Abstract
In many neurons, trains of action potentials show frequency-dependent broadening. This broadening results from the voltage-dependent inactivation of K+ currents that contribute to action potential repolarisation. In different neuronal cell types these K+ currents have been shown to be either slowly inactivating delayed rectifier type currents or rapidly inactivating A-type voltage-gated K+ currents. Recent findings show that inactivation of a Ca2+-dependent K+ current, mediated by large conductance BK-type channels, also contributes to spike broadening. Here, using whole-cell recordings in acute slices, we examine spike broadening in lateral amygdala projection neurons. Spike broadening is frequency dependent and is reversed by brief hyperpolarisations. This broadening is reduced by blockade of voltage-gated Ca2+ channels and BK channels. In contrast, broadening is not blocked by high concentrations of 4-aminopyridine (4-AP) or α-dendrotoxin. We conclude that while inactivation of BK-type Ca2+-activated K+ channels contributes to spike broadening in lateral amygdala neurons, inactivation of another as yet unidentified outward current also plays a role.
As initially shown in the squid axon (Hodgkin, 1976) action potentials in central neurons result from the activity of voltage-dependent ion currents. The depolarising phase of the action potential results from activation of voltage-dependent Na+ and Ca2+ currents (Llinás, 1988), while the time course of repolarisation is determined by a combination of Na+ current inactivation and activation of a number of different K+ currents (Storm, 1990). During repetitive activity action potential repolarisation is often slower for subsequent spikes. Such broadening of action potentials has been observed in a wide variety of invertebrate and vertebrate neurons both in vitro and in vivo. Spike broadening occurs in all compartments of the neuron in which it has been examined: soma (Aldrich et al. 1979; Ma & Koester, 1996; Shao et al. 1999), dendrite (Andreasen & Lambert, 1995) and axon terminal (Jackson et al. 1991; Geiger & Jonas, 2000). In most neurons, action potentials are accompanied by rises in cytosolic Ca2+ initiated by the opening of voltage-gated Ca2+ channels. As the resulting Ca2+ influx during action potentials largely occurs during repolarisation (Llinás et al. 1981), the duration of the action potential is a key determinant of the amount of Ca2+ influx. Rises in intracellular Ca2+ are responsible for initiating a range of cellular processes, from transmitter release to gene transcription (Ghosh & Greenberg, 1995). For example, at the presynaptic nerve terminal, the width of the action potential determines the amount transmitter release (Augustine, 1990; Sabatini & Regehr, 1997; Geiger & Jonas, 2000) and in regions outside the terminal, the amplitude and kinetics of Ca2+-activated K+ currents, which play a role in the frequency of action potential firing, are dependent on the amount of Ca2+ influx (Sah, 1992, 1996). Thus action potential broadening has an important role in many aspects of neuronal activity.
The mechanisms underlying spike broadening during repetitive firing have been examined in a number of different neural systems. In invertebrates, where it was first studied, broadening is frequency dependent and is due to cumulative inactivation of voltage-gated K+ currents that contribute to action potential repolarisation (Aldrich et al. 1979; Quattrocki et al. 1994; Ma & Koester, 1996). A similar conclusion was reached in experiments on mammalian nerve terminals in the pituitary (Jackson et al. 1991) and at mossy fibre terminals in the hippocampus (Geiger & Jonas, 2000). These K+ currents include slowly inactivating, delayed rectifier type currents (Aldrich et al. 1979; Geiger & Jonas, 2000) and the low threshold, rapidly inactivating A-type K+ currents (Ma & Koester, 1996; Giese et al. 1998). In a recent study, spike broadening in hippocampal pyramidal neurons was also found to involve inactivation of a Ca2-activated K+ current mediated by rapidly inactivating large conductance BK channels (Shao et al. 1999).
In the present study we have examined spike broadening during repetitive firing in the lateral nucleus of the amygdala (LA). The amygdala is part of the limbic system that plays a key role in assigning emotional significance to sensory input, and has been shown to be particularly important for emotional learning, such as during fear conditioning (LeDoux, 2000; Davis & Whalen, 2001). The LA is a cortical-like structure that receives sensory information from cortical and thalamic areas and projects to other nuclei within the amygdala, as well as sending recurrent connections to the cortex and thalamus (Sah et al. 2003). Two types of electrophysiologically distinct neuron are present within this structure, pyramidal-like projection neurons and local circuit inhibitory interneurons (McDonald, 1984; Paré & Gaudreau, 1996; Mahanty & Sah, 1998). As in other cell types, a number of voltage- and Ca2+-dependent K+ currents contribute to the action potential time course in LA projection neurons (Faber & Sah, 2002). We have previously reported that following somatic current injections spike broadening occurs during trains of action potentials fired by projection neurons (Faber et al. 2001). Here we show that this broadening is a frequency-dependent phenomenon that occurs during firing evoked by both somatic current injections and synaptic activation. This broadening results in part from inactivation of large conductance BK-type Ca2+-activated K+ channels.
METHODS
All experiments were performed on rat brain slices maintained in vitro. Wistar rats (unsexed, 21-28 days old) were anaesthetised with intraperitoneal pentobarbitone (50 mg kg−1) and were killed by decapitation. Rat brains were rapidly removed and placed in ice cold artificial cerebral spinal fluid (aCSF) containing (mM): NaCl 118, KCl 2.5, NaHCO3 25, glucose 10, MgCl2 2.5, CaCl2 2.5 and NaH2PO4 1.2. Coronal slices (400 μm thick) containing the amygdala were cut using a microslicer DTK-1000 (Dosaka). The slices were allowed to recover in oxygenated (95 % O2/5 % CO2) aCSF at 34 °C for 30 min and then kept at room temperature for a further 30 min before experiments were performed. Slices were then transferred to the recording chamber as required. These procedures were in accordance with the guidelines of the Institutional Animal Ethics Committee. Within the recording chamber slices were held in position using a nylon net, stretched over a flattened U-shaped platinum wire, and were continuously perfused with oxygenated aCSF maintained at 30 °C. When using Cd2+ to block voltage-gated Ca2+ channels, NaHPO4 was omitted from the aCSF to prevent precipitation of cadmium phosphate.
Whole-cell recordings were made from neurons in the LA using IR/differential interference contrast (DIC) techniques. Electrodes (3-6 MΩ) were coated with beeswax to reduce capacitance and filled with pipette solution containing (mM): KMeSO4 135, NaCl 8, Hepes 10, Mg2ATP 2 and Na3GTP 0.3 (pH 7.2 with KOH, osmolality 300 mosmol kg−1). On some occasions 10 mM BAPTA was included in the internal pipette solution. Signals were recorded using a patch clamp amplifier (Axon instruments, Axopatch 1-D or Multiclamp 700A, Foster City, CA, USA). No differences in spike broadening were seen when using the two different amplifiers. Responses were filtered at 5 kHz and digitised at 10 kHz (Instrutech, Greatneck, NY, USA, ITC-16). All data were acquired, stored and analysed on a Macintosh G4 using Axograph 4.6 (Axon Instruments). Only cells with a membrane potential greater than -55 mV were included in this study. Access resistance was 5-30 MΩ and was monitored throughout the experiment. In all experiments, drugs were applied by diluting to the correct concentration in the superfusate.
To investigate the firing properties of neurons, six to eight current injection steps (600 ms) were applied from -100 to +600 pA in 100 pA increments. Spike half-widths were measured at 50 % of the peak using the event detection macro in Axograph. This analysis was carried out on spikes evoked either using the prolonged current injections described above, or by brief 2-5 ms current injections of 0.8-2 nA at varying frequencies. When examining the action of drugs on broadening, action potentials were either evoked by a train of five brief (5 ms) current injections (0.8 nA) at 50 Hz, or by a prolonged (600 ms) depolarising current injection (300 pA), and spike half-widths were measured before and after drug applications. Afterhyperpolarisations (AHPs) were evoked by giving a 50 ms 400 pA current injection from a holding potential of -50 mV. For nucleated patch recordings, 10 mM EGTA or BAPTA was included in the internal solution in half of the experiments and on some occasions TTX (1 μM) and Cd2+ (250 μM) or Ni+ (5 mM) were included in the superfusate. However no differences in the outward current were seen with and without EGTA or BAPTA, and with and without TTX, Cd2+ and Ni+, and for this reason the results have been pooled. To obtain nucleated patches, after obtaining a whole-cell recording, negative pressure was applied to the pipette and the pipette was slowly withdrawn from the cell (Sather et al. 1992). Capacitance transients were compensated for using a P/4 protocol and 80 % series resistance compensation was used, with a lag of < 3 μs. To examine outward currents evoked by brief depolarisations, 5 ms voltage steps to +40 mV from a holding potential of -60 mV were applied at varying intervals. To examine the outward currents activated during action potentials, a voltage waveform of a train of spikes evoked by either 600 or 5 ms depolarising steps, recorded in current clamp, were used as the voltage clamp command. All K+ currents shown are averages from five to ten sweeps. For afferent stimulation, a bipolar stimulating electrode was placed in the external capsule. Tetanic stimulation was given as 10 stimuli at 50-100 Hz. Results are expressed as means ± S.E.M. Student's t tests were used for statistical comparisons between groups.
Drugs and chemicals
Iberiotoxin (Ibtx) and α-dendrotoxin (α-DTX) were obtained from Alomone Laboratories. Ni+, Cd2+, paxilline, BAPTA, EGTA, tetraethylammonium (TEA) and 4-aminopyridine (4-AP) were obtained from Sigma. All drugs were applied by directly adding them to the superfusate from stock solutions. Drugs were applied until their action was maximal. However, in instances where washout of drugs was tested (e.g. Fig. 5I), drugs were only applied until they started to have an effect when washout was started.
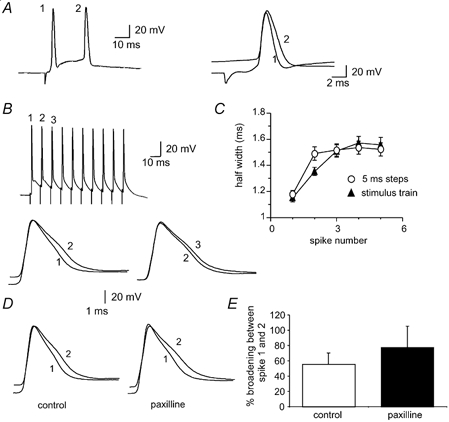
Figure 5. Contribution of BK channels to spike broadening.
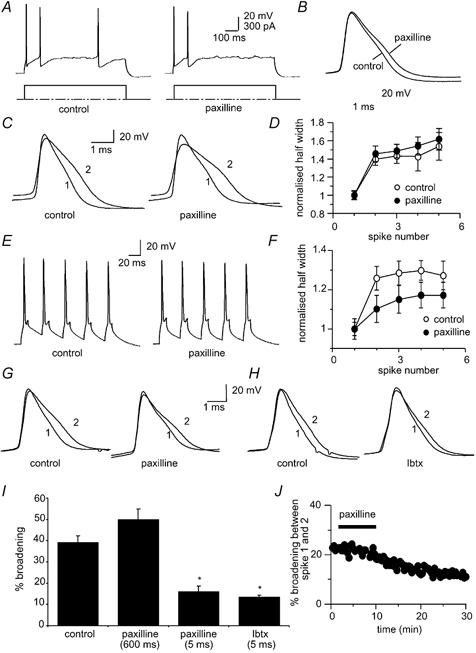
A, action potential train evoked by a 300 pA current injection before (left) and after (right) blockade of BK channels with the specific blocker paxilline (10 μM). B, the first action potential before and after paxilline has been superimposed and shows that paxilline significantly broadens the spike compared to the control. C and D, spike broadening is still present in the presence of paxilline. E, action potential train evoked by brief current injections before (left) and after (right) application of 10 μM paxilline. F, mean data for 6 cells comparing the extent of broadening over the five action potentials in control (open circles) and in the presence of paxilline (filled circles). G, the first two action potentials superimposed before and after paxilline show that spike broadening in response to brief current injections is reduced by paxilline. H, the first two action potentials evoked by brief current injections superimposed before (left) and after (right) iberiotoxin (50 nM) show that broadening is attenuated by iberiotoxin. I, average data showing broadening between the first two spikes following application of paxilline and iberiotoxin when action potentials have been evoked with long or short current injections. Control values for the long (300 pA) and short current injections have been averaged. J, time course of reduction of spike broadening of spikes evoked by brief current injections by paxilline.
RESULTS
We have previously shown that LA projection neurons exhibit a continuum of firing properties, ranging from cells that fire one to five spikes in response to a prolonged (600 ms) depolarising current injection before fully accommodating, to those that fire repetitively throughout the current injection (Faber et al. 2001). In the present study we have concentrated only on cells that accommodate, which comprise approximately 80 % of LA projection neurons. In all cells the second action potential in a train was significantly broader than the first (P < 0.0001; n = 266, Fig. 1). The mean half-widths of the first and second spikes evoked in response to a prolonged 400 pA current injection were 1.09 ± 0.03 and 1.54 ± 0.04 ms respectively, giving a mean percentage increase between the first and second spike of 43 ± 3 % (P < 0.001, n = 49, Fig. 1). However, no further significant broadening occurred after the second spike, with the mean half-width of the third spike being 1.64 ± 0.04 ms (P > 0.05, 6 ± 1 % versus the second spike, n = 49). The measured spike half-widths of the first spike and spike broadening between the first two spikes (Fig. 1F) remained stable over a recording period of 20 min.
Figure 1. Action potential broadening in projection neurons in the lateral amygdala.
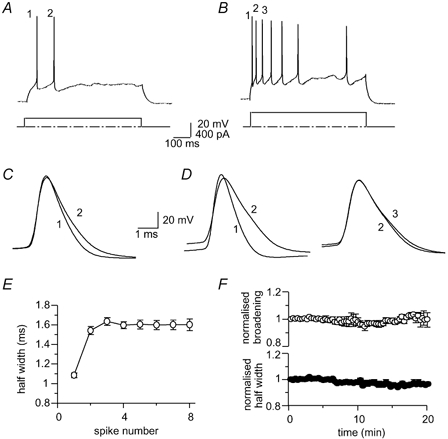
Trains of action potentials evoked in the same cell in response to a long (600 ms), 200 pA (A) and 400 pA (B) depolarising current injection. C and D, the numbered action potentials in (A and B) have been superimposed to show the extent of spike broadening between the first three action potentials. E, average data from 49 cells showing action potential half-width for the first seven action potentials in response to a 400 pA current injection. F, action potential half-width of the first spike (filled circles) and broadening between the first two spikes (open circles) are stable over 20 min of whole-cell recording (n = 4).
Frequency dependence of broadening
Spike broadening has been shown to be frequency dependent in a number of different neurons (Aldrich et al. 1979; Quattrocki et al. 1994; Ma & Koester, 1996; Shao et al. 1999). To test whether broadening in the LA was also frequency dependent, action potentials were evoked with increasingly larger current injections. Action potentials evoked by larger current injections fired at higher frequencies and showed greater broadening (Fig. 2). The mean frequency of discharge between the first two spikes was 25 ± 4 Hz with a 200 pA current injection, 45 ± 4 Hz for a 300 pA current injection and 69 ± 5 Hz for a 400 pA current injection (n = 49). It was noted that action potentials that were broader showed a more depolarised threshold, slower rate of rise and smaller amplitudes. These effects are due to inactivation of the underlying voltage-dependent Na+ current (E. Faber and P. Sah, unpublished observations). On average, action potential half-width increased by 24 ± 2 % (P < 0.001, n = 49) with a 200 pA current injection, by 36 ± 3 % (P < 0.001, n = 49) with a 300 pA current injection and by 43 ± 3 % (P < 0.001, n = 49) with a 400 pA current injection.
Figure 2. Frequency dependence of spike broadening.
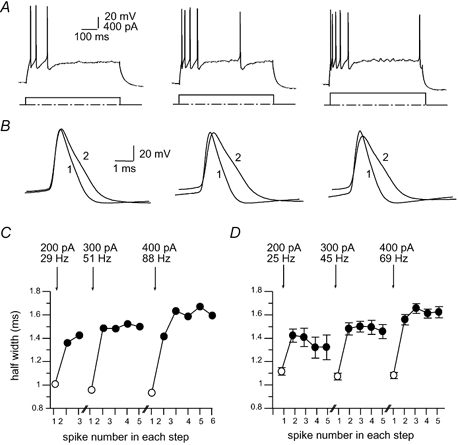
A, action potential trains evoked by 600 ms current injections of 200, 300 and 400 pA. Note the increase in the frequency of action potential discharge with increasing current injections. B, the first two action potentials have been superimposed for each current injection. C, action potential half-width is plotted for each action potential for the three current injections shown in A. The frequency of discharge for the first two spikes is shown for each current injection. The averaged data for 49 neurons is shown in D, together with the mean firing frequency of the first two spikes.
We next sought to ascertain whether the enhanced broadening with larger current injections is due to the higher frequency of firing or results from the more sustained and larger depolarisation of the membrane associated with long current injections. To test this we applied trains of brief (5 ms) current steps (0.8-1.5 nA) to evoke single spikes at a fixed frequency. Broadening still occurred between the first two spikes when action potentials were evoked by brief current injections at 50 Hz (26 ± 3 %, P < 0.001, n = 25, Fig. 3A and B). This broadening showed a similar pattern to that seen when action potentials were evoked at an equivalent frequency by prolonged current injections in the same cells (Fig. 3B). The extent of broadening with the brief steps was frequency dependent with greater broadening observed at higher frequencies of stimulation (n = 11, Fig. 3C).
Figure 3. Action potential broadening does not require prolonged depolarising current injections.
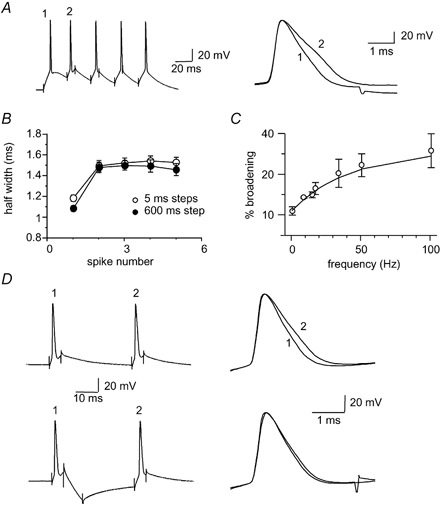
A, action potentials evoked by repetitive 5 ms current injections at 50 Hz. The first two action potentials have been superimposed on the right to show the spike broadening. B, average data from 25 neurons comparing the action potential broadening for five action potentials evoked at 50 Hz (open circles) with a prolonged (600 ms) 300 pA current injection giving a mean discharge frequency of 45 Hz for the first two spikes (filled circles). C, frequency dependence of spike broadening. Percentage broadening of the first two action potentials at different frequencies shows that broadening is more pronounced at higher stimulation frequencies (n = 11). D, spike broadening can be reversed with a hyperpolarising current injection. Upper traces show the action potential broadening evoked by two 5 ms current injections delivered at 33 Hz. The action potentials have been superimposed on the right. In the lower panel a hyperpolarising current injection (400-600 pA, 20 ms) is delivered between the two depolarising voltage steps (same neuron). The two action potentials superimposed on the right show no significant broadening (n = 9).
Such frequency-dependent broadening is consistent with inactivation of a K+ current(s) that contributes to action potential repolarisation (Aldrich et al. 1979; Jackson et al. 1991; Quattrocki et al. 1994; Ma & Koester, 1996). As recovery from inactivation is voltage dependent, being faster at more negative membrane potentials, we next tested whether broadening could be reversed by membrane hyperpolarisation. A hyperpolarising current (400-600 pA) was applied between two depolarising current injections delivered at 33 Hz. At this stimulation frequency the increase in half-width between the first and second spikes was 26 ± 5 % (P < 0.05, n = 9). Following insertion of a hyperpolarising step between the depolarising steps no significant broadening occurred between the two spikes (0 ± 3 %, n = 9, P > 0.05, Fig. 3D). These results suggest that broadening in LA neurons is due to voltage-dependent inactivation of currents active during spike repolarisation.
Currents underlying spike broadening
As in many other neurons (Storm, 1990; Sah, 1996), BK channels also play a role in action potential repolarisation in LA neurons (Faber & Sah, 2002). In hippocampal pyramidal neurons, inactivation of BK channels has been shown to contribute to broadening during repetitive activity (Shao et al. 1999). We therefore tested whether a similar mechanism is active in LA neurons. We first blocked activation of BK channels by reducing Ca2+ influx by applying the Ca2+ channel blockers Ni+ (5 mM) or Cd2+ (250 μM). When action potentials were evoked by prolonged current injections (300 pA), application of Ni+ (Fig. 4) or Cd2+ broadened action potentials, presumably due to blockade of BK channel activation (Faber & Sah, 2002). However, spike broadening was not significantly altered. In the presence of Ni+ (5 mM), the first and second spikes broadened by 40 ± 6 % compared with 45 ± 5 % in control (P > 0.05, n = 7, Fig. 4C). Similarly, the effect of Cd2+ (250 μM, data not shown) on broadening did not reach statistical significance, where broadening during the first two spikes evoked by a 300 pA current injection was 36 ± 5 % in control conditions and 25 ± 9 % in the presence of Cd2+ (P > 0.05, n = 4). Lowering the concentration of extracellular Ca2+ to 0.5 mM also failed to significantly prevent broadening with a 25 ± 5 % broadening in 0.5 mM Ca2+ containing aCSF and 35 ± 6 % broadening in the control (P > 0.05, n = 6).
Figure 4. Ca2+ influx contributes to spike broadening.
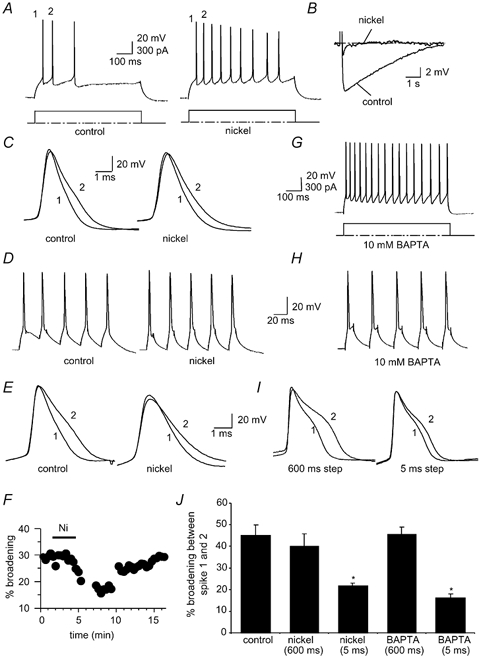
A, action potential train evoked by a 300 pA current injection before (left) and after (right) blockade of Ca2+ influx with 5 mM Ni+. Note the reduction in spike frequency adaptation as the slow afterhyperpolarisation (AHP) activated by a current injection is blocked in the presence of Ni+ (B). C, the first two action potentials before and after Ni+ have been superimposed. Ni+ does not reduce broadening with this protocol. D, action potential train (50 Hz) evoked by brief current injections before (left) and after (right) blockade of Ca2+ influx with 5 mM Ni+. E, the first two action potentials before and after Ni+ nickel have been superimposed showing that Ni+ attenuated broadening between the first two spikes. F, time course showing the reversibility of the effect of Ni+ on broadening. G, action potential train evoked by a prolonged 300 pA current injection in a cell loaded with 10 mM BAPTA. H, action potentials evoked by 5 ms current injections in a BAPTA loaded cell. I, superimposed first and second spikes evoked by prolonged (left) and brief (right) current injections in a BAPTA-loaded cell; broadening is reduced when the spikes are evoked by brief current injections. J, average data illustrating that spike broadening is unaffected by Ni+ or BAPTA when action potentials are evoked by prolonged current injection but is reduced when action potentials are evoked by brief current steps. Note that the interspike interval for prolonged current injections is shorter in the presence of Ni+ due to the reduction in accommodation. Control values for the long (300 pA) and short current injections have been averaged.
As expected (Faber & Sah, 2002), application of Ca2+ channels blockers attenuated the AHP and reduced spike frequency adaptation (n = 6, Fig. 4A and B). Thus it is possible that the broadening seen when BK channels are blocked results from the increase in spike firing frequency. To test for this we examined the action of Ni+ on broadening when action potentials were evoked with a train of 5 ms current injections at 50 Hz (Fig. 4D). In contrast with the long current injections, Ni+ significantly reduced spike broadening between the first and second spikes when they were evoked at a fixed frequency (Fig. 4E, F and J). Under control conditions the first and second spikes had mean half-widths of 1.2 ± 0.05 ms and 1.6 ± 0.1 ms respectively, a broadening of 33 ± 3 % (n = 8). In contrast, in the presence of Ni+ (5 mM), the first two spikes had half-widths of 1.5 ± 0.1 and 1.8 ± 0.2 ms respectively, a mean increase of 21 ± 1 % (P < 0.005, n = 8, Fig. 4E). This effect of Ni+ was reversible upon washout (Fig. 4F). In addition to blocking Ca2+ influx we also tested the effect on spike broadening when cells were loaded with the fast Ca2+ chelator BAPTA (10 mM) to block BK channel activation. Upon depolarisation with a 600 ms current injection, cells loaded with BAPTA fired repetitively due to blockade of the slow AHP (Faber & Sah, 2002), and the spikes were broader due to blockade of BK channels (Fig. 4G and I). With a 300 pA current injection, spike broadening was similar to that under control conditions, with a broadening between the first two spikes of 45 ± 4 % (P > 0.05, n = 5, Fig. 4I and J). However, when spikes were evoked with brief current injections at 50 Hz, broadening was significantly reduced to 16 ± 2 % in the presence of BAPTA, with half-widths of the first and second spikes of 1.5 ± 0.1 and 1.7 ± 0.04 ms respectively (P < 0.005, n = 4, Fig. 4I and J).
As BK channels are both voltage- and Ca2+-sensitive (Cui et al. 1997), it is possible that in the presence of Ni+ and BAPTA, Ca2+ concentrations at BK channels were not low enough to fully block their activation at the peak of the action potential. Thus we further tested the possible involvement of BK channels in spike broadening by using the selective BK channel blockers iberiotoxin (Ibtx) and paxilline. Application of BK channel blockers significantly slowed spike repolarisation (Faber & Sah, 2002). However, as with the Ca2+ channel blockers (Fig. 4), spike broadening during repetitive firing was unaffected when measured during a prolonged current injection (Fig. 5A-D). Broadening between the first and second spikes in control conditions was 40 ± 3 and 45 ± 4 % in the presence of 10 μM paxilline (1.4 ± 0.1 to 2.0 ± 0.1 ms, n = 18, Fig. 5C and D). However, with brief current injections to evoke spikes at a fixed frequency, spike broadening was attenuated by paxilline. In the presence of paxilline, broadening between the first two spikes was significantly reduced from 26 ± 3 to 16 ± 3 % (P < 0.05, n = 13, Fig. 5E-G). As with paxilline, Ibtx (50 nM) also reduced broadening from 21 ± 2 % in control conditions to 13 ± 1 % in Ibtx (1.4 ± 0.1 to 1.6 ± 0.1 ms, P < 0.05, n = 5, Fig. 5H and I). Together, these results indicate that while inactivation of BK channels contributes to spike broadening, a second mechanism, independent of BK channels, also contributes to spike broadening. This second mechanism is more prominent following prolonged depolarising steps than brief depolarising steps.
In addition to BK channel inactivation, inactivation of the A-current (IA), a low threshold rapidly inactivating K+ current that contributes to action potential repolarisation in some neurons (Storm, 1987; Kang et al. 2000), has also been shown to contribute to spike broadening in hippocampal pyramidal neurons (Giese et al. 1998). To test for the possible involvement of the A-current in LA neurons we used the blocker 4-aminopyridine (4-AP, 5 mM). At this concentration, 4-AP blocks a number of K+ channels, including those mediating IA (Coetzee et al. 1999). Application of 4-AP significantly broadened action potentials (Fig. 6). However, 4-AP had no effect of spike broadening, measured using brief current injections to keep the firing frequency constant (Fig. 6). The extent of broadening between the first two spikes in control conditions was 51 ± 7 and 46 ± 10 % in the presence of 5 mM 4-AP (P > 0.05, n = 4). It should be noted that the effect of 4-AP in action potential repolarisation is likely to be due to blockade of currents other than the A-current because presence of such currents have not been evident under whole-cell voltage clamp (E. Faber & P. Sah, unpublished observations). In addition, action potentials initiated from -80 mV were not significantly broader (half-width 1.4 ± 0.1 ms) than those evoked from a holding potential of -55 mV (half-width 1.3 ± 0.03; P > 0.05, n = 3), further showing that A-currents are not active during action potential repolarisation in LA pyramidal-like neurons.
Figure 6. The A-current is not involved in spike broadening.
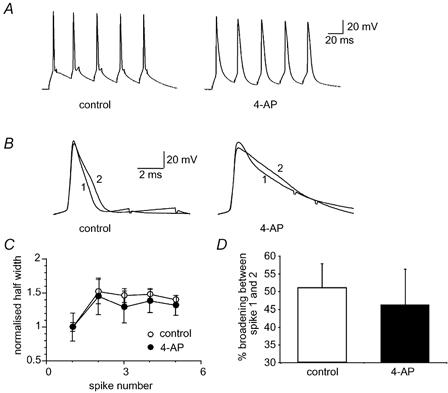
IA (the A-current, a low threshold rapidly inactivating K+ current) was blocked by application of 4-aminopyridine (4-AP, 5 mM). A, action potential train evoked by brief current injections before (left) and after (right) 4-AP application. B, the first two action potentials evoked by a brief current injection have been superimposed before and after application of 4-AP. C, average data from 4 neurons showing the normalised broadening in control (open circles) and in 4-AP (filled circles) over the five action potentials. D, mean data for 4 cells showing the percentage broadening between the first two spikes is not reduced by 4-AP.
In mossy fibre terminals, α-dendrotoxin (α-DTX), a toxin that blocks K+ channels containing Kv1.1, 1.2 and 1.6 subunits (Coetzee et al. 1999), was found both to broaden spikes and to prevent broadening during repetitive firing (Geiger & Jonas, 2000). We therefore examined the effects of α-DTX on spike broadening. In LA pyramidal cell neurons, application of α-DTX (100 nM) failed to prevent spike broadening (Fig. 7). The mean half-widths of the first and second spikes evoked by brief current injections were 1.2 ± 0.05 and 1.6 ± 0.03 ms under control conditions, and 1.2 ± 0.05 and 1.6 ± 0.1 ms in the presence of α-DTX (P > 0.05, n = 4). This result is perhaps not surprising since α-DTX did not significantly broaden action potentials in LA neurons, indicating that the α-DTX-sensitive current is not a major contributor to action potential repolarisation in these neurons (Faber & Sah, 2002).
Figure 7. Spike broadening does not require α-dendrotoxin (α-DTX)-sensitive K+ channels.
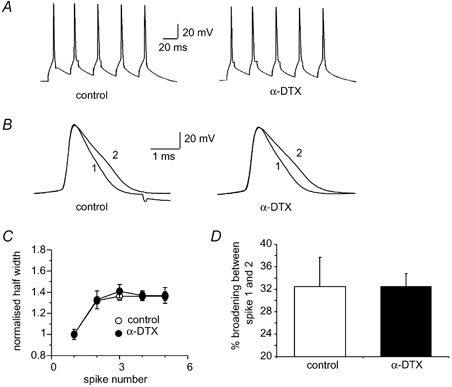
A, action potentials evoked by brief current injections before (left) and after 100 nM α-DTX (right). B, the first two superimposed action potentials in control and following application of α-DTX. C, average broadening is shown for the five action potentials before (open circles) and after addition of α-DTX (filled circles, n = 4). D, mean percentage broadening between the first two spikes is unchanged in the presence of α-DTX (n = 4).
Finally, we examined the effect of TEA (10 mM), a broad spectrum voltage-gated K+ channel blocker. TEA had no significant effect on spike frequency discharge (91 ± 7 Hz in control versus 89 ± 12 Hz in TEA, P > 0.05, n = 3) when spikes were elicited by a prolonged 300 pA current injection. TEA significantly broadened spikes, but did not prevent spike broadening during repetitive firing (not shown). Under control conditions, the first and second spikes had mean half-widths of 1.37 ± 0.2 and 2 ± 0.2 ms respectively, giving a mean increase of 46 ± 7 % (n = 3). In contrast, in the presence of TEA, the first and second spikes had half-widths of 2.1 ± 0.2 and 6.9 ± 1.3 ms, giving a mean increase in half-width of 117 ± 27 % (n = 3).
K+ currents measured in nucleated somatic patches
These results show that as in hippocampal pyramidal neurons (Shao et al. 1999), spike broadening in LA projection neurons is partly mediated by BK channel inactivation. However, it is clear that inactivation of a voltage-dependent K+ current(s) other than that mediated by BK channels also contributes, and is likely to account for the residual spike broadening in the presence of BK channel blockers. Unlike in hippocampal neurons or mossy fibre terminals (Giese et al. 1998) this residual broadening cannot be accounted for by inactivation of IA (Shao et al. 1999) or α-DTX-sensitive channels (Geiger & Jonas, 2000). To test for the presence of outward currents that show frequency-dependent inactivation we excised nucleated patches. Depolarisation of nucleated patches from a holding potential of -60 mV activated TTX-sensitive inward Na+ currents and large outward currents. These currents did not show any inactivation aver 5 ms and were insensitive to α-DTX (100 nM, n = 7, data not shown). These outward currents showed marked frequency-dependent inactivation that plateaued after the first three repetitions (Fig. 8A). When evoked at 50 Hz, the peak amplitude of the outward current evoked by the second voltage step was 86 ± 2 % of the first (P < 0.001, n = 9), while that evoked by the third step was 79 ± 3 % of the first (P < 0.05 for second versus third step, n = 9). No further significant reduction in the amplitude of the outward currents evoked by the fourth and fifth depolarisations was observed (P > 0.05, n = 9). This voltage-dependent inactivation was relieved by brief hyperpolarising steps to -100 mV (Fig. 8B and C). With hyperpolarising voltage steps the outward current elicited by the second step was 12 % greater than that elicited by the first (P > 0.05, n = 6). The frequency dependence of inactivation of outward currents evoked by two depolarising voltage steps was examined over a range of inter-stimulus frequencies up to 100 Hz. Similar to spike broadening (see Fig. 3C), inactivation was greater at higher frequencies, and recovery from inactivation at -60 mV was almost fully achieved at a 2 Hz inter-stimulus frequency, with the current evoked by the second step recovering to 96 ± 1 % of control (n = 8, Fig. 8D).
Figure 8. The K+ current in nucleated patches shows cumulative inactivation.
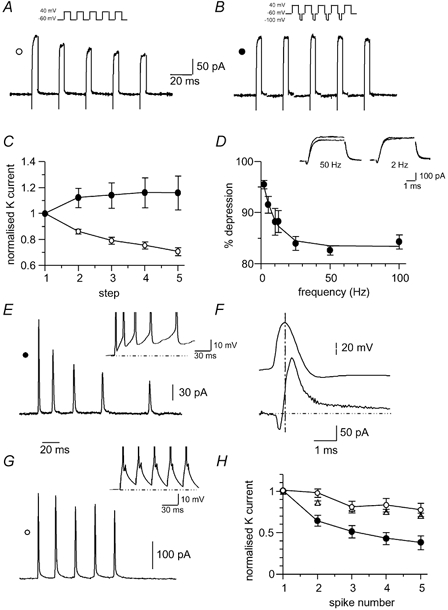
A, nucleated patch outward currents were evoked by 5 ms depolarising voltage steps to +40 mV from a holding potential of -60 mV (inset). B, same patch as shown in (A) but with a 10 ms hyperpolarising step to -100 mV between the depolarising voltage steps. C, average data from 9 neurons showing normalised peak outward current with (filled circles) and without (open circles) a hyperpolarising voltage step. D, frequency dependence of outward current inactivation (n = 8). Outward currents were evoked with a pair of depolarising steps to +40 mV, at frequencies from 2 to 100 Hz. The percentage reduction of the outward current during the second pulse is plotted against the interpulse frequency. Superimposed outward currents recorded at inter-stimulus frequencies of 50 Hz (left) and 2 Hz (right) are shown in the inset. E, outward currents evoked by action potential train command waveforms from -60 mV elicited by a prolonged (600 ms) depolarising step in the presence of TTX (1 μM) and Cd2+ (250 μM) undergo inactivation. F, the current evoked by a single action potential waveform. The vertical dashed line marks the peak of the action potential. Note that as expected, the K+ current is maximal during membrane repolarisation. G, outward currents evoked by action potential train command waveforms from -60 mV elicited by brief (5 ms, see inset) depolarising steps. H, cumulative inactivation of the outward currents activated by action potential waveforms shown in E and G (n = 6). Note the greater inactivation of outward current with the prolonged depolarising action potential waveform (filled circles) compared with the brief depolarising action potential waveform (open circles). The data for inactivation during 5 ms voltage steps from (C) have been superimposed (open triangles).
To confirm that these outward currents are also active during action potentials, we used trains of action potentials that had been evoked in current clamp by either prolonged 600 ms or brief 5 ms depolarising steps as the voltage command. The outward current elicited by the 600 ms step-evoked action potential waveform underwent a similar pattern of inactivation to that seen with the voltage step commands (n = 6, Fig. 8E and H). In contrast, inactivation of the outward current was substantially attenuated when evoked by a spike waveform recorded using 5 ms depolarising steps (n = 3, P < 0.05, Fig. 8G and H). These results demonstrate the presence of an outward current in LA neurons that undergoes voltage-dependent inactivation. This outward current shows significantly more inactivation when evoked by action potentials activated by a prolonged depolarising step as compared with action potentials generated by brief depolarising steps. It can be seen that the inactivation of this current continues during the first four step depolarisations (Fig. 8C, E and H), similar to the broadening of action potentials evoked when BK channels have been blocked (cf. Fig. 5F). Application of TEA reduced the outward current evoked by brief depolarising steps to 47 ± 10 % of control and further addition of 4-AP reduced it to 26 ± 8 % (n = 3). However, in the presence of these compounds the frequency-dependent inactivation of the remaining current was little affected, consistent with the spike broadening seen in the presence of TEA or 4-AP.
Spike broadening during synaptic stimulation
Finally, to examine the extent of broadening under more physiological conditions, action potentials were initiated by synaptic activation. In some instances two spikes could be evoked by a single shock applied to the external capsule (Fig. 9A). The first spike had a mean half-width of 1.2 ± 0.1 ms and the second of 1.7 ± 0.2 ms (n = 5, P < 0.05, Fig. 9A). This broadening is similar to that seen with somatic current injections (P > 0.05). To examine spike broadening during trains of more than two spikes, tetani of 10 stimuli at 100 Hz were applied to the external capsule (Fig. 9B). Following synaptic stimulation, broadening of action potentials during repetitive firing was similar to that seen with somatic current injections (Fig. 9C). However, following a tetanus the broadening between the first three spikes was more graded. Thus, the second spike was significantly broader than the first (1.35 ± 0.03 and 1.15 ± 0.03 ms respectively, n = 53, P < 0.001) and the third spike was significantly broader than the second (1.5 ± 0.05 and 1.35 ± 0.03 ms, n = 53, P < 0.01). This is in contrast with the somatic current injections, where most of the broadening occurs between the first two spikes, and the third spike is not significantly broader than the second (see Figs 1–3). The graded broadening of action potentials evoked by synaptic stimulation more closely resembles that seen in the presence of BK channel blockers. One possible explanation of this result is that inactivation of BK channels plays a lesser role in spike broadening when action potentials are evoked by synaptic stimulation, possibly due a different locus of spike initiation during somatic current injection as compared to EPSPs (see below). To test for this possibility we also examined spike broadening during synaptic stimulation when BK channels were blocked with paxilline. For these experiments tetanic stimulation was applied at 50 Hz to enable direct comparison with the brief current injections, which were also given at 50 Hz. Application of paxilline increased the amplitude of evoked EPSPs, presumably due to blockade of presynaptic BK channels and the resultant spike broadening (Hu et al. 2001). However, spike broadening of the action potentials evoked by repetitive synaptic stimulation was largely unaffected in the presence of paxilline. Under control conditions the first and second spikes had mean half-widths of 1.3 ± 0.05 and 2.0 ± 0.2 respectively, giving a mean increase of 56 ± 15 %, whereas in paxilline the first and second spikes had mean half-widths of 1.4 ± 0.06 and 2.4 ± 0.3 ms respectively, a mean increase of 78 ± 28 % (P > 0.05, n = 5; Fig. 9D). This is similar to the effect of paxilline when action potentials were evoked with prolonged current injections (Fig. 5) and is perhaps not surprising since the summating EPSPs would be expected to generate a sustained depolarisation not unlike that during a prolonged current injection. Thus the other inactivating K+ current that contributes to spike broadening (see above) would be more likely to play a greater role than BK channel inactivation in spike broadening under these conditions.
Figure 9. Action potentials evoked by synaptic stimulation show spike broadening.
A, two action potentials evoked by synaptic stimulation of the external capsule (traces on left). The two action potentials, superimposed on the right, show spike broadening. B, high-frequency train of action potentials evoked by tetanic afferent stimulation (100 Hz) evokes action potentials that show spike broadening for the first three action potentials. The first two and the second and third action potentials have been superimposed in the lower traces. C, average spike broadening data during tetanic stimulation (filled triangles, n = 53). For comparison the data obtained from cells in which action potentials were evoked by a brief, 5 ms current injection (open circles) have been superimposed. D, action potentials evoked during a train of synaptic stimulation (50 Hz) before and after application of paxilline. Action potentials are broader in the presence of paxilline but continue to show broadening during the first two spikes. E, average data from 6 cells showing spike broadening between the first two action potentials in a train following application of paxilline.
DISCUSSION
As reported previously for pyramidal neurons in both the cortex and hippocampus, we have shown that action potentials fired by projection neurons in the lateral nucleus of the amygdala show broadening during repetitive firing. This broadening is apparent during trains of spikes evoked by either prolonged or brief somatic current injections as well as following synaptic stimulation. Two lines of evidence point to voltage-dependent inactivation of a repolarising K+ current mediating spike broadening. Firstly, the broadening is frequency dependent, consistent with the frequency-dependent inactivation displayed by several K+ currents. Secondly, broadening is relieved by hyperpolarising the membrane between action potentials, which enhances recovery from inactivation. These properties are characteristic of spike broadening in other neurons where it has been shown to be due to voltage-dependent inactivation of K+ currents active during action potential repolarisation (Aldrich et al. 1979; Jackson et al. 1991; Quattrocki et al. 1994; Giese et al. 1998; Shao et al. 1999; Geiger & Jonas, 2000; Van Goor et al. 2000).
Broadening in LA neurons plateaus rapidly, requiring at most three action potentials, similar to the broadening described in hippocampal CA1 pyramidal neurons, where the increase in spike duration largely results from inactivation of BK-type Ca2+-activated K+ channels (Shao et al. 1999). Similarly in LA neurons, when action potentials are evoked by brief current injections at fixed interspike intervals, spike broadening is also markedly reduced when BK channels are blocked. Thus, under these conditions inactivation of BK-type Ca2+-activated K+ channels contributes to spike broadening. BK channels are well suited to fulfil such a role in mediating spike broadening due to their dependence on both voltage and Ca2+ for activation (Sah & Faber, 2002) and IC, the macroscopic current mediated by BK channels, can show rapid inactivation (Hicks & Marrion, 1998).
Although a significant attenuation of broadening was seen when BK channels were blocked, a residual broadening remained. This result indicates that currents other than those mediated by BK channels also contribute to spike repolarisation (Faber & Sah, 2002) and undergo significant inactivation. A similar conclusion has also been reached for action potential broadening in hippocampal pyramidal neurons where both IC (Shao et al. 1999) and IA (Giese et al. 1998) contribute to spike broadening. We have shown that outward currents that undergo frequency-dependent inactivation are present on the somata of LA pyramidal neurons. However, the exact identities of the currents that underlie the residual broadening under physiological conditions remain elusive. The residual spike broadening is unlikely to be due to inactivation of either the A-current (Giese et al. 1998) or α-DTX sensitive channels (Geiger & Jonas, 2000) as neither 4-AP nor α-DTX blocked spike broadening. It should be noted that under normal conditions, rapidly activating K+ currents are partially responsible for action potential repolarisation. However, as action potentials broaden when some of these conductances are blocked (as with TEA or 4-AP) currents that activate more slowly can also contribute to repolarisation and thus the currents responsible for action potential broadening under these conditions may not be the same as those under control conditions. Furthermore, due to the much longer duration of the action potential, currents that are normally active during repolarisation would undergo more inactivation than under control conditions (Ma & Koester, 1996). Thus for example, action potentials evoked in the presence of 10 mM TEA had a half-width of 2.1 ms and broadening between the first two spikes was 117 % compared with 46 % under control conditions where the action potential had a half-width of 1.4 ms.
The outward currents that undergo frequency dependent in LA projection neurons do not appear to show any inactivation over 5 ms. Thus these channels would have to undergo substantial voltage inactivation from closed states (Jerng et al. 1999; Ding & Lingle, 2002) to contribute to spike broadening. It is clear from the data shown in Fig. 8 that Ca2+-independent outward currents activated during action potentials undergo inactivation between spikes. Moreover, the extent of inactivation of the outward currents activated by action potentials was significantly larger when using spikes generated by prolonged current injections (Fig. 8), correlating with the discrepancies in the pharmacology of spike broadening of spikes evoked by long and short current injections. One explanation for this difference could be that the K+ currents that inactivate during the action potential recover much less when the interspike trajectory is more depolarised (inset Fig. 8). In agreement with this, spike broadening between the first two spikes when evoked with the short current injections at 50 Hz (26 ± 3 %) is less than that seen with a prolonged current injection of 300 pA (36 ± 3 %) where the interspike frequency for the first two spikes is 45 ± 4 Hz. As shown in Fig. 5, when action potentials were evoked by prolonged current injections, blockade of BK channels had little effect on broadening. This difference is likely to result from more depolarised interspike trajectory, and thus greater inactivation of voltage-dependent K+ currents (Fig. 8) under these conditions. Given these considerations, the relative contribution of BK channel versus non-BK channel inactivation to broadening under physiological conditions, when the spike has not been artificially broadened, remains unclear.
Spike broadening in LA neurons was saturated by the third spike, suggesting that the repolarising currents are fully inactivated within the first few spikes. This behaviour is similar to that seen in BK-channel-mediated broadening in somatic recordings from CA1 hippocampal neurons, where spike broadening was maximal after the second or third spike (Giese et al. 1998; Shao et al. 1999). However, this contrasts with spikes recorded in the dendrites of hippocampal neurons and in mossy fibre terminals. In these neurons there was a progressive cumulative broadening from the first spike up to the 100th spike. Furthermore, recovery from inactivation took several seconds (Jung et al. 1997; Geiger & Jonas, 2000). This discrepancy indicates that there is much variability in the types of K+ currents that contribute to action potential repolarisation in different cells. Voltage-gated and Ca2+-activated K+ channel densities and subtypes have been shown to vary along the somatodendritic axis in a number of neurons (Sah & Bekkers, 1996; Hoffman et al. 1997; Magee et al. 1998; Poolos & Johnston, 1999; Bekkers, 2000; Hausser et al. 2000; Bekkers & Delaney, 2001). As K+ channels have a varied repertoire of biophysical properties (Coetzee et al. 1999), variations in K+ channel distribution can lead to different types of broadening in different cell compartments. For example, recordings made directly from cortical presynaptic elements showed a greater degree of broadening than at the somata (Geiger & Jonas, 2000). Similarly, dendritic recordings in hippocampal pyramidal neurons revealed broader action potentials and a greater degree of broadening than at the soma (Poolos & Johnston, 1999). Thus, the extent of broadening seen in somatic recordings in LA pyramidal neurons may be an underestimation of the broadening that occurs at the terminals and in the dendrites of these cells. These differences may also explain the more graded spike broadening seen during synaptic stimulation compared with somatic current injections in LA neurons.
Physiological consequences of spike broadening
As Ca2+ influx during action potentials mainly occurs during repolarisation, changes in spike half-width have a large impact on the amount of Ca2+ influx, which has important implications for many neuronal processes. In most pyramidal neurons influx of Ca2+ during action potentials activates a number of Ca2+-activated K+ currents that control action potential repolarisation and the frequency of cell spiking. Both the amplitude and the decay kinetics of these currents depend on the amount of Ca2+ influx (Sah, 1992). Thus spike broadening would contribute to the early spike frequency adaptation seen in many neurons (Connors & Gutnick, 1990; Schwindt et al. 1992; Stocker et al. 1999). In presynaptic terminals, the steep Ca2+ dependence of transmitter release indicates that broader spikes would lead to enhanced transmitter release. This has been clearly demonstrated at the squid giant synapse (Augustine, 1990) and several mammalian terminals (Jackson et al. 1991; Sabatini & Regehr, 1997; Geiger & Jonas, 2000). Such an enhancement of transmitter release and the resultant enhancement of excitatory postsynaptic potentials with spike broadening may boost plasticity, particularly during high-frequency activity where the broadening is greater (Regehr et al. 1994; Geiger & Jonas, 2000). This may be relevant to LA projection neurons, which can fire up to 100 Hz in vivo. Consistent with this, a clear impairment of several learning tasks was apparent in transgenic mice lacking the Kvβ1.1 subunit, where spike broadening was attenuated (Giese et al. 1998). In addition to presynaptic enhancement of transmitter release with spike broadening in the axon terminals, spike broadening in dendrites may also play an important functional role postsynaptically in synaptic integration. Broader back propagating action potentials may increase the window of opportunity for plasticity achieved by pairing of EPSPs and postsynaptic action potentials (Magee & Johnston, 1997). In the amygdala, and in particular in the LA, such plasticity is likely to play an important role in fear conditioning (LeDoux, 2000).
Acknowledgments
This work was supported by grants from the NH&MRC and recurrent funding from JCSMR. We would like to thank anonymous referees for suggesting experiments on the initial version of this manuscript.
REFERENCES
- Aldrich RW, Getting PA, Thompson SH. Mechanism of frequency-dependent broadening of molluscan neuron soma spikes. J Physiol. 1979;291:531–544. doi: 10.1113/jphysiol.1979.sp012829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen M, Lambert JD. Regenerative properties of pyramidal cell dendrites in area CA1 of the rat hippocampus. J Physiol. 1995;483:421–441. doi: 10.1113/jphysiol.1995.sp020595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine GJ. Regulation of transmitter release at the squid giant synapse by presynaptic delayed rectifier potassium current. J Physiol. 1990;431:343–364. doi: 10.1113/jphysiol.1990.sp018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers JM. Distribution and activation of voltage-gated potassium channels in cell-attached and outside-out patches from large layer 5 cortical pyramidal neurons of the rat. J Physiol. 2000;525:611–620. doi: 10.1111/j.1469-7793.2000.t01-2-00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers JM, Delaney AJ. Modulation of excitability by alpha-dendrotoxin-sensitive potassium channels in neocortical pyramidal neurons. J Neurosci. 2001;21:6553–6560. doi: 10.1523/JNEUROSCI.21-17-06553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–103. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Cui J, Cox DH, Aldrich RW. Intrinsic voltage dependence and Ca2+ regulation of mslo large conductance Ca-activated K+ channels. J Gen Physiol. 1997;109:647–673. doi: 10.1085/jgp.109.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Ding JP, Lingle CJ. Steady-state and closed-state inactivation properties of inactivating BK channels. Biophys J. 2002;82:2448–2465. doi: 10.1016/S0006-3495(02)75588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ESL, Callister RJ, Sah P. Morphological and electrophysiological properties of principal neurons in the rat lateral amygdala in vitro. J Neurophysiol. 2001;85:714–723. doi: 10.1152/jn.2001.85.2.714. [DOI] [PubMed] [Google Scholar]
- Faber ESL, Sah P. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J Neurosci. 2002;22:1618–1628. doi: 10.1523/JNEUROSCI.22-05-01618.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JR, Jonas P. Dynamic control of presynaptic Ca2+ inflow by fast-inactivating K+ channels in hippocampal mossy fiber boutons. Neuron. 2000;28:927–939. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Greenberg ME. Calcium signalling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268:239–246. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- Giese KP, Storm JF, Reuter D, Fedorov NB, Shao LR, Leicher T, Pongs O, Silva AJ. Reduced K+ channel inactivation, spike broadening, and after-hyperpolarization in Kvbeta1. 1-deficient mice with impaired learning. Learn Mem. 1998;5:257–273. [PMC free article] [PubMed] [Google Scholar]
- Hausser M, Spruston N, Stuart GJ. Diversity and dynamics of dendritic signaling. Science. 2000;290:739–744. doi: 10.1126/science.290.5492.739. [DOI] [PubMed] [Google Scholar]
- Hicks GA, Marrion NV. Ca2+-dependent inactivation of large conductance Ca2+-activated K+ (BK) channels in rat hippocampal neurones produced by pore block from an associated particle. J Physiol. 1998;508:721–734. doi: 10.1111/j.1469-7793.1998.721bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL. The Pursuit of Nature. Informal Essays on the History of Physiology. Cambridge: Cambridge University Press; 1976. Chance and design in electrophysiology: an informal account of certain experiments on nerve carried out between 1934 and 1952; pp. 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Hu H, Shao L-R, Chavoshy S, Gu N, Trieb M, Behrens R, Laake P, Pongs O, Knaus HG, Ottersen OP, Storm JF. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci. 2001;21:9585–9597. doi: 10.1523/JNEUROSCI.21-24-09585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Konnerth A, Augustine GJ. Action potential broadening and frequency-dependent facilitation of calcium signals in pituitary nerve terminals. Proc Natl Acad Sci U S A. 1991;888:380–384. doi: 10.1073/pnas.88.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerng HH, Shahidullah M, Covarrubias M. Inactivation gating of Kv4 potassium channels: molecular interactions involving the inner vestibule of the pore. J Gen Physiol. 1999;113:641–660. doi: 10.1085/jgp.113.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HY, Mickus T, Spruston N. Prolonged sodium channel inactivation contributes contributes to dendritic action potential attenuation in hippocampal pyramidal neurons. J Neurosci. 1997;17:6639–6646. doi: 10.1523/JNEUROSCI.17-17-06639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Huguenard JR, Prince DA. Voltage-gated potassium channels activated during action potentials in layer V neocortical pyramidal neurons. J Neurophysiol. 2000;83:70–80. doi: 10.1152/jn.2000.83.1.70. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Llin ás R. The intrinsic electrophysiological properties of mammalian neurons: Insights into central nervous system function. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Llin ás R, Steinberg IZ, Walton K. Presynaptic calcium currents in squid giant synapse. Biophys J. 1981;33:289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Koester J. The role of K+ currents in frequency-dependent spike broadening in Aplysia R20 neurons: a dynamic-clamp analysis. J Neurosci. 1996;16:4089–4101. doi: 10.1523/JNEUROSCI.16-13-04089.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Neuronal organisation of the lateral and basolateral amygdaloid nuclei of the rat. J Comp Neurol. 1984;222:589–606. doi: 10.1002/cne.902220410. [DOI] [PubMed] [Google Scholar]
- Magee J, Hoffman D, Colbert C, Johnston D. Electrical and calcium signaling in dendrites of hippocampal pyramidal neurons. Annu Rev Physiol. 1998;60:327–346. doi: 10.1146/annurev.physiol.60.1.327. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275:209–213. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- Par é D, Gaudreau H. Projection cells and interneurons of the lateral and basolateral amygdala: Distinct firing patterns and differential relation to theta and delta rhythms in conscious cats. J Neurosci. 1996;16:3334–3350. doi: 10.1523/JNEUROSCI.16-10-03334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolos NP, Johnston D. Calcium-activated potassium conductances contribute to action potential repolarization at the soma but not the dendrites of hippocampal CA1 pyramidal neurons. J Neurosci. 1999;19:5205–5212. doi: 10.1523/JNEUROSCI.19-13-05205.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrocki EA, Marshall J, Kaczmarek LK. A Shab potassium channel contributes to action potential broadening in peptidergic neurons. Neuron. 1994;12:73–86. doi: 10.1016/0896-6273(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Regehr WG, Delaney KR, Tank DW. The role of presynaptic calcium in short-term enhancement at the hippocampal mossy fiber synapse. J Neurosci. 1994;14:523–537. doi: 10.1523/JNEUROSCI.14-02-00523.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini BL, Regehr WG. Control of neurotransmitter release by presynaptic waveform at the granule cell to Purkinje cell synapse. J Neurosci. 1997;17:3425–3435. doi: 10.1523/JNEUROSCI.17-10-03425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P. The role of calcium influx and calcium buffering in the kinetics of a Ca2+ activated K+ current in rat vagal motoneurons. J Neurophysiol. 1992;68:2237–2248. doi: 10.1152/jn.1992.68.6.2237. [DOI] [PubMed] [Google Scholar]
- Sah P. Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996;19:150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- Sah P, Bekkers JM. Apical dendritic location of slow-afterhyperpolarization current in hippocampal pyramidal neurons: implications for the integration of LTP. J Neurosci. 1996;16:4537–4542. doi: 10.1523/JNEUROSCI.16-15-04537.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ESL. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol. 2002;66:345–353. doi: 10.1016/s0301-0082(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ESL, Lopez De Armentia M, Power JM. The amygdaloid complex, anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sather W, Dieudonne S, MacDonald JF, Ascher P. Activation and desensitization of N-methyl-D-aspartate receptors in nucleated outside-out patches from mouse neurones. J Physiol. 1992;450:643–672. doi: 10.1113/jphysiol.1992.sp019148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt PC, Spain WJ, Crill WE. Calcium-dependent potassium currents in neurones from cat sensorimotor cortex. J Neurophysiol. 1992;67:216–226. doi: 10.1152/jn.1992.67.1.216. [DOI] [PubMed] [Google Scholar]
- Shao LR, Halvorsrud R, Borg-Graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol. 1999;521:135–146. doi: 10.1111/j.1469-7793.1999.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 1999;96:4662–4667. doi: 10.1073/pnas.96.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- Van Goor F, Le Beau AP, Krsmanovic LZ, Sherman A, Catt KJ, Stojilkovic SS. Amplitude-dependent spike-broadening and enhanced Ca(2+) signaling in GnRH-secreting neurons. Biophys J. 2000;79:1310–1323. doi: 10.1016/S0006-3495(00)76384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


