Abstract
In rabbit portal vein myocytes noradrenaline activates a non-selective cation current (Icat) which involves a transient receptor potential protein (TRPC6). Previously we have shown that the diaylglycerol (DAG) analogue 1-oleoyl-2-acetyl-sn-glycerol (OAG) stimulates Icat via a protein kinase C (PKC)-independent mechanism, and in the present study we have investigated the interaction between inositol phosphates (InsPs) and OAG on Icat. With whole-cell recording of Icat from freshly isolated rabbit portal vein myocytes the amplitude and rate of activation of noradrenaline-evoked Icat were much greater than those of OAG-induced Icat. Inclusion of inositol 1,4,5-trisphosphate (Ins(1,4,5)P3) in the pipette solution did not evoke Icat but greatly potentiated the amplitude and rate of activation of OAG-induced Icat. With isolated outside-out patches Ins(1,4,5)P3 markedly increased the rate of activation and the open probability of OAG-evoked channel activity, with no change in unitary conductance, channel mean open times or burst durations. The effects of Ins(1,4,5)P3 were mimicked by Ins(2,4,5)P3, 3-F-Ins(1,4,5)P3 and Ins(1,4)P2 but not by Ins(1,3,4,5)P4 and the potentiating effects of InsPs were not inhibited by heparin. Therefore it is concluded that both DAG and InsPs are necessary for full activation of Icat by noradrenaline and the effect of InsPs is via a heparin-insensitive mechanism and represents a novel action of InsPs.
In rabbit portal vein smooth muscle cells, noradrenaline activates a Ca2+-permeable non-selective cation current (Icat) which is mediated by α1-adrenoceptors (Byrne & Large, 1988; Wang & Large, 1991). This conductance is not stimulated by a rise in intracellular Ca2+ concentration ([Ca2+]i) or by depletion of internal Ca2+ stores and therefore it is a classical receptor-operated channel (Wang & Large, 1991). The proposed physiological role of Icat is to mediate noradrenaline-evoked membrane depolarisation and open voltage-dependent Ca2+ channels (VDCCs) and to produce direct influx of Ca2+ ions to evoke vasoconstriction (Wang & Large, 1991; Inoue et al. 2001). Recently it has been shown that a member of the transient receptor potential family of proteins (TRPC6) is a central component of Icat in rabbit portal vein myocytes (Inoue et al. 2001).
Previously we investigated the transduction mechanism of Icat and showed that noradrenaline activates Icat by G-protein stimulation of phospholipase C (PLC) and hydrolysis of phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2). With regard to the two products of this reaction, intracellular dialysis of inositol 1,4,5-trisphosphate (Ins(1,4,5)P3) did not evoke Icat but the diacylglycerol (DAG) analogue 1-oleoyl-2-acetyl-sn-glycerol (OAG) stimulated Icat (Helliwell & Large, 1997). Therefore the transduction mechanism involved the classical phosphoinositide pathway of G-protein coupled to PLC and DAG stimulation of Icat, but a significant observation was that DAG evoked Icat by a protein kinase C (PKC)-independent mechanism (Helliwell & Large, 1997). However a difficulty in the proposed model was that the amplitude and rate of activation of Icat evoked by OAG were considerably reduced compared to noradrenaline-stimulated responses (Helliwell & Large, 1997). In the present work we have investigated the effect of inositol phosphates (InsPs) on the response to OAG and the results show that Ins(1,4,5)P3 and Ins(1,4)P2 markedly increase the amplitude and rate of activation of OAG-induced Icat. These results indicate that noradrenaline utilises DAG, Ins(1,4,5)P3 and Ins(1,4)P2 for activation of Icat.
METHODS
Cell isolation
New Zealand White rabbits (2–3 kg) were killed by an i.v. injection of sodium pentobarbitone (120 mg kg−1, in accordance with the UK Animals (Scientific Procedure Act, 1986) and the portal vein was removed into normal physiological salt solution (PSS). The tissue was enzymatically dispersed using techniques described previously (Albert et al. 2001). The normal PSS contained (mm): NaCl 126, KCl 6, CaCl2 1.5, MgCl2 1.2, glucose 10, and Hepes 11; pH adjusted to 7.2 with 10 m NaOH.
Electrophysiology
Whole-cell currents and single channel currents in outside-out patches were recorded at room temperature as previously described (Albert et al. 2001; Albert & Large, 2001). The bath chamber (volume of approximately 250 μl) was perfused by solution from reservoirs under gravity feed with flow rates of approximately 5 ml min−1.
Whole-cell currents and single channel currents were acquired and analysed as previously described (see above references for a detailed account). It was not possible to determine accurately the numbers of channels in a patch and therefore we calculated open probability (NPo) using the equation:
Solutions and drugs
The myocytes were perfused in K+-free external solution containing (mm): NaCl 126, CaCl2 1.5, Hepes 10, glucose 11; pH adjusted to 7.2 with NaOH (267 ± 5 mosmol l−1). The patch pipette solution contained (mm): CsCl 18, caesium aspartate 108, MgCl2 1.2, Hepes 10, glucose 11, BAPTA 10, CaCl2 1, (free internal calcium concentration approximately 14 nm, calculated using EQCAL software), Na2ATP 1, NaGTP 0.2; pH 7.2 with Tris (300 ± 5 mosmol l−1).
All drugs including heparin (sodium salt from bovine intestinal mucosa) were from Sigma (UK). The values are the means ±s.e.m. of n cells, and for any series of experiments the cells from at least three animals were used. Statistical analysis was carried out initially on data groups using a one-way ANOVA and if a level of significance was found between these groups then a Student's t test was used. The level of significance for the ANOVA and t tests was set at P < 0.05.
RESULTS
Comparison of whole-cell Icat evoked in response to noradrenaline and OAG
Traces i and ii in Fig. 1A show representative records of whole-cell currents evoked in response to bath application of, respectively, noradrenaline and OAG in rabbit portal vein myocytes. It can be seen that the amplitude and activation rate of the noradrenaline-evoked responses were significantly larger than those of the OAG-induced currents (mean data in Fig. 1Bi and ii). Moreover the latency (time between start of application and onset of current) of the current was markedly larger for OAG than noradrenaline (Fig. 1Biii).
Figure 1. Effect of Ins(1,4,5)P3 on OAG-induced whole-cell currents.
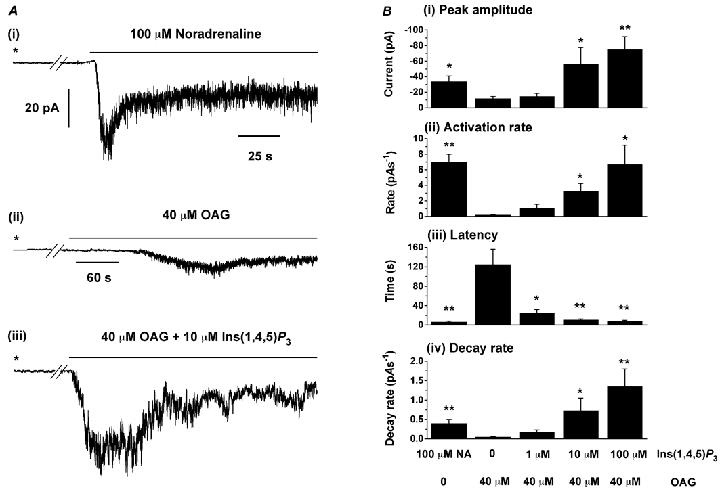
Ai, typical response to bath application of 100 μm noradrenaline recorded in the absence of Ins(1,4,5)P3 in the patch pipette solution. Aii and iii show records of OAG-evoked whole-cell currents in the absence and presence respectively of 10 μm Ins(1,4,5)P3 in the pipette solution. In all cases the holding potential was −50 mV and breaks in the traces correspond to 2 min intervals. The asterisk denotes when whole-cell configuration was obtained. B, mean data for peak amplitude (i), activation rate (ii), latency (iii) and decay rate rate (iv) for noradrenaline-evoked (n = 9) and OAG-induced whole-cell currents activated in the absence (n = 5) or presence of 1 μm (n = 5), 10 μm (n = 9) and 100 μm Ins(1,4,5)P3 (n = 6) in the pipette solution. Activation rate (pA s−1) was calculated from 10–90 % of the rise time of the currents. *P < 0.05, **P < 0.01, compared to OAG-evoked currents in the absence of Ins(1,4,5)P3. Note that with noradrenaline Ins(1,4,5)P3 was not included in the pipette solution.
Ins(1,4,5)P3 potentiates OAG-induced whole-cell Icat
Inclusion of Ins(1,4,5)P3 (1–100 μm) in the patch pipette solution alone did not activate an inward current, but Fig. 1Aiii shows that with 10 μm Ins(1,4,5)P3 in the pipette solution bath-applied OAG induced much larger whole-cell inward currents which activated faster than control responses, and moreover these effects were associated with a large reduction in the latency. The mean data for 1, 10 and 100 μm Ins(1,4,5)P3 are shown in Fig. 1B. At 10 μm and 100 μm, Ins(1,4,5)P3 significantly potentiated the amplitude of OAG-induced currents (Fig. 1Bi) which was associated with a significant reduction in latency and increase in activation rate (Fig. 1Bii and iii). At 1 μm, Ins(1,4,5)P3 did not alter the amplitude and activation rate of OAG-induced currents but greatly reduced the latency of the responses (Fig. 1Biii). In addition it appeared that Ins(1,4,5)P3 had an inhibitory action, since after reaching peak value OAG-evoked currents decayed more rapidly with 10 and 100 μm Ins(1,4,5)P3 in the pipette than in control conditions (Fig. 1Biv).
Ins(1,4,5)P3 increases the activity of single OAG-induced cation channel currents
We carried out experiments in outside-out patches to investigate whether the properties of the OAG-evoked channel currents were similar in the presence and absence of Ins(1,4,5)P3 in the pipette solution. Figure 2A and B are typical records of single channel currents induced by OAG in the absence and presence of 10 μm Ins(1,4,5)P3, respectively. It can be seen quite clearly that in the presence of Ins(1,4,5)P3 OAG evoked channel currents of much higher activity and the time to peak NPo was significantly reduced compared to OAG-evoked responses in the absence of Ins(1,4,5)P3. The mean data for 1, 10 and 100 μm Ins(1,4,5)P3 are shown in Fig. 2C and D.
Figure 2. Effect of Ins(1,4,5)P3 on OAG-induced single channel currents in outside-out patches.
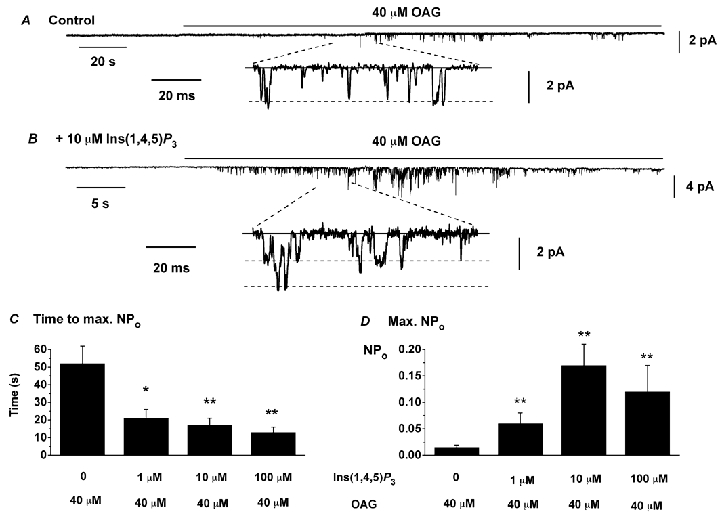
A, effect of 40 μm OAG in the absence of Ins(1,4,5)P3 in the pipette solution; B, effect of 40 μm OAG with 10 μm Ins(1,4,5)P3 in the pipette solution. The continuous lines represent closed states and the dashed lines open states in the expanded traces. Holding potential was −50 mV. Note the multiple openings in B due to higher channel activity and the different time scales for the upper records in A and B. C and D show mean data from OAG-evoked channel currents in the absence (n = 8) and presence of Ins(1,4,5)P3 (n = 5–9). *P < 0.05, **P < 0.01, compared to OAG-evoked channel currents in the absence of Ins(1,4,5)P3.
Figure 3A shows OAG-induced channel activity recorded at different membrane potentials from the same outside-out patch with a pipette solution containing 10 μm Ins(1,4,5)P3. The activity of these OAG-evoked channels was voltage dependent, with a NPo of 0.36 at −50 mV and a NPo of 0.84 at +50 mV (Fig. 3A), which is a characteristic of OAG-evoked channel currents described previously (Albert & Large, 2001). Figure 3B shows the current-voltage relationship (I-V) of the OAG-induced channel currents shown in Fig. 3A. These channel currents had a linear I-V relationship between −70 mV and +50 mV, with a slope conductance of 25 pS and a reversal potential (Erev) of +6 mV. In six patches, OAG-induced channel currents recorded in the presence of 10 μm Ins(1,4,5)P3 had a mean slope conductance of 26 ± 2 pS and a mean Erev of +9 ± 3 mV. Figure 3B also shows an I-V relationship for the OAG-evoked channel currents recorded in the absence of Ins(1,4,5)P3, which had a slope conductance of 25 pS and an Erev of +9 mV. In five patches the mean slope conductance of these channel currents was 24 ± 1 pS (n = 5) and the mean Erev was +7 ± 2 mV (n = 5). In experiments carried out with pipette solutions containing 1 μm or 100 μm Ins(1,4,5)P3, OAG-induced channel currents with similar slope conductances and Erev to those evoked in the presence of 10 μm Ins(1,4,5)P3 (data not shown).
Figure 3. Properties of single OAG-induced channel currents activated in the presence of 10 μm Ins(1,4,5)P3.

A, OAG-induced channel currents evoked in the presence of 10 μm Ins(1,4,5)P3 recorded from the same patch at different membrane potentials. Note the higher activity and longer openings at +50 mV. B, I-V relationship from two different patches of OAG-induced channel currents activated in the presence (•) and absence (○) of 10 μm Ins(1,4,5)P3 and both had unitary conductances of 25 pS and Er of +6 mV and +9 mV respectively. C and D, open time and burst duration distributions of OAG-evoked channel currents induced in the presence of 10 μm Ins(1,4,5)P3 at −50 mV. Both distributions could be fitted by the sum of two time exponentials.
Figure 3C and D illustrates typical distributions of open times and burst durations of OAG-evoked channel currents in the presence of 10 μm Ins(1,4,5)P3. The mean open times were 0.89 ± 0.09 ms (Oτ1, n = 9) and 6.4 ± 1.7 ms (Oτ2, n = 9) and mean burst durations were 4.2 ± 0.4 ms (Bτ1, n = 9) and 19 ± 5.8 ms (Bτ2, n = 9). These values are similar to those obtained in the absence of Ins(1,4,5)P3 (Albert & Large, 2001 and present data not shown).
These data suggest that Ins(1,4,5)P3 potentiates OAG-induced channel currents which have unitary conductance and kinetic properties similar to OAG-evoked channel currents recorded in the absence of Ins(1,4,5)P3. These data therefore suggest that Ins(1,4,5)P3 is potentiating the activity of the same OAG-evoked channel currents and not activating a different cation conductance.
Effect of other InsPs on OAG-induced channel activity
It was of interest to investigate whether the effects of Ins(1,4,5)P3 were a direct action or due to metabolic products. Figure 4A and B shows that inclusion in the patch pipette solution of the relatively stable analogues 3-F-Ins(1,4,5)P3 and Ins(2,4,5)P3 had similar effects to Ins(1,4,5)P3 (compare mean data in Fig. 4E and F with Fig. 2C and D). These results indicate that Ins(1,4,5)P3 potentiates the responses to OAG. However, surprisingly, Ins(1,4)P2, a major metabolite of Ins(1,4,5)P3, also produced similar effects (Fig. 4C, E and F). In contrast Ins(1,3,4,5)P4 did not enhance OAG-induced channel activity (Fig. 4E and F). The OAG-evoked channel currents in the presence of 3-F-Ins(1,4,5)P3, Ins(2,4,5)P3 and Ins(1,4)P2 had a unitary conductance, Erev and kinetic parameters similar to those channel channels evoked by OAG alone (data not shown). Therefore both Ins(1,4,5)P3 and Ins(1,4)P2 potentiate the effects of OAG on single channel currents.
Figure 4. Effect of InsPs on OAG-induced channel currents in outside-out patches.
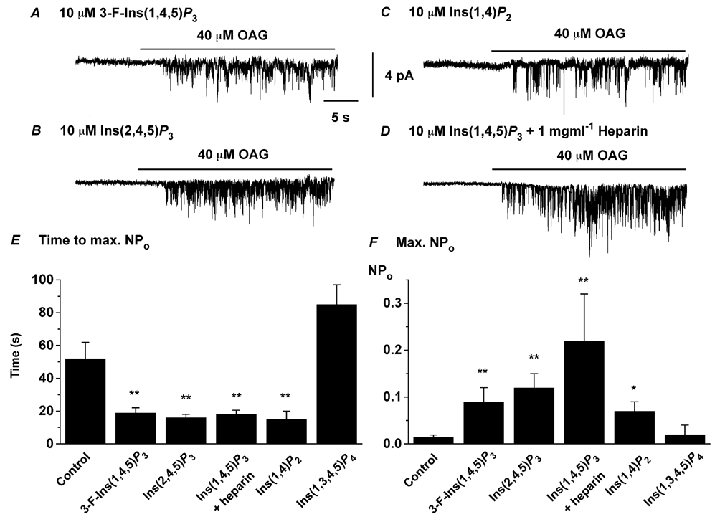
A, B and C show that inclusion of, respectively, 3-F-Ins(1,4,5)P3, Ins(2,4,5)P3 and Ins(1,4)P2 in the pipette solution produced similar effects to Ins(1,4,5)P3 on OAG-induced channel currents. D, inclusion of 1 mg ml−1 heparin in the pipette solution did not prevent the effect of Ins(1,4,5)P3 of potentiating OAG-induced single channel currents. E and F show mean data of time to maximum NPo and maximum NPo of OAG-evoked channel activity in the presence of InsPs (n = 5–8). *P < 0.05, **P < 0.01, compared to OAG-evoked channel currents in the absence of InsPs in the pipette solution.
To investigate whether these effects were mediated by classical Ins(1,4,5)P3 receptors, we tested the effect of the antagonist heparin. It can be seen clearly that 1 mg ml−1 heparin did not prevent the action of Ins(1,4,5)P3 in potentiating OAG-induced channel currents (Fig. 4D-F).
In order to study whether these effects of OAG and Ins(1,4,5)P3 are due to a direct action on the channels we carried out experiments in inside-out patches. In 10 patches we bath-applied OAG in the presence and absence of Ins(1,4,5)P3, but could not evoke channel activity. This result is perhaps predictable from previous work in which we provided evidence to indicate that phosphorylation by a kinase similar to myosin light chain kinase is required for OAG and noradrenaline to activate Icat (Aromolaran et al. 2000). This intermediate mechanism presumably is not present in inside-out patches but is intact in larger outside-out patches. These results suggest that the effects of OAG and/or Ins(1,4,5)P3 may not be directly on the channel protein.
DISCUSSION
The present work shows that intracellular Ins(1,4,5)P3 and Ins(1,4)P2 markedly potentiate the amplitude and rate of activation of DAG-evoked Icat in rabbit portal vein myocytes. Intracellular application of InsPs did not themselves stimulate channel opening but had a profound synergistic interaction with OAG which has weak agonist activity when applied on its own (Helliwell & Large, 1997; Albert & Large, 2001; present study). Therefore it appears that the InsPs prime the channel to allow more efficacious activation by OAG. It has been noted previously that the amplitude and rate of activation of OAG-induced currents were smaller than noradrenaline-evoked responses (Helliwell & Large, 1997). Quantitative comparisons show that the presence of Ins(1,4,5)P3 in the intracellular solution brings the characteristics of the OAG-induced Icat closer to those of the currents stimulated by noradrenaline (see Fig. 1). Therefore it appears that noradrenaline utilises both DAG and Ins(1,4,5)P3 for full and rapid activation of Icat. The observation that Ins(1,4)P2, a major metabolite of Ins(1,4,5)P3, markedly potentiated Icat increases the potential physiological relevance of the mechanism described in this report.
The present study does not reveal the mechanism by which Ins(1,4,5)P3 potentiates the action of OAG but it is unlikely to be due to Ca2+ release from the sarcoplasmic reticulium (SR). The high concentration of BAPTA (10 mm) would be expected to buffer any Ca2+ released from internal stores and, anyway, an increase of [Ca2+]i does not increase the amplitude of Icat (Helliwell & Large, 1996). Also any effect mediated by typical Ins(1,4,5)P3 receptors on the SR would be expected to be blocked by heparin, which did not occur.
In addition to potentiating OAG-evoked Icat, Ins(1,4,5)P3 also increased the decay rate of the OAG-induced whole-cell currents. Interestingly, Ins(1,4,5)P3 also accelerated the decay rate of the noradrenaline-induced Icat, which was not commented on previously (see Fig. 5 in Helliwell & Large, 1997). At present we have no further insight on this inhibitory action of Ins(1,4,5)P3 but it warrants further investigation.
Previously it has been shown that Ins(1,4,5)P3 activates Ca2+-permeable cation channels in human T-lymphocytes (Kuno & Gardner, 1987) and human carcinoma A431 cells (Kaznacheyera et al. 2000). More pertinent to the present work, since TRPC6 has been shown to be an essential component of Icat in portal vein myocytes, it has been demonstrated that Ins(1,4,5)P3 activates expressed human TRPC3 channels in HEK 293 cells (Kiselyov et al. 1998), although these results have been contradicted in a recent study (Trebak et al. 2003). It has also been proposed that the cation current activated by brain-derived nerve growth factor in neonatal rat pontine neurones is a TRPC3 channel activated by Ins(1,4,5)P3 (Li et al. 1999). In our work, Ins(1,4,5)P3 did not activate channels and moreover the potentiating effect of Ins(1,4,5)P3 on OAG-induced currents was not blocked by heparin whereas the Ins(1,4,5)P3-induced currents were inhibited by heparin in both HEK 293 cells (Kiselyov et al. 1998) and rat pontine neurones (Li et al. 1999). The work of Kiselyov et al. (1998) and others has been used to support the conformational-coupling model of store-operated Ca2+ entry, but Icat in rabbit portal vein myocytes is not a store-operated conductance (Byrne & Large, 1988; Wang & Large, 1991; Large, 2002). Therefore the effect of Ins(1,4,5)P3 reported in the present work represents a novel heparin-insensitive action of this intracellular mediator.
Finally it is relevant to comment on the transduction mechanism linking the α1-adrenoceptor to Icat in rabbit portal vein myocytes. In some respects the classical G-protein-PI-PLC pathway is involved, but there are two notable differences. First, DAG activates Icat via a PKC-independent mechanism (Helliwell & Large, 1997), and second, it appears that both DAG and Ins(1,4,5)P3 converge on the same target protein in the sarcolemma (present study). In many tissues Ins(1,4,5)P3 and DAG follow divergent routes with different targets (DAG activates PKC and Ins(1,4,5)P3 acts on the sarco/endoplasmic reticulum). It will be interesting to see if this synergism between Ins(1,4,5)P3 and DAG is involved in the activation of other TRP channels in other tissues.
Acknowledgments
This work was supported by The Wellcome Trust.
REFERENCES
- Albert AP, Aromolaran AS, Large WA. Agents that increase tyrosine phosphorylation activate a non-selective cation current in single rabbit portal vein smooth muscle cells. J Physiol. 2001;530:207–217. doi: 10.1111/j.1469-7793.2001.0207l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert AP, Large WA. Comparison of spontaneous and noradrenaline-evoked non-selective cation channels in rabbit portal vein myocytes. J Physiol. 2001;530:457–468. doi: 10.1111/j.1469-7793.2001.0457k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aromolaran AS, Albert AP, Large WA. Evidence for myosin light chain kinase mediating noradrenaline-evoked cation current in rabbit portal vein myocytes. J Physiol. 2000;524:853–863. doi: 10.1111/j.1469-7793.2000.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne NG, Large WA. Membrane ionic mechanisms activated by noradrenaline in cells isolated from the rabbit portal vein. J Physiol. 1988;404:557–573. doi: 10.1113/jphysiol.1988.sp017306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell RM, Large WA. Dual effect of external Ca2+ on noradrenaline-activated cation current in rabbit portal vein smooth muscle cells. J Physiol. 1996;492:75–88. doi: 10.1113/jphysiol.1996.sp021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell RM, Large WA. α1-Adrenoceptor activation of a non-selective cation current in rabbit portal vein by 1, 2-diacyl-sn-glycerol. J Physiol. 1997;499:417–428. doi: 10.1113/jphysiol.1997.sp021938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular α1-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res. 2001;88:325–337. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- Kaznacheyeva E, Zubov A, Nikolaev A, Alexeenko V, Bezprozvanny I, Mozhayeva GN. Plasma membrane calcium channels in human carcinoma A431 cells are functionally coupled to inositol 1, 4, 5-trisphosphate receptor-phosphatidylinositol 4, 5-bisphosphate complexes. J Biol Chem. 2000;275:4561–4564. doi: 10.1074/jbc.275.7.4561. [DOI] [PubMed] [Google Scholar]
- Kiselyov KI, Xu X, Mozhayeva GN, Kuo T, Pessah I, Mignery GA, Zhu X, Birnbaumer L, Muallem S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- Kuno M, Gardner P. Ion channels activated by inositol 1, 4, 5-trisphosphate in plasma membrane of human T-lymphocytes. Nature. 1987;326:301–304. doi: 10.1038/326301a0. [DOI] [PubMed] [Google Scholar]
- Large WA. Receptor-operated Ca2+-permeable non-selective cation channels in vascular smooth muscle: a physiologic perspective. J Cardiovasc Electrophysiol. 2002;13:493–501. doi: 10.1046/j.1540-8167.2002.00493.x. [DOI] [PubMed] [Google Scholar]
- Li H-S, Xu X-Z, Montell C. Activation of a TRPC-dependent cation current through the neurotrophin BDNF. Neuron. 1999;24:261–273. doi: 10.1016/s0896-6273(00)80838-7. [DOI] [PubMed] [Google Scholar]
- Trebak M, Bird GSTJ, McKay RR, Birnbaumer L, Putney JW. Signalling mechansim for receptor-activated canonical transient receptor potential 3 (TRPC3). channels. J Biol Chem. 2003;278:16244–16252. doi: 10.1074/jbc.M300544200. [DOI] [PubMed] [Google Scholar]
- Wang Q, Large WA. Noradrenaline-evoked cation conductance recorded with the nystatin whole-cell method in rabbit portal vein cells. J Physiol. 1991;435:21–39. doi: 10.1113/jphysiol.1991.sp018496. [DOI] [PMC free article] [PubMed] [Google Scholar]


