Abstract
Electrophysiological and computational evidence indicate that the excitatory current from the synapses on the somato-dendritic membrane is not large enough to drive the motoneurones to the firing frequencies actually attained under normal motor activity. It has been proposed that this paradox could be explained if the voltage-dependent persistent inward currents (PICs) present in the dendrites of motoneurones served to amplify synaptic excitation. We report here that dendritic PICs cause a large amplification of synaptic excitation, and that this amplification is enhanced when the background firing by current injection is increased. Moreover the frequency reduction by synaptic inhibition is greatly enhanced at higher firing frequencies, when the current through the recording electrode has activated the dendritic PICs, as is the case when the current-to-frequency slope suddenly becomes steeper. We also demonstrate that synaptic inhibition is several times more effective in reducing the firing caused by synaptic excitation than firing evoked by current injection through the recording microelectrode. That would be explained if motoneuronal discharge by synaptic excitation – but not by current injection in the soma – is always supported by dendritic PICs. We conclude that dendritic PICs contribute dynamically to the transformation of synaptic input into a motoneuronal frequency code.
As the final common path, spinal motoneurones integrate input from descending motor tracts and sensory feedback systems and transform it into a discharge frequency, which determines the contraction level of the muscles during movement. The efficiency of this transformation was addressed in the 1960s by recording the relation between injected current and firing frequency in the motoneurones (the I-f relation, Granit et al. 1963; Kernell, 1965a,b; for an up-to-date review see Powers & Binder, 2001). More recently, Powers, Binder and their colleagues recorded the ‘effective synaptic currents’ close to the spike-initiating region (Powers & Binder, 1995) during activation of excitatory inputs from a large number of sources (summarized in Powers & Binder, 2001). From the relation between synaptic current and firing frequency of the motoneurones they found that there is not enough excitatory current to bring the firing frequency to the level seen during natural motor activity. Through simulations of the behaviour of anatomically realistic models of motoneurones, Rose & Cushing (1999) reached a similar conclusion. A solution to this paradox could be the existence of intrinsic motoneuronal mechanisms that amplify the ‘raw’ synaptic excitation. In the 1970s Schwindt & Crill (for a review, Schwindt & Crill, 1984) discovered a persistent inward current (PIC) in spinal motoneurones that was uncovered when outward currents were blocked. Due to these PICs, short-lasting depolarizing current pulses could result in long-lasting self-sustained firing, or plateau potentials if the Na+ spikes were blocked. The physiological significance of these PICs was highlighted when it was discovered that their expression was contingent on an appropriate neuromodulation by monoamines (Hounsgaard et al. 1988). Ever since their discovery, it has been assumed that the PICs serve to amplify synaptic inputs to motoneurones (see reviews: Powers & Binder, 2001; Hornby et al. 2002; Hultborn et al. 2003; Binder, 2003), and this has also been demonstrated experimentally (e.g. Bennett et al. 1998; Lee & Heckman, 2000; Prather et al. 2001; Lee et al. 2003).
The further cellular analysis of the PICs and their transmitter control was accomplished in in vitro preparations of the turtle and mouse spinal cords (Hounsgaard & Kiehn, 1989, Svirskis & Hounsgaard, 1998, Carlin et al. 2000). It has been established that these currents are located mainly to the dendritic part of the membrane, i.e. overlapping with the synaptic input to the neurone (Hounsgaard & Kiehn, 1993; Carlin et al. 2000; Svirskis et al. 2001; Powers & Binder, 2003).
The purpose of the present study was to investigate (1) whether the PICs contribute to a variable gain amplification of synaptic current and (2) to determine the amount of amplification as measured from the analysis of firing frequency of the neurone. We demonstrate that synaptic excitation, as well as inhibition, is amplified by the plateau properties, and that this amplification is graded and quantitatively important. It is likely to explain the paradoxical mismatch between synaptic current and actual firing during natural motor tasks.
METHODS
The experimental protocol was approved by the Danish Animal Experimentation Inspectorate. Intracellular recordings were made from lumbar motoneurones in 14 male cats. During surgery the animals were kept under deep anaesthesia with isoflurane (2–2.5 %) mixed with nitrous oxide (50 %) and oxygen (50 %). During anaesthesia the cats were anaemically decerebrated by ligating the basilar and both common carotid arteries, a procedure that has been shown to produce a decerebration that involves all cortical tissue above the pons, where the basilar ligature is placed (Crone et al. 1988). The anaesthetic was removed 5–6 h after ligating the vessels, and the decerebration was verified to be clinically complete by the development of tonic extensor muscle tone (alpha rigidity), lack of spontaneous movements, and large non-reactive pupils (Crone et al. 1988). In some cats stereotyped stepping movements developed, and in these cases anaesthesia was immediately reinstated and the brain removed rostral to an intercollicular section. In these cases the brain was found to be necrotic, and it was concluded that the stepping movements must have originated from the caudal brainstem centres. Following these procedures and tests, pancuronium bromide (0.6 mg h−1) was given to block neuromuscular transmission, and artificial respiration was initiated. The general maintenance of the preparation, including the monitoring of blood pressure and temperature, is further described in Bennett et al. (1998). At the end of the experiments the animals were killed by an overdose of Membumal.
Nerve preparation, laminectomy, pyramidal tract activation, stimulation and recording
Nerve dissection, laminectomy, stimulation and recording followed routine procedures (see Bennett et al. 1998 for details). In seven experiments the L6-S2 dorsal roots were sectioned in order to obtain a pure recurrent inhibition upon stimulation of the dissected muscle nerves. The fourth ventricle was exposed (mid-cerebellum removed) to allow the placement of a stimulating electrode in the pyramid (coordinates: L 1.0; A 8; H −10, the exact placement relying on the low threshold for evoking a typical slow negative pyramidal cord dorsum potential). The intracellular recordings were made with potassium acetate-filled electrodes with an Axoclamp 2B amplifier (Axon Instruments) in either standard bridge mode, or in discontinuous current clamp (DCC) mode. The DCC mode allowed for more accurate measurements of membrane potential despite changes in electrode resistance with injected current.
Experimental protocol
Figure 1A gives a schematic outline of the experimental arrangement as well as the conceptual model underlying the experiments. On penetration (likely to be into the soma), basic properties were measured, including antidromic identification, spike height, afterhyperpolarization (AHP) duration (Fig. 1B), resting membrane potential, input resistance (RIN), rheobase, and the I-f slopes for primary and secondary ranges (Granit et al. 1963, Kernell, 1965a,b) as seen with slow depolarizing current ramps (Fig. 1C). In Fig. 1C it is seen that the I-f slope slowly increases for a while before ‘taking off’ for the secondary range. This is variable on repeated trials in the same motoneurone. We believe that this gradual onset may be explained by stepwise recruitment of the plateau current in different parts of the dendritic tree. Cells with resting membrane potentials more negative than −55 mV and spike height of > 65 mV were included in the results. Plateau properties were inferred from (1) self-sustained firing following depolarizing current pulses, (2) frequency acceleration during constant current injection and (3) a counter-clockwise f-I hysteresis in response to triangular current pulses (Fig. 1D). During the ascending phase of the slow depolarizing current injection, synaptic excitation (pyramidal excitation, muscle stretch, crossed extensor reflex), or inhibition (recurrent inhibition, pyramidal inhibition, crossed extensor reflex in flexor motoneurones) were added at fixed intervals. The increase or decrease in mean firing frequency by the synaptic excitation and inhibition was analysed.
Figure 1. Schematic drawing of the experimental arrangement and firing response to current injected through the recording electrode.
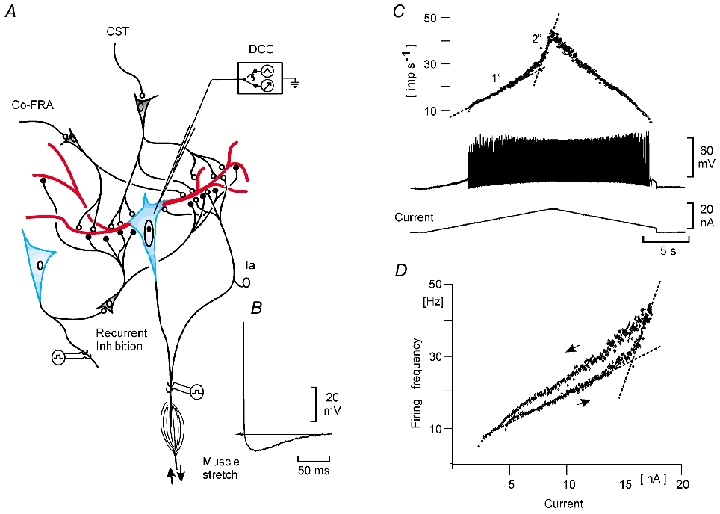
A, the motoneurone soma/initial segment (blue) where the summated inward current/depolarization is converted into a frequency code, with the post-spike afterhyperpolarization (B) as a key element for the current–frequency relation (C). A also shows the excitatory input from the corticospinal tract (CST), the crossed extensor reflex (co-FRA) and the muscle spindle Ia afferents, as well as the inhibitory input from motor axon collaterals, via Renshaw cells (recurrent inhibition). In all cases, the dominating part of the synaptic input is directed to the dendritic area (red). The major part of the non-inactivating voltage-dependent persistent inward currents (PICs) is also localized to the dendritic compartments (red). C, firing pattern and membrane potentials during injection of triangular current pulses. Upper plot, instantaneous firing frequency; middle trace, spike activity; third trace, triangular profile of the injected current. The thin interrupted lines in the upper part approximate in the current-frequency slopes (I–f slopes) corresponding to Kernell's (1965a,b) primary (1o) and secondary (2o) ranges of firing. The steeper relation with the secondary range is likely to be explained by PICs recruited from the dendritic compartments with stronger currents through the somatic recording electrode (see Bennett et al. 1998). Recording was done in DCC mode (see Methods). D, plot of spike frequency vs. injected current for the data in C. Note the counter-clockwise hysteresis that is a reflection of the PICs recruited at the initiation of the secondary range (see Bennett et al. 1998).
RESULTS
Figure 2A and B illustrates how pyramidal synaptic excitation (following a train of stimuli to the pyramidal tract, of constant stimulation strength and duration) caused a depolarization of the membrane below firing threshold, and then contributed to a variable increase in firing frequency as the firing of the motoneurone was slowly increased by a current ramp through the recording microelectrode. In the analysis of this frequency increase we adopted an experimental scheme similar to the one introduced by Powers & Binder (1995) in measuring the ‘effective synaptic current’. The train of stimuli given to the pyramidal tract caused a net depolarization of about 7 mV, when recorded close to firing threshold (* in Fig 2A). The input resistance (RIN) of the motoneurone at this membrane potential was 0.9 MΩ, i.e the effective synaptic current (as seen from the soma) corresponded to 7.8 nA. Figure 2A and B show that the increase of firing frequency evoked by pyramidal excitation became larger as the injected current (and thus the background firing frequency) was increased. The frequency increase caused by the pyramidal stimulus train was 6 imp s−1 at the first trial shortly following the recruitment of the motoneurone (Fig. 2A and B). This corresponded well to the expected frequency increase by the pyramidal EPSP with a calculated current of 7.8 nA at firing threshold and a f-I slope of 0.7 imp s−1 nA−1 for the primary range. However, the frequency increase to the subsequent stimuli far exceeded the expected value, and must be explained by amplification at a stage before the I-f transduction. In a sample of 13 motoneurones this ‘amplification ratio’ was 3.1 ± 1.9 (mean ±s.d.).
Figure 2. Amplification of the frequency response to corticospinal tract stimulation (A and B) and muscle stretch (C and D) during increasing firing frequencies evoked by gradually increasing depolarizing current through the recording microelectrode.
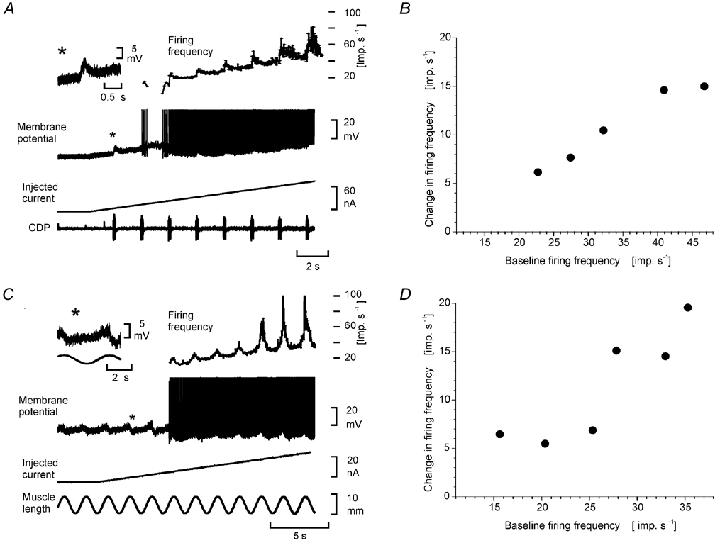
A, recordings from a posterior biceps motoneurone. The inset (*) shows the CST EPSP evoked by a train (50 stimuli at 300 Hz) to the pyramidal tract, recorded at a membrane potential close to firing threshold. The main recordings show the cell's firing frequency (upper plot), spike train (second trace) the injected current (third trace), and the cord dorsum potential (CDP, fourth trace). B, the increased firing frequency during the CST excitation (ordinate) as a function of firing frequency induced by the injected current through the recording microelectrode (abscissa). C, recordings from a triceps surae motoneurone. The inset (*) shows the stretch evoked EPSP recorded at a membrane potential close to firing threshold. The main recordings show the cell's firing frequency (upper plot), spike train (second trace), injected current (third trace), and muscle stretch (fourth trace). D, the increased frequency response during the stretch excitation (ordinate) as a function of firing frequency induced by the current through the recording microelectrode (abscissa).
Similar experiments were made for excitation from two other sources: the excitation from muscle spindle afferents (mainly the monosynaptic Ia excitation) elicited by sinusoidal stretches of the triceps surae muscles (in triceps motoneurones) and the crossed extensor reflex (by short trains of volleys in high threshold afferents in the contralateral common peroneal nerve). Figure 2C and D illustrates similar increases in the added discharge to muscle stretch as the ‘baseline’ firing rates grew higher. In 10 triceps surae motoneurones the ‘amplification ratio’ was 4.3 ± 1.7.
In our opinion the simplest interpretation of the results presented above is that synaptic excitation, causing strong local depolarization close to the synapses, is able to recruit the PICs locally. With more depolarizing current through the recording microelectrode – and thus higher firing frequencies – the number of PIC channels recruited gradually increases, but still at a level far below the threshold needed to recruit the plateau current in an all-or-none manner by current injection alone. If this interpretation is correct, it would be predicted that synaptic inhibition would only decrease the firing frequency corresponding to the calculated inhibitory current at the soma – and that the amount of frequency reduction would stay constant all along the primary range (‘algebraic subtraction’). Within the secondary range, reflecting the recruitment of the ‘plateau current’, the effect of the synaptic inhibition would be expected to increase, as it would act by ‘turning off’ this plateau current, effectively getting the motoneurone back to the primary range. This prediction was fully verified as illustrated in Figure 3.
Figure 3. Amplification of the reduction in firing frequency by recurrent inhibition during increasing firing frequencies evoked by gradually increasing depolarizing current through the recording microelectrode.
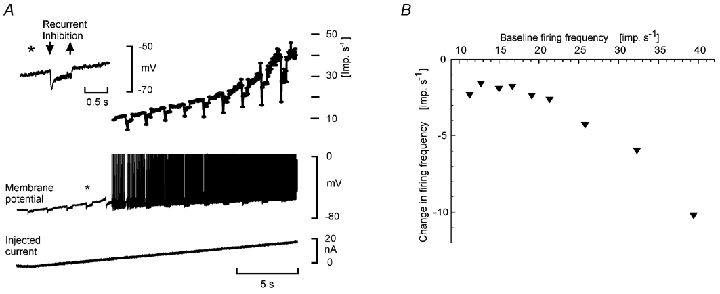
Recordings from a flexor digitorum longus motoneurone. A, inset (*), the recurrent inhibition evoked by a train (50 stimuli at 100 Hz, 5 ×T) of antidromic volleys in the nerve to lateral gastrocnemious–soleus, recorded at a membrane potential close to firing threshold. Same format as in Fig. 2. Note that the reduction is constant along the primary range, but increases markedly in the secondary range. B, the reduced firing frequency during the recurrent inhibition (ordinate) as a function of firing frequency induced by current through the recording microelectrode (abscissa).
Figure 3A illustrates the recurrent inhibition evoked by antidromic volleys in motor axons, at a membrane potential close to the firing threshold. With the RIN estimated to be 2.6 MΩ, the effective synaptic current is about 3.1 nA and 1.4 nA for the initial peak and the following plateau phase, respectively. It is seen that the decrease in instantaneous frequency (peak and plateau) was constant (around 6 and 2 imp s−1 for the peak and plateau, respectively) as the firing frequency due to the current injection increased from 10 to 25 imp s−1 (Fig. 3A). With an I-f slope of 1.4 imp s−1 nA−1 this reduction was very close to the predicted values. In contrast, when the injected current was increased, so that the discharge frequency approached 40 imp s−1, the decrease in frequency evoked by recurrent inhibition was enhanced. In Fig 3A it is seen that this increased efficiency of recurrent inhibition actually occurred as the discharge turned from the primary to the secondary range. When claiming that the inhibition is the same all along the primary range we only refer to the linear segment, thus avoiding the transitional segment with a gradually increasing slope (cf. Fig. 1C, and Methods). The results illustrated in Fig. 3 for recurrent inhibition were seen in all of 22 tested motoneurones. The ‘amplification factor’ along the primary range was 1.0, while the decrease in frequency increased by a factor of 3.0 ± 0.8 (comparing the effect at the peak of the secondary range with that in primary range). Similar results were also obtained for inhibition originating from pyramidal tract stimulation and inhibition evoked from high threshold afferents in the contralateral limb (4 motoneurones).
Our interpretation of the constant reduction in firing frequency all along the primary range followed by the dramatic increase as the firing entered the secondary range is that synaptic inhibition effectively shut off the potential dependent dendritic PICs underlying the secondary range firing. As we have already proposed that the amplification of the discharge by synaptic excitation is due to recruitment of local PICs (cf. Fig. 2) even at low firing rates, it would be expected that synaptic inhibition is more effective in reducing a discharge evoked by synaptic excitation as compared to injected current. In order to test this prediction we have compared the efficiency of recurrent inhibition in reducing a comparable firing frequency evoked either by a current pulse or by synaptic excitation. The prediction was supported by the results as illustrated in Fig. 4.
Figure 4. Recurrent inhibition is more effective in reducing firing evoked by synaptic excitation than by current injection.
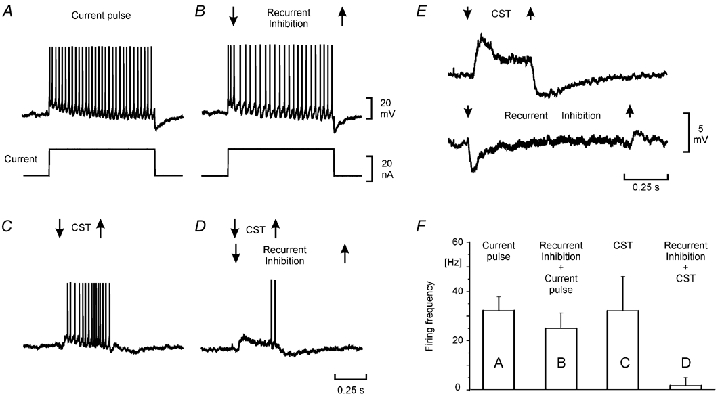
Recordings from a semitendinousus motoneurone. A and B, repetitive firing elicited by a rectangular current pulse alone (A) and together with recurrent inhibition (B). C and D, repetitive firing evoked by a train of impulses to the CST alone (C) and together with recurrent inhibition (D). Each alternative was repeated 20 times and the means and s.d. are shown as a bar graph in F. E, the recurrent inhibition (90 stimuli at 100 Hz, 5 ×T) and the EPSP evoked by the CST stimulation (14 stimuli at 200 Hz) used in B–D, but here recorded at a membrane potential close to firing threshold. The records were low frequency (1 Hz) filtered.
In Fig. 4 the efficiency of recurrent inhibition was thus compared to the firing caused by a current pulse alone (Fig. 4A and B) and the firing of comparable frequency elicited by pyramidal synaptic excitation (Fig. 4C and D). It is seen that the discharge caused by the pyramidal excitation was more sensitive to the synaptic inhibition. The tests illustrated in Fig. 4A-D were repeated (n = 20 for each alternative) and the results displayed as a bar graph in Fig. 4F. Similar results were obtained in five motoneurones.
DISCUSSION
The present study demonstrates that PICs in the spinal motoneurones may amplify the firing frequency response to synaptic excitation and inhibition. The magnitude of this amplification is sufficient to explain the gap pointed out in previous studies (Rose & Cushing, 1999; Powers & Binder, 2001) between the strength of synaptic excitation to the motoneurones and their firing frequencies during natural movements. We suggest that one important function of the PICs is to ensure that the transformation of synaptic drive to motoneuronal output is dynamically amplified contingent on the state of neuromodulation.
Comments on the effect of recurrent inhibition on motoneuronal discharge
We have interpreted the effect of recurrent inhibition on secondary range firing, as well as on the discharge by synaptic excitation, as due to turning off, or preventing the opening of, the PIC channels by local hyperpolarization. This interpretation is in line with the observation of an enhanced efficacy of inhibition under conditions in which an excitatory input has activated a PIC (Powers & Binder, 2000, see their Fig. 10). An alternative interpretation could be that the synaptic inhibition is localized to the stem dendrites, effectively shunting the amplified synaptic excitation on its way towards the soma. Similarly, such a shunting would prevent the current injected through the somatic recording electrode to reach the dendrites, thus preventing the activation of PICs and the development of secondary range firing. Although the latter possibility is difficult to exclude from our present experiments, the earlier work on the termination of Renshaw cells onto motoneurones suggests that they terminate widely over the dendritic tree (Fyffe, 1991) thus supporting our primary interpretation. The two views do not exclude each other, and both mechanisms could contribute to the same result.
The possibility could be raised that the more efficient recurrent inhibition of pyramidal evoked discharge (as compared to pulse evoked discharge) could be due to a pyramidal facilitation of recurrent inhibition (excitation of Renshaw cells). This seems unlikely for two reasons. Firstly, the Renshaw cells were activated by supramaximal stimulation of all motor axons at 100 imp s−1 of the stimulated nerve, not giving much of a ‘subliminal fringe’ from which the pyramidal stimulation could facilitate firing of the Renshaw cells to antidromic volleys. Actually the pyramidal stimulation did indeed discharge several motoneurones (recorded as a discharge from the peripheral nerve) and thus certainly recruited several Renshaw cells. It therefore seems more likely that the additional recurrent inhibition by antidromic volleys during pyramidal stimulation could have been smaller (occlusion) than without pyramidal stimulation. Secondly, we tested the possibility experimentally by recording the amount of recurrent inhibition to single antidromic volleys when preceded by a conditioning train of pyramidal volleys (with the membrane potential of the motoneurone either hyperpolarized to prevent firing, or depolarized to a level at which the spikes were inactivated). We found no evidence for a pyramidal facilitation of the recurrent inhibition in the five motoneurones in which this was tested. We therefore conclude that the stronger effect of recurrent inhibition on the discharge by synaptic excitation than by current injection is more likely to depend on a specific action on the dendritic amplification of the synaptic excitation.
Are PICs graded or of ‘all-or-none’ character?
As described in recent reviews (Powers & Binder, 2001; Hultborn et al. 2003) plateau potentials in motoneurones were recorded as all-or-none phenomena in current clamp mode in the original publications. Correspondingly, in voltage clamp mode a strong negative resistance slope in the I-V curve was seen to have an amplitude that would be regenerative and lead to a stable membrane potential (the plateau potential) in current clamp mode. Much later the effect of tonic synaptic excitation and inhibition on the threshold of the plateau potentials was investigated (evoked by current injection to the soma; Bennett et al. 1998). With synaptic excitation the plateau threshold was reduced, as seen from the recording microelectrode (presumably positioned in the cell's soma), while synaptic inhibition had the opposite effect. This was interpreted as being due to the dendritic localization of the PICs – the membrane depolarization and hyperpolarization by the synaptic input were closer to the PIC channels than the somatic electrode. In most studies up to this date the experimental protocol in which the plateau currents were elicited by current through the microelectrode (soma) are likely to have had a tendency to over-emphasize the ‘all-or-none’ character of the current producing the plateau potential. But are plateau potentials normally seen as an all-or none phenomenon? It seems functionally more realistic to imagine that a local synaptic excitation (predominately in the dendrites) would activate PICs only in its immediate environment – and lasting only as long as the local active synaptic excitation. The present results strongly support this view of a gradual activation.
Comparison to previous data on transformation of synaptic input into motoneuronal activity
Granit et al. (1963, 1966a,b) were the first to study the effect of superimposing synaptic excitation or inhibition on motoneuronal firing evoked by current pulses injected through the recording microelectrode. In contrast to us they observed that the synaptic excitation added to the motoneuronal discharge in a linear fashion when the motoneurones fired within the primary range. A likely explanation of this discrepancy is that the preparations in the studies by Granit et al. were under light barbiturate anaesthesia, which effectively depresses the dendritic PICs (Guertin & Hounsgaard, 1999). This is also the most likely explanation for why they only rarely found motoneurones which showed secondary range firing. Nevertheless, in these rare cases Granit et al. also observed that the increase in firing by excitation and the decrease by inhibition were larger than expected from a linear summation. A recent study by Prather et al. (2001) on the summation of synaptic excitation to a discharge evoked by current through the recording microelectrode also described amplification, but differing from our present results, they did not find a variable and gradually increasing amplification with increasing firing rate. From their illustrations it seems that there was a tendency for increased amplification at higher firing rates, but it did not reach significance. This apparent difference may depend on differences in the experimental procedure as Prather et al. (2001) measured steady state changes in firing rate, while we measured ‘dynamic’ changes during slow triangular ramps. Lee et al. (2003) investigated the inward currents of various EPSPs during different holding potentials (at the soma) in voltage-clamp mode. They demonstrated a large – and graded – increase in the inward current during synaptic excitation at increasingly depolarizing holding potentials, in full accordance with our present findings with synaptic excitation. From their voltage-clamp data they estimated the amplification factor (caused by the PICs) to be ≈2.7 times. With the increase in mean firing frequency following synaptic excitation as an outcome measure we found amplification factors of 3.1 (pyramidal excitation) and 4.3 (stretch evoked excitation). In the case of synaptic inhibition we only found an amplification (with a factor of 3.0) as the motoneurone entered the secondary range, where the PICs are developing.
Functional considerations
In decerebrate unanaestethized cats Bennett et al. (1998) demonstrated that the threshold for the PICs to current pulses were similar to the recruitment level of the motoneurone, when a tonic subthreshold synaptic input was provided. Even more relevant, but still with more indirect evidence, experiments on freely moving rats (Gorassini et al. 1999) and voluntary movement in humans (Kiehn & Eken, 1997, Gorassini et al. 2002) suggest that PICs contribute to the firing pattern already at recruitment level. The present evidence therefore suggests that the PICs are part and parcel of the normal recruitment process and frequency regulation. Given the large amplitude of amplification demonstrated in this report it seems urgent to proceed with the experiments on intact preparations and provide more and stronger evidence for the contribution of PICs during actual behaviour. It is obvious that this mechanism causing a variable amplification will have a bearing on the global relation between cortical activity and muscle force, a question that can be approached in chronic primate experiments.
Acknowledgments
We thank Lillian Grondahl for her expert, long-provided technical assistance in our Copenhagen laboratory, and Gilles Detillieux, Winnipeg, Canada, for expert help with the data collection/analysis system. The research has been supported by grants from the Danish Medical Research Council, the NOVO Nordisk Foundation, the Ludvig & Sara Elsass Foundation, the Michaelsen Foundation, and the Sofus & Olga Friis Foundation.
REFERENCES
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998;80:2023–2037. doi: 10.1152/jn.1998.80.4.2023. [DOI] [PubMed] [Google Scholar]
- Binder MD. Integration of synaptic and intrinsic dendritic currents in cat spinal motoneurons. Brain Res Rev. 2003;40:1–8. doi: 10.1016/s0165-0173(02)00183-2. [DOI] [PubMed] [Google Scholar]
- Carlin KP, Jones KE, Jiang Z, Jordan LM, Brownstone RM. Dendritic L-type calcium currents in mouse spinal motoneurons: implications for bistability. Eur J Neurosc. 2000;12:1635–1646. doi: 10.1046/j.1460-9568.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Kiehn O, Mazieres L, Wigstrom H. Maintained changes in motoneuronal excitability by short-lasting synaptic inputs in the decerebrate cat. J Physiol. 1988;405:321–343. doi: 10.1113/jphysiol.1988.sp017335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe RE. Spatial distribution of recurrent inhibitory synapses on spinal motoneurons in the cat. J Neurophysiol. 1991;65:1134–1149. doi: 10.1152/jn.1991.65.5.1134. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Bennett DJ, Kiehn O, Eken T, Hultborn H. Activation patterns of hindlimb motor units in the awake rat and their relation to motoneuron intrinsic properties. J Neurophysiol. 1999;82:709–717. doi: 10.1152/jn.1999.82.2.709. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol. 2002;87:1850–1858. doi: 10.1152/jn.00024.2001. [DOI] [PubMed] [Google Scholar]
- Granit R, Kernell D, Lamarre Y. Algebraical summation in synaptic activation of motoneurones firing within the ‘primary range’ to injected currents. J Physiol. 1966a;187:379–399. doi: 10.1113/jphysiol.1966.sp008097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granit R, Kernell D, Lamarre Y. Synaptic stimulation superimposed on motoneurones firing in the ‘secondary range’ to injected current. J Physiol. 1966b;187:401–415. doi: 10.1113/jphysiol.1966.sp008098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granit R, Kernell D, Shortess GK. Quantitative aspects of repetitive firing of mammalian motoneurons, caused by injected currents. J Physiol. 1963;168:911–931. doi: 10.1113/jphysiol.1963.sp007230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin PA, Hounsgaard J. Non-volatile general anaesthetics reduce spinal activity by suppressing plateau potentials. Neuroscience. 1999;88:353–358. doi: 10.1016/s0306-4522(98)00371-6. [DOI] [PubMed] [Google Scholar]
- Hornby TG, McDonagh JC, Reinking RM, Stuart DG. Motoneurons: A preferred firing range across vertebrate species. Muscle Nerve. 2002;25:632–648. doi: 10.1002/mus.10105. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Calcium spikes and calcium plateaux evoked by differential polarization in dendrites of turtle motoneurones in vitro. J Physiol. 1993;468:245–259. doi: 10.1113/jphysiol.1993.sp019769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol. 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Brownstone RB, Toth TI, Gossard J-P. Mori S, Stuart DG, Wiesendanger M, editors. Key mechanisms for setting the input-output gain across the motoneuron pool. In Brain Mechanisms for the Integration of Posture and Movement. Progr Brain Res. 2003;143:77–94. doi: 10.1016/s0079-6123(03)43008-2. [DOI] [PubMed] [Google Scholar]
- Kernell D. The adaptation and the relation between discharge frequency and current strength of cat lumbosacral motoneurones stimulated by long-lasting injected currents. Acta Physiol Scand. 1965a;65:65–73. doi: 10.1111/j.1748-1716.1965.tb04081.x. [DOI] [PubMed] [Google Scholar]
- Kernell D. High-frequency repetitive firing of cat lumbosacral motoneurones stimulated by long-lasting injected currents. Acta Physiol Scand. 1965b;65:74–86. doi: 10.1111/j.1748-1716.1965.tb04081.x. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons. J Neurophysiol. 1997;78:3061–3068. doi: 10.1152/jn.1997.78.6.3061. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci. 2000;20:6734–6740. doi: 10.1523/JNEUROSCI.20-17-06734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Kuo JJ, Jiang MC, Heckman CJ. Influence of active dendritic currents on input-output processing in spinal motoneurons in vivo. J Neurophysiol. 2003;89:27–39. doi: 10.1152/jn.00137.2002. [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Effective synaptic current and motoneuron firing rate modulation. J Neurophysiol. 1995;74:793–801. doi: 10.1152/jn.1995.74.2.793. [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Summation of effective synaptic currents and firing rate modulation in cat spinal motoneurons. J Neurophysiol. 2000;83:483–500. doi: 10.1152/jn.2000.83.1.483. [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol. 2001;143:137–263. doi: 10.1007/BFb0115594. [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Persistent sodium and calcium currents in rat hypoglossal motoneurons. J Neurophysiol. 2003;89:615–24. doi: 10.1152/jn.00241.2002. [DOI] [PubMed] [Google Scholar]
- Prather JF, Powers RK, Cope TC. Amplification and linear summation of synaptic effects on motoneuron firing rate. J Neurophysiol. 2001;85:43–53. doi: 10.1152/jn.2001.85.1.43. [DOI] [PubMed] [Google Scholar]
- Rose PK, Cushing S. Non-linear summation of synaptic currents on spinal motoneurons: lessons from simulations of the behaviour of anatomically realistic models. Prog Brain Res. 1999;123:99–107. doi: 10.1016/s0079-6123(08)62847-2. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Membrane properties of cat spinal motoneurones. In: Davidoff RA, editor. Handbook of the Spinal Cord. 2 and 3. New York: Blackwell Science Inc; 1984. pp. 199–246. [Google Scholar]
- Svirskis G, Gutman A, Hounsgaard J. Electrotonic structure of motoneurons in the spinal cord of the turtle: inferences for the mechanisms of bistability. J Neurophysiol. 2001;85:391–398. doi: 10.1152/jn.2001.85.1.391. [DOI] [PubMed] [Google Scholar]
- Svirskis G, Hounsgaard J. Transmitter regulation of plateau properties in turtle motoneurons. J Neurophysiol. 1998;79:45–50. doi: 10.1152/jn.1998.79.1.45. [DOI] [PubMed] [Google Scholar]


