Abstract
Intestinal inflammation induces hyperexcitability of dorsal root ganglia sensory neurons, which has been implicated in increased pain sensation. This study examined whether alteration of sodium (Na+) and/ or potassium (K+) currents underlies this hyperexcitability. Ileitis was induced in guinea pig ileum with trinitrobenzene sulphonic acid (TBNS) and dorsal root ganglion neurons innervating the site of inflammation were identified by Fast Blue or DiI fluorescence labelling. Whole cell recordings were made from acutely dissociated small-sized neurons at 7–10 days. Neurons exhibited transient A-type and sustained outward rectifier K+ currents. Compared to control, both A-type and sustained K+ current densities were significantly reduced (42 and 34 %, respectively; P < 0.05) in labelled neurons from the inflamed intestine but not in non-labelled neurons. A-type current voltage dependence of inactivation was negatively shifted in labelled inflamed intestine neurons. Neurons also exhibited tetrodotoxin-sensitive and resistant Na+ currents. Tetrodotoxin-resistant sodium currents were increased by 37 % in labelled neurons from the inflamed intestine compared to control (P < 0.01), whereas unlabelled neurons were unaffected. The activation and inactivation curves of these currents were unchanged by inflammation. These data suggest ileitis increases excitability of intestinal sensory neurons by modulating multiple ionic channels. The lack of effect in non-labelled neurons suggests signalling originated at the nerve terminal rather than through circulating mediators and, given that Na+ currents are enhanced whereas K+ currents are suppressed, one or more signalling pathways may be involved.
Abdominal pain is a major cause of morbidity for patients who suffer from intestinal disorders, such as inflammatory bowel disease (IBD) and irritable bowel syndrome. Antecedent infection or inflammation has been implicated in the visceral hyperalgesia associated with the irritable bowel syndrome (IBS). The cell bodies of sensory neurons which initiate these sensations originate in the dorsal root ganglia (DRG) (Mayer & Gebhart, 1994). Sub-populations of DRG neurons respond to innocuous and noxious mechanical stimulation, as well as chemical and thermal stimulation, and thus are thought to be polymodal sensory neurons (Mayer & Gebhart, 1994). These polymodal neurons play a central role in responding to conditions that are potentially injurious to tissues. Inflammatory mediators cause a reduction in the threshold and an increase in the gain of the transduction process of these neurons in a process referred to as ‘peripheral sensitization’. Also, under certain pathological conditions, there is evidence that an additional population of normally mechanically insensitive nociceptive fibres can be recruited (Gebhart, 2000). Following inflammatory stimulation these ‘silent afferent’ fibres become active at rest and begin respond to mechanical stimulation. Together, these afferent pathways have been suggested to contribute to disproportionate pain states in response to injury and may persist even after the inflammatory state has resolved (Al Chaer et al. 2000; Collins et al. 2001).
In studies in the somatic nervous system, peripheral sensitization appears to involve both acute and chronic mechanisms (Woolf & Costigan, 1999). Acute mechanisms of increased nociceptive stimulation include both direct depolarization of nerve terminals by neuroactive agents and alterations in ionic currents in the membrane terminals (Rang et al. 1991). Also, later and longer lasting transcription-dependent changes occur and appear to be evoked by either signalling molecules, such as nerve growth factor and/or activity-dependent second messenger cascades (McCleskey & Gold, 1999). These transcription-dependent events can result in altered expression of voltage-gated ion channels, such as increased expression of TTX-resistant sodium channels, and increased TTX-resistant currents (TTX-R INa) (Khasar et al. 1998; Tanaka et al. 1998) or decreased availability of voltage-gated K+ channels (Yoshimura & de Groat, 1999).
Compared to the somatic nervous system, there is considerably less known about the ionic mechanisms underlying inflammation-induced changes in sensory neurons innervating the gastrointestinal tract. Recent studies in the rat stomach have shown that experimentally induced gastric ulcers increase TTX-R INa in vagal and DRG neurons innervating the stomach (Bielefeldt et al. 2002a) and to a lesser degree in mild gastritis (Bielefeldt et al. 2002b). This latter study, however, found that outward K+ currents were unchanged. In contrast, studies in viscera outside the gastrointestinal (GI) tract suggest that inflammation may also modulate K+ currents (Yoshimura & de Groat, 1999) Taken together, it is likely that changes in ionic mechanisms underlying inflammation-induced plasticity depend on the organ involved and the inflammatory repertoire which follows the initiation of inflammation.
We have recently used trinitrobenzene sulphonic acid (TNBS) ileitis in the guinea pig, as a model of inflammatory bowel disease (Moore et al. 2002), to examine the effects of intestinal inflammation on DRG neurons. We found hyperexcitability of dissociated DRG neurons innervating the intestine, manifested by decreased threshold and repetitive firing, properties consistent with hyperalgesia and allodynia. This study employs whole cell voltage clamp techniques in this model to determine if changes in Na+ and/ or K+ currents underlie this hyperexcitability.
METHODS
Animal model and neuron isolation
Guinea pigs (140–225 g) of either sex were obtained from Charles River Laboratories (Montreal, Quebec, Canada). Experiments were performed according to the guidelines of the Canadian Council of Animal Care and approved by the Queen's University animal care committee.
Animals were anaesthetized using a combination of Hypnorm (0.315 mg ml−1 fentanyl citrate and 10 mg ml−1 fluanisone) and midazolam (5 mg ml−1) (0.0025 ml g−1 each, i.p.), and surgery was performed under aseptic conditions to exteriorize the terminal ileum as described previously (Moore et al. 2002). Ileal-projecting neurons were labelled by injecting the retrograde tracer Fast Blue (dissolved in distilled water) or DiI (dissolved in EtOH) into the ileal wall using a Hamilton syringe fitted with a 30 Ga needle. A total injection volume of 15–20 μl was given via 10–15 injection sites. DiI was used in later experiments since Fast Blue became unavailable. Ileitis was induced by injection of 0.5 ml of TNBS (25 mg ml−1 in 25 % EtOH; TNBS kindly provided by Dr G. Morris, Department of Biology, Queen's University, Kingston, Ontario, Canada K7L 5G2) into the ileal lumen using a 30 Ga needle. The intestine was replaced in the abdominal cavity and the wound sutured with 4–0 silk. Buprenex (Buprenophine 0.0225 mg g−1i.p., Reckitt and Colman) was given to all animals to control post-operative pain. Animals recovered from the anaesthetic in a quiet environment, on an electric thermal blanket to maintain normothermia. The post-operative condition of the animals was monitored at least twice daily by trained animal care staff. Animals that were failing to thrive or showed behaviour suggestive of persistent pain were killed. After 7–10 days, animals were anaesthetized by inhalation of isofluorane and killed by cervical dislocation and exsanguination.
The terminal ileum was removed from each animal to establish the degree of TNBS induced ileitis. The ileum was opened along the mesenteric border and pinned flat with the mucosal surface uppermost. Inspection of the mucosa revealed mucosal hemorrhage, ulceration and thickening of the tissue, as described in our previous studies (Moore et al. 2002). In the current study, inspection of the mucosa revealed similar damage following inflammation.
DRG neurons were isolated from thoracic vertebra Th10–13 as described previously (Moore et al. 2002). Briefly, isolated DRG were dissected free of adherent connective tissue and then incubated at 37°C in HBSS with 0.2 mg ml−1 papain and 0.4 mg ml−1 cysteine for 10 min. This was followed by washing in L-15 medium (GIBCO-BRL) with 10 % fetal bovine serum. The DRGs were then incubated in HBSS containing 1 mg ml−1 Type 1 collagenase (Worthington) and 4 mg ml−1 dispase II (Boeringer Manneheim). The ganglia were then titurated with a fire-polished Pasteur pipette. Neurons were maintained in MEM culture medium with Earle's salts and HCO3 (GIBCO-BRL) containing 1 % penicillin-streptomycin, 2 mm glutamine and 0.2 % (w/v) glucose. The cell suspension was plated onto rat tail collagen-coated glass coverslips and stored in a humidified incubator at 37°C under 95 % air and 5 % CO2 until they were retrieved for use in electrophysiological experiments 4–24 h later.
Solutions
For current clamp experiments, the control solution used to superfuse the cells contained (mm): NaCl, 140; KCl, 5; MgSO4, 1; CaCl2, 1; Hepes, 5; pH adjusted to 7.4 using NaOH. Identical control solutions were used to superfuse the cells in K+ current voltage clamp experiments, except for the equimolar replacement of NaCl with N-methyl-d-glucamine. Potassium channel recording solutions were adjusted to pH 7.4 using HCl. The control filling solution contained (mm): KCl, 140; Hepes, 5; MgSO4, 1; EGTA, 1; pH adjusted to 7.2 using KOH. For the recording of sodium currents, solutions of the following composition were used: extracellular solution (mm): NaCl, 100; NMDG, 50; Hepes, 10; MgCl2, 10; d-Glucose, 10; pH adjusted to 7.4 with HCl. Pipette solution (mm): Cs, 115; NaCl, 25; Hepes, 10; MgCl2, 3; EGTA, 11; pH adjusted to 7.2 with CsOH.
Electrophysiological procedures
Coverslips supporting adherent DRG neurons were placed in a RC-26 recording chamber (Warner Instrument Corporation) and mounted on an inverted microscope (IX-70, Olympus, Japan) fitted for both fluorescence and bright field microscopy. Labelled neurons were identified by their fluorescence under brief (< 15 s) exposure to ultraviolet light, using a U-MWIG2 filter for Fast Blue or a U-MWU2 (Olympus, Japan) for DiI, after which cells were viewed under bright field illumination. Whole cell recordings were made using variations of the patch clamp technique (Hamill et al. 1981) and an Axopatch-200B amplifier (Axon Instruments). Patch electrodes were fabricated using thin-walled borosilicate glass (Kimble Products) and a PP-830 electrode puller (Narishige Instruments) or P-97 (Sutter Instruments). After fire polishing, pipettes used for whole cell voltage clamp experiments had DC resistances of 1.5–3.0 MΩ when filled with the control filling solution. Electronic compensation of total series resistance was used in all experiments. Capacitive transients were corrected using analog circuitry. Drugs were added to the bath solution, or delivered by a fast-flow solution switching system (VC-6, Warner Instrument Corporation). Membrane currents were filtered at 2 kHz and sampled at 5 kHz and stored on a microcomputer for later analysis using pCLAMP 8.2 software (Axon Instruments). Cells were included for analysis if in current clamp mode the resting membrane potential was more negative than −40 mV and the cells displayed overshooting action potentials or in voltage clamp mode the series resistance error (after compensation) was less than 5 mV and seal and access resistances remained stable. The mean number of cells studied per animal was 3 (range 1–10).
Drugs and chemicals
All chemicals and drugs were from Sigma Chemical Co. 4-Aminopyridine (4-AP) was dissolved in distilled water to a stock concentration of 100 mm and the pH was adjusted to 7.4 using 2 n HCl. Tetrodotoxin (TTX) was dissolved in water at a stock concentration of 1 mm. Drugs were diluted to their final concentration in the bath solution. Drugs were administered either by direct addition to the bath solution, or directly to the cell under study using a fast-flow solution switching system (Warner Instruments).
Statistical analysis
Student's t test or ANOVA with Student-Newman-Keuls post hoc test were used where appropriate. P values < 0.05 were considered significant.
RESULTS
General properties
Successful recordings were obtained from 149 DRG neurons. Three groups of neurons were examined in this study; Fast Blue- or DiI-labelled neurons from control animals (Control) or TNBS-treated animals (TNBS) and neurons from TNBS-treated animals which were not labelled (non-labelled TNBS). Approximately 3–5 % of the dissociated cells were labelled with Fast Blue or DiI. The mean resting membrane potential in current clamp recordings from labelled control neurons was −58.1 ± 1.1 mV (range −48 to −64 mV; n = 13). Our previous studies of TNBS ileitis using intracellular recording techniques demonstrated that small cell size correlated very closely with the following properties: TTX-resistant action potentials with inflections on the repolarizing phase and capsaicin sensitivity (Moore et al. 2002). These properties have been shown to be present in small-diameter somatic and visceral unmyelinated afferents, a proportion of which are nociceptive afferents (Sengupta & Gebhart, 1994; Blackshaw & Gebhart, 2002). Using whole cell current clamp recordings, we found that small neurons had similar properties, i.e. TTX-resistant action potentials with a prominent inflection of the repolarizing phase (5/5). Measurements of cell capacitance were used to monitor size, and only cells with capacitance < 40 pF were examined, as these cells consistently expressed these properties. Larger cells (mean capacitance 81 ± 7.4 pF) had narrow, TTX-sensitive action potentials (4/4). In our previous work using the same model we demonstrated inflammation-induced changes in excitability of this sub-population of small neurons. In these studies, intracellular recordings were obtained from neurons from control (n = 12) and animals with ileitis (n = 17) and we found that TNBS ileitis caused significant reductions in rheobase, and increased the number of action potentials evoked at 2 × rheobase. Furthermore TNBS ileitis resulted in increased action potential upstroke velocity and increased input resistance. (Moore et al. 2002). In the present study, using whole cell recording techniques, we demonstrated a similar effect of TNBS ileitis on rheobase (> 65 % decrease compared to control and non-labelled cells) and number of action potentials elicited at twice rheobase (> 2.7 times compared to control and non-labelled cells) (Fig. 1).
Figure 1. Effects of TNBS ileitis on intestinal sensory neuron excitability.
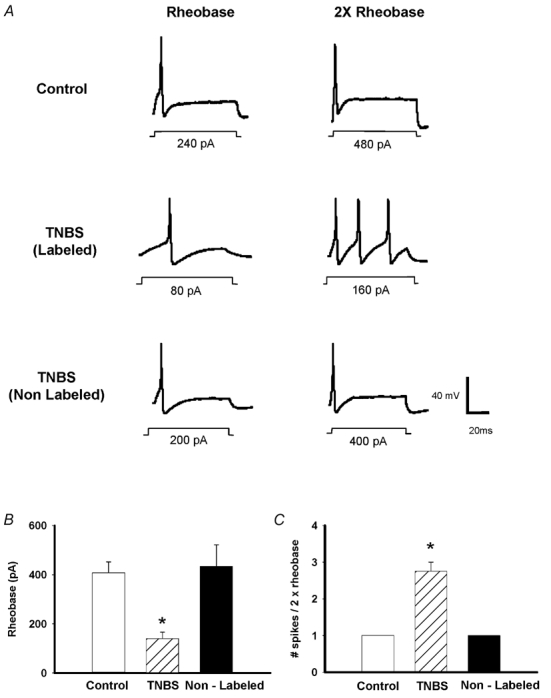
A, representative current clamp traces of action potentials elicited at rheobase and two times rheobase in control labelled, TNBS labelled and TNBS non-labelled neurons. TNBS results in a reduction of the rheobase, while increasing the number of spikes evoked by a 2 × rheobase current injection. Inflections on the falling phase of the action potential are not obvious due to the time scale. B, mean rheobase in control labelled (n = 13), TNBS labelled (n = 4) and TNBS non-labelled (n = 5) neurons (*P < 0.05). C, mean number of spikes elicited by a 2 × rheobase current injection in these same groups of neurons (*P < 0.05).
Characterization of voltage-gated K+ currents
Previous studies of unidentified DRG neurons have identified two kinetically distinct voltage-dependent K+ currents, a transient ‘A-type’ current (IA) and ‘sustained delayed rectifier type’ (IK) (McFarlane & Cooper, 1991; Akins & McCleskey, 1993; Gold et al. 1996; Everill et al. 1998). In the present study, using a voltage clamp protocol with 5 mV steps (400 ms) from a holding potential of −100 mV, both the IA and sustained IK were evident (Fig. 2A). The reversal potential of these currents was −75.5 ± 2.2 mV, near the predicted reversal potential for potassium (−84 mV). Inactivating IA currents were adequately fitted by a monoexponential decay with a mean time constant of 171 ± 16 ms (range 133–210 ms).
Figure 2. Voltage-gated potassium currents and the effect of TNBS ileitis on current density.
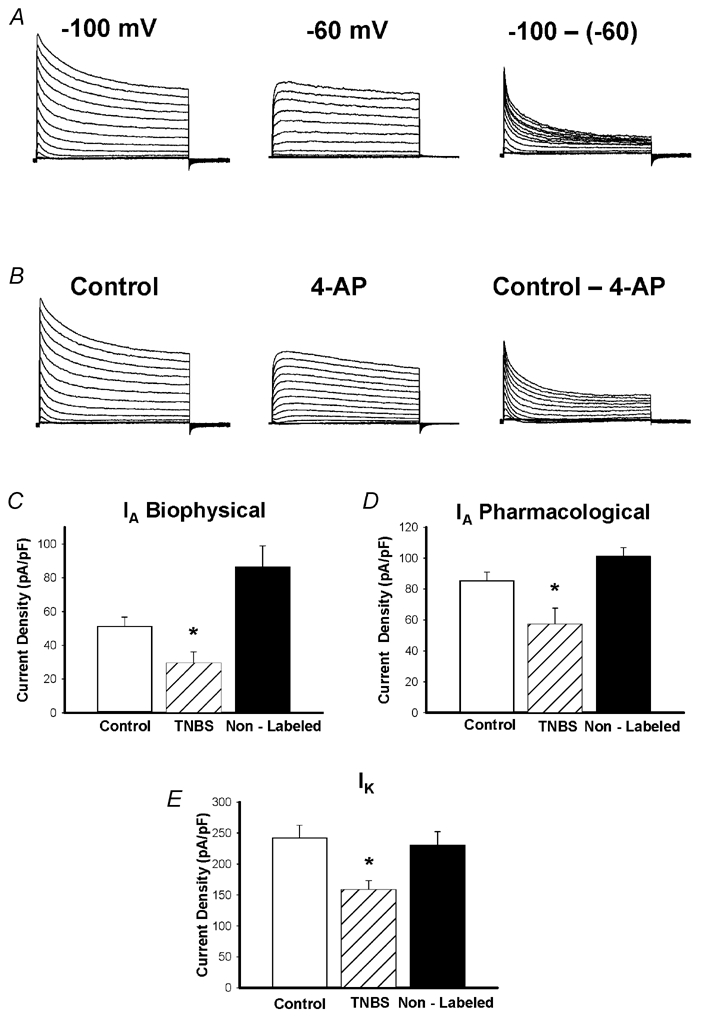
Representative traces from one cell showing currents separated biophysically by manipulating the holding potential (A) and pharmacologically by 4-AP (B). Currents were elicited in response voltage steps from −90 mV to +50 mV in 5 mV increments. A, left: at holding potentials of −100 mV, two currents were apparent, a transient, inactivating ‘A’ type current, and a non-inactivating sustained IK type current. A, middle: resultant current when membrane potential is held at −60 mV. Note disappearance of the transient component. Only the sustained component remains, and was measured at 400 ms. A, right: subtraction of the sustained from the total current yields IA. IA amplitude was measured as the peak of the transient component. B, left: in the pharmacological experiments, the holding potential was −100 mV. B, middle: when the voltage steps were repeated in the presence of 1 mm 4-AP the transient component was significantly inhibited. B, right: results of subtracting current obtained in the presence of 4-AP from control, revealing the 4-AP-sensitive IA current. C, effect of TNBS ileitis on mean peak IA density obtained using either biophysical (left) or pharmacological separation (right). TNBS ileitis results in a significant reduction in IA density compared to control, or non-labelled TNBS neurons (*P < 0.05). D, effect of TNBS ileitis on mean peak IK density. TNBS ileitis results in a significant reduction in peak IK density, compared to control and non-labelled cells (*P < 0.05).
IA and IK currents were isolated based on their contrasting biophysical and pharmacological properties. In preliminary experiments, we established that neither current was significantly inactivated when the membrane potential was held at −100 mV (Fig. 2). However, the IA was selectively inactivated when the membrane potential was held at −60 mV, whereas inactivation of IK at this holding potential was minimal (Fig. 2). Thus, the sustained current was isolated by holding the membrane potential at −60 mV, with the sustained IK measured isochronally, 400 ms after the onset of the pulse, at which time IA was largely inactivated, minimizing contamination by this current (Fig. 2). The IA was isolated by subtracting the sustained IK from the total K+ current recorded from a holding potential of −100 mV. Peak IA was measured as the peak of the transient component of this subtracted current (Fig. 2).
IA was also isolated pharmacologically using the K+ channel blocker 4-aminopyridine (4-AP) (Fig. 2B). Preliminary experiments demonstrated that 100 μm 4-AP (n = 6), and 600 μm 4-AP (n = 3) caused partial suppression of the IA currents whereas 2 mm (n = 3) completely suppressed IA and caused significant suppression of the IK currents. 4-AP most selectively blocked the IA at a concentration of 1 mm. Therefore the IA was isolated by subtracting the current in the presence of 1 mm 4-AP from the total control current, yielding the 4-AP-sensitive IA (Fig. 2B). Experiments using both of these approaches in the same control neuron revealed no significant difference in the IA current density obtained by either method (80.3 ± 19.7 vs. 99.1 ± 10.0 pA pF−1, n = 5)
IA and IK current density following TNBS-induced inflammation
The density of IA and IK elicited by a depolarizing pulse to +50 mV was compared in labelled neurons from control animals and both labelled and unlabelled neurons from TNBS animals (Fig. 2C and D). Current density was determined by normalizing the current amplitude by the cell's capacitance. The IK amplitude was measured 400 ms following the onset of a depolarizing pulse from a holding potential of −60 mV. The IA amplitude was measured as the peak current amplitude of the subtracted current as described above (Fig. 2A and B). Figure 2C-E illustrates the reduction of IA and IK in labelled neurons from TNBS animals. When IA was isolated biophysically, the IA density was 42 % less (P < 0.05) in labelled neurons from TNBS animals (n = 22) than in labelled neurons from control animals (n = 14) and 65 % less than in unlabelled neurons from TNBS animals (n = 19) (Fig. 2C). IA was also isolated pharmacologically using 4-AP (1 mm). The 4-AP-sensitive IA in labelled neurons was reduced by 33 % in labelled TNBS neurons (n = 6, P < 0.05) compared to the currents in labelled neurons from control animals (n = 4) and was 43 % less than in non-labelled TNBS neurons (n = 7, P < 0.01, Fig. 2D). The current density of IK from labelled TNBS neurons (n = 20) was 34 % less (P < 0.05) than in labelled neurons from control animals (n = 15) and 31 % less (P < 0.05) than in unlabelled neurons from TNBS animals (n = 20) (Fig. 2E).
We also attempted to isolate IK pharmacologically, using TEA. However, we found, as have others (Everill et al. 1998; Gold et al. 1996) that TEA in doses sufficient to block IK, also cause significant blockade of the IA.
Voltage dependence of activation and inactivation
Voltage dependencies of activation and inactivation were compared in labelled control cells and labelled TNBS cells to determine if changes in TNBS animals could contribute to the reduced current density.
To determine IA K+ conductance and IK K+ conductance, peak IA amplitudes and isochronally measured end-pulse current amplitudes were measured, respectively. Conductance was then determined using the relation G = I/(Vm−EK), where G is the conductance, I is the measured membrane current, Vm is the voltage step, and EK is the equilibrium K+ potential, which was calculated to be −84 mV in control solutions. Average K+ conductance was plotted against membrane potential (Fig. 4) and the continuous line is an average of individual fits to a Boltzmann function of the form:
where G is the conductance, Gmax is the fitted maximal conductance, V1/2 is the membrane potential for half-activation, Vm is the command potential, and k is the slope factor. No differences in the voltage dependencies of activation for IA or IK were found between control and TNBS neurons (Fig. 3A and B).
Figure 4. Voltage-gated sodium currents and the effects of TNBS ileitis.
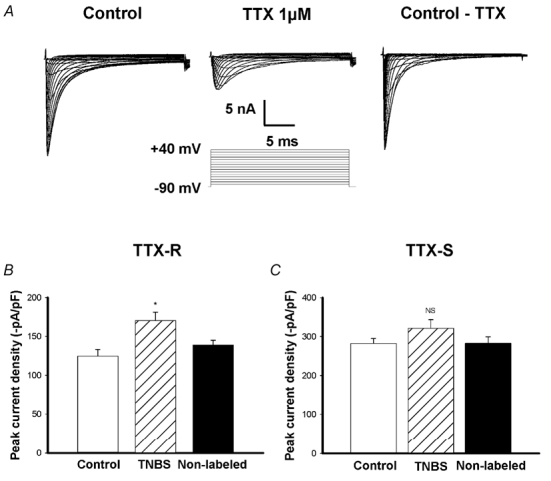
A, representative voltage-gated sodium currents from a small (25 pF) intestinal DRG neuron. Left: total inward current generated by stepwise 30 ms voltage pulses between −80 and +40 mV from a holding potential of −90 mV. Middle: TTX-R INa currents obtained using the same protocol in the presence of 1 μm TTX. Right: TTX-S INa currents obtained by subtracting TTX-R current from total. B, mean peak TTX-R INa current density. TTX-R INa density was increased by TNBS ileitis, *P < 0.05 compared to control and non-labelled neurons (n = 7 cells for each group). C, TTX-S INa current density was not significantly affected by TNBS ileitis (P > 0.05, n = 7 cells for each group).
Figure 3. Activation and inactivation curves of voltage-gated K+ currents.
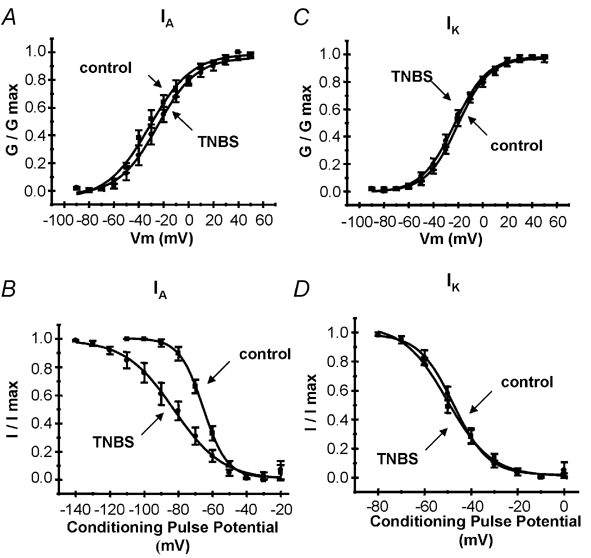
Activation curves were generated by voltage pulses in 5 mV steps from −80 to +50 mV. Each curve represents the mean of curves fitted to the Boltzmann equation (n = 8 cells each). A, activation curves for IA in control and TNBS neurons. TNBS did not affect the activation properties of IA. B, inactivation curves for IA. Inactivation curves were constructed using a two-pulse protocol, a 1 s prepulse varying between −120 and 0 mV, followed by a 400 ms test pulse of +50 mV. The peak of the transient component was measured. TNBS treatment resulted in a slight leftward shift in the inactivation curve of IA. Activation (C) and inactivation (D) curves for IK. Inactivation curves for IK were generated using a two-pulse protocol, with an 8 s prepulse varying between −80 and 0 mV, followed by a 1 s test pulse of +50 mV. End-pulse IK amplitude was measured. TNBS had no effect on voltage dependence of either activation or inactivation of IK.
To examine the voltage dependencies of inactivation for IA and IK, we employed two-pulse voltage protocols as described by others (Philipson et al. 1991; Yoshimura & de Groat, 1999). Residual IA currents were measured following short conditioning pulses (1 s duration) which allowed inactivation of only the rapidly inactivating IA currents. Longer conditioning pulses (8 s duration) allowed inactivation of both IA and IK currents. Therefore residual currents were isochronally measured at the end of a 1 s test pulse to minimize the contribution of IA currents to the measured residual current. The residual current amplitude was plotted against conditioning pulse potential and the continuous line is an average of fits to a negative Boltzmann function:
where I is the current, Imax is the maximal current, V1/2 is the membrane potential for half-activation, Vm is the command potential, and k is the slope factor (Philipson et al. 1991). Significant differences in IA were found between control and TNBS neurons with V1/2 (control −65.4 ± 2.0 mV, TNBS 85.2 ± 2.4 mV, P < 0.05) and slope factors (control 6.9 ± 0.9, TNBS 14.3 ± 1.1, P < 0.05) (Fig. 3C). However, the voltage dependence and voltage sensitivity of inactivation for IK were not different between control and TNBS neurons (V1/2: control −47.6 ± 2.8 mV, TNBS −50.2 ± 3.0 mV; slope: control 7.5 ± 1.5, TNBS 9.4 ± 1.2, P > 0.05; Fig. 3D).
We also employed a pharmacological approach using 4-AP (1 mm) to isolate IA and examine its voltage dependence. Using the above two-pulse protocol, in the absence and presence of 4-AP, the currents obtained in the presence of 4-AP were subtracted from those obtained in its absence (n = 10). These experiments indicated that sensitivity to 4-AP varied significantly with the prepulse voltage thus making analysis of the voltage dependence of inactivation of the subtracted 4-AP sensitive current impractical. This finding has been reported by others (Thompson, 1982; Rasmusson et al. 1995).
Characterization of voltage-gated sodium currents
Large inward currents were evoked in all neurons (n = 60) by depolarizing +5 mV voltage steps from −80 to +40 mV from a holding potential of −90 mV (Fig. 4A). These currents were separated into a TTX-resistant (TTX-R INa) and TTX-sensitive (TTX-S INa) components using TTX (1 μm). TTX-S INa was obtained by subtracting the inward currents in the presence of 1 μm TTX from the currents recorded in the absence of TTX (Fig. 4A). All cells examined exhibited both the TTX-R INa and TTX-S INa currents.
Effects of TNBS ileitis on Na+ current density
TTX-R INa and TTX-S INa peak current densities were obtained by normalizing the current amplitude by the individual cell's capacitance. TTX-R INa was increased significantly in the labelled TNBS group (n = 10) compared to labelled control neurons (n = 10) (37 %, P < 0.01) or non-labelled control neurons (n = 10) (32 %, P < 0.05) (Fig. 4B). TTX-S INa was not significantly different between inflamed labelled neurons (282.0 ± 12.7 pA pF−1, n = 10), labelled control neurons (312.2 ± 22.4 pA pF−1, n = 10, P > 0.05) or TNBS non-labelled neurons (282.3 ± 17.0 pA pF−1, n = 10, P > 0.05) (Fig. 4C).
Activation and inactivation properties of TTX-R INa
We examined the voltage dependence of activation and inactivation of TTX-R INa to determine if changes in these properties could contribute to the increase in current density induced by inflammation. Activation curves were generated using depolarizing voltage steps in the presence of TTX (1 μm). Normalized conductance (G/Gmax) was plotted against test pulse voltage and the data were fitted using a Boltzmann function of the form:
Voltage of half-activation and slope were calculated from the average of the fitted activation curves. Inflammation did not alter the slope (control 4.0 ± 0.50, TNBS 5.2 ± 0.32, n = 7, P > 0.05) or the voltage of half-activation of TTX-R INa (control −12.8 ± 2.0, TNBS −16.9 ± 0.38, n = 7, P > 0.05) (Fig. 5A).
Figure 5. Activation and inactivation curves for TTX-R INa.
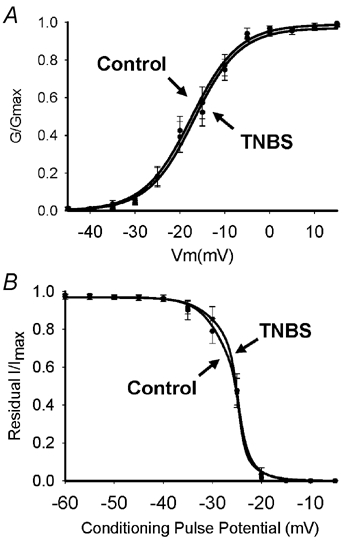
Activation curves in A were generated using voltage steps from −80 to +20 mV. Normalized conductance (G/Gmax) was plotted against test pulse voltage and fitted to a Boltzmann function. Lines represent average of the individual curve fits. TNBS ileitis did not affect the activation properties of the TTX-R INa. (P > 0.05, n = 7) B, inactivation curves for TTX-R INa. Inactivation curves were generated using a two-pulse protocol, with a 1 s prepulse between −120 and 0 mV, followed by a 30 ms test pulse to 0 mV. Normalized current (I/Imax) was plotted against prepulse voltage and fitted to a negative Boltzmann function. Line represents the average of individual curve fits. Inactivation properties were not significantly affected by TNBS ileitis (n = 7, P > 0.05).
Steady-state inactivation curves were generated using a 1 s prepulse from −120 to 0 mV, followed by a 30 ms test pulse to 0 mV. The resultant normalized peak current (I/Imax) was plotted against the prepulse voltage, and the data were fitted to a negative Boltzmann function of the form:
Mean voltage of half-inactivation and slope were calculated from the average of the individual fitted curves (Fig. 5B). Inflammation had no effect on the voltage of half-inactivation of the TTX-R INa (control 26.0 ±1.6 mV, TNBS 25.6 ± 2.3 mV, n = 7, P > 0.05), or the slope (Control −2.03 ± 0.45 mV−1, TNBS −1.7 ± 0.42 mV−1).
DISCUSSION
Voltage-gated Na+ and K+ channels play a fundamental role in controlling neuronal excitability (Hille, 1992) and exhibit significant diversity in sensory neurons, depending upon their innervation and functional properties. The present study characterized K+ and Na+ currents in small-diameter sensory neurons and tested the hypothesis that alteration of one or more currents underlies the hyperexcitability seen in these neurons during inflammation. DRG neurons were functionally identified by retrograde labelling from the intestine, and only small-sized cells were selected for study, as we had previously shown that small cell size correlates with properties such as TTX-resistant action potentials, capsaicin sensitivity, and inflections on the repolarizing phase of the action potential (Moore et al. 2002). Sensory neurons innervating the intestine exhibit transient IA and sustained outward IK K+ currents and both TTX-resistant and TTX-sensitive inward Na+ currents. The major finding was that TNBS ileitis, a model of inflammatory bowel disease caused a marked reduction in the voltage-gated potassium currents, IA and IK, whereas TTX-R INa currents were significantly increased. These changes were not observed in unlabelled neurons from animals with ileitis, suggesting that signalling occurred at the nerve terminal rather than as result of circulating inflammatory mediators. Moreover, the opposing effects on K+ and Na+ currents would suggest that multiple signalling pathways are involved.
Voltage-gated K+ currents in neurons innervating control and inflamed ileum
The IA current plays an important role in setting high thresholds for spike activation and suppression of repetitive firing (Rudy, 1988; Hille, 1992; Yoshimura & de Groat, 1999). Several types of IA current have been described in DRG sensory neurons (Gold et al. 1996; Yoshimura & de Groat, 1999) and can be separated into those with relatively fast and slow inactivation rates based on their biophysical and pharmacological properties (Gold et al. 1996; Yoshimura & de Groat, 1999). Several studies have shown that the fast inactivating IA currents are confined to the large-sized neurons with TTX-sensitive spikes (Yoshimura et al. 1996; Gold et al. 1996). The slowly inactivating IA current has an inactivation time constant of between 150 and 300 ms and the voltage of half-maximal inactivation is displaced more positively than the fast inactivating IA (Akins & McCleskey, 1993; Fjell et al. 1999). In the present study the transient IA inactivation was fitted by a single monoexponential function with a time constant of 171 ms, typical of that described for the slowly inactivating IA. These slowly inactivating IA currents, in contrast to the fast inactivating currents, have been found to be selectively expressed in the small-sized neurons exhibiting TTX-resistant action potentials (Yoshimura et al. 1996). Taken together, the data suggest that slowly inactivating IA current is the dominant form of IA in sensory afferent DRG neurons innervating the small intestine.
In addition to the transient IA current, studies of DRG sensory neurons have identified a sustained delayed rectifier current in all cells (Akins & McCleskey, 1993; Gold et al. 1996). In the present study, these currents could be separated from the IA currents by prepulse inactivation or by 1 mm 4-AP which blocked the IA currents but had little effect on the sustained current, as reported by others (Gold et al. 1996). Some studies have suggested that this sustained current may represent several different currents, based largely on their steady-state inactivation properties (Akins & McCleskey, 1993; Gold et al. 1996). These sustained currents, however, often could not be separated by voltage protocols or pharmacological agents (Gold et al. 1996) making the study of changes in individual sustained currents impractical.
TNBS ileitis caused a significant reduction in both IA and sustained IK currents identified in this study and we have previously shown that pharmacological suppression of K+ currents such as IA can significantly increase the excitability of sensory neurons innervating the intestine (Moore et al. 2002). It is unclear whether these changes in K+ currents occur elsewhere in the GI tract because there has been relatively little work done in this area and in studies conducted in a gastritis model of inflammation similar changes were not observed (Bielefeldt et al. 2002b). It is possible that this difference can be explained by variations in the degree of inflammation or experimental protocols. Our finding that visceral inflammation suppresses IA and correspondingly increases excitability of sensory neurons is similar to previous studies examining the inflamed urinary bladder (Yoshimura & de Groat, 1999). This reduction in peak current density was associated with a hyperpolarizing shift in the inactivation curve, as described in the current study, suggesting there was an associated change in the biophysical properties of the channels. The shift of the inactivation curve in a hyperpolarizing direction would make fewer IA channels available, at or near resting membrane potentials, and lead to a further increase in excitability of the cell, leading to repetitive firing. In contrast to the study in the rat urinary bladder, we also observed a marked reduction in the current density of IK. Whether these differences also reflect degrees of inflammation in these organs or are unique to the sensory neurons and the organs they innervate remains to be established. Given that the role of these voltage-gated potassium currents is to raise the action potential threshold and limit firing (Hille, 1992) suppression of these currents would be expected to increase excitability.
Voltage-gated Na+ currents in neurons innervating control and inflamed ileum
Three types of sodium channels predominate in DRG neurons; NaV1.7 channels, which are responsible for the fast, rapidly inactivating TTX-S INa current, NaV1.8 channels, which result in a more slowly inactivating TTX-R INa and are relatively selectively expressed in nociceptors, and the recently described NaV1.9 which is responsible for a TTX-R persistent current that is ultra-slowly inactivating (Dib-Hajj et al. 2002).The activation and inactivation properties of our TTX-R INa currents fit the known properties of the NaV1.8 channel, although a detailed molecular or immunocytochemical characterization of guinea pig DRG sodium channels has yet to be performed.
We observed a significant increase in TTX-R INa in sensory neurons innervating the inflamed ileum, as has been implicated in other viscera (Yoshimura et al. 2001; Bielefeldt et al. 2002a,b) and the somatic nervous system. In the somatic nervous system (Khasar et al. 1998; Tanaka et al. 1998) these changes were also accompanied by neuronal hyperexcitability and molecular evidence of increased expression of sodium channels (Gould et al. 1998,1999), specifically the TTX-R channel subtype, NaV1.8 (Tanaka et al. 1998). Moreover, mice deficient in Nav1.8 exhibit decreased neuronal excitability (Renganathan et al. 2001) and decreased visceral pain (Laird et al. 2002). Antisense NaV1.8 oligodeoxynucleotide can also prevent inflammation-induced mechanical hyperalgesia (Khasar et al. 1998) and cyclophosphamide cystitis-induced bladder hyperreflexia (Yoshimura et al. 2001). Taken together, these studies suggest that inflammation-induced increased expression of TTX-R INa in nociceptive neurons is common to inflammation in both the somatic and visceral organs, including the GI tract, and is an important mechanism underlying neuronal hyperexcitability and visceral hyperalgesia.
Potential mechanisms mediating inflammation-induced changes in ion channels
The inflammatory milieu surrounding the nerve terminal contains numerous inflammatory mediators, such as PGE2, and 5-HT, which have been shown to augment TTX-R Na+ currents in vitro, in both visceral (Gold et al. 2002) and somatic afferents (Cardenas et al. 2001; Gold et al. 2002). PGE2 has also been shown to decrease IK potassium currents through the same cyclic AMP-protein kinase C-dependent mechanism which alters Na+ currents, in unidentified DRG neurons (Nicol et al. 1997; Evans et al. 1999). These changes occur within minutes and are dependent on the activation of intracellular second messenger systems, and probably subsequent phosphorylation of ion channels or other regulatory cell signalling proteins. These actions cannot account for the findings in the present study because recordings were obtained from cell bodies of DRG neurons located in ganglia outside the inflammatory milieu of the intestine and recordings were made at least 6–8 h after removal of the neurons from the animal at a time when unlabelled neurons were unaffected. Consequently, changes in ionic currents appear to result from longer term alterations such as transcriptional events altering the number of channels, their subunits or other biophysical properties of the membrane channels themselves.
Both activity-dependent and growth factor signalling pathways have been proposed to activate transcription factors which can alter ion channel expression. In particular there is considerable evidence that the neurotrophin nerve growth factor (NGF) can increase expression of TTX-R INa (Fjell et al. 1999; Bielefeldt et al. 2003). In addition NGF and other neuotrophins have been shown to modulate various potassium channels, via activation of tyrosine kinases (Yang et al. 2001) or via activation of ceramide (Zhang et al. 2002). Thus NGF appears to be an important candidate for modulating ion channel activity in visceral inflammation. However, experiments specifically designed to test this hypothesis are needed. It is also unknown whether the diverging effects on these currents are due to signalling through a single pathway or whether multiple pathways are involved.
Conclusions
This study demonstrates that an animal model of inflammatory bowel disease is associated with a suppression of transient and sustained K+ currents and augmentation of TTX-R INa currents in intestinal sensory neurons. The implications are that these mechanisms are important in the increased neuronal excitability seen in these neurons as well as in the increased nociceptive trafficking which occurs during intestinal inflammation, such as in Crohn's disease. Furthermore, there is increasing evidence that visceral hyperalgesia underlies many functional bowel disorders, such as post-infectious irritable bowel syndrome. Whether the findings observed in the current study are present following the resolution of macroscopic inflammation, or whether low levels of microscopic inflammation, which are suggested to persist in these conditions (Collins et al. 2001; Chadwick et al. 2002; Tornblom et al. 2002), are sufficient to sustain these changes remains to be established.
Acknowledgments
This work was supported by a grant from The Crohn's and Colitis Foundation of Canada. M. J. Beyak was supported by a CIHR/CAG/Astra-Zeneca Research Initiative Award. The authors wish to thank Margaret O'Reilly and Iva Kosatka for their expert technical assistance in the performance of these experiments.
REFERENCES
- Akins PT, McCleskey EW. Characterization of potassium currents in adult rat sensory neurons and modulation by opioids and cyclic AMP. Neuroscience. 1993;56:759–769. doi: 10.1016/0306-4522(93)90372-m. [DOI] [PubMed] [Google Scholar]
- Al Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Ozaki N, Gebhart GF. Experimental ulcers alter voltage-sensitive sodium currents in rat gastric sensory neurons. Gastroenterology. 2002a;122:394–405. doi: 10.1053/gast.2002.31026. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Ozaki N, Gebhart GF. Mild gastritis alters voltage-sensitive sodium currents in gastric sensory neurons in rats. Gastroenterology. 2002b;122:752–761. doi: 10.1053/gast.2002.31901. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Ozaki N, Gebhart GF. Role of nerve growth factor in modulation of gastric afferent neurons in the rat. Am J Physiol Gastrointest Liver Physiol. 2003;284:G499–507. doi: 10.1152/ajpgi.00356.2002. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Gebhart GF. The pharmacology of gastrointestinal nociceptive pathways. Curr Opin Pharmacol. 2002;2:642–649. doi: 10.1016/s1471-4892(02)00211-4. [DOI] [PubMed] [Google Scholar]
- Cardenas LM, Cardenas CG, Scroggs RS. 5HT increases excitability of nociceptor-like rat dorsal root ganglion neurons via cAMP-coupled TTX-resistant Na(+) channels. J Neurophysiol. 2001;86:241–248. doi: 10.1152/jn.2001.86.1.241. [DOI] [PubMed] [Google Scholar]
- Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–1783. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- Collins SM, Piche T, Rampal P. The putative role of inflammation in the irritable bowel syndrome. Gut. 2001;49:743–745. doi: 10.1136/gut.49.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib-Hajj S, Black JA, Cummins TR, Waxman SG. NaN/Nav1. 9: a sodium channel with unique properties. Trends Neurosci. 2002;25:253–259. doi: 10.1016/s0166-2236(02)02150-1. [DOI] [PubMed] [Google Scholar]
- Evans AR, Vasko MR, Nicol GD. The cAMP transduction cascade mediates the PGE2-induced inhibition of potassium currents in rat sensory neurones. J Physiol. 1999;516:163–178. doi: 10.1111/j.1469-7793.1999.163aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everill B, Rizzo MA, Kocsis JD. Morphologically identified cutaneous afferent DRG neurons express three different potassium currents in varying proportions. J Neurophysiol. 1998;79:1814–1824. doi: 10.1152/jn.1998.79.4.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell J, Cummins TR, Davis BM, Albers KM, Fried K, Waxman SG, Black JA. Sodium channel expression in NGF-overexpressing transgenic mice. J Neurosci Res. 1999;57:39–47. doi: 10.1002/(SICI)1097-4547(19990701)57:1<39::AID-JNR5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Gebhart GF. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications IV. Visceral afferent contributions to the pathobiology of visceral pain. Am J Physiol Gastrointest Liver Physiol. 2000;278:G834–838. doi: 10.1152/ajpgi.2000.278.6.G834. [DOI] [PubMed] [Google Scholar]
- Gold MS, Shuster MJ, Levine JD. Characterization of six voltage-gated K+ currents in adult rat sensory neurons. J Neurophysiol. 1996;75:2629–2646. doi: 10.1152/jn.1996.75.6.2629. [DOI] [PubMed] [Google Scholar]
- Gold MS, Zhang L, Wrigley DL, Traub RJ. Prostaglandin E(2) modulates TTX-R I(Na). in rat colonic sensory neurons. J Neurophysiol. 2002;88:1512–1522. doi: 10.1152/jn.2002.88.3.1512. [DOI] [PubMed] [Google Scholar]
- Gould HJ, III, England JD, Liu ZP, Levinson SR. Rapid sodium channel augmentation in response to inflammation induced by complete Freund's adjuvant. Brain Res. 1998;802:69–74. doi: 10.1016/s0006-8993(98)00568-x. [DOI] [PubMed] [Google Scholar]
- Gould HJ, III, Gould TN, Paul D, England JD, Liu ZP, Reeb SC, Levinson SR. Development of inflammatory hypersensitivity and augmentation of sodium channels in rat dorsal root ganglia. Brain Res. 1999;824:296–299. doi: 10.1016/s0006-8993(99)01218-4. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. Sunderland, MA, USA: Blackwell Science Inc; 1992. [Google Scholar]
- Khasar SG, Gold MS, Levine JD. A tetrodotoxin-resistant sodium current mediates inflammatory pain in the rat. Neurosci Lett. 1998;256:17–20. doi: 10.1016/s0304-3940(98)00738-1. [DOI] [PubMed] [Google Scholar]
- Laird JM, Souslova V, Wood JN, Cervero F. Deficits in visceral pain and referred hyperalgesia in Nav1. 8 (SNS/PN3)-null mice. J Neurosci. 2002;22:8352–8356. doi: 10.1523/JNEUROSCI.22-19-08352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleskey EW, Gold MS. Ion channels of nociception. Annu Rev Physiol. 1999;61:835–856. doi: 10.1146/annurev.physiol.61.1.835. [DOI] [PubMed] [Google Scholar]
- McFarlane S, Cooper E. Kinetics and voltage dependence of A-type currents on neonatal rat sensory neurons. J Neurophysiol. 1991;66:1380–1391. doi: 10.1152/jn.1991.66.4.1380. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Moore BA, Stewart TM, Hill C, Vanner SJ. TNBS ileitis evokes hyperexcitability and changes in ionic membrane properties of nociceptive DRG neurons. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1045–1051. doi: 10.1152/ajpgi.00406.2001. [DOI] [PubMed] [Google Scholar]
- Nicol GD, Vasko MR, Evans AR. Prostaglandins suppress an outward potassium current in embryonic rat sensory neurons. J Neurophysiol. 1997;77:167–176. doi: 10.1152/jn.1997.77.1.167. [DOI] [PubMed] [Google Scholar]
- Philipson LH, Hice RE, Schaefer K, Lamendola J, Bell GI, Nelson DJ, Steiner DF. Sequence and functional expression in Xenopus oocytes of a human insulinoma and islet potassium channel. Proc Natl Acad Sci U S A. 1991;88:53–57. doi: 10.1073/pnas.88.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang HP, Bevan S, Dray A. Chemical activation of nociceptive peripheral neurones. Br Med Bull. 1991;47:534–548. doi: 10.1093/oxfordjournals.bmb.a072491. [DOI] [PubMed] [Google Scholar]
- Rasmusson RL, Zhang Y, Campbell DL, Comer MB, Castellino RC, Liu S, Strauss HC. Bi-stable block by 4-aminopyridine of a transient K+ channel (Kv1. 4). cloned from ferret ventricle and expressed in Xenopus oocytes. J Physiol. 1995;485:59–71. doi: 10.1113/jphysiol.1995.sp020712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renganathan M, Cummins TR, Waxman SG. Contribution of Na(v)1. 8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol. 2001;86:629–640. doi: 10.1152/jn.2001.86.2.629. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988;25:729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Gebhart GF. Characterization of mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat. J Neurophysiol. 1994;71:2046–2060. doi: 10.1152/jn.1994.71.6.2046. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Cummins TR, Ishikawa K, Dib-Hajj SD, Black JA, Waxman SG. SNS Na+ channel expression increases in dorsal root ganglion neurons in the carrageenan inflammatory pain model. Neuroreport. 1998;9:967–972. doi: 10.1097/00001756-199804200-00003. [DOI] [PubMed] [Google Scholar]
- Thompson S. Aminopyridine block of transient potassium current. J Gen Physiol. 1982;80:1–18. doi: 10.1085/jgp.80.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornblom H, Lindberg G, Nyberg B, Veress B. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972–1979. doi: 10.1053/gast.2002.37059. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Costigan M. Transcriptional and posttranslational plasticity and the generation of inflammatory pain. Proc Natl Acad Sci U S A. 1999;96:7723–7730. doi: 10.1073/pnas.96.14.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Feng L, Zheng F, Johnson SW, Du J, Shen L, Wu CP, Lu B. GDNF acutely modulates excitability and A-type K(+). channels in midbrain dopaminergic neurons. Nat Neurosci. 2001;4:1071–1078. doi: 10.1038/nn734. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci. 1999;19:4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, Seki S, Novakovic SD, Tzoumaka E, Erickson VL, Erickson KA, Chancellor MB, De Groat WC. The involvement of the tetrodotoxin-resistant sodium channel Na(v)1. 8 (PN3/SNS). in a rat model of visceral pain. J Neurosci. 2001;21:8690–8696. doi: 10.1523/JNEUROSCI.21-21-08690.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, White G, Weight FF, de Groat WC. Different types of Na+ and A-type K+ currents in dorsal root ganglion neurones innervating the rat urinary bladder. J Physiol. 1996;494:1–16. doi: 10.1113/jphysiol.1996.sp021471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Vasko MR, Nicol GD. Ceramide, a putative second messenger for nerve growth factor, modulates the TTX-resistant Na(+) current and delayed rectifier K(+) current in rat sensory neurons. J Physiol. 2002;544:385–402. doi: 10.1113/jphysiol.2002.024265. [DOI] [PMC free article] [PubMed] [Google Scholar]


