Abstract
The activity pattern of low-threshold human trapezius motor units was examined in response to brief, voluntary increases in contraction amplitude (‘EMG pulse’) superimposed on a constant contraction at 4–7% of the surface electromyographic (EMG) response at maximal voluntary contraction (4–7% EMGmax). EMG pulses at 15–20% EMGmax were superimposed every minute on contractions of 5, 10, or 30 min duration. A quadrifilar fine-wire electrode recorded single motor unit activity and a surface electrode recorded simultaneously the surface EMG signal. Low-threshold motor units recruited at the start of the contraction were observed to stop firing while motor units of higher recruitment threshold stayed active. Derecruitment of a motor unit coincided with the end of an EMG pulse. The lowest-threshold motor units showed only brief silent periods. Some motor units with recruitment threshold up to 5% EMGmax higher than the constant contraction level were recruited during an EMG pulse and kept firing throughout the contraction. Following an EMG pulse, there was a marked reduction in motor unit firing rates upon return of the surface EMG signal to the constant contraction level, outlasting the EMG pulse by 4 s on average. The reduction in firing rates may serve as a trigger to induce derecruitment. We speculate that the silent periods following derecruitment may be due to deactivation of non-inactivating inward current (‘plateau potentials’). The firing behaviour of trapezius motor units in these experiments may thus illustrate a mechanism and a control strategy to reduce fatigue of motor units with sustained activity patterns.
Most established knowledge of motor unit firing behaviour is based on experiments performed on extremity muscles and usually not exceeding 1 min duration. Studies with longer recording periods have been performed, notably control and training of individual motor units, with experimental feedback given by the observed motor unit (e.g. Basmajian, 1963), and experiments collecting a large number of firings for statistical evaluation of motor unit synchrony (Sears & Stagg, 1976). These experimental protocols are not designed to ascertain the ‘natural’ firing behaviour of motor units active for long periods of time, such as low-threshold motor units in postural muscles. The extensive motor control literature has therefore not yet addressed those aspects that could be unique to the control of motor units in postural muscles. In an earlier publication we documented the phenomenon of motor unit substitution in the human trapezius muscle (Westgaard & De Luca, 1999), i.e. the recruitment of higher-threshold motor units to replace lower-threshold, putative fatigued motor units that stop firing. Motor unit substitution is an old concept that has been reported in some scientific papers and is often mentioned within the clinical community (Person, 1974; Kato et al. 1981; Fallentin et al. 1993), but few reports exist that properly document the phenomenon. In the earlier publication where motor unit firing was monitored for 10 min, we noted instances of substitution coinciding with brief periods of reduced excitatory drive to the motoneuron pool, manifest as a brief period of reduced activity in the electromyographic signal recorded by surface electrodes (‘EMG gaps’). When substitution occurred in a few motor units, the rest remained active throughout the recording period.
In another study we showed that the firing rates of the human trapezius motor units were relatively stable and independent of the overall activity level in the muscle, at least in the case of slowly augmenting contractions or contractions maintained at a set contraction level (Westgaard & De Luca, 2001). An observation of particular interest was that the firing rate of continuously active motor units tended to decrease below the level of stable firing after the first few seconds of a temporary increase in surface electromyographic (EMG) activity, despite the root mean square (RMS)-detected amplitude of the surface EMG staying equal to or higher than the constant EMG value. We considered that the temporary depression in firing could be helpful in inducing substitution in that the net excitatory input to already active motor units was reduced and the motor units were thereby closer to recruitment threshold. The aim of the present study is to test this hypothesis. A secondary aim is to generate new knowledge regarding the physiological processes that promote substitution in the trapezius muscle. A procedure of contractions at a constant level by the trapezius muscle, but with short-duration increases in voluntary EMG superimposed every minute (‘EMG pulse’) was implemented. Most experiments were carried out for 10 min, but some experiments were extended to 30 min to look for evidence of more frequent or longer-duration substitution in putative fatigued motor units. The effect of EMG pulse amplitude on substitution was examined in experiments of 5 min duration, with EMG pulses of increasing amplitude imposed every minute.
METHODS
Ten healthy subjects, three males and seven females, volunteered for the study. The age ranged from 20 to 56 years. The experiments were performed according to the Declaration of Helsinki. Each subject read and signed an informed consent form approved by the local Institutional Review Board prior to participating in the study. We studied the trapezius muscle, and detected the surface and intramuscular EMG signals. The indwelling EMG signal was used to study firing behaviour of trapezius motor units. Force developed by the trapezius cannot be reliably monitored due to the complex biomechanics of the muscle synergies controlling shoulder movement. The root-mean-square (RMS)-detected trapezius surface EMG signal was therefore used as a proxy indicator of trapezius force development. The surface EMG signal was calibrated as a percentage of the RMS-detected EMG activity at maximal voluntary contraction (%EMGmax).
Experimental procedures
Three experimental procedures were performed. The first consisted of a contraction of 10 min duration with constant EMG amplitude, with brief periods (nominally of 2-4 s duration) of voluntary increase in muscle activity (‘EMG pulses’) superimposed every minute. The constant contraction amplitude ranged from 4 to 7 % EMGmax in all experiments, determined by the observation of a suitable number of motor units in brief trial contractions before the start of the experiment proper. The peak amplitude of the EMG pulses was targeted to 15-20 % EMGmax. In the second procedure the contraction period was extended to 30 min; the first 10 min was performed in the same manner as in the first procedure. It continued with a 10-min constant EMG amplitude contraction without EMG pulses and finally the procedure of the first 10 min was repeated from 20 to 30 min. The third procedure consisted of a 5-min constant EMG amplitude contraction with a series of four EMG pulses at 1 min intervals, performed at increasing strength from 10 to 25 % EMGmax. At the end of each procedure the contraction level was briefly reduced and a ramp contraction was performed to re-examine recruitment threshold of the motor units. The procedures were carried out in a fixed order: first the 10-min contraction, then the 5-min contraction, followed by the 30-min contraction and finally another 10-min contraction, similar to the first. Between each procedure, rest periods of at least 2 min were allowed. The experiment was carried out with the subject seated. Straps placed over the shoulders provided resistance to the attempted movement of elevating the shoulders. The shoulder elevations were performed bilaterally, but with EMG data always collected from the left trapezius.
The EMG pulses were voluntary contractions controlled by the subject and initiated by a vocal cue from the experimenter, who watched a wall-mounted clock also visible to the subject. The subjects attempted to elevate the shoulders until the trapezius surface EMG signal, displayed on a visual display monitor in front of them, reached the required level and then the contraction was immediately reduced to the constant EMG amplitude. It was emphasized in the instructions to the subjects that the EMG response should not drop below the constant EMG amplitude. The timing in the execution of the contractions was not controlled except for the instruction that the EMG pulses should be brief. The need for control in reaching the high point and the return to the constant contraction baseline nevertheless necessitated feedback control in the execution of the EMG pulses, which were exercised in test contractions until an acceptable performance was achieved.
EMG recordings and analyses
The surface EMG signal was detected with an active differential electrode with two circular areas, 6 mm in diameter and centre-to-centre distance 20 mm, in skin contact. The electrode was positioned with the medial recording area 20 mm lateral to the midpoint of the line between the C7 spinous process and the acromion (Jensen et al. 1993). The surface EMG signal was band-pass filtered at 10-1000 Hz. The RMS-detected surface EMG signal was averaged at a time resolution of 0.2 s for graphical presentation purposes. The intramuscular EMG signal was recorded with specialized quadrifilar wire electrodes. These electrodes were constructed by bonding together four 50-μm nylon-coated nickel-chrome alloy wires (‘Stablohm 800A’, California Fine Wire Co, Grover Beach, CA, USA). The wire bundle was cut transversely, exposing only the cross-section of the wires. The wire bundle was placed in a 27-gauge needle and a hook was formed at approximately 1 mm from the exposed end of the wire. The needle was inserted to a depth of approximately 10 mm at a location approximately 10 mm medial to the midpoint of a line between the C7 spinous process and the acromion. The needle was removed and the wire bundle remained lodged in the muscle. Three pairs were chosen as the differential input to the amplifiers. The signals were band-pass filtered from 1 to 10 kHz. All EMG signals were stored on a digital recorder (DATaRec-A160, Racal-Heim Systems GmbH, Bergisch Gladbach, Germany). The signals were subsequently reconverted to an analog form and digitized at a sampling rate of 50 kHz on a PC.
The intramuscular EMG signals were resolved into the individual motor unit firing trains using the Precision Decomposition technique (LeFever et al. 1982; De Luca & Adam, 1999). This technique uses template matching, template updating, firing probabilities and superposition resolution to identify the individual firing times of the motor units with up to 100 % accuracy (Mambrito & De Luca, 1984). The firing rates of the motor units were obtained by inverting the time series of the inter-pulse intervals. The firing rates were subsequently low-pass filtered at 0.5 Hz. Inspection of the trains of firing rates showed that the filtering tended to reduce the peak firing rates by 1-2 pulses per second (p.p.s.), but did not affect the estimates of the post-pulse depression in firing.
RESULTS
Three 30-min contractions, four 10-min contractions and four 5-min contractions from five subjects were successful in providing data that could be studied. In these contractions, between two and six motor units were recruited within 1 min of the start of the contraction and were followed until the end of the contraction (‘stable motor units’). If a stable motor unit was temporarily derecruited at the constant-EMG-amplitude contraction level, it still fired in the EMG pulses. Thirty-four stable motor units were followed in the 11 experiments; an additional 25 motor units were either followed for part of these experiments and were lost before the end of contraction (‘not identified with certainty’) or the motor unit was only observed during the EMG pulses. The mean firing rate of the stable motor units during the constant EMG amplitude contraction was 10.0 p.p.s. (S.D. 0.8 p.p.s., range 9-12 p.p.s.). Peak firing rate in the EMG pulses was 18.2 p.p.s. (S.D. 1.9 p.p.s., range 13-22 p.p.s.). The EMG pulses increased on average 13.7 % EMGmax above the constant contraction level, range 10.9-16.7 % EMGmax (excluding the procedure with EMG pulses of variable amplitude).
Twenty-three of the 34 stable motor units were potentially able to show threshold reversal between motor units, since higher-threshold motor units were observed in the same experiment. Twelve of these (52 %) were derecruited with higher-threshold motor units active. This included 3 of 6 motor units in the 30-min contractions, 7 of 9 motor units in the 10-min contractions and 2 of 8 motor units in the 5-min contractions. The periods of no firing (‘silent periods’) ranged from a few seconds to a few minutes and started 3 min or later into the contraction, except for one motor unit that was recruited near the constant contraction level and was derecruited 1 min later.
One of the 10-min contractions is shown in Fig. 1. The figure shows the RMS of the surface EMG signal (Fig. 1A) and firing rates of three motor units with stable firing, presented in the order of increasing recruitment threshold (Fig. 1B). The insets adjacent to the plots of firing rates present templates of the motor unit action potential as detected by the three channels of the quadrifilar wire electrode. The templates were extracted at times marked by asterisks in the firing rate plots and were essentially unchanged during the contraction, indicating that the electrode did not move and the same motor units were recorded. Even minute changes in the electrode position will cause dramatic changes in the shape of the template in at least one of the channels, as was observed for some motor units that were rejected as not having stable recording.
Figure 1. RMS-detected surface electromyogram (EMG) response (A) and firing pattern of three motor units (B) during a constant amplitude, 10-min contraction with brief periods of elevated EMG activity (‘EMG pulses’) superimposed every minute.
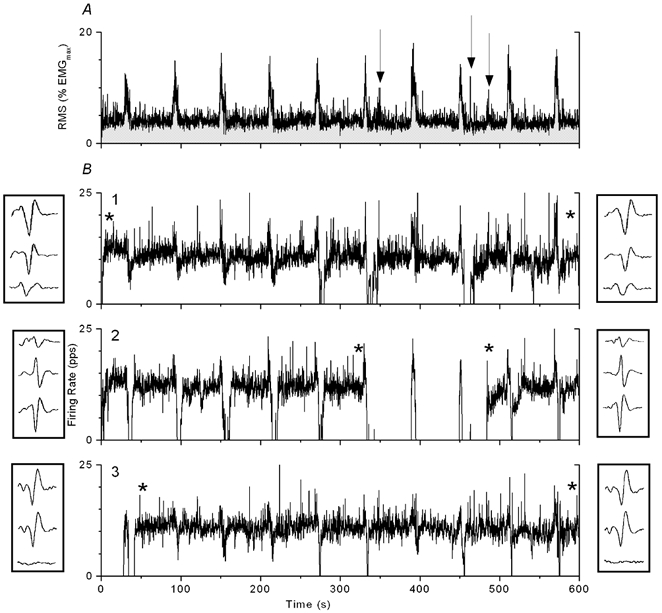
Firing rates were low-pass filtered at 0.5 Hz. Two examples of motor unit templates for each motor unit (shown next to the firing rate plot) were extracted at times marked by asterisks. In the case of motor unit no. 2, it is shown that the templates upon derecruitment and re-recruitment match each other closely. The motor units are ordered by increasing recruitment threshold. Arrows mark times of re-recruitment of derecruited motor units, coinciding with small, spontaneously evoked EMG pulses.
In the experiment of Fig. 1, motor unit no. 2 stopped firing for 150 s, except for two brief periods of firing during the EMG pulses. Motor unit no. 1 had brief periods without firing after EMG pulses in the same time period and motor unit no. 3 was continuously active. Thus, the two motor units with the lowest thresholds were derecruited while the motor unit with the highest threshold (no. 3), recruited on the first EMG pulse, showed sustained firing in this experiment. The re-recruitment of the inactive motor units was associated with brief periods of elevated activity in the surface EMG (arrows). These smaller pulses occurred spontaneously, as feedback adjustments to the display of the surface EMG signal, and were not intended to be part of the procedure. Finally, Fig. 1B illustrates a consistent, but variable depression in motor unit firing rates following the EMG pulses.
Figure 2 shows the results of a 30-min experiment where four motor units were followed. The data are presented the same way as in Fig. 1. In this experiment the motor unit with the highest threshold, no. 4, fired intermittently during all EMG pulses, with a few minutes of continuous firing at the beginning of the experiment and during the second period of EMG pulses that occurred 20 min later. This motor unit was clearly activated by a pulse and likewise turned off following another pulse that occurred 3 min later (time-expanded view in Fig. 3). During this period the motor unit with the lowest threshold did not fire for 145 s (except for activity during pulses) and was re-recruited soon after motor unit no. 4 stopped firing, at the time of a small pulse in the surface EMG. This can be considered an example of simultaneous derecruitment and re-recruitment of motor units (‘motor unit substitution’). However, derecruitment and re-recruitment of the two motor units were separated by many seconds and are therefore unlikely to be part of a reciprocal interaction between the two motor units. Only one other instance of such juxtaposition of firing rate events was observed, and then with a similar separation of the derecruitment and re-recruitment times.
Figure 2. A 30-min recording showing surface EMG response (A) and firing pattern of four motor units, ordered by increasing recruitment threshold (B).
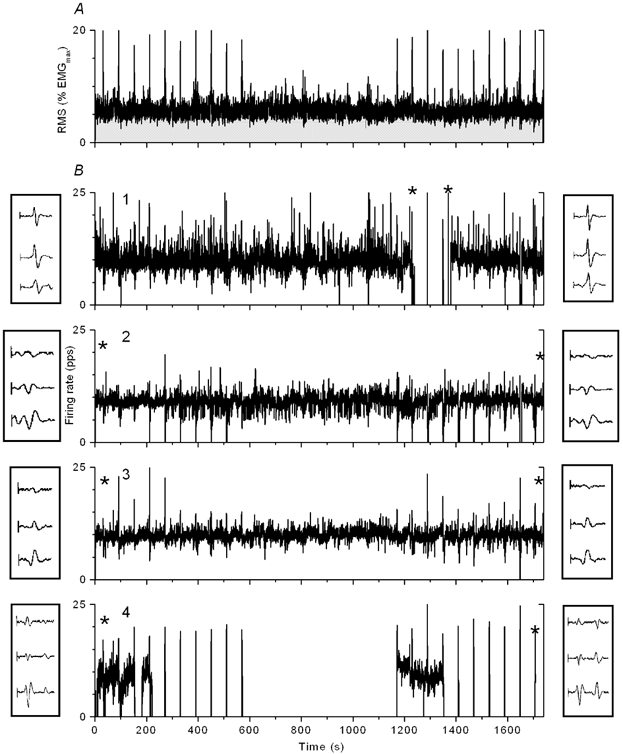
Firing rates are low-pass filtered at 0.5 Hz. Two examples of motor unit templates, shown next to the corresponding firing rate plot, were extracted at times marked by asterisks. EMG pulses were superimposed on the constant contraction at 1-min intervals during the first and the last 10 min.
Figure 3. Time-expanded view of recording in Fig. 2 to show details in the threshold reversal of motor units nos 1 and 4.
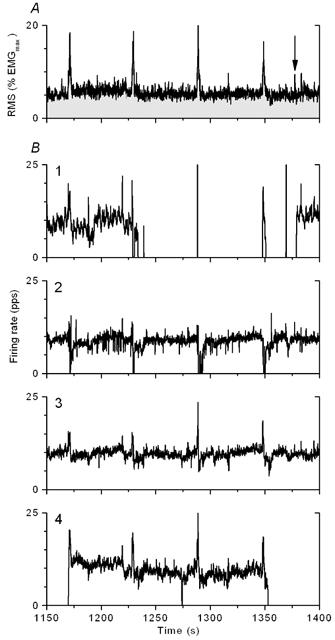
Arrow marks time of re-recruitment of motor unit. Derecruitment and re-recruitment of the motor units are separated by several seconds, making it unlikely that the threshold reversal is governed by an interactive physiological process.
Re-recruitment of motor units was generally found to coincide with planned or spontaneous small EMG pulses. Derecruitment always coincided with the post-EMG pulse depression whenever procedures with EMG pulses were performed. Silent periods were further observed during the interval without EMG pulses in two of three successful 30-min experiments. In both cases the motor units were derecruited at the time of a short depression in the surface EMG. They were re-recruited either at the first EMG pulse 20 min into the experiment or at a small spontaneous EMG pulse. These two instances of silent periods are thereby similar to observations previously reported (Westgaard & De Luca, 1999). The 5-min experiments did not reveal any consistent effect of increasing the amplitude of the EMG pulse on motor unit firing behaviour.
Figure 4 shows firing behaviour of motor units with sustained firing and motor units only active during the EMG pulses in the experiments of Fig. 1 (Fig. 4A-C) and Fig. 2 (Fig. 4D-F). The surface EMG is averaged over four EMG pulses and five motor units were identified in both experiments. The top panels (A and D) show averaged firing rates of motor units 1-3 from Fig. 1 and Fig. 2. Motor units only firing during the EMG pulse showed more variable firing behaviour. The firing patterns in the four pulses are therefore superimposed and shown in separate panels for motor units no. 4 and 5 of the two experiments. The motor units with sustained firing show a consistent, but variable depression in the firing pattern following the EMG pulse (Fig. 4A and D). The new recruited motor units had higher peak firing rates than the motor units with sustained firing and were derecruited in the downward phase of the EMG pulse, at a time with lowered firing rates for the motor units with sustained firing. This firing behaviour was consistently observed for all motor units active only during the EMG pulse; in particular, new recruited motor units were not observed to fire throughout the period with reduction in firing rates of the early recruited motor units following the EMG pulse.
Figure 4. Motor unit firing behaviour in the experiments shown in Fig. 1 (A-C) and Fig. 2 (D-F).
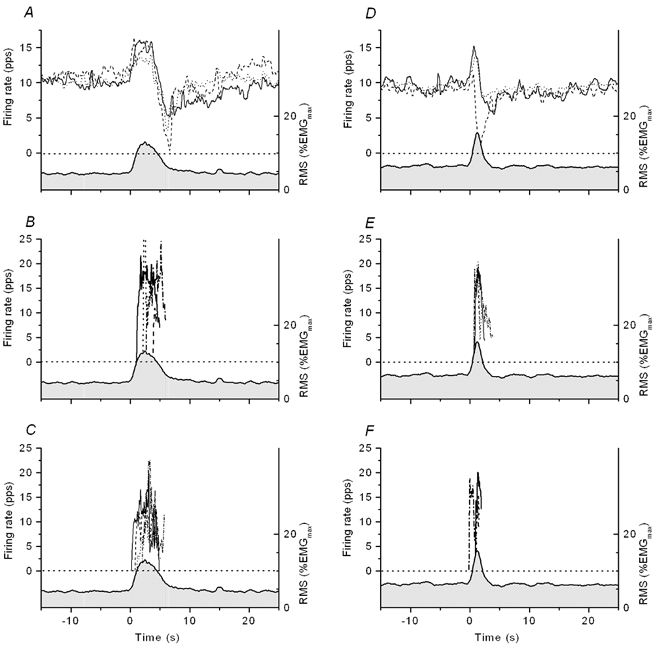
Firing rate calibration is shown on the left vertical axis; right axis shows calibration of the surface EMG response. The surface EMG responses, traced by the lower curve with grey shading underneath, are averaged from pulses 6, 7, 9 and 10 in Fig. 1 (panels A-C) and from pulses 7-10 in Fig. 2 (panels D-F). Pulse 8 in Fig. 1 was skipped due to an atypical execution of the EMG pulse. Averaged firing rates of motor units 1-3 are shown in panels A and D. The continuous line represents motor unit no. 1, followed by dashed (no. 2) and dotted (no. 3) curves. Panels B and C and E and F show activity patterns of four motor units only active during the EMG pulses. These motor units showed considerable variation in their firing patterns between consecutive EMG pulses and traces of the different responses are therefore overlaid. The motor unit in panel E is motor unit no. 4 of Fig. 2, with sustained activity between EMG pulses early in the experiment and after 20 min. The small increase in firing of some motor units prior to the EMG pulse is most likely chance occurrences due to the slow modulation of firing typical of these motor units (Westgaard et al. 2002).
The apparent discrepancy between reduced motor unit firing following the EMG pulse vs. stable or elevated amplitude of the surface EMG prompted further analysis of the experimental results. Histograms were constructed to show all motor unit firings detected by the intramuscular electrode, regardless of whether the event was identified by the decomposition algorithm or not. Figure 5 shows the averaged surface EMG and histogram of motor unit firings at a resolution of 0.5 s for the experiment in Fig. 5A and the first 10 min of the experiment in Fig. 5B. The surface EMG and the histogram of motor unit firings are based on 10 events of EMG pulses, each centred at the start of the pulse. Insets above the histograms show the surface EMG representation of the three lowest-threshold motor units before and after the EMG pulse, derived by spike triggered averaging. The time periods used for spike triggered averaging are marked by horizontal bars above the histograms. The number of firings in the histograms corresponds to observing an average of about five motor units for the top panel and four motor units for the bottom panel, assuming a firing rate of 10 p.p.s. for the constant-amplitude part of the contraction. The time course of the histograms is influenced by the observed silent periods; however, the marked depression in motor unit firing following the EMG pulse was a consistent finding in all experiments.
Figure 5. Averaged surface EMG response (line with light grey shading underneath) and histogram of motor unit firings for the experiment illustrated in Fig. 1 and for the first 10 min of the experiment shown in Fig. 2.
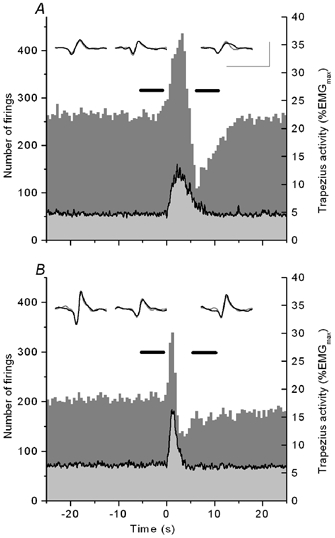
Ten EMG pulses are included, with responses centred at the start of the EMG pulse. Insets show surface representations of motor units nos 1-3 in the two experiments, derived by spike-triggered averaging before (grey) and after (black) the EMG pulse. Calibration bars indicate 100 μV and 50 ms (A and B). The triggering pulses are located to intervals marked by horizontal bars above the histograms. The marked dip in firings starts halfway into the down phase of the EMG pulse.
Figure 6 (filled symbols) shows the duration of silent periods following derecruitment as a percentage of observation time after first recruitment for the 34 stable motor units during the first 10 min of the contractions (5 min in the case of contractions with EMG pulses of variable amplitude). The duration of the silent periods is plotted as a function of recruitment threshold (A) and recruitment threshold relative to the constant contraction amplitude (B). Motor units were recruited on the leading ramp at the start of contraction, on brief overshoots before the constant level was established, or on the up-phase of the first EMG pulse. Few motor units were recruited at very low threshold; however, the three motor units recruited at thresholds < 2 % EMGmax only registered a minimal amount of silent periods (Fig. 6A). The six motor units with thresholds lower than 2 % EMGmax below the constant contraction level showed very short silent periods in the first 5 or 10 min of the contraction (Fig. 6B). Two of these were observed for 30 min and had silent periods of 2-3 min in the period from 20 to 30 min (open circles in Fig. 6B). Conversely, some motor units with recruitment threshold substantially higher than the constant contraction level maintained firing throughout the 10-min observation period, once they were recruited. Motor units only active during the EMG pulses are not included in the figure. They would register with silent periods of 90-95 %, depending on the time course of the EMG pulse.
Figure 6. Duration of silent periods after first recruitment of motor units (quantified as a percentage of observation time after first recruitment) vs. recruitment threshold.
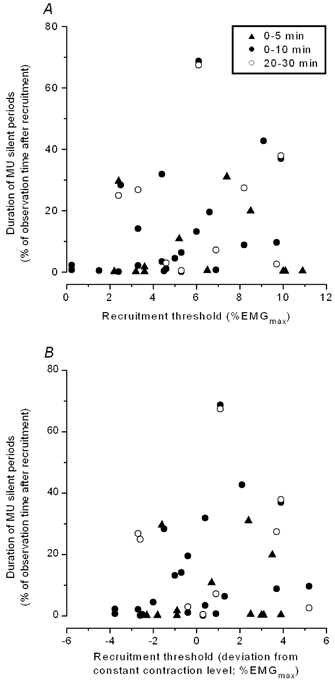
Recruitment thresholds are shown as absolute values (A) and as the difference between recruitment threshold and the constant contraction level (B). Negative values on horizontal axis in B indicate recruitment thresholds lower than the constant contraction level. Values are shown for the first 5 or 10 min of contraction (filled symbols) and in the case of 30-min contractions, the period from 20 to 30 min (open circles). Motor units with recruitment thresholds more than 2 % EMGmax below the constant contraction level show little silent periods in the first 10 min of contraction.
Recruitment thresholds were checked by ramp contractions at the end of the constant contraction and compared to the initial thresholds. The thresholds for the same motor units on repeated measurements varied typically by ≈2 % EMGmax (mean difference 1.9 %, S.D. 1.2 % EMGmax); but four motor units (excluded from the statistics) were recruited at a substantially different recruitment threshold in the ramp relative to the initial recruitment (difference 5-7 % EMGmax; two motor units had elevated and two reduced thresholds). Motor units recruited in the EMG pulses showed more variation in their thresholds. This may in part be due to the steep onset of an EMG pulse, possibly augmented by intra-individual variation in the execution of the pulses. Some motor units were excluded because they were not observed in all EMG pulses, either due to recording failure or elevated recruitment thresholds. Recruitment thresholds of these motor units were therefore not further examined.
Some of the motor units in the present series showed a marked depression in firing rate following an EMG pulse. The depression in firing varied considerably between motor units in the same experiment, but was relatively constant for the same motor unit in repeated pulses (see Fig. 1 and Fig. 2 for examples). The firing rates were not re-established at the pre-pulse level until several seconds after the surface EMG signal returned to the constant contraction level. There was also variation between experiments in the duration of the EMG pulse, see Fig. 4, but the execution was generally consistent within each experiment. A scatter plot of the duration of firing rate perturbation by RMS response width, i.e. the response durations from the start of the EMG pulse until the surface EMG response and motor unit firing rates were re-established at pre-pulse levels, is shown in Fig. 7. The line of identity and a linear regression line are also shown. The recovery of motor unit firing rates is delayed relative to the surface EMG signal, on average by 4.3 s (S.D. 3.0 s, 95 % CI ±0.6 s). A Pearson correlation analysis showed that the slope of the regression line was significantly different from zero (P < 0.001) and from the line of identity (P < 0.0001). Thus, the firing rate recovery was more delayed for brief than long EMG pulses, relative to the pulse duration.
Figure 7. Scatter plot to show duration of the perturbation in motor unit firing (from start of the EMG pulse until the pre-pulse firing rate is re-established) vs. duration of surface EMG pulse (from start of the EMG pulse until the pre-pulse activity level is re-established).
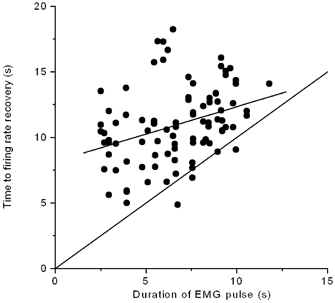
The time of recovery of firing rates was determined as the time a moving average of the firing rate (duration 2 s) was re-established at the pre-pulse level. The first four pulses at the start of contraction and after 20 min for the 30-min contractions are included. Line of identity is shown and a linear regression line is fitted to the data.
Motor units with strong post-pulse depression in firing stopped firing for several seconds. Conceivably, this may initiate a silent period of longer duration (Westgaard & De Luca, 1999). The post-pulse depression in firing may depend on motor unit recruitment threshold, in absolute terms or relative to the constant contraction level. A numeric integration of the ‘area of depression’ (i.e. the area traced by the post-pulse depression in firing, below the constant firing rate) was carried out and examined for associations with motor unit recruitment threshold or duration of silent periods, but no such associations were found.
The motor unit firing behaviour in the EMG pulses was further explored to see if specific features were interrelated. The first four responses in the periods 0-10 and 20-30 min were used in this analysis, excluding five responses where the post-pulse depression was sufficiently deep and long (>15 s) to be considered a silent period. The low-pass filtered instantaneous firing rate was down to zero in a further 19 of 94 responses, but the duration was comparable to other post-pulse depressions in the same experiment and they were therefore retained in the material. Firing rate depression level and depression duration did not correlate (r2 = 0.004, P = 0.54). The duration of firing rate depression showed a weak, but statistically significant association with firing rate elevation during the EMG pulse (r2 = 0.05, P = 0.04). We choose not to emphasize this result as there was a clear lack of such correlation in some of the individual experiments (see Fig. 4). There were no other statistically significant associations between variables characterizing firing behaviour during the EMG pulse. The mean firing rate depression was 5.6 p.p.s. (S.D. 2.9 p.p.s., range 1-13 p.p.s.). The mean duration of the firing rate depression, below the constant firing level, was 7.2 s (S.D. 2.7 s, range 1.2-14.4 s).
DISCUSSION
To our knowledge, this is the first study to investigate the influence of brief periods of elevated EMG activity on motor unit firing during sustained contractions of several minutes’ duration. We purposefully chose to study the trapezius muscle because it participates in postural trunk and arm movement and may also be activated by mental demands (Wærsted et al. 1996). Low-threshold motor units of this muscle commonly experience long periods of sustained firing even though the trapezius activity in common tasks of daily living is low, between 1 and 10 % EMGmax (Westgaard et al. 2001; Holte & Westgaard, 2002). This is therefore the range of muscle activity explored in the present series.
The trapezius motor units featured abrupt changes in recruitment threshold, with derecruitment always coinciding with a depression in firing rate following an EMG pulse. The silent periods following derecruitment were typically of 2-3 min duration; they started several minutes into the contraction and were more evident in contractions of more than 5 min duration. The silent periods were restricted to a few seconds for motor units with very low threshold (lower than 2-3 % EMGmax below the constant contraction level). Conversely, some motor units recruited on the first EMG pulse, with recruitment threshold substantially higher than the constant contraction amplitude (up to 5 % EMGmax higher), showed sustained firing during the experiment.
The changes in recruitment threshold during sustained contractions demonstrate a potential for motor unit substitution. In this study the substitution term is avoided as we did not observe concurrent recruitment and derecruitment events in this experimental series. The important common aspect of the classic motor unit substitution concept and the present results is that individual motor units, which initially have recruitment thresholds below the constant contraction amplitude as measured by surface EMG, present firing patterns with silent periods following derecruitment. The order of recruitment is thereby reversed relative to other motor units concurrently active. This may represent a motor control adaptation to reduce fatigue in low-threshold motor units during sustained contractions.
Non-inactivating inward current (‘plateau potentials’; Hounsgaard et al. 1988) is a possible physiological mechanism to explain the ability of low-threshold trapezius motoneurons to abruptly increase and lower their recruitment threshold at the start and the end of a silent period. The elevation of recruitment threshold in the present series is shown to be genuine and specific by the consistent re-recruitment of silenced motor units at the higher contraction level in the EMG pulses. Plateau potentials are traditionally detected by a sudden jump in firing rate (e.g. Eken & Kiehn, 1989). However, non-inactivating inward current can be triggered near or even before recruitment threshold if motoneurons are recruited through synaptic activation (Bennett et al. 1998). Gorassini and coworkers (2002) introduced an indirect method of observing possible plateau potential contribution to motoneuron excitation by using the firing rate of a low-threshold control unit as a measure of synaptic drive to a higher-threshold test unit. This approach is difficult to use for the testing of trapezius motor units in slowly decreasing contractions due to the low firing rate modulation (Westgaard & De Luca, 2001). However, the modulation of firing rates during the EMG pulses allowed the observation that motor units only firing during the pulse were derecruited at a time of marked reduction in firing of lower-threshold motor units. This observation, as well as motor units showing sustained firing at a lower central drive than necessary to recruit the motor unit, is consistent with the triggering of plateau potentials in trapezius motoneurons. Inactivation of plateau potentials by synaptic inhibitory activity may explain the post-pulse derecruitment of motor units (Bennett et al. 1998). Alternative mechanisms for modulation of recruitment threshold include differential depression of synaptic input to motoneurons (Rekling et al. 2000), and a state change in segmental interneurons that control neural input to the motoneurons (Burke, 1999; Jankowska, 2001; Chen et al. 2001).
The experimental protocol with control of contraction level by monitoring surface EMG activity introduces some difficulties in the interpretation of results. First, output from the trapezius motor nuclei (rather than net excitatory input) is controlled. Excitatory input to the motoneurons may be continuously adjusted to compensate for changes in intrinsic motoneuron properties; e.g. if plateau potentials account for the firing behaviour of motoneurons in sustained contractions, plateau potentials are also likely to be triggered when contractions first start. Second, the intramuscular EMG recording is highly restricted in its pick-up area. A precondition for a successful decomposition of motor units with the wire electrode and decomposition algorithm is that no more than 6-8 motor units are recorded on average. The overall reduction in post-EMG pulse firing rates, at a time when the surface EMG signal was similar to or higher than the constant contraction level, was a consistent result throughout this experimental series. If the contraction level had been force-controlled, post-tetanic potentiation of the twitch force would have been a viable explanation of this phenomenon (Klein et al. 2001). We found by use of spike-triggered averaging that the electromyographic representation of the motor units in the surface EMG was unchanged before vs. after the EMG pulse. The surface EMG signal represents a linear summation of motor unit potentials (Day & Hulliger, 2001) and a mismatch between surface EMG signal power and motor unit activity immediately below the surface EMG electrode is thereby indicated. The small number of motor units recorded by the intramuscular electrode may introduce a selection bias. Alternatively, a compensatory increase in surface EMG signal power may be generated by motor unit activity detected by the surface, but not the intramuscular EMG electrode (Westgaard et al. 1996; Jensen & Westgaard, 1997). This point requires further study; at present we conclude that a temporary net reduction in the activation of the motor units underneath the surface EMG electrode following the EMG pulse cannot be excluded (even allowing for the partial fill-in of firing by the transient recruited motor units).
Thus, the short-duration silent periods in motor unit firing immediately following an EMG pulse may take place in a condition of reduced excitatory drive to the motoneurons. This may explain the occurrence of short-duration silent periods following the EMG pulse without invoking mechanisms such as inactivation of plateau potentials. However, the silent periods of several minutes’ duration, covering times when firing rates recorded by the intramuscular electrode have recovered and with the elevated recruitment threshold tested by subsequent EMG pulses, are not explained by this reasoning. Furthermore, threshold reversal between motor units, as illustrated in Fig. 3, was observed in several experiments. This emphasizes the distinct changes to motoneuron thresholds in sustained, low-level contractions.
The post-pulse depression in firing rates may represent a complex interplay of motor control synergies, as already discussed. Even plateau potentials may contribute by level-setting of thresholds; however, the all-or-nothing manner of plateau potentials cannot alone explain the graded firing rate response. In an earlier study we showed low firing rate modulation of trapezius motor units in slowly augmenting contractions in contrast to the firing behaviour of FDI motor units, but trapezius motor units were observed to fire at a higher rate than FDI motor units in relatively fast contractions (Westgaard & De Luca, 2001). This may indicate competing excitatory and inhibitory influences that balance each other to maintain a stable firing rate. If inhibition takes effect and withdraws at a slower time course than the excitatory inputs, excitation would dominate in fast contractions to cause increased firing rates. Withdrawal of excitation in the down-phase of the EMG pulse would contribute to the post-pulse depression in firing. The long duration of the post-pulse depression may point to interactions within the segmental neuronal network that is not purely based on classic ionotropic synaptic signalling. Both GABA and monoamine transmitters have metabotropic effects on motoneurons (Rekling et al. 2000).
A candidate system to initiate post-pulse depression in firing is the Renshaw cells, which are proposed to serve as a variable gain regulator at the motoneuronal level (Hultborn et al. 1979; Baldissera et al. 1981). Recurrent inhibition in the human is more powerful in proximal than distal muscles, during weak than strong contractions, and during phasic than tonic contractions of similar force (Katz & Pierrot-Deseilligny, 1998), consistent with a strong Renshaw influence under the present experimental circumstances. Alternative explanatory schemes may involve segmental peripheral input. Spindle afferents generally respond with excitation to muscle activation, but some spindle afferents reduce their firing rates in contractions (Edin & Vallbo, 1990). The trapezius has a multi-pennate fibre orientation, which makes the proprioceptive input from the periphery less predictable.
The present results supplement the earlier demonstration that motor unit substitution during constant contractions was promoted by short depressions in contraction amplitude (Westgaard & De Luca, 1999). Taken together, it appears that force (i.e. EMG) variation in either direction promotes motor unit derecruitment during sustained contractions of the trapezius muscle. From a behavioural standpoint, if silent periods are important in the control strategy of postural motor units, it seems that this mechanism relies on force variation to facilitate it.
Finally, the findings in this study do not negate the concept of a ‘common drive’ from supraspinal centres to the motoneurons in a motor nucleus (De Luca et al. 1982). Both the derecruitment and the regulation of firing rates of trapezius motor units (Westgaard & De Luca, 2001) can be understood as control features operating at the segmental level, representing a local control of firing that modulates a descending command signal including the common drive phenomenon.
Acknowledgments
The experiments and analysis of motor unit recordings were performed at the Norwegian University of Science and Technology, Trondheim, using equipment and software developed by the NeuroMuscular Research Center of Boston University. This work was partially supported by a NICHD Biomedical Research Partnership Grant. C.W. was supported by the Research Council of Norway.
REFERENCES
- Baldissera F, Hultborn H, Illert M. Intergration in spinal neuronal systems. In: Brooks VB, editor. Handbook of Physiology, section 1, The Nervous System, Motor Control. II. Bethesda, MD, USA: American Physiological Society; 1981. pp. 509–595. [Google Scholar]
- Basmajian JV. Control and training of individual motor units. Science. 1963;141:440–441. doi: 10.1126/science.141.3579.440. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998;80:2023–2037. doi: 10.1152/jn.1998.80.4.2023. [DOI] [PubMed] [Google Scholar]
- Burke RE. The use of state-dependent modulation of spinal reflexes as a tool to investigate the organization of spinal interneurons. Exp Brain Res. 1999;128:263–277. doi: 10.1007/s002210050847. [DOI] [PubMed] [Google Scholar]
- Chen D, Theiss RD, Ebersole K, Miller JF, Rymer WZ, Heckman CJ. Spinal interneurons that receive input from muscle afferents are differentially modulated by dorsolateral descending systems. J Neurophysiol. 2001;85:1005–1008. doi: 10.1152/jn.2001.85.2.1005. [DOI] [PubMed] [Google Scholar]
- Day SJ, Hulliger M. Experimental simulation of cat electromyogram: evidence for algebraic summation of motor-unit action-potential trains. J Neurophysiol. 2001;86:2144–2158. doi: 10.1152/jn.2001.86.5.2144. [DOI] [PubMed] [Google Scholar]
- De Luca CJ, Adam A. Decomposition and analysis of intramuscular electromyographic signals. In: Windhorst U, Johansson H, editors. Modern Techniques in Neuroscience Research. Heidelberg: Springer; 1999. pp. 757–776. [Google Scholar]
- De Luca CJ, Le Fever RS, McCue MP, Xenakis AP. Control scheme governing concurrently active human motor units during voluntary contractions. J Physiol. 1982;329:129–142. doi: 10.1113/jphysiol.1982.sp014294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin BB, Vallbo ÅB. Muscle afferent responses to isometric contractions and relaxations in humans. J Neurophysiol. 1990;63:1307–1313. doi: 10.1152/jn.1990.63.6.1307. [DOI] [PubMed] [Google Scholar]
- Eken T, Kiehn O. Bistable firing properties of soleus motor units in unrestrained rats. Acta Physiol Scand. 1989;136:383–394. doi: 10.1111/j.1748-1716.1989.tb08679.x. [DOI] [PubMed] [Google Scholar]
- Fallentin N, Jørgensen K, Simonsen EB. Motor unit recruitment during prolonged isometric contractions. Eur J Appl Physiol. 1993;67:335–341. doi: 10.1007/BF00357632. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol. 2002;87:1850–1858. doi: 10.1152/jn.00024.2001. [DOI] [PubMed] [Google Scholar]
- Holte KA, Westgaard RH. Daytime trapezius muscle activity and shoulder-neck pain of service workers with work stress and low biomechanical exposure. Am J Ind Med. 2002;41:393–405. doi: 10.1002/ajim.10039. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Lindström S, Wigström H. On the function of recurrent inhibition in the spinal cord. Exp Brain Res. 1979;37:399–403. doi: 10.1007/BF00237722. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal systems: identification, multifunctional character and reconfigurations in mammals. J Physiol. 2001;533:31–40. doi: 10.1111/j.1469-7793.2001.0031b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen C, Vasseljen O, Westgaard RH. The influence of electrode position on bipolar surface electromyogram recordings of the upper trapezius muscle. Eur J Appl Physiol. 1993;67:266–273. doi: 10.1007/BF00864227. [DOI] [PubMed] [Google Scholar]
- Jensen C, Westgaard RH. Functional subdivision of the upper trapezius muscle during low-level activation. Eur J Appl Physiol. 1997;76:335–339. doi: 10.1007/s004210050257. [DOI] [PubMed] [Google Scholar]
- Kato M, Murakami S, Takahashi K, Hirayama H. Motor unit activities during maintained voluntary muscle contraction at constant levels in man. Neurosci Let. 1981;25:149–154. doi: 10.1016/0304-3940(81)90323-2. [DOI] [PubMed] [Google Scholar]
- Katz R, Pierrot-Deseilligny E. Recurrent inhibition in humans. Prog Neurobiol. 1998;57:325–355. doi: 10.1016/s0301-0082(98)00056-2. [DOI] [PubMed] [Google Scholar]
- Klein CS, Ivanova TD, Rice CL, Garland SJ. Motor unit discharge rate following twitch potentiation in human triceps brachii muscle. Neurosci Lett. 2001;316:153–156. doi: 10.1016/s0304-3940(01)02389-8. [DOI] [PubMed] [Google Scholar]
- LeFever RS, Xenakis AP, De Luca CJ. A procedure for decomposing the myoelectric signal into its constituent action potentials. Part II. Execution and test for accuracy. IEEE Trans Biomed Eng. 1982;29:158–164. doi: 10.1109/TBME.1982.324882. [DOI] [PubMed] [Google Scholar]
- Mambrito B, De Luca CJ. A technique for the detection, decomposition and analysis of the EMG signal. Electroencephalogr Clin Neurophysiol. 1984;58:175–188. doi: 10.1016/0013-4694(84)90031-2. [DOI] [PubMed] [Google Scholar]
- Person RS. Rythmic activity of a group of human motoneurones during voluntary contraction of a muscle. Electroencephalogr Clin Neurophysiol. 1974;36:585–595. doi: 10.1016/0013-4694(74)90225-9. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong X-W, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears TA, Stagg D. Short-term synchronization of intercostal motoneurone activity. J Physiol. 1976;263:357–381. doi: 10.1113/jphysiol.1976.sp011635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wærsted M, Eken T, Westgaard RH. Activity of single motor units in attention-demanding tasks: firing pattern in the human trapezius muscle. Eur J Appl Physiol. 1996;72:323–329. doi: 10.1007/BF00599692. [DOI] [PubMed] [Google Scholar]
- Westgaard RH, Bonato P, Holte KA. Low-frequency oscillations (< 0. 3 Hz) in the electromyographic (EMG) activity of the human trapezius muscle during sleep. J Neurophysiol. 2002;88:1177–1184. doi: 10.1152/jn.2002.88.3.1177. [DOI] [PubMed] [Google Scholar]
- Westgaard RH, De Luca CJ. Motor unit substitution in long-duration contractions of the human trapezius muscle. J Neurophysiol. 1999;82:501–504. doi: 10.1152/jn.1999.82.1.501. [DOI] [PubMed] [Google Scholar]
- Westgaard RH, De Luca CJ. Motor control of low-threshold motor units in the human trapezius muscle. J Neurophysiol. 2001;85:1777–1781. doi: 10.1152/jn.2001.85.4.1777. [DOI] [PubMed] [Google Scholar]
- Westgaard RH, Jansen T, Jensen C. EMG of neck and shoulder muscles: the relationship between muscle activity and muscle pain in occupational settings. In: Kumar S, Mital A, editors. Electromyography in Ergonomics. London: Taylor & Francis; 1996. pp. 227–258. [Google Scholar]
- Westgaard RH, Vasseljen O, Holte KA. Trapezius muscle activity as a risk indicator for shoulder and neck pain in female service workers with low biomechanical exposure. Ergonomics. 2001;44:339–353. doi: 10.1080/00140130119649. [DOI] [PubMed] [Google Scholar]


