One of the most impressive adaptive physiological responses is that of muscle to high intensity exercise, as espoused by power athletes and body builders, which results in increases in muscle mass. Athletics and vanity aside, there are many reasons for wishing to know more about the mechanisms underlying this hypertrophy, not least being the possibility of pharmacologically enhancing it in sarcopenia. The work of Bolster and colleagues in this issue of The Journal of Physiology brings us nearer to a complete understanding of the relevant subcellular events occurring in response to resistance exercise by providing a time course of activities of signalling proteins involved in regulating the translational phase of muscle protein synthesis.
A seminal paper in the field (Baar & Esser, 1999) showed that the activity of a major regulatory stimulator of the synthesis of proteins involved in the ribosomal machinery of protein translation, p70S6kinase (p70 S6k), was enhanced by electrical stimulation of rat muscle to mimic resistance exercise. After this it was demonstrated that translation - the actual process of protein synthesis using mRNA as the template - and the signal transduction pathways that regulate it, are selectively activated by resistance exercise but not by the mode of exercise that increases the proportion of slow twitch oxidative muscle fibres (Nader & Esser, 2001). In subsequent work, it was shown that the upstream activators of p70 S6k, i.e. protein kinase B (PKB) and mammalian target of rapamycin (mTOR) (Bodine et al. 2001; Rommel et al. 2001), were crucial for skeletal muscle hypertrophy, that signalling to PKB via PI3 kinase was probably involved (Rommel et al. 2001) and that, in vitro at least, the muscle growth factor insulin-like growth factor (IGF)-1 activated the pathway (Pallafacchina et al. 2002).
Bolster and colleagues aimed to obtain information about the temporal responses of the signalling pathways stimulated by intense exercise and about the links between them; this should provide insight into about the uniqueness or multiplicity of the routes carrying the signals to the protein synthetic machinery. To this end they conditioned rats, carrying 60 % of their body weight in vests, to stretch up on their hind legs to touch an illuminated bar, a task the rats could do 50 times in divided sets. The animals were then terminally anaesthetized and the gastrocnemius muscles of the leg of each animal were sampled at intervals of 5-60 min.
The results showed that the pathways of translational regulation are activated immediately after resistance exercise. There was significantly increased phosphorylation of PKB at 5 min, and at 10 min there was phosphorylation of the eukaryotic initiation factor binding protein eIF4-BP1, which allows the association of the ribosomal scaffolding proteins eIF4E and eIF4G; the phosphorylation of ribosomal protein S6 was also seen from 10 min onwards after exercise. The data presented strongly suggested that mTOR, a protein situated at the heart of the signalling cascade (Fig. 1), was activated by phosphorylation, which would make sense as it lies centrally in the pathway between PKB and p70 S6k or eIF4-BP1, but the changes were too variable to be statistically significant. The activation of all these proteins was transient, suggesting that there may be an early short lived response in translational up-regulation after resistance exercise, possibly occurring in parallel to increased transcriptional regulation.
Figure 1. Scheme of the pathways investigated.
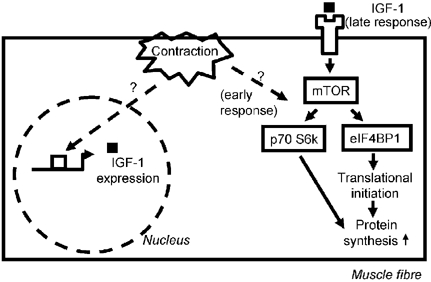
Contraction may induce a transient activation of the translational machinery via the proteins shown and others. Since contraction also leads to an increase in the expression of IGF-1 variants, these may trigger a late sustained response. All activations should lead to increased muscle protein synthesis and hypertrophy.
The results are important not only in focusing attention on the involvement of the PI3K/PKB-mTOR-p70 S6k or eIF4-BP1 pathway, but also they tend to rule out the involvement of a pathway acting through glycogen synthase kinase 3 (GSK3) and eIF2B/eIF2α, because there were no changes at all in the activation of the latter.
The findings are disappointing in one regard since they do not include measures of muscle protein synthesis: thus the endpoint of the activation cannot be tied to a functional result. However, we know that in human muscle phosphorylation of eIF4-BP1 and p70 S6k are stimulated in parallel with both myofibrillar and sarcoplasmic protein synthesis after intense isometric exercise (Rennie, 2001). These latter data illustrate one of the odd differences between the responses in human and rat muscle: in human muscle the response of p70 S6k is very long lasting - at least 12 h in our hands (Cuthbertson et al. 2002). Obviously there is a requirement for more latency and dose-response studies, enabling us to further tease out the relative importance and redundancy of the pathways involved.
What are the implications of the work? The transient nature of the responses is important in suggesting that the regulatory system ‘resets’ quickly during rest, which makes physiological sense and may give clues to the existence or not of a ‘golden period’ after resistance exercise during which enhanced amino acid availability is most effective at promoting hypertrophy (Esmarck et al. 2001). Also, like all novel work it raises as many new questions as it answers - such as what is the upstream event activating PI3K/PKB? It cannot be IGF-1 or even the muscle variant mechano-growth factor (MGF) since the response is too fast. There are many candidates - from calcium release to integrin signalling: the smart money is divided between the two.
References
- Baar K, Esser K. Am J Physiol. 1999;276:C120–127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- Bodine SC, et al. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Bolster DR, et al. J Physiol. 2003;553:213–220. doi: 10.1113/jphysiol.2003.047019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson DJR, et al. J Physiol. 2002;539.P:P160. [Google Scholar]
- Esmarck B, et al. J Physiol. 2001;535:301–311. doi: 10.1111/j.1469-7793.2001.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader GA, Esser KA. J Appl Physiol. 2001;90:1936–1942. doi: 10.1152/jappl.2001.90.5.1936. [DOI] [PubMed] [Google Scholar]
- Pallafacchina G, et al. Proc Natl Acad Sci USA. 2002;99:9213–9218. doi: 10.1073/pnas.142166599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie MJ. Int Sport Nutr Exerc Metab. 2001;11(suppl.):S170–S176. doi: 10.1123/ijsnem.11.s1.s170. [DOI] [PubMed] [Google Scholar]
- Rommel C, et al. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]


