Abstract
The physiological significance of the presence of GLUT2 at the food-facing pole of intestinal cells is addressed by a study of fructose absorption in GLUT2-null and control mice submitted to different sugar diets. Confocal microscopy localization, protein and mRNA abundance, as well as tissue and membrane vesicle uptakes of fructose were assayed. GLUT2 was located in the basolateral membrane of mice fed a meal devoid of sugar or containing complex carbohydrates. In addition, the ingestion of a simple sugar meal promoted the massive recruitment of GLUT2 to the food-facing membrane. Fructose uptake in brush-border membrane vesicles from GLUT2-null mice was half that of wild-type mice and was similar to the cytochalasin B-insensitive component, i.e. GLUT5-mediated uptake. A 5 day consumption of sugar-rich diets increased fructose uptake fivefold in wild-type tissue rings when it only doubled in GLUT2-null tissue. GLUT5 was estimated to contribute to 100 % of total uptake in wild-type mice fed low-sugar diets, falling to 60 and 40 % with glucose and fructose diets respectively; the complement was ensured by GLUT2 activity. The results indicate that basal sugar uptake is mediated by the resident food-facing SGLT1 and GLUT5 transporters, whose mRNA abundances double in long-term dietary adaptation. We also observe that a large improvement of intestinal absorption is promoted by the transient recruitment of food-facing GLUT2, induced by the ingestion of a simple-sugar meal. Thus, GLUT2 and GLUT5 could exert complementary roles in adapting the absorption capacity of the intestine to occasional or repeated loads of dietary sugars.
Human diet, in Western countries, has been considerably enriched in readily absorbable sugar. Fructose, glucose and sucrose syrups are now commonly used in soft drinks. A high sugar diet increases the expression of the proteins required for sugar absorption: sucrase isomaltase (the enzyme that cleaves sucrose into glucose and fructose moieties) (Inukai et al. 1993; Burant & Saxena, 1994; Shu et al. 1997; Kishi et al. 1999) and GLUT2 (Miyamoto et al. 1993). This adaptation of the small intestine to sugar ingestion is rapid and reversible (Ferraris, 2001). Nevertheless, the underlying mechanisms of these acute and long-term adaptations are not fully understood.
The uptake of glucose in the small intestine is a sodium-dependent transport process mediated by SGLT1 (Wright, 1993), whereas that of fructose is specifically due to facilitated transport by GLUT5 in human (Burant et al. 1992), rat (Rand et al. 1993) and mouse (Corpe et al. 2002) small intestine. SGLT1 and GLUT5 proteins are mainly located in the food-facing membrane of mature, absorbing intestinal cells (Davidson et al. 1992; Mahraoui et al. 1992). Furthermore, a facilitative glucose transport activity has been described in the brush-border membrane of guinea pig enterocytes (Brot-Laroche et al. 1986); this transport component called BBS2 is regulated by diet (Brot-Laroche et al. 1988). Recently, GLUT2, a very efficient fructose and glucose facilitative transporter, was shown to be transiently associated with the apical membrane of enterocytes in ex vivo perfusion of rat jejunum (Helliwell et al. 2000a,b; Kellett, 2001; Au et al. 2002). On the other pole of the cell, glucose and fructose exit to the bloodstream through GLUT2 (Thorens, 1992; Cheeseman, 1993). In addition, GLUT5 in basolateral membranes (Mahraoui et al. 1992; Blakemore et al. 1995) might participate in fructose efflux. Nevertheless, the recent demonstration that mice lacking GLUT2 can absorb glucose normally (Stumpel et al. 2001) suggests that some other mechanisms may exist to allow sugar exit. Indeed, an exocytotic-related outflow of glucose is proposed to contribute to glucose exit from intestinal cells. This alternative pathway may contribute up to 15 % of the overall transepithelial transport of glucose in normal mice (Stumpel et al. 2001). In enterocytes, fructose can be converted into glucose (Bismut et al. 1993) and a glucose 6-phosphatase (G6Pase) activity converts glucose 6-phosphate to glucose in the endoplasmic reticulum (Rajas et al. 1999; Croset et al. 2001); the exocytosis process might therefore be relevant in case of prolonged fructose ingestion. It is still unclear to what extent GLUT2 and GLUT5 isoforms have overlapping biological roles in intestinal cells.
The physiological significance of the presence of GLUT2 and GLUT5, at the food-facing pole of intestinal cells, is addressed by a study of fructose absorption. To address this question, wild-type and GLUT2-null mice were fed fructose- or glucose-rich diets to stimulate GLUT2 or GLUT5 gene expression selectively. Our aims were to determine whether the lack of GLUT2 expression was deleterious to intestinal fructose absorption and to evaluate the relative contribution of GLUT2 and GLUT5 to sugar absorption capacities.
METHODS
Mice
Wild-type mice were from the C57Bl/6 strain (Janvier, France). GLUT2-null mice (RIP GLUT1 × GLUT2-/-) (Guillam et al. 1997) were bred in the transgenic animal facilities of the IFR58 (Paris). All animal procedures complied with published recommendations for the use of laboratory animals by the French government. Mice were fed for 5 days with a low carbohydrate-high protein, or a glucose- or fructose-rich-low protein diet (Table 1). Fed mice were taken at the end of the feeding period and the presence of food in the stomach was verified. In some experiments, mice received a gastric bolus (0.4 ml) of 40 % glucose, fructose or sucrose. After 30 min, blood samples from the tail vein were analysed with the Glucotrend plus test (Roche Diagnostics GmbH, Manheim, Germany). The diets did not cause any significant differences in body weights, the values were: for protein fed 23.5 ± 0.5 g, n = 19; for glucose fed 25.1 ± 0.3 g, n = 19; and for fructose fed 24.8 ± 0.4 g, n = 19 wild-type mice; and for protein fed 24.3 ± 1.0 g, n = 14; for glucose fed 24.1 ± 0.7 g, n = 17; and for fructose fed 22.4 ± 0.6 g, n = 18 GLUT2-null mice. Intestinal samples were taken rapidly after the mice were killed by cervical dislocation.
Table 1.
Composition of the various diets given to the mice
| Diet | Standard chow | Protein-rich LC | Glucose-rich HG | Fructose-rich HF |
|---|---|---|---|---|
| Protein or casein | 23 | 80 | 19 | 19 |
| Methionine | 0.45 | — | 0.3 | 0.3 |
| Cellulose | 7 | 6 | 2.7 | 2.7 |
| Carbohydrate | 51 | — | — | — |
| Dextrose | — | a | 65 | — |
| Fructose | — | — | — | 65 |
| Vitamin | 3 | 1 | 1 | 1 |
| Mineral salts | 6 | 7 | 7 | 7 |
| Lipid | 5 | 6 | 5 | 5 |
The components of the diets are expressed as percentage weight (kg) of the pellet. Lipids are provided as a 3:1 mix of corn and colza oil. The mineral salt mixture contains 29% starch. aLC diets were supplemented with 25 g kg−1 dextrose in order to keep GLUT2-null mice alive.
Messenger RNA analysis
Total RNA was extracted using TRI reagent (MRC, Cincinnati, USA) according to manufacturer's protocol. Messenger RNAs were analysed by Northern blot and/or real-time PCR. For Northern blots, rat GLUT5 (2.1 kb EcoRI insert, kind gift of G. I. Bell, University of Chicago, IL, USA), mouse GLUT2 cDNA (600 bp PvuII-EcoRI; Suzue et al. 1989) and human SGLT1 (2.0 kb EcoRI insert, G. I. Bell; Chantret et al. 1994) cDNA probes were used. Density analysis (Gel Analyst 3.02 software) was performed and results expressed as the ratio of mRNA to 18S rRNA. Reverse transcription (RT) was performed with 2 μg total RNA in the presence of 400 U MMLV reverse transcriptase (Boehringer), 13 μm pol (N) 6 random hexamer oligonucleotide (Amersham Pharmacia Biotech Europe), 40 U RNAse inhibitor (Boehringer), 0.5 mm dNTP, 10 μm DTT, 8 μl of RT 5× buffer in a total volume of 40 μl. Messenger RNA was quantified using the Light-Cycler System according to the manufacturer's procedures (Roche Molecular Biochemicals I primer Indianapolis, IN, USA). PCR reactions were performed with a 1:400 dilution of the RT in the SYBR Green I master mix with the specific primers for the cDNA of interest: mouse GLUT2 forward primer 5′-GTCCAGAAAGCCCCAGATACC-3′ and reverse primer 5′-GTGACATCCTCAGTTCCTCTTAG-3′ (Guillemain et al. 2002); glucose 6-phosphatase forward primer 5′-TTACCAAGACTCCCAGGACTG-3′, reverse primer 5′-GAGCTGTTGCTGTAGTAGTCG-3′, L19 forward primer 5′-AAGATCGATCGCCACATGTATCA-3′ and reverse primer 5′-TGCGTGCTTCCTTGGTCTTAGA-3′ (Guillemain et al. 2002). 18S rRNA (Applied Biosystem) was measured using a 1:2000 dilution of the RT. L19, a mRNA encoding a ribosomal subunit protein, and 18S rRNA were used as internal controls.
Uptake measurements
Tissue uptakes
Fructose uptake was measured in the jejunum. The intestine was everted and washed rapidly in ice-cold Ringer solution (mm: NaCl, 115; NaHCO3, 25; MgCl2, 1.2; CaCl2, 1.2; K2HPO4, 2.4; and KH2PO4, 0.4; pH 7.3) gassed with O2/CO2 5 %/95 % for 15 min. Rings were produced and kept in cold-gassed Ringer solution before incubation with the substrate at 37°C in a stirring water bath (1200 r.p.m.). Incubations were for 2 min in oxygenated Ringer solution containing 50 mmd-[U-14C]fructose (10 GBq mmol−1). l-[1-3H]glucose (133.6 MBq mmol−1) (New England Nuclear/Dupont de Nemours) was used to evaluate the non-specific uptake. The time was chosen to be within the linear region of uptake and sufficiently short to keep metabolism insignificant. Uptakes were stopped with ice-cold Ringer containing 300 mm mannitol and rinsed before extraction of radioactivity with 10 % HNO3. Counts were expressed in nanomoles per milligram wet weight per 2 min ±s.e.m. (n).
Brush border membrane vesicle transport assay
Cell homogenates were prepared from snap-frozen or fresh jejunum in 10 mm Hepes Tris-HCl, pH 7.4, containing 400 mm mannitol, 5 mm EGTA and 1 % protease inhibitors (Sigma). Brush-border membrane vesicles were prepared by MgCl2 EGTA precipitation (Brot-Laroche et al. 1985, 1988). Membranes were stored in liquid nitrogen until use. Uptake rates were measured according to the rapid filtration technique essentially as described in (Brot-Laroche et al. 1985, 1988) using 10 mm[U-14C]fructose as substrate. [1-3H]l-glucose (10 mm) was measured in parallel to determine non-specific uptake. Cytochalasin B (CB, 10 μm) was used to inhibit facilitative GLUT2 transport but not GLUT5, which is insensitive to this inhibitor (Burant et al. 1992; Corpe et al. 2002). Counts were expresssed in picomoles per milligram protein per 5 s ±s.e.m. (n).
Western blots
Intestinal total membrane fractions and brush-border membranes were prepared from the wild-type and GLUT2-null mice. Protein samples (40 μg) were solubilized in the Laemmli buffer (0.125 m Tris-HCl pH 6.8, 20 % glycerol, 4 % SDS, 10 % β-mercaptoethanol) and electrophoresed under denaturing conditions in 10 % SDS gels. Proteins were electro-transferred to Hybond-ECL membranes (Amersham Pharmacia Biotech Europe). The membranes were blocked with fat-free milk (5 %) and then allowed to react with 1/500 dilution of antibodies directed against peptide SHYRHVLGVPLDDRRA of the first extracellular loop between (TM1-TM2) of GLUT2; against peptide RNSTEERIDLDA of SGLT1; and against peptide AEIEAQFDEDEKKK of PepT1. All antibodies were raised in the rabbit and they were targeted against peptide antigens that are identical in the human, rat and mouse sequences. The antibody ECCD2 (Zymed, San Francisco, USA) was against E-cadherin. The antibody raised against the first extracellular loop of GLUT2 was compared with an antibody targeting the C-terminus peptide of mouse GLUT2 (Thorens, 1992) and gave identical immunoblot results (not shown). The horseradish-peroxidase-coupled, secondary antibody was produced in the donkey (Santa Cruz Biotech, California, USA). The luminol ECL detection system (Amersham Pharmacia Biotech Europe) was used to reveal the peroxidase label. The relative abundance of the proteins was assayed by densitometry using the Gelanalyst 3.01 software (Greystone-Iconix).
Immunofluorescence studies
The small intestine was rapidly everted and washed in ice-cold O2/CO2 (5 %/95 %)-gassed Krebs Ringer buffer pH 7.4, supplemented with 50 mmd-fructose, d-glucose or l-glucose as required. The samples were then fixed for 30 min in ice-cold paraformaldehyde 4 % containing the appropriate 50 mm sugar and then incubated overnight in 20 % sucrose-PBS at 4°C. Samples were frozen in the Cryomatrix protector (Shandon) with isopentane at −80°C and stored. Cryosections (7 μm) were taken. After washes with 100 mm glycine in PBS and saturation in 3 % BSA, 0.2 % Tween 20 in PBS for 30 min, the samples were incubated for 1 h at 37°C with the GLUT2-L1 antibody (1/100). The label was revealed with a secondary anti-rabbit antibody CY3 or CY2 (1/200) in donkey. Nuclei were labelled with TOTO-3 (Molecular Probes) after an RNAse A treatment to eliminate RNA labelling. Control sections were labelled with anti-GLUT2 that was neutralized for 30 min prior to use with an excess of the peptide antigen, giving no fluorescent signal. Fluorescence analysis was performed with a ZeissLSM510 confocal microscope.
Statistical analyses
For multiple comparisons, statistical analyses were performed using a one-way analysis of variance (ANOVA). Data analysis was performed using GraphPad Prism software and considered statistically significant at P values <0.05.
RESULTS
This study was designed to evaluate GLUT2- and GLUT5-related intestinal fructose absorption. We compared and contrasted results obtained in wild-type and GLUT2-null mice at the expression and function levels. The physiological characteristics of the GLUT2-null mice have already been described (Guillam et al. 1997; Stumpel et al. 2001) and our aim was to extend the investigations towards short, i.e. after a meal, or prolonged impact of dietary manipulation.
The levels of glycaemia (Table 2) of wild-type mice fed high-glucose and high-fructose pellets (c; P < 0.001) were identical and both were higher than that of mice fed a low-carbohydrate-high protein diet (a). By contrast, the level of glycaemia of GLUT2-null mice fed a high-fructose diet was lower than that of the corresponding wild-type mice (P < 0.001) and the difference was maintained after a gastric fructose load, when glycaemia increased twofold (d; P < 0.001) in wild-type mice and GLUT2-null mice (h; P < 0.001) respectively. The blood glucose levels were identical in GLUT2-null and wild-type mice fed a glucose-rich diet or the standard chow. Overall these levels of glycaemia indicate that the regulation of glycaemia is altered in the GLUT2-null mice.
Table 2.
Effect of dietary manipulation on mice glycaemia
| Diet | Wild-type mice mm±s.e.m.(n) | GLUT2-null mice mm±s.e.m.(n) | Wild/null |
|---|---|---|---|
| LC | 5.10 ± 0.72(26)a | 4.43 ± 0.83(14)e | * |
| HG | 7.04 ± 0.94(25)c | 6.60 ± 1.44(32)f | n.s. |
| HF | 7.63 ± 1.27 (34)c | 5.44 ± 1.10(41)g | *** |
| HF + fructose bolus | 11.93 ± 2.38(18)d | 8.27 ± 1.60(20)h | *** |
| Standard chow | 5.93 ± 0.66 (13)b | 6.71 ± 1.05 (6)f | n.s. |
Mice had free access to the different diets for 5 days. Glucose was measured from tail blood at 9:00 a.m. The levels of glycaemia of fructose-fed mice were measured before (HF) and 30 min after a fructose bolus in the stomach (HF + fructose bolus) and were statistically different P < 0.001 in both GLUT2-null and wild-type mice. Statistical significance
p < 0.001 and
P < 0.05 comparing wild-type and GLUT2-null mice for each diet. Letters indicate statistically significant differences relative to sugar (HG, HF, HF + fructose bolus and standard chow) in the diet as compared with low carbohydrate supply (LC) in wild-type animals: bP < 0.01, a,c,dP < 0.001; and GLUT2-null mice: e,g,hP < 0.0001, fP < 0.05.
Dietary glucose or fructose manipulation affects facilitative-transporter mRNA expression
Sugar-rich diets efficiently upregulate intestinal GLUT5 and GLUT2 mRNA. We performed Northern blots and quantitative RT-PCR assays (Fig. 1) on total RNA extracts from the mucosa of the upper jejunum. In wild-type mice, GLUT2 and SGLT1 mRNA levels were increased to the same extent by glucose and fructose feeding, whereas the fructose feeding specifically stimulated GLUT5 mRNA. Glucose 6-phosphatase (G6Pase) mRNA was threefold higher in fructose-fed than in glucose-fed mice. GLUT2-null mice exhibited very similar dietary responses to wild-type mice for SGLT1 and GLUT5 mRNA. GLUT5 mRNA was expressed in the ileum of wild-type animals fed a high fructose diet but was undetectable when glucose-rich or low carbohydrate diets were applied (Fig. 1). Interestingly, GLUT5 mRNA was increased threefold in the ileum of GLUT2-null mice as compared with wild-type animals, indicating a higher stimulation when GLUT2 expression is absent. Moreover, in GLUT2-null mice, a large increase in G6Pase mRNA was observed when compared with wild-type mice, probably relevant to the exocytotic pathway.
Figure 1. Transporter mRNA expression upon adaptation to carbohydrate diets.
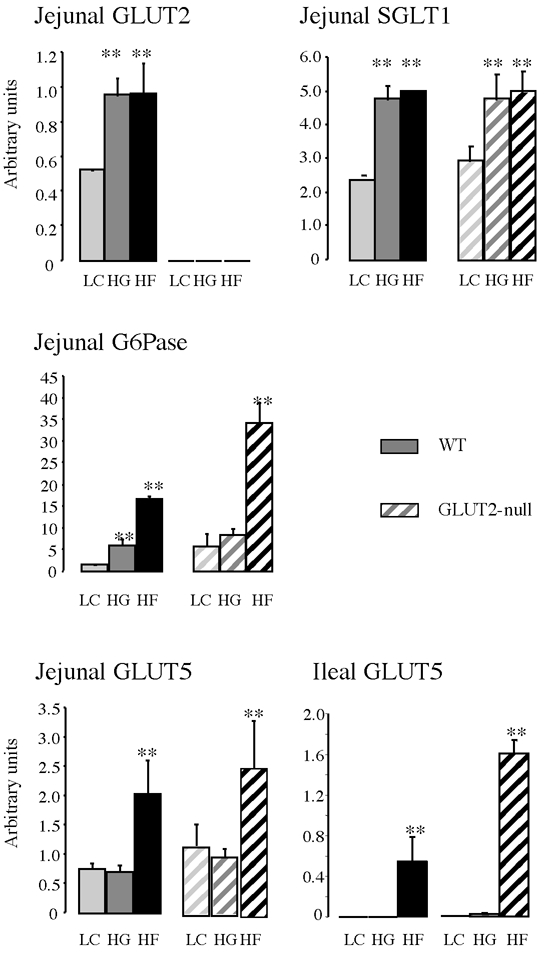
Intestinal RNA samples were extracted from mucosal scrapping of the upper jejunum or of the ileum of wild-type (WT, filled bars) or GLUT-2 null (hatched bars) mice. Messenger abundance was quantified either by density scanning of Northern blots (GLUT2, SGLT1, GLUT5) or by RT-PCR in real time with a Light-Cycler (GLUT2, glucose 6-phosphatase (G6Pase)). Results were obtained with an average 6 mice fed low carbohydrate (LC, light grey), glucose-rich (HG, grey) or fructose-rich (HF, dark grey) diets for 5 days. All quantifications were normalized against L19 or the 18S ribosomal RNA. Results are expressed in arbitrary units ±s.e.m.; ** significant difference (P < 0.01) of data when compared with LC diet.
GLUT2 protein expression in intestinal brush-border membranes
GLUT2 protein was found in the crude membrane fraction (CM) and was enriched in the brush-border membrane (BBM) in our experimental conditions (Fig. 2A). E-cadherin, a protein associated with the basolateral membrane domain of enterocytes was used to verify the purity of our vesicle preparations (Fig. 2A); it was below the level of detection in the 40 μg BBM protein sample, although it was readily detected in 40 μg of crude membrane preparations. SGLT1 and PepT1, two markers of the apical domain of enterocytes, were strongly enriched in BBM preparations of wild-type mice (Fig. 2A). A similar enrichment for SGLT1 and PepT1 in BBM was obtained with GLUT2-null-mice (data not shown). As expected, GLUT2 protein was not detected in the BBM of GLUT2-null mice (Fig. 2B) whereas the GLUT2 level was very high in mice fed a glucose- or fructose-rich diet (Fig. 2B). In chow-fed mice or in fructose-fed mice that have been starved overnight, the amount of GLUT2 protein was low in the BBM (Fig. 2C). By contrast, a strong GLUT2 signal was obtained in the brush border from starved mice that had received a fructose, glucose, fructose and glucose, or sucrose bolus for 30 min and from mice that still had free access to fructose food and that had free fructose remaining in their intestine. Unfortunately, we could not evaluate the amount of GLUT5 protein in the BBM due to lack of mouse-specific antibodies. Nevertheless, we could quantify GLUT5 function by using a biochemical approach.
Figure 2. GLUT2 protein expression in purified brush-border membrane.
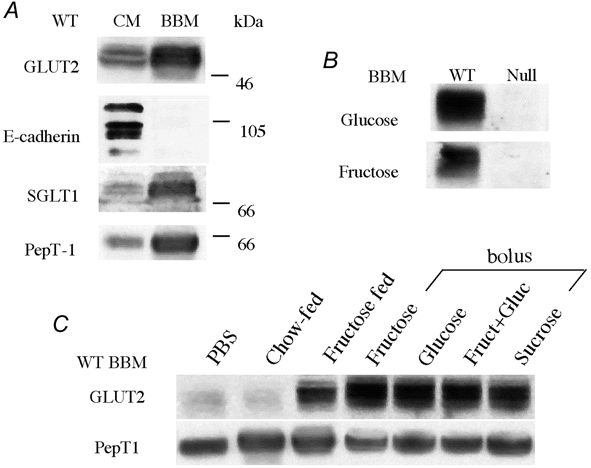
Intestinal brush-border membranes (BBM, 40 μg) were prepared from the jejunum of wild-type (WT) or GLUT2-null (Null) mice. Western blots were then performed as described in Methods. ECL-films were exposed for an average 3 min. A, presence of GLUT2 in purified BBM preparation. The purity of BBM vesicles is shown. E-cadherin, a basolateral membrane marker, is present only in crude membrane fractions (CM) and absent in corresponding purified BBM. SGLT-1 and PepT-1 brush membrane markers are enriched in the BBM fraction. B, presence of GLUT2 in BBM from wild-type and absence from GLUT2-null mice fed a glucose-rich or a fructose-rich diet. C, expression of GLUT2 in BBM preparations from wild-type mice submitted to dietary manipulations. GLUT2 was absent from the BBM of mice receiving a PBS bolus after an overnight fast or mice fed a standard chow ad libitum. GLUT2 was present in the BBM of mice fed a fructose diet ad libitum, or in BBM of mice that had received a 40 % glucose, fructose, glucose + fructose or sucrose gastric bolus after an overnight fast. Experiments represent 2–3 independent BBM preparations with 6–9 mice.
Fructose uptake in purified BBM vesicles
We then measured fructose uptake in BBM vesicles from wild-type mice expressing high GLUT2 levels and from GLUT2-null mice expressing high GLUT5 levels, i.e. in fructose-fed mice. Fructose was used as a test substrate to distinguish between GLUT5 and GLUT2. Cytochalasin B selectively inhibits GLUT2 but not GLUT5 fructose transport activity in human and mouse (Burant et al. 1992; Corpe et al. 2002). We could thus calculate the relative contribution of GLUT2 and GLUT5 to fructose absorption at the apical membrane. Fructose uptake was inhibited by 60 % by cytochalasin B in BBM vesicles from wild-type mice whereas it was unchanged in vesicles from GLUT2-null mice (Fig. 3). In addition, the residual uptake level measured in the presence of cytochalasin B in wild-type mice was identical to that measured in GLUT2-null mice vesicle preparations (Fig. 3). Fructose uptake through the apical membrane of enterocytes is thus probably the sum of fructose uptake by a cytochalasin B-insensitive (GLUT5) and a cytochalasin B-sensitive fructose transporter (GLUT2).
Figure 3. Fructose uptake in jejunal BBM vesicles.
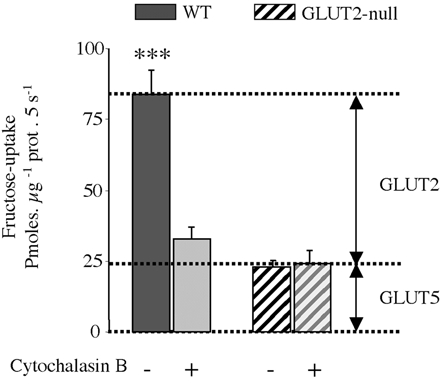
The uptake of 10 mm fructose was measured in isolated BBM vesicles at 35°C for 5 s in the presence or absence of 10 μm cytochalasin B. Wild-type and GLUT2-null mice were fed a fructose diet for 5 days, and 30 min after a fructose bolus the intestine was dissected. We used 3 membrane-vesicle preparations from 3 independent experiments with 5 mice per membrane preparation. Results are expressed as picomoles per microgram of protein per 5 seconds ±s.e.m., (n = 6–10 determinations; ***P < 0.001). Data were corrected for non-carrier mediated diffusion as estimated by 10 mml-glucose uptakes, measured in parallel.
Dietary regulation of the contribution of GLUT2 and GLUT5 to fructose uptake
Fructose transport assays in tissue rings were designed to evaluate uptake at the luminal side of the intestine in mice fed various sugar diets. Because the insertion of GLUT2 in the BBM declines rapidly in the absence of sugar (Kellett, 2001), incubation sequences were randomized to avoid an unwanted effect of the time after intestinal preparation. In all mice, non-specific uptake and binding, as estimated by [1-3H]l-glucose label, were similar. The fructose concentration was chosen to compare the uptake at a concentration appropriate to the Km value for GLUT5 in the range of 6–12 mm (Burant et al. 1992; Corpe et al. 2002) and for GLUT2 in the range of 16–25 mm (Gould et al. 1991). A 50 mm concentration was suitable to assay both transport activities.
Fructose uptake in the intestine of wild-type and GLUT2-null mice fed the low carbohydrate diet was identical (Fig. 4) suggesting that GLUT5 is the operating transporter. The GLUT5 contribution to fructose uptake varied thus from 100 % of total uptake in low-sugar-fed mice, dropping down to 60 % in glucose- and to 40 % in fructose-fed mice. Fructose uptake was significantly higher in wild-type mice fed glucose- (2.1-fold, P < 0.001) or fructose- (5.7-fold, P < 0.001) rich diet when compared with the low carbohydrate diet. In sharp contrast, fructose uptake was not stimulated in the intestine of GLUT2-null mice fed the glucose diet (Fig. 4) as compared with the low carbohydrate diet, indicating some loss of transport capacity in mice lacking GLUT2. Interestingly, feeding GLUT2-null mice with fructose, upregulated fructose uptake twofold (Fig. 4), a result relevant to the stimulation of GLUT5 mRNA expression by fructose in mice (Fig. 1). The most striking difference observed was between wild-type and GLUT2-null mice fed the fructose-rich diet. When challenged by fructose feeding, GLUT2-null mice exhibited a 60 % reduction in transport capacity as compared with wild-type mice, suggesting that the lack of GLUT2 strongly diminishes fructose uptake in the jejunum of GLUT2-null mice.
Figure 4. Fructose uptake in jejunal rings.
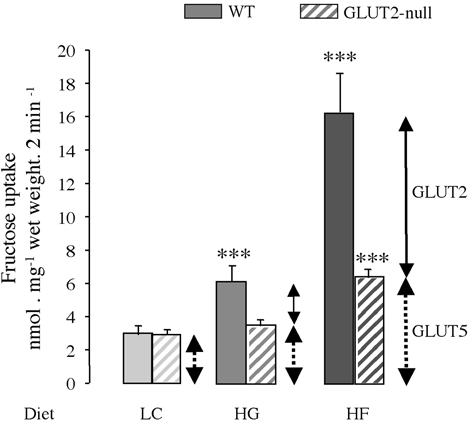
Wild-type (filled bars) and GLUT2-null (hatched bars) mice were fed a low carbohydrate (LC, light grey), glucose-rich (HG, medium grey) or fructose-rich (HF, dark grey) diet for 5 days. Everted intestinal rings were incubated for 2 min in 50 mm fructose. Results are expressed as nanomoles per milligram wet weight per 2 minutes ±s.e.m. obtained in 3 separate experiments with at least 15 uptake determinations. Each condition has been performed with at least 12 mice per group. Statistical significance, ***P < 0.001.
Basolateral and apical localization of GLUT2
We further localized GLUT2 in the jejunum of sugar-deprived or fructose-fed wild-type and GLUT2-null mice by immunofluorescence and confocal microscopy (Fig. 5). As expected, the GLUT2 protein was found in the villi but not in the crypts of the jejunum (data not shown). It was always located in the basolateral membranes up to the terminal web and to a lower extent in the cytoplasm of wild-type cells. GLUT2 could not be detected in GLUT2-null mice, which were used as non-specific controls (Fig. 5A). Similar low backgrounds were obtained using the peptide-blocked antibody indicating that the antibody did not cross-react with other intestinal proteins (Fig. 5 compare A and E). Importantly, and in addition to its basolateral expression, the GLUT2 protein was located in the apical membrane domain of enterocytes after fructose- (Fig. 5C and D) or glucose-bolus (data not shown). Apical GLUT2 label was observed over the whole length of the villus (Fig. 5C).
Figure 5. Localization of GLUT2 by immunohistochemistry in the membrane domain of enterocytes.
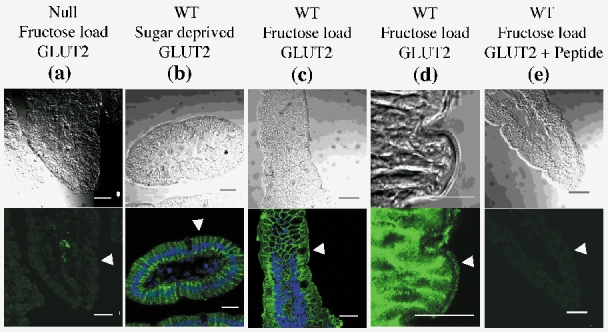
Mice were fed either the low carbohydrate (sugar deprived, B) or the fructose-rich diet together a fructose load (A, C, D, E). Antibody against the extracellular loop of GLUT2 (green) was used in GLUT2-null (A) and wild-type (B-E) mice. The TOTO-3 label of nuclei is in blue (B and C). A cross-section of a villus from a sugar-deprived animal is shown (B). Note the fluorescence of GLUT2 in the basolateral membrane but not in the apical membrane domains of the cells. GLUT2 is present in the apical and basolateral membranes of fructose-loaded mice (C). A higher magnification of an intestinal villus lining three enterocytes in fructose-stimulated mice is shown (D). Figures are representative of at least three immunolabelling experiments. Scale (white bars) 20 μm. Note the similarity between GLUT2-null background (A) and that obtained with peptide-blocked GLUT2 antibodies (E). Arrowheads point to the apical membrane domain of enterocytes.
DISCUSSION
The scheme of two entry-transporters, GLUT5 and SGLT1, and one exit-transporter, GLUT2, for sugar transfer across enterocytes now appears oversimplified. We used wild-type and GLUT2-null mice fed various diets to study fructose uptake and to show that GLUT2 has an important role to play at the BBM. Our studies demonstrate for the first time how long-term dietary adaptation impacts the acute induction of apical GLUT2-mediated fructose absorption. We also show that GLUT2 has a strong, positive impact on intestinal fructose absorption in mice fed a sugar-rich diet. Absorption in GLUT2-null mice was reduced to less than half the value measured in wild-type mice indicating that the missing transport capacity could not be compensated fully by GLUT5.
GLUT5 accounts for 100 % of fructose uptake in low carbohydrate conditions. The upregulation of GLUT5 expression by fructose feeding is well documented (Ferraris, 2001). Fructose adaptation is developed in order to meet the intestinal requirement for total absorption of sugars. It is worth emphasizing that GLUT5 mRNA was expressed in the distal part of the small intestine of fructose-fed animals suggesting that fructose transport by GLUT5 is saturated in the proximal part of the intestine. These results indicate some degree of fructose malabsorption when wild-type mice are fed a fructose-rich diet. Thus, the threefold higher expression of GLUT5 in the ileum of GLUT2-null mice is probably a consequence of increased luminal fructose in order to compensate for the lower capacity of absorption due to the lack of GLUT2.
In animals fed the low carbohydrate diet, the lack of GLUT2, the other fructose transporter in the jejunum, does not affect fructose absorption. In previous work, we have reported that the kinetics glucose of transport in GLUT2-null mice were indistinguishable from those in control mice (Stumpel et al. 2001). At first sight, such a result seems at variance with the demonstration that glucose and fructose cause rapid trafficking of GLUT2 to the apical membrane in wild-type mice, resulting in the large differences in fructose transport compared with GLUT2-null mice described above. The explanation of the apparent paradox lies in the differences in diet on which the mice are maintained. In the previous studies, mice were maintained on a complex carbohydrate diet. In this case, the luminal load of readily absorbable sugars is low, so that the GLUT2 level at the apical membrane is minimal and therefore the two high affinity transporters, GLUT5 or SGLT1, are able to provide the whole of sugar uptake necessary in GLUT2-null mice. By contrast, we show that GLUT2 can account for up to 60 % of the total fructose uptake across the food-facing (apical) membrane of enterocytes in animals challenged with high loads of simple sugar nutrients; these data therefore suggest that GLUT2 is an essential contributor to sugar absorption in such circumstances. Accordingly, we propose that sugar-induced trafficking of GLUT2 to the apical membrane provides rapid and precise regulation of absorptive capacity to match dietary intake in conditions of sugar abundance.
GLUT2-dependent and GLUT2-independent pathways mediate the release of sugar into the portal vein. Indeed GLUT2-null mice can absorb sugars to some extent and thrive provided some sugar is supplied, indicating that the export of glucose and fructose exists (Thorens et al. 2000; Stumpel et al. 2001). In addition, the intestine of GLUT2-null mice adapts to diminished transport capacity by increasing intestinal length (data not shown). However, our data indicate that alternative pathways for release of sugar in blood are probably overloaded in case of high sugar ingestion.
We showed that the consumption of a fructose-, a glucose- or a sucrose-rich (glucose-fructose) meal is sufficient to promote the insertion of GLUT2 in the food-facing membrane of enterocytes within 30 min. GLUT2 insertion has also been observed ex vivo in rat intestines (Helliwell et al. 2000b; Kellett, 2001; Au et al. 2002). We could show that this process occurred in vivo, i.e. in physiological conditions in non-anaesthetized mice. Little is known about the mechanism of GLUT2 insertion at the food-facing membrane. Activation of the protein kinase C (PKC) and MAP kinase transduction pathways (Helliwell et al. 2000b; Kellett, 2001), as well as GLP-2 entero-endocrine signalling (Au et al. 2002), are likely to be stimuli. Nevertheless, a sugar meal could activate other pathways including metabolic, endocrine and nervous signals.
In other species, rat (Kellett & Helliwell, 2000; Kellett, 2001; Au et al. 2002) or mice (this study), the presence of GLUT2 at the apical membrane of enterocytes has been documented. Moreover, a facilitative glucose transport system has been functionally described in guinea pigs (Brot-Laroche et al. 1985, 1988) and pigs (unpublished work of E. Brot-Laroche). This transport system called brush border system 2 (BBS2) has the functional features of GLUT2, i.e. low affinity (25–50 mm) and inhibition by cytochalasin B. Moreover, BBS2 was able to be measured in vesicles prepared from intestine of fed but not in fasted guinea pigs (Brot-Laroche et al. 1988). However, the direct demonstration that BBS2 is GLUT2 remains to be established because of the need for species-specific antibodies. In human small intestine, the difficulty of demonstrating the expression of GLUT2 in the BBM is most likely to be because of the biopsy practice for which intestines must be empty from food traces. In these conditions, GLUT2 expression is restricted to the basolateral membrane of enterocytes (Dyer et al. 2002).
Several malabsorption syndromes provide important information concerning the relative contribution of GLUT2 and GLUT5 in humans. The Fanconi-Bickel syndrome (FBS) is the first example of a human genetic disorder caused by a mutation of the GLUT2 gene, resulting in an inactive truncated protein (Santer et al. 1998). Patients suffering FBS have clinical symptoms of fasting hypoglycaemia, postprandial hyperglycaemia and hepatomegaly secondary to glycogen storage. The patients exhibit glycosuria and glucose-galactose intolerance associated with intestinal malabsorption (Santer et al. 1998; Sakamoto et al. 2000), but they tolerate fructose in their food indicating that the lack of functional GLUT2 does not impair the function of GLUT5 in humans. It is noteworthy that the first patient identified with FBS was a shepherd, who was essentially eating milk products probably containing low amounts of free glucose or fructose and so consuming a suitable diet in view of his genetic defect and the present data.
Fructose malabsorption is frequent in infants who are given purified fructose-rich juice. These malabsorbing toddlers exhibit a positive hydrogen breath test upon fructose ingestion, indicating that the colonic flora can ferment the sugar. These toddlers do not suffer from GLUT5 genetic deficiency (Hoekstra et al. 1993; Wasserman et al. 1996) indicating that some other mechanism is involved. We can therefore speculate that some fructose-intolerant toddlers might exhibit intestinal transport defaults, characterized by defective GLUT2 insertion in intestinal membranes.
If luminal concentrations of fructose are low, the absorptive capacity provided by GLUT5 alone appears sufficient to cope with dietary intake. However, when the diet contains a large concentration of simple sugars, additional absorptive capacity is provided by the rapid recruitment of GLUT2 to the food-facing membrane. The relative roles of GLUT5 and GLUT2 in short-term regulation of sugar uptake will depend on the level of expression of both transporters related to the nature of long-term diet. Our understanding of the respective roles of GLUT2 and GLUT5 in the physiology of intestinal absorption of sugars has increased. It paves the way for the establishment of strategies for pharmacological and nutritional intervention to reduce, or increase, the capacity of intestinal absorption of sugars.
Acknowledgments
We thank Professor J. Chambaz for constant support and fruitful discussion. We are grateful to Dr M. Vasseur for access to the rapid thermostatic incubation machine in vesicle uptake assays. We wish to acknowledge the contribution of C. Lasne and M. Seau for animal care. G.L.K. is grateful to The Wellcome trust for support. G.L.K. is the recipient of a Leverhulme Trust Research Fellowship. F.G. is a fellow of the French MRT. A.L. is the recipient of a grant No. 5531 from ARC.
REFERENCES
- Au A, Gupta A, Schembri P, Cheeseman CI. Rapid insertion of GLUT2 into the rat jejunal brush-border membrane promoted by glucagon-like peptide 2. Biochem J. 2002;367:247–254. doi: 10.1042/BJ20020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bismut H, Hers HG, Van Schaftingen E. Conversion of fructose to glucose in the rabbit small intestine. A reappraisal of the direct pathway. Eur J Biochem. 1993;213:721–726. doi: 10.1111/j.1432-1033.1993.tb17812.x. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Aledo JC, James J, Campbell FC, Lucocq JM, Hundal HS. The GLUT5 hexose transporter is also localized to the basolateral membrane of the human jejunum. Biochem J. 1995;309:7–12. doi: 10.1042/bj3090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brot-Laroche E, Dao MT, Alcalde AI, Delhomme B, Triadou N, Alvarado F. Independent modulation by food supply of two distinct sodium-activated D-glucose transport systems in the guinea pig jejunal brush-border membrane. Proc Natl Acad Sci U S A. 1988;85:6370–6373. doi: 10.1073/pnas.85.17.6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brot-Laroche E, Serrano MA, Delhomme B, Alvarado F. Different temperature sensitivity and cation specificity of two distinct D-glucose/Na+ cotransport systems in the intestinal brush-border membrane. Ann N Y Acad Sci. 1985;456:47–50. doi: 10.1111/j.1749-6632.1985.tb14843.x. [DOI] [PubMed] [Google Scholar]
- Brot-Laroche E, Serrano MA, Delhomme B, Alvarado F. Temperature sensitivity and substrate specificity of two distinct Na+- activated D-glucose transport systems in guinea pig jejunal brush border membrane vesicles. J Biol Chem. 1986;261:6168–6176. [PubMed] [Google Scholar]
- Burant CF, Saxena M. Rapid reversible substrate regulation of fructose transporter expression in rat small intestine and kidney. Am J Physiol. 1994;267:G71–79. doi: 10.1152/ajpgi.1994.267.1.G71. [DOI] [PubMed] [Google Scholar]
- Burant CF, Takeda J, Brot-Laroche E, Bell GI, Davidson NO. Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem. 1992;267:14523–14526. [PubMed] [Google Scholar]
- Chantret I, Rodolosse A, Barbat A, Dussaulx E, Brot-Laroche E, Zweibaum A, Rousset M. Differential expression of sucrase-isomaltase in clones isolated from early and late passages of the cell line Caco-2: evidence for glucose-dependent negative regulation. J Cell Sci. 1994;107:213–225. doi: 10.1242/jcs.107.1.213. [DOI] [PubMed] [Google Scholar]
- Cheeseman CI. GLUT2 is the transporter for fructose across the rat intestinal basolateral membrane. Gastroenterology. 1993;105:1050–1056. doi: 10.1016/0016-5085(93)90948-c. [DOI] [PubMed] [Google Scholar]
- Corpe CP, Bovelander FJ, Munoz CM, Hoekstra JH, Simpson IA, Kwon O, Levine M, Burant CF. Cloning and functional characterization of the mouse fructose transporter, GLUT5. Biochim Biophys Acta. 2002;1576:191–197. doi: 10.1016/s0167-4781(02)00284-1. [DOI] [PubMed] [Google Scholar]
- Croset M, Rajas F, Zitoun C, Hurot JM, Montano S, Mithieux G. Rat small intestine is an insulin-sensitive gluconeogenic organ. Diabetes. 2001;50:740–746. doi: 10.2337/diabetes.50.4.740. [DOI] [PubMed] [Google Scholar]
- Davidson NO, Hausman AM, Ifkovits CA, Buse JB, Gould GW, Burant CF, Bell GI. Human intestinal glucose transporter expression and localization of GLUT5. Am J Physiol. 1992;262:C795–800. doi: 10.1152/ajpcell.1992.262.3.C795. [DOI] [PubMed] [Google Scholar]
- Dyer J, Wood IS, Palejwala AEA, Shirazi-Beechey SP. Expression of monosaccharide transporters in intestine of diabetic humans. Am J Physiol Gastrointest Liver Physiol. 2002;282:G241–248. doi: 10.1152/ajpgi.00310.2001. [DOI] [PubMed] [Google Scholar]
- Ferraris RP. Dietary and developmental regulation of intestinal sugar transport. Biochem J. 2001;360:265–276. doi: 10.1042/0264-6021:3600265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould GW, Thomas HM, Jess TJ, Bell GI. Expression of human glucose transporters in Xenopus oocytes: kinetic characterization and substrate specificities of the erythrocyte, liver, and brain isoforms. Biochemistry. 1991;30:5139–5145. doi: 10.1021/bi00235a004. [DOI] [PubMed] [Google Scholar]
- Guillam MT, Hummler E, Schaerer E, Yeh JI, Birnbaum MJ, Beermann F, Schmidt A, Deriaz N, Thorens B, Wu JY. Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nat Genet. 1997;17:327–330. doi: 10.1038/ng1197-327. [DOI] [PubMed] [Google Scholar]
- Guillemain G, Munoz-Alonso MJ, Cassany A, Loizeau M, Faussat AM, Burnol AF, Leturque A. Karyopherin alpha2: a control step of glucose-sensitive gene expression in hepatic cells. Biochem J. 2002;364:201–209. doi: 10.1042/bj3640201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell PA, Richardson M, Affleck J, Kellett GL. Regulation of GLUT5, GLUT2 and intestinal brush-border fructose absorption by the extracellular signal-regulated kinase, p38 mitogen-activated kinase and phosphatidylinositol 3-kinase intracellular signalling pathways: implications for adaptation to diabetes. Biochem J. 2000a;350:163–169. [PMC free article] [PubMed] [Google Scholar]
- Helliwell PA, Richardson M, Affleck J, Kellett GL. Stimulation of fructose transport across the intestinal brush-border membrane by PMA is mediated by GLUT2 and dynamically regulated by protein kinase C. Biochem J. 2000b;350:149–154. [PMC free article] [PubMed] [Google Scholar]
- Hoekstra JH, van Kempen AA, Kneepkens CM. Apple juice malabsorption: fructose or sorbitol. J Pediatr Gastroenterol Nutr. 1993;16:39–42. doi: 10.1097/00005176-199301000-00008. [DOI] [PubMed] [Google Scholar]
- Inukai K, Asano T, Katagiri H, Ishihara H, Anai M, Fukushima Y, Tsukuda K, Kikuchi M, Yazaki Y, Oka Y. Cloning and increased expression with fructose feeding of rat jejunal GLUT5. Endocrinology. 1993;133:2009–2014. doi: 10.1210/endo.133.5.8404647. [DOI] [PubMed] [Google Scholar]
- Kellett GL. The facilitated component of intestinal glucose absorption. J Physiol. 2001;531:585–595. doi: 10.1111/j.1469-7793.2001.0585h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellett GL, Helliwell PA. The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem J. 2000;350:155–162. [PMC free article] [PubMed] [Google Scholar]
- Kishi K, Tanaka T, Igawa M, Takase S, Goda T. Sucrase-isomaltase and hexose transporter gene expressions are coordinately enhanced by dietary fructose in rat jejunum. J Nutr. 1999;129:953–956. doi: 10.1093/jn/129.5.953. [DOI] [PubMed] [Google Scholar]
- Mahraoui L, Rousset M, Dussaulx E, Darmoul D, Zweibaum A, Brot-Laroche E. Expression and localization of GLUT-5 in Caco-2 cells, human small intestine, and colon. Am J Physiol. 1992;263:G312–318. doi: 10.1152/ajpgi.1992.263.3.G312. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Hase K, Takagi T, Fujii T, Taketani Y, Minami H, Oka T, Nakabou Y. Differential responses of intestinal glucose transporter mRNA transcripts to levels of dietary sugars. Biochem J. 1993;295:211–215. doi: 10.1042/bj2950211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajas F, Bruni N, Montano S, Zitoun C, Mithieux G. The glucose-6 phosphatase gene is expressed in human and rat small intestine: regulation of expression in fasted and diabetic rats. Gastroenterology. 1999;117:132–139. doi: 10.1016/s0016-5085(99)70559-7. [DOI] [PubMed] [Google Scholar]
- Rand EB, Depaoli AM, Davidson NO, Bell GI, Burant CF. Sequence, tissue distribution, and functional characterization of the rat fructose transporter GLUT5. Am J Physiol. 1993;264:G1169–1176. doi: 10.1152/ajpgi.1993.264.6.G1169. [DOI] [PubMed] [Google Scholar]
- Sakamoto O, Ogawa E, Ohura T, Igarashi Y, Matsubara Y, Narisawa K, Iinuma K. Mutation analysis of the GLUT2 gene in patients with Fanconi-Bickel syndrome. Pediatr Res. 2000;48:586–589. doi: 10.1203/00006450-200011000-00005. [DOI] [PubMed] [Google Scholar]
- Santer R, Schneppenheim R, Suter D, Schaub J, Steinmann B. Fanconi-Bickel syndrome-the original patient and his natural history, historical steps leading to the primary defect, and a review of the literature. Eur J Pediatr. 1998;157:783–797. doi: 10.1007/s004310050937. [DOI] [PubMed] [Google Scholar]
- Shu R, David ES, Ferraris RP. Dietary fructose enhances intestinal fructose transport and GLUT5 expression in weaning rats. Am J Physiol. 1997;272:G446–453. doi: 10.1152/ajpgi.1997.272.3.G446. [DOI] [PubMed] [Google Scholar]
- Stumpel F, Burcelin R, Jungermann K, Thorens B. Normal kinetics of intestinal glucose absorption in the absence of GLUT2: evidence for a transport pathway requiring glucose phosphorylation and transfer into the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2001;98:11330–11335. doi: 10.1073/pnas.211357698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzue K, Lodish HF, Thorens B. Sequence of the mouse liver glucose transporter. Nucleic Acids Res. 1989;17:10099. doi: 10.1093/nar/17.23.10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B. Molecular and cellular physiology of GLUT-2, a high-Km facilitated diffusion glucose transporter. Int Rev Cytol. 1992;137:209–238. doi: 10.1016/s0074-7696(08)62677-7. [DOI] [PubMed] [Google Scholar]
- Thorens B, Guillam MT, Beermann F, Burcelin R, Jaquet M. Transgenic reexpression of GLUT1 or GLUT2 in pancreatic beta cells rescues GLUT2-null mice from early death and restores normal glucose-stimulated insulin secretion. J Biol Chem. 2000;275:23751–23758. doi: 10.1074/jbc.M002908200. [DOI] [PubMed] [Google Scholar]
- Wasserman D, Hoekstra JH, Tolia V, Taylor CJ, Kirschner BS, Takeda J, Bell GI, Taub R, Rand EB. Molecular analysis of the fructose transporter gene (GLUT5) in isolated fructose malabsorption. J Clin Invest. 1996;98:2398–2402. doi: 10.1172/JCI119053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright EM. The Intestinal Na+/Glucose Cotransporter. Annu Rev Physiol. 1993;55:575–589. doi: 10.1146/annurev.ph.55.030193.003043. [DOI] [PubMed] [Google Scholar]


