Abstract
Long-term potentiation (LTP) is supposed to be a cellular mechanism involved in memory formation. Similar to distinct types of memory formation, LTP can be separated into a protein synthesis-independent early phase (early-LTP) and a protein synthesis-dependent late phase (late-LTP). An important question is whether the transformation from early- into late-LTP can be elicited by behavioural conditions such as the attention to novel events. Therefore, we investigated the effect of exploration of a novel environment (novelty-exploration) on subsequently induced early-LTP in the dentate gyrus of freely moving rats. While a delay of 60 min between exploration onset and LTP induction had no effect, intervals of 30 or 15 min led to a reinforcement of early- to late-LTP. Exploration of a familiar environment failed to prolong LTP maintenance. The novelty-induced LTP reinforcement was blocked when the translation inhibitor anisomycin or the β-adrenergic antagonist propranolol were applied intracerebroventricularly before exploration onset. These findings support the hypothesis that the synergistic interplay of novelty-triggered noradrenergic activity and weak tetanic stimulation promotes the synthesis of certain proteins that are required for late-LTP. Such a cellular mechanism may underlie novelty-dependent enhancement of memory formation.
Hippocampal long-term potentiation (LTP), the use-dependent strengthening of synaptic efficacy after brief tetanic stimulation of afferent pathways, is the most prominent model for cellular mechanisms, which may underlie learning and memory formation. Several studies have shown that LTP can be influenced by certain behavioural factors, which also affect memory formation such as stress, or emotional arousal (Xu et al. 1997; Seidenbecher et al. 1997, Korz & Frey 2003). A behaviourally relevant parameter that is decisive for the enhancement of memory formation is novelty perception (for review see Knight & Nakada, 1998). Insights into the underlying cellular mechanisms might arise from the examination of the interaction of exploration of a novel environment and subsequently induced LTP. A recent study conducted in hippocampal area CA1 found a dopamine-dependent facilitation of LTP induction by preceding novelty-exploration but did not examine the influence of pre-tetanic novelty-exposure on LTP maintenance (Li et al. 2003).
The present study focused on long-lasting effects of novelty on hippocampal LTP. We investigated whether preceding exploration of a novel environment can prolong the early phase of LTP (early-LTP) in the dentate gyrus (DG) of freely moving rats. Similar to protein synthesis-dependent and -independent stages of memory formation (Grecksch & Matthies, 1980; Bourtchouladze et al. 1998; Quevedo et al. 1999), LTP persistence consists of at least two distinct phases. Early-LTP lasts for about 4–6 h and is largely independent of protein synthesis, whereas late-LTP, with a duration of more than 4–6 h, depends on translation processes (Krug et al. 1984; Frey et al. 1988; Matthies et al. 1990; Frey et al. 2001). The investigation of processes leading to a reinforcement of early- into late-LTP may be of great importance for the understanding of memory consolidation at the cellular level. To test whether the influence of novelty-exploration on early-LTP is characterized by the requirement of protein synthesis, we examined the effect of the translation inhibitor anisomycin on LTP maintenance under different experimental conditions.
Among the mechanisms that have been implicated in the detection of a novel stimulus (novelty-detection) β-adrenergic activation plays a prominent role. Novelty-detection was found to be accompanied by increased hippocampal noradrenergic activity, driven by enhanced firing of the locus coeruleus, the main source of noradrenaline (norepinephrine; NA) in the brain (Klukowski & Harley, 1994; Sara et al. 1994; Vankov et al. 1995; Kitchigina et al. 1997). Furthermore, NA and the activation of β-adrenergic receptors, respectively, were reported to be crucial to the enhancement of LTP and memory by emotional arousal (Griffin & Taylor, 1995; Seidenbecher et al. 1997; Cahill & McGaugh, 1998). In freely moving rats, we have recently shown that distinct types of late-LTP in the DG depend on β-adrenergic receptor activation (Straube & Frey, 2003). Therefore, we tested whether the β-adrenergic antagonist propranolol specifically prevents the novelty-induced LTP modulation.
METHODS
Subjects and surgery
The experiments conformed with the Institutional, Federal German and International guidelines on the ethical use of animals approved by the Land Saxony Anhalt. Under Nembutal anaesthesia (40 mg kg−1, i.p.) male Wistar rats (8–9 weeks old, 200–300 g) underwent stereotaxical implantation of a bipolar stimulation electrode in the medial perforant path (coordinates: AP −6.9 mm from bregma, L 4.1 mm from bregma, V 2.4–2.7 mm from dura) and a monopolar recording electrode in the hilus of the DG (AP −2.8 mm, L 1.8 mm, V 3.2–3.5 mm) in the right hemisphere (Fig. 1). Insulated stainless steel wires of 125 μm diameter were used as electrodes, positioned and fixed at that depth where the population spike amplitude (PSA) reached a maximum. Chronic brass cannulae were implanted into the right lateral ventricle (AP −0.8 mm, L 1.6 mm, V 3.5 mm). The entire assembly of electrodes and cannulae was fixed to the skull with dental acrylic. The rats were allowed to recover for at least 6 days after surgery.
Figure 1. Schematic illustration of electrode localization in the angular bundle for stimulation and the hilar region of the dentate gyrus for recording.
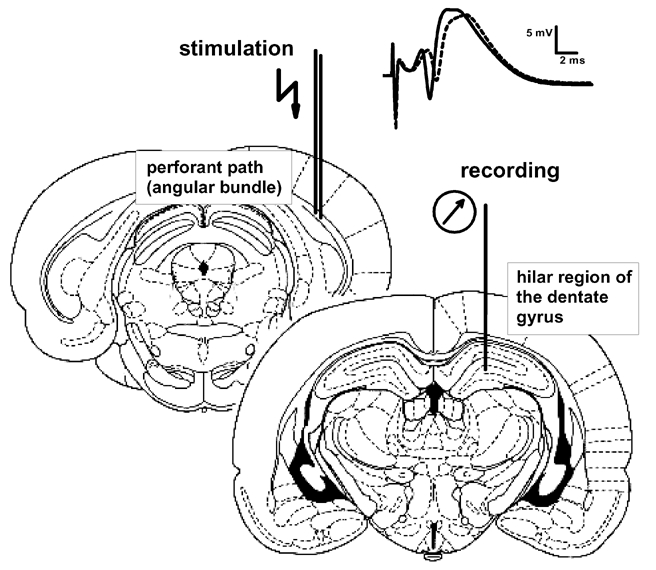
Insets show analogue examples of recordings obtained before (dashed line) and after LTP-induction (continuous line) of the perforant path (upper right). Notice the increase in the PSA (downwards spike-like deflection) and the small increase in the slope of the population excitatory postsynaptic potential (first upward deflection). The PSA was used as an indicator of potentiation.
Electrophysiology
The animals were habituated to the experimental conditions on the day before the electrophysiological experiment and remained in the recording boxes (40 × 40 × 40 cm) until the end of the recording session. During recordings rats were connected with a flexible cable allowing them to move freely with ad libitum access to water and food. Biphasic constant current pulses (0.1 ms per half-wave) were applied to the medial perforant path to evoke extracellular field potentials in the DG of about 40 % of the maximal PSA for the PSA measurements or of about 50 % of the maximal slope of the field excitatory post-synaptic potential (fEPSP) for the fEPSP measurements. The PSA was measured from the initial positive peak to the maximum negative deflection of the potential. The fEPSP was obtained by determining the slope during the first millisecond of the potential.
During baseline recording (1 h) five single responses (10 s interpulse interval) were averaged every 15 min. Baseline recording ended 1 h before tetanization to avoid interference with the behavioural manipulations. Early-LTP was induced by three bursts of 15 pulses at a frequency of 200 Hz with 10 s interburst intervals and a 0.1 ms width per pulse half wave. For tetanization the same stimulus intensity was used as for testing the PSA. With the exception of a test pulse given at 1 min post-tetanus, the field potentials were recorded at 15 min intervals after tetanization and were averaged as 1 h values.
Generally, PSA-recording and analysis were favoured against the slope of fEPSPs since the latter is relatively unstable in freely moving animals, especially considering that the perforant path stimulation intensity was adjusted to obtain an optimal population spike, which negatively influences the fEPSP in the hilus. During high-frequency stimulation, i.e. during LTP induction, the spike is required to induce late-LTP. We have previously studied the relationships between PSA potentiation and EPSP potentiation and found a positive correlation between both variables over a wide range of tetanization frequencies in the rat DG (Lopez Planes et al. 1999; Frey et al. 2001). However, to verify that the long-term changes in the PSA were accompanied by similar changes of the fEPSP, we conducted an additional experiment using a test stimulation more suited to detect changes in the fEPSP slope (see above). Analysis of this experiment revealed that the time course of the fEPSP resembled the PSA measurements. This supports our assumption that the long-lasting changes of the PSA reflect synaptic modifications (see also Lopez Planes et al. 1999; Frey et al. 2001).
Hippocampal EEG signals were recorded every 10 s as 2 s samples by using the same electrode as for recording field potentials. The power spectrum of the EEG was obtained using fast Fourier transforms. The power values were averaged for 5 min blocks and are given as the percentage change from baseline (= mean power during the 30 min before exploration).
Behaviour
Voluntary exploration was elicited by opening a door between the initial, normally lightened box and an adjacent, novel box. The novel box was slightly darkened by covering half of the normally open ceiling with a non-transparent plastic board and the upper half of the transparent front window with a black plastic sheet. Most animals moved into the new box within 90 s. Animals that did not enter the new box within this period were gently placed at the border between the two boxes on the familiar side, which led in all cases to an immediate, voluntary entry into the new box. The results from these rats did not differ from the mean of the group (data not shown). During exploration we could not observe any signs of stress like defecation, freezing or piloerection. Furthermore, all animals remained voluntarily within the novel box after the end of the exploratory phase, which lasted about 15 min. The door between the two boxes was closed 90 min after tetanization while the rats were resting in the novel box. In the ‘familiar environment’ group, rats were allowed to become familiar with the second recording box on the previous day (three times for 1 h, separated by 3 h intervals). Locomotor activity was assessed by counting crossings per minute between the two boxes and the two halves of the novel box.
Brain temperature recording
Brain temperature was measured in an independent sample of rats that were implanted with small-bead thermistors (41 A23, VECO, Springfield, NJ, USA) in the hilus of the DG. The temperature values were recorded every minute and averaged for 5 min blocks.
Serum corticosterone measurement
Serum corticosterone levels were evaluated from animals without implanted electrodes that were treated identically to the groups used for electrophysiology. Fifteen minutes after the onset of exploration the animals were taken from the recording box, decapitated using a guillotine within 20 s and trunk blood was collected within 60 s. This procedure was performed in all groups (including a group of non-exploring animals) between 9:30 a.m. and 10:30 a.m. Blood was collected in an Eppendorf tube and was allowed to coagulate at room-temperature. After 30 min, the blood was centrifuged (10000 r.p.m., 10 min) and the serum was stored at −84°C. The samples were analysed using radioimmunoassays within 8 weeks.
Drug application
Propranolol (6.76 nmol in 0.9 % NaCl), isoproterenol (48.44 nmol in 0.9 % NaCl), or vehicle (0.9 % NaCl) were injected 30 min before the tetanus. Anisomycin (0.905 μmol) was injected 60 min before tetanization to avoid baseline effects shortly after injection that could interfere with LTP induction (Frey et al. 2001). Anisomycin was first dissolved in 15 μl HCl solution (1 n) and then adjusted with NaOH (1 n) to a pH of 7.0. The final volume of 50 μl was obtained by adding NaCl (0.9 %). Drugs or saline were applied in a 5-μl volume over a 4 min period into the right lateral ventricle through the chronically implanted cannula (Seidenbecher et al. 1997; Frey et al. 2001). The injector was inserted 1 h before and removed 1 h after the tetanus. There were no observed effects of drug application on exploratory behaviour.
Data analysis
Multiple comparisons between the control group and experimental groups were conducted with the Dunnett test. For simple comparisons the Student's t test or, when normal distribution and variance-homogeneity were not given, the Mann-Whitney U-test was used. All tests were two-tailed and a probability level of P < 0.05 was considered statistically significant. The electrophysiological data are expressed as means of the percentage change from baseline ±s.e.m.
RESULTS
Time-dependent reinforcement of early- into late-LTP by preceding novelty-exploration
In the first set of experiments, we examined whether and how exploration of a novel environment affects early-LTP when starting at different time points before LTP induction. Weak tetanization in the absence of behavioural manipulation resulted in a transient early-LTP decaying from 213.4 ± 11.7 % (1 min post-tetanus) to baseline level within 8 h (114.7 ± 14.5 %; P > 0.05 compared to baseline; n = 14; shown as control group in Fig. 2). A baseline control group, which received the same test stimulation but no tetanus, indicated the stability of the evoked potentials over the same time period (n = 8; ‘no cannula group’ in Fig. 3D). When the animals were allowed to explore a novel environment 60 min before LTP induction, no effect on the LTP time course was observed (n = 9, Fig. 2A). However, exploration starting 30 and 15 min pre-tetanus transformed the early-LTP into a longer lasting potentiation with a duration of at least 8 h (8h: 162.9 ± 24.2 % and 194.4 ± 17.2 %; all P < 0.05; all n = 9; Fig. 2B and C). Whereas in the 30 min group the PSA had returned to baseline after 24 h, in the 15 min group the potentiation remained significantly different from baseline and controls for at least 24 h (166.4 ± 13.4 % versus 101.5 ± 12.4 % in controls; P < 0.05; Fig. 2C). In additional groups we evaluated the effect of exploration starting 15 min before the tetanus on the time course of the fEPSP potentiation. Similar to the PSA, the fEPSP was statistically significantly different at the 24 h time point compared to baseline (114.2 ± 5.2 %; P < 0.05; n = 7) and to non-exploring controls (controls: 97.3 ± 5.4 %; P < 0.05; n = 10). For all experiments, differences between experimental groups and controls were not seen for the magnitude of LTP induction measured 1 min post-tetanus indicating that all effects were specific for LTP maintenance.
Figure 2. Effects on early-LTP by preceding exploration of a novel environment.
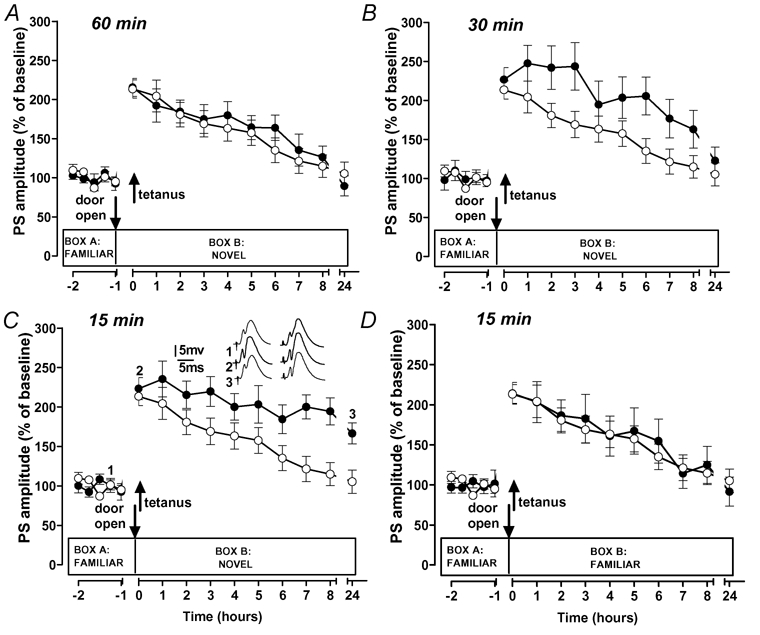
○, time course of early-LTP induced by weak tetanization in the absence of behavioural manipulations (n = 14). •, experimental groups (n = 9 for all groups). A, exploration starting 60 min before tetanization did not affect early-LTP. B, C, exploration onset 30 or 15 min before tetanization prolonged early-LTP to last for at least 8 h or, in the case of the 15 min delay, for 24 h (C). The insets show representative analogue traces recorded at the indicated time points from an individual of the control group (left) and the exploration group (right). D, a significant effect on the LTP time course was not observed when exploration of a familiar environment started 15 min before the weak tetanization.
Figure 3. Involvement of protein synthesis and β-adrenergic receptors in novelty-induced LTP reinforcement.
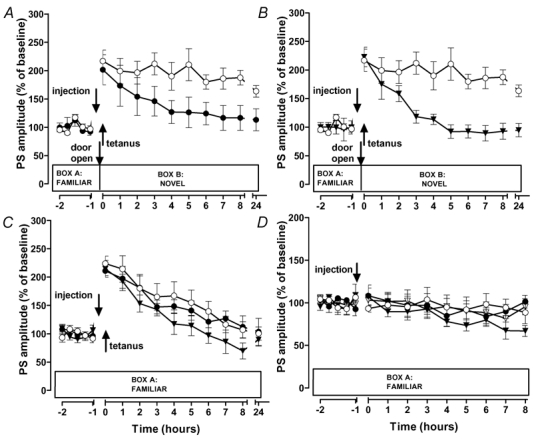
Number of animals per group was n = 8 (saline-group in C and all groups in D), or n = 6 (all other groups). Saline-groups (○), propranolol-groups (•), anisomycin-groups (▾), no cannula group (▿). A,B, the β-adrenergic antagonist propranolol as well as the protein synthesis inhibitor anisomycin blocked the LTP-reinforcing effect of novelty-exploration that started 15 min before the tetanus. Propranolol and anisomycin did not significantly affect early-LTP (C) or baseline (D).
The relationship of novelty-detection or exploratory behaviour on LTP reinforcement
In order to evaluate whether the obtained results depended on the novelty of the second recording box, we tested effects of exploration of a familiar environment starting 15 min pre-tetanus. In contrast to a novel environment, the exploration of a familiar box had no effect on early-LTP (8 h time point: 120.2 ± 11.7 %; P > 0.05; n = 9; Fig. 2D). Furthermore, we investigated whether the obtained reinforcement of LTP could have been influenced by general factors such as brain temperature, corticosterone level, locomotor activity and EEG, which have been reported to affect hippocampal recordings in vivo (Greenstein et al. 1988; Moser et al. 1994, Xu et al. 1998a,b; Orr et al. 2001). Differences between the ‘novel’-and the ‘familiar’ -group were not found during and after exploration when comparing basal PSA, brain temperature, and serum corticosterone levels. There was a transient, non-significant decrease of the PSA (measured every 5 min) under both conditions that lasted for about 45 min and reached about 75–80 % compared to the pre-exploration level (at the 15 min time point: 79.6 ± 13.0 % in the ‘novel’ condition and 75.2 ± 9.9 % in the ‘familiar’ condition; both groups n = 7). The change in the baseline potentials was accompanied by a transient increase in brain temperature (see also Moser et al. 1994) by about 1°C (at the 15 min time point: 0.9 ± 0.2°C in the ‘novel’ condition and 0.8 ± 0.1°C in the ‘familiar’ condition; both groups n = 6). Serum corticosterone levels, measured 15 min after door-opening, showed the same statistically non-significant increase under both exploration conditions (136.9 ± 44.9 ng ml−1 in the ‘novel’ condition and 134.5 ± 81.8 ng ml−1 in the ‘familiar’ condition; both groups n = 6) when compared to resting animals (62.1 ± 39.1 ng ml−1; n = 6). Overall locomotor activity during the 15 min after door-opening was significantly higher in the novelty group compared to the re-exploration group (31.1 ± 2.2 versus 17.7 ± 3.2 crossings; P < 0.05; both groups n = 7). However, at the 15 min time point, active exploration had finished and locomotor activity was virtually absent under both conditions (0.1 ± 0.1 versus 0.2 ± 0.2 crossings). The spectral power of 6–8 Hz EEG oscillations, the dominant hippocampal EEG frequency during exploratory behaviour (Xu et al. 1998a, Vinogradova, 2001), was different between the novel and familiar environment group at the 5 min time point (364.8 ± 38.1 % versus 239.5 ± 20.6 %; P < 0.05; both groups n = 7), but not at the 10 min and 15 min time points (at the 15 min time point: 180.8 ± 20.6 % versus 154.0 ± 15.6 %; P > 0.05).
Requirement of protein synthesis and β-adrenergic receptor activation for novelty-induced late-LTP
In this series of experiments we examined whether the pronounced prolongation of early-LTP, that was obtained when novelty-exploration preceded the tetanus by 15 min, requires β-adrenergic receptor activation and protein synthesis. In the saline group, novelty-exploration led to LTP lasting for at least 24 h (at 24 h: 163.5 ± 10.0 %; P < 0.05; n = 6; saline group in Fig. 3A,B). Application of the β-adrenergic antagonist propranolol or of the protein synthesis inhibitor anisomycin, respectively, prevented the LTP reinforcement as indicated by the absence of potentiation in the drug-treated groups after 8 h (propranolol group: 116.6 ± 21.9 %; anisomycin-group: 92.8 ± 14.1 %; P > 0.05; both groups n = 6; Fig. 3A,B) and 24 h (propranolol group: 113.2 ± 19.5 %; anisomycin-group: 94.8 ± 11.6 %; P > 0.05; Fig. 3A,B). When the drugs were applied to groups of non-exploring animals with either weak or no tetanization, propranolol showed no effect on potentials, and anisomycin caused a mild, but statistically non-significant depression at late time points (Fig. 3C,D). Thus, propranolol and anisomycin specifically blocked the novelty-induced LTP reinforcement. In addition, application of the β-adrenergic agonist isoproterenol 30 min before LTP induction was sufficient to transform early- into late-LTP. Isoproterenol transformed early- into a lasting LTP with a duration of at least 24 h (at 24 h: 161.9 ± 19.5 % versus 94.5 ± 11.1 % in saline controls; P < 0.05; Fig. 4A). Isoproterenol had no effect on baseline potentials, which remained stable over time (Fig. 4B). Thus, pharmacological activation of β-adrenergic receptors mimicked the effect of novelty.
Figure 4. Pharmacological activation of β-adrenergic receptors mimics the effect of novelty on LTP.
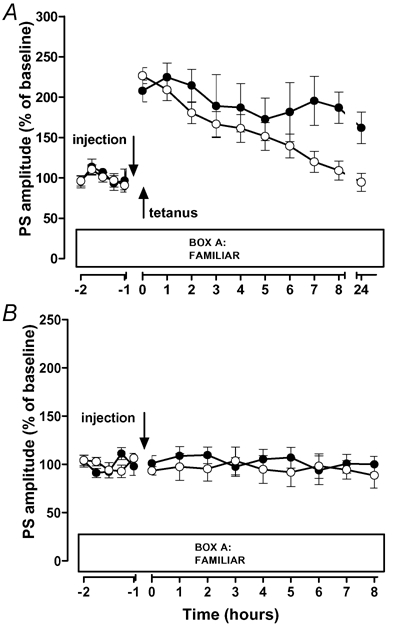
○, saline-groups (n = 14 in A; n = 8 in B); •, isoproterenol-groups (n = 6 in A; n = 8 in B). A, isoproterenol (ISO) injected 30 min before LTP induction reinforced early- to late-LTP. B, baseline potentials in isoproterenol-treated animals remained stable over time.
DISCUSSION
We have shown that protein synthesis-independent early-LTP can be transformed into long-lasting late-LTP in the DG by preceding exploration of a novel environment. This reinforcement could be blocked by the application of a translation inhibitor, anisomycin and required the activation of β-adrenergic receptors. It was specific to novelty. The exploration of a novel but not a familiar environment affected LTP maintenance. We ruled out a direct influence of several factors that accompany exploratory behaviour such as changes in brain temperature, corticosterone level, locomotor activity and hippocampal EEG.
LTP reinforcement was only observed when the exploration started within a time window of about 30 min before LTP induction. This result closely corresponds to a previous study showing that stimulation of the basolateral amygdala (BLA) 5 and 30 min but not 60 min before induction of early-LTP led to late-LTP (Frey et al. 2001). Stimulation of the BLA is supposed to increase neuromodulatory activity in the hippocampus (Frey et al. 2001), an effect which has also been described to accompany novelty-detection (Sara et al. 1994; Vankov et al. 1995; Kitchigina et al. 1997). This gives rise to the assumption that similar molecular mechanisms within the DG could underlie different forms of LTP reinforcement. Furthermore, it can be supposed that the mechanisms for the novelty-induced LTP prolongation are partially comparable to LTP reinforcement by arousing behavioural manipulations described by Seidenbecher et al. (1997). In this study, post-tetanic mild foot shocks or access to water in water-deprived animals prolonged LTP. The same stimuli given before tetanization provoked a tendency towards a reinforcement of LTP. Compared to the latter results, novelty-exploration seems to interact more effectively with a subsequent LTP induction. The data of the present study indicate that the LTP-reinforcing power of novelty-detection declines with increasing delays between exploration onset and tetanization. Similar results of a time constraint were found for area CA1 previously, although the time-window described by Li et al. (2003) appears to be more confined than that observed in our study. These authors found that novelty-exploration starting 10 min but not 30 min before tetanization facilitated LTP induction.
In the present study, LTP prolongation was blocked by application of the β-adrenergic antagonist propranolol. Thus, an increased noradrenergic activity seems to be one important factor for the novelty-induced transformation of early- into late-LTP. This assumption is supported by the fact that activation of β-adrenergic receptors 30 min before the weak tetanus mimicked the LTP-reinforcing effect of novelty-exploration. Furthermore, it is well documented that novelty may enhance the activity of noradrenergic neurons of the locus coeruleus (Sara et al. 1994; Vankov et al. 1995; Kitchigina et al. 1997), whose fibres densely innervate the DG (Milner et al. 2000). Activation of the locus coeruleus (Klukowski & Harley 1994) and of β-adrenergic receptors can induce LTP-like phenomena in the DG (Dahl & Sarvey, 1989; Stanton et al. 1989; Dahl & Li, 1994) and the blockade of β-adrenergic receptors blocks distinct types of late-LTP in the DG of freely moving animals (Straube & Frey, 2003). Moreover, NA and the activation of β-adrenergic receptors have been shown to enhance memory formation and LTP maintenance (Griffin & Taylor, 1995; Seidenbecher et al. 1997; Cahill & McGaugh, 1998). For example, the reinforcement of DG-LTP by emotional arousal or stimulation of the BLA was dependent on β-adrenergic activation (Seidenbecher et al. 1997; Frey et al. 2001). Interestingly, LTP-enhancing effects of novelty-exploration in the CA1 area were dependent on dopaminergic but not noradrenergic neurotransmission (Li et al. 2003). These results support models of area-specific effects of NA and dopamine in the hippocampus (Swanson-Park et al. 1999). While dopaminergic neurotransmission is required for late-LTP in the CA1 region (Frey et al. 1993; Frey & Morris 1998; Swanson-Park et al.. 1999), NA seems to be important for the induction and maintenance of LTP in the DG (see also Stanton & Sarvey, 1985; Frey & Morris 1998; Swanson-Park et al. 1999).
The activation of β-adrenergic receptors is known to stimulate the cAMP/PKA cascade (Stanton & Sarvey, 1985; Watabe et al. 2000), one of the major pathways to induce protein synthesis-dependent late-LTP (Frey et al. 1993). Therefore, it was tempting to test whether novelty-induced LTP reinforcement is dependent on protein synthesis. Our finding that anisomycin did not significantly affect early-LTP but blocked the transformation of early-LTP into a longer-lasting LTP is in accordance with the widely accepted distinction of at least two phases of LTP, of which only the late phase was shown to be protein synthesis-dependent (Krug et al. 1984; Frey et al. 1988; Matthies et al. 1990; Frey et al. 2001). Our results demonstrate that behavioural manipulations can specifically transfer early-LTP into late-LTP, which was abolished by the application of the translation inhibitor anisomycin during its initiation. This is in accordance with recently obtained data investigating protein synthesis-dependent reinforcement of early- into late-LTP by drinking in water-deprived rats (Bergado et al. 2003). New plasticity-related proteins which where synthesized under the influence of behavioural manipulations could have reinforced early-LTP by mechanisms such as ‘synaptic tagging’ (Frey & Morris, 1997, 1998). The setting of a synaptic tag by the weak tetanus would allow tagged synapses to profit from proteins whose synthesis was not or not only induced by the homosynaptic, glutamatergic tetanization but also by novelty-coupled heterosynaptic inputs.
In conclusion, novelty-exploration can lead to the reinforcement of subsequently induced early-LTP in the DG. This process requires β-adrenergic receptor activation and was blocked by the application of a translation inhibitor. The LTP reinforcement described here may illustrate how hippocampus-dependent memory is strengthened in response to non-stressful but behaviourally important environmental changes.
Acknowledgments
We thank Dr Stefan Leutgeb for his critical comments. Furthermore, we are grateful to Professor Dr D. V. Holst and Dr V. Stefanski for analysing the blood samples in the Animal Department of Physiology, Bayreuth, Germany. This work was supported by funds of the LSA 3121A/0029H awarded to J.U.F. and D.B., as well as by the EU Framework V ‘NAPPY’ awarded to J.U.F.
REFERENCES
- Bergado JA, Almaguer-Melian W, Kostenko S, Frey S, Frey JU. Behavioral reinforcement of long-term potentiation in rat dentate gyrus in vivo is protein synthesis-dependent. Neurosci Lett. 2003 doi: 10.1016/s0304-3940(03)00943-1. in press. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem. 1998;5:365–374. [PMC free article] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Dahl D, Li J. Long-lasting potentiation and depression by novel isoproterenol and cholecystokinin 8-S interactions in the dentate gyrus. Exp Brain Res. 1994;100:155–159. doi: 10.1007/BF00227288. [DOI] [PubMed] [Google Scholar]
- Dahl D, Sarvey JM. Norepinephrine induces pathway-specific long-lasting potentiation and depression in the hippocampal dentate gyrus. Proc Natl Acad Sci U S A. 1989;86:4776–4780. doi: 10.1073/pnas.86.12.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Bergado-Rosado J, Seidenbecher T, Pape HC, Frey JU. Reinforcement of early long-term potentiation (early-LTP) in dentate gyrus by stimulation of the basolateral amygdala: heterosynaptic induction mechanisms of late-LTP. J Neurosci. 2001;21:3697–3703. doi: 10.1523/JNEUROSCI.21-10-03697.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Frey U, Krug M, Reymann KG, Matthies H. Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging: implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci. 1998;21:181–188. doi: 10.1016/s0166-2236(97)01189-2. [DOI] [PubMed] [Google Scholar]
- Grecksch G, Matthies H. Two sensitive periods for the amnesic effect of anisomycin. Pharmacol Biochem Behav. 1980;12:663–665. doi: 10.1016/0091-3057(80)90145-8. [DOI] [PubMed] [Google Scholar]
- Greenstein YJ, Pavlides C, Winson J. Long-term potentiation in the dentate gyrus is preferentially induced at theta rhythm periodicity. Brain Res. 1988;438:331–334. doi: 10.1016/0006-8993(88)91358-3. [DOI] [PubMed] [Google Scholar]
- Griffin MG, Taylor GT. Norepinephrine modulation of social memory: evidence for a time- dependent functional recovery of behavior. Behav Neurosci. 1995;109:466–473. doi: 10.1037//0735-7044.109.3.466. [DOI] [PubMed] [Google Scholar]
- Kitchigina V, Vankov A, Harley C, Sara SJ. Novelty-elicited, noradrenaline-dependent enhancement of excitability in the dentate gyrus. Eur J Neurosci. 1997;9:41–47. doi: 10.1111/j.1460-9568.1997.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Klukowski G, Harley CW. Locus coeruleus activation induces perforant path-evoked population spike potentiation in the dentate gyrus of awake rat. Exp Brain Res. 1994;102:165–170. doi: 10.1007/BF00232449. [DOI] [PubMed] [Google Scholar]
- Knight RT, Nakada T. Cortico-limbic circuits and novelty: a review of EEG and blood flow data. Rev Neurosci. 1998;9:57–70. doi: 10.1515/revneuro.1998.9.1.57. [DOI] [PubMed] [Google Scholar]
- Korz V, Frey JU. Stress-related modulation of hippocampal long-term potentiation in rats: involvement of adrenal steroid receptors. J Neurosci. 2003;23:7281–7287. doi: 10.1523/JNEUROSCI.23-19-07281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug M, Lossner B, Ott T. Anisomycin blocks the late phase of long-term potentiation in the dentate gyrus of freely moving rats. Brain Res Bull. 1984;13:39–42. doi: 10.1016/0361-9230(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- López Planes J, Almaguer Melian W, Jas García J, Bergado Rosado JA. Influencia de la frecuencia de estimulación sobre procesos de plasticidad sináptica en el giro dentado de la rata. Arch Neurosci.(Mex) 1999;4:9–20. [Google Scholar]
- Matthies H, Frey U, Reymann K, Krug M, Jork R, Schroeder H. Different mechanisms and multiple stages of LTP. Adv Exp Med Biol. 1990;268:359–368. doi: 10.1007/978-1-4684-5769-8_39. [DOI] [PubMed] [Google Scholar]
- Milner TA, Shah P, Pierce JP. Beta-adrenergic receptors primarily are located on the dendrites of granule cells and interneurons but also are found on astrocytes and a few presynaptic profiles in the rat dentate gyrus. Synapse. 2000;36:178–193. doi: 10.1002/(SICI)1098-2396(20000601)36:3<178::AID-SYN3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Moser EI, Moser MB, Andersen P. Potentiation of dentate synapses initiated by exploratory learning in rats: dissociation from brain temperature, motor activity, and arousal. Learn Mem. 1994;1:55–73. [PubMed] [Google Scholar]
- Orr G, Rao G, Houston FP, McNaughton BL, Barnes CA. Hippocampal synaptic plasticity is modulated by theta rhythm in the fascia dentata of adult and aged freely behaving rats. Hippocampus. 2001;11:647–654. doi: 10.1002/hipo.1079. [DOI] [PubMed] [Google Scholar]
- Quevedo J, Vianna MR, Roesler R, De Paris F, Izquierdo I, Rose SP. Two time windows of anisomycin-induced amnesia for inhibitory avoidance training in rats: protection from amnesia by pretraining but not pre-exposure to the task apparatus. Learn Mem. 1999;6:600–607. doi: 10.1101/lm.6.6.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ, Vankov A, Herve A. Locus coeruleus-evoked responses in behaving rats: a clue to the role of noradrenaline in memory. Brain Res Bull. 1994;35:457–465. doi: 10.1016/0361-9230(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Seidenbecher T, Reymann KG, Balschun D. A post-tetanic time window for the reinforcement of long-term potentiation by appetitive and aversive stimuli. Proc Natl Acad Sci U S A. 1997;94:1494–1499. doi: 10.1073/pnas.94.4.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton PK, Mody I, Heinemann U. A role for N-methyl-D-aspartate receptors in norepinephrine-induced long-lasting potentiation in the dentate gyrus. Exp Brain Res. 1989;77:517–530. doi: 10.1007/BF00249605. [DOI] [PubMed] [Google Scholar]
- Stanton PK, Sarvey JM. The effect of high-frequency electrical stimulation and norepinephrine on cyclic AMP levels in normal versus norepinephrine-depleted rat hippocampal slices. Brain Res. 1985;358:343–348. doi: 10.1016/0006-8993(85)90981-3. [DOI] [PubMed] [Google Scholar]
- Straube T, Frey JU. Involvement of β-adrenergic receptors in protein synthesis-dependent late long-term-potentiation (LTP) in the dentate gyrus of freely moving rats: the critical role of the LTP induction strength. Neuroscience. 2003;119:473–579. doi: 10.1016/s0306-4522(03)00151-9. [DOI] [PubMed] [Google Scholar]
- Swanson-Park JL, Coussens CM, Mason-Parker SE, Raymond CR, Hargreaves EL, Dragunow M, Cohen AS, Abraham WC. A double dissociation within the hippocampus of dopamine D1/D5 receptor and beta-adrenergic receptor contributions to the persistence of long-term potentiation. Neuroscience. 1999;92:485–497. doi: 10.1016/s0306-4522(99)00010-x. [DOI] [PubMed] [Google Scholar]
- Vankov A, Herve-Minvielle A, Sara SJ. Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur J Neurosci. 1995;7:1180–1187. doi: 10.1111/j.1460-9568.1995.tb01108.x. [DOI] [PubMed] [Google Scholar]
- Vinogradova OS. Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus. 2001;11:578–598. doi: 10.1002/hipo.1073. [DOI] [PubMed] [Google Scholar]
- Watabe AM, Zaki PA, O'Dell TJ. Coactivation of beta-adrenergic and cholinergic receptors enhances the induction of long-term potentiation and synergistically activates mitogen-activated protein kinase in the hippocampal CA1 region. J Neurosci. 2000;20:5924–5931. doi: 10.1523/JNEUROSCI.20-16-05924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Anwyl R, Rowan MJ. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature. 1997;387:497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- Xu L, Anwyl R, Rowan MJ. Spatial exploration induces a persistent reversal of long-term potentiation in rat hippocampus. Nature. 1998a;394:891–894. doi: 10.1038/29783. [DOI] [PubMed] [Google Scholar]
- Xu L, Holscher C, Anwyl R, Rowan MJ. Glucocorticoid receptor and protein/RNA synthesis-dependent mechanisms underlie the control of synaptic plasticity by stress. Proc Natl Acad Sci U S A. 1998b;95:3204–3208. doi: 10.1073/pnas.95.6.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]


