Abstract
There is evidence in rodents that Ca2+-calmodulin-dependent protein kinase II (CaMKII) activity is higher in contracting skeletal muscle, and this kinase may regulate skeletal muscle function and metabolism during exercise. To investigate the effect of exercise on CaMKII in human skeletal muscle, healthy men (n = 8) performed cycle ergometer exercise for 40 min at 76 ± 1 % peak pulmonary O2 uptake (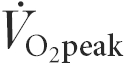 ), with skeletal muscle samples taken at rest and after 5 and 40 min of exercise. CaMKII expression and activities were examined by immunoblotting and in vitro kinase assays, respectively. There were no differences in maximal (+ Ca2+, CaM) CaMKII activity during exercise compared with rest. Autonomous (- Ca2+, CaM) CaMKII activity was 9 ± 1 % of maximal at rest, remained unchanged at 5 min, and increased to 17 ± 1 % (P < 0.01) at 40 min. CaMKII autophosphorylation at Thr287 was 50–70 % higher during exercise, with no differences in CaMKII expression. The effect of maximal aerobic exercise on CaMKII was also examined (n = 9), with 0.7- to 1.5-fold increases in autonomous CaMKII activity, but no change in maximal CaMKII activity. CaMKIV was not detected in human skeletal muscle. In summary, exercise increases the activity of CaMKII in skeletal muscle, suggesting that it may have a role in regulating skeletal muscle function and metabolism during exercise in humans.
), with skeletal muscle samples taken at rest and after 5 and 40 min of exercise. CaMKII expression and activities were examined by immunoblotting and in vitro kinase assays, respectively. There were no differences in maximal (+ Ca2+, CaM) CaMKII activity during exercise compared with rest. Autonomous (- Ca2+, CaM) CaMKII activity was 9 ± 1 % of maximal at rest, remained unchanged at 5 min, and increased to 17 ± 1 % (P < 0.01) at 40 min. CaMKII autophosphorylation at Thr287 was 50–70 % higher during exercise, with no differences in CaMKII expression. The effect of maximal aerobic exercise on CaMKII was also examined (n = 9), with 0.7- to 1.5-fold increases in autonomous CaMKII activity, but no change in maximal CaMKII activity. CaMKIV was not detected in human skeletal muscle. In summary, exercise increases the activity of CaMKII in skeletal muscle, suggesting that it may have a role in regulating skeletal muscle function and metabolism during exercise in humans.
Calcium (Ca2+) is an important second messenger involved in regulating many cellular events (Berridge et al. 2000). In skeletal muscle, increases in intracellular Ca2+ due to release from the sarcoplasmic reticulum play a pivotal role in excitation-contraction coupling as well as other cellular events (Berchtold et al. 2000).
One action of the ubiquitously expressed Ca2+ sensor protein calmodulin (CaM) is to bind to and activate a family of Ser/Thr protein kinases known as the Ca2+-CaM-dependent protein kinases (CaMKs). One of these proteins is CaMKII, which is encoded by four homologous but distinct genes (α, β, γ and δ), and at least one gene product is expressed in all tissues including skeletal muscle (Tobimatsu & Fujisawa, 1989). CaMKII activity is detectable in skeletal muscle (Pelosi & Donella-Deana, 2000; Fluck et al. 2000b) and immunoblotting has revealed the presence of the γ and δ isozymes and a splice variant of the β isozyme designated βM (Bayer et al. 1998; Sacchetto et al. 2000). A variant of the α isozyme is also expressed in skeletal muscle, but is non-functional as a kinase and is likely to be an anchoring protein (Bayer et al. 1996, 1998).
Unlike CaMKI and CaMKIV, CaMKII can be fully activated by Ca2+-CaM, independently of an upstream kinase (Hudmon & Schulman, 2002). A unique feature of the CaMKII is that upon activation by CaM binding, the heterotrimeric kinase undergoes intersubunit phosphorylation at a conserved amino acid residue (Thr286/7), making the kinase partially independent of Ca2+-CaM (Hudmon & Schulman, 2002). Thus, when a Ca2+ transient is over, the kinase retains heightened activity above basal (Hudmon & Schulman, 2002). Importantly, this characteristic is conserved in the CaMKII of skeletal muscle (Pelosi & Donella-Deana, 2000). Another important feature is that the activation of CaMKII is sensitive to the frequency of Ca2+ oscillations (De Koninck & Schulman, 1998).
Given that skeletal muscle CaMKII is not active at basal levels, but active at free Ca2+ concentrations seen during a twitch (Pelosi & Donella-Deana, 2000) it is likely that contractile activity activates skeletal muscle CaMKII. While there are studies examining the effect of contractile activity on CaMKII in skeletal muscle of rodents (Antipenko et al. 1999; Fluck et al. 2000b), no studies have examined the effect of exercise on CaMKII in skeletal muscle of humans.
METHODS
Experimental protocol
Healthy, active but untrained, men (n = 17; 24 ± 5 years; body mass index (BMI) = 24 ± 2 kg m−2; mean ± s.d.) were recruited for two separate studies. Written and verbal information about the purpose, nature, and potential risks relating to the experimental procedures was given to the subjects before they provided consent to participate. The protocol was reviewed and approved by the Deakin University Human Research Ethics Committee and conformed to the standards set by the Declaration of Helsinki (last modified in 2000). One to two weeks prior to testing, subjects completed an incremental exercise test to volitional exhaustion on an electromagnetically braked cycle ergometer (Lode, Groningen, The Netherlands) to determine their peak pulmonary O2 uptake (O2peak), which averaged 51 ± 2 ml kg−1 min−1 (mean ± s.e.m.). Expired air was analysed by O2 and CO2 analysers (AEI Technologies, Pittsburgh, PA, USA) and expired volume by a turbine ventilometer. The gas analysers were calibrated against gases of known composition prior to each test.
Subjects were asked to refrain from exercise as well as caffeine, nicotine and alcohol ingestion for 24 h prior to the study. Subjects were provided with a standardised meal (≈80 % CHO) for the evening prior to testing and reported to the laboratory in the morning after an overnight fast. In both studies, subjects rested for at least 20 min in the supine position before a muscle sample was obtained from the vastus lateralis by percutaneous needle biopsy under local anaesthesia and immediately frozen in liquid N2. In one study subjects (n = 8) exercised for 40 min at 76 ± 1 % O2peak with biopsies taken at 5 and 40 min of exercise. In another study, subjects (n = 9) performed bicycle exercise for 10 min at 50 % O2peak, after which the resistance was increased to elicit a power output requiring 100 % O2peak and this was continued until volitional fatigue (3.5 ± 0.2 min) upon which a muscle sample was taken. During exercise, biopsy samples were taken and frozen within 20 s after the last contraction. Muscle samples were stored in liquid N2 until analysis.
Analytical techniques
All chemicals were purchased from Sigma-Aldrich unless otherwise stated. To solubilise tissue protein, muscle samples were homogenised (Polytron X-100, Kinematica) in 1:12 (w/v) of ice-cold buffer (Buffer A) containing 50 mm Tris-HCl (pH 7.5), 250 mm sucrose, 1 mm EDTA, 1 mm EGTA, 1 mm phenylmethylsulphonic fluoride (PMSF), 1 mm dithiothreitol, 5 mm sodium fluoride, 5 mm sodium pyrophosphate, 10 % glycerol, 1 mm benzamidine, and 5 µl ml−1 protease inhibitor cocktail. Samples were spun at 700g for 5 min, and the supernatant was taken and supplemented with Buffer A containing Nonidet P-40 at a final concentration of 1 % and mixed well at 4 °C. The pellet was solublised with an appropriate extraction buffer to yield a fraction of enriched nuclear proteins (McGee et al. 2003). Aliquots of both fractions were taken for total protein assay (Pierce BCA, Rockford, IL, USA) and the remaining lysate was stored at -80 °C until analysis.
To detect CaMKII activity, samples were assayed in vitro as described previously (Fluck et al. 2000b), with minor modifications. To detect CaMKII activity in skeletal muscle lysates, 10-20 µg of protein (5 µl) was added to a preheated reaction mix composed of (final concentrations): 10 mm Hepes (pH 7.2), 5 mm MgCl2, 1 mm EGTA, 0.1 mm sodium pyrophosphate, 0.1 mm ATP (0.2 Ci mmol−1 5′-[γ32P]ATP; Amersham Biosciences, Uppsala, Sweden), 25 µm autocamtide-2 substrate peptide (Hanson et al. 1989; Upstate Biotech., Lake Placid, NY, USA) with (maximal) or without (autonomous) 1.2 mm CaCl2 and 1.2 µm calmodulin in a final reaction volume of 25 µl. In preliminary experiments, autocamtide-2 related inhibitory peptide (AIP) was added to a final concentration of 10 µm (Ishida et al. 1995) to confirm that the assay was specific for CaMKII. The reaction proceeded at 30 °C for 2.5 min and was terminated by spotting 10 µl of the reaction mix onto a P81 phosphocellulose filter paper (Whatman, Kent, UK). The reaction mix was absorbed for 2-3 s before washing in 75 mm phosphoric acid for at least 3 × 10 min. All reactions were run in duplicate (CV = 3.1 ± 0.5 %), and background reactions (without addition of peptide) were run for each sample and subtracted from maximal and autonomous counts. The incorporation of γ-32P onto the peptide was measured by liquid scintillation counting (Wallac 1409, Turku, Finland), and enzyme activity was determined as described by Goueli et al. (2001). In order to directly measure CaMKII activity, CaMKII was immunopurified (IP) from muscle lysate protein. To do this 2 µg of polyclonal CaMKII antibody (M-176, Santa Cruz Biotech., CA, USA) were incubated with 375 µg of lysate protein in a final volume of 600 µl with gentle mixing for 2 h at 4 °C. Following this 40 µl of 50 % (v/v) of protein-A-sepharose (Amersham Biosciences) were added and further incubated with mixing for 2 h at 4 °C. The bead-antibody-antigen complex was separated by centrifugation and washed three times with a buffer containing 10 mm Tris (pH 7.2), 1 mm sodium pyrophosphate and 1 mm EGTA, then resuspended with 55 µl of buffer and aliquots were assayed for 10 min as described above. The activity of immunopurified CaMKII was ≈30 % of that of the lysate, mainly because of an inability to completely purify the kinase from the total amount of protein added (data not shown). The assay time and protein added were within a linear range for lysate and IP assays (data not shown). Preliminary testing revealed that within-sample and between-assay CVs for the CaMKII assay were 4.5 ± 0.7 % and 3.8 ± 0.5 %, respectively.
To provide a positive control for the assay, L6 skeletal myotubes were treated with Ca2+ ionophore A23187. All media solutions were purchased from Invitrogen. L6 myoblasts were grown in αMEM containing 10 % horse serum and differentiated in αMEM containing 2 % horse serum in standard culture conditions (37 °C, 5 % CO2) until 80-90 % confluent. L6 myotubes were serum starved overnight, and 1 h before experimentation serum-free media was replaced with Hank's Balanced Salt Solution (HBSS) containing 10 mm Hepes (pH 7.4), 1.26 mm CaCl2 and 0.5 mm MgCl2. Cells (n = 3 dishes per treatment) were treated with 1 µm A23187 and control cells were treated with vehicle (0.1 % ethanol) for 30 s. Media were aspirated and cells were lysed immediately and collected with Buffer A containing 1 % Nonidet P-40 after 10 min on ice. Protein concentration was determined (range = 0.9-1.0 mg ml−1) and CaMKII activity and expression were measured from lysates as described above. There was a 3-fold increase (vehicle = 5.6 ± 0.6 % maximal, A23187 = 16.0 ± 1.0 % maximal; P = 0.001) in autonomous (with no change in maximal activity) CaMKII activity with ionophore treatment, as observed in other studies (MacNicol & Schulman, 1992).
To determine CaMKII expression and autophosphorylation, equal amounts of proteins in muscle lysate or IP samples were subjected to SDS-PAGE, transferred onto nitrocellulose membranes and blocked (5 % skim milk in TBS-T, pH 7.4) for 1 h at room temperature. Membranes were washed and incubated with anti-CaMKII or anti-pThr286-CaMKII (Cell Signaling Tech., Beverly, MA, USA) antibodies (1:500) for 14-16 h at 4 °C, after which they were washed. CaMKIV expression was detected using a monoclonal antibody (BD Biosciences, NJ, USA). Membranes were then incubated with peroxidase-conjugated secondary antibodies (Chemicon, Temecula, CA, USA; 1:5000 dilution) and washed thoroughly and proteins were visualised with chemiluminescence (Western Lightning, PerkinElmer, Boston, MA, USA) and light detection (Kodak Image Station 440CF).
Calculations and statistics analyses
Autonomous CaMKII activity was also expressed as a percentage of maximal CaMKII activity. Arbitrary units for protein abundance were expressed as a ratio of sample band intensity relative to an internal control band intensity. Statistical testing was done with t tests (MS Excel) or one-way ANOVA, with repeated measures with post hoc (Student-Newman-Keuls) testing being performed when differences were significant, as appropriate (GraphPad Prism, v.2.01). Data are expressed as means ± s.e.m. and differences were considered to be significant when P < 0.05.
RESULTS
CaMKII is expressed in human skeletal muscle
Similar to animal studies (Bayer et al. 1998; Sacchetto et al. 2000), immunoblotting of human skeletal muscle for CaMKII yielded a band at 72 kDa corresponding to the βM isozyme, and a strong band at approximately 55 kDa which is likely to be either or both the δ and γ isozymes (Fig. 1A). Despite being able to detect CaMKIV in rat brain, no signal was detected for CaMKIV in human skeletal muscle (Fig. 1B). In resting muscle, Ca2+-CaM-stimulated activity was 8- to 10-fold higher than activity measured without Ca2+ and CaM as has been observed in rat skeletal muscle (Fluck et al. 2000a). In preliminary experiments, maximal and autonomous activity were inhibited by 75-90 % when 10 µm AIP was added to the reaction, indicating that the predominant kinase contributing to phosphate transfer in the reaction is likely to be CaMKII. Taken together, these data suggest that CaMKII is expressed in human skeletal muscle.
Figure 1. CaMKII, but not CaMKIV, is expressed in human skeletal muscle.
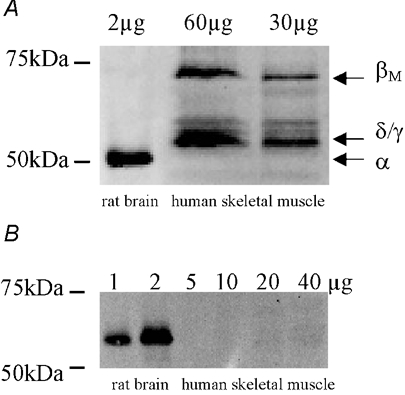
Solublised protein from rat brain and human skeletal muscle tissue was subjected to immunoblotting procedures and probed using polyclonal CaMKII (A) and monoclonal CaMKIV (B) antibodies. Indicated are the various isoforms of CaMKII that are expressed.
Skeletal muscle CaMKII is activated by exercise
As can be seen in Fig. 2, autonomous CaMKII activity during submaximal, high-intensity exercise was not higher at 5 min, but was 74 ± 24 % higher at 40 min when compared with basal activity. There was a slight decrease in maximal CaMKII activity, but this was not statistically significant. To examine whether this decrease was due to movement of the CaMKII to the nuclei, immunoblotting was performed on the lysates from a fraction enriched in nuclei (data not shown). No differences in the abundance of 55 kDa CaMKII abundance between time points in this fraction were seen (the 72 kDa band was barely detectable). In addition, there were no differences in the abundance of CaMKII in the crude lysate preparations used for activity measurements (data not shown). However, phosphorylation of CaMKII-δ/γ at Thr287 was 67 ± 12 % and 52 ± 14 % higher at 5 and 40 min, respectively (P < 0.01), while no differences in CaMKII-βM phosphorylation were seen, when compared with the basal sample (Fig. 3). As can be seen in Fig. 4, autonomous CaMKII activity was 0.7- to 1.5-fold higher after 3-5 min of maximal aerobic exercise, but there were no differences in maximal CaMKII activity. Taken together, these data suggest that CaMKII is activated in contracting skeletal muscle during exercise in humans.
Figure 2. Submaximal exercise increases autonomous, but not maximal, in vitro CaMKII activity in human skeletal muscle.
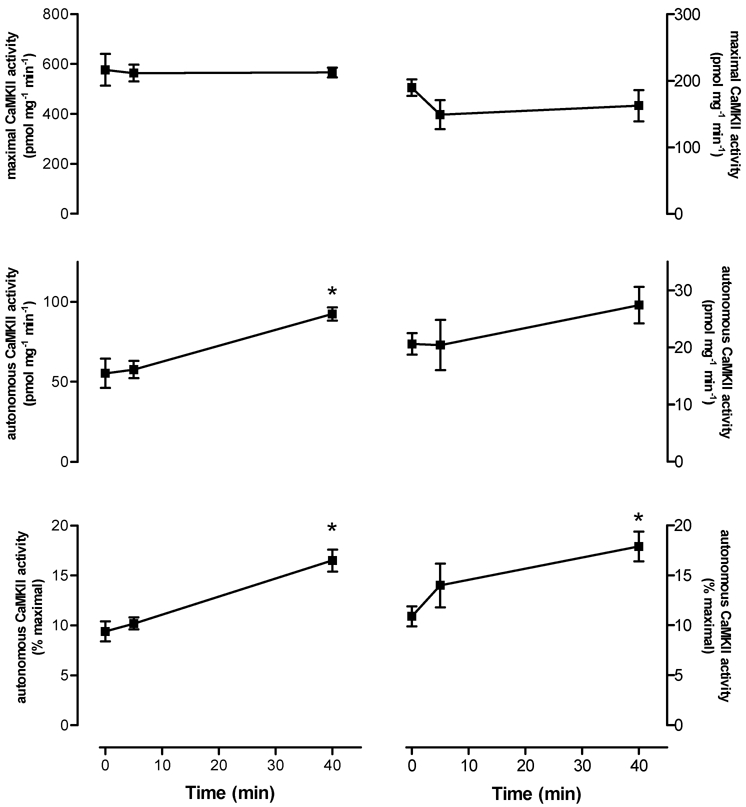
Shown are the activities measured from tissue lysates (left column) and immunoprecipitates (IP, right column). Data are means ± s.e.m.n = 8. * Significantly different from 0, P < 0.01.
Figure 3. Submaximal exercise increases CaMKII autophosphorylation at Thr287.
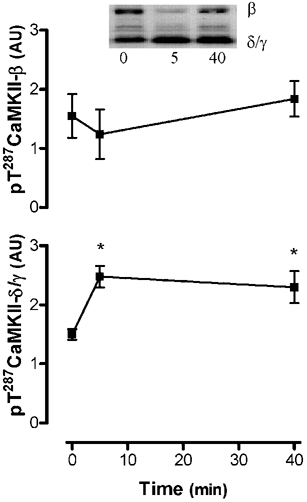
Solubilised proteins from crude skeletal muscle tissue extracts were subjected to immunoblotting procedures and probed using a polyclonal pT287-CaMKII antibody. Data are means ± s.e.m.n = 8. * Significantly different from 0, P < 0.05.
Figure 4. Maximal aerobic exercise (3.5 ± 0.2 min) increases autonomous, but not maximal, in vitro CaMKII activity in human skeletal muscle.
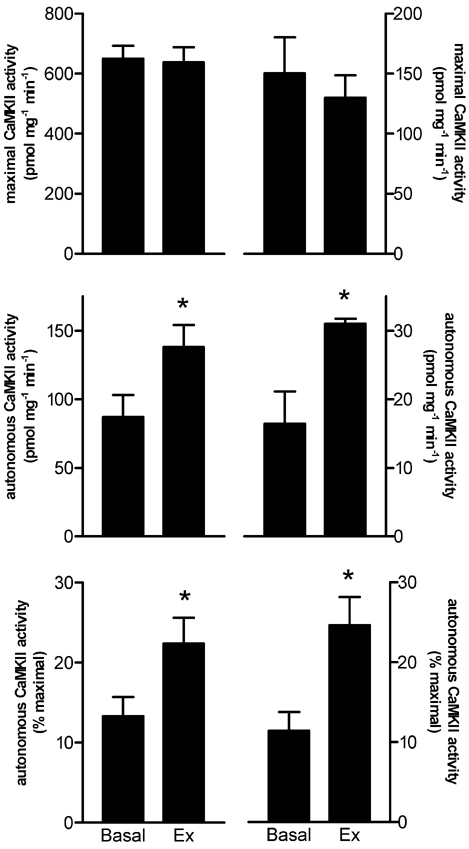
Shown are the activities measured from tissue lysates (left column) and immunoprecipitates (IP, right column). Data are means ± s.e.m.n = 9 (lysate), n = 6 (IP). * Significantly different from Basal, P < 0.05.
DISCUSSION
This is the first study to directly demonstrate activation of CaMKII in contracting human skeletal muscle when measured immediately post exercise. This finding is in agreement with a study of isolated mouse skeletal muscle fibres that presented indirect evidence that CaMKII activity is increased by repeated contractions (Tavi et al. 2003). Furthermore, Fluck et al. (2000b) have demonstrated increases in autonomous CaMKII activity in overloaded avian muscle and in rodent skeletal muscle after 14 days of voluntary wheel running. In the present study, there were no significant changes in maximal in vitro CaMKII activity, while autonomous CaMKII activity increased from approximately 9-11 % of maximal in basal samples to 16-25 % with exercise. This appears to be very modest activation considering that autonomous activity can reach up to 50-60 % of maximal in an in vitro preparation of rabbit skeletal muscle (Pelosi & Donella-Deana, 2000). However, it has been observed that the generation of autonomous activity through mobilisation of intracellular Ca2+ in cells is consistently lower than that which can be generated by incubation of enzyme with Ca2+in vitro (Hudmon & Schulman, 2002). When comparing the two different exercise intensities, it appears that there is generally a larger increase in autonomous activity with maximal aerobic exercise (≈1-fold increase) when compared with high-intensity submaximal exercise (≈0.7-fold increase). This could be the result of a greater proportion of active muscle fibres or greater Ca2+ levels in individual fibres with the higher exercise intensity. Regardless, the effect of exercise intensity was not directly examined and warrants further investigation.
The mechanism for increased autonomous activity of CaMKII appears to be related to increased phosphorylation at Thr287. In the present study, a 50–70 % increase in Thr287 phosphorylation of CaMKII in contracting muscle during submaximal exercise was observed. Similarly, a preliminary report demonstrated 2-fold higher CaMKII autophosphorylation with tetanic stimulation of rat hindlimb muscle (Wright et al. 2003). The increase in autonomous activity is likely to be caused by a direct effect of Ca2+, whereby the generation of transient Ca2+ ‘spikes’ by depolarisation induces CaM binding, and subsequent activation and autophosphorylation, as has been observed in a cell-free system (De Koninck & Schulman, 1998) and intact neurons (Eshete & Fields, 2001). The functional consequence of autophosphorylation is believed to be maintenance of activity between Ca2+ transients, thereby allowing persistent phosphorylation of downstream substrates during repeated stimulation (Hudmon & Schulman, 2002).
While CaMKIV is expressed in murine skeletal muscle (J. T. Treebak, A. J. Rose & M. Hargreaves, unpublished observations), it was not detected in human skeletal muscle in the present study. Although immunopurified maximal in vitro CaMKIV activity was detected in human skeletal muscle, albeit at levels much lower than CaMKII, it was not influenced by exercise (data not shown). CaMKI is also expressed in rat skeletal muscle (Picciotto et al. 1993), and is expressed at the mRNA level in human skeletal muscle (S. L. McGee, A. J. Rose & M. Hargreaves, unpublished observations). Further study is needed to reveal the effect of contractions on skeletal muscle CaMKI.
Although no functional effects of CaMKII activation were examined in the present study, there is evidence from other studies that CaMKII may be involved in modification of skeletal muscle function with exercise, including the regulation of Ca2+ homeostasis, metabolism and gene expression. A recent study revealed that injection of a specific CaMKII inhibitor into intact mouse fast-twitch skeletal muscle fibres resulted in a decrease in the force production in response to a tetanic electrical stimulus which was associated with a blunted increase in Ca2+ (Tavi et al. 2003). Given that CaMKII inhibition had no effect on sarcoplasmic reticulum (SR) Ca2+ uptake or the force-Ca2+ relationship, it was concluded that CaMKII activation during exercise is likely to affect SR Ca2+ release by phosphorylating and further activating the SR Ca2+ release channels during tetanic stimulation (Tavi et al. 2003). Indeed, there is evidence in rabbit skeletal muscle that CaMKII inhibition can blunt the activation of the Ca2+ release channel (Dulhunty et al. 2001). There is also evidence that CaMKII can phosphorylate the SR Ca2+ pumps in rabbit slow-twitch, but not fast-twitch, skeletal muscle and accelerate SR Ca2+ uptake (Hawkins et al. 1994). However, while Ca2+ pumps and channels appear to be substrates of CaMKII in animal skeletal muscle, the only identified substrate of endogenous CaMKII (other than itself) in human skeletal muscle is phospholamban (PLB; Margreth et al. 2000). Phosphorylation of PLB by CaMKII is believed to relieve the inhibition of the SR Ca2+ pumps by disrupting the physical interaction between the Ca2+ pumps and PLB (for review see Simmerman & Jones, 1998). Thus, in human muscle, CaMKII may regulate Ca2+ homeostasis during contraction by altering SR Ca2+ uptake through PLB-Ca2+ pump phosphorylation, although no studies have directly investigated this.
Several studies suggest that Ca2+ spikes may be involved in the stimulation of glucose transport during muscle contraction (Richter et al. 2003). There is evidence that CaMKII inhibition can block hypoxia- and insulin-stimulated glucose transport in skeletal muscle (Brozinick et al. 1999), and a preliminary report has demonstrated a 50 % inhibition of glucose transport with tetanic stimulation of rat hindlimb muscle (Wright et al. 2003). There are several lines of evidence that increases in Ca2+ may mediate some adaptive responses to repeated exercise (Berchtold et al. 2000) and that CaMKs are potentially involved in this response (Fluck et al. 2000b; Ojuka et al. 2002). Indeed, CaMKII can phosphorylate transcription factors such as cAMP response element binding protein (Sheng et al. 1991) and serum response factor (Fluck et al. 2000a). Thus, the activation of skeletal muscle CaMKII during exercise describes a potential mechanism by which muscle contractions increase glucose transport and gene transcription in skeletal muscle. Further studies are needed to confirm the functional significance of CaMKII activation.
In summary, exercise increases the autonomous, but not the maximal, activity of CaMKII in skeletal muscle of humans. The mechanism for increased autonomous CaMKII activity is related to higher CaMKII autophosphorylation at Thr287.
Acknowledgments
The technical assistance of Dr Andrew Garnham, Ms Janelle Mollica, Dr George Kraniou and Mr Sean McGee is gratefully acknowledged. The authors also acknowledge the scientific input of Dr Belinda Michell and Professor Bruce Kemp in the development of assay procedures.
REFERENCES
- Antipenko A, Frias JA, Parra J, Cadefau JA, Cusso R. Effect of chronic electrostimulation of rabbit skeletal muscle on calmodulin level and protein kinase activity. Int J Biochem Cell Biol. 1999;31:303–310. doi: 10.1016/s1357-2725(98)00112-5. [DOI] [PubMed] [Google Scholar]
- Bayer KU, Harbers K, Schulman H. α KAP is an anchoring protein for a novel CaM kinase II isoform in skeletal muscle. EMBO J. 1998;17:5598–5605. doi: 10.1093/emboj/17.19.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer KU, Lohler J, Harbers K. An alternative, nonkinase product of the brain-specifically expressed Ca2+/calmodulin-dependent kinase II α isoform gene in skeletal muscle. Mol Cell Biol. 1996;16:29–36. doi: 10.1128/mcb.16.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold MW, Brinkmeier H, Muntener M. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol Rev. 2000;80:1215–1265. doi: 10.1152/physrev.2000.80.3.1215. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Brozinick JT, Jr, Reynolds TH, Dean D, Cartee G, Cushman SW. 1-[N, O-bis-(5-isoquinolinesulphonyl)-N-methyl-l-tyrosyl]-4- phenylpiperazine (KN-62), an inhibitor of calcium-dependent camodulin protein kinase II, inhibits both insulin- and hypoxia-stimulated glucose transport in skeletal muscle. Biochem J. 1999;339:533–540. [PMC free article] [PubMed] [Google Scholar]
- De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF, Laver D, Curtis SM, Pace S, Haarmann C, Gallant EM. Characteristics of irreversible ATP activation suggest that native skeletal ryanodine receptors can be phosphorylated via an endogenous CaMKII. Biophys J. 2001;81:3240–3252. doi: 10.1016/S0006-3495(01)75959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshete F, Fields RD. Spike frequency decoding and autonomous activation of Ca2+-calmodulin-dependent protein kinase II in dorsal root ganglion neurons. J Neurosci. 2001;21:6694–6705. doi: 10.1523/JNEUROSCI.21-17-06694.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluck M, Booth FW, Waxham MN. Skeletal muscle CaMKII enriches in nuclei and phosphorylates myogenic factor SRF at multiple sites. Biochem Biophys Res Commun. 2000a;270:488–494. doi: 10.1006/bbrc.2000.2457. [DOI] [PubMed] [Google Scholar]
- Fluck M, Waxham MN, Hamilton MT, Booth FW. Skeletal muscle Ca2+-independent kinase activity increases during either hypertrophy or running. J Appl Physiol. 2000b;88:352–358. doi: 10.1152/jappl.2000.88.1.352. [DOI] [PubMed] [Google Scholar]
- Goueli SA, Hsiao K, Goueli BS. Assaying activity of individual protein kinases in crude tissue or cellular extracts. Methods Enzymol. 2001;333:16–27. doi: 10.1016/s0076-6879(01)33040-9. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Kapiloff MS, Lou LL, Rosenfeld MG, Schulman H. Expression of a multifunctional Ca2+/calmodulin-dependent protein kinase and mutational analysis of its autoregulation. Neuron. 1989;3:59–70. doi: 10.1016/0896-6273(89)90115-3. [DOI] [PubMed] [Google Scholar]
- Hawkins C, Xu A, Narayanan N. Sarcoplasmic reticulum calcium pump in cardiac and slow twitch skeletal muscle but not fast twitch skeletal muscle undergoes phosphorylation by endogenous and exogenous Ca2+/calmodulin-dependent protein kinase. Characterization of optimal conditions for calcium pump phosphorylation. J Biol Chem. 1994;269:31198–31206. [PubMed] [Google Scholar]
- Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H. A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochem Biophys Res Commun. 1995;212:806–812. doi: 10.1006/bbrc.1995.2040. [DOI] [PubMed] [Google Scholar]
- McGee SL, Howlett KF, Starkie RL, Cameron-Smith D, Kemp BE, Hargreaves M. Exercise increases nuclear AMPK α2 in human skeletal muscle. Diabetes. 2003;2:926–928. doi: 10.2337/diabetes.52.4.926. [DOI] [PubMed] [Google Scholar]
- MacNicol M, Schulman H. Cross-talk between protein kinase C and multifunctional Ca2+/calmodulin-dependent protein kinase. J Biol Chem. 1992;267:12197–12201. [PubMed] [Google Scholar]
- Margreth A, Pallanca A, Damiani E. Calmodulin kinase-mediated phosphorylation of phospholamban in skeletal muscle sarcoplasmic reticulum. A critical reappraisal of the state of the problem in the light of new findings with normal and diseased muscle. Basic Appl Myol. 2000;10:151–157. [Google Scholar]
- Ojuka EO, Jones TE, Nolte LA, Chen M, Wamhoff BR, Sturek M, Holloszy JO. Regulation of GLUT4 biogenesis in muscle: evidence for involvement of AMPK and Ca2+ Am J Physiol Endocrinol Metab. 2002;282:E1008–1013. doi: 10.1152/ajpendo.00512.2001. [DOI] [PubMed] [Google Scholar]
- Pelosi M, Donella-Deana A. Localization, purification, and characterization of the rabbit sarcoplasmic reticulum associated calmodulin-dependent protein kinase. Biochemistry (Mosc) 2000;65:259–268. [PubMed] [Google Scholar]
- Picciotto MR, Czernik AJ, Nairn AC. Calcium/calmodulin-dependent protein kinase I. cDNA cloning and identification of autophosphorylation site. J Biol Chem. 1993;268:26512–26521. [PubMed] [Google Scholar]
- Richter EA, Nielsen JN, Jorgensen SB, Frosig C, Wojtaszewski JF. Signalling to glucose transport in skeletal muscle during exercise. Acta Physiol Scand. 2003;178:329–335. doi: 10.1046/j.1365-201X.2003.01153.x. [DOI] [PubMed] [Google Scholar]
- Sacchetto R, Damiani E, Pallanca A, Margreth A. Coordinate expression of Ca2+-ATPase slow-twitch isoform and of beta calmodulin-dependent protein kinase in phospholamban-deficient sarcoplasmic reticulum of rabbit masseter muscle. FEBS Lett. 2000;481:255–260. doi: 10.1016/s0014-5793(00)01993-1. [DOI] [PubMed] [Google Scholar]
- Sheng M, Thompson MA, Greenberg ME. CREB: a Ca2+-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- Simmerman HK, Jones LR. Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol Rev. 1998;78:921–947. doi: 10.1152/physrev.1998.78.4.921. [DOI] [PubMed] [Google Scholar]
- Tavi P, Allen DG, Niemela P, Vuolteenaho M, Westerblad H. Calmodulin kinase modulates Ca2+ release in mouse skeletal muscle. J Physiol. 2003;551:5–12. doi: 10.1113/jphysiol.2003.042002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobimatsu T, Fujisawa H. Tissue-specific expression of four types of rat calmodulin-dependent protein kinase II mRNAs. J Biol Chem. 1989;264:17907–17912. [PubMed] [Google Scholar]
- Wright DC, Holloszy JO, Han DG. The role of calmodulin kinase (CAMK). in calcium and contraction induced muscle glucose transport. Diabetes. 2003;52:A12. [Google Scholar]


