Abstract
The neurogenic sensory vascular responses of the dura mater encephali are considered to contribute significantly to the mechanisms of meningeal nociception and headache. Although the fundamental role of capsaicin-sensitive afferent nerves in the development of the neurogenic inflammatory responses of a variety of tissues is well established, their participation in meningeal vascular reactions is unclear. In the present study, the effects of the topical application of capsaicin on the dural blood flow and on the morphology of the dural nerve fibres were examined in control and capsaicin-pretreated rats by means of laser Doppler flowmetry and electron microscopy, respectively. In the control rats, the dural application of capsaicin at concentrations of 50 and 100 nm induced significant increases in blood flow in the branches of the medial meningeal artery. This capsaicin-induced vasodilatation was abolished by capsazepine, a transient receptor potential vanilloid 1 (TRPV1) receptor antagonist, and by hCGRP8–37, a calcitonin gene-related peptide (CGRP) receptor antagonist. Administration of capsaicin at higher concentrations (1 and 10 μm) resulted in marked, dose-dependent decreases in dural blood flow. The capsaicin-induced vasodilatation was abolished, whereas vasoconstriction was augmented, by systemic pretreatment of the animals with capsaicin. Electron microscopy revealed degenerating unmyelinated axons in the dura mater after an acute exposure to capsaicin (10 μm), providing support for the existence and possible functional role of capsaicin-sensitive dural afferent nerves. The results indicate that capsaicin-induced vasodilatation in the rat dura mater is mediated by the release of CGRP from the sensory nerves, whereas the vasoconstrictor response may be attributed to a direct action of capsaicin on the vascular smooth muscle. The present study demonstrates for the first time that capsaicin-sensitive nociceptive afferent nerves contribute significantly to the dural vasodilatory responses and suggests an important role in meningeal nociception.
The sensory innervation of the dura mater encephali mediates both nociceptive and vascular responses, which are implicated in the generation of headaches. As the major pain-sensitive intracranial structure, the dura mater encephali has become the preferential target for studies of the peripheral mechanisms of meningeal nociception and headache (Strassman et al. 1986; Davis & Dostrovsky, 1988; Bolay et al. 2002). The dura mater is innervated by trigeminal sensory nerve fibres which contain vasoactive neuropeptides such as calcitonin gene-related peptide (CGRP), substance P (SP) and neurokinin A (NKA) (Mione et al. 1992; Messlinger et al. 1993; Edvinsson & Goadsby, 1998; Edvinsson et al. 1998). Most of the peptidergic nerve fibres are closely associated with dural blood vessels (Edvinsson & Uddman, 1981; Messlinger et al. 1993). Dural afferents not only convey nociceptive information to the central nervous system but, through the release of vasoactive peptides, also promote the sterile inflammatory process of neurogenic inflammation in the innervated tissue (Dimitriadou et al. 1992). CGRP released by trigeminal sensory fibres causes arteriolar vasodilatation, whereas SP and NKA increase the vascular permeability in the dura mater (Brain et al. 1985; Goadsby et al. 1988; Moskowitz & Cutrer, 1994). Neuropeptides may also act on the dural mast cells, resulting in the release of vasoactive substances, e.g. histamine (Dimlich et al. 1991; Ottosson & Edvinsson, 1997). Neurogenic inflammation has been proposed as an important mechanism in the generation of pain and changes in blood flow in headache patients (Moskowitz, 1984). Clinical observations support the significance of neuropeptide release in the pathophysiology of primary headaches; jugular venous blood collected from the affected side during migraine and cluster headache attacks contained increased levels of the vasodilator sensory neuropeptide CGRP (Goadsby et al. 1990; Goadsby & Edvinsson, 1994).
Under experimental conditions, neurogenic inflammation can be induced via the activation of chemosensitive primary sensory neurones by either antidromic electrical stimulation of afferent nerves or direct (orthodromic) chemical stimulation, e.g. by capsaicin (Jancsóet al. 1968; Jancsóet al. 1977, 1980; Maggi et al. 1986; Chahl, 1988; Holzer, 1991). Therefore, it is conceivable that the activation of capsaicin-sensitive afferent nerves innervating intracranial tissues may contribute to local vascular reactions and the generation of pain in headache patients.
Capsaicin-sensitve primary afferent neurones comprise a morphologically, neurochemically and functionally well characterized population of sensory ganglion cells (Jancsóet al. 1977; Buck & Burks, 1986; Holzer, 1991; Jancsó, 1992) which express the TRPV1 or capsaicin receptor (Caterina et al. 1997). These nociceptive neurons have a dual function: they are involved in the transmission of nociceptive impulses generated by chemical, heat or mechanical stimuli and, by the release of neuropeptides from the stimulated sensory nerve endings, they also fulfil a local regulatory function resulting in changes in smooth muscle contraction/relaxation, vasodilatation, plasma extravasation and other cellular functions (Jancsóet al. 1968; Jancsóet al. 1980, 1987; Buck & Burks, 1986; Holzer, 1991; Szallasi & Blumberg, 1999). Vasodilatory responses elicited by the stimulation of capsaicin-sensitive sensory nerves are mediated mainly by CGRP whereas SP, NKA and vasoactive intestinal polypeptide are involved in the mediation of most other cellular responses (Maggi & Meli, 1988; Holzer, 1991; Szallasi & Blumberg, 1999).
Capsaicin may have direct or indirect effects on vascular smooth muscle. It acts on the vanilloid (TRPV1) receptors of perivascular sensory nerve fibres and releases their neuropeptide content, resulting in vasodilatation, while capsaicin-induced vasoconstriction is probably a direct effect on blood vessels by calcium inflow into the smooth muscle cells (Toda et al. 1972; Edvinsson et al. 1990).
Repeated administration of capsaicin, under both in vivo and in vitro conditions, results in the development of characteristic functional impairments originally termed capsaicin desensitization (Jancsó, 1968). This term refers to changes that involve both functional and pharmacological desensitization of capsaicin-sensitive afferent neurons. The term pharmacological desensitization, involving acute desensitization and tachyphylaxis, has been proposed to denote the calcium-dependent changes in the responsiveness of sensory neurons to frequent or prolonged application of capsaicin at low (nanomolar) concentrations, resulting in a reduction or loss of cellular responses to the drug but not to other stimuli (Bevan & Docherty, 1993). Hence, tachyphylaxis and acute desensitization to capsaicin applied at low concentrations for a short duration are regarded as physiological phenomena (Koplas et al. 1997; Liu & Simon, 1998) probably unrelated to structural changes (Király et al. 1991). Applications of capsaicin at higher concentrations produce functional desensitization resulting in a reduction or loss of neural responses, involving nociceptive reflexes and peptide release, elicited by not only capsaicin but also other types of stimuli (Bevan & Docherty, 1993). Functional desensitization appears to be associated with or even caused by degenerative structural alterations (Buck & Burks, 1986; Jancsóet al. 1987; Király et al. 1991; Jancsó, 1992; Simone et al. 1998; Dux et al. 1999) and depletion of neuropeptides (Jessel et al. 1978; Buck & Burks, 1986; Saito & Goto, 1986; Jancsóet al. 1987; Holzer, 1991) from the sensory neuron.
Previous experiments have shown that local electrical stimulation of the dura mater evokes vasodilatation mediated by the release of CGRP from trigeminal nerve fibres (Kurosawa et al. 1995). However, the involvement of capsaicin-sensitive sensory nerves in this reaction could not be demonstrated (Peitl et al. 1999); the vasodilatory response to antidromic electrical stimulation of dural afferent fibres was not inhibited significantly by prior systemic treatment with capsaicin, which is known to result in a depletion of neuropeptides, including CGRP (Ferdinandy et al. 1997), from sensory nerves. It is well established that CGRP, the principal peptide mediating the vasodilatory effect of sensory nerve stimulation (Brain et al. 1985), is contained in both capsaicin-sensitive and capsaicin-insensitive afferent nerves (Carr et al. 1990). Since the amount of CGRP released from capsaicin-insensitive afferents upon electrical stimulation may be substantial, it may mask the effect of CGRP release from capsaicin-sensitive fibres. Accordingly, the present experiments were initiated in an attempt to reveal a possible contribution of capsaicin-sensitive afferent nerves to meningeal vasodilatory responses by an experimental approach utilizing selective chemical stimulation of the dural sensory nerves and an established rat cranial window technique (Kurosawa et al. 1995; Dux et al. 2002; Strecker et al. 2002). The possible involvement of TRPV1 receptor activation and consequent CGRP release was also studied, using a competitive TRPV1 receptor antagonist and a specific CGRP receptor antagonist, respectively. Further, electron microscopy was used to demonstrate the presence of capsaicin-sensitive afferent nerves in the dura mater encephali.
METHODS
Experimental animals and surgery
The experiments were approved by the Ethical Committee for Animal Care of the University of Szeged and efforts were made to keep the number of animals as low as possible.
Adult male Wistar rats weighing 300–400 g were used. One group of animals was given subcutaneous injections of capsaicin on 3 consecutive days at increasing doses of 10, 20 and 100 mg kg−1 (capsaicin desensitization; Ferdinandy et al. 1997). Intact animals and rats given the solvent for capsaicin (6 % ethanol and 8 % Tween 80 in saline) served as controls. Four days after the last injection, the rats were anaesthetized with thiopentone (150 mg kg−1, i.p.; Thiopental, Biochemie GmbH, Austria). Additional doses of thiopentone (25 mg kg−1, i.p.) were administered to maintain the appropriate level of anaesthesia, as assessed by the absence of changes in systemic blood pressure or nociceptive reflexes to noxious stimuli. Systemic blood pressure was recorded with a pressure transducer via a cannula inserted into the right femoral artery. The animals were tracheotomized and breathed spontaneously (Dux et al. 2002). The body temperature of the animals was recorded with a thermoprobe inserted into the rectum, and was kept at 37–37.5 oC with a heating pad. A cranial window for the measurement of dural blood flow was prepared according to Kurosawa et al. (1995). Briefly, the head was fixed in a stereotaxic frame, the scalp was removed and the left parietal bone was exposed. A cranial window of 4 mm × 6 mm was drilled into the exposed parietal bone. To avoid thermal lesions, a saline-cooled drill was used. At the end of the experiments, the animals were killed with an overdose of thiopentone (250 mg kg−1, i.p.).
Drug application
Substances were applied topically onto the exposed dura mater. The cranial window was carefully filled from a micropipette with 50 μl of a modified synthetic interstitial fluid (SIF) containing (mm): 135 NaCl, 5 KCl, 1 MgCl2, 5 CaCl2, 10 glucose and 10 Hepes (Levy & Strassman, 2002). All drugs but capsaicin and capsazepine were dissolved in SIF. Stock solutions of capsaicin (32 mm, Sigma) and capsazepine (1 mm, Sigma) were prepared with the aid of 6 % ethanol and 8 % Tween 80 in saline and were further diluted with SIF. In some experiments, the spontaneous recovery of the dural blood flow after applications of capsaicin at 100 nm and 10 μm was studied. In these experiments, capsaicin was removed only after the dural blood flow had returned to the basal level. In other experiments, the solutions containing capsaicin were removed after 5 min and the dura mater was washed repeatedly with SIF to allow the blood flow to return to the basal level, which usually occurred within 10–15 min. In the same animal, two to three different concentrations of capsaicin were tested. Capsaicin was applied at increasing concentrations, except for those experiments where the effects of capsaicin at 100 nm were tested before and after its administration at 10 μm. In some experiments, the effects of repeated capsaicin applications were tested: the same concentrations of capsaicin were applied three times, separated by wash-out periods. To determine the contribution of TRPV1 receptors and the role of CGRP in the capsaicin-induced changes of blood flow, the TRPV1 receptor antagonist capsazepine (Sigma) and the CGRP receptor antagonist hCGRP8–37 (Sigma), respectively, were applied onto the exposed surface of the dura. After 5 min, capsaicin (100 nm) was administered for 5 min. In capsaicin-desensitized animals, histamine at 10 μm was applied to the dura mater for 5 min after completion of the measurement of the capsaicin-induced blood flow changes.
Measurement of dural blood flow and evaluation of data
Dural blood flow was measured with a needle-type probe of a laser Doppler flowmeter (Perimed, Sweden) directed towards a branch of the medial meningeal artery. To minimize flow signals from the cortical blood vessels, recording sites were selected along the larger branches of the medial meningeal artery lying distant from the visible cortical blood vessels. Under these circumstances, the registered laser Doppler signal almost exclusively reflects the meningeal blood flow (Kurosawa et al. 1995). Blood flow values were recorded on-line with a time constant of 1 s. Data on the meningeal blood flow (measured in perfusion units, PU), systemic blood pressure and body temperature were stored and processed with the Perisoft program (Perimed, Sweden). The basal flow was the mean flow value measured during a 5 min period prior to drug application. The percentage changes induced in the blood flow by topical application of capsaicin or other drugs were determined as mean flow values within the 5 min application period (calculated separately for each minute) relative to the basal flow. The effects of TRPV1 and CGRP receptor antagonists on the capsaicin-induced blood flow changes were determined by comparing the changes in blood flow in response to capsaicin before and after the application of the respective antagonist. All flow values are expressed as means ±s.e.m. Statistical analysis of the data was performed with one-way analysis of variance (ANOVA) followed by the Tukey test. A probability level of P < 0.05 was regarded as a statistically significant difference between groups.
Electron microscopy
Adult male Wistar rats weighing 350–400 g were anaesthetized deeply with thiopentone (150 mg kg−1, i.p.) and then decapitated. The skin and muscles of the skull were removed and the jaw was separated at the temporomandibular joint. The skull was divided in half by a cut with a fine saw along the sagittal suture (Ebersberger et al. 1999). The skull halves were placed into carbogen-gassed Krebs solution (composition (mm): 119 NaCl, 25 NaHCO3, 1.2 KH2PO4, 1.5 MgSO4, 4.7 KCl, 2.5 CaCl2 and 11 glucose). For the identification of capsaicin-sensitive afferent axons in the dura mater, the technique introduced by Király et al. (1991) was utilized. Briefly, after a preincubation period of 10 min at 37 oC, capsaicin at 10 μm or an equivalent amount of its solvent was added to the solution. After 10 min, the capsaicin-containing solution was replaced with fresh physiological solution and the skull halves were incubated for a further period of 60 min. The specimens were fixed in situ with a fixative containing 2 % paraformaldehyde and 1 % glutaraldehyde in 0.1 m phosphate buffer (pH 7.4) for 2 h. Small samples of the dura mater encephali containing branches of the medial meningeal artery were cut out, post-fixed in a 2 % buffered solution of osmium tetroxide for 2 h, dehydrated in graded alcohols and embedded in Araldite. Ultrathin sections were cut on a Reichert-Jung Ultracut E ultrotome, stained with uranyl acetate and lead citrate and examined under a Jeol Jem 1010 electron microscope.
RESULTS
Effect of topical application of capsaicin on dural blood flow
Administration of capsaicin at concentrations of 50 and 100 nm, but not 10 nm, produced significant increases in blood flow. The highest increases in dural blood flow were observed during the second and third minute of the application period (Fig. 1A and Fig. 2). The capsaicin-induced increases in dural blood flow amounted to 10–15 % and lasted for about 9 min, whereafter the blood flow returned to the basal level. In contrast, topical application of capsaicin at 1 or 10 μm elicited an immediate decrease in dural blood flow (Fig. 1B and Fig. 2). The blood flow reduction was dose dependent and peaked during the second minute of capsaicin application. Following the administration of capsaicin at 1 and 10 μm, the blood flow returned to the control level after 5 ± 0.6 and 12 ± 0.8 min, respectively. The effects of capsaicin were reproducible at all concentrations: no significant differences could be observed in the blood flow-increasing or -decreasing effects of three consecutive applications of the same capsaicin concentration. However, a single exposure of the dura mater to capsaicin at 10 μm significantly and permanently inhibited the effect of a subsequent application of capsaicin at 100 nm, which normally elicited an increase in blood flow (Fig. 3).
Figure 1. Effect of topical application of capsaicin on meningeal blood flow.
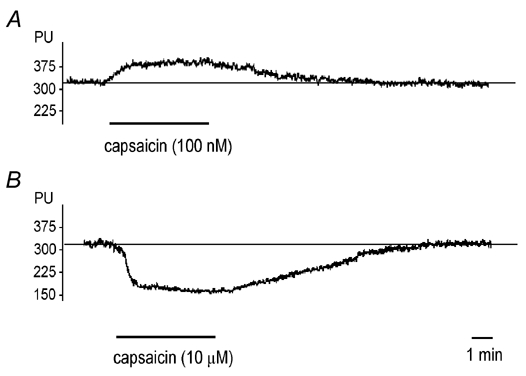
Original recordings indicating the blood flow-increasing effect of capsaicin (100 nm, A) and the vasoconstriction induced by capsaicin (10 μm, B) applications to the dura mater. Blood flow is measured in perfusion units (PU). The horizontal line indicates the mean basal flow measured before capsaicin application.
Figure 2. Effects of topical application of various concentrations of capsaicin on meningeal blood flow.
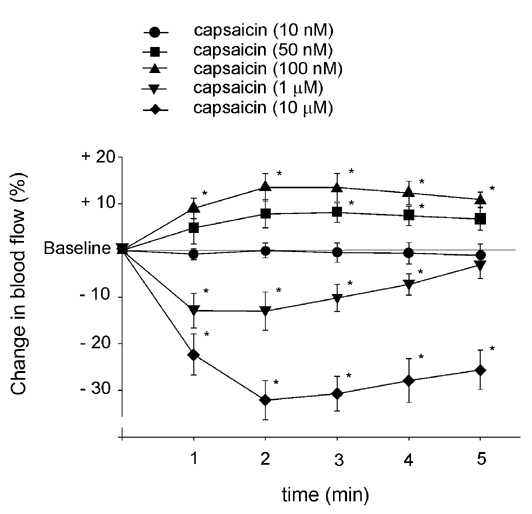
The changes in blood flow induced by capsaicin are calculated as mean percentage changes ±s.e.m. for five consecutive 1 min periods relative to the basal flow prior to capsaicin application (n = 6–11). * Significantly different from the basal flow, P < 0.05.
Figure 3. Effect of acute capsaicin desensitization on capsaicin-induced blood flow changes in dura mater.
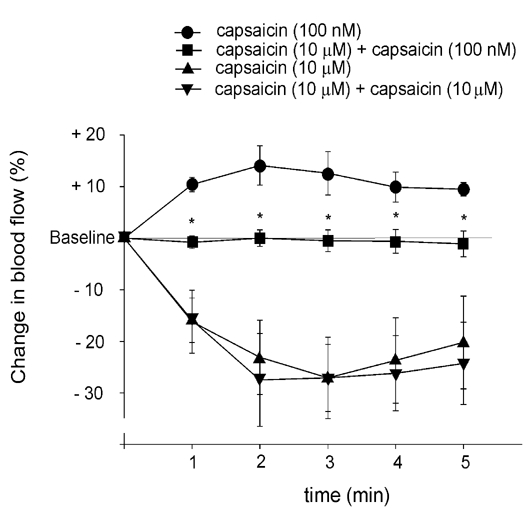
Changes in blood flow induced by capsaicin application (100 nm and 10 μm) before and after 10 μm capsaicin are calculated as mean percentage changes ±s.e.m. for five consecutive 1 min periods relative to the basal flow (n = 5). * Significantly different from the effect of the same concentration of capsaicin before the application of the desensitizing concentration (10 μm) of capsaicin, P < 0.05.
Local applications of SIF or the vehicle for capsaicin onto the dura mater failed to induce significant changes in blood flow (3 ± 2.3 and 3 ± 1.5 % increases, respectively). The mean arterial blood pressure was not affected by capsaicin applied onto the dura mater encephali: it was 112 ± 4 mmHg before and 115 ± 7 mmHg during application of the highest concentration of capsaicin (10 μm). Intact animals and rats given the solvent for capsaicin did not differ in any of the above reactions.
Effect of systemic capsaicin desensitization on capsaicin-induced changes in blood flow
In capsaicin-desensitized animals, the vasodilatory effect of capsaicin applied at 50 or 100 nm was significantly inhibited. In contrast, the vasoconstriction produced by the dural application of capsaicin at 1 μm was significantly augmented (Fig. 4). The application of histamine (10 μm) induced a significant meningeal vasodilatation (15.7 ± 1.4 % blood flow increase) in all the capsaicin-desensitized animals.
Figure 4. Effects of systemic capsaicin desensitization on capsaicin-induced changes in meningeal blood flow.
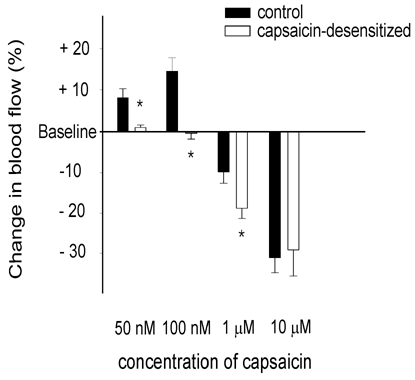
Bars represent changes induced in meningeal blood flow by application of capsaicin at 50 nm (n = 6), 100 nm (n = 7), 1 μm (n = 7) and 10 μm (n = 5) in control rats and after systemic capsaicin desensitization. Changes in blood flow are calculated as mean percentage changes ±s.e.m. for the third 1 min period of capsaicin application (when effect of capsaicin desensitization was most pronounced) relative to the basal flow. * Significantly different from the effect of capsaicin in the control rats, P < 0.05.
Effects of capsazepine and hCGRP8–37 on capsaicin-induced increase of dural blood flow
The failure of capsaicin to evoke an increase in dural blood flow in rats pretreated with capsaicin was circumstantial evidence of the involvement of TRPV1 receptors in the mediation of capsaicin-induced dural vasodilatation. To obtain direct pharmacological evidence of the involvement of TRPV1 receptors in dural vasodilatation, capsazepine, a specific vanilloid type 1 receptor antagonist, was used. Topical application of capsazepine (10 μm) did not have a significant effect on the basal blood flow (2.7 ± 1.8 % increase). After pre-application of capsazepine, a significant inhibition of the capsaicin-induced increase in blood flow was observed (Fig. 5): the original vasodilatory effect of 100 nm capsaicin was turned into vasoconstriction.
Figure 5. Effect of TRPV1 receptor antagonist capsazepine and CGRP receptor antagonist hCGRP8–37 on capsaicin-induced vasodilatation in dura mater.
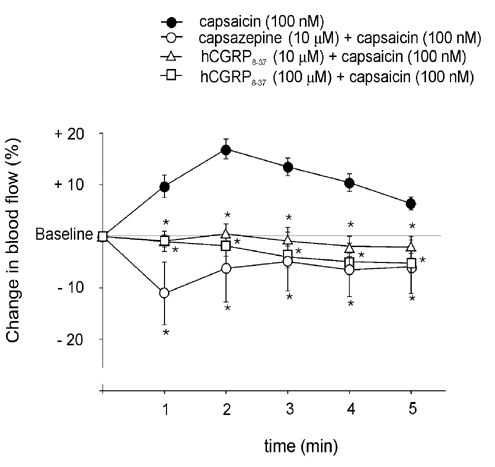
Changes induced in blood flow by capsaicin (100 nm) before and after the application of the TRPV1 receptor antagonist capsazepine (10 μm, n = 5) or hCGRP8–37 (10 and 100 μm, n = 6), calculated as mean percentage changes ±s.e.m. for five consecutive 1 min periods relative to the basal flow. * Significantly different from the effect of capsaicin before the application of the respective antagonist, P < 0.05.
hCGRP8–37 at 10 and 100 μm did not induce significant changes in meningeal blood flow (1.9 ± 0.8 and 2.1 ± 0.6 % increases, respectively). However, pre-application of hCGRP8–37 resulted in a significant inhibition of the capsaicin-induced vasodilatation (Fig. 5). Further, after the pre-application of hCGRP8–37, capsaicin at 100 nm, which produced significant increases in dural blood flow in the control animals, led to a moderate decrease in blood flow. The mean arterial blood pressure of the animals was not influenced significantly by capsazepine or hCGRP8–37 applied onto the dura mater encephali.
Electron microscopy
In the control specimens of the dura mater encephali incubated with the vehicle for capsaicin, the ultrastructure of the nerve fibres and other elements of the tissue were preserved. Many myelinated and unmyelinated axons were observed in small nerve bundles which exhibited a normal appearance. In contrast, in specimens that were exposed to capsaicin at 10 μm for 10 min, some unmyelinated axons displayed severe structural alterations in all dura mater samples. These changes are characteristic of osmiophilic axonal degeneration; the axoplasm of the affected unmyelinated nerve fibres contained amorphous or lamellated electron-dense material and demonstrated disorganization of the cellular organelles. None of the myelinated axons underwent similar structural alterations, and other structural elements of the tissue also appeared normal (Fig. 6).
Figure 6. Ultrastructural changes induced in dural unmyelinated axons by topical application of capsaicin.
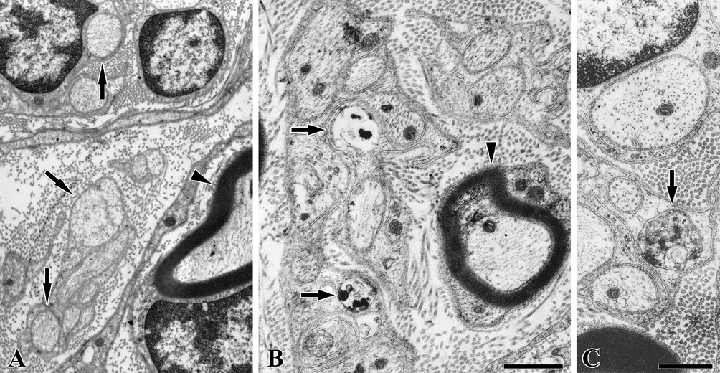
Electron micrographs showing myelinated (arrowheads) and unmyelinated (arrows) axons of the rat dura mater encephali following a 10 min exposure to capsaicin (10 μm, B and C) or its vehicle (A) in vitro. Following the exposure to capsaicin, some unmyelinated (arrows in B and C), but no myelinated axons exhibited severe ultrastructural damage indicative of the osmiophilic degeneration of these axons. A higher power electron micrograph (C) illustrates the normal appearance of the microtubules, neurofilaments and mitochondria in the intact axons and the disorganized axoplasm of an affected axon containing osmiophilic amorphous material (arrow). The scale bar in B indicates 0.5 μm and applies to both A and B; the scale bar in C indicates 1 μm.
DISCUSSION
The present experiments revealed a hitherto unrecognized vasodilatory function of the capsaicin-sensitive afferent nerves that innervate the dura mater encephali of the rat. The results indicate that chemical stimulation of the meningeal sensory fibres by capsaicin at low concentrations elicits a moderate, but significant, vasodilatory response as assessed by laser Doppler flowmetry. This response is inhibited or even completely abolished by prior acute local capsaicin desensitization, i.e. application of capsaicin at a concentration that has been shown to produce a rapid, selective degeneration of capsaicin-sensitive afferent nerves (Király et al. 1991). Similarly, systemic pretreatment with capsaicin at a dose that results in a profound depletion of sensory neuropeptides, e.g. SP and CGRP, from afferent nerves, markedly inhibited or even abolished the vasodilatory effect of capsaicin (Jancsóet al. 1977, 1987; Gamse et al. 1982; Jancsó & Such, 1985; Buck & Burks, 1986; Holzer, 1991; Jancsó, 1992). In contrast, capsaicin desensitization did not affect histamine-induced vasodilatation, which is mediated by a direct action on endothelial and smooth muscle cell histamine receptors (Dux et al. 2002). The present findings therefore strongly suggest that sensory nerve-mediated vasodilatation of the dura mater involves in part capsaicin-sensitive afferent fibres. This is supported by the finding that capsazepine, a specific TRPV1 receptor antagonist, inhibited the capsaicin-induced vasodilatation. Although the sensory innervation of the rat dura mater has been extensively studied, the contribution of capsaicin-sensitive afferent nerves has not been revealed (Andres et al. 1987). Indeed, our electron microscopic findings provide the first direct morphological evidence of a capsaicin-sensitive innervation of the dura mater of the rat.
Many of the sensory fibres innervating the dura mater contain vasoactive peptides, and in particular CGRP (Keller & Marfurt, 1991; Messlinger et al. 1993; Knyihar-Csillik et al. 1995). This peptide has been shown to play a crucial role in sensory nerve-mediated vascular reactions (Brain et al. 1985; Louis et al. 1989) and has been implicated in the mechanisms of dural circulation and meningeal nociception (Edvinsson & Uddman, 1981). In line with these findings, the present experiments have provided evidence for a fundamental role of CGRP released by the activation of TRPV1 receptors in the vascular effects of capsaicin in the dura mater encephali of the rat. The specific CGRP antagonist hCGRP8–37 inhibited the vasodilatory effect of capsaicin, indicating that this effect may be primarily mediated by CGRP. However, the findings additionally revealed that CGRP may also play an important modulatory role in the mechanism of capsaicin-induced vasoconstriction, an effect generally regarded as a direct vascular action of capsaicin (Toda et al. 1972; Duckles, 1986; Edvinsson et al. 1990, Pórszász et al. 2002). Hence, both systemic or local capsaicin desensitization and administration of the TRPV1 or CGRP receptor antagonists resulted in augmented vasoconstrictor responses of the dural vessels to capsaicin. In fact, low concentrations of capsaicin, normally producing vasodilatation, elicited significant vasoconstrictor responses in rats pretreated systemically with capsaicin or topically with the TRPV1 and CGRP antagonists. Modulation by CGRP of the vasoconstrictor responses that can be elicited by circulating vasoactive agents and tisssue metabolites may contribute to meningeal vascular and nociceptive mechanisms. Capsaicin-induced vasoconstriction may be a mechanism that is involved in the regulation of vascular tone under physiological/pathophysiological conditions. Further studies are needed to clarify whether chemical agents that are released in normal or inflammed tissue and stimulate sensory nerves or endogenous vanilloids may contribute to local vasomotor regulation.
The present findings are to some extent at variance with those of earlier studies suggesting that dural vasodilatation evoked by stimulation of sensory nerves may be solely mediated by capsaicin-insensitive afferent nerves in the rat (Peitl et al. 1999). The most likely explanation for this discrepancy may be that antidromic electrical stimulation of the trigeminal nerve in capsaicin-desensitized rats produced a robust vasodilatory response due to the release of CGRP from capsaicin-insensitive fibres, which masked the effect of CGRP normally released from a smaller population of capsaicin-sensitive afferent fibres. Hence, in our experiments, with selective chemical stimulation of CGRP-containing capsaicin-sensitive afferent nerves by capsaicin, we were able to demonstrate the contribution of these particular afferent nerves to the meningeal vasodilatory responses.
In conclusion, the present experiments have revealed a hitherto unrecognized vasodilatory mechanism which involves CGRP-containing capsaicin-sensitive afferent nerves, and have provided direct morphological evidence for the existence of such nerves in the rat dura mater. It is suggested that capsaicin-sensitive afferent nerves may contribute significantly not only to the vascular reactions but also to the nociceptive mechanisms of the dura mater possibly associated with the pathomechanism of headaches.
Acknowledgments
The authors are grateful to Dr Antal Nógrádi for the use of the electron microscope, to Éva Hegyeshalmi for excellent technical assistance and to Mihály Dezsö for preparing photographic artwork. This work was supported in part by research grants OTKA T032507 and ETT 569/2003.
REFERENCES
- Andres KH, von Düring M, Muszynski K, Schmidt RF. Nerve fibres and their terminals of the dura mater encephali of the rat. Anat Embriol. 1987;175:289–301. doi: 10.1007/BF00309843. [DOI] [PubMed] [Google Scholar]
- Bevan SJ, Docherty RJ. Cellular mechanisms of the action of capsaicin. In: Wood JN, editor. Capsaicin in the Study of Pain. London: Blackwell Science Inc; 1993. pp. 27–44. [Google Scholar]
- Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nature Medicine. 2002;8:136–142. doi: 10.1038/nm0202-136. [DOI] [PubMed] [Google Scholar]
- Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- Buck SH, Burks TF. The neuropharmacology of capsaicin: review of some recent observations. Pharmacol Rev. 1986;38:179–226. [PubMed] [Google Scholar]
- Carr PA, Yamamoto T, Nagy JI. Calcitonin gene-related peptide in primary afferent neurons of rat: co-existence with fluoride-resistant acid phosphatase and depletion by neonatal capsaicin. Neuroscience. 1990;36:751–760. doi: 10.1016/0306-4522(90)90017-x. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chahl LA. Antidromic vasodilatation and neurogenic inflammation. Pharm Ter. 1988;37:275–300. doi: 10.1016/0163-7258(88)90029-0. [DOI] [PubMed] [Google Scholar]
- Davis KD, Dostrovsky JO. Responses of feline trigeminal spinal tract nucleus neurons to stimulation of the middle meningeal artery and sagittal sinus. J Neurophysiol. 1988;59:648–665. doi: 10.1152/jn.1988.59.2.648. [DOI] [PubMed] [Google Scholar]
- Dimitriadou V, Buzzi MG, Theoharides TC, Moskowitz MA. Ultrastructural evidence for neurogenically mediated changes in blood vessels of the rat dura mater and tongue following antidromic trigeminal stimulation. Neuroscience. 1992;48:187–203. doi: 10.1016/0306-4522(92)90348-6. [DOI] [PubMed] [Google Scholar]
- Dimlich RVW, Keller JT, Strauss TA, Fritts MJ. Linear arrays of homogenous mast cells in the dura mater of the rat. J Neurocytol. 1991;20:485–503. doi: 10.1007/BF01252276. [DOI] [PubMed] [Google Scholar]
- Duckles SP. Effects of capsaicin on vascular smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1986;333:59–64. doi: 10.1007/BF00569661. [DOI] [PubMed] [Google Scholar]
- Dux M, Sann H, Schemann M, Jancsó G. Changes in fibre populations of the rat hairy skin following selective chemodenervation by capsaicin. Cell Tissue Res. 1999;296:471–477. doi: 10.1007/s004410051307. [DOI] [PubMed] [Google Scholar]
- Dux M, Schwenger N, Messlinger K. Possible role of histamine (H1- and H2-) receptors in the regulation of meningeal blood flow. Br J Pharmacol. 2002;137:874–880. doi: 10.1038/sj.bjp.0704946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersberger A, Averbeck B, Messlinger K, Reeh PW. Release of substance P, calcitonin gene-related peptide and prostaglandin E2 from rat dura mater encephali following electrical and chemical stimulation in vitro. Neuroscience. 1999;89:901–907. doi: 10.1016/s0306-4522(98)00366-2. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Goadsby PJ. Neuropeptides in headache. Eur J Neurol. 1998;5:329–341. [Google Scholar]
- Edvinsson L, Gulbenkian S, Barroso CP, Cunhae Sá M, Polak JM, Mortensen A, Jorgensen L, Jansen-Olesen I. Innervation of the human middle meningeal artery: immunohistochemistry, untrastructure, and role of endothelium for vasomotility. Peptides. 1998;19:1213–1225. doi: 10.1016/s0196-9781(98)00066-7. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Jansen I, Kingman TA, McCulloch J. Cerebrovascular responses to capsaicin in vitro and in situ. Br J Pharmacol. 1990;100:312–318. doi: 10.1111/j.1476-5381.1990.tb15801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L, Uddman R. Adrenergic, cholinergic and peptidergic nerve fibres in dura mater – involvement in headache? Cephalalgia. 1981;1:175–179. doi: 10.1046/j.1468-2982.1981.0104175.x. [DOI] [PubMed] [Google Scholar]
- Ferdinandy P, Csont T, Csonka Cs, Török M, Dux M, Németh J, Horváth IL, Dux L, Szilvássy Z, Jancsó G. Capsaicinsensitive local sensory innervation is involved in pacing-induced preconditioning in rat hearts: role of nitric oxide and CGRP. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:356–363. doi: 10.1007/pl00005062. [DOI] [PubMed] [Google Scholar]
- Gamse R, Petsche U, Lembeck F, Jancsó G. Capsaicin applied to peripheral nerve inhibits axoplasmic transport of substance P and somatostatin. Brain Res. 1982;239:447–462. doi: 10.1016/0006-8993(82)90521-2. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L. Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain. 1994;117:427–434. doi: 10.1093/brain/117.3.427. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol. 1988;23:193–196. doi: 10.1002/ana.410230214. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- Jancsó G. Pathobiological reactions of C-fibre primary sensory neurones to peripheral nerve injury. Exp Physiol. 1992;77:405–431. doi: 10.1113/expphysiol.1992.sp003603. [DOI] [PubMed] [Google Scholar]
- Jancsó G, Király E, Jancsó-Gábor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977;270:741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- Jancsó G, Király E, Jancsó-Gábor A. Chemosensitive pain fibres and inflammation. Int J Tiss Reac. 1980;2:57–66. [Google Scholar]
- Jancsó G, Király E, Such G, Joó F, Nagy A. Neurotoxic effect of capsaicin in mammals. Acta Physiol Hung. 1987;69:295–314. [PubMed] [Google Scholar]
- Jancsó G, Such G. Evidence for a capsaicin-sensitive vasomotor mechanism in the ventral medullary chemosensitive area of the cat. Naunyn Schmiedebergs Arch Pharmacol. 1985;329:56–62. doi: 10.1007/BF00695193. [DOI] [PubMed] [Google Scholar]
- Jancsó N. Desensitization with capsaicin as a tool for studying the function of pain receptors. In: Lim RKS, editor. Pharmacology of Pain. Oxford: Blackwell Science Inc; 1968. pp. 33–55. [Google Scholar]
- Jancsó N, Jancsó-Gábor A, Szolcsányi J. The role of the sensory nerve endings in neurogenic inflammation induced in human skin and in the eye and paw of the rat. Br J Pharmacol Chemoter. 1968;33:32–41. doi: 10.1111/j.1476-5381.1968.tb00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM, Iversen LL, Cuello AC. Capsaicin-induced depletion of substance P from primary sensory neurones. Brain Res. 1978;152:183–188. doi: 10.1016/0006-8993(78)90146-4. [DOI] [PubMed] [Google Scholar]
- Keller JT, Marfurt CF. Peptidergic and serotoninergic innervation of the rat dura mater. J Comp Neurol. 1991;309:515–534. doi: 10.1002/cne.903090408. [DOI] [PubMed] [Google Scholar]
- Király E, Jancsó G, Hajós M. Possible morphological correlates of capsaicin desensitization. Brain Res. 1991;540:279–282. doi: 10.1016/0006-8993(91)90518-z. [DOI] [PubMed] [Google Scholar]
- Knyihar-Csillik E, Tajti J, Mohtasham S, Sari G, Vecsei L. Electrical stimulation of the Gasserian ganglion induces structural alterations of calcitonin gene-related peptide-immunoreactive perivascular sensory nerve terminals in the rat cerebral dura mater: a possible model of migraine headache. Neurosci Lett. 1995;184:189–192. doi: 10.1016/0304-3940(94)11203-u. [DOI] [PubMed] [Google Scholar]
- Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci. 1997;17:3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa M, Messlinger K, Pawlak M, Schmidt RF. Increase of meningeal blood flow after electrical stimulation of rat dura mater encephali: mediation by calcitonin gene-related peptide. Br J Pharmacol. 1995;114:1397–1402. doi: 10.1111/j.1476-5381.1995.tb13361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Strassman AM. Distinct sensitizing effects of the cAMP-PKA second messenger cascade on rat dural mechanonociceptors. J Physiol. 2002;538:483–493. doi: 10.1113/jphysiol.2001.013175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Simon SA. The influence of removing extracellular Ca2+ in the desensitization responses to capsaicin, zingeron and olvanil in rat trigeminal ganglion neurons. Brain Res. 1998;809:246–252. doi: 10.1016/s0006-8993(98)00853-1. [DOI] [PubMed] [Google Scholar]
- Louis SM, Jamieson A, Russell NJ, Dockray GJ. The role of substance P and calcitonin gene-related peptide in neurogenic plasma extravasation and vasodilatation in the rat. Neuroscience. 1989;32:581–586. doi: 10.1016/0306-4522(89)90281-9. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Manzini S, Giuliani S, Santicioli P, Meli A. Extrinsic origin of the capsaicin-sensitive innervation of rat duodenum: possible involvement of calcitonin gene-related peptide (CGRP) in the capsaicin-induced activation of intramural non-adrenergic non-cholinergic neurons. Naunyn Schmiedebergs Arch Pharmacol. 1986;334:172–180. doi: 10.1007/BF00505818. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Meli A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen Pharmacol. 1988;19:1–43. doi: 10.1016/0306-3623(88)90002-x. [DOI] [PubMed] [Google Scholar]
- Mione MC, Cavanagh JFR, Kirkpatrick KA, Burnstock G. Plasticity in expression of calcitonin gene-related peptide and substance P immunoreactivity in ganglia and fibres following guanethidine and/or capsaicin denervation. Cell Tissue Res. 1992;268:491–504. doi: 10.1007/BF00319156. [DOI] [PubMed] [Google Scholar]
- Messlinger K, Hanesch U, Baumgärtel M, Trost B, Schmidt RF. Innervation of the dura mater encephali of cat and rat: ultrastructure and calcitonin gene-related peptide-like and substance P-like immunoreactivity. Anat Embryol. 1993;188:219–237. doi: 10.1007/BF00188214. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA. The neurobiology of vascular head pain. Ann Neurol. 1984;16:157–168. doi: 10.1002/ana.410160202. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA, Cutrer FM. Possible importance of neurogenic inflammation within the meninges to migraine headache. In: Feilds HL, Liebeskind JC, editors. Progress in Pain Research and Management. Vol. 1. Seattle: Blackwell Science Inc; 1994. pp. 43–49. [Google Scholar]
- Ottosson A, Edvinsson L. Release of histamine from dural mast cells by substance P and calcitonin gene-related peptide. Cephalalgia. 1997;17:166–174. doi: 10.1046/j.1468-2982.1997.1703166.x. [DOI] [PubMed] [Google Scholar]
- Peitl B, Pethö G, Pórszász R, Németh J, Szolcsányi J. Capsaicin-insensitive sensory-efferent meningeal vasodilatation evoked by electrical stimulation of trigeminal nerve fibres. Br J Pharmacol. 1999;127:457–467. doi: 10.1038/sj.bjp.0702561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pórszász R, Porkoláb Á, Ferencz A, Pataki T, Szilvássy Z, Szolcsányi J. Capsaicin-induced nonneural vasoconstriction in canine mesenteric arteries. Eur J Pharmacol. 2002;441:173–175. doi: 10.1016/s0014-2999(01)01596-5. [DOI] [PubMed] [Google Scholar]
- Saito A, Goto K. Depletion of calcitonin gene-related peptide (CGRP) by capsaicin in cerebral arteries. J Pharmacobiodyn. 1986;9:613–619. doi: 10.1248/bpb1978.9.613. [DOI] [PubMed] [Google Scholar]
- Simone DA, Nolano M, Johnson T, Wendelschafer-Crabb G, Kennedy WR. Intradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibers: correlation with sensory function. J Neurosci. 1998;18:8947–8959. doi: 10.1523/JNEUROSCI.18-21-08947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman AM, Mason P, Moskowitz MA, Maciewicz RJ. Response of brainstem trigeminal neurons to electrical stimulation of the dura. Brain Res. 1986;379:242–250. doi: 10.1016/0006-8993(86)90777-8. [DOI] [PubMed] [Google Scholar]
- Strecker T, Dux M, Messlinger K. Increase in meningeal blood flow by nitric oxide – interaction with calcitonin gene-related peptide receptor and prostaglandin synthesis inhibition. Cephalalgia. 2002;22:233–241. doi: 10.1046/j.1468-2982.2002.00356.x. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Toda N, Usui H, Nishino N, Fujiwara M. Cardiovascular effects of capsaicin in dogs and rabbits. J Pharmacol Exp Ther. 1972;181:512–521. [PubMed] [Google Scholar]


