Abstract
Despite the multiple effects on mammals during development, the effectiveness of the insulin-like growth factor-1 (IGF-1) to sustain cell function and structure in the brain of senescent mammals is almost completely unknown. To address this issue, we investigated whether the effects of IGF-1 on specific targets are preserved at later stages of life. Voltage-gated Ca2+ channels (VGCC) are well-characterized targets of IGF-1. VGCC regulate membrane excitability and gene transcription along with other functions that have been found to be impaired in the brain of senescent rodents. As the voluntary control of movement has been reported to be altered in the elderly, we investigated the expression, function and responsiveness of high (HVA)- and low-voltage-activated (LVA) Ca2+ channels to IGF-1, using the whole-cell configuration of the patch-clamp and RT-PCR in the specific region of the rat motor cortex that controls hindlimb muscle movement. We detected the expression of α1A, α1B and α1E genes encoding the HVA Ca2+ channels P/Q, N and R, respectively, but not α1C, α1D, α1S encoding the L-type Ca2+ channel in this region of the brain cortex. IGF-1 enhanced Ca2+ channel currents through P/Q- and N-type channels but not significantly through the R-type or LVA channels. IGF-1 enhanced the amplitude but did not modify the voltage dependence of Ca2+ channel currents in young (2- to 4-week-old), young adult (7-month-old) and senescent (28- to 29-month-old) rats. These results support the concept that despite the reported decrease in circulating (liver) and local (central nervous system) production of IGF-1 with ageing, key neuronal targets such as the VGCC remain responsive to the growth factor throughout life.
The insulin-like growth factor-1 (IGF-1) and its receptor IGF-1R are expressed in the central nervous system (Marks et al. 1991; Bondy et al. 1992) and play a central role in dendritic growth (Niblock et al. 2000), myelination (Florini et al. 1996; Ye et al. 2002), neuronal survival (Chrysis et al. 2001; Niikura et al. 2001) and adult stem-cell differentiation (Brooker et al. 2000), among other functions. Systemic IGF-1 exerts an effect on the brain as demonstrated by its ability to cross the blood-brain barrier and induce neurogenesis in adult rats (Aberg et al. 2000). The age-related decrease in pulsatile secretion of growth hormone in humans (Ho et al. 1987) and rodents (Sonntag et al. 1980) is paralleled by decreased levels of IGF-1 in blood (Ho et al. 1987). This may result in impairments of the IGF-1-dependent effects described above on the central nervous system at later stages of life. The requirement for a prolonged administration of growth hormone (GH) to ameliorate the age-related decline in cognitive function in rats (Sonntag et al. 2000) suggests that alterations in the GH-IGF1-IGF1R axis occur in mammalian ageing brain. Despite the multiple effects of IGF-1 during development, its role and effectiveness to sustain function in the ageing brain from senescent mammals is almost completely unknown. To address this issue, it is relevant to determine whether effects of IGF-1 on specific targets are preserved at later stages of life.
Voltage-gated Ca2+ channels (VGCC) are well-characterized targets of IGF-1. VGCC regulate membrane excitability and gene transcription among other functions (Berridge et al. 2000). In the present study, we investigated the ability of IGF-1 to modulate VGCC expressed in pyramidal neurons of the layer V of the brain motor cortex involved in the control of hindlimb movement in young (2- to 4-week-old), adult (7-month-old) and senescent (28- to 29-month-old) rats. These neurons encode functional muscle synergies in primates (Holdefer & Miller, 2002) with obvious effects on voluntary control of movement and posture by their influence on spinal cord motor neurons located in the lateral part of the lamina IX or interneurons that project to them. Whether motor cortex controls either high level features of limb movement (Georgopoulos et al. 1982) or muscle activation directly in voluntary movement (Todorov, 2000) is debatable at the present time (Scott, 2000). However, the influence of neocortex neurons on various targets depends on the firing behaviour of pyramidal neurons (Chagnac-Amitai et al. 1990; Connors & Gutnick, 1990) that depends in turn on the ionic currents that shape action potentials (Stewart & Foehring, 2001). As there is specificity in the interaction of VGCC with Ca2+ firing behaviour in neocortical pyramidal neurons (Pineda et al. 1998), it is obvious that changes in the population of VGCC or their sensitivity to IGF-1 can account for alterations in limb movement or directly on muscle activation in voluntary movement. Functional and structural decline in the neuromuscular system with ageing have been recognized as causes of impairment in physical performance and loss of independence in the elderly (Delbono, 2003). As the voluntary control of movement and posture have been reported to be altered in the elderly (Leonard et al. 1997; Krampe, 2002), we investigated the expression, function and responsiveness of high- and low-threshold VGCC to IGF-1. For this, we used the whole-cell configuration of the patch-clamp and molecular techniques in the specific region of the rat motor cortex that controls hindlimb muscle movement.
METHODS
Localization of hindlimb cortex area of the brain by electrical stimulation mapping
Fisher344XBN rats (2-3 months old, n = 5) were anaesthetized using a combination of ketamine (100 mg ml−1) and xylazine (20 mg ml−1). A volume of 0.1-0.15 ml of the mixture per 100 g rat was injected i.p. with supplemental doses of one-quarter of the initial dose as needed. No assisted respiration was needed during the procedure. Animals did not exhibit spontaneous movements or evidence of pain. Animal handling and procedures followed an approved protocol by the Animal Care and Use Committee of Wake Forest University School of Medicine. At the end of the experiments, rats were killed by an overdose of the anaesthetics. Procedures to determine the brain cortex region involved in the control of hindlimb movement followed published methods (Neafsey et al. 1986).
Identification of pyramidal neurons from layer V of the rat brain motor cortex
The identification of pyramidal neurons from layer V of the brain motor cortex was performed in whole-cell voltage-clamp configuration by delivering Lucifer yellow via the patch-clamp glass pipette. We stained 15, 10 and 12 cells from 2- to 4-week-old, 7- and 28- to 29-month-old rats, respectively. Fluorescent images were digitized using a CCD camera EEV37 (Photometrix, Tucson, AZ, USA) controlled by Isee software (Inovision, Durham, NC, USA). This procedure was applied to a limited number of cells. The remaining cortical motor neurons were identified by their typical location in motor cortex, pyramidal shape and large size visualized with an infrared camera and monitor (DAGE-MTI, Michigan City, IN, USA).
Patch-clamp recording of pyramidal motor cortical neurons Ca2+channel currents
Young (2- to 4-week-old), young adult (7-month-old) and old (28- to 29-month-old) F344XBN rats have been used for patch-clamp recordings. The inclusion of these age groups in the study allows us to differentiate the effects of maturation and senescence on our measurements. Rats were decapitated and the area of brain cortex dissected rapidly and placed in ice-cold saline. Transverse slices (300 µm thick) were prepared using a vibrating slicer (Vibratome Series 10000, TPI, St Louis, MO, USA). Slices were incubated at 32 °C for 30 min and thereafter at room temperature (20-21 °C) until they were transferred to the recording chamber. The volume of the recording chamber was 300 µl. This volume was replaced approximately four times per minute. Brain slices were treated with 0.25 mg pronase E-actinase E enzyme (protease from Streptomyces griseus, Sigma) per millilitre of ACSF (see below) for 30 min before recording to get rid of debris and facilitate membrane seal. Pyramidal motor neuron Ca2+ channel currents were measured using the whole-cell configuration of the patch-clamp technique (Hamill et al. 1981) with an Axopatch 200A amplifier and pClamp 8 software (Axon Instruments, Union City, CA, USA). A Digidata 1200 interface (Axon Instruments) was used for A/D conversion. Patch pipettes were pulled from borosilicate glass (Boralex) using a Flaming Brown micropipette puller (P97; Sutter Instrument Co., Novato, CA, USA) and then fire-polished to obtain electrode resistance ranging from 2 to 4 MΩ when filled with the internal solution. The internal solution contained (mm): 130 CsCl, 20 TEACl, 1 EGTA, 4 MgATP, 0.4 GTP and 10 Hepes (titrated to pH 7.2 with CsOH) (Plant et al. 1998). Membrane currents during a voltage pulse, P, were initially corrected by analog subtraction of linear components. The remaining linear components were digitally subtracted on-line using hyperpolarizing control pulses of one-quarter of the test pulse amplitude (−P/4 procedure) as described (Delbono, 1992; Delbono et al. 1997). We verified that subpulses elicited no ionic currents. Four control pulses were applied before the test pulse. Potential voltage errors associated with whole-cell recording in large cells were minimized by adequate compensation for whole-cell capacitance transients. The voltage errors were compensated to 70-80 %. Pipette series resistance and linear capacitance of the cell membrane were calculated as described (McCobb et al. 1989; Shaefer et al. 2003). Cell capacitance was calculated as the integral of the transient current in response to a brief hyperpolarization pulse from -80 (holding potential) to -90 mV. Ca2+ channel currents were evoked by depolarizing voltage steps from the holding potential to command potentials ranging from -70 to 50 mV.
The inward Ca2+ channel current (ICa)-voltage relationship was fit to the following equation:
| (1) |
where Gmax is the maximal conductance, V is the membrane potential, Vr is the reversal potential, V1/2 is the half-activation potential, z is the effective valence and 25.3 is the value for RT/F (R is the gas constant, T is the absolute temperature and F is Faraday's constant) at 20 °C.
Solutions and chemicals
The artificial cerebrospinal fluid (ACSF) contained (mm): 124 NaCl, 2.5 KCl, 2 CaCl2, 2 MgCl2, 1.25 NaH2PO4, 26 NaHCO3 and 10 dextrose. The pH was equilibrated to 7.3 by gassing the solution with a mixture of 95 % O2 and 5 % CO2. To record Ca2+ channel currents, the saline solution was replaced with one containing (mm): 105 NaCl, 20 TEACl, 2.5 KCl, 1 BaCl2, 1 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, 20 glucose, 0.5 µm tetrodotoxin (TTX), 1 µm strychnine and 10 µm bicuculline (pH 7.3 when gassed with 95 % O2 and 5 % CO2) (Plant et al. 1998). Ca2+ channel currents were blocked using the following toxins: ω-conotoxin (CTx)-GVIA, ω-agatoxin IVA/ω-conotoxin MVIIC and ω-Aga-IIIA, all purchased to Alomone Labs (Jerusalem, Israel). SNX-482 was purchased to Peptides International (Louisville, KT, USA). Toxins were prepared as stocks in water and kept frozen at -20 °C. Nisoldipine and nifedipine (Sigma, St Louis, MO, USA) stocks solutions were prepared in 100 % ethanol. Toxins were applied using different methods according to specific experiments, as indicated below. The IGF-1R tyrosine kinase inhibitor I-OMe-AG538 (Blum et al. 2000; Zheng et al. 2002a) was purchased to Calbiochem (San Diego, CA, USA). The experiments were carried out at room temperature (20-21 °C).
PCR amplification of the rat brain isoforms RNA encoding the α1-subunit of the HVA and LVA Ca2+ channels
The mRNA encoding the α1 subunit of the HVA and LVA Ca2+ channels expressed in the brain cortex area controlling hindlimb motility was transcribed into cDNA as described (Lambolez et al. 1992; Plant et al. 1998) with some modifications. RNA was isolated from fresh brain with TRI reagent RNA extraction solution (MRC, OH, USA) as described (Zheng et al. 2002b). Total RNA preparation was treated with DNase I to get rid of genomic DNA contamination. Reverse transcription was performed in a solution containing 5 µm hexamer random primers (Roche, Indianapolis, IN, USA), 10 mm dithiothreitol, 0.5 mm of four deoxyribonucleotides triphosphate (Pharmacia, Peapack, New Jersey, USA), 20 U of ribonuclease inhibitor (Promega, Madison, WI, USA) and 100 U of Moloney murine leukaemia virus reverse transcriptase (BRL, Gaithersburg, MD, USA). The cDNA encoding HVA and LVA Ca2+ channels was amplified using partially degenerate primers that amplified fragments of the rat brain isoforms of the HVA (α1A-α1E and α1S) (Plant et al. 1998) and LVA (α1G-α1I) Ca2+ channel α1-subunits. For the detection of α1A-E and α1S, cDNA was digested with the following enzymes DrdI, BpmI, HincII, AflII, AccI and ClaI, respectively. Ca2+ channels cDNA digestion gave rise to the following fragments 406/184, 268/322, 153/476, 99/542, 178/421 and 413/240 bp corresponding to α1A-E and α1S in agarose gel, according to restriction analysis (Plant et al. 1998). The primers for LVA Ca2+ channel α1-subunits were: sense 5′-ATCTTCCTCAACTGTATCACC-3′ and antisense 5′-A(G/T)GTCCAGGTAGTGGCTGGT-3′. The position of the primers on the individual sequences was: sense 3906-3926 and antisense 4842-4861 on α1G (GeneBank access number AF290112, rat brain), sense 3975-3995 and antisense 4890-4909 on α1H (GeneBank AF290213, rat brain) and sense 3421-3441 and antisense 4327-4346 on α1I (GeneBank 290214, rat brain). The sizes of the amplified fragments calculated from the reported sequences were 955, 935 and 926 bp for α1G, α1H and α1I, respectively. A single PCR amplification was performed using FailSafe PCR System kit (Epicentre, Madison, WI, USA). Digestion of LVA Ca2+ channels DNA with StuI, SacI or ClaI generated two fragments of 794 and 162, 606 and 329, and 706 and 220 bp for α1G, α1H and α1I genes, respectively.
Statistical analysis
Data were analysed using Student's t test or analysis of variance (ANOVA). A value of P < 0.05 was considered significant. Data are expressed as means ± s.e.m. with the number of observations (n).
RESULTS
Localization of the recording area
Whole-cell patch-clamp recordings were performed on the brain motor cortex involved in controlling the movement of hindlimbs. This area was mapped in a separate group of rats from those used for patch-clamp experiments. Penetration microstimulation mapping confirmed the location and extension of the area described previously (Neafsey et al. 1986; Liang et al. 1991). The hindlimb motor cortex began at bregma and extended 2-3 mm further caudally. The rostral, caudal, medial and lateral borders of this area were found similar to those described (Neafsey et al. 1986).
IGF-1 enhances Ca2+ channel currents through HVA Ca2+ channels in 2- to 4-week-old rats
Ca2+ channel currents carried by Ba2+ ions were recorded in pyramidal neurons located on layer V of the brain motor cortex area as described above. Large pyramidal-shaped neurons were identified using an infrared camera and monitor, as described above. To confirm the pyramidal shape of the soma and extension and complexity of the dendrites we filled the neurons with Lucifer yellow via the patch pipette as described (see Methods) in a subset of the cells studied (n = 15). Figure 1 illustrates a typical cortical pyramidal motor neuron used for our recordings. The soma and thick apical dendrite (arrow) are the parts of the neuron on focus. Ca2+ channel currents were evoked by depolarizing voltage steps from the holding potential (−80 mV) to command potentials ranging from -70 to 50 mV. Brain slices were incubated for 15 min in either IGF-1 (20 ng ml−1) or a combination of the IGF-1R tyrosine kinase inhibitor AG538 (25 µm) (Blum et al. 2000) and IGF-1, before recording started. In this case, slices were incubated for 15 min in AG538 followed by 15 min in IGF-1 plus AG538. Figure 2 shows representative Ca2+ channel currents from -30 to 30 mV recorded in control (A), IGF-1 (B) and AG538 plus IGF-1 (C). It appears that 20 ng ml−1 IGF-1 significantly enhances HVA Ca2+ channel currents, an effect that is prevented by preincubating the brain slice in 25 µm AG538. No interactions between IGF-1 and AG538 were detected other than those reported here and in previous publications (Blum et al. 2000, Zheng et al. 2002a,b). Figure 2D shows the current-voltage relationship for control (filled circles, 20 cells from 13 rats), IGF-1 (open circles, 17 cells, 11 rats) and AG538 plus IGF-1 (triangles, 15 cells, 9 rats). The IGF-1 effect on channel current amplitude was statistically significant from -30 to 10 mV (P < 0.05). Data were fitted to eqn (1) and the best-fit parameters are included in Table 1. It is apparent that IGF-1 increases maximal conductance but does not modify the voltage dependence of the Ca2+ channel currents. The low-voltage-activated (LVA) Ca2+ channel currents did not exhibit statistically significant changes in the amplitude after exposure to IGF-1 (Fig. 2D, see also Fig. 6). LVA Ca2+ channel currents were inactivated by changing the holding potential from -80 to -60 mV. The effect of IGF-1 on HVA Ca2+ channel currents was also tested in voltage-clamped neurons by rapid solution change. The rate of solution change was maintained at 1.0-1.5 ml min−1 to avoid stimulation of mechanosensitive N-type Ca2+ channels (Calabrese et al. 2002). Figure 2E illustrates a representative experiment in which a pyramidal motor neuron was pulsed to -10 mV for 250 ms every 5 s, in control conditions and during IGF-1 perfusion (bar). Figure 2F shows Ca2+ channel current traces corresponding to control (a), the lowest current amplitude recorded in the cell (probably associated with run-down) (b) and IGF-1-dependent potentiation (c) in Fig. 2E. The initial fast peak depicted in Fig. 2F corresponds to Na+ currents resistant to 0.5 µm tetrodotoxin. This current was completed blocked by incubation in 5 µm tetrodotoxin (data not shown). A lag of 1-2 min in the appearance of IGF-1-evoked current potentiation is evident. This delay may result from activation of a phosphorylation cascade leading to HVA Ca2+ channels phosphorylation (Delbono et al. 1997) (see below).
Figure 1. Pyramidal motor neuron from layer V of the rat brain cortex.
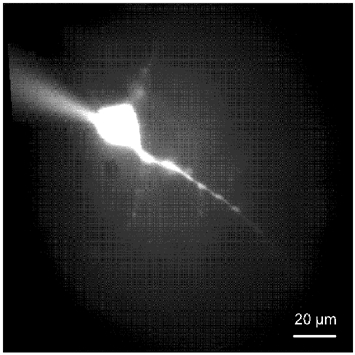
Digitized image of a pyramidal neuron from layer V of the brain motor cortex filled with Lucifer yellow via the glass electrode used for whole-cell patch-clamp (upper left corner). The cortical motor neuron has a pyramidal-shaped soma with gradually emerging, thick apical dendrite, and basilar dendrites.
Figure 2. Insulin-like growth factor-1 (IGF-1) enhances Ca2+ channel currents through HVA Ca2+ channels in 2- to 4-week-old rats.

Representative Ca2+ channel currents from -30 to 30 mV with 20 mV intervals in control (A), IGF-1-treated (B) and AG538 plus IGF-1 (C). D, Ca2+ channel current-voltage relationship in control (filled circles), 20 ng ml−1 IGF-1 (open circles) and AG538 plus IGF-1 (open triangles). Experimental data were fitted to eqn (1) (see Methods). Asterisks indicate statistically significant differences compared with control (P < 0.05). E, effect of rapid perfusion of IGF-1 (bar) on high-voltage-activated (HVA) Ca2+ channel currents tested in voltage-clamped pyramidal motor neurons pulsed to -10 mV for 250 ms every 5 s. The rate of solution perfusion was 1.0-1.5 ml min−1. F, Ca2+ channel currents corresponding to control (a), the lowest current amplitude recorded in the cell (b) and IGF-1-dependent potentiation (c) are illustrated. G, RT-PCR for HVA Ca2+ channel α1 expression in the brain cortex region used for electrophysiological recordings. M and A-S indicate the lanes in which the marker and undigested Ca2+ channel cDNAs, respectively, have been loaded.
Table 1.
Average of best-fit parameters describing the voltage dependence of Ca2+ channel currents in brain pyramidal motor neurons
| Parameters | 2- to 4-week-old rat | 7-month-old rat | 28- to 29-month-old rat | ||||
|---|---|---|---|---|---|---|---|
| Control | IGF-1 | AG538 + IGF-1 | Control | IGF-1 | Control | IGC-1 | |
| Gmax (PS) | 12 ± 1 | 19 ± 2* | 13 ± 2 | 3.3 ± 0.4 | 7.0 ± 0.9* | 4.4 ± 0.5 | 8.1 ± 0.9* |
| Vr(mV) | 50 ± 7 | 50 ± 6 | 51 ± 5 | 50 ± 3 | 51 ± 4 | 52 ± 6 | 52 ± 4 |
| z | 2.9 ± 0.2 | 3.0 ± 0.2 | 2.8 ± 0.3 | 3.0 ± 0.2 | 3.0 ± 0.4 | 2.7 ± 0.2 | 2.9 ± 0.3 |
| V1/2 (mV) | −36 ± 4 | −36 ± 3 | −34 ± 4 | −44 ± 5* | −40 ± 3 | −35 ± 3 | −37 ± 5 |
Statistically significant (P < 0.05) differences between IGF-1 treated group and the remaining group(s). Gmax is the maximal conductance, V is the membrane potential, Vr is the reversal potential, V1/2 is the half-activation potential, z is the effective valence. Values are expressed as means ± s.e.m.
Figure 6. Effects of IGF-1 on LVA Ca2+channel currents.

A, agarose gel showing LVA Ca2+ channel expression in brain motor cortex from young (3-week-old), young adult (7-month-old) and senescent (28-month-old) rats. G-I, indicate the three T-type Ca2+ channel isoforms. The first lane on the left shows the marker. B, peak channel current amplitude-time relationship. Pyramidal motor neuron from a 3-week-old rat, voltage-clamped at a holding potential of -80 mV and pulsed to a command voltage of -50 mV for 250 ms every 5 s. The bar corresponds to the time the cell was exposed to IGF-1. C, illustration of the current traces corresponding to the first (a) and last (b) pulse in B.
The molecular expression of the HVA Ca2+ channels was investigated by RT-PCR in the block of the brain cortex used for electrophysiological recordings (Fig. 2G). The α1A, α1B and α1E mRNAs encoding the HVA Ca2+ channels P/Q, N and R, respectively, were detected by the presence of the following fragments: 406/184, 322/268 and 421/178, respectively, in agarose gels after enzymatic treatment (Plant et al. 1998). Expected fragments for α1C (476/153), α1D (542/99) and α1S (413/240) were not found in any of the five preparations from the five rats studied. These results suggest that IGF-1 enhances Ca2+ channel currents through P/Q- and/or N- and/or R-type, but not through L-type Ca2+ channels.
To identify the Ca2+ channel(s) mediating the IGF-1-induced HVA current potentiation, we blocked the Ba2+ conductance through different Ca2+ channel subtypes using specific toxins or channel antagonists in acutely perfused brain slices. Figure 3A shows the ratio between the peak Ca2+ channel currents recorded in the presence and absence of a channel blocker (grey bars), and the ratio between the effect of a Ca2+ channel blocker plus IGF-1 and a Ca2+ channel blocker alone on the peak Ca2+ channel currents (black bar). Ca2+ currents were normalized to the extrapolated control value. The dashed lines in Fig. 3B-H represent the extrapolation of the Ca2+ channel currents recorded during the first 150 s to the end of the experiment. For the statistical analysis, the peak Ca2+ channel current before and after the application of a channel blocker or the peak currents recorded in the presence of the channel antagonist and in the presence of the antagonist plus IGF-1, were compared. P < 0.05 was considered statistically significant (asterisks). The peak Ca2+ channel current was determined by stimulating the cell with command pulses from -70 to 70 mV, with a 10 mV interval. The effects of IGF-1 and/or Ca2+ channel toxins were studied on the peak Ca2+ current. To this end, the voltage pulse that elicited the maximum current amplitude was applied repetitively afterwards. The exposure time to these compounds is indicated in Fig. 3B-G (bars). Figure 3H illustrates the time course of the run-down of a control Ca2+ channel current. The IGF-1R tyrosine kinase inhibitor AG538 (50 µm) prevented IGF-1-dependent Ca2+ channel current potentiation (Fig. 3A and B). Pre-exposure of the slice to the P/Q-channel toxin ω-Aga-IVA (100 nm) or to the N-type channel toxin ω-CTx-GVIA (2 µm) resulted in partial inhibition of the current (Fig. 3A, C and D) by 20 ± 2.1 and 24 ± 2.7 %, respectively, whereas the combination of these two toxins completely prevented the IGF-1-dependent current potentiation (Fig. 3A and E). The inhibitor of the R-type Ca2+ channel SNX-482 (0.5 µm) did not prevent the IGF-1-evoked potentiation of the Ca2+ channel current (Fig. 2A and F). Similarly, the L-type Ca2+ channel blockers nisoldipine (2 µm) (Fig. 3A and G) or nifedipine (2 µm) (data not shown) did not inhibit Ca2+ channel current nor prevent IGF-1-dependent current potentiation, consistent with the lack of detectable expression of L-type Ca2+ channel in this brain region (Fig. 2G). Figure 3H illustrates the time course of the Ca2+ channel current in the absence of both Ca2+ channel toxins and IGF-1. These experiments confirm that IGF-1 potentiation is not related to spontaneous changes in Ca2+ current amplitude. The perfusion of the T-channel blocker Ni2+ (50 µm) did not prevent IGF-1-dependent current potentiation (Fig. 3A and Fig. 6). These experiments support the conclusion that P/Q- and N-type Ca2+ channels mediate the IGF-1-induced enhancement of HVA Ca2+ channel currents. To determine whether the IGF-1-dependent potentiation of HVA Ca2+ channel currents is preserved at later ages, we investigated the effect of this growth factor on brain slices from young adult and senescent rats.
Figure 3. Effects of channel blockers on HVA Ca2+ channel currents recorded in 2- to 4-week-old rats.

A, effects of acute perfusion of a channel blocker alone or in combination with IGF-1 on the peak Ca2+ channel current. Bars represent means ± s.e.m. of the ratio between the peak Ca2+ channel current recorded in the presence and absence of a channel blocker (grey bars) and the ratio between the effect of the combination of a Ca2+ channel blocker plus IGF-1 and a Ca2+ channel blocker alone on the peak Ca2+ channel current (black bars). Asterisks indicate the statistical significance (P < 0.05). B-G, representative experiments included in A. H, time course of the run-down of a control Ca2+ channel current. Dashed lines represent the extrapolation of the current run-down (see text). Bars on top of each graph indicate the exposure time to a drug and/or IGF-1.
IGF-1 enhances HVA Ca2+ channel currents in young adult rats
Ca2+ channel currents recorded in young adult rats (7 months old) had a similar voltage distribution but lower amplitude than that recorded in pyramidal motor neurons from 2- to 4-week-old rats (Fig. 4A-C). To determine whether the difference in Ca2+ channel current amplitude results from changes in pyramidal motor neuron size and/or Ca2+ channel density with age, cell capacitance was measured. The mean pyramidal motor neuron capacitance was 14.1 ± 1.9 and 12.2 ± 1.7 pF for 2- to 4-week-old and 7-month-old rats, respectively (P > 0.05), supporting the concept that there is no significant changes in cell membrane surface with maturation (see below). Figure 4C shows that in young adult rats, similarly to younger animals, IGF-1 (open circles, 18 cells, 14 rats) significantly enhanced Ca2+ channel current amplitude compared to control (filled circles, 17 cells, 15 rats) in the -30 to 20 mV voltage range (P < 0.05) but did not modify the voltage dependence of the current. The half-activation potential of the current is shifted to more negative potentials compared to the value recorded in 2- to 4-week-old rats (Table 1). The Ca2+ channel profile in this brain region demonstrated the expression of α1A, α1B and α1E and the absence of α1C, α1D and α1S (Fig. 4D), similar to that described for 2- to 4-week-old rats. The analysis of the Ca2+ channels involved in the IGF-1-induced potentiation of the peak Ca2+ current (Fig. 4E) showed a similar pattern to that described for younger rats (Fig. 3A), but the amplitude of the IGF-1 evoked Ca2+ current potentiation was more obvious than in younger animals. The magnitude of the current potentiation was 99 and 46 %, respectively.
Figure 4. IGF-1 enhances HVA Ca2+ channel currents in young adult rats.

Representative Ca2+ channel current records from -30 to 30 mV with 20 mV intervals in control (A) and IGF-1-treated (B) pyramidal motor neurons. C, Ca2+ channel current-voltage relationship in control (filled circles) and after treatment with 20 ng ml−1 IGF-1 (open circles). Experimental data were fitted to eqn (1). Average best-fit parameters are shown in Table 1. Asterisks indicate statistically significant difference (P < 0.05). D, RT-PCR for HVA Ca2+ channel α1 expression in brain motor cortex. M and A-S indicate the lanes in which the marker and undigested Ca2+ channel cDNAs, respectively, were loaded. E, effects of acute perfusion of a channel blocker alone or in combination with IGF-1 on the peak Ca2+ channel current. Bars represent means ± s.e.m. of the ratio between the peak Ca2+ channel current recorded in the presence and absence of a channel blocker (grey bars) and the ratio between the effect of the combination of a Ca2+ channel blocker plus IGF-1 and a Ca2+ channel blocker alone on the peak Ca2+ channel current (black bar). Asterisks indicate the statistical significance (P < 0.05).
IGF-1 enhances HVA Ca2+ channel currents in senescent rats
Ca2+ channel currents recorded in senescent (28-29 month-old) rats (Fig. 5A and B) have a similar voltage dependence and amplitude to those recorded in neurons from 7-month-old rats, however, the amplitude was significantly lower than in motor neurons from 2- to 4-week-old rats. The mean motor neuron capacitance for 28-month-old rats was 11.8 ± 1.6 pF, a value that is not significantly different to that recorded in 2- to 4-week-old and 7-month-old rats (P > 0.05), suggesting that there are not significant changes in cell membrane surface with senescent in this cell population. Preincubation in IGF-1 of the brain slice from 28-month-old rats significantly enhanced Ca2+ channel current amplitude in the -30 to 10 mV voltage range and had no effects on the voltage dependence of the currents (Fig. 5A-C and Table 1). The Ca2+ channel expression profile is similar to that described for 2- to 4-week-old and 7-month-old rats. The expression of α1A, α1B and α1E and the absence of α1C, α1D and α1S (Fig. 5D) indicates that the pattern of HVA channels expression does not change with senescence in this brain region. The analysis of the Ca2+ channels involved in the acute response to IGF-1 (Fig. 5E) is similar to that described for young adult rats (Fig. 4E) but the magnitude of the peak Ca2+ channel current potentiation is more pronounced than that recorded in neurons from 2- to 4-week-old rats (Fig. 3A). The IGF-1-dependent current potentiation was 95 and 46 %, respectively, for 28- to 29-month-old and 2- to 4-week-old rats.
Figure 5. IGF-1 enhances HVA Ca2+ channel currents in senescent rats.

Representative Ca2+ channel current records from -30 to 30 mV with 20 mV intervals in control (A) and IGF-1-treated (B) pyramidal motor neurons from 28-month-old rats. C, IGF-1 significantly enhanced Ca2+ channel current amplitude in the -30 to 20 mV voltage range and no effects on the current-voltage relationship was observed. The average best-fit parameters are shown in Table 1. D, agarose gel showing the expression of α1A, α1B and α1E and absence of α1C, α1D and α1S in brain motor cortex. E, effects of acute perfusion of a channel blocker alone or in combination with IGF-1 on the peak Ca2+ channel currents. Bars represent means ± s.e.m. of the ratio between the peak Ca2+ channel currents recorded in the presence and absence of a channel blocker (grey bars) and the ratio between the effect of the combination of a Ca2+ channel blocker plus IGF-1 and a Ca2+ channel blocker alone on the peak Ca2+ channel currents (black bar). Asterisks indicate the statistical significance.
IGF-1 does not enhance significantly LVA Ca2+ channel currents
The expression of LVA Ca2+ channels and their response to the incubation in IGF-1 has been shown above (Figs 2D, 4C and 5C). The effects of acute exposure to the growth factor were studied in a separate group of experiments. Figure 6A shows that the rat brain T-type Ca2+ channel family (α1G, α1H and α1I) is expressed throughout the lifespan in the motor cortex involved in hindlimb motility. Although these results do not necessarily indicate that pyramidal motor neurons express T-type channels, the three channel subtypes have been detected by in situ hybridization in layer V of the cerebral cortex (Talley et al. 1999). The T-type α1I isoform predominates in the three age groups, showing cDNA fragments in agarose gel at 706 and 220 bp after enzymatic digestion. Considerably lower amounts of the T-type channels α1G and α1H were detected in the three age groups in five preparations from five rats. The cDNA fragments corresponding to α1G and α1H are 794/162 and 606/329 bp, respectively. Figure 6B illustrates an experiment in which a brain slice was acutely exposed to IGF-1 for the time indicated (bar). Pyramidal motor neurons from 2- to 4-week-old rats were voltage-clamped at a holding potential of -80 mV and pulsed to a command voltage of -50 mV. Figure 6C illustrates the first (a) and last (b) currents plotted in Fig. 6B. It appears that IGF-1 did not enhance the current amplitude. No potentiation was recorded in any of the cells tested (n = 11 slices from four rats). Changes in the holding potential (to -90 or -110 mV) or command pulse (at -60, -40 mV) did modify the effect of IGF-1 on the peak Ca2+ channel current. No potentiation was observed in either slices from young adult (n = 7 slices from four rats) or from senescent (10 slices from five rats) rats (data not shown).
DISCUSSION
To design rational interventions to prevent and/or delay functional impairment of movement control, it is crucial to determine whether central nervous system neurons remain responsive to growth factors at later stages of life. IGF-1 is a key factor involved in maintenance of brain structure and function throughout life based on its role in neuronal differentiation and maturation (Arsenijevic & Weiss 1998; Ye et al. 2002), ion channel function and cell excitability (see above). Here, we show that HVA Ca2+ channels expressed in pyramidal motor neurons of layer V of the brain motor cortex from 2- to 4-week-old rats are responsive to IGF-1 and retain this capacity during maturation and senescence.
Decline of motor control in senescent mammals
The paucity of data involving the cortical pyramidal motor neuron is in contrast with the substantial evidence for alterations in the structure and function of the nerve and muscle in the decline in strength and mobility of the elderly (for a review see Delbono, 2003). Technical difficulties in assessing function have probably hindered a more comprehensive understanding of the role of the central nervous system in the age-dependent decline in motor function. Slowing of movements and loss of fine motor skills in human ageing are thought to reflect alterations in brain systems subserving motor function (Smith et al. 1999). It has been reported that elderly subjects show decreased activation of task-specific canonical areas in comparison with young subjects (Mattay et al. 2002). Elderly subjects demonstrated greater activation in both spatial extent and magnitude in not only the contralateral primary motor cortex, an area critical for motor execution, but in other cortical and subcortical regions involved in motor processing, and also in ipsilateral areas (Mattay et al. 2002). A steep decline in motor performance (Smith et al. 1999) coincides approximately with the reported decrease in peripheral motor function in individuals after age 60 years (Hurley, 1995).
Ca2+ channels type expression in pyramidal neurons of the brain motor cortex
It is known that the level of expression of the Ca2+ channel subtypes in the central nervous system varies according to the region explored (Mynlieff & Beam, 1992; Randall & Tsien, 1995; Umemiya & Berger, 1995), but information about the Ca2+ channel population in pyramidal neurons from layer V of the brain cortex involved in hindlimb control is not available. In the present study, we report that pyramidal motor neurons from this area express HVA Ca2+ channels. N-, P/Q- and R-type Ca2+ channels, encoded by α1B, α1A and α1E genes, respectively, contribute to the recorded HVA whole-cell currents. The expression of N- and P/Q-channels in this region is consistent with previous studies in neocortical pyramidal neurons (Pineda et al. 1998). The use of the tarantula venom SNX-482 allowed us to characterize the expression of R-type Ca2+ channels. L-type Ca2+ channels have been reported in acutely dissociated pyramidal neurons from sensorimotor cortex (Lorenzon & Foehring, 1995a); however, they have not been found in the RT-PCR analysis of brain and brain stem motor nuclei (Plant et al. 1998). These results are consistent with the lack of pharmacological and molecular evidence for L-type Ca2+ channel expression in our preparation.
Ca2+ channel currents recorded in the present study represent a fraction of the Ca2+ channels expressed in the cortical motor neurons because they are missing those expressed in distal dendritic compartments. It is not known whether qualitative or quantitative differences exist in the population of Ca2+ channels expressed in the soma and proximal dendrites compared to distal dendrites of brain cortex pyramidal motor neurons. Electrophysiological recordings in acutely dissociated brain cortex pyramidal neurons suggest a differential expression of voltage-gated Ca2+ channels (Lorenzon & Foehring 1995a,b). The expression of T-, N-, L- and R-type Ca2+ channels in the soma and P-type channels in distal axons of facial motor neurons has been reported (Plant et al. 1998). Immunostaining procedures in rat spinal cord motor neurons showed expression of P/Q-type Ca2+ channels in nerve terminals located along the cell bodies and dendrites, N-type Ca2+ channels along the cell surface membrane and nerve terminals, and L-type Ca2+ channels in the soma and proximal dendrites (Westenbroek et al. 1998).
We have found that the maximal conductance of HVA channels decreased from 2- to 4-week-old to 7-month-old rats, whereas no significant difference was detected between young adult and senescent rats. The decline in the peak Ca2+ channel conductance with maturation occurs in the absence of significant changes in membrane capacitance, which suggests that Ca2+ channel density and/or regulation changes across ages. The lack of changes in membrane capacitance reported here is consistent with previous reports on hippocampal CA1 pyramidal neurons from 2- to 3-month-old rabbits and those older than 36 months (Power et al. 2002) and neocortical neurons from neonatal to 4- to 5-week-old rats (Lorenzon & Foehring, 1995b). The HVA current density and current-voltage relationship were reported unchanged in Fisher 344 rats aged from 1 to 26.5 months in acutely dissociated basal forebrain neurons (Murchison & Griffith, 1996). However, an increase in Ca2+ current density from neonatal to adult followed by a decline in senescent rats has been described in dorsal root ganglion neurons (Kostyuk et al. 1993). Studies on the expression, modulation and properties of HVA Ca2+ channel function with ageing are needed to fully address differences in Ca2+ channel conductance with ageing among different brain regions.
Ca2+ channel conductances of 7, 14, 20 and 28 pS have been reported in rat hypoglossal motor neurons using 110 mm Ba2+ as a charge carrier (Umemiya & Berger, 1995). These conductance values correspond to T-, N-, P- and L-type Ca2+ channels, respectively. The absence of L-type Ca2+ channel expression in our preparation together with a considerably lower Ba2+ concentration in the bathing solution account for the lower maximal conductance recorded. Although RT-PCR experiments did not allow us to locate the specific cell population expressing the Ca2+ channel subtypes mentioned above, they confirmed electrophysiological data on the channels that do not express in pyramidal motor neurons.
Although the population of pyramidal motor neurons studied here is mainly involved in the control of hindlimb motility, this is probably a heterogeneous group of cells. The percentage contribution of each Ca2+ channel population to the whole-cell Ca2+ channel current varies from neuron to neuron (Lorenzon & Foehring, 1995a; Plant et al. 1998). Also, probably not all of the pyramidal motor neurons recorded project to the spinal cord, other projections are corticostriatal, corticotectal, corpus callosum, etc. and probably the population of Ca2+ channels varies from one to another pyramidal cell (Stewart & Foehring, 2000). The heterogeneity in motor neuron projections and Ca2+ channel expression can contribute to their firing pattern (Connors & Gutnick, 1990; Chagnac-Amitai et al. 1990) and to their ultimate functional role in motility control.
The three subtypes of T-channels (α1G, α1H and α1I) that contribute to neuronal excitability (Chemin et al. 2002) have been found in the region of the brain cortex explored in the present study. Meanwhile α1G is expressed predominantly in cerebellum, hippocampus, thalamus and olfactory bulb (Klugbauer et al. 1999). It has been reported that α1G and α1H mRNAs are expressed in various regions of the adult rat brain, while α1I mRNA is restricted to the striatum (McRory et al. 2001). In contrast, a more diffuse expression of α1I, including brain cortex, has been described by other groups (Talley et al. 1999). As a non-significant trend of IGF-1 to enhance Ca2+ channel current amplitude in the low-voltage range (−70 to −40 mV) has been detected, no further characterization of the contribution of any of the three channel subtypes to the whole-cell current was carried out. An explanation for the differential effect of IGF-1 on HVA and LVA Ca2+ channels in the pyramidal motor neurons is not obvious.
Mechanism of action of IGF-1 on voltage-activated Ca2+ channels
Most of our current knowledge on IGF-1-evoked regulation of VGCC derives from studies in skeletal muscle cells in which IGF-1 enhances L-type Ca2+ channel expression by regulating gene transcription (Zheng et al. 2002a,b). In addition, IGF-1 modulates channel function by activating a protein kinase cascade that results in channel phosphorylation (Kleppisch et al. 1992; Delbono et al. 1997). IGF-1 also enhances Ca2+ channel currents through L- and N-type Ca2+ channels in clonal GH4C1 pituitary cells and cultured cerebellar neurons from postnatal day (P)5-P7 rats (Selinfreund & Blair, 1994; Blair & Marshall, 1997). The enhancement of Ca2+ entry through VGCC has been reported to fail in muscle cells from ageing rodents, a phenomenon that can be explained by alterations in channel phosphorylation (Delbono et al. 1997).
N-, P/Q- and R-type Ca2+ channels mediate the IGF-1-induced enhancement of HVA Ca2+ channel currents. IGF-1 interaction with IGF-1R might result in HVA Ca2+ channel phosphorylation by activating a signalling cascade involving tyrosine kinase and probably other protein kinase(s), as demonstrated for the L-type Ca2+ channel α1 subunit expressed in skeletal muscle fibres from the adult mouse (Delbono et al. 1997). The prevention of Ca2+ current potentiation by tyrosine kinase inhibitors, together with the involvement of protein kinase C (PKC) in L-type Ca2+ channel phosphorylation (Delbono et al. 1997), support the concept that the activation of a phosphorylation cascade may modulate HVA Ca2+ channels conductance in cortical motor neurons.
It can be postulated that despite the decrease in circulating levels of liver IGF-1, autocrine and paracrine effects of IGF-1 on the brain, resulting from local production by neurons, meninges and blood vessels, are maintained in senescent rodents (Lund et al. 1986; Niblock et al. 1998; Sonntag et al. 1999). However, a significant decrease in IGF-1 and IGF-1R proteins in rat brain between 11 and 32 months of age in cortical layer V has been reported (Sonntag et al. 1999). A role for local, in contrast to systemic IGF-1, in the preservation of brain function finds support in the lack of correlation between the plasma levels of IGF-1 and Ca2+ channel function. We report here a decrease in the Ca2+ channel current amplitude with maturation but not with senescence, whereas the plasma IGF-1 declines from adulthood to senescence. D'Acosta et al. (1993) have reported mean plasma concentrations of IGF-1 (in nanograms per millilitre) of 114 and 97 in 10- and 29-month-old rats, respectively. Shimokawa et al. (2002) reported an IGF-1 plasma concentration of 157.3 ± 55 ng ml−1 for 6-month-old rats. The plasma IGF-1 concentrations reported by Velasco and collaborators (2001) (mean values) were 82 and 47 % for 11- and 27-month-old rats compared with 3-month-old rats, respectively.
The magnitude of Ca2+ channel current potentiation evoked by IGF-1 is more obvious in young adults (99 %) and senescent (95 %) than in 2- to 4-week-old rats (46 %). We speculate that this could be an indication that basal HVA Ca2+ channel phosphorylation is higher, and consequently further phosphorylation is limited in very young rodents. The reduction or suppression of IGF-1-dependent transcriptional activity over a prolonged period in brain might have multiple effects in addition to L-type Ca2+ channel expression. Whether IGF-1 activates a tyrosine kinase-PKC cascade as postulated for skeletal muscle (Delbono et al. 1997), an Akt-PI-3 kinase signalling, as postulated for cerebellar granule neurons (Blair et al. 1999) or some other mechanism leading to phosphorylation of HVA Ca2+ channels in brain cortex layer V pyramidal motor neurons, is not known at the present time.
The levels of and interaction with insulin-like growth factor binding proteins (IGFBPs) determines the bioavailability and activity of IGF-1. The relation between IGF-1 and IGF-1 binding proteins is very complex on various tissues. IGFBP2 inhibits IGF-1, both in vivo and in vitro (Jones & Clemmons 1995; Clemmons et al. 1995). IGFBP5 and IGFBP6 are IGF-1 inhibitors (Babajko et al. 1997; D'Acosta et al. 1998). Upregulation of IGFBP-2, -5 and/or -6 might prevent binding of IGF-1 to IGF-1R leading to a compensatory increase in IGF-1R. However, this mechanism can be argued based on the reported decrease in IGF-1R in cortical layer V with age (Sonntag et al. 1999). Local isoforms of IGF-1 seem to play a major role in tissue trophism, function, maintenance and repair (Hill & Goldspink, 2003). It has been shown that IGF-1 is differentially spliced in response to local demands in skeletal muscle (Hill & Goldspink, 2003); however, this is not known in brain. It seems that the large molecular weight IGF-1 mRNAs predominate in brain, and although not tested, post-transcriptional regulation of IGF-1 synthesis may be particularly relevant in this tissue (Lund, 1994).
Changes in the neuroendocrine system have been postulated as a mechanism of ageing together with cellular and intercellular stochastic theories (for a review see Holliday, 1996; Arking, 1998). IGF-1 signalling plays a role in both life span and tissue structure and function (Wolkow et al. 2000). IGF-1 has several targets and is involved in a number of neuronal functions (see above). Considerable evidence support the tenet that changes in the neuroendocrine system may result in age-related changes in organ function (for a review see Delbono, 2003). The age-related decline in pulsatile secretion of growth hormone leads to significant decrease in circulating IGF-1 with potential impact on those targets. Therefore, the preservation of pyramidal neuron responsiveness to IGF-1 throughout life substantiates a role for its use in intervention of the age-related decline in brain motor function.
Acknowledgments
The present study was supported by the National Institutes of Health/National Institute on Aging (AG18755, AG13934, and AG15820), and Muscular Dystrophy Association of America grants to Osvaldo Delbono.
REFERENCES
- Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arking R. Biology of Aging: Observations and Principles. Sunderland, Massachusetts: Sinauer Associates, Inc.; 1998. [Google Scholar]
- Arsenijevic Y, Weiss S. Insulin-like growth factor-I is a differentiation factor for postmitotic CNS stem cell-derived neuronal precursors: distinct actions from those of brain-derived neurotrophic factor. J Neurosci. 1998;18:2118–2128. doi: 10.1523/JNEUROSCI.18-06-02118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babajko S, Leneuve P, Loret C, Binoux M. IGF-binding protein-6 is involved in growth inhibition in SH-SY5Y human neuroblastoma cells: its production is both IGF- and cell density-dependent. J Endocrinol. 1997;152:221–227. doi: 10.1677/joe.0.1520221. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nature Rev. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Blair LA, Bence-Hanulec KK, Mehta S, Franke T, Kaplan D, Marshall J. Akt-dependent potentiation of L channels by insulin-like growth factor-1 is required for neuronal survival. J Neurosci. 1999;19:1940–1951. doi: 10.1523/JNEUROSCI.19-06-01940.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair LAC, Marshall J. IGF-1 modulates N and L calcium channels in a PI 3-kinase-dependent manner. Neuron. 1997;19:421–429. doi: 10.1016/s0896-6273(00)80950-2. [DOI] [PubMed] [Google Scholar]
- Blum G, Gazit A, Levitzki A. Substrate competitive inhibitors of IGF-1 receptor kiinase. Biochem. 2000;39:15705–15712. doi: 10.1021/bi001516y. [DOI] [PubMed] [Google Scholar]
- Bondy C, Werner H, Roberts CT, Jr, Leroith D. Cellular pattern of type-I insulin-like growth factor receptor gene expression during maturation of the rat brain: comparison with insulin-like growth factors I and II. Neurosci. 1992;46:909–923. doi: 10.1016/0306-4522(92)90193-6. [DOI] [PubMed] [Google Scholar]
- Brooker GJ, Kalloniatis M, Russo VC, Murphy M, Werther GA, Bartlett PF. Endogenous IGF-1 regulates the neuronal differentiation of adult stem cells. J Neurosci Res. 2000;59:332–341. doi: 10.1002/(sici)1097-4547(20000201)59:3<332::aid-jnr6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Calabrese B, Tabarean IV, Juranka P, Morris CE. Mechanosensitivity of N-type calcium channel currents. Biophys J. 2002;83:2560–2574. doi: 10.1016/S0006-3495(02)75267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnac-Amitai Y, Luhmann HJ, Prince DA. Burst generating and regular spiking layer 5 pyramidal neurons of rat neocortex have different morphological features. J Comp Neurol. 1990;296:598–613. doi: 10.1002/cne.902960407. [DOI] [PubMed] [Google Scholar]
- Chemin J, Monteil A, Perez-Reyes E, Bourinet E, Nargeot J, Lory P. Specific contribution of human T-type calcium channel isotypes (alpha(1G), alpha(1H) and alpha(1I)) to neuronal excitability. J Physiol. 2002;540:3–14. doi: 10.1113/jphysiol.2001.013269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysis D, Calikoglu AS, Ye P, D'Ercole AJ. Insulin-like growth factor-I overexpression attenuates cerebellar apoptosis by altering the expression of Bcl family proteins in a developmentally specific manner. J Neurosci. 2001;21:1481–1489. doi: 10.1523/JNEUROSCI.21-05-01481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons DR, Busby WH, Arai T, Nam TJ, Clarke JB, Jones JI, Ankrapp DK. Role of insulin-like growth factor binding proteins in the control of IGF actions. Prog Growth Factor Res. 1995;6:357–366. doi: 10.1016/0955-2235(95)00013-5. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- D'Costa AP, Lenham JE, Ingram RL, Sonntag WE. Moderate caloric restriction increases type 1 IGF receptors and protein synthesis in aging rats. Mech Ageing Dev. 1993;71:59–71. doi: 10.1016/0047-6374(93)90035-p. [DOI] [PubMed] [Google Scholar]
- D'Costa AP, Prevette DM, Houenou LJ, Wang S, Zackenfels K, Rohrer H, Zapf J, Caroni P, Oppenheim RW. Mechanisms of insulin-like growth factor regulation of programmed cell death of developing avian motoneurons. J Neurobiol. 1998;39:379–374. [PubMed] [Google Scholar]
- Delbono O. Calcium current activation and charge movement in denervated mammalian skeletal muscle fibres. J Physiol. 1992;451:187–203. doi: 10.1113/jphysiol.1992.sp019160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbono O. Neural control of aging skeletal muscle. Aging Cell. 2003;2:21–29. doi: 10.1046/j.1474-9728.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- Delbono O, Renganathan M, Messi ML. Regulation of mouse skeletal muscle L-type Ca2+ channel by activation of the insulin-like growth factor-1 receptor. J Neurosci. 1997;17:6918–6928. doi: 10.1523/JNEUROSCI.17-18-06918.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florini JR, Ewton DZ, Coolican SA. Growth hormone and insulin growth factor system in myogenesis. Endocr Rev. 1996;17:481–517. doi: 10.1210/edrv-17-5-481. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J Neurosci. 1982;2:1527–1537. doi: 10.1523/JNEUROSCI.02-11-01527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hill M, Goldspink G. Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage. J Physiol. 2003;549:409–418. doi: 10.1113/jphysiol.2002.035832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho KY, Evans WS, Blizzard RM, Veldhuis JD, Merriam GR, Samojlik E, Furlanetto R, Rogol AD, Kaiser DL, Thorner MO. Effects of sex and age on the 24-hour profile of growth hormone secretion in man: importance of endogenous estradiol concentrations. J Clin Endocrinol Metab. 1987;64:51–58. doi: 10.1210/jcem-64-1-51. [DOI] [PubMed] [Google Scholar]
- Holdefer RN, Miller LE. Primary motor cortical neurons encode functional muscle synergies. Exp Brain Res. 2002;146:233–243. doi: 10.1007/s00221-002-1166-x. [DOI] [PubMed] [Google Scholar]
- Holliday R. Understanding ageing. New York: Cambridge University Press; 1996. [Google Scholar]
- Hurley BF. Age, gender, and muscular strength. J Gerontol A Biol Sci Med Sci. 1995;50:41–44. doi: 10.1093/gerona/50a.special_issue.41. [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Kleppisch T, Klinz F-Z, Hescheler J. Insulin-like growth factor I modulates voltage-dependent Ca2+ channels in neuronal cells. Brain Res. 1992;591:283–288. doi: 10.1016/0006-8993(92)91709-n. [DOI] [PubMed] [Google Scholar]
- Klugbauer N, Marais E, Lacinova L, Hofmann F. A T-type calcium channel from mouse brain. Pflugers Arch. 1999;437:710–715. doi: 10.1007/s004240050836. [DOI] [PubMed] [Google Scholar]
- Kostyuk P, Pronchuk N, Savchenko A, Verkhratsky A. Calcium currents in aged rat dorsal root ganglion neurones. J Physiol. 1993;461:467–483. doi: 10.1113/jphysiol.1993.sp019523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krampe RT. Aging, expertise and fine motor movement. Neurosci Biobehav Rev. 2002;26:769–776. doi: 10.1016/s0149-7634(02)00064-7. [DOI] [PubMed] [Google Scholar]
- Lambolez B, Audinat E, Bochet P, Crepel F, Rossier J. AMPA receptor subunits expressed by single Purkinje cells. Neuron. 1992;9:247–258. doi: 10.1016/0896-6273(92)90164-9. [DOI] [PubMed] [Google Scholar]
- Leonard CT, Matsumoto T, Diedrich PM, McMillan JA. Changes in neural modulation and motor control during voluntary movement of older individuals. J Gerontol A Biol Sci Med Sci. 1997;52:M320–325. doi: 10.1093/gerona/52a.5.m320. [DOI] [PubMed] [Google Scholar]
- Liang FY, Moret V, Wiesendanger M, Rouiller EM. Corticomotoneuronal connections in the rat: evidence from double-labeling of motoneurons and corticospinal axon arborizations. J Comp Neurol. 1991;311:356–366. doi: 10.1002/cne.903110306. [DOI] [PubMed] [Google Scholar]
- Lorenzon NM, Foehring RC. Characterization of pharmacologically identified voltage-gated calcium channel currents in acutely isolated rat neocortical neurons. I. Adult neurons. J Neurophysiol. 1995a;73:1430–1442. doi: 10.1152/jn.1995.73.4.1430. [DOI] [PubMed] [Google Scholar]
- Lorenzon NM, Foehring RC. Characterization of pharmacologically identified voltage-gated calcium channel currents in acutely isolated rat neocortical neurons. II. Postnatal development. J Neurophysiol. 1995b;73:1443–1451. doi: 10.1152/jn.1995.73.4.1443. [DOI] [PubMed] [Google Scholar]
- Lund PK. Insulin-like growth factor I: molecular biology and relevance to tissue-specific expression and action. Recent Prog Horm Res. 1994;49:125–148. doi: 10.1016/b978-0-12-571149-4.50010-6. [DOI] [PubMed] [Google Scholar]
- Lund PK, Moats-Staats BM, Hynes MA, Simmons JG, Jansen M, D'Ercole AJ, Van Wyk JJ. Somatomedin-C/insulin-like growth factor-I and insulin-like growth factor-II mRNAs in rat fetal and adult tissues. J Biol Chem. 1986;261:14539–14544. [PubMed] [Google Scholar]
- McCobb DP, Best PM, Beam KG. Development alters the expression of calcium currents in chick limb motoneurons. Neuron. 1989;2:1633–1643. doi: 10.1016/0896-6273(89)90052-4. [DOI] [PubMed] [Google Scholar]
- McRory JE, Santi CM, Hamming KS, Mezeyova J, Sutton KG, Baillie DL, Stea A, Snutch TP. Molecular and functional characterization of a family of rat brain T-type calcium channels. J Biol Chem. 2001;276:3999–4011. doi: 10.1074/jbc.M008215200. [DOI] [PubMed] [Google Scholar]
- Marks JL, Porte D, Jr, Baskin DG. Localization of type I insulin-like growth factor receptor messenger RNA in the adult rat brain by in situ hybridization. Mol Endocrinol. 1991;5:1158–1168. doi: 10.1210/mend-5-8-1158. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002;58:630–635. doi: 10.1212/wnl.58.4.630. [DOI] [PubMed] [Google Scholar]
- Murchison D, Griffith WH. High-voltage-activated calcium currents in basal forebrain neurons during aging. J Neurophysiol. 1996;76:158–174. doi: 10.1152/jn.1996.76.1.158. [DOI] [PubMed] [Google Scholar]
- Mynlieff M, Beam KG. Characterization of voltage-dependent calcium currents in mouse motoneurons. J Neurophysiol. 1992;68:85–92. doi: 10.1152/jn.1992.68.1.85. [DOI] [PubMed] [Google Scholar]
- Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF, Terreberry RR. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res. 1986;396:77–96. doi: 10.1016/s0006-8993(86)80191-3. [DOI] [PubMed] [Google Scholar]
- Niblock MM, Brunso-Bechtold JK, Lynch CD, Ingram RL, McShane T, Sonntag WE. Distribution and levels of insulin-like growth factor I mRNA across the life span in the Brown Norway × Fischer 344 rat brain. Brain Res. 1998;804:79–86. doi: 10.1016/s0006-8993(98)00645-3. [DOI] [PubMed] [Google Scholar]
- Niblock MM, Brunso-Bechtold JK, Riddle DR. Insulin-like growth factor I stimulates dendritic growth in primary somatosensory cortex. J Neurosci. 2000;20:4165–4176. doi: 10.1523/JNEUROSCI.20-11-04165.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niikura T, Hashimoto Y, Okamoto T, Abe Y, Yasukawa T, Kawasumi M, Hiraki T, Kita Y, Terashita K, Kouyama K, Nishimoto I. Insulin-like growth factor I (IGF-I) protects cells from apoptosis by Alzheimer's V642I mutant amyloid precursor protein through IGF-I receptor in an IGF-binding protein-sensitive manner. J Neurosci. 2001;21:1902–1910. doi: 10.1523/JNEUROSCI.21-06-01902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda JC, Waters RS, Foehring RC. Specificity in the interaction of HVA Ca2+ channel types with Ca2+-dependent AHPs and firing behavior in neocortical pyramidal neurons. J Neurophysiol. 1998;79:2522–2534. doi: 10.1152/jn.1998.79.5.2522. [DOI] [PubMed] [Google Scholar]
- Plant TD, Schirra C, Katz E, Uchitel O, Konnerth A. Single-cell RT-PCR and functional characterization of Ca2+ channels in motoneurons of the rat facial nucleus. J Neurosci. 1998;18:9573–9584. doi: 10.1523/JNEUROSCI.18-23-09573.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JM, Wu WW, Sametsky E, Oh MM, Disterhoft JF. Age-related enhancement of the slow outward calcium-activated potassium current in hippocampal CA1 pyramidal neurons in vitro. J Neurosci. 2002;22:7234–7243. doi: 10.1523/JNEUROSCI.22-16-07234.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall A, Tsien RW. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J Neurosci. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AT, Helmstaedter M, Sakmann B, Korngreen A. Correction of conductance measurements in non-space-clamped structures: 1. Voltage-gated K(+) channels. Biophys J. 2003;84:3508–3528. doi: 10.1016/S0006-3495(03)75086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SH. Population vectors and motor cortex: neural coding or epiphenomenon? Nat Neurosci. 2000;3:307–308. doi: 10.1038/73859. [DOI] [PubMed] [Google Scholar]
- Selinfreund RH, Blair LAC. Insulin-like growth factor-1 induces a rapid increase in calcium currents and spontaneous membrane activity in clonal pituitary cells. Mol Pharmacol. 1994;45:1215–1220. [PubMed] [Google Scholar]
- Shimokawa I, Higami Y, Utsuyama M, Tuchiya T, Komatsu T, Chiba T, Yamaza H. Life span extension by reduction in growth hormone-insulin-like growth factor-1 axis in a transgenic rat model. Am J Pathol. 2002;160:2259–2265. doi: 10.1016/S0002-9440(10)61173-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Umberger GH, Manning EL, Slevin JT, Wekstein DR, Schmitt FA, Markesbery WR, Zhang Z, Gerhardt GA, Kryscio RJ, Gash DM. Critical decline in fine motor hand movements in human aging. Neurology. 1999;53:1458–1461. doi: 10.1212/wnl.53.7.1458. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Lynch C, Bennett SA, Khan AS, Thornton PL, Cooney PT, Ingram RL, Mcshane T, Brunso-Bechtold JK. Alterations in insulin-like growth factor-1 gene and protein expression and type 1 insulin-like growth factor receptors in the brains of ageing rats. Neuroscience. 1999;88:269–279. doi: 10.1016/s0306-4522(98)00192-4. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Lynch C, Thornton P, Khan A, Bennett S, Ingram R. The effects of growth hormone and IGF-1 deficiency on cerebrovascular and brain ageing. J Anat. 2000;197:575–585. doi: 10.1046/j.1469-7580.2000.19740575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Steger RW, Forman LJ, Meites J. Decreased pulsatile release of growth hormone in old male rats. Endocrinology. 1980;107:1875–1879. doi: 10.1210/endo-107-6-1875. [DOI] [PubMed] [Google Scholar]
- Stewart A, Foehring RC. Calcium currents in retrogradely labeled pyramidal cells from rat sensorimotor cortex. J Neurophysiol. 2000;83:2349–2354. doi: 10.1152/jn.2000.83.4.2349. [DOI] [PubMed] [Google Scholar]
- Stewart AE, Foehring RC. Effects of spike parameters and neuromodulators on action potential waveform-induced calcium entry into pyramidal neurons. J Neurophysiol. 2001;85:1412–1423. doi: 10.1152/jn.2001.85.4.1412. [DOI] [PubMed] [Google Scholar]
- Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci. 1999;19:1895–1911. doi: 10.1523/JNEUROSCI.19-06-01895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov E. Direct cortical control of muscle activation in voluntary arm movements: a model. Nat Neurosci. 2000;3:391–398. doi: 10.1038/73964. [DOI] [PubMed] [Google Scholar]
- Umemiya M, Berger AJ. Single-channel properties of four calcium channel types in rat motoneurons. J Neurosci. 1995;15:2218–2224. doi: 10.1523/JNEUROSCI.15-03-02218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco B, Cacicedo L, Melian E, Fernandez-Vazquez G, Sanchez-Franco F. Sensitivity to exogenous GH and reversibility of the reduced IGF-I gene expression in aging rats. Eur J Endocrinol. 2001;145:73–85. doi: 10.1530/eje.0.1450073. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Hoskins L, Catterall W A. Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons and nerve terminals. J Neurosci. 1998;18:6319–6330. doi: 10.1523/JNEUROSCI.18-16-06319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkow CA, Kimura KD, Lee MS, Ruvkun G. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science. 2000;5489:147–150. doi: 10.1126/science.290.5489.147. [DOI] [PubMed] [Google Scholar]
- Ye P, Li L, Richards RG, Diaugustine RP, D'Ercole AJ. Myelination is altered in insulin-like growth factor-I null mutant mice. J Neurosci. 2002;22:6041–6051. doi: 10.1523/JNEUROSCI.22-14-06041.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Wang ZM, Delbono O. Insulin-like growth factor-1 increases skeletal muscle DHPR alpha 1S transcriptional activity by acting on the cAMP-response element-binding protein element of the promoter region. J Biol Chem. 2002a;277:50535–50542. doi: 10.1074/jbc.M210526200. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Wang Z-M, Delbono O. Charge movement and transcription regulation of L-type calcium channel alpha-1S in skeletal muscle cells. J Physiol. 2002b;540:397–409. doi: 10.1113/jphysiol.2001.013464. [DOI] [PMC free article] [PubMed] [Google Scholar]


