Abstract
Proteinase-activated receptor 2 (PAR2) is a receptor for mast cell tryptase and trypsins and might participate in brain-gut communication. However, evidence that PAR2 activation can lead to afferent impulse generation is lacking. To address this issue, we examined the sensitivity of jejunal afferent nerves to a hexapeptide agonist of PAR2, SLIGRL-NH2, and the modulation of the resulting response to treatment with drugs and vagotomy. Multiunit recordings of jejunal afferent activity were made using extracellular recording techniques in anaesthetised male rats. SLIGRL-NH2 (0.001–1 mg kg−1, I.V.) increased jejunal afferent firing and intrajejunal pressure. The reverse peptide sequence (1 mg kg−1, I.V.), which does not stimulate PAR2, was inactive. Naproxen (10 mg kg−1, I.V.), but not a cocktail of ω-conotoxins GVIA and SVIB (each at 25 μg kg−1, I.V.), curtailed both the afferent response and the intrajejunal pressure rise elicited by the PAR2 agonist. Although neither treatment modulated the peak magnitude of the afferent firing, they each altered the intestinal motor response, unmasking an initial inhibitory component. Nifedipine (1 mg kg−1, I.V.) reduced the peak magnitude of the afferent nerve discharge and abolished the initial rise in intrajejunal pressure produced by SLIGRL-NH2. Vagotomy did not significantly influence the magnitude of the afferent response to the PAR2 agonist, which involves a contribution from capsaicin-sensitive fibres. In conclusion, intravenous administration of SLIGRL-NH2 evokes complex activation of predominantly spinally projecting extrinsic intestinal afferent nerves, an effect that involves both direct and indirect mechanisms.
Immunological mechanisms present in the intestinal mucosa are implicated in the recognition process that ultimately leads to the triggering of secretomotor events, which expel potentially dangerous material from the gut. A functional interaction between immunocompetent mucosal mast cells and intestinal afferent nerves could contribute to this defence process and also initiate illness behaviours associated with the ingestion of harmful matter. For example, histological studies have shown that mucosal mast cells are situated in close proximity to the terminals of extrinsic afferent nerves (Williams et al. 1997). In addition, the extrinsic afferent nerve activity evoked by intestinal anaphylaxis is sensitive to selective H1 and 5-HT3 receptor antagonists, suggesting that the mast cell mediators histamine and 5-HT are involved in orchestrating the mucosal response to antigenic matter (Jiang et al. 2000). However, in addition to these mast cell products, the serine proteinase mast cell tryptase could function in neuro-immune signalling through interaction with a novel class of cell surface proteins known as proteinase-activated receptors or PARs.
PARs are heptahelical molecules that are members of the G-protein-coupled superfamily and molecular cloning has revealed the existence of four subtypes (PAR1-4) (Schmidlin & Bunnett, 2001; Macfarlane et al. 2001; Vergnolle et al. 2001b) that have contrasting sensitivities to endogenous serine proteinases. For instance, PAR1 and PAR4 are stimulated by thrombin, with PAR3 functioning as a co-factor in the activation of the latter (Nakanishi-Matsui et al. 2000). PAR2 and PAR4 are sensitive to the digestive enzyme trypsin, whereas mast cell tryptase selectively activates PAR2. Serine proteinases activate PARs by a unique mechanism that initially involves recognition and then cleavage of the receptor by the enzyme at a specific site in from the extracellular N-terminus (Schmidlin & Bunnett, 2001; Macfarlane et al. 2001; Vergnolle et al. 2001b). This creates a new N-terminus that acts as a tethered ligand and activates the cleaved receptor molecule. In addition, hexapeptide sequences corresponding to the tethered ligand can selectively activate PARs 1, 2 and 4 without the need for receptor cleavage (Schmidlin & Bunnett, 2001; Macfarlane et al. 2001; Vergnolle et al. 2001b; Lan et al. 2001) and are useful tools for receptor characterisation.
From the perspective of neuro-immune signalling, PAR2 was of initial great interest as it is this receptor that is sensitive to mast cell tryptase and PAR2 is expressed on the cell bodies of spinal extrinsic afferent nerves, some of which may project to the intestines (Steinhoff et al. 2000). Moreover, there is evidence that functional PAR2 is present on the terminals of some of these fibres and is involved in the genesis of neurogenic inflammation and hyperalgesia in somatic structures and the large bowel (Steinhoff et al. 2000; Vergnolle et al. 2001a; Coelho et al. 2002). However, the peripheral mechanisms invoked by PAR2 activation that lead to altered sensitivity in extrinsic sensory fibres innervating the small intestine remain to be established. To elaborate these, we therefore investigated the effects of the PAR2-activating peptide SLIGRL-NH2 on the discharge of mesenteric afferent nerves supplying the jejunum. To characterise the mechanisms and sensory fibres involved, we additionally investigated whether (1) the production of prostaglandins, (2) synaptic neurotransmission within the intestine, and (3) the release of substance P modified any of the resulting responses. Finally we investigated the subpopulations of extrinsic mesenteric afferents underlying the response to PAR2 activation. Some of these observations have been reported previously in abstract form (Kirkup et al. 2000).
METHODS
Animals
Experiments were conducted using 51 Sheffield-strain male Wistar rats (300-450 g) that were allowed, unless otherwise stated, free access to both regular solid food and water. All surgical and experimental procedures were in accordance with the UK Animals (Scientific Procedures) Act 1986.
Recovery surgical procedures
A sub-diaphragmatic vagotomy or sham procedure was performed on 14 pentobarbitone sodium (60 mg kg−1)-anaesthetised rats; our experimental protocol is fully described in an earlier publication (Booth et al. 2001). Following recovery from anaesthesia, animals were returned to their housing and fed on a liquid diet (Complan Original, Heinz Ltd, Uxbridge, UK; in full fat pasteurised milk) to which they had been accustomed prior to surgery, for a further 5-10 days before afferent recording experiments were performed. The success of the vagotomy was confirmed by electrical stimulation of the cervical vagal nerve trunks as described previously (Kirkup et al. 1998).
Non-recovery surgical procedures
These procedures have also been extensively described elsewhere (Kirkup et al. 1999). General anaesthesia was produced in rats with an intraperitoneal injection of pentobarbitone sodium (60 mg kg−1) and was sustained by intravenous (I.V.) infusion (0.5-1 mg kg−1 min−1) of the anaesthetic. The trachea was intubated with a short length of tubing to facilitate spontaneous respiration. The right external jugular vein was cannulated with two saline-filled cannulae to facilitate maintenance anaesthesia and systemic administration of drugs. The left common carotid artery was cannulated with a heparinised catheter (200 units ml−1 heparin in saline) to record blood pressure (Neurolog NL108, Digitimer, Welwyn Garden City, Herts, UK). The heart rate was obtained from the arterial pressure record. Body temperature was monitored with a rectal thermometer and maintained at around 37 °C by means of a heating blanket.
A midline laparotomy was performed and the caecum was excised. A 10 cm loop of proximal jejunum was isolated and cannulated at the oral and anal ends. The oral cannula served as a drain. The aboral cannula contained two smaller bore saline-filled catheters, one of which was for the local administration of saline, the other was connected to a pressure transducer (Neurolog NL108) to measure intrajejunal pressure (the aboral and oral cannulae were closed to provide isovolumetric recording conditions). The intestinal loop was filled with saline up to a baseline pressure of 4-6 mmHg and this was maintained by intraluminal infusion of saline (0.4-1.0 ml h−1) through the aboral cannula connected to the pressure transducer. The abdominal incision was sutured to a 5 cm diameter steel ring to form a well that was subsequently filled with pre-warmed (37 °C) light liquid paraffin.
Nerve preparation and afferent recording
A mesenteric arcade was placed on a black Perspex platform and a single nerve bundle was dissected from the surrounding tissue. This was severed distal from the wall of the jejunum (approximately 1-1.5 cm) to eliminate efferent nerve activity. It was then attached to one of a pair of platinum electrodes, with a strand of connective tissue wrapped around the other to act as a differential. The electrodes were connected to a Neurolog head stage (Neurolog NL100) and the signal from them was amplified (Neurolog NL104) and filtered (Neurolog NL125). The neurogram was displayed on a storage oscilloscope (Dual Beam Storage 5113, Tektronix, Guernsey, UK) and relayed, together with the signals from the arterial and intrajejunal pressure transducers, into a 1401 plus interface (Cambridge Electronic Design (CED), Cambridge, UK). A PC running Spike2 software (CED) sampled these signals on-line.
Experimental protocols
After a 45-60 min stabilisation period, the sensitivity of the nerve bundle was assessed with an I.V. dose of 2-methyl-5-hydroxytryptamine (2-methyl-5-HT, 30 μg kg−1). After a further 5 min, the jejunal loop was distended for 15 s with the rapid introduction (< 1 s) of 0.5 ml of saline. The preparation was left for a further 5-10 min stabilisation period before any other treatments were administered. Nerve preparations from vagus-intact preparations that failed to respond to these two stimuli were discarded and a fresh bundle was dissected out. These two test stimuli were used as selection criteria to ensure that recordings were obtained from bundles containing subpopulations of vagal and spinal extrinsic afferent nerves and thus minimise variability (Hillsley et al. 1998). In preliminary experiments, a sequential dose-response curve to the PAR2 agonist was produced with the interval between each successive dose being sufficient to allow recovery of variables to their baseline values. Only one dose-response curve was generated per preparation. From these pilot studies, a dose of PAR2 agonist that produced a robust response was determined (1 mg kg−1). To ascertain the specificity of this response, the effects of this dose of the PAR-2 agonist and the same dose of the reverse peptide sequence were compared in three more preparations. Following on from this, the effects of several inhibitors and vagotomy upon the chosen dose of PAR2-activating peptide were evaluated in three series of experiments. As the reproducibility of the responses to this dose of PAR2 agonist was not ascertained, in the first series of studies its effects were assessed in separate groups of vagus-intact animals that were pretreated I.V. (for 5 min) with either vehicle (saline or 25 % v/v dimethyl sulfoxide (DMSO) in saline) or inhibitor (naproxen, 10 mg kg−1; ω-conotoxins GVIA and SVIB, each at 25 μg kg−1 or nifedipine, 1 mg kg−1). The ensuing response to the PAR2 agonist was then recorded for a period of 15 min. In the second series of investigations, a substance P (SP) desensitisation protocol was employed to investigate the involvement of neurokinin-1 (NK1) receptors in the responses to PAR2 agonist in vagus-intact preparations. Before PAR2 application, substance P (30 μg kg−1, I.V.) was administered twice, with a period of 10 min between each dose. Preparations were then challenged with SLIGRL-NH2 15 min after the second SP administration. The third dose of SP was applied once the resulting response to PAR2 activation recovered to baseline. The third series of experiments was conducted in two groups of preparations that had undergone either a chronic sub-diaphragmatic vagotomy or a sham procedure. In these animals, SLIGRL-NH2 was administered 5-10 min after the initial test stimuli were given and the response was recorded for 15 min. One group of vagotomised animals was treated with naproxen (10 mg kg−1, I.V.) and nifedipine (1 mg kg−1, I.V.) before administration of the PAR2 agonist. A femoral artery was also cannulated in these animals and the catheter was passed through the iliac artery and abdominal aorta to 5 mm rostral to the coeliac axis in order to permit close intra-arterial (I.A.) injection of capsaicin (0.5-1.0 μg kg−1). Agonist drugs were administered (I.V.) in a 0.1 ml volume whereas inhibitors were injected (I.V.) over a period of 60 s in a dose volume of 2.0 ml kg−1. Administration of either type of agent was followed by a 0.35 ml saline flush. On completion of an experiment, animals were killed by anaesthetic overdose followed by exsanguination.
Analysis of data
Afferent neurograms were analysed using Spike2 software in order to count the total number of action potentials crossing a preset threshold in sequential time bins. The mean baseline value for each of the measured variables of afferent activity (in spikes s−1), intrajejunal pressure (in mmHg), blood pressure (in mmHg) and heart rate (in beats min−1) was determined over a 30 s period prior to any treatment. For the dose-response studies, the peak change in the variable following administration of the PAR2 agonist was measured and the mean baseline value was subtracted in order to deduce the change evoked by the agonist in that variable. In all other studies, the mean level of each variable was measured in consecutive 5 s bins for a period of 5 min from the time of administration of the PAR2 agonist. The respective mean baseline level of these variables was subtracted from these values to calculate the change elicited by the agonist. Additionally, for certain experimental groups, the area under the curve of the afferent and intrajejunal pressure responses was measured for the first 5 min following administration and corrected for baseline activity. The time of administration of the agonist was determined from the perturbation of the blood pressure trace. Data are presented as the arithmetic mean ± S.E.M. from three or more animals per vehicle- or antagonist-treated or untreated group. Where n values are given, they refer to the number of animals. Significant differences between group means were determined by appropriate use of parametric or non-parametric analysis of variance followed by Student's paired or unpaired t test (with Bonferroni corrections) or Dunn's analysis, respectively. A probability of P < 0.05 was considered to be indicative of a statistically significant difference.
Single unit analysis of the whole mesenteric nerve discharge was performed using the template matching algorithm in Spike2 as described previously (Hillsley et al. 1998). This analysis was restricted to bundles from vagotomised animals treated with naproxen and calcium channel blocks in order to determine the capsaicin sensitivity of PAR2-sensitive afferent fibres.
Drugs
Capsaicin, naproxen (sodium salt), nifedipine (hydrochloride) and substance P (acetate salt) were obtained from Sigma Chemical Co. (Poole, Dorset, UK). 2-Methyl-5-HT (hydrochloride salt) was purchased from Research Biochemicals Inc. (Poole, Dorset, UK). ω-Conotoxin GVIA and ω-conotoxin SVIB were purchased from Alomone Laboratories (Jerusalem, Israel). Pentobarbitone (sodium salt, Sagatal) was obtained from Rhône Mérieux Ltd (Harlow, UK). The PAR2 agonist (SLIGRL-NH2) and the reverse sequence peptide (LRGILS-NH2) were synthesised and purified as described previously (Steinhoff et al. 2000).
Apart from nifedipine and capsaicin, all of the compounds were dissolved in 0.9 % w/v NaCl solution (saline). 2-Methyl-5-HT and naproxen were made up fresh each day in saline whereas the PAR2 peptide and the toxins were dissolved in saline and frozen in aliquots that were thawed when required. For the dose-response experiments, serial dilutions of the PAR2 agonist were made in saline. Nifedipine was dissolved in 25 % v/v DMSO in saline on the day of the experiment and protected from light. Capsaicin was dissolved in 50 % v/v DMSO in saline at a concentration of 100 μg ml−1 and diluted in saline to the required concentration for injecting.
RESULTS
Effects of SLIGRL-NH2
Dose-response studies were initially conducted in three preparations. SLIGRL-NH2 (0.001-1.0 mg kg−1, I.V.) elicited dose-dependent increases in mesenteric afferent nerve discharge and intrajejunal pressure and dose-dependent decreases in blood pressure (Fig. 1). These effects did not achieve a maximum over the dose range tested (Fig. 1). From these studies it was deduced that a dose of the peptide agonist of 1 mg kg−1 (I.V.) evoked a robust activation of mesenteric afferents and this was used for the subsequent experiments. To demonstrate the specificity of the response to SLIGRL-NH2, the effects of the peptide and its reverse sequence were examined in a further three preparations. Administration of the reverse sequence peptide (1 mg kg−1, I.V.), which does not activate PAR2, produced no discernible effect in these animals, whereas subsequent administration of SLIGRL-NH2 stimulated marked increases in afferent discharge and intrajejunal pressure and a hypotension (Fig. 2). In these six experiments the effect of SLIGRL-NH2 on afferent activity was initiated 4.1 ± 1.6 s after administration. The peptide-evoked rises in intrajejunal pressure occurred 5.2 ± 1.6 s after injection, a latency that was not significantly different from that for the effect on afferent discharge. The pattern of activation of afferent nerves and rises in intrajejunal pressure evoked by SLIGRL-NH2 was somewhat variable. The afferent response was characterised by an initial abrupt and intense increase in the firing of afferent nerves. This level of discharge continued for between 40 and 60 s before decaying towards the baseline level of firing. In four of these six experiments, the elevation in discharge was sustained and remained 8 ± 4 spikes s−1 above baseline 15 min after administration of SLIGRL-NH2. In the other two preparations, the increase in afferent activity returned to baseline levels after a mean time of 376 s (range 323-428 s). The intrajejunal pressure response was also characterised by an abrupt initial rise that was sometimes followed by phasic elevations in pressure which occurred over the first 40-60 s of the response. This activity settled to a level sustained above baseline that decayed slowly. In three of these six experiments, the elevation in pressure did not recover within the 15 min recording period and remained 1.2 ± 0.7 mmHg above baseline at this time point. In the two preparations in which the elevation in afferent activity recovered, the pressure increases decayed to baseline after a mean time period of 623 s (range 550-696 s) whereas in the one remaining preparation, no increase in intrajejunal pressure was observed. In contrast to these variables, the pattern of hypotension evoked by SLIGRL-NH2 was consistent and was characterised by an abrupt fall that recovered towards baseline pressure monophasically. At the end of the time period measured, the blood pressure in four out of these six preparations was 19 ± 6 mmHg below baseline. In the other two animals in which both the afferent and pressure response waned, blood pressure recovered to baseline 523 s (range 482-563 s) after administration of the PAR2 agonist.
Figure 1. Dose-response relationships for the effects of SLIGRL-NH2 on jejunal afferent discharge (AD), intrajejunal pressure (IP) and blood pressure (BP) in the anaesthetised rat.
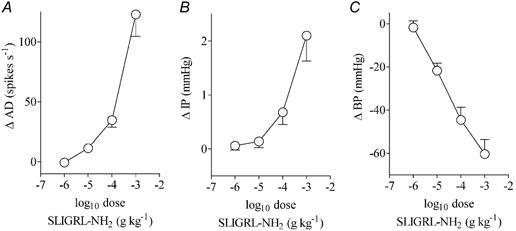
Data are expressed as means ± S.E.M. of the peak change evoked by the agonist (n = 3).
Figure 2. Effects of SLIGRL-NH2 and its reverse peptide LRGILS-NH2 on both jejunal afferent activity and intraluminal pressure.
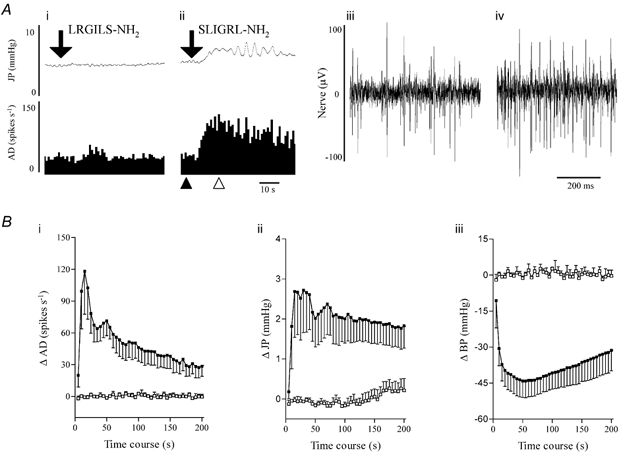
A, an illustrative example of concomitant recordings of the effects of LRGILS-NH2 (1 mg kg−1, I.V., i) and SLIGRL-NH2 (1 mg kg−1, I.V., ii) on intrajejunal pressure (JP, upper traces) and jejunal afferent nerve activity (AD, lower traces, shown as sequential rate histograms). The downward pointing arrows indicate the point of injection of the peptides. To the left are excerpts of the extracellular recording of baseline jejunal afferent discharge (iii, pertaining to the time point on the sequential rate histogram indicated by ▴ in ii) and the action potential firing obtained during the response evoked by SLIGRL-NH2 (iv, from the timepoint on the sequential rate histogram denoted by ▵ in ii). B, mean data for the effects of SLIGRL-NH2 (▪, 1 mg kg−1) and LRGILS-NH2 (□, 1 mg kg−1) on jejunal afferent discharge (i), intrajejunal pressure (ii) and blood pressure (BP, iii). Data are expressed as means ± S.E.M. of each variable in a 5 s period minus the baseline of six experiments in the case of SLIGRL-NH2 (data pooled from the responses evoked by the activating peptide in the dose-response and reverse peptide studies) and three experiments in the case of LRGILS-NH2.
Effects of pretreatment with naproxen or calcium channel inhibitors
To delineate the mechanisms that contributed to the afferent and intrajejunal responses evoked by SLIGRL-NH2, the effects of several inhibitory agents on these phenomena were investigated. PAR2 stimulation is coupled to the generation of prostaglandins (Kong et al. 1997; Vergnolle et al. 1998). We therefore investigated the effects of cyclo-oxygenase (COX) inhibition on the responses elicited by the PAR2 agonist. Pretreatment with naproxen (10 mg kg−1, I.V.), a non-selective COX inhibitor, produced no significant effect on the baseline variables (Table 1). However, the responses to SLIGRL-NH2 were significantly altered compared with the vehicle-treated group (Fig. 3). The COX inhibitor produced no significant effect on the afferent activity evoked by the PAR2 agonist over the first 100 s of the response (Fig. 3). However, the duration of the sustained elevation in afferent nerve firing was significantly curtailed (Fig. 3). Naproxen also modulated the pattern of the rise in intrajejunal pressure provoked by SLIGRL-NH2, with the agonist initially provoking a decrease in intrajejunal pressure (Fig. 3). The peak increase in pressure was not significantly reduced; however, as for the afferent response, the duration of the rise in intrajejunal pressure was significantly shortened (Fig. 3).
Table 1.
Effects of i.v. administration of vehicle and inhibitors on the baseline values of the variables of jejunal activity (AD), intrajejunal pressure (JP), blood pressure (BP) and heart rate (HR)
| AD | JP | BP | HR | |
|---|---|---|---|---|
| (spikes s−1) | (mmHg) | (mmHg) | (beats min−1) | |
| Vehicle; saline (n = 9) | ||||
| Pre | 40 ± 5 | 5.6 ± 1.0 | 114 ± 4 | 382 ± 8 |
| Post | 37 ± 3 | 5.4 ± 1.0 | 114 ± 3 | 386 ± 10 |
| Naproxen (10 mg kg−1, i.v., n = 5) | ||||
| Pre | 33 ± 1 | 4.5 ± 0.2 | 114 ± 7 | 397 ± 17 |
| Post | 34 ± 6 | 5.0 ± 0.4 | 111 ± 8 | 395 ± 13 |
| ω-onotoxins GVIA and SVIB (each at 25 μg kg-1, i.v., n = 7) | ||||
| Pre | 32 ± 11 | 4.6 ± 0.7 | 116 ± 5 | 372 ± 10 |
| Post | 53 ± 14** | 4.8 ± 0.7 | 71 ± 4*** | 328 ± 8*** |
| Vehicle; 25% DMSO in saline (n = 4) | ||||
| Pre | 30 ± 7 | 4.8 ± 0.6 | 113 ± 6 | 367 ± 10 |
| Post | 28 ± 7 | 4.8 ± 0.5 | 118 ± 8 | 368 ± 9 |
| Nifedipine (1 mg kg-1, i.v., n = 6) | ||||
| Pre | 40 ± 9 | 4.5 ± 0.4 | 118 ± 4 | 394 ± 12 |
| Post | 29 ± 8* | 3.0 ± 0.3** | 69 ± 2*** | 406 ± 11 |
Pre, value 30 s before treatment with vehicle or inhibitor; Post, value 5 min after treatment with vehicle or inhibitor. Values are means ±s.e.m. (n, number of animals).
P < 0.001
P < 0.01
P < 0.05, significantly different from values calculated before inhibitor.
Figure 3. Response profiles show the effects of naproxen and a cocktail of ω-conotoxins GVIA and SVIB on PAR2 agonist-evoked jejunal afferent discharges.
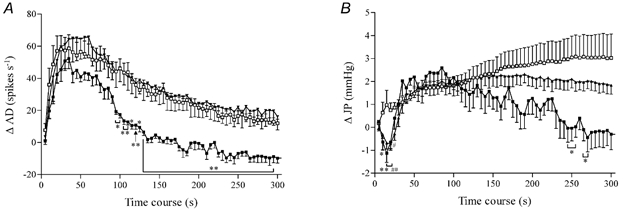
A, the afferent response (AD) to SLIGRL-NH2 (1 mg kg−1) was unchanged by the combination of toxins (each at 25 μg kg−1, I.V.) and only the duration of the evoked neural activity was curtailed by naproxen (10 mg kg−1, I.V.). B, naproxen and the toxins each unmasked an initial reduction of intrajejunal pressure (JP) provoked by administration of SLIGRL-NH2 and the former also shortened the duration of the evoked intrajejunal pressure increase. □, saline, n = 9; ▪, naproxen, n = 6; ▴, cocktail of ω-conotoxins GVIA and SVIB, n = 7. Data represent the mean ± S.E.M. of each variable in a 5 s period minus the baseline. *P < 0.05, **P < 0.01 naproxen- compared with vehicle-treated group. #P < 0.05, ##P < 0.01 toxin compared with vehicle-treated group.
To ascertain the involvement of neurogenic mechanisms in the responses mediated by PAR2 stimulation, we investigated the effects of pretreatment with the neuronal calcium channel inhibitors ω-conotoxin GVIA and ω-conotoxin SVIB. This cocktail of toxins inhibits N-type and non-N-type neuronal calcium channels and would serve to minimise synaptic transmission within the intestine. Administration of this combination of toxins produced a significant modulation of the baseline variables (Table 1). Nevertheless, the burst of afferent firing produced by SLIGRL-NH2 was not significantly altered compared with the vehicle-treated group (Fig. 3). As was observed following naproxen treatment, injection of the PAR2 agonist produced a significant acute reduction in intrajejunal pressure in those preparations previously exposed to the cocktail of conotoxins (Fig. 3). However, neither the peak increase nor the duration of the intrajejunal pressure response was significantly altered in animals that were pretreated with the toxins (Fig. 3).
As the jejunal afferent discharge evoked by SLIGRL-NH2 occurred in conjunction with rises in intrajejunal pressure, the contribution of the rises in pressure to afferent discharge was investigated in a series of experiments with the L-type calcium channel inhibitor nifedipine. This compound attenuates intestinal smooth muscle and secretory activity that might contribute to alterations in intrajejunal pressure in our manometric system. Injection of nifedipine produced significant effects on the baseline values of the variables and also significantly modulated the responses evoked by SLIGRL-NH2. Compared with the vehicle-treated group, nifedipine treatment reduced (between 5 and 20 s, and 95 and 125 s) but did not abolish the elevation in afferent discharge elicited by PAR2 receptor stimulation (Fig. 4Ai and Bii). Nifedipine markedly attenuated the evoked increases in intrajejunal pressure. The initial phasic rises in pressure evoked by SLIGRL-NH2 were abolished over the first 30 s after administration. Furthermore, the sustained component of the pressure response was significantly reduced compared with the vehicle-treated group between 30 and 75 s after injection of the PAR2 agonist (Fig. 4Aii and Bii).
Figure 4. Effects of nifedipine on both intestinal motor and mesenteric afferent responses to PAR2 agonist.
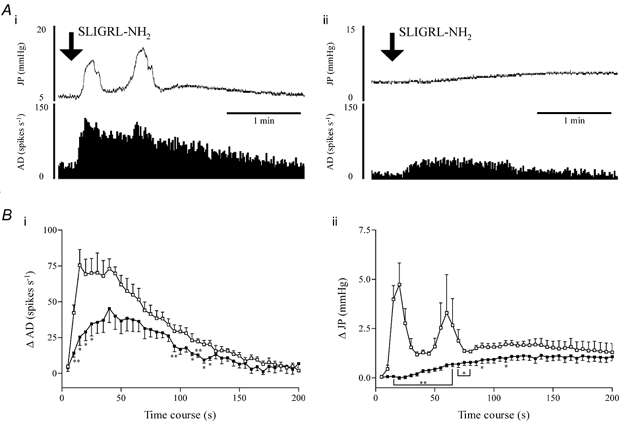
A, representative traces of the effects of SLIGRL-NH2 (1 mg kg−1) on intrajejunal pressure (JP, upper traces) and jejunal afferent activity (AD, lower traces) in animals pretreated with either vehicle (i) or nifedipine (1 mg kg−1, I.V., ii). They clearly show that PAR2-evoked phasic increases in intrajejunal pressure and profound afferent bursting were markedly reduced after treatment with nifedipine. B, graphs summarising the pooled data for the elevations in afferent activity (i) and intrajejunal pressure (ii) evoked by SLIGRL-NH2 after treatment with either vehicle or nifedipine and showing the sensitivity of these responses to the calcium channel inhibitor (□, 25 % v/v DMSO in saline, n = 4; ▪, nifedipine, n = 6). Data represent the mean ± S.E.M. of each variable in a 5 s period minus the baseline. *P < 0.05, **P < 0.01.
Effects of pretreatment with substance P
Substance P released from afferent endings mediates some of the effects of PAR2 agonists in cutaneous and visceral tissues (Steinhoff et al. 2000; Coelho et al. 2002). To determine whether this mechanism contributed to the responses we observed, we investigated the sensitivity of mesenteric afferent bundles to substance P and the extent to which desensitisation of the peptide's receptors affected the elevations in afferent activity and intrajejunal pressure evoked by SLIGRL-NH2 (1 mg kg−1, I.V.). Injection of substance P (30 μg kg−1, I.V.) induced increases in afferent activity and intrajejunal pressure in three of the seven and seven of the seven preparations tested, respectively (Fig. 5A). A subsequent injection of the same dose of substance P elicited a similar rise in intrajejunal pressure but a significantly reduced level of afferent discharge in those nerve bundles previously responsive to the peptide. The PAR2 agonist was injected 15 min after the second application of substance P and induced elevations in afferent nerve discharge and intrajejunal pressure (data not shown) that were no different from those observed in preparations pretreated with saline, irrespective of whether the afferent nerve bundle was sensitive to substance P or not (Fig. 5B). To verify that the afferents were still desensitised to substance P during the SLIGRL-NH2 challenge, a third treatment with the same dose of substance P was applied once the PAR2 agonist response had recovered completely. Both afferent and motor responses to the second and third administrations of substance P were comparable (Fig. 5A).
Figure 5. Influence of substance P on both intestinal motor and afferent responses to PAR2 agonist.
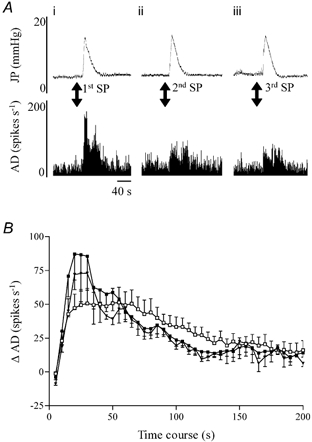
A, representative traces of the effects of substance P (30 μg kg−1, I.V.) on intrajejunal pressure (JP, upper traces) and jejunal afferent nerve activity (AD, lower traces) in a preparation whose mesenteric nerve bundle exhibited sensitivity to the tachykinin. The peptide elicited marked increases in intrajejunal pressure and afferent activity, but only the pressure response was reproducible when the peptide was administered 10 min later (compare i with ii). After treatment with the PAR2 agonist, a third exposure to substance P (iii) induced a similar rise in intrajejunal pressure compared with the previous challenges but the afferent response remained diminished. B, graphs showing that irrespective of whether or not the mesenteric nerve bundle was sensitive to substance P, SLIGRL-NH2 evoked a comparable afferent discharge (□, saline vehicle, n = 6; ▪, substance P pretreatment, n = 7; ▾, substance P-sensitive only, n = 3). Data represent the mean ± S.E.M. of each variable in a 5 s period minus the baseline.
Effects of sub-diaphragmatic vagotomy
To identify the subpopulation of jejunal afferent nerves that exhibited sensitivity to administration of SLIGRL-NH2 (1 mg kg−1, I.V.), experiments were performed in groups of animals that had undergone either a sham operation or a sub-diaphragmatic vagotomy. The bursts of afferent discharge and increases in intrajejunal pressure elicited by the PAR2 agonist were not significantly different between the sham and vagotomised groups of preparations, irrespective of whether the responses were compared at discrete time points or as the area under either the afferent or motor response curve during the first 5 min of the response period (Fig. 6).
Figure 6. PAR2 agonist-evoked jejunal afferent and motor responses in sham-operated and vagotomised preparations.
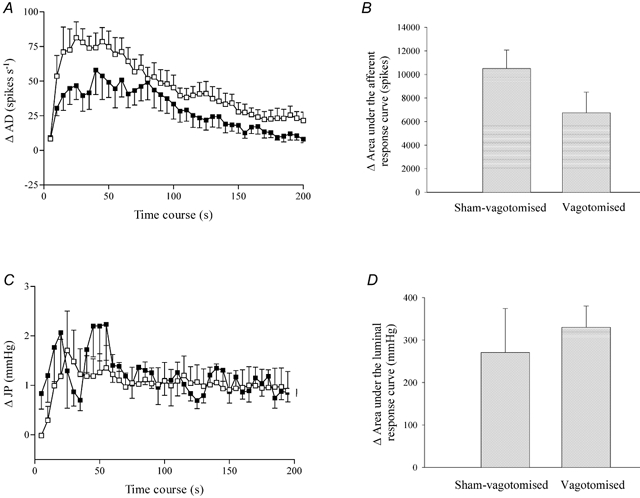
A, the intestinal afferent response profile to PAR2 agonist in sham-vagotomised and vagotomised animals. B, area under the afferent response curve during the initial 5 min period following administration of the PAR2 agonist in sham-vagotomised and vagotomised animals. C, the intrajejunal pressure response profile to PAR2 agonist in sham-vagotomised and vagotomised animals. D, area under the intraluminal pressure response curve to PAR2 agonist in sham-vagotomised and vagotomised animals. □, sham-operated, n = 6; ▪, vagotomised, n = 5. Data in A and C represent the mean ± S.E.M. of each variable in consecutive 5 s periods minus the baseline. Note that the afferent response to PAR2 agonist, while reduced in vagotomised animals, was not significantly different from the sham treatment controls.
In three vagotomised preparations pretreated with a cocktail of naproxen (10 mg kg−1, I.V.) and nifedipine (1 mg kg−1, I.V.), SLIGRL-NH2 evoked a modest increase in afferent discharge and a markedly attenuated rise in intrajejunal pressure (Fig. 7A), and the duration of each of these responses was significantly curtailed with respect to the sham-operated and vagotomised groups of animals (Fig. 6A and C). Using waveform discrimination software, four units were resolved from two of these three recordings that were each activated by both SLIGRL-NH2 and then capsaicin (0.5-1.0 μg kg−1, I.A.). An example of a unit demonstrating these characteristics is given in Fig. 7B.
Figure 7. Effects of PAR2 agonist (1 mg kg−1, I.V.) on afferent discharge (AD) and intrajejunal pressure (JP) obtained from vagotomised animals pretreated with a cocktail of naproxen and nifedipine.
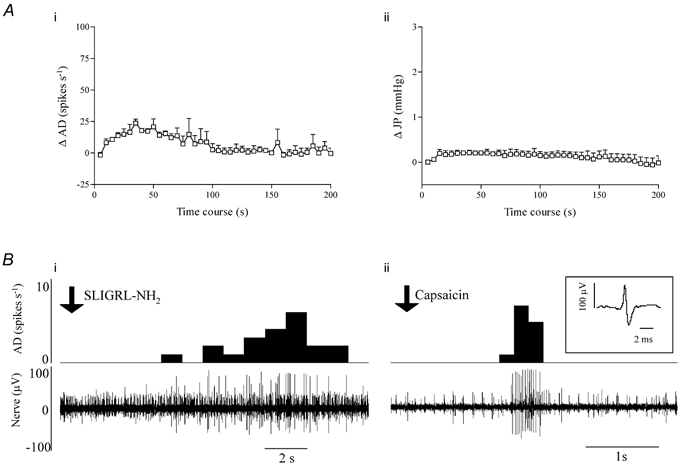
A, prior administration of these agents clearly attenuated the excitatory effects on extrinsic sensory nerves (i) and abolished the increase in intrajejunal pressure (ii) evoked by the PAR2 agonist (compare with Fig. 6). Waveform discrimination analysis of a multiunit extracellular recording of jejunal afferent activity taken from one of these vagotomised preparations. Bi, sequential rate histogram (upper) and corresponding segment of nerve recording (lower) illustrating the activation of a large amplitude unit by SLIGRL-NH2 (time of administration indicated by the downward pointing arrow) after pretreatment with naproxen and nifedipine. Bii, the same large amplitude unit, whose waveform is shown in the inset, was clearly sensitive to capsaicin (0.5 μg kg−1, I.A.; time of administration indicated by the downward pointing arrow).
DISCUSSION
Mechanisms responsible for the excitatory effect of the PAR2 agonist
The principal observation from the present study is that administration to anaesthetised rats of the tethered ligand of PAR2, SLIGRL-NH2, evokes abrupt increases in the activity of jejunal afferent nerves and intrajejunal pressure. A series of investigations was performed to discriminate the mechanisms accounting for the observed excitation of jejunal afferent nerves.
Firstly it was noted that effects of SLIGRL-NH2 on jejunal afferent activity and intrajejunal pressure occurred in conjunction with a decrease in systemic blood pressure. The mechanisms responsible for the latter effect were not investigated but it presumably reflects PAR2-mediated release of nitric oxide from the endothelium and subsequent relaxation of vascular smooth muscle. Irrespective of the actual processes involved, we have shown previously that falls in blood pressure of this magnitude and time course do not acutely affect the baseline activity of jejunal afferent nerves (Kirkup et al. 1998). Indeed, complete interruption of the blood flow to the intestinal loop during ischaemia-reperfusion studies evokes an afferent response only after a latency of 45-60 s (W. Jiang & D. Grundy, unpublished observation) at which time point in the present study the afferent response to the PAR2 agonist had peaked and was returning towards baseline. Therefore it is unlikely that changes in intestinal blood flow contributed to the observed profound afferent firing that ensued subsequent to injection of the PAR2 agonist.
As rises in intrajejunal pressure were evoked by PAR2 stimulation, it was a real possibility that the accompanying excitation of jejunal afferents represented purely the stimulation of mechanosensitive fibres present in the nerve bundles. However, it is clear, particularly following treatment with naproxen and neuronal calcium channel blockers, that afferent activity and rises in intestinal pressure can be uncoupled, especially in the early phase of the response to PAR2 stimulation. To determine systematically the contribution of mechanical changes to the elevation in afferent nerve activity, experiments were performed in preparations that were pretreated with the L-type calcium channel inhibitor nifedipine. This compound attenuates smooth muscle contractility and also dampens epithelial cell secretion; elevations in either (or both) of these two activities would result in a rise in intrajejunal pressure and a contemporaneous stimulation of mechanosensitive nerves. Additionally, this substance might retard the evoked release of some mediators from other cellular sources in the gut wall that subsequently excite jejunal extrinsic afferent nerves. Nifedipine treatment indeed resulted in diminished increases in pressure in the jejunum and afferent discharge elicited by SLIGRL-NH2. These observations clearly suggested that a component of the afferent nerve excitation may be secondary to either stimulation of mechanosensitive fibres following raised intrajejunal pressure or release of other mediators that activate receptors on the afferent nerve terminal. However, it is clear that despite treatment with nifedipine, abrupt increases in afferent discharge were still induced by the PAR2 agonist, unrelated to increases in pressure in the jejunum. This result therefore indicates that a component of the initial phase of afferent nerve excitation provoked by PAR2 stimulation was not secondary to changes in pressure in the jejunum and could therefore comprise an effect directly on the afferent terminal.
PAR2 is expressed on neurons present within the myenteric and submucosal plexuses (Corvera et al. 1999; Green et al. 2000; Linden et al. 2001). By implication, the effects of SLIGRL-NH2 on intrajejunal pressure observed in the present study might have occurred secondary to activation of these enteric neurons. Moreover, the PAR2-mediated excitations of extrinsic jejunal afferents may have featured indirect mechanisms that arose following augmented transmission within the enteric nervous system (ENS). For example, some of the afferent firing may have represented the discharge of intestinofugal fibres. These fibres project along mesenteric paravascular nerve bundles to prevertebral ganglia where they synapse with postganglionic sympathetic efferent nerves and they are thus considered to function in the initiation of peripheral reflexes (Szurszewski et al. 2002). Significantly, the cell bodies of these second order neurones reside in the myenteric plexus and their activity is regulated by the synaptic inputs they receive from a myriad enteric neurones (Tassicker et al. 1999). Additionally, the processes of some vagal extrinsic primary afferent nerves, which have their cell bodies in the nodose ganglia, are located in mesenteric nerve bundles. Some of these fibres form intraganglionic laminar endings around ganglia in the myenteric plexus (Phillips & Powley, 2000) and might therefore be excited by substances discharged into the synaptic neuropil from enteric neurones. We therefore investigated whether such mechanisms were at play by pretreating preparations with a cocktail of conotoxins, which inhibit neuronal calcium channels and would therefore assuage synaptic transmission. Strikingly, treatment with the toxins produced no significant effect on the burst of afferent firing produced by the PAR2 agonist across the time course measured. However, the abrupt increase in intrajejunal pressure evoked by SLIGRL-NH2 in vehicle-treated preparations was replaced with a transient decrease in intrajejunal pressure, which lasted for between 25 and 30 s, before an increase in pressure above baseline was observed. This finding suggests that the initial rise in intrajejunal pressure induced by PAR2 stimulation is an integrated response and involves the activation of enteric excitatory motor nerves in conjunction with inhibitory mechanisms. In the absence of toxins, the former of these is presumably the dominant stimulus of the two, thus explaining why an abrupt rise is initially seen following administration of SLIGRL-NH2 in vehicle-treated preparations. The mechanisms responsible for the initial reduction in intrajejunal pressure in toxin-treated preparations was not investigated but might represent PAR2-mediated release of inhibitory neurotransmitters that is insensitive to the cocktail of toxins we used. Nevertheless, it would appear from these series of experiments that the initial burst of jejunal afferent nerve discharge in response to PAR2 activation is unlikely to represent predominantly the excitation of intestinofugal fibres or subpopulations of vagal afferent nerves secondary to enhanced activity within the ENS. This conclusion adds further weight to our contention that a component of the initial excitation of jejunal afferent nerves evoked by SLIGRL-NH2 involves a direct effect at the level of the extrinsic afferent nerve ending.
PAR2 stimulation is linked to the release of several substances, which have multiple effects on diverse cell types (Kong et al. 1997; Vergnolle et al. 1998; Steinhoff et al. 2000). Such evoked mediator release could have contributed to the jejunal afferent discharge elicited by SLIGRL-NH2 in the current study. We examined whether the generation of prostaglandins contributed to the PAR2-mediated afferent stimulation, as these receptors are coupled to arachidonic acid release (Kong et al. 1997; Vergnolle et al. 1998) and, additionally, because we have recently shown that prostanoids have profound excitatory effects on jejunal extrinsic afferents (Haupt et al. 2000). We found that exposure to naproxen, a non-selective COX inhibitor, modulated the effects of SLIGRL-NH2 on jejunal extrinsic afferent activity and intrajejunal pressure. Interestingly, naproxen did not affect the initial burst of afferent discharge elicited by the PAR2 agonist but shortened significantly the duration of the afferent response. This suggested that prostaglandin formation did not contribute to the initial burst of firing evoked by SLIGRL-NH2 but participated in the prolongation of the increased afferent nerve discharge. In addition, it would seem that liberation of prostaglandins contributes to the extended elevation in intrajejunal pressure induced by administration of the PAR2 ligand. However, it is not immediately clear why inhibition of prostaglandin synthesis would attenuate the initial increase in intrajejunal pressure produced by SLIGRL-NH2. One possible explanation is that PAR2-mediated activation of enteric excitatory motor nerves is dependent on the presence of certain prostaglandins in the external milieu of the myenteric plexus similar to that suggested for secretomotor reflexes (Green et al. 2000).
Substance P is stored in the endings of primary afferent nerves present in both somatic and visceral tissues (Steinhoff et al. 2000; Vergnolle et al. 2001a; Coelho et al. 2002). In the cutaneous system, PAR2-mediated release of SP from primary afferent endings is involved in the neurogenic inflammation that the former produces. We were therefore interested to determine whether such a mechanism contributed to the effects of the PAR2 agonist witnessed in the current study. As substance P-selective receptors (or NK1 receptors) are notorious for exhibiting desensitisation (Bowden et al. 1994; McConalogue et al. 1998), we used a desensitisation protocol to examine whether activation of these receptors was involved in the PAR2-induced elevations in jejunal afferent activity and intrajejunal pressure. Interestingly, recurrent administration of substance P did not alter increases in intrajejunal pressure evoked by the peptide, suggesting that the NK1 receptors, presumably on intestinal smooth muscle, do not readily desensitise in our experimental model. However, there was a marked desensitisation of the NK1 receptors that mediated excitation of jejunal afferents although the sensitivity to substance P was not an exclusive property of these jejunal paravascular nerve bundles. Indeed, the magnitude of afferent firing in response to administration of SLIGRL-NH2 was independent of whether or not an afferent response to substance P was observed. Furthermore, the degree of afferent discharge observed in these experiments was no different from that evoked by the PAR2 agonist in the saline-treated group. Such findings strongly indicate that NK1 receptors were not involved in the excitation of jejunal afferent nerves following administration of the PAR2 agonist. However, it is plausible that PAR2-evoked substance P release might be involved in long-term changes in the excitability of extrinsic afferent nerves in gastrointestinal inflammatory disorders. Coelho et al. (2002) observed changes in the sensitivity of rectal afferents to distension 10-24 h after intraluminal administration of PAR2-activating peptide which was attenuated by NK1 receptor blockade. However, such long-term changes are very different from the rapid and transient activation by PAR2 ligand in the present study. It has recently been recognised that mast cells express PAR2, raising the possibility that mast cell protease may play a feedback role in an amplified mast cell activation cascade (D'Andrea et al. 2000).
Sensory fibres mediating the excitatory effect of the PAR2 agonist
As briefly alluded to above, the paravascular nerve bundles from which we made our recordings consist of heterogeneous populations of sensory fibres. In addition to the vagal primary afferents and second order intestinofugal fibres, the processes of spinal primary afferents, which have their cell bodies located in the dorsal root ganglia, are present within mesenteric nerves (Cervero et al. 1988). In order to elucidate which of these populations of fibres displayed sensitivity to the PAR2 agonist, we compared the responses to SLIGRL-NH2 in vagus-intact and vagotomised animals. The afferent response to PAR2 stimulation was attenuated in vagotomised animals but the magnitude of the reduction in either peak afferent firing or area under the response curve was not significant. Thus it remains possible that the terminals of some populations of vagal afferents may express PAR2 receptors although this has yet to be demonstrated. However, what is striking from the present observation is that the majority of jejunal sensory fibres that manifest sensitivity to SLIGRL-NH2 are not vagal and are therefore either spinal afferents or intestinofugal fibres. The latter is unlikely for a number of reasons. Firstly, as discussed above, the response to PAR2 stimulation was unaffected by synaptic blockade. Intestinofugal fibres receive a prominent synaptic input for enteric sensory neurones which would have been attenuated by synaptic blockade (Szurszewski et al. 2002). Intestinofugal fibres may themselves be mechanosensory but the response to PAR2 appears to be largely independent of any mechanical response arising from changes in intraluminal pressure. Finally, in a few vagotomised preparations pretreated with naproxen and nifedipine (to minimise indirect excitation of jejunal afferents), we identified several fibres that exhibited sensitivity to capsaicin and were, in addition, abruptly activated following administration of SLIGRL-NH2. Such observations support the contention that PAR2 stimulation leads to the direct excitation of the endings of spinal primary afferent nerves. As such, these observations strongly suggest that PAR2 stimulation might be involved in the rapid transmission of stimuli arising in the intestines to higher centres.
Conclusions
In summary, we have shown that the PAR2 agonist SLIGRL-NH2 evokes a specific excitation of jejunal extrinsic afferent nerves and elevation in intrajejunal pressure in the anaesthetised rat. Multiple mechanisms contribute to these effects. Prostaglandin generation underlies the prolonged activation of jejunal sensory fibres and sustained rises in pressure within the jejunum. However, a component of the initial burst of jejunal afferent excitation evoked by SLIGRL-NH2 reflects a direct action on the endings of jejunal extrinsic afferent nerves of spinal origin. Given the accepted protective role of these fibres, this finding implies that PAR2 stimulation might be involved in initiating mucosal defence mechanisms. Additionally, as spinal pathways convey painful signals that arise in the viscera, we tentatively speculate that this receptor mechanism might be involved in the sensitisation of nociceptive stimuli from the gut to the brain.
Acknowledgments
This work was supported by the BBSRC and by NIH grants DK43207 and DK57840. A.J.K. was a Glaxo Wellcome Research Fellow during the tenure of these studies. W.J. is a Research Fellow supported by the BBSRC.
REFERENCES
- Booth CE, Kirkup AJ, Hicks GA, Humphrey PP, Grundy D. Somatostatin sst2 receptor-mediated inhibition of mesenteric afferent nerves of the jejunum in the anesthetized rat. Gastroenterology. 2001;121:358–369. doi: 10.1053/gast.2001.26335. [DOI] [PubMed] [Google Scholar]
- Bowden JJ, Garland AM, Baluk P, Lefevre P, Grady EF, Vigna SR, Bunnett NW, McDonald DM. Direct observation of substance P-induced internalization of neurokinin 1 (NK1) receptors at sites of inflammation. Proc Natl Acad Sci U S A. 1994;91:8964–8968. doi: 10.1073/pnas.91.19.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero F, Sharkey KA. An electrophysiological and anatomical study of intestinal afferent fibres in the rat. J Physiol. 1988;401:381–397. doi: 10.1113/jphysiol.1988.sp017168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho AM, Vergnolle N, Guiard B, Fioramonti J, Bueno L. Proteinases and proteinase-activated receptor 2, a possible role to promote visceral hyperalgesia in rats. Gastroenterology. 2002;122:1035–1047. doi: 10.1053/gast.2002.32387. [DOI] [PubMed] [Google Scholar]
- Corvera CU, Déry O, McConalogue K, Gamp P, Thoma M, Al-Ani B, Caughey GH, Hollenberg MD, Bunnett NW. Thrombin and mast cell tryptase regulate guinea-pig myenteric neurons through proteinase-activated receptors-1 and -2. J Physiol. 1999;517:741–756. doi: 10.1111/j.1469-7793.1999.0741s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea MR, Rogahn CJ, Andrade-Gordon P. Localization of protease-activated receptors-1 and -2 in human mast cells, indications for an amplified mast cell degranulation cascade. Biotech Histochem. 2000;75:85–90. doi: 10.3109/10520290009064152. [DOI] [PubMed] [Google Scholar]
- Green BT, Bunnett NW, Kulkarni-Narla A, Steinhoff M, Brown DR. Intestinal type 2 proteinase-activated receptors, expression in opioid-sensitive secretomotor neural circuits that mediate epithelial ion transport. J Pharmacol Exp Ther. 2000;295:410–416. [PubMed] [Google Scholar]
- Haupt W, Jiang W, Kreis ME, Grundy D. Prostaglandin EP receptor subtypes have distinctive effects on jejunal afferent sensitivity in the rat. Gastroenterology. 2000;119:1580–1589. doi: 10.1053/gast.2000.20337. [DOI] [PubMed] [Google Scholar]
- Hillsley K, Kirkup AJ, Grundy D. Direct and indirect actions of 5-hydroxytryptamine on the discharge of mesenteric afferent fibres innervating the rat jejunum. J Physiol. 1998;506:551–561. doi: 10.1111/j.1469-7793.1998.551bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Kreis ME, Eastwood C, Kirkup AJ, Humphrey PP, Grundy D. 5-HT3 and histamine H1 receptors mediate afferent nerve sensitivity to intestinal anaphylaxis in rats. Gastroenterology. 2000;119:1267–1275. doi: 10.1053/gast.2000.19461. [DOI] [PubMed] [Google Scholar]
- Kirkup AJ, Booth CE, Chessell IP, Humphrey PP, Grundy D. Excitatory effect of P2X receptor activation on mesenteric afferent nerves in the anaesthetised rat. J Physiol. 1999;520:551–563. doi: 10.1111/j.1469-7793.1999.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkup AJ, Bunnett NW, Grundy D. A proteinase-activated receptor subtype 2 agonist stimulates mesenteric afferent discharge. Gastroenterology. 2000;118:A173. [Google Scholar]
- Kirkup AJ, Eastwood C, Grundy D, Chessell IP, Humphrey PP. Characterization of adenosine receptors evoking excitation of mesenteric afferents in the rat. Br J Pharmacol. 1998;125:1352–1360. doi: 10.1038/sj.bjp.0702202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W, McConalogue K, Khitin LM, Hollenberg MD, Payan DG, Bohm SK, Bunnett NW. Luminal trypsin may regulate enterocytes through proteinase-activated receptor 2. Proc Natl Acad Sci U S A. 1997;94:8884–8889. doi: 10.1073/pnas.94.16.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan RS, Knight DA, Stewart GA, Henry PJ. Role of PGE2 in protease-activated receptor-1, -2 and -4 mediated relaxation in the mouse isolated trachea. Br J Pharmacol. 2001;132:93–100. doi: 10.1038/sj.bjp.0703776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DR, Manning BP, Bunnett NW, Mawe GM. Agonists of proteinase-activated receptor 2 excite guinea pig ileal myenteric neurons. Eur J Pharmacol. 2001;431:311–314. doi: 10.1016/s0014-2999(01)01447-9. [DOI] [PubMed] [Google Scholar]
- McConalogue K, Corvera CU, Gamp PD, Grady EF, Bunnett NW. Desensitization of the neurokinin-1 receptor (NK1-R) in neurons, effects of substance P on the distribution of NK1-R, Galphaq/11, G-protein receptor kinase-2/3, and beta-arrestin-1/2. Mol Biol Cell. 1998;9:2305–2324. doi: 10.1091/mbc.9.8.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- Nakanishi-Matsui M, Zheng YW, Sulciner DJ, Weiss EJ, Ludeman MJ, Coughlin SR. PAR3 is a cofactor for PAR4 activation by thrombin. Nature. 2000;404:609–613. doi: 10.1038/35007085. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle, re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Rev. 2000;34:1–26. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- Schmidlin F, Bunnett NW. Protease-activated receptors, how proteases signal to cells. Curr Opin Pharmacol. 2001;1:575–582. doi: 10.1016/s1471-4892(01)00099-6. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, Trevisani M, Hollenberg MD, Wallace JL, Caughey GH, Mitchell SE, Williams LM, Geppetti P, Mayer EA, Bunnett NW. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- Szurszewski JH, Ermilov LG, Miller SM. Prevertebral ganglia and intestinofugal afferent neurones. Gut. 2002;51(suppl. 1):i6–10. doi: 10.1136/gut.51.suppl_1.i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassicker BC, Hennig GW, Costa M, Brookes SJ. Rapid anterograde and retrograde tracing from mesenteric nerve trunks to the guinea-pig small intestine in vitro. Cell Tissue Res. 1999;295:437–452. doi: 10.1007/s004410051250. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, Bunnett NW, Sharkey KA, Brussee V, Compton SJ, Grady EF, Cirino G, Gerard N, Basbaum AI, Andrade-Gordon P, Hollenberg MD, Wallace JL. Proteinase-activated receptor-2 and hyperalgesia, a novel pain pathway. Nat Med. 2001a;7:821–826. doi: 10.1038/89945. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, Macnaughton WK, Al-Ani B, Saifeddine M, Wallace JL, Hollenberg MD. Proteinase-activated receptor 2 (PAR2)-activating peptides, identification of a receptor distinct from PAR2 that regulates intestinal transport. Proc Natl Acad Sci U S A. 1998;95:7766–7771. doi: 10.1073/pnas.95.13.7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnolle N, Wallace JL, Bunnett NW, Hollenberg MD. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol Sci. 2001b;22:146–152. doi: 10.1016/s0165-6147(00)01634-5. [DOI] [PubMed] [Google Scholar]
- Williams RM, Berthoud HR, Stead RH. Vagal afferent nerve fibres contact mast cells in rat small intestinal mucosa. Neuroimmunomodulation. 1997;4:266–270. doi: 10.1159/000097346. [DOI] [PubMed] [Google Scholar]


