Abstract
The effect of extracellular ATP (10–100 μm) on intracellular Ca2+ concentration ([Ca2+]i) and firing rate has been studied in single pacemaker cells isolated from the sinus venosus of cane toads. In spontaneously firing cells, ATP initially increased peak [Ca2+]i by 43 ± 5 %, increased diastolic [Ca2+]i by 20 + 3 % and increased the firing rate by 58 ± 8 %. These early effects were followed by a late phase in which both the peak [Ca2+]i and the firing rate declined. Adenosine, and UTP (respectively, P1- and P2Y2,4,6-selective agonists) caused no significant change in [Ca2+]i or firing rate, while αβ-methylene ATP (a P2X1,3 agonist) caused a small increase in firing rate but no changes in [Ca2+]i. In contrast the P2Y1-selective agonist 2-MesADP (1 μm) mimicked the biphasic effects of ATP and these effects were inhibited by the purinoceptor antagonists suramin and PPADS and by the P2Y1-selective antagonist MRS 2179. Immunohistochemistry established that P2Y1 purinoceptors were present on the cell surface. Western blotting analysis demonstrated that the P2Y1 antibody recognised a 57 kDa protein. After sarcoplasmic reticulum Ca2+ release was prevented with caffeine or ryanodine, ATP no longer had any effect on [Ca2+]i or firing rate. Furthermore, the SR Ca2+ store content was decreased during the late phase of 2-MesADP application. The effect of ATP was coupled to phospholipase C (PLC) activity because the PLC inhibitor U-73122 eliminated the effects of ATP. Our study shows that in toad pacemaker cells, the biphasic effects of ATP on pacemaker activity are mainly through P2Y1 purinoceptors, which are able to modulate Ca2+ release from the SR Ca2+ store.
The classic study by Drury & Szent-Gyorgyi (1929) established that ATP and its derivatives could exert chronotropic effects on the intact heart. Later investigations in frog atrial tissue showed that exogenous ATP had a biphasic action on force production and spontaneous firing rate with an initial positive inotropic and chronotropic phase followed by a negative phase (Burnstock & Meghji, 1981). It was suggested that the positive phase was mediated by P2 purinoceptors, activated by ATP, while the negative phase was mediated by P1 purinoceptors, activated by adenosine. It has also been established that ATP is produced by a range of sources within the heart. ATP is a cotransmitter released from sympathetic nerve terminals with adrenaline in the amphibian heart (Hoyle & Burnstock, 1986). ATP is also released by myocytes during hypoxia and ischaemia (Forrester & Williams, 1977). The sympathetically released ATP appears to be functionally important in the cane toad pacemaker region because some of the effects of sympathetic nerve stimulation, including the tachycardia, can be mimicked by application of ATP (Bramich et al. 1990). In addition, the early phase of sympathetic tachycardia can be abolished after P2 purinoceptor desensitisation (Bramich et al. 1990). These results establish the involvement of ATP and purinoceptors in the regulation of the heart rate.
Based on recent molecular cloning studies, a number of P2 subtypes are now recognised. P2Xn represent ligand-gated ion channels and P2Yn represent G protein-coupled receptors (for review see Burnstock, 1996; von Kugelgen & Wetter, 2000). Many of these receptor subtypes have been found in the heart (for review see Vassort, 2001). Several members of the P2Y family such as P2Y1,2,4,6 are found in the heart tissues and are coupled to phospholipase C (PLC) though a Gq protein pathway (Morris et al. 1990; Filtz et al. 1994). In many cell types activation of these P2Y receptor subtypes mediates inositol 1,4,5-trisphosphate (IP3) formation and causes release of Ca2+ from intracellular stores (for review see Berridge, 1997).
ATP has now been shown to produce a wide variety of effects in cardiac preparations (for review see Vassort, 2001). For instance, the positive inotropic responses observed in whole hearts can be observed in isolated ventricular myocytes and have been shown to involve increased systolic intracellular Ca2+ concentration (Danziger et al. 1988). At a more mechanistic level, extracellular ATP has been reported to both increase and decrease the L-type Ca2+ current (Alvarez et al. 1990; Qu et al. 1993), to open a non-specific cation current (Friel & Bean, 1988), to affect a variety of K+ currents (Matsuura & Ehara, 1997), and to affect several ion exchangers including the Na+/H+ and the Cl−/HCO3− exhangers (Pucéat & Vassort, 1995).
Studies on the mechanism of pacemaker firing and its regulation by neurotransmitters have made considerable progress in recent years. A number of laboratories have demonstrated that interventions which affect intracellular Ca2+ concentration ([Ca2+]i) also influence firing rate (Zhou & Lipsius, 1993; Ju & Allen, 1998; Bogdanov et al. 2001). A particularly clear example is that ryanodine, which reduces sarcoplasmic reticulum (SR) Ca2+ release, generally slows the firing rate (Rigg & Terrar, 1996; Ju & Allen, 1998). Normally some of the Ca2+ released from the SR is extruded from the cell on the Na+/Ca2+ exchanger, generating an inward current which acts as a pacemaker current; thus when the systolic [Ca2+]i is reduced by ryanodine, the firing rate is reduced (Zhou & Lipsius, 1993; Ju & Allen, 1998). β-adrenergic stimulation increases firing rate and several studies have now suggested that the enhanced firing rate arises partly through its effects on [Ca2+]i and the Na+/Ca2+ exchanger in addition to the phosphorylation of conventional pacemaker currents (Ju & Allen, 1999; Vinogradova et al. 2002). β-adrenergic stimulation is generally thought to increase [Ca2+]i by cAMP-dependent phosphorylation of proteins but a recent study suggested that there is an atypical β-receptor which operates through a G protein/PLC/IP3 pathway to elevate [Ca2+]i (Bramich et al. 2001). In a previous study these workers suggested the involvement of the SR Ca2+ by showing that depletion of intracellular Ca2+ stores with caffeine abolished the response to sympathetic nerve stimulation (Bramich & Cousins, 1999).
So far there have been no detailed studies of the effects of ATP on pacemaker cells. It is not known which subtypes of purinoceptors are involved nor whether ATP affects [Ca2+]i. Studies on the intact heart are complicated by the presence of ecto-ATPases which rapidly degrade ATP to adenosine, and by the possibility that exogenous drugs may affect autonomic nerves or intracardiac neurons in addition to the various cardiac myocytes. To avoid these problems we have isolated single cane toad pacemaker cells and applied drugs directly to the pacemaker cells. We confirm earlier studies showing that ATP produces an early acceleration of firing rate and a later decline. We show for the first time that ATP also affects the intracellular Ca2+ concentration ([Ca2+]i) in pacemaker cells. Based on pharmacological, immunohistochemical and Western blotting studies, we show that the effects of ATP on [Ca2+]i and heart rate are primarily through P2Y1 purinoceptors. We also show that changes in the Ca2+ content of the SR play a role in the response of pacemaker cells to ATP.
METHODS
Preparation of pacemaker cells
These experiments were approved by the Animal Ethical Committee of the University of Sydney and conform to Australian guidelines. Toads (Bufo marinus) were killed by decapitation and pithed. Single pacemaker cells were enzymatically isolated from the sinus venosus as previously described (Ju et al. 1995; Ju & Allen, 1998). The cells were routinely superfused with the following (standard) solution (mm): NaCl 110, KCl 2.5, MgSO4 0.5, CaCl2 2, NaHepes 10, glucose 10; pH 7.3; equilibrated with air. Drugs were applied from a fine tube positioned within 200 μm of the cell to ensure a rapid onset of action. All experiments were performed at room temperature (22°C) which is close to the physiological temperature for these semi-tropical amphibia.
Fluorescence measurements
After isolation, cells were incubated with 5 μm indo-1-AM for 10–15 min (Ju & Allen, 1998). Loaded cells were illuminated at 360 ± 5 nm with a UV light source whose intensity was reduced 30-fold with a neutral density filter. The emitted fluorescence was directed to two photomultiplier tubes via either a 400 ± 5 nm or a 510 ± 5 nm interference filter. The analogue signals were digitised and the ratio of fluorescence signals at 400 nm/510 nm (R) was calculated and converted to [Ca2+]i using the following equation (Grynkiewicz et al. 1985):
An in vivo calibration method was used and gave the following values: Rmin, 0.11; Rmax, 1.94; β, 2.84; Kd, 606 nm; where Rmin is the ratio at zero [Ca2+]i, Rmax is the ratio at saturating [Ca2+]i and β is the ratio of fluorescence at 510 nm at zero and saturating [Ca2+]i. [Ca2+]i signals were filtered at 10 Hz which does not distort the signals from this preparation (Ju & Allen, 1998).
Voltage-clamp procedure
The perforated-patch technique was used to record membrane currents. The pipette solution contained (mm) 100 KCl, 10 KH2PO4, 10 Tes, 5 NaH2PO4, 2 MgSO4, pH 7.3. Nystatin (240 μg ml−1) was added to the pipette solutions. The series resistance of perforated patches were < 20 MΩ; cell capacitances were 30–50 pF. Series resistance and capacitance compensation were made once cell access had been achieved.
Immunohistochemistry
Antisera were raised in sheep against a non-homologous, extracellular epitope from the rat P2Y1 receptor sequence (Yao et al. 2001). Antibody specificity was established using enzyme-linked immunosorbent assays (ELISA), Western blotting and affinity purification. Antisera were purified using a Protein G column to separate IgG fractions and were affinity purified using Affi-Gel 10 (Bio-Rad).
Isolated single pacemaker cells were attached to poly-l-lysine-coated slides for 1 h before being fixed using 1 % paraformaldehyde for 1 min at room temperature. Fixed cells were washed with physiological solution for 10 min then treated with 10 % BSA for 30 min. The cells were then incubated with sheep anti-P2Y1 antiserum at 1:100 dilution for 2 h. Thereafter the cells were washed (3 × 10 min) and then incubated with cyanine 3-labelled donkey anti-sheep secondary antiserum (Jackson Immunoresearch) at 1:250 dilution for 1 h. The slides were washed (3 × 10 min) and then mounted and examined using a Leica TCS-NT laser scanning confocal microscope. Standard controls were obtained in the absence of either primary or secondary serum to establish specific labelling.
Western blotting
Total protein was extracted from toad tissues and rat aorta (as positive control) in a lysis buffer containing NaH2PO4 10 mm, pH 7.4, EDTA 4 mm, DTT 1 mm, protease inhibitors cocktail 5 μl ml−1, phenylmethylsulphonyl fluoride (PMSF) 1 mm and SDS 1 %. After centrifugation, the supernatants were boiled and subjected to 10 % sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were then transferred onto a PVDF membrane and probed with sheep anti-P2Y1 antiserum in the absence or presence of the epitope peptide that was used to generate the antibody. The epitope peptide was pre-incubated with P2Y1 antiserum at 4° C overnight prior to application. Anti-sheep IgG-HRP (Santa Cruz, USA) was used for the secondary detection of the immune bands. The images were processed using a UVISAVE apparatus (UVItec Limited, Cambridge, England).
Drugs
The following drugs were used: adenosine 5′-triphosphate (ATP), adenosine 5′-diphosphate (ADP), uridine 5′-triphosphate (UTP), 2-methylthioadenosine 5′-diphosphate (2-MesADP), adenosine, pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid tetrasodium (PPADS), α,β-methylene-ATP, suramin (all from Sigma), U-73122 (from Calbiochem-Novabiochem), MRS 2179 (from Tocris).
Statistics
All statistical data are presented as means ±s.e.m. with the number of cells studied as n. Statistical tests were Student's paired or unpaired t tests and P < 0.05 was taken as the level of significance.
RESULTS
Effects of ATP on [Ca2+]i and firing rate in isolated cane toad pacemaker cells
[Ca2+]i was recorded from single isolated toad pacemaker cells and showed spontaneous elevations (Ca2+ transients) which accompanied each action potential (Ju & Allen, 1998). Application of ATP 10–100 μm caused biphasic changes in both [Ca2+]i and firing rate as shown in Fig. 1. The initial positive phase (fast phase) occurred within several seconds of application of ATP and reached a peak in 5–10 s. The peak systolic [Ca2+]i increased by 43 ± 5 % from 624 ± 33 nm to 880 ± 49 nm (n = 22, P < 0.0001) and was accompanied by a 20 ± 3 % increase in diastolic [Ca2+]i from 230 ± 13 nm to 275 ± 16 nm (P < 0.0001). The spontaneous firing rate also increased by 58 ± 8 % from 24 ± 1 min−1 to 36 ± 2 min−1 (P < 0.0001). In the inhibitory phase (slow phase, 10– 40 s after application of ATP) the diastolic [Ca2+]i declined 12 ± 2 % compared to the control and 25 ± 7 % compare to the positive phase (P < 0.0001). The spontaneous firing rate also declined to 12 ± 1 min−1 which was 52 ± 6 % of control (P < 0.0001); in some cases the cells became quiescent (e.g. Fig. 3B). The peak [Ca2+]i declined significantly compared to the initial positive phase (P < .0001) but was not significantly different from the original control (P > 0.05). The effects of ATP were concentration-dependent and reversible. When concentration was 1 μm or lower, ATP had no significant effects on either [Ca2+]i or firing rate (n = 5). Maximal responses were observed at 100 μm (n = 10) and a concentration of 10 μm gave a near maximal response.
Figure 1. Biphasic effect of ATP on [Ca2+]i and firing rate in a toad pacemaker cell.
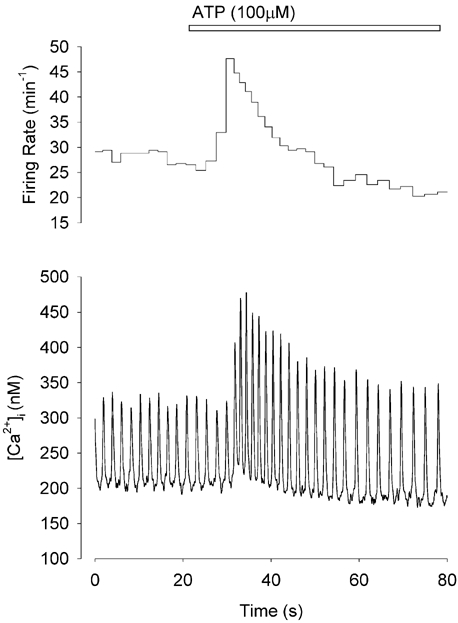
Continuous record of [Ca2+]i from a spontaneously firing toad pacemaker cell before and during 100 μm ATP application (lower panel). Upper panel shows the instantaneous firing rate calculated from the interval between each Ca2+ transient. Initially (fast phase) both [Ca2+]i and firing rate increase. Subsequently (slow phase) both [Ca2+]i and firing rate decline.
Figure 3. The effect of 2-MesADP on [Ca2+]i and firing rate.
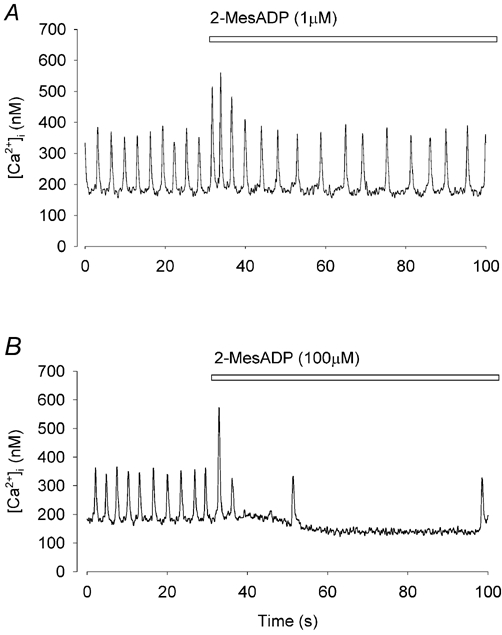
A, application of 1 μm 2-MesADP caused biphasic changes in both [Ca2+]i and firing rate. B, application of a high concentration of 2-MesADP(100 μm) caused a brief fast phase (single large Ca2+ transient) followed by a pronounced slow phase.
Determination of the subtype of purinoceptor involved
The effects of ATP on single toad pacemaker cells were similar to an early study on intact frog atrial tissue (Burnstock & Meghji, 1981). They showed that the positive effects of ATP could be mimicked by αβ-methylene ATP, a P2X1,3 receptor agonist (for review see Harden et al. 1995). To test this possibility, we applied αβ-methylene ATP (100–500 μm) to toad pacemaker cells and observed a small increase in firing rate of 26 ± 8 % (n = 7, P < 0.02). However, as shown in Fig. 2A, there was no significant change in peak or diastolic [Ca2+]i (peak systolic [Ca2+]i in the fast phase increased by 1 ± 4 %, diastolic [Ca2+]i decreased by 5 ± 3 %; P > 0.05, n = 7). In contrast, when ATP (100 μm) was applied to the same cell, the typical biphasic response was observed (not shown). In voltage clamp experiments, we found no inward current induced by ATP at a holding potential of −60 mV (data not shown). These results suggest that the increased [Ca2+]i caused by ATP is not through a P2X receptor.
Figure 2. The effect of αβ-methylene ATP, adenosine and carbachol on [Ca2+]i and firing rate.
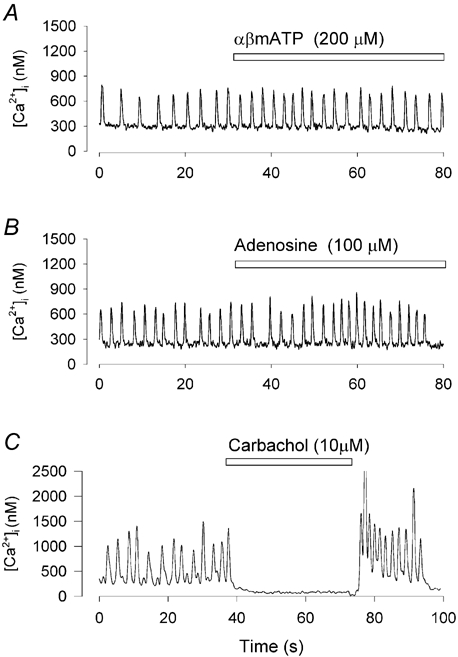
All records show [Ca2+]i recorded in a spontaneously firing toad pacemaker cell. A, αβ-methylene ATP (αβmATP, 200 μm) caused no change in [Ca2+]i although it slightly increased firing rate. B, adenosine (100 μm) caused no significant change in either [Ca2+]i or firing rate. C, carbachol (10 μm) caused cessation of firing.
In frog atria the P1 agonist adenosine mimicked the negative chronotropic effect of ATP and this effect was blocked by the P1 receptor antagonist theophylline (Burnstock & Meghji, 1981). In mammalian pacemaker cells adenosine evokes a time-independent potassium current which modulates pacemaker activity (Belardinelli et al. 1988). To determine the involvement of P1 receptors in our preparation we applied adenosine on single toad pacemaker cells. In 15 cells, adenosine (1–1000 μm) caused no significant change in either firing rate or [Ca2+]i (Fig. 2B). However, the same cell responded to ATP application (not shown). In addition, these cells also responded to the muscarinic agonist carbachol (10 μm). Carbachol slowed or stopped spontaneous pacemaker firing in eight out of eight cells tested (Fig. 2C). It is believed that carbachol activates a potassium channel (KAch) (Pfaffinger et al. 1985) and that the same channel is activated by adenosine in mammalian sino-atrial node pacemaker cells (for review see Belardinelli et al. 1988). These results suggest that P1 receptors are not the cause of the slow phase of ATP actions in toad pacemaker cells.
So far, we have found no evidence that effects of ATP on [Ca2+]i in toad pacemaker cells were linked to either P1 or P2X receptors. It is known that [Ca2+]i can be modulated through P2Y receptors which are G protein-coupled and involve the mobilisation of Ca2+ from intracellular stores. While P2Y1 receptors are adenine nucleotide-specific, P2Y2,4,6 receptors respond to uridine nucleotide triphosphates (Lustig et al. 1993). To identify the subtype of P2Y receptors we applied UTP (100–300 μm) to pacemaker cells. No significant change in either [Ca2+]i or firing rate (data not shown, n = 11, P > 0.05) was observed. However, 2-MesADP, a selective P2Y1 receptor agonist, mimicked the effects of ATP but at a lower concentration (1 μm) (Fig. 3A). Note that 2-MesADP produces both positive and negative phases of activity. At high concentrations (100 μm) the fast phase could be reduced to a single large Ca2+ transient followed by a pronounced inhibition of firing rate (Fig. 3B). In voltage clamp experiments 2-MesADP induced no significant inward or outward current when membrane potential was held at −60 mV (n = 9, data not shown) suggesting that there is no ligand-activated current (P2X receptor). These results are consistent with the profile for P2Y1 receptors (for review see Vassort, 2001).
Inhibition of the effects of ATP by purinoceptor antagonists
To confirm that the effect of ATP was through a P2 receptor, 10 μm suramin, a relatively non-specific P2 receptor antagonist, was used (Fig. 4) (Ralevic & Burnstock, 1998). Suramin completely blocked the effects of ATP on firing rate in both fast and slow phases (P < 0.01, n = 5). In the fast phase, the percentage increase in the peak [Ca2+]i and the resting [Ca2+]i by ATP was also significantly reduced by suramin (P < 0.05, P < 0.01 respectively). The diastolic [Ca2+]i did not change significantly in the presence of suramin. Figure 4D and E shows pooled data of these comparisons. PPADS (10 μm), a P2 purinoceptor antagonist, also significantly blocked the effects of ATP on peak Ca2+ and firing rate. In four control experiments, ATP increased systolic [Ca2+]i by 28 ± 10 % and increased the firing rate by 78 ± 21 %. In the presence of PPADS, ATP had no significant effect either on systolic [Ca2+]i (7 ± 11 %) or on firing rate (20 ± 18 %).
Figure 4. Suramin inhibits ATP-induced changes in [Ca2+]i and firing rate.
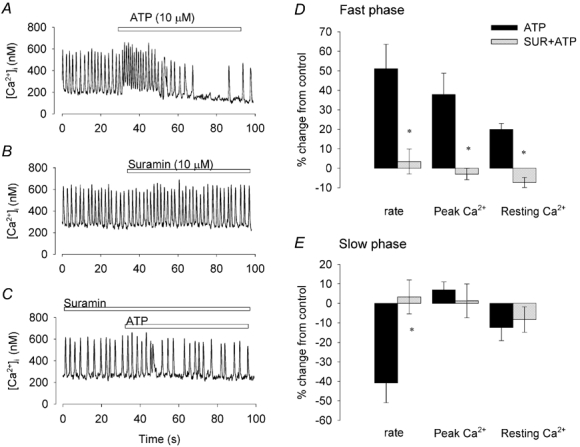
[Ca2+]i recorded from a spontaneously firing pacemaker cell when ATP was applied (A), and when suramin was applied (B). After 2 min in the presence of suramin, ATP was reapplied (C, all records from the same cell). D and E, bar graphs of the percentage changes caused by ATP and suramin (SUR) plus ATP, calculated using the pre-ATP or pre-suramin periods as control. Bars show ± 1 s.e.m. (n = 5). *P < 0.05 from preceding control (Student's paired t test).
The most potent and selective P2Y1 antagonist is the bisphosphate derivative 2′-deoxy-N6-methyladenosine-3′,5′-bisphosphate (MRS 2179) (von Kugelgen & Wetter, 2000). MRS 2179 (10 μm) transiently reduced the peak [Ca2+]i and firing rate as demonstrated in Fig. 5B. However, after 2 min exposure these transient effects became insignificant. In the presence of MRS 2179, the effect of 2-MesADP on [Ca2+]i and firing rate were significantly reduced (P < 0.02, P < 0.01 respectively, n = 7). During the slow phase, the reduction of firing rate was also significantly reduced compared to 2-MesADP alone (P < 0.05). ATP-induced changes in [Ca2+]i and firing rate were also blocked by MRS 2179 (n = 5, Fig. 5). The results with suramin, PPADS and MRS 2179 are all consistent with a P2Y1 receptor being involved in both the fast and slow phases of the ATP response.
Figure 5. MRS 2179 inhibits 2-MesADP-induced changes in [Ca2+]i and firing rate.
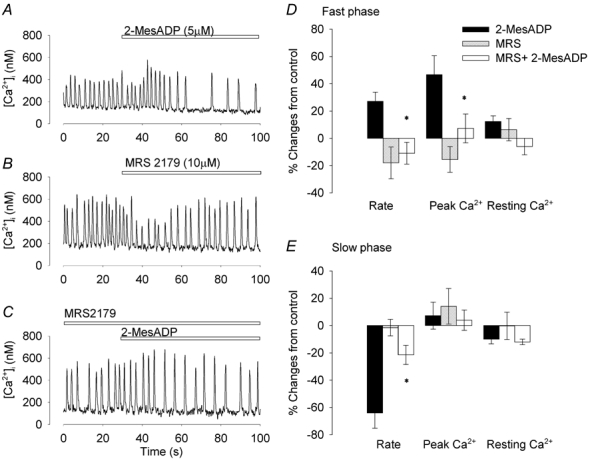
[Ca2+]i recorded from a spontaneously firing pacemaker cell before and during application of 2-MesADP (A) or MRS 2179 (B). After 2 min in the presence of MRS 2179, ATP was applied in the same cell (C). D and E, bar graphs of the percentage changes caused by ATP, MRS 2179 and ATP in the presence of MRS 2179, calculated using the pre-ATP or pre-MRS 2719 periods as control. Bars show ± 1 s.e.m. (n = 7). *P < 0.05 from preceding control (Student's paired t test).
Detection of P2Y1 receptors by immunohistochemistry and Western blotting
The existence of a P2Y1 receptor was further confirmed by using a P2Y1 receptor antibody (Yao et al. 2001; Fong et al. 2002). Strong fluorescence signals of cyanine 3-labelled donkey anti-sheep secondary antibody were detected in cells labelled with P2Y1 primary antibody as shown in Fig. 6. There was no fluorescence signal detected when only the fluorescent secondary antibody was used (data not shown). P2Y1 signals were mainly distributed in the surface of the pacemaker cells provided the initial fixation procedure did not permeabilise the surface membrane. The results confirm the presence of P2Y1 receptors on the surface membrane of toad pacemaker cells.
Figure 6. Immunohistochemistry and Western blotting of P2Y1 purinoceptors.
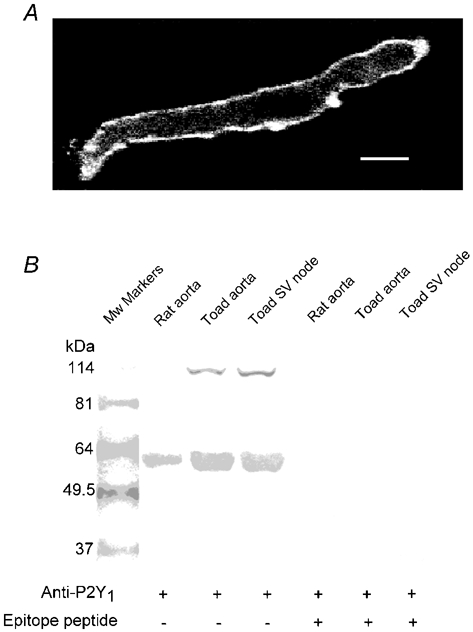
A, pacemaker cell fixed and stained with P2Y1 antibody and fluorescent secondary antibody. Bar indicates 6 μm. B, Western blots demonstrating P2Y1 expression in toad tissues. Total protein extracts were subject to 10 % SDS-PAGE and probed with P2Y1 antiserum in the absence (lanes 2–4) and presence (lanes 5–7) of epitope peptide. One band of about 57 kDa was observed in the rat aorta sample that is known to express P2Y1 receptors. This band is probably the glycosylated form of P2Y1. The same band was present in the toad samples, whereas an additional band of about 114 kDa was also seen in these samples. This band may be a dimer of the P2Y1 receptor. Preincubation with the epitope peptide greatly attenuated the 114 kDa band and abolished the 57 kDa band, suggesting specific labelling.
To establish whether the antibody specifically recognises the P2Y1 receptor, we performed Western blot analysis on isolated sinus venosus tissue from cane toad. Rat aorta and toad aorta tissue were used as positive controls since it is known that P2Y1 receptors are present on smooth muscle cells (Pediani et al. 2003). Total protein extracts were subjected to 10 % SDS-PAGE and probed with the P2Y1 antibody. Figure 6B showed one major band of approximately 57 kDa was observed in the rat aorta sample. This band is most probably the glycosylated form of P2Y1 as reported by others (Moore et al. 2000; Fong et al. 2002). The same band was present in the toad aorta and sinus venosus tissue, together with an additional band of about 114 kDa. The molecular mass of this additional band suggests it might represent a dimer of the P2Y1 receptor. However, the reason why the dimer only appeared in toad tissue is unknown. After P2Y1 antibody was pre-absorbed with the epitope peptide, the 57 kDa band was completely abolished in all of three tissues. Preincubation with epitope peptide also greatly attenuated the 114 kDa band. These results suggest that both bands were related to the P2Y1 receptors.
The effects of ATP and 2-MesADP were dependent on the loading of the SR Ca2+store
The changes in [Ca2+]i mediated by P2Y receptors generally arise through IP3-dependent release of Ca2+ from intracellular stores (Vassort, 2001). To explore the mechanism by which ATP increased [Ca2+]i in toad pacemaker cells we used caffeine to deplete the SR of Ca2+ (Callewaert et al. 1989). Figure 7A shows the [Ca2+]i response to rapid application of caffeine (10 mm) in a spontaneously firing pacemaker cell. Caffeine caused a large and rapid rise in [Ca2+]i as described previously (Ju & Allen, 1998). The spontaneous [Ca2+]i signals ceased for about 20 s before spontaneous firing gradually recovered. We have proposed that the cessation of firing was a consequence of the depletion of the SR Ca2+ store (Ju & Allen, 1999). Here we show that after depletion of the SR store with caffeine, ATP no longer had any effect on either firing rate or [Ca2+]i as shown in Fig. 7B. In five cells, ATP under control conditions increased resting [Ca2+]i by 20 ± 5 % but when reapplied after application of caffeine the increase was no longer significant (decrease of 2 ± 2 %). It could be argued that, because caffeine generally stops firing completely, the effect of ATP is still present but not detectable when the cell is not firing. However this interpretation is made less likely by the observation that the effects of ATP were also prevented by pretreatment of the cells with ryanodine (10–20 μm) which slowed but did not stop cell firing. Before application of ryanodine, ATP caused 39 ± 4 % increase in peak [Ca2+]i (P < 0.02, n = 6). However, after 20–30 min in the present of ryanodine, ATP failed to produce a significant change in peak [Ca2+]i (P < 0.05). These results suggest that the effect of ATP in the fast phase was dependent on release of Ca2+ from the SR store.
Figure 7. Inhibition of ATP effect after SR Ca2+ store is depleted with caffeine.
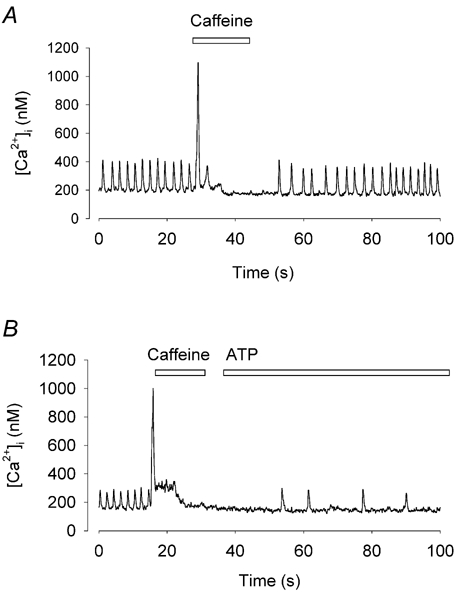
A, a rapid application of caffeine evoked a rise in [Ca2+]i. The cell stopped firing for a period of time after application of caffeine. B, ATP was applied immediately after caffeine application. ATP no longer produced any of its characteristic effects on [Ca2+]i or firing rate.
Is the slow phase of the ATP response also related to SR Ca2+ release? We showed above that spontaneous firing slows or ceases when the SR stores is depleted of Ca2+ by caffeine. It seemed possible that ATP or 2-MesADP partially emptied the SR of Ca2+ as a consequence of the increased Ca2+ release in the rapid phase. This possibility is supported by the similarity between the effects of a high concentration of 2-MesADP (Fig. 3B) and caffeine (Fig. 7A and Fig. 8A). To test this hypothesis we applied caffeine during the slow phase response. In the control period, the peak of the caffeine-induced [Ca2+]i signal was 1359 ± 202 nm. During the slow phase following 2-MesADP application, the caffeine-induced Ca2+ signal was reduced to 641 ± 100 nm (Fig. 8, n = 10, P < 0.002). After 3–5 min in standard solution, the caffeine-induced [Ca2+]i signal recovered to control (Fig. 8D, 1209 ± 210 nm, n = 7). This result suggests that the reduction of systolic [Ca2+]i observed in the slow phase was caused by partial depletion of the SR Ca2+ store.
Figure 8. SR Ca2+ store is partially depleted by 2-MesADP.
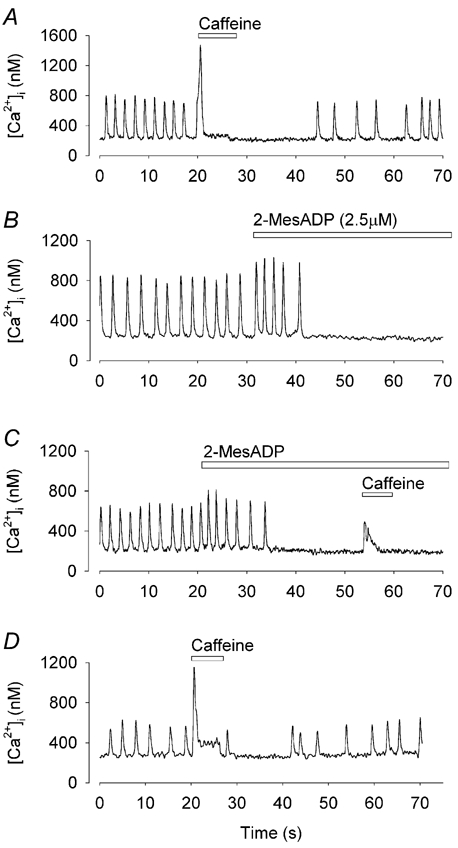
[Ca2+]i recorded in a single toad pacemaker cells; all records from the same cell. A, rapid application of caffeine caused a transient increase in [Ca2+]i, which was followed by a short period of quiescence and a gradual recovery of firing. B, 2-MesADP caused a characteristic increase followed by a decrease in firing rates. C, caffeine was applied during the quiescent period caused by 2-MesADP. D, re-application of caffeine after wash off of 2-MesADP.
Involvement of PLC in the ATP-induced [Ca2+]i responses
P2Y receptors are generally coupled to phospholipase C (PLC) (Vassort, 2001). To test whether inhibition of PLC could also block the effect of ATP, we used the PLC inhibitor U-73122 (1 μm). U-73122 was applied to the cells that responded to ATP application as shown in Fig. 9. Application of U-73122 alone caused a transient increased firing rate and [Ca2+]i (Fig. 9B), which may be explained by the observation that U-73122 can cause the release of Ca2+ from intracellular stores (Mogami et al. 1997). After 3–5 min exposure to U-73122, the ATP-induced [Ca2+]i changes were significantly reduced (Fig. 9C). In five cells after using U-73122, ATP did not produce significant changes in either firing rate or [Ca2+]i (P > 0.05). This result suggests the involvement of PLC in the coupling of the P2Y1 receptor to Ca2+ release.
Figure 9. U-73122 inhibits the effect of ATP on [Ca2+]i and firing rate.
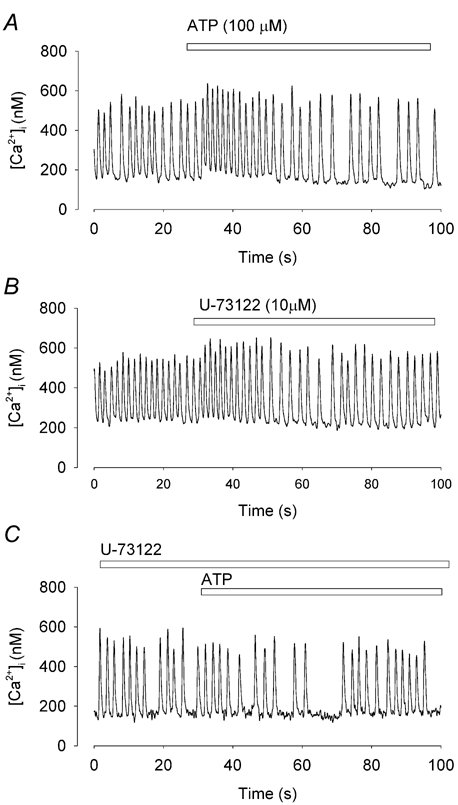
Spontaneous Ca2+ transients recorded from the same pacemaker cell. A, ATP induced a typical biphasic effect. B, application of U-73122 to the cell. Note that there is a small acceleration of the firing rate. C, After 2 min application of U-73122, ATP caused no significant change in [Ca2+]i or firing rate.
DISCUSSION
The involvement of P2Y1 purinoceptors in modulation of cardiac pacemaker activity
In the present study we have demonstrated that ATP induces a biphasic effect on [Ca2+]i associated with changes in firing rate in isolated toad pacemaker cells. These results are consistent with early studies in intact heart tissue, in which ATP produced a transient tachycardia followed by a slower heart rate (Hollander & Webb, 1957; Burnstock & Meghji, 1981). Early studies suggested that these responses to ATP were through P2X or P1 receptors (Burnstock & Meghji, 1981; Belardinelli et al. 1988). However, in our preparation the P2X1,3 agonist αβ-methylene ATP caused only a small monophasic increase in rate which cannot explain the response to ATP. The P1 agonist adenosine had no effect on firing rate or [Ca2+]i. In contrast the P2Y1-selective agonist 2-MesADP mimicked both the increase and the decrease in firing rate produced by ATP but was more potent than ATP. In addition, MRS 2179, a specific P2Y1 purinoceptor antagonist, inhibited the effect of 2-MesADP and ATP. Our results demonstrate that a P2Y1 receptor is the principal receptor involved in modulation of [Ca2+]i and firing rate in toad pacemaker cells. These findings were supported by the presence of a surface membrane-located P2Y1 purinoceptor judged by antibody binding. Furthermore, immunoblotting revealed that the sinus venosus of cane toad contains a protein with a molecular mass similar to that of P2Y1 receptors. The apparent molecular mass of 57 kDa is close to previous reports of P2Y1 receptors in rat and human brain tissue (Moore et al. 2000; Fong et al. 2002). Although this molecular mass is higher than the 42 kDa predicted from the amino acid sequence (Ayyanathan et al. 1996), it is believed that the difference can be explained by glycosylation of sites on the extracellular C-terminal (Moore et al. 2000).
The primary P2Y1 antibody used in this study was raised against an epitope in the rat P2Y1 receptor. The epitope sequence chosen was: CALIKTGFQFYYLPAVY and the homologous region in the Xenopus P2Y1 receptor is CSLTKTGFQFYYLPAVY. Thus only two residues close to the Cys link residue differ between the two mammalian and amphibian species and would not be expected to seriously interfere with antibody binding. Therefore, it is not surprising that good cross-reactivity between species was achieved.
Although our data point clearly to the P2Y1 receptor as the main purinoceptor underlying the effects of ATP on [Ca2+]i and firing rate, we do not exclude minor roles for other receptors. A wide range of P1, P2X and P2Y receptors have been described in mammalian hearts (Vassort, 2001). Our experiments suggest some role for P2X1,3 receptors because αβ-methylene ATP slightly increased firing rate. Furthermore P2X receptor antibodies also showed substantial binding to our cells (not shown).
More surprising was the lack of effect of adenosine on toad pacemaker cells. It is conceivable that our isolation procedure destroyed P1 receptors in these cells. However, similar results have been observed in intact toad sinus venosus tissue in which no proteases were used (D. Hirst and N. Bramich, personal communication). Furthermore, our cells showed a consistent response to carbachol, demonstrating that muscarinic receptors were functional. Since adenosine and carbachol receptors are thought to be coupled to the same potassium channels (KAch,Ado) this also establishes that the channel itself is functional (Pfaffinger et al. 1985).
Involvement of PLC and IP3 in the response to ATP
The coupling of P2Y1 purinoceptors to PLC is well established from many previous studies (Morris et al. 1990; Filtz et al. 1994). We found that the effects of ATP or 2-MesADP were eliminated by the PLC inhibitor U-73122. This result is consistent with ATP acting through a P2Y1 receptor coupled to PLC activation. It has also been shown that U-73122 abolished the membrane depolarisation evoked by sympathetic nerve stimulation in intact toad pacemaker preparations (Bramich et al. 2001). Thus ATP released from the sympathetic terminals appears to act through the same pathway. In many tissues, PLC activation is linked to increased IP3 production which binds to IP3 receptors causing Ca2+ release from intracellular stores (for review see Berridge, 1997). In ventricular myocytes, the role of IP3 in cardiac excitation-contraction coupling is controversial (Bers, 2001) although recent studies have demonstrated IP3 receptor expression in atrial myocytes and suggest that IP3 facilitates SR Ca2+ release (Lipp et al. 2000). As yet we do not have direct evidence for a role for IP3 in toad pacemaker cells. Assuming that IP3 levels are enhanced by ATP, IP3 may cause a slow release of Ca2+, leading to the increase in resting [Ca2+]i which lasts 10–20 s. This increase in resting [Ca2+]i may then enhance Ca2+-induced Ca2+ release leading to the increased peak [Ca2+]i.
Does ATP affect firing rate through its effects on [Ca2+]i?
The mechanisms by which ATP first accelerates and then slows the firing rate are not established. One possibility is that one or more of the conventional pacemaker currents are affected by ATP and this possibility needs direct testing. However an intriguing possibility is that the [Ca2+]i changes we observed are part of the cause of the firing changes. In support of this possibility is fact that all the purinoceptor agonists and antagonists changed firing rate and [Ca2+]i in parallel suggesting that the two are closely associated.
The relationship between SR Ca2+ stores and the effects of ATP is also most easily understood if [Ca2+]i can affect the firing rate. One observation is that ATP has no effect on diastolic or systolic [Ca2+]i or on firing rate when the SR Ca2+ is depleted by caffeine, or SR Ca2+ release is inhibited by ryanodine. Our hypothesis to explain this is that ATP can no longer cause the increased Ca2+ release from a Ca2+-depleted SR store and consequently the increased peak [Ca2+]i and increased firing rate are absent. A second observation is the reduced store content and firing rate during the slow phase of the action of ATP. We assume this arises because when SR release is enhanced, in the absence of other changes, the cell will gradually lose Ca2+ through increased Na+/Ca2+ exchanger activity until reduced loading of the store causes the peak [Ca2+]i to return to its previous level. The quantitative aspects of this process have been explored in detail in ventricular cells (Trafford et al. 2000; Eisner et al. 2000).
It has previously been shown that the acceleration of firing rate caused by sympathetic nerve stimulation is also dependent on the SR Ca2+ load (Bramich & Cousins, 1999). We have also shown that some effects of isoprenaline on toad pacemaker cells were dependent on the SR Ca2+ store (Ju & Allen, 1999). A reduced SR Ca2+ store content was also related to slowing and irregularity of pacemaker activity during metabolic inhibition (Ju & Allen, 2003). Thus there is increasing evidence to suggest that SR Ca2+ loading exerts an important role in determining the heart rate. Two possible mechanisms by which this might occur are as follows: (i) increase in the SR Ca2+ store will tend to cause larger Ca2+ release and therefore larger systolic [Ca2+]i. This will increase the efflux of Ca2+ on the Na+/Ca2+ exchanger causing additional inward current and more rapid depolarisation of the pacemaker potential; (ii) alternatively, an increased Ca2+ store might cause an increased frequency of Ca2+ sparks during diastole (Ju & Allen, 2000; Huser et al. 2000; Bogdanov et al. 2001). Sparks occurring close to the surface membrane may cause localised Na+/Ca2+ exchanger activity and enhance the firing rate (Bogdanov et al. 2001). The attraction of this mechanism is that it can potentially explain changes in the firing rate even when the amplitude of the Ca2+ transients is unchanged, as occurs in the late phase of the action of ATP.
Conclusions
This study establishes that ATP affects firing rate and [Ca2+]i in toad pacemaker cells through a P2Y1 purinoceptor. The effects on both firing rate and [Ca2+]i are biphasic with an initial increase followed by a later decrease. The coupling between the P2Y1 purinoceptor and the [Ca2+]i and firing changes involves PLC and probably operates by increasing Ca2+ release from the SR which subsequently becomes depleted. It is likely that these changes in Ca2+ handling have an important role in the changes in firing rate.
Acknowledgments
We are grateful to the National Health and Medical Research Council of Australia for financial support.
REFERENCES
- Alvarez JL, Mongo K, Scamps F, Vassort G. Effects of purinergic stimulation on the Ca current in single frog cardiac cells. Pflugers Arch. 1990;416:189–195. doi: 10.1007/BF00370241. [DOI] [PubMed] [Google Scholar]
- Ayyanathan K, Webb TE, Sandhu AK, Athwal RS, Barnard EA, Kunapul SP. Cloning and chromosomal localisation of the human P2Y1 purinoceptor. Biochem Biophys Res Comm. 1996;218:783–788. doi: 10.1006/bbrc.1996.0139. [DOI] [PubMed] [Google Scholar]
- Belardinelli L, Giles WR, West A. Ionic mechanisms of adenosine actions in pacemaker cells from rabbit heart. J Physiol. 1988;405:615–633. doi: 10.1113/jphysiol.1988.sp017352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Elementary and global aspects of calcium signalling. J Physiol. 1997;499:291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM. Excitation-contraction Coupling and Cardiac Contractile Force. 2. Dordrecht: Blackwell Science Inc; 2001. pp. 1–249. [Google Scholar]
- Bogdanov KY, Vinogradova TM, Lakatta EG. Sinoatrial nodal cell ryanodine receptor and Na+-Ca2+ exchanger: molecular partners in pacemaker regulation. Circ Res. 2001;88:1254–1258. doi: 10.1161/hh1201.092095. [DOI] [PubMed] [Google Scholar]
- Bramich NJ, Cousins HM. Effects of sympathetic nerve stimulation on membrane potential, [Ca2+]i, and force in the toad sinus venosus. Am J Physiol. 1999;276:H115–128. doi: 10.1152/ajpheart.1999.276.1.H115. [DOI] [PubMed] [Google Scholar]
- Bramich NJ, Cousins HM, Edwards FR, Hirst GD. Parallel metabotropic pathways in the heart of the toad, Bufo marinus. Am J Physiol. 2001;281:H1771–1777. doi: 10.1152/ajpheart.2001.281.4.H1771. [DOI] [PubMed] [Google Scholar]
- Bramich NJ, Edwards FR, Hirst GDS. Sympathetic nerve stimulation and applied transmitters on the sinus venosus of the toad. J Physiol. 1990;429:349–375. doi: 10.1113/jphysiol.1990.sp018261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. P2 purinoceptors: historical perspective and classification. Ciba Found Symp. 1996;198:1–28. doi: 10.1002/9780470514900.ch1. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Meghji P. Distribution of P1- and P2-purinoceptors in the guinea-pig and frog heart. Br J Pharmacol. 1981;73:879–885. doi: 10.1111/j.1476-5381.1981.tb08741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert G, Cleemann L, Morad M. Caffeine-induced Ca2+ release activates Ca2+ extrusion via Na+-Ca2+ exchanger in cardiac myocytes. Am J Physiol. 1989;257:C147–152. doi: 10.1152/ajpcell.1989.257.1.C147. [DOI] [PubMed] [Google Scholar]
- Danziger RS, Raffaeli S, Moreno-sanchez R, Sakai M, Capogrossi MC, Spurgeon HA, Hansford RG, Lakatta EG. Extracellular ATP has a potent effect to enhance cytosolic calcium and contractility in single ventricular myocytes. Cell Calcium. 1988;9:193–199. doi: 10.1016/0143-4160(88)90023-1. [DOI] [PubMed] [Google Scholar]
- Drury AB, Szent-Gyorgyi A. The physiological activity of adenine compounds with special reference to their action upon the mammalian heart. J Physiol. 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner DA, Choi HS, Diaz ME, O'neill SC, Trafford AW. Integrative analysis of calcium cycling in cardiac muscle. Circ Res. 2000;87:1087–1094. doi: 10.1161/01.res.87.12.1087. [DOI] [PubMed] [Google Scholar]
- Filtz TM, Li Q, Boyer JL, Nicholas RA, Harden TK. Expression of a cloned P2Y purinergic receptor that couples to phospholipase C. Mol Pharmacol. 1994;46:8–14. [PubMed] [Google Scholar]
- Fong AY, Krstew EV, Barden J, Lawrence AJ. Immunoreactive localisation of P2Y1 receptors within the rat and human nodose ganglia and rat brainstem: comparison with [α33P]deoxyadenosine 5′-triphosphate autoradiography. Neuroscience. 2002;113:809–823. doi: 10.1016/s0306-4522(02)00237-3. [DOI] [PubMed] [Google Scholar]
- Forrester T, Williams CA. Release of adenosine triphosphate from isolated adult heart cells in response to hypoxia. J Physiol. 1977;268:371–390. doi: 10.1113/jphysiol.1977.sp011862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel DD, Bean BP. Two ATP-activated conductances in bullfrog atrial cells. J Gen Physiol. 1988;91:1–27. doi: 10.1085/jgp.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Harden TK, Boyer JL, Nicholas RA. P2-purinergic receptors: subtype-associated signaling responses and structure. Annu Rev Pharmacol Toxicol. 1995;35:541–579. doi: 10.1146/annurev.pa.35.040195.002545. [DOI] [PubMed] [Google Scholar]
- Hollander PB, Webb JL. Effects of adenine nucleotides on the contractility and membrane potentials of rat atrium. Circ Res. 1957;5:349–353. doi: 10.1161/01.res.5.4.349. [DOI] [PubMed] [Google Scholar]
- Hoyle CHV, Burnstock G. Evidence that ATP is a neurotransmitter in the frog heart. Eur J Pharmacol. 1986;124:285–289. doi: 10.1016/0014-2999(86)90229-3. [DOI] [PubMed] [Google Scholar]
- Huser J, Blatter LA, Lipsius SL. Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells. J Physiol. 2000;524:415–422. doi: 10.1111/j.1469-7793.2000.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YK, Allen DG. Intracellular calcium and Na+-Ca2+ exchange current in isolated toad pacemaker cells. J Physiol. 1998;508:153–166. doi: 10.1111/j.1469-7793.1998.153br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YK, Allen DG. How does β-adrenergic stimulation increase heart rate? The role of intracellular Ca2+ release in amphibian pacemaker cells. J Physiol. 1999;516:793–804. doi: 10.1111/j.1469-7793.1999.0793u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YK, Allen DG. The distribution of calcium in toad cardiac pacemaker cells during spontaneous firing. Pflugers Arch. 2000;441:219–227. doi: 10.1007/s004240000418. [DOI] [PubMed] [Google Scholar]
- Ju YK, Allen DG. Early effects of metabolic inhibition on intracellular Ca2+ in toad pacemaker cells: involvement of Ca2+ stores. Am J Physiol. 2003;284:H1087–1094. doi: 10.1152/ajpheart.00755.2002. [DOI] [PubMed] [Google Scholar]
- Ju YK, Saint DA, Hirst GD, Gage PW. Sodium currents in toad cardiac pacemaker cells. J Membr Biol. 1995;145:119–128. doi: 10.1007/BF00237370. [DOI] [PubMed] [Google Scholar]
- Lipp P, Laine M, Tovey SC, Burrell KM, Berridge MJ, Li W, Bootman MD. Functional InsP3 receptors that may modulate excitation-contraction coupling in the heart. Curr Biol. 2000;10:939–942. doi: 10.1016/s0960-9822(00)00624-2. [DOI] [PubMed] [Google Scholar]
- Lustig KD, Shiau AK, Brake AJ, Julius D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc Natl Acad Sci U S A. 1993;90:5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura H, Ehara T. Selective enhancement of the slow component of delayed rectifier K+ current in guinea-pig atrial cells by external ATP. J Physiol. 1997;503:45–54. doi: 10.1111/j.1469-7793.1997.045bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogami H, Lloyd MC, Gallacher DV. Phospholipase C inhibitor, U73122, releases intracellular Ca2+, potentiates Ins(1,4,5)P3-mediated Ca2+ release and directly activates ion channels in mouse pancreatic acinar cells. Biochem J. 1997;324:645–651. doi: 10.1042/bj3240645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D, Chambers J, Waldvogel H, Faull R, Emson P. Regional and cellular distribution of the P2Y1 purinergic receptor in the human brain; striking neuronal localisation. J Comp Neurol. 2000;421:374–384. doi: 10.1002/(sici)1096-9861(20000605)421:3<374::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Morris AJ, Waldo GL, Downes CP, Harden TK. A receptor and G-protein-regulated polyphosphoinositide-specific phospholipase C from turkey erythrocytes. II. P2Y-purinergic receptor and G-protein-mediated regulation of the purified enzyme reconstituted with turkey erythrocyte ghosts. J Biol Chem. 1990;265:13508–13514. [PubMed] [Google Scholar]
- Pediani JD, McGrath JC, Wilson SM. P2Y receptor-mediated Ca2+ signalling in cultured rat aortic smooth muscle cells. Br J Pharmacol. 2003;126:1660–1666. doi: 10.1038/sj.bjp.0702470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffinger PJ, Martin JM, Hunter DD, Nathanson NM, Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985;317:536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Pucéat M, Vassort G. Neurohumoral modulation of intracellular pH in the heart. Cardiovasc Res. 1995;29:178–183. doi: 10.1016/s0008-6363(96)88567-1. [DOI] [PubMed] [Google Scholar]
- Qu Y, Campbell DL, Strauss HC. Modulation of L-type Ca2+ current by extracellular ATP in ferret isolated right ventricular myocytes. J Physiol. 1993;471:295–317. doi: 10.1113/jphysiol.1993.sp019902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Rigg L, Terrar DA. Possible role of calcium release from the sarcoplasmic reticulum in pacemaking in guinea-pig sino-atrial node. Exp Physiol. 1996;81:877–880. doi: 10.1113/expphysiol.1996.sp003983. [DOI] [PubMed] [Google Scholar]
- Trafford AW, Sibbring GC, Diaz ME, Eisner DA. The effects of low concentrations of caffeine on spontaneous Ca release in isolated rat ventricular myocytes. Cell Calcium. 2000;28:269–276. doi: 10.1054/ceca.2000.0156. [DOI] [PubMed] [Google Scholar]
- Vassort G. Adenosine 5′-triphosphate: a P2-purinergic agonist in the myocardium. Physiol Rev. 2001;81:767–806. doi: 10.1152/physrev.2001.81.2.767. [DOI] [PubMed] [Google Scholar]
- Vinogradova TM, Bogdanov KY, Lakatta EG. β-Adrenergic stimulation modulates ryanodine receptor Ca2+ release during diastolic depolarization to accelerate pacemaker activity in rabbit sinoatrial nodal cells. Circ Res. 2002;90:73–79. doi: 10.1161/hh0102.102271. [DOI] [PubMed] [Google Scholar]
- von Kugelgen I, Wetter A. Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- Yao ST, Barden JA, Lawrence AJ. On the immunohistochemical distribution of ionotropic P2X receptors in the nucleus tractus solitarius of the rat. Neuroscience. 2001;108:673–685. doi: 10.1016/s0306-4522(01)00438-9. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Lipsius SL. Na+-Ca2+ exchange current in latent pacemaker cells isolated from cat right atrium. J Physiol. 1993;466:263–285. [PMC free article] [PubMed] [Google Scholar]


