Abstract
Previous studies have demonstrated that group II muscle afferents exert powerful actions on contralateral motoneurones and that these actions are mediated primarily via lamina VIII commissural interneurones. We examined whether dorsal horn interneurones also contribute to these actions, as they have been shown to contribute to the actions of group II afferents on ipsilateral motoneurones. We tested the susceptibility of IPSPs and EPSPs evoked from group II afferents in contralateral motoneurones to presynaptic inhibition as an indicator of the relative contribution of dorsal horn interneurones to these PSPs, since the monosynaptic activation of dorsal horn interneurones is more weakly and more briefly depressed by presynaptic inhibition than is the monosynaptic activation of lamina VIII and other intermediate zone and ventral horn interneurones. While the earliest components of IPSPs and EPSPs evoked by group II afferents were abolished by conditioning stimulation of group II afferents, consistent with them being evoked disynaptically by commissural interneurones, trisynaptic components of these PSPs were only partly reduced and are therefore attributed to dorsal horn interneurones. The same conditioning stimuli depressed the disynaptic excitation of lamina VIII commissural interneurones by group II afferents much less effectively than they depressed monosynaptic excitation, indicating that dorsal horn interneurones contribute to this disynaptic excitation. On the basis of these observations we conclude that that dorsal horn interneurones contribute to the late actions of group II muscle afferents on contralateral motoneurones through their disynaptic actions on commissural interneurones.
Activation of group II muscle afferent fibres may result in two kinds of responses in motoneurones innervating contralateral limb muscles. Depending on the experimental situation it may be followed by either crossed inhibition or crossed excitation and hence contribute to quite different movement synergies (Sherrington, 1906; Aggelopoulos & Edgley, 1995; Aggelopoulos et al. 1996). Classically, the most potent actions of group II muscle afferents result in ipsilateral flexion reflexes which are associated with crossed extension. However, with the spinal cord intact and under general anaesthesia, stimulation of group II afferents produces a widespread inhibition, rather than excitation of contralateral extensor motoneurones. The inhibition has a short central latency and is evoked principally by relays in the middle lumbar segments of the spinal cord (Arya et al. 1991; Aggelopoulos & Edgley, 1995). Since the latency of IPSPs evoked in contralateral motoneurones is only marginally longer than the latency of disynaptic actions of group II afferents on ipsilateral motoneurones, and must involve axons that cross the cord, it was proposed that disynaptic pathways, i.e. single commissural interneurones, mediate these IPSPs (see Arya et al. 1991). The crossed inhibition of extensors disappears after a transverse spinal transection in the thoracic cord, being replaced by excitation, but reappears after systemic administration of a selective 5HT1A and 5HT7 receptor agonist (Aggelopoulos & Edgley, 1995). The selection of either the crossed excitatory or crossed inhibitory alternative pathways from group II afferents may thus critically depend on neurones which are under modulatory serotoninergic control. Two distinct populations of group II-activated interneurones of this type have been described (see Edgley & Jankowska, 1987b). One of these populations includes interneurones located in the dorsal horn, that do not project to motor nuclei and the responses of which are consistently depressed by 5HT. Interneurones of the other population are located in the intermediate zone or the ventral horn (including lamina VIII) and interneurones at these locations may be either depressed or facilitated by 5HT (Jankowska et al. 2000). Some of the intermediate zone and ventral horn interneurones with monosynaptic input from group II afferent fibres project to the ipsilateral motor nuclei (Cavallari et al. 1987; Edgley & Jankowska, 1987b) and other lamina VIII commissural interneurones with group II input (Jankowska & Noga, 1990) project to contralateral motoneurones (for references see Discussion). Modulatory actions of 5HT upon commissural interneurones would thus be expected to be particularly important for switching between the crossed excitatory and inhibitory reflex actions of group II afferents. However, commissural interneurones, like other intermediate zone and ventral horn neurones, may be excited by group II afferents both monosynaptically and disynaptically (Jankowska et al. 2002b), the disynaptic input via other interneurones making their activation more secure. Activation of commissural interneurones may also be weakened by the actions of other inhibitory interneurones with group II input. Modulatory 5HT actions upon the interneurones that provide excitatory and/or inhibitory input to commissural interneurones may thus be as important for the selection of group II actions as are the actions of 5HT upon the commissural interneurones themselves. Nevertheless, it remained an open question which of the several known subpopulations of interneurones with group II input contribute to the disynaptic excitation and inhibition of commissural interneurones. We addressed this question, considering specifically the involvement of dorsal horn interneurones. There is evidence that these interneurones provide an additional input from group II afferents to the intermediate zone interneurones which project to ipsilateral motoneurones, as indicated in Fig. 1A (Jankowska et al. 2002b). Our hypothesis was that they have similar actions on lamina VIII commissural interneurones affecting contralateral motoneurones, as indicated in Fig. 1B. We investigated this possibility by comparing the effects of presynaptic inhibition on disynaptic and trisynaptic components of postsynaptic potentials evoked in contralateral motoneurones and on monosynaptic and disynaptic input from group II afferents to commissural interneurones.
Figure 1. Relationships between dorsal horn and commissural lamina VIII interneurones.
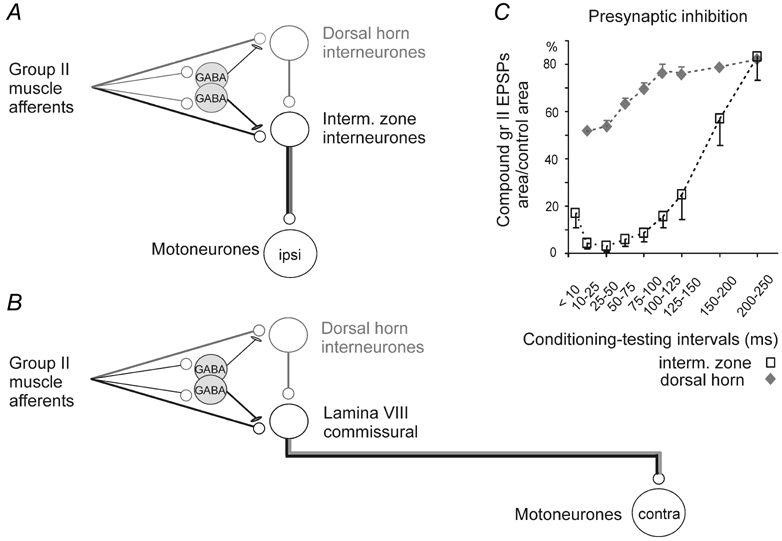
A, the neuronal network underlying the actions of group II afferents on ipsilateral motoneurones (from Jankowska et al. 2002b). Pathways between group II afferents and ipsilateral motoneurones run either directly through intermediate zone neurones (pathway shown in black) or indirectly through dorsal horn neurones (pathway shown in grey). B, the hypothesised connections between the dorsal horn interneurones and lamina VIII commissural interneurones, following the same scheme as in A. C, diagram showing stronger and longer lasting presynaptic inhibition of transmission from group II afferents to neurones in the intermediate zone than to neurones in the dorsal horn, as estimated from changes in monosynaptic components of group II evoked field potentials at the two locations (from Jankowska et al. 2002a). Note that the dorsal horn field potentials recovered to > 50 % of control within 50 ms whereas the intermediate zone field potentials remained < 20 % of control for more than 100 ms.
The rationale for using presynaptic inhibition for this purpose was based on previous findings, summarised in Fig. 1C, that presynaptic inhibition induced by conditioning stimulation of group II afferents may prevent the monosynaptic activation of intermediate zone interneurones by subsequently stimulated group II afferents, by abolishing input from these afferents over a period of ≈30–50 ms after the conditioning stimulus (open symbols). In contrast, the group II input to dorsal horn neurones is only partially reduced at these intervals (filled symbols), which might allow activation of a considerable proportion of these neurones, as subsequently demonstrated (see Jankowska et al. 2002b). In addition, the depression of group II input to intermediate zone interneurones persists for up to 100 ms whereas the activation of dorsal horn interneurones recovers earlier (Jankowska et al. 2002a). The synaptic actions of group II afferents seen 50–100 ms after a train of conditioning stimuli are thus much more likely due to actions of dorsal horn interneurones, by themselves or via intermediate zone/ventral horn interneurones, than to non-assisted monosynaptic actions of the latter.
METHODS
Preparation
The results were obtained during experiments on eight deeply anaesthetised adult cats. Anaesthesia was induced with sodium pentobarbital (40 mg kg−1i.p.) and maintained with several doses of α-chloralose (3–5 mg kg−1 h−1i.v. up to 50 mg kg−1 Rhone Poulenc Santé, France). During recording neuromuscular transmission was blocked by pancuronium bromide (Pavulon, Organon about 0.2 mg kg−1 h−1i.v.) and the animals were artificially ventilated. Blood pressure and heart rate were continuously monitored and if they changed or if the pupils began to dilate in response to noxious stimuli, additional doses of anaesthetic were given. The mean blood pressure was kept at 100–130 mm Hg and the end-tidal concentration of CO2 at about 4 % by adjusting the parameters of artificial ventilation and the rate of a continuous infusion of a bicarbonate buffer solution with 5 % glucose (1–2 ml h−1 kg−1). The core body temperature was kept at about 38°C by servo-controlled infrared lamps. At the end of the experiment the animals were killed with an overdose of anaesthetic (until cardiac arrest). All of the experimental procedures were approved by Göteborg ethics committee and followed NIH and EU guidelines for animal care.
During the preliminary dissection the third to seventh lumbar segments of the spinal cord (L3-L7) were exposed by laminectomy and a number of hindlimb peripheral nerves were dissected and transected. The nerves were mounted on stimulating electrodes: subcutaneous cuff electrodes (for the quadriceps, Q, sartorius, Sart, and saphenus, Saph, nerves, which were taken on both sides) or pairs of silver hook electrodes in a paraffin oil pool (for the posterior biceps and semitendinosus, PBST, anterior biceps and semimembranosus, ABSM, sural, Sur, gastrocnemius and soleus, GS (also taken on both sides), anterior tibial and extensor digitorum longus nerves jointly referred to as deep peroneal, DP, and cutaneous branches of the superficial peroneal, SP, nerve). Branches of the femoral nerve (Q, Sart and Saph nerves) and the GS from the right hindlimb were also prepared for stimulation.
Recording and stimulation
Intracellular records from motoneurones were made using microelectrodes filled with 2 m potassium citrate solution (tip about 2 μm, resistance about 4 MΩ). Extracellular and/or intracellular records from commissural neurones were made with similar glass microelectrodes (tip diameter about 1.5–2 μm) filled with a 2 % rhodamine dextran and neurobiotin in 0.9 % NaCl solution, since the successfully penetrated neurones were labelled for a subsequent analysis of their morphology and immunocytochemistry (Bannatyne et al. 2003a). Simultaneous with the unit recordings, records of afferent volleys from the surface of the spinal cord were made using one silver electrode in contact with the dura and another in a back muscle. Both single sweep records and averages of 10–20 potentials were recorded on-line for subsequent comparison of test and conditioned potentials. For further details see Results and Jankowska et al. (2002a).
Peripheral nerves were stimulated with rectangular current pulses (0.1 ms duration, 3–5 times threshold, T, for the most sensitive fibres in a nerve). Single or double (5 ms apart) stimuli were used as test stimuli and trains of 3–5 stimuli (3.3 ms apart, 4T or 5T) were used as conditioning stimuli to induce presynaptic inhibition. For all neurones stimuli of 1.8–2T were used to assess any effects of group I afferents. Conditioning stimulation of group I afferents alone (< 1.8T) was ineffective in all cases. The conditioning stimuli preceded the test stimuli at intervals between 40 and 100 ms. Q or Sart group II afferents were used as the source of presynaptic inhibition. Axons of ascending tract fibres were activated with two pairs of silver ball electrodes in contact with the dura on both sides of the spinal cord at the Th 12–13 level. This allowed the interneurones (not activated by such stimuli) and ascending tract neurones (antidromically activated) to be distinguished. Commissural neurones were recorded from the left side of the spinal cord and were identified by antidromic activation by stimuli (up to 50 μA) delivered through a varnished tungsten electrode inserted into the contralateral (right) GS motor nuclei in the L7 segment.
Sampling
Motoneurones used for the analysis included any in which relatively short latency (3–4.8 ms) IPSPs or EPSPs with a sharp onset and steep rising phase (Arya et al. 1991) were evoked by stimulation of group II afferents from the contralateral hindlimb. The sample included 18 motoneurones in which IPSPs and/or EPSPs were evoked from Q group II afferents. The sample of commissural interneurones consisted of neurones located in the L4 or L5 segments which were antidromically activated from the contralateral GS motor nucleus. In all cases, collisions between short latency spikes evoked from the motor nucleus (0.8–1.2 ms; distance 25–30 mm) and synaptically induced responses were used to verify that the activation was indeed antidromic. All of the interneurones were located within the areas where distinct ventral horn field potentials were evoked from group II afferents, but not by group I afferents (Edgley & Jankowska, 1987a), at depths corresponding to lamina VIII. All of these interneurones were monosynaptically excited by group II afferents from Q or Sart nerves (at a stimulus intensity of 5T) but were not activated by stimuli that activated group I afferents alone (1.8–2T).
Analysis
The amplitudes of EPSPs and IPSPs were quantified by measuring the areas of their rising phases (the initial 2.5 ms). Grouped data are presented as means and standard errors (s.e.m.) of the mean areas. Student's paired and unpaired t tests were used for evaluating statistical significance of the observed differences. For extracellularly recorded spikes from interneurones, responses were quantified using peristimulus time histograms (PSTHs), which were constructed from the responses to series of stimuli (usually 20), alternating series of conditioned and unconditioned stimuli.
RESULTS
The results are in two sections. In the first we report observations based on recordings from motoneurones located contralateral to the group II afferents that were stimulated. In the second part we report observations based on recordings from commissural interneurones, located on the same side of the spinal cord as the group II afferents that were stimulated. These commissural neurones had axons that crossed the midline and descended to the contralateral motornuclei (Fig. 1B).
Effects of presynaptic inhibition on IPSPs evoked in contralateral motoneurones by group II afferents
On the basis of previous studies (see Jankowska et al. 2002a and Discussion) effects of conditioning stimulation of group II afferents will be considered primarily to be due to presynaptic inhibition and will be referred to as such. These effects were examined in 15 contralateral motoneurones in which IPSPs were evoked from group II afferents at segmental latencies of 3.0–4.5 ms. The sample included two Q, one Sart, nine GS, and three PBST motoneurones recorded over five experiments. All of the IPSPs evoked either by single or by the first of two test stimuli near-maximal for group II afferents (5T) were strongly depressed when preceded by a short train of conditioning stimuli. Figure 2 illustrates this. The early components of the IPSPs (within 2–2.5 ms from the onset) were abolished by the conditioning stimuli. Depending on the intervals between the conditioning and test stimuli the later components of the IPSPs were either abolished (as in Fig. 2C and D) or were considerably depressed (as in Fig. 2A and B).
Figure 2. Effects of conditioning stimulation of contralateral group II afferents on short latency IPSPs evoked from contralateral group II afferents in motoneurones.
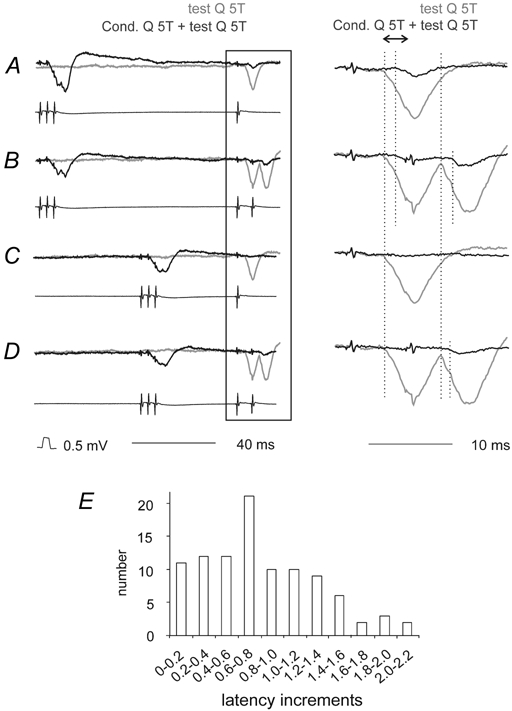
A and B, IPSPs evoked in a GS motoneurone by single and double stimuli to contralateral group II afferents. Upper records are intracellular potentials; the lower parallel records show afferent volleys from the cord dorsum. Control (unconditioned) responses are shown in grey and conditioned responses in black. The conditioning–testing interval was 94 ms. The panels on the right show expanded records of the responses to the test stimuli (from the parts of the left hand records within the box). C and D, as A and B but with a conditioning–testing interval of 45 ms. For all traces the vertical dotted lines indicate the onsets of the conditioned and unconditioned IPSPs. The double arrow above the right panel in A shows the time window within which the areas of the IPSPs were measured. F, increments in the latency of IPSPs evoked by conditioning stimulation of Q in all cases in which IPSPs with a distinct onset were evoked (n = 101). In this and the following figures, the negativity is downwards in the microelectrode records and upwards in the records from the cord dorsum.
When both test and conditioning stimuli were applied to Q, the depression of the early components of the IPSPs evoked in the whole sample of contralateral motoneurones at 40–50 ms conditioning-testing intervals was to 18 % of the control IPSP size (range 0–63 %, n = 9, statistically significant at P < 0.001, paired t test). At 80–100 ms conditioning-test intervals the depression was somewhat weaker, to 27 % of the control size (range 0–72 %, n = 14, statistically significant at P < 0.001, paired t test). When conditioning stimuli were applied to Sart the depression was weaker than that evoked from Q, to 69 % for intervals of 80–100 ms (range 32–88 %, n = 4), but still statistically significant (paired t test, P < 0.05). In contrast, no significant depression of these crossed IPSPs followed conditioning stimulation of the contralateral Q (i.e. Q on the same side as the motoneurones) in keeping with the reported weak crossed presynaptic inhibition of group II afferents (Enríquez-Denton et al. 2000).
In addition to the reductions in amplitude, IPSPs evoked following conditioning stimuli had longer latencies. This is again illustrated in Fig. 2 by the dotted lines, and also by the first pair of dotted lines in Fig. 3B. The distribution of increments in latency is shown in Fig. 2E. Following conditioning stimulation of Q the mean increment was 0.83 ± 0.05 ms (range: 0–2.4 ms, n = 101; pooled data for effects evoked with various conditioning parameters). The most frequently seen increments (0.6–0.8 ms) would correspond with one additional synaptic delay. Changes in the latencies of IPSPs following conditioning stimuli are thus consistent with the possibility that the earliest parts of the IPSPs were evoked by direct (monosynaptic) activation of lamina VIII commissural interneurones by group II afferents, which are under strong presynaptic inhibitory control, while the somewhat less powerfully depressed longer latency components were due to disynaptic activation of the same interneurones (as outlined in Fig. 1B) via dorsal horn interneurones which are more resistant to presynaptic inhibition.
Figure 3. Effects of conditioning stimulation of contralateral group II afferents on EPSPs evoked from contralateral group II afferents in motoneurones.
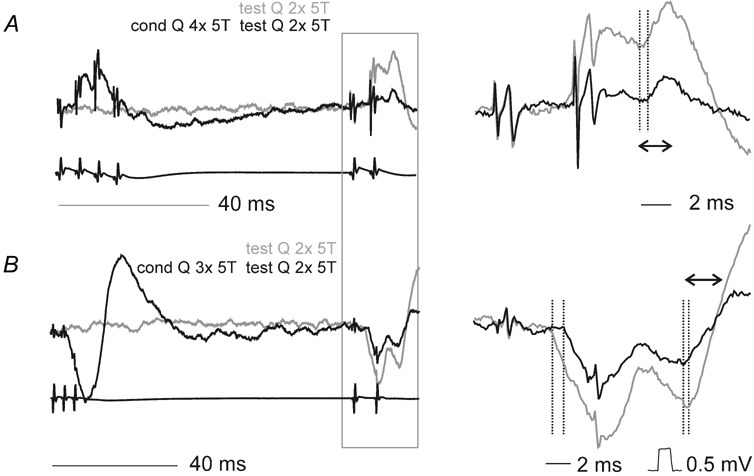
A and B, records from Q and PBST motoneurones respectively (upper traces) and cord dorsum potentials (lower traces). Unconditioned responses are shown in grey and conditioned (cond) responses in black. The panels on the left show records at a slow timebase, the panels to the right show the responses to the test stimuli (from the box in the lefthand panels) expanded (twice vertically four times horizontally). Dotted lines in the panels on the right indicate the onset of the unconditioned and conditioned PSPs. The double arrows in the right panels show the time windows within which the areas of the PSPs were compared.
In order to obtain further support for this possibility, we used temporal facilitation. To achieve this, the stimulus parameters were adjusted so that the conditioning stimuli practically abolished IPSPs evoked by single test stimuli, for example by reducing the test stimulus intensity from 5T to 4T or by increasing the number of conditioning stimuli from three to four. If dorsal horn interneurones have excitatory actions on lamina VIII commissural interneurones they should reach threshold for generating spike potentials in commissural interneurones (and for subsequent IPSPs in contralateral motoneurones) when these actions were strengthened by temporal facilitation, i.e. when paired test stimuli were used instead of single stimuli. The effects of single and paired test stimuli can be compared in Fig. 2C and D. After conditioning stimulation a single test stimulus did not evoke an IPSP (Fig. 2C), whereas the second of a pair second test stimulus did, but at longer latency than the unconditioned IPSP (Fig. 2D, black and grey traces, respectively). When small IPSPs were present after the first stimulus (as in Fig. 2B) the amplitude of these IPSPs increased after the second stimulus. In 10 contralateral motoneurones in which the comparison was made, when IPSPs following the first stimulus were depressed to on average 3 % or 4 % of the control (after 40–50 and 80–100 conditioning testing intervals respectively), those following a second stimulus were depressed to only 45 % or 40 % of control. The latencies of IPSPs induced under these conditions exceeded the latencies of unconditioned IPSPs by a mean of 0.47 ± 0.5 ms (±s.e.m.; n = 101), as indicated by the dotted lines in the panels on the right of Fig. 2.
Effects of presynaptic inhibition on EPSPs evoked by group II afferents in contralateral motoneurones
In three of the 18 contralateral motoneurones that were tested the principal responses to stimulation of group II afferents were EPSPs (exemplified in Fig. 3A; grey traces). These were evoked at segmental latencies of 2.3, 3.6 and 4.4 ms, which overlapped with the latencies of the IPSPs described in the section above, and might therefore have been mediated in a similar way. At conditioning-testing intervals of 60–100 ms the areas of the early components of these EPSPs (time windows of 2 ms following the onset) were depressed to 0, 20 and 12 % of control values and the latencies were increased by 0.6–1.3 ms. In nine additional contralateral motoneurones, EPSPs were superimposed on shorter latency IPSPs, as illustrated in Fig. 3B. The latencies of these EPSPs could not be measured with confidence but the areas of conditioned and unconditioned responses could be compared within an arbitrary time window (beginning at the points where the records crossed the base line, as indicated by double headed arrows in Fig. 3B). After conditioning stimulation these areas were reduced to 30 ± 4 % of the control values. Note that this decrease in EPSP amplitude occurs despite the preceding IPSP also being depressed in amplitude, so any EPSP shunting associated with the IPSP should also be reduced. The effects of the presynaptic inhibition appear thus to be similar on crossed EPSPs and crossed IPSPs.
Evidence for disynaptically evoked excitation of commissural neurones by group II afferents
Previous observations on lamina VIII commissural neurones have focused on the obvious monosynaptic excitation in response to stimulation of group II afferents (Jankowska & Noga, 1990). However, closer inspection of the input to these neurones reveals additional disynaptic excitation from group II afferents. The disynaptic excitation is indicated either by the presence of components appearing at longer latencies, or by later spikes which become particularly distinct following the second or third stimulus of a train. For instance, in two commissural neurones illustrated in Fig. 4 (A and D) the first stimulus evoked early monosynaptic EPSPs, but subsequent stimuli evoked additional later EPSPs. In both A and D the first dotted line indicates the monosynaptic EPSP evoked by the second stimulus and the second dotted line shows the onset of a later component. Both the temporal facilitation of these later components after successive stimuli and the longer latencies indicate their mediation via additional interneurones; the differences in latencies of the monosynaptic and later components (about 1.3 and 1 ms in A and D, respectively), leaving time for one (or maximally two) additional central relays. Similar time differences were found between early and late components of EPSPs evoked in all three intracellularly recorded commissural interneurones and in the extracellularly recorded spike potentials of nine neurones.
Figure 4. Examples of early and late excitation of commissural neurones by group II afferents, attributable to monosynaptic and disynaptic actions.
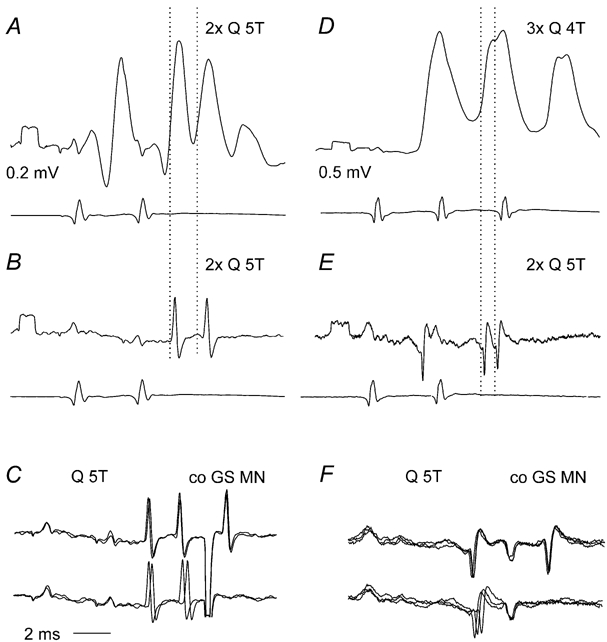
A–C and D–F, illustrate records from two different commissural neurones respectively. A and D show EPSPs evoked by two and three stimuli applied to the quadriceps nerve (Q) (upper traces) and cord dorsum potentials (lower traces). B and E show extracellular spikes, recorded just before penetration of the neurones, with cord dorsum potentials. Note the likely disynaptically induced EPSPs (the second of the dotted lines) following the monosynaptically evoked ones (first dotted lines) after the second stimulus. C and F, antidromic activation from the contralateral GS motor nucleus (co GS MN) and collision between synaptically and antidromically induced extracellular spikes confirming antidromic activation; two and three superimposed records. Voltage calibrations are 0.2 mV for A and B and 0.5 mV for D and E.
Presynaptic inhibition of activation of commissural interneurones by group II afferents
Effects of presynaptic inhibition were tested on 10 commissural interneurones (either extracellularly or intracellularly recorded) from five experiments. The main purpose was to examine activation of these neurones after conditioning stimuli delivered 50–100 ms earlier, at a time when monosynaptic activation of intermediate zone and ventral horn neurones is still abolished by presynaptic inhibition, while activation of dorsal horn neurones has already recovered from its effects (see Fig. 1C).
Figure 5 shows that the same conditioning stimuli that depressed EPSPs and IPSPs of group II origin in contralateral motoneurones potently depressed the responses of a commissural interneurone. In the control situation two stimuli to Q at 4T both evoked discharges on most occasions seen in the unit records (A), and in the PSTH (D). Following conditioning stimulation (five stimuli, 5T) to Q (B) or to Sart (C) the neurone responded mainly to the second test stimulus. When Q was the conditioning stimulus the first test stimulus was ineffective, and the second test stimulus evoked fewer spikes and at longer latencies than the unconditioned case (dotted lines after the second test stimulus in E). After conditioning stimuli applied to Sart (F) the first test stimulus evoked a few spikes at longer latency than those evoked by unconditioned test stimuli (onsets marked by the dotted lines after the first test stimulus in F).
Figure 5. Depression of extracellularly recorded spike potentials and intracellularly recorded EPSPs in a commissural interneurone by conditioning stimulation.
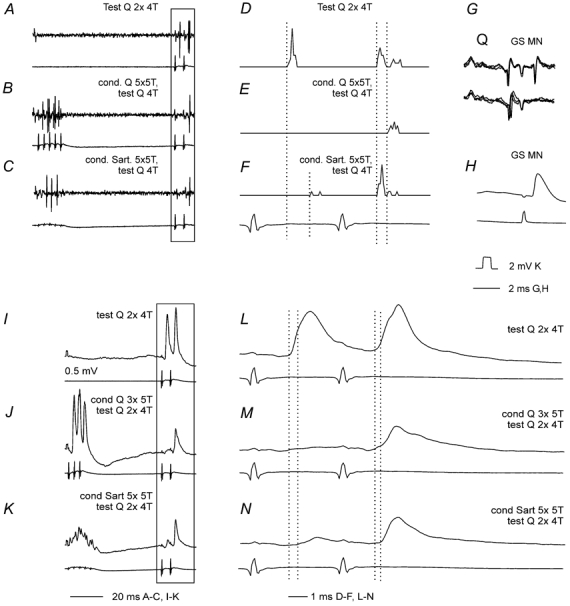
A–C, extracellularly recorded spike potentials evoked by stimulation of group II afferents in Q and the corresponding cord dorsum records. These were preceded by conditioning simulation of group II afferents (5 stimuli, 5T) to Q (B) or Sart (C), conditioning-testing interval of 80 ms. D-F, peristimulus time histograms (PSTH) of responses evoked by 20 consecutive test stimuli, including those in the areas marked by the box in A–C, unconditioned (D) and conditioned by stimulation of Q (E) or Sart (F). G, responses to stimuli applied in the contralateral GS motor nucleus (GS MN, upper trace) and collision by synaptically evoked spike potentials (lower trace) confirming antidromic activation. H, blocked antidromically evoked spike potential after penetration of the neurone. I–K, EPSPs evoked by Q group II afferents after penetration of the neurone, unconditioned (I), conditioned by Q group II afferents (3 stimuli, 5T) in J, and conditioned by sart; group II afferents (5 stimuli 5T, K). Conditioning-testing intervals were 56 ms. L–N show the responses to test stimuli of the records in I–K (in the box) on an expanded timebase. The first and second dotted lines in D–F and L–N indicate the latencies of the unconditioned spikes and EPSPs and of those induced after conditioning stimuli, respectively.
In the whole sample of neurones tested, the number of spikes evoked by a series of 20 single stimuli at 50–80 ms conditioning-testing intervals was reduced from 15.4 ± 0.8 (mean and s.e.m.) to 2.0 ± 0.64 (i.e. to 13 % of control, data from 33 tests involving various combinations of stimuli). The depression was from 15.85 ± 1.18 to 1.76 ± 0.87 (to 11 % of control in 15 tests) by conditioning stimuli which were applied to the same nerve as the test stimuli (homogenetic presynaptic inhibition) and from 15.06 ± 1.05 to 2.91 ± 1.11 (to 19 % of control in 18 tests) by stimulation of another nerve (heterogenetic presynaptic inhibition). The differences between the numbers of responses preceded and not preceded by conditioning stimuli were in both these cases highly statistically significant at P < 0.0001 (Student's paired t test). However there were no statistically significant differences between effects of the homogenetic and heterogenetic presynaptic inhibition (Student's unpaired t test).
All responses seen following conditioning stimuli appeared at longer latencies than responses to unconditioned stimuli. These latencies were increased from a mean of 3.7 ± 0.5 to 5.5 ± 0.4 ms (i.e. by 2.1 ± 0.6 ms) by homogenetic presynaptic inhibition and from 3.6 ± 0.60 to 6.2 ± 0.1 ms (i.e. by 2.5 ± 0.6 ms) by heterogenetic presynaptic inhibition. In both cases the increases were statistically significant (P = 0.015 and P < 0.001, respectively, Student's paired t test). Both homogenetic and heterogenetic presynaptic inhibition were stronger when evoked by conditioning stimulation of Q (to 5 % and 4 % in the two cases) than by conditioning stimulation of Sart (to 17 % and 31 %) but no statistically significant differences were found between effects of these nerves when their stimulation preceded test stimuli applied to the same or to another nerve.
The specificity of the depression could be examined in one neurone in which short latency responses were evoked not only from group II afferents but also by stimuli applied within the reticular formation. In this case the early responses evoked from group II afferents were depressed by preceding conditioning stimulation of group II afferents, while monosynaptically evoked responses from the reticular formation were only slightly reduced by even by stronger conditioning stimuli (Fig. 6). This differential effect of the conditioning stimuli supports the conclusion (see Discussion) that the depression of responses evoked by conditioning stimulation of group II afferents was evoked by presynaptic inhibition of transmission from these afferents rather via postsynaptic inhibition of the commissural neurones.
Figure 6. Differential effects of conditioning stimulation on activation of a commissural interneurone by group II and reticulospinal afferents.
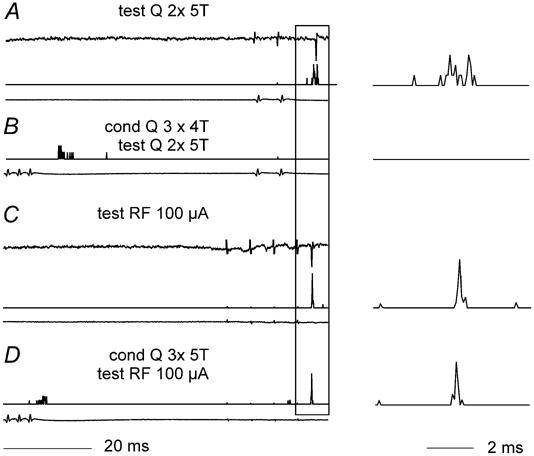
A shows three traces, from top to bottom, extracellular records from an interneurone discharged by two test stimuli to Q at 5T, the PSTH derived from a series of 20 such stimuli and the cord dorsum volleys. B, PSTH and volleys traces as in A, showing that the same test stimuli are ineffective when preceded by conditioning stimulation (Q, 4 stimuli, 4T, 56 ms interval). C, traces as in A showing that the same interneurone was discharged on stimulation of reticulospinal fibres (RF, 4 stimuli 100 μA). D shows that the reticulospinal response was not substantially altered by the same conditioning stimuli as in B. Only the PSTH and the volleys are shown. On the right the PSTH records of responses to the test stimuli from A–D are shown on an expanded timebase to facilitate comparison.
Intracellular records from commissural interneurones that were sufficiently stable to allow analysis of presynaptic inhibition on postsynaptic potentials evoked from group II afferents could be obtained in only three interneurones. In two of the cells group II afferents evoked EPSPs at segmental latencies of 2.3 and 2.9 ms with respect to the onset of the Q group I afferent volley. Previous analysis of the timing of the volleys in slower conducting group II afferents relative to the group I volleys of the Q nerve in the midlumbar segments suggests that the earliest components of both of these can be attributed to monosynaptic connections (Edgley & Jankowska, 1987b). When conditioning stimuli preceded the test stimuli, the earliest components of the EPSPs were abolished (Fig. 5M and N; Fig. 7B and D). The remaining components had onsets with latencies about 0.5 ms (Fig. 5) and 0.9 ms (Fig. 7) longer than the original EPSPs, compatible with an additional synaptic delay. The effects of conditioning stimuli on EPSPs evoked by a second test stimulus was dependant on the stimulus parameters; the amplitudes of earliest components of these EPSPs could be depressed as effectively as were those of the first EPSPs (Fig. 5N and Fig. 7D) or could be less somewhat weaker (Fig. 5F).
Figure 7. Differential effects of presynaptic inhibition on early and later components of EPSPs evoked from group II afferents in a commissural interneurone.
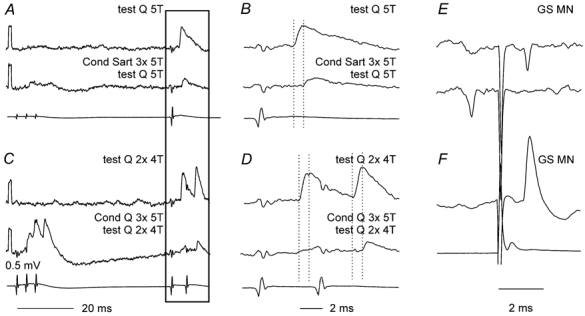
A, control EPSP (upper trace) evoked by near maximal stimulation of Q group II afferents, which shows a decrease in amplitude and increase in latency following heterogenetic conditioning stimuli (Sart, second trace, conditioning–testing interval of 56 ms). B, horizontally expanded parts of records in boxed areas in A. C, as A but for homogenetic depression (Q) and for EPSPs evoked by double instead of single test stimuli. D, expanded parts of records in C. Dotted lines in the expanded records indicate the onset of the control and conditioned EPSPs. E, extracellular records of antidromically evoked spike potentials in the interneurone and collision by synaptically evoked spikes. F, intracellular record of the blocked antidromic spike. Bottom traces in A–D and F are from the cord dorsum. Negativity is downwards in the microelectrode records and upwards in the records from the cord dorsum. Voltage calibration in C is for all the microelectrode records.
Early components of the IPSPs evoked by group II afferents in the other commissural neurone, were also preferentially depressed by the conditioning stimulation. The IPSPs were evoked at segmental latencies of 3.7–4.2 ms, which were 1–1.5 ms longer than the latencies of EPSPs attributable to monosynaptic actions of group II afferents in other neurones of the same region (see Edgley & Jankowska, 1987b). The earliest components of these IPSP were thus most likely to have been evoked disynaptically while the later components can be attributed to tri- or polysynaptic actions. As shown in Fig. 8B, C, E, F and G, the early components of these IPSPs (reversed in the intracellular recording) were either abolished or decreased following conditioning stimulation of group II afferents, leading to the conclusion that they were probably mediated by intermediate zone interneurones. In contrast, the later components of these IPSPs were more resistant to the presynaptic inhibition but were affected when the number of conditioning stimuli was increased (Fig. 8C and G). The later components may therefore be attributed to actions of dorsal horn neurones, possibly via the same intermediate zone interneurones that mediated the disynaptic inhibition. The records in Fig. 8 also illustrate the dependence of the depression on conditionin- testing intervals since conditioning stimuli that abolished the early and late IPSPs at a conditioning-test interval of 53 ms (Fig. 8C) were much less effective when the interval was 96 ms (Fig. 8E) and a similar sized effect required twice as many conditioning stimuli (Fig. 8G).
Figure 8. Differential effects of presynaptic inhibition on early and later components of IPSPs evoked from group II afferents in a commissural interneurone.
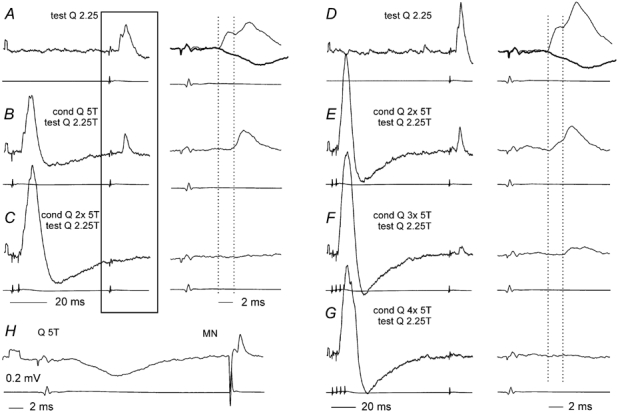
Records A–G were taken after the neurone was filled with an intracellular marker during which the IPSP reversed. The IPSP recorded soon after the penetration of the neurone (segmental latency 3.5–4 ms) and an antidromic spike evoked from the contralateral GS motor nucleus (latency 1 ms), are shown in H. This hyperpolarizing IPSP is superimposed (grey trace) on the reversed IPSPs in the expanded records in A and D. A, a control reversed IPSP evoked by a near threshold stimulation of group II afferents on a slow timebase in the left panel and an the expanded timebase in the right panel. B and C, reduction in the amplitude (B), and disappearance (C) of this IPSP following single and double conditioning stimuli (conditioning–testing interval 53 ms). The mean depression within a time window of 2 ms from the onset of the PSP was 4 % and 3 %. D, a control IPSP evoked at the same stimulus intensity (but larger because of the increasing depolarisation of the neurone), similarly at two timebases. E–G, increasing effects of an increasing number of conditioning stimuli (conditioning–testing interval 96 ms). The mean depression within 2 ms from the onset of the unconditioned PSP was to 6 %, 4 % and 0 %. Note that the depression of the early components was stronger than the effect on the later components, which began 0.8–2.5 ms after the onset of the control IPSPs. Bottom traces in each pair of records are from the cord dorsum. Voltage calibration in H applies to all of the microelectrode records. Note the different time scales for A–C and D–G.
DISCUSSION
Our results demonstrate that nerve impulses in group II afferent fibres strongly depress the synaptic actions of these afferent fibres on both lamina VIII commissural interneurones and contralateral motoneurones. Since the depression of the earliest actions of group II afferents is particularly strong and long lasting it is consistent with the depression of monosynaptic activation of the interneurones and of their direct actions upon contralateral motoneurones. The combination of delayed disynaptic activation of the commissural interneurones and similarly delayed excitation and inhibition of contralateral motoneurones that are seen under the same conditions are, on the other hand, compatible with disynaptic activation of lamina VIII interneurones via other neurones which are less affected by the conditioning stimuli.
Reasons for attributing the depressive effects of conditioning stimulation of group II afferents to presynaptic inhibition
Previous studies have demonstrated that short trains of stimuli applied to group II afferent fibres evoke very strong presynaptic inhibition of transmission from the same or other group II afferent fibres, in particular in the intermediate zone and in the ventral horn in the midlumbar segments (Riddell et al. 1995; Jankowska et al. 2002a). Presynaptic inhibition would therefore reduce the probability of activating lamina VIII commissural interneurones and hence weaken excitation and inhibition of contralateral motoneurones evoked through these interneurones. If some interneurones activated by the conditioning stimuli evoked a sufficiently powerful and long-lasting postsynaptic inhibition of commissural interneurones, the depression of activation of lamina VIII commissural interneurones might be secondary to this, as well as to presynaptic inhibition. However, a number of observations show that the effects of postsynaptic inhibition, if any, are much less significant than those of presynaptic inhibition. In particular, the early components of EPSPs and IPSPs evoked in either commissural interneurones or contralateral motoneurones are abolished not only at relatively short (40–50 ms) but also at fairly long (80–100 ms) intervals between the conditioning and testing stimuli: postsynaptic inhibition would be expected to decline within 50 ms and be negligible after 100 ms. In addition, the depressive effects of stimulation of group II afferents were seen when the conditioning stimuli induced postsynaptic inhibition of commissural interneurones (e.g. as illustrated in Fig. 7C and Fig. 8B and C) as well as when no postsynaptic effects were seen (e.g. as in Fig. 7A), and whether the test stimuli were applied at the end of the declining phase of the IPSPs (as in Fig. 7C and Fig. 8B and C) or a few tens of milliseconds later (as in Fig. 8F and G). Finally, the responsiveness of the neurone illustrated in Fig. 6 to reticular formation stimulation was only very slightly reduced by conditioning stimuli that abolished responses to group II stimulation. This is in keeping with the lack of modulation of the terminals of descending fibres by presynaptic inhibition (Rudomin et al. 1975)
Post-activation depression following conditioning stimulation of group II afferents might be another factor contributing to the depressive effects of conditioning stimulation. However, although post-activation depression might be as effective on group II afferents in the dorsal horn as it is on group I afferents in motor nuclei, its effects are much weaker on group II afferents in the intermediate zone (Hammar et al. 2002). The principal action of post-activation depression might thus be to decrease the probability of activation of dorsal horn interneurones, rather than to affect lamina VIII commissural interneurones. In addition, key evidence against post-activation depression playing a major role is the observation that conditioning stimuli to one nerve depressed effects evoked from another nerve (heterogenetic) as effectively as those evoked from the same nerve (homogenetic). Another relevant observation is that the degree of post-activation depression was similar during, just after and about 1 s after a train of conditioning stimuli (Fig. 6 in Hultborn et al. 1996), while the degree of the depression found in this study changed with relatively small changes in the conditioning-test intervals (Fig. 2), or when the numbers of conditioning stimuli were altered (Fig. 8). The depression we describe is thus more consistent with the effects of conditioning stimuli at an interneuronal level, rather than with post-activation depression.
Indications that lamina VIII commissural interneurones mediate both early and late actions of group II afferents upon contralateral motoneurones
On the basis of morphological studies several populations of commissural neurones could mediate the crossed actions of group II afferents in motoneurones. However, the disynaptic actions of these afferents (Arya et al. 1991) would be likely to be mediated only by lamina VIII commissural interneurones since these are the only interneurones which have been found to project to contralateral motor nuclei in adult cats (Harrison et al. 1986; Jankowska & Skoog, 1986; Alstermark & Kummel, 1990; Hoover & Durkovic, 1992). Tri- and polysynaptic actions could also be mediated by the same lamina VIII commissural interneurones, via axon collaterals given off in the contralateral grey matter outside motor nuclei (Scheibel & Scheibel, 1966; Matsuyama & Mori, 1998; Mori et al. 1998; Bannatyne et al. 2003 a,b) and via other premotor interneurones. However, contralateral premotor interneurones could also be excited by commissural interneurones located in the dorsal horn (Cajal, 1953; Scheibel & Scheibel, 1966; Eide et al. 1999; Stokke et al. 2002), since some dorsal horn interneurones with powerful input from group II afferents have been found to have crossed projections (Bras et al. 1989; Bannatyne et al. 2003a). If dorsal horn commissural interneurones are involved in mediating tri- and/or polysynaptic actions of group II afferent fibres to contralateral motoneurones independently of lamina VIII interneurones, i.e. only via contralaterally located premotor interneurones, then their effects would be depressed to a much lesser extent than the effects mediated by lamina VIII interneurones by presynaptic inhibition. Previous observations showed that a considerable proportion of dorsal horn interneurones may be activated by single, and all by double test stimuli at the ranges of conditioning-testing intervals used in this study (Jankowska et al. 2002b). Furthermore, presynaptic effects of these conditioning stimuli on contralateral premotor interneurones would be expected to be weak, if not negligible, taking into account that group II afferents evoke only very weak crossed presynaptic inhibition (Enríquez-Denton et al. 2000)while both the early and late components of EPSPs and IPSPs evoked by group II afferents fibres in contralateral motoneurones are very strongly depressed by conditioning stimuli. Major actions of dorsal horn commissural interneurones independent of lamina VIII commissural interneurones therefore appear to be unlikely and we propose that both the early and late crossed actions of group II afferents on contralateral motoneurones are mediated by lamina VIII commissural interneurones. The earliest of these actions would be mediated by monosynaptic activation of lamina VIII commissural interneurones by group II afferents. These would be followed, around 1 ms later, by actions that can be attributed to the activation of lamina VIII commissural interneurones by dorsal horn interneurones located on the same side. Less direct actions of lamina VIII commissural interneurones (via other premotor interneurones located at the same side as the motoneurones) might also follow.
Indications that disynaptic excitation of lamina VIII commissural interneurones is mediated via dorsal horn group II interneurones
Evidence for disynaptic excitation of lamina VIII commissural neurones is based on direct records from these interneurones. As shown in the Results (Fig. 4), group II muscle afferents may excite these interneurones disynaptically as well as monosynaptically. The pertinent question is to what extent the disynaptic actions of group II afferents are mediated by other intermediate zone or ventral horn interneurones, including lamina VIII interneurones, and to what extent by dorsal horn interneurones. Since intermediate zone and ventral horn interneurones are strongly affected by presynaptic inhibition at conditioning test intervals of up to 100 ms (Jankowska et al. 2002b) it is unlikely that they could be responsible for the disynaptic excitation, which remains when the monosynaptic excitation is depressed. In addition, although ipsilaterally projecting premotor neurones may have mutual interconnections (Edgley & Jankowska, 1987b), no evidence has been found for ipsilateral axon collaterals of lamina VIII commissural interneurones which would be necessary for such interactions (Bannatyne, 2003a). Disynaptic actions via other lamina VIII interneurones could therefore also be excluded, considering their morphology.
On the other hand, dorsal horn interneurones could contribute to the disynaptic excitation of lamina VIII commissural interneurones, since the disynaptic actions of group II afferents on lamina VIII commissural interneurones and the activation of dorsal horn interneurones are both depressed with a similar time course and to a similar extent by conditioning stimuli. The disynaptic excitation of commissural lamina VIII neurones therefore resembles the disynaptic excitation of ipsilaterally projecting intermediate zone interneurones activated by group II afferents (Jankowska et al. 2002b), and the arguments made in favour of dorsal horn neurones mediating the disynaptic excitation in the previous study would therefore apply to their actions on lamina VIII commissural interneurones. These include the demonstration that the conditioning stimuli applied 80–100 ms prior to test stimuli did not prevent monosynaptic activation of dorsal horn interneurones by group II afferents and that these neurones were activated at sufficiently shorter latencies. Taking into account the progressively longer conduction times of nerve impulses in thin axon collaterals of group II afferent fibres projecting into deeper parts of the ventral horn (Fu & Schomburg, 1974; Edgley & Jankowska, 1987a,b; Lundberg et al. 1987a) the differences between the segmental latencies of activation of dorsal horn interneurones (1.5–2.5 ms) and of disynaptic activation of lamina VIII commissural interneurones (3–4 ms) would leave sufficient time for a synaptic relay, even if the conduction times along the axons of dorsal horn interneurones descending to lamina VIII are longer than those to laminae VI-VII. Recent analysis of terminal axon collateral arbours of dorsal horn interneurones has also revealed that they extend sufficiently far ventrally to be able to mediate disynaptic excitation of lamina VIII commissural neurones (Bannatyne et al. 2003a).
How can presynaptic inhibition contribute to left–right limb coordination?
Our results show that presynaptic control of the crossed actions of group II afferents may be as effective as is the presynaptic control of ipsilateral actions. Presynaptic inhibition may thus be used to adjust the balance between the synaptic actions of group II afferents on both sides of the body and to select appropriate synergies of muscle contractions of the left and right extremities. Differential effects of presynaptic inhibition on different subpopulations of interneurones with group II input may be particularly useful in this respect, especially if different motor synergies were subserved by lamina VIII commissural neurones in pathways from group II afferents, by other commissural interneurones (e.g. those activated from the reticular formation, S. A. Edgley, I. Hammar & E. Jankowska, unpublished observations) and by various dorsal horn interneurones.
In the original description of crossed actions of group II afferents it was argued that the latency of the crossed IPSPs seen in the majority of motoneurones was consistent with disynaptic mediation via midlumbar commissural neurones, although the delays involved in conduction from dorsal root entry to midlumbar neurones and in conduction caudally to the motor nuclei complicate the estimate of total conduction delay (Arya et al. 1991). Our results indicate that the proposed disynaptic pathway via lamina VIII commissural interneurones operates in parallel with a trisynaptic pathway which uses the dorsal horn neurones as well as the commissural neurones. The use of such parallel pathways may greatly increase the probability of activation of commissural interneurones by doubling their synaptic input. They may increase the flexibility of activation of these neurones, since different combinations of afferents converge onto intermediate zone or ventral horn neurones and dorsal horn neurones. For example group II and cutaneous afferents from broad receptive fields would preferentially act through dorsal horn neurones, whereas a more restricted set of group II afferents and group I afferents may contribute to processing in intermediate zone and ventral horn subpopulations. It may also be particularly important for the selection of different motor synergies that activation of neurones in the intermediate zone/lamina VIII and in the dorsal horn may be independently modulated by monoamines. As shown previously, the activation of dorsal horn interneurones by group II afferents is depressed by serotonin whereas the activation of intermediate zone interneurones is depressed by noradrenaline (Bras et al. 1990; Jankowska et al. 2000). Our preliminary observations suggest that lamina VIII interneurones with group II input behave like the previously described ipsilaterally projecting intermediate zone interneurones.
Selection of specific subpopulations of lamina VIII and dorsal horn interneurones by their patterns of input, differential presynaptic inhibition in monosynaptic and disynaptic input pathways, and modulation by descending monoaminergic neurones could greatly increase the possibilities for selection of networks involved in different movements (Lundberg, 1975; Lundberg et al. 1987b). It may be still premature to attempt to explain how networks of neurones in pathways from group II afferents are used to switch between the two main synergies of activation of muscles on both sides of the body: ipsilateral flexion associated with crossed extension or bilateral flexion or extension. New information on the identity of the neurones that are involved and on the differential modulation of their activation nevertheless brings this explanation closer.
Acknowledgments
The study was supported by a grant from the National Institutes of Health (NS 40 863). We wish to thank Mrs Rauni Larsson for her invaluable assistance during the experiments.
REFERENCES
- Aggelopoulos NC, Burton MJ, Clarke RW, Edgley SA. Characterization of a descending system that enables crossed group II inhibitory reflex pathways in the cat spinal cord. J Neurosci. 1996;16:723–729. doi: 10.1523/JNEUROSCI.16-02-00723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggelopoulos NC, Edgley SA. Segmental localisation of the relays mediating crossed inhibition of hindlimb motoneurones from group II afferents in the anaesthetized cat spinal cord. Neurosci Lett. 1995;185:60–64. doi: 10.1016/0304-3940(94)11225-8. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Kummel H. Transneuronal transport of wheat germ agglutinin conjugated horseradish peroxidase into last order spinal interneurones projecting to acromio- and spinodeltoideus motoneurones in the cat. 1. Location of labelled interneurones and influence of synaptic activity on the transneuronal transport. Exp Brain Res. 1990;80:83–95. doi: 10.1007/BF00228850. [DOI] [PubMed] [Google Scholar]
- Arya T, Bajwa S, Edgley SA. Crossed reflex actions from group II muscle afferents in the lumbar spinal cord of the anaesthetized cat. J Physiol. 1991;444:117–131. doi: 10.1113/jphysiol.1991.sp018869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Networks of inhibitory and excitatory commissural interneurons mediating crossed reticulospinal actions identified by immunocytochemistry. Eur J Neurosci. 2003a doi: 10.1046/j.l460-9568.2003.02973.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Maxwell DJ, Edgley SA, Hammar I, Jankowska E. Commissural interneurons in cat spinal motor pathways: identification of excitatory and inhibitory cells. J Physiol. 2003b;548P:P116. [Google Scholar]
- Bras H, Cavallari P, Jankowska E, Kubin L. Morphology of midlumbar interneurones relaying information from group II muscle afferents in the cat spinal cord. J Comp Neurol. 1989;290:1–15. doi: 10.1002/cne.902900102. [DOI] [PubMed] [Google Scholar]
- Bras H, Jankowska E, Noga B, Skoog B. Comparison of effects of various types of NA and 5-HT agonists on transmission from group II muscle afferents in the cat. Eur J Neurosci. 1990;2:1029–1039. doi: 10.1111/j.1460-9568.1990.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Cavallari P, Edgley SA, Jankowska E. Post-synaptic actions of midlumbar interneurones on motoneurones of hind-limb muscles in the cat. J Physiol. 1987;389:675–689. doi: 10.1113/jphysiol.1987.sp016677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. J Physiol. 1987a;385:393–413. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. J Physiol. 1987b;389:647–674. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide AL, Glover J, Kjaerulff O, Kiehn O. Characterization of commissural interneurons in the lumbar region of the neonatal rat spinal cord. J Comp Neurol. 1999;403:332–345. [PubMed] [Google Scholar]
- Enríquez-Denton M, Scott DT, Riddell JS. Modulation of synaptic transmission from group II muscle afferents by crossed pathways in the spinal cord. Soc Neurosci Abstr. 2000;26:57–12. [Google Scholar]
- Fu TC, Schomburg ED. Electrophysiological investigation of the projection of secondary muscle spindle afferents in the cat spinal cord. Acta Physiol Scand. 1974;91:314–329. doi: 10.1111/j.1748-1716.1974.tb05687.x. [DOI] [PubMed] [Google Scholar]
- Hammar I, Slawinska U, Jankowska E. A comparison of post-activation depression of synaptic actions evoked by different afferents and at different locations in the feline spinal cord. Exp Brain Res. 2002;145:126–129. doi: 10.1007/s00221-002-1098-5. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Jankowska E, Zytnicki D. Lamina VIII interneurones interposed in crossed reflex pathways in the cat. J Physiol. 1986;371:147–166. doi: 10.1113/jphysiol.1986.sp015965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover JE, Durkovic RG. Retrograde labeling of lumbosacral interneurons following injections of red and green fluorescent microspheres into hindlimb motor nuclei of the cat. Somatosens Mot Res. 1992;9:211–226. doi: 10.3109/08990229209144772. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res. 1996;108:450–462. doi: 10.1007/BF00227268. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Chojnicka B, Heden CH. Effects of monoamines on interneurons in four spinal reflex pathways from group I and/or group II muscle afferents. Eur J Neurosci. 2000;12:701–714. doi: 10.1046/j.1460-9568.2000.00955.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Noga BR. Contralaterally projecting lamina VIII interneurones in middle lumbar segments in the cat. Brain Res. 1990;535:327–330. doi: 10.1016/0006-8993(90)91618-q. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Skoog B. Labelling of midlumbar neurones projecting to cat hindlimb motoneurones by transneuronal transport of a horseradish peroxidase conjugate. Neurosci Lett. 1986;71:163–168. doi: 10.1016/0304-3940(86)90552-5. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Slawinska U, Hammar I. Differential presynaptic inhibition of actions of group II afferents in di- and polysynaptic pathways to feline motoneurones. J Physiol. 2002a;542:287–299. doi: 10.1113/jphysiol.2001.014068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Slawinska U, Hammar I. On organization of a neuronal network in pathways from group II muscle afferents in feline lumbar spinal segments. J Physiol. 2002b;542:301–314. doi: 10.1113/jphysiol.2001.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A. Control of spinal mechanisms from the brain. In: Tower DB, editor. The Basic Neurosciences. New York: Blackwell Science Inc; 1975. pp. 253–265. [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 1. Distribution and linkage of reflex actions to alpha-motoneurones. Exp Brain Res. 1987a;65:271–281. doi: 10.1007/BF00236299. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 3. Secondary spindle afferents and the FRA: a new hypothesis. Exp Brain Res. 1987b;65:294–306. doi: 10.1007/BF00236301. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Mori S. Lumbar interneurons involved in the generation of fictive locomotion in cats. Ann NY Acad Sci. 1998;860:441–443. doi: 10.1111/j.1749-6632.1998.tb09070.x. [DOI] [PubMed] [Google Scholar]
- Mori S, Matsui T, Kuze B, Asanome M, Nakajima K, Matsuyama K. Cereballar-induced locomotion: reticulospinal control of spinal rhythm generating mechanisms in cats. In: Kiehn O, Harris-Warrick RM, Jordan LM, Hultborn H, Kudo N, editors. Neuronal Mechanisms for Generating Locomotor Activity. NY: Blackwell Science Inc; 1998. pp. 94–106. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Histology of the Nervous System of Man and Vertebrates. Oxford: Blackwell Science Inc; 1953. English translation, 1995. [Google Scholar]
- Riddell JS, Jankowska E, Huber J. Organization of neuronal systems mediating presynaptic inhibition of group II muscle afferents in the cat. J Physiol. 1995;483:443–460. doi: 10.1113/jphysiol.1995.sp020596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudomin P, Nunez R, Madrid J. Modulation of synaptic effectiveness of Ia and descending fibers in cat spinal cord. J Neurophysiol. 1975;38:1181–95. doi: 10.1152/jn.1975.38.5.1181. [DOI] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. Spinal motorneurons, interneurons and Renshaw cells. A Golgi study. Arch Ital Biol. 1966;104:328–353. [Google Scholar]
- Sherrington CS. The Integrative Action of the Nervous System. New Haven and London: Blackwell Science Inc; 1906. [Google Scholar]
- Stokke MF, Nissen UV, Glover JC, Kiehn O. Projection patterns of commissural interneurons in the lumbar spinal cord of the neonatal rat. J Comp Neurol. 2002;446:349–359. doi: 10.1002/cne.10211. [DOI] [PubMed] [Google Scholar]


