the Swedish Research Council (Project 10842 and 14402), the Swedish National Center for Sports Research, the Lars Hierta's Memorial Fund, and funds at the Karolinska Institutet.
REFERENCES
- Allen DG, Westerblad H. Role of phosphate and calcium stores in muscle fatigue. J Physiol. 2001;536:657–665. doi: 10.1111/j.1469-7793.2001.t01-1-00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol. 1998;509:565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock DF, Herrington J, Goodwin PC, Park YB, Hille B. Mitochondrial participation in the intracellular Ca2+ network. J Cell Biol. 1997;136:833–844. doi: 10.1083/jcb.136.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balog EM, Fruen BR, Kane PK, Louis CF. Mechanisms of Pi regulation of the skeletal muscle SR Ca2+ release channel. Am J Physiol Cell Physiol. 2000;278:C601–611. doi: 10.1152/ajpcell.2000.278.3.C601. [DOI] [PubMed] [Google Scholar]
- Bruton JD, Tavi P, Aydin J, Westerblad H, Lännergren L. Mitochondrial and myoplasmic Ca2+ in single fibres from mouse limb muscles during repeated tetanic contractions. J Physiol. 2003;551:179–190. doi: 10.1113/jphysiol.2003.043927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R, Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J. 1985;48:789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortassa S, Aon MA, Marban E, Winslow RL, O'Rourke B. An integrated model of cardiac mitochondrial energy metabolism and calcium dynamics. Biophys J. 2003;84:2734–2755. doi: 10.1016/S0006-3495(03)75079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstedt AJ, Katz A, Tavi P, Westerblad H. Creatine kinase injection restores contractile function in creatine-kinase-deficient mouse skeletal muscle fibres. J Physiol. 2003;547:395–403. doi: 10.1113/jphysiol.2002.034793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstedt AJ, Katz A, Westerblad H. Role of myoplasmic phosphate in contractile function of skeletal muscle: studies on creatine kinase-deficient mice. J Physiol. 2001;533:379–388. doi: 10.1111/j.1469-7793.2001.0379a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstedt AJ, Katz A, Wieringa B, Westerblad H. Is creatine kinase responsible for fatigue? Studies of skeletal muscle deficient of creatine kinase. FASEB J. 2000;14:982–990. doi: 10.1096/fasebj.14.7.982. [DOI] [PubMed] [Google Scholar]
- De Groof AJ, Oerlemans FT, Jost CR, Wieringa B. Changes in glycolytic network and mitochondrial design in creatine kinase-deficient muscles. Muscle Nerve. 2001;24:1188–1196. doi: 10.1002/mus.1131. [DOI] [PubMed] [Google Scholar]
- Duchen MR, Leyssens A, Crompton M. Transient mitochondrial depolarizations reflect focal sarcoplasmic reticular calcium release in single rat cardiomyocytes. J Cell Biol. 1998;142:975–988. doi: 10.1083/jcb.142.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke AM, Steele DS. Mechanisms of reduced SR Ca2+ release induced by inorganic phosphate in rat skeletal muscle fibers. Am J Physiol Cell Physiol. 2001;281:C418–429. doi: 10.1152/ajpcell.2001.281.2.C418. [DOI] [PubMed] [Google Scholar]
- Dzeja PP, Terzic A, Wieringa B. Phosphotransfer dynamics in skeletal muscle from creatine kinase gene-deleted mice. Mol Cell Biochem. 2003 doi: 10.1023/b:mcbi.0000009856.23646.38. (in press) [DOI] [PubMed] [Google Scholar]
- Gorselink M, Drost MR, Van Der Vusse GJ. Murine muscles deficient in creatine kinase tolerate repeated series of high-intensity contractions. Pflugers Arch. 2001;443:274–279. doi: 10.1007/s004240100687. [DOI] [PubMed] [Google Scholar]
- Han JW, Thieleczek R, Varsanyi M, Heilmeyer LM., Jr Compartmentalized ATP synthesis in skeletal muscle triads. Biochemistry. 1992;31:377–384. doi: 10.1021/bi00117a010. [DOI] [PubMed] [Google Scholar]
- Hennig R, Lømo T. Firing patterns of motor units in normal rats. Nature. 1985;314:164–166. doi: 10.1038/314164a0. [DOI] [PubMed] [Google Scholar]
- Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci U S A. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasik A, Veksler V, Boehm E, Novotova M, Ventura-Clapier R. From energy store to energy flux: a study in creatine kinase deficient fast skeletal muscle. FASEB J. 2003;17:708–710. doi: 10.1096/fj.02-0684fje. [DOI] [PubMed] [Google Scholar]
- Kavanagh NI, Ainscow EK, Brand MD. Calcium regulation of oxidative phosphorylation in rat skeletal muscle mitochondria. Biochim Biophys Acta. 2000;1457:57–70. doi: 10.1016/s0005-2728(00)00054-2. [DOI] [PubMed] [Google Scholar]
- Kay L, Nicolay K, Wieringa B, Saks V, Wallimann T. Direct evidence for the control of mitochondrial respiration by mitochondrial creatine kinase in oxidative muscle cells in situ. J Biol Chem. 2000;275:6937–6944. doi: 10.1074/jbc.275.10.6937. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AV, Tiivel T, Sikk P, Kaambre T, Kay L, Daneshrad Z, Rossi A, Kadaja L, Peet N, Seppet E, Saks VA. Striking differences between the kinetics of regulation of respiration by ADP in slow-twitch and fast-twitch muscles in vivo. Eur J Biochem. 1996;241:909–915. doi: 10.1111/j.1432-1033.1996.00909.x. [DOI] [PubMed] [Google Scholar]
- Lamb GD. Voltage-sensor control of Ca2+ release in skeletal muscle: insights from skinned fibers. Front Biosci. 2002;7:d834–d842. doi: 10.2741/A815. [DOI] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. Effects of intracellular pH and [Mg2+] on excitation-contraction coupling in skeletal muscle fibres of the rat. J Physiol. 1994;478:331–339. doi: 10.1113/jphysiol.1994.sp020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J, Westerblad H. The temperature dependence of isometric contractions of single, intact fibres dissected from a mouse foot muscle. J Physiol. 1987;390:285–293. doi: 10.1113/jphysiol.1987.sp016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J, Westerblad H, Bruton JD. Changes in mitochondrial Ca2+ detected with Rhod-2 in single frog and mouse skeletal muscle fibres during and after repeated tetanic contractions. J Muscle Res Cell Motil. 2001;22:265–275. doi: 10.1023/a:1012227009544. [DOI] [PubMed] [Google Scholar]
- Litsky ML, Pfeiffer DR. Regulation of the mitochondrial Ca2+ uniporter by external adenine nucleotides: the uniporter behaves like a gated channel which is regulated by nucleotides and divalent cations. Biochemistry. 1997;36:7071–7080. doi: 10.1021/bi970180y. [DOI] [PubMed] [Google Scholar]
- Loew LM, Carrington W, Tuft RA, Fay FS. Physiological cytosolic Ca2+ transients evoke concurrent mitochondrial depolarizations. Proc Natl Acad Sci U S A. 1994;91:12579–12583. doi: 10.1073/pnas.91.26.12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald WA, Stephenson DG. Effects of ADP on sarcoplasmic reticulum function in mechanically skinned skeletal muscle fibres of the rat. J Physiol. 2001;532:499–508. doi: 10.1111/j.1469-7793.2001.0499f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marechal G, Beckers-Bleukx G. Force-velocity relation and isomyosins in soleus muscles from two strains of mice (C57 and NMRI) Pflugers Arch. 1993;424:478–487. doi: 10.1007/BF00374911. [DOI] [PubMed] [Google Scholar]
- Raymackers JM, Gailly P, Schoor MC, Pette D, Schwaller B, Hunziker W, Celio MR, Gillis JM. Tetanus relaxation of fast skeletal muscles of the mouse made parvalbumin deficient by gene inactivation. J Physiol. 2000;527:355–364. doi: 10.1111/j.1469-7793.2000.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackhouse SK, Reisman DS, Binder-Macleod SA. Challenging the role of pH in skeletal muscle fatigue. Phys Ther. 2001;81:1897–1903. [PubMed] [Google Scholar]
- Steeghs K, Benders A, Oerlemans F, De Haan A, Heerschap A, Ruitenbeek W, Jost C, Van Deursen J, Perryman B, Pette D, Bruckwilder M, Koudijs J, Jap P, Veerkamp J, Wieringa B. Altered Ca2+ responses in muscles with combined mitochondrial and cytosolic creatine kinase deficiencies. Cell. 1997;89:93–103. doi: 10.1016/s0092-8674(00)80186-5. [DOI] [PubMed] [Google Scholar]
- Steeghs K, Oerlemans F, De Haan A, Heerschap A, Verdoodt L, De Bie M, Ruitenbeek W, Benders A, Jost C, Van Deursen J, Tullson P, Terjung R, Jap P, Jacob W, Pette D, Wieringa B. Cytoarchitectural and metabolic adaptations in muscles with mitochondrial and cytosolic creatine kinase deficiencies. Mol Cell Biochem. 1998;184:183–194. [PubMed] [Google Scholar]
- Veksler VI, Kuznetsov AV, Anflous K, Mateo P, Van Deursen J, Wieringa B, Ventura-Clapier R. Muscle creatine kinase-deficient mice. II. Cardiac and skeletal muscles exhibit tissue-specific adaptation of the mitochondrial function. J Biol Chem. 1995;270:19921–19929. doi: 10.1074/jbc.270.34.19921. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J. 1992;281:21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh B, Tonkonogi M, Soderlund K, Hultman E, Saks V, Sahlin K. The role of phosphorylcreatine and creatine in the regulation of mitochondrial respiration in human skeletal muscle. J Physiol. 2001;537:971–978. doi: 10.1111/j.1469-7793.2001.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. The influence of intracellular pH on contraction, relaxation and [Ca2+]i in intact single fibres from mouse muscle. J Physiol. 1993;466:611–628. [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Mechanisms underlying changes of tetanic [Ca2+]i and force in skeletal muscle. Acta Physiol Scand. 1996;156:407–416. doi: 10.1046/j.1365-201X.1996.196000.x. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG, Lännergren J. Muscle fatigue: lactic acid or inorganic phosphate the major cause. News Physiol Sci. 2002;17:17–21. doi: 10.1152/physiologyonline.2002.17.1.17. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Dahlstedt AJ, Lännergren J. Mechanisms underlying reduced maximum shortening velocity during fatigue of intact, single fibres of mouse muscle. J Physiol. 1998;510:269–277. doi: 10.1111/j.1469-7793.1998.269bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Lännergren J, Allen DG. Slowed relaxation in fatigued skeletal muscle fibers of Xenopus and mouse. Contribution of [Ca2+]i and cross-bridges. J Gen Physiol. 1997;109:385–399. doi: 10.1085/jgp.109.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Yoshioka T. Profiles of creatine kinase isoenzyme compositions in single muscle fibres of different types. J Muscle Res Cell Motil. 1991;12:37–44. doi: 10.1007/BF01781172. [DOI] [PubMed] [Google Scholar]


