Abstract
This study examined stumbling corrective (tripping) responses to mechanical disturbances applied to the foot during stepping in healthy human infants, in whom independent walking had not yet developed. During treadmill-elicited stepping, a foam-padded baton instrumented with a force transducer was used to deliver light touches to either the dorsum or the side of the foot at various times of the step cycle. Disturbances to the dorsum of the foot during the swing phase resulted in a general enhancement of flexor activity, including a significant facilitation in the tibialis anterior muscle and an increase in knee flexion during swing. There was also an increase in step cycle duration. Stance phase disturbances resulted in a significant inhibitory effect in the quadriceps muscle and after early stance disturbances, a significant prolongation of the stance phase duration. Disturbances applied to the side of the foot during the swing phase of forward stepping did not result in any effect on the kinematic pattern. If the infant was stepping sideways, however, disturbances to the side of the foot resulted in an increase in flexion during the swing phase. The results show that infants have a phase-dependent reflex response to light touches applied to the foot. Furthermore, the reflex response is location specific and task specific. Thus, much of the sophistication necessary for controlling sensory inputs in walking is present well before the onset of independent walking.
During locomotion, strategies must be in place to rapidly compensate for any obstruction or perturbation that could interfere with progression. For example, when the trajectory of the limb is altered, such as when we hit an unexpected obstacle during the swing phase, rapid corrective responses are needed to prevent a fall. This type of response has been termed a ‘stumbling corrective reaction’ (Forssberg et al. 1975). These corrective responses must be carefully controlled by the nervous system in a phase-dependent manner such that they help and do not hinder forward progression. In intact cats, for example, a touch to the dorsum of the paw during the swing phase elicits a flexion response at the hip and knee so that the limb is lifted over the obstacle (Wand et al. 1980). The same touch during the stance phase of walking elicits less consistent responses, presumably because a light touch to the dorsum of the foot at this time is unlikely to hinder walking. Several studies have reported these corrective responses to trip-inducing stimuli in spinal, decerebrate and intact cats as well as adult humans (Miller et al. 1977; Prochazka et al. 1978; Forssberg, 1979; Drew & Rossignol 1987; Zehr et al. 1997, 1998; Schillings et al. 2000). The fact that they are even observed in spinal animals demonstrates the capacity of spinal locomotor networks to appropriately respond to disturbances during stepping.
The functional aspect of these reflexes has been demonstrated in a number of ways. For example, the area of the foot touched or stimulated affects the type of response observed, a characteristic known as ‘local sign’ (Sherrington, 1947). Electrical stimulation of nerves supplying different areas of the foot elicits very different responses during forward walking in adult humans (van Wezel et al. 1997; Zehr et al. 1997, 1998). Presumably, touch to different areas signal different types of problems that would require different corrective responses. In contrast, during standing, stimulation to different areas of the foot generates the same response (Komiyama et al. 2000). Thus, reflexes are gated differently depending on the location of the stimulus as well as the task that is being performed. The task specificity of the response also extends to the direction of progression (Buford & Smith, 1993; Duysens et al. 1996). Touch to the dorsum of the foot or paw elicits a flexion response during forward walking, but not during backward walking. Touch to the sole of the foot or paw elicits a flexion response during backward walking, but not during forward walking.
Are corrective responses to trip-inducing stimuli present in the locomotor system before the onset of independent walking? Moreover, does the locomotor system in infants possess the sophistication required for location- and task-specific responses to disturbances? Responses in the leg of the very young (under 1 year of age) are much less likely to be influenced by volition because the primary pathway controlling volitional movements to the lower limbs remain immature during this period (Yakovlev & Lecours, 1967; Eyre et al. 1991; Müller et al. 1991). We found that infants respond to touch of the foot dorsum in a similar way to that observed in decerebrate and intact cats and adult humans. In addition, touch to different regions of the foot induced different responses (location-specificity). We also found that the response to touches to a given region of the foot is dependent on the direction of walking, showing the characteristic of task-specificity.
METHODS
A total of 33 infants between the ages of 5.5 and 13 months (mean age: 9 months) were recruited from local public health clinics to participate in the study. None of the infants could walk independently. Suitability for participation in the study was assessed over the telephone. Parents were give verbal instruction on methods to practice the stepping response with their infant as described in Yang et al. (1998). Only infants who could make at least 10 consecutive steps at a time, as reported by the parent, were brought in for study. Parents or guardians provided written voluntary consent on behalf of their infant for participation in the study. All procedures were conducted in accordance with the ethical guidelines set out by the Declaration of Helsinki and were approved by the ethics committees of the University of Alberta and the local health authority.
This study was subdivided into three separate sections. In section 1, 12 infants were used to investigate the stumbling corrective response to mechanical disturbances to the dorsum of the foot during forwards stepping. In section 2, eight infants were used to investigate whether the stumbling corrective response showed location-specificity, by comparing mechanical disturbances to the dorsum of the foot to those to the medial or lateral side of the foot during forwards stepping. Finally, in section 3, 13 infants were used to investigate the task-specificity of the stumbling corrective response by comparing mechanical disturbances to the lateral side of the foot between forwards and sideways stepping.
Recording procedures
Infants were completely disrobed except for a diaper and shirt. Pairs of infant-sized silver-silver chloride recording electrodes (Kendall LTP, Chicopee, MA, USA) were affixed on the skin overlying the tibialis anterior (TA), gastrocnemius/soleus (Sol), quadriceps (Quad), and hamstrings (Hams) muscles on the left leg after cleaning the skin with rubbing alcohol. Twin-axis electrogoniometers (Penny & Giles Computer Products, Biometrics, Blackwood Gwent, UK) were placed over the hip and knee joints of the left leg (16 infants), or just the left knee joint (17 infants) to measure knee motion in the sagittal plane (flexion/extension) and hip motion in the sagittal plane and the coronal plane (abduction/adduction). The goniometer measuring hip joint angle was placed so that one arm was aligned to the mid-axillary line of the trunk and the other was aligned along the long axis of the femur. The arms of the goniometer measuring knee joint angle were aligned along the long axis of the femur and the long axis of the tibia. Adhesive joint markers were placed over the head of the fifth metatarsal, the lateral malleolus, the knee joint line, the greater trochanter and the top of the iliac crest on the left side.
To elicit stepping, infants were held under their arms with their feet touching a slowly moving treadmill belt (Gaitway treadmill system, Kistler Instruments, Amherst, NY, USA). In all infants except two, the treadmill belt was moving at 0.23 m s−1. In the other two infants, the treadmill belt was moving at 0.31 m s−1. The treadmill is instrumented with force plates under the belt, which allows for the determination of foot contact. The infant was allowed to bear as much of its own weight as possible, the rest being supported by the investigator holding the infant. Short trials (up to 3 min, depending on the infant's endurance and tolerance) were recorded with rest breaks in between. A baton with a foam-padded end was used to deliver short touches to either the dorsal surface or the medial or lateral surface of the foot. The baton was instrumented with a uniaxial force transducer so positioned to measure forces directed through the long axis of the baton. The disturbance was applied to the foot with the foam-padded end of the baton, which produced compressive forces directed longitudinally along the long axis of the baton. After a period of control (undisturbed) stepping, disturbances were delivered approximately every third to tenth step. Only steps with disturbances lasting between 100 and 400 ms and between 2 and 10 N were included in the analysis.
In section 1 of the study, disturbances were applied at a variety of times throughout the step cycle. Stepping was always in the forward direction. Note that there was no goniometer signal measured for the hip in these 12 infants. Hip angle was thus measured from the video data (see below).
For sections 2 and 3 of the study, the analysis focused only on disturbances that occurred during the early swing phase of the step cycle. For the results obtained for section 2, both hip and knee flexion/extension movements were measured by goniometers. In three infants, side disturbances were applied to the lateral aspect of the foot. To minimize the chance of obstructing the markers placed on the foot, side disturbances were applied to the medial aspect of the foot in subsequent infants. We did not observe any difference in the kinematics of the swing phase when comparing the effects of medial disturbances with lateral disturbances. There was also no statistically significant difference in the electromyographic amplitude from any of the muscles between the two types of side disturbances. Thus, for the purposes of this study, data from steps with disturbances to the medial and lateral sides of the foot were grouped together under the side disturbances category.
To test the task-specificity of the response (section 3), disturbances to the lateral aspect of the left foot were applied during both forward and sideways stepping. The orientation of the infant relative to the direction of the treadmill belt was changed to elicit either forwards or sideways stepping. During forwards stepping, the infant was held so that the left side of the body was facing the camera. During sideways stepping, the infant's back faced the video camera. In these infants, a marker was also placed over the posterior aspect of the left heel to estimate the foot trajectory during sideways stepping. Abduction/adduction angles as well as flexion/extension angles were also measured from the hip goniometer in 6 of these 13 infants.
Signals from the electromyography (EMG), goniometer, force plate and instrumented baton were recorded on analog tape. A video camera (PV-950; Panasonic, Secaucus, NJ, USA) was used to record stepping. A digital timer synchronized the analog and video data by generating a light signal on video and a pulse on analog tape at a rate of 1 Hz.
Data analysis
The EMG data were high-pass filtered at 10 Hz, full-wave rectified, and low-pass filtered at 30 Hz. The goniometer, force plate, and instrumented baton data were low-pass filtered at 30 Hz. All analog data from the EMGs, goniometers, force plates, instrumented baton and synchronization light were converted to digital form at 250 Hz using a computer software program (Axoscope, Axon Instruments Inc., Foster City, CA, USA). Video data were examined for sequences of sustained, alternating stepping (at least four consecutive steps) and the corresponding analog data were then identified. EMG data were examined for artifact. Signals with artifact were discarded. We also eliminated trials in which the region of the foot that was touched was inaccurate, as judged by the video image. The stance and swing phase durations were estimated by the time of left foot contact and toe off, using the force plate signals in conjunction with the video image. Step cycle duration was measured between successive foot contact times. Thus, for stance phase disturbances, the step cycle duration included that stance phase and the following swing phase. For swing phase disturbances, the step cycle duration included the preceding stance phase and the current swing phase. The EMG, goniometer signals, and timing data of all of the undisturbed steps were selected and averaged using a customized software program.
Control steps were selected from stepping sequences that occurred before the introduction of the baton. Between 10 and 20 control steps were selected for subsequent averaging of the EMG, goniometer, timing and kinematic (video) data from each infant. The peak force from the baton was used to determine the phase of the step cycle in which the disturbance occurred (early stance, late stance, early swing or late swing). Responses that occurred in the same phase were averaged together. For each disturbed step, the durations of the stance and swing phase were measured using the force plate signals in conjunction with the video data. Step cycle duration was measured between successive foot contact times. EMG amplitudes from each infant were normalized to the peak EMG obtained from the average of the control steps for each muscle. To measure EMG responses to the disturbances, the average amplitude of the EMG over a 200 ms window centred about the peak of the baton signal was calculated. The corresponding period of EMG in control steps was then identified and subtracted from the disturbed step, yielding a subtracted signal in which positive values indicate facilitation and negative values indicate inhibition.
To assess whether swing phase disturbances interfered with limb trajectory during the swing phase, the ankle angle was computed from the video data. The analog video data were converted at 30 Hz to a digital form suitable for subsequent computer analysis using Adobe Premiere (Adobe Systems Inc., San Jose, CA, USA). The markers overlying the head of the fifth metatarsal, the lateral malleolus and the knee joint were then digitized manually using custom-written software (D. Garand, Garand International Telecommunications Ltd, Edmonton, Canada). The coordinate position data of the markers were digitally filtered using a fourth-order dual pass Butterworth filter at a low-pass of 6 Hz (Winter, 1990) with custom-written software in Matlab (Mathworks, Natick, MA, USA). Only trials where the apparent length of the foot and shank segments did not change by more than 10 % were accepted for subsequent analysis. Based on these criteria, a total of 62 trials from 20 infants were used.
Foot trajectory and hip angle were also computed from the video data as described above. The foot trajectory was plotted by the position of the marker over the head of the fifth metatarsal for forwards stepping trials in section 1. During sideways stepping trials (section 2), the foot trajectory was plotted by the position of the marker over the posterior aspect of the heel. For the comparison of foot trajectory between forwards and sideways stepping (in section 3) the ankle and heel markers ideally should have been used to plot foot trajectory. However, during forwards stepping, the position of the ankle marker was often blocked during disturbed steps by the approach taken to apply the lateral disturbances. Thus, we used a more distal marker (over the head of the fifth metatarsal) to plot foot trajectory during forwards stepping. Student's paired t tests or repeated measures ANOVAs were used to evaluate differences between control stepping parameters and those measured during disturbed steps (P < 0.05). For repeated measures tests, the P value was corrected for post hoc comparisons using the Dunn-Bonferroni method.
RESULTS
Section 1. Stumbling corrective response to disturbances to the dorsum of the foot
Temporal data
The results from 12 infants are presented in this section. On average (±s.e.m.), early swing disturbances started 132.8 (±11.4) ms after the start of the swing phase (corresponding to 24 % of the swing phase) while late swing disturbances started after 369.8 (±28) ms (71 % of the swing phase). Early stance disturbances started 335.9 (±38.6) ms after the start of the stance phase (32 % of the stance phase) and late stance disturbances started after 712.7 (±79.1) ms (73 % of the stance phase). The number of infants in each disturbance category is as follows: early swing, 11; late swing, 10; early stance, 10; and late stance, 7. Each infant served as its own control. The amount of force applied with each type of disturbance was comparable across the four conditions (ANOVA, P > 0.05). The average (±s.e.m.) force of early swing disturbances was 4.8 (±0.35) N and that of late swing disturbances was 4.6 (±0.42) N. The average (±s.e.m.) force of early stance disturbances was 5.2 (±0.44) N and that of late stance was 5.6 (±0.58) N. On the other hand, disturbances applied during the swing phase tended to have a steeper rate of rise of the force signal compared to those applied during the stance phase (ANOVA, P < 0.05). The average (±s.e.m.) slope of the disturbances applied during the swing phase was 0.09 (±0.01) N ms−1 while that during the stance phase was 0.05 (±0.01) N ms−1. This is likely to be due to the fact that during the swing phase, the foot is moving against the baton while during the stance phase, the foot is stationary.
Rapid disturbances to the ankle during the swing phase may have resulted in rapid ankle plantarflexor movements which could have initiated a stretch reflex response. To address this possibility, ankle angular velocity during disturbances applied in the swing phase was calculated from the video data. In 28 of the 62 trials, swing phase disturbances caused a plantarflexion movement during the swing phase at an average (±s.d.) speed of 50 (±41) deg s−1. The large variability in the plantarflexor responses is due to the wide range (from 5 to 185 deg s−1). Nevertheless, even in the infants who showed the more rapid plantarflexor responses (from 70 to 185 deg s−1), no stretch reflex response was observed in the TA muscle. In the remaining trials (34 out of 62), ankle motion continued through dorsiflexion.
Disturbances applied during early swing phase significantly increased the duration of the ongoing swing phase in all infants by, on average, 200 ms (paired t test, P < 0.05, Fig. 1A). This increase in swing phase duration corresponded to an overall increase in step cycle duration. During late swing, disturbances also caused a significant increase in the duration of the ongoing swing phase (on average by 258 ms) as well as the step cycle (paired t test, P < 0.05, Fig. 1B). Disturbances applied during the early stance phase significantly increased the duration of the ongoing stance phase (on average by 129 ms) as well as the step cycle (paired t test, P < 0.05, Fig. 1C). Late stance phase disturbances caused a small (on average by 60 ms) but significant increase in the duration of the subsequent swing phase (paired t test, P < 0.05) but had no effect on the duration of the step cycle (Fig. 1D).
Figure 1. Effect of disturbances on the timing of locomotion.
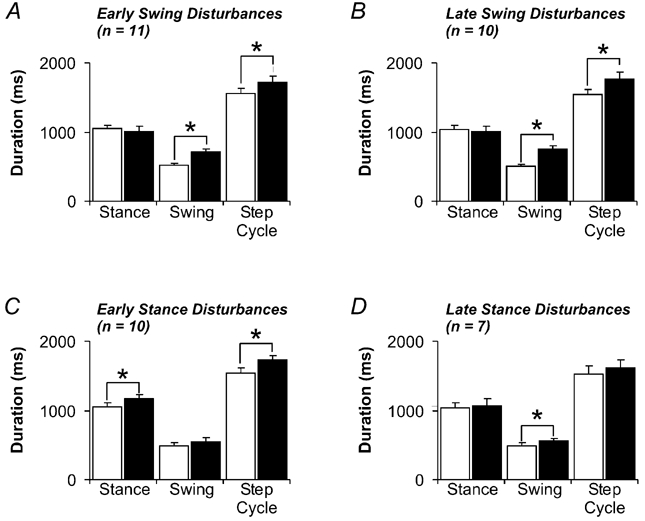
The durations of the stance phase, swing phase, and step cycle during control steps (white bars) and disturbed steps (black bars) are shown for early swing (A), late swing (B), early stance (C) and late stance (D) disturbances. Error bars represent 1 s.e.m. * P < 0.05; n, the number of infants contributing data to each figure.
Kinematic data
Figure 2 illustrates typical responses to early swing disturbances during stepping. Figure 2A shows an example of swing phase movement of the leg during an undisturbed step. The interval between each stick figure corresponds to 33 ms. Figure 2B illustrates an example of a step during which a disturbance was applied during the early swing phase in the same infant depicted in Fig. 2A. The thick bar represents the time of the disturbance. The average toe trajectory across all infants during steps with early swing disturbances is shown in Fig. 2C (left panel). Note the increase in the toe trajectory height during the swing phase of the disturbed steps (thick black line) compared to control steps (thin black line). The maximum toe height after early swing disturbances was significantly greater than that during control stepping (paired t test, P < 0.05). Disturbances occurring during the late swing phase tended to result in a slight increase in toe trajectory height although this increase was not significant. There was also an increase in knee flexion after early swing as well as late swing disturbances (Fig. 2D). The peak knee flexion during the swing phase was significantly higher compared to control in both of these conditions (paired t test, P < 0.05).
Figure 2. Kinematic data from steps with swing phase disturbances.
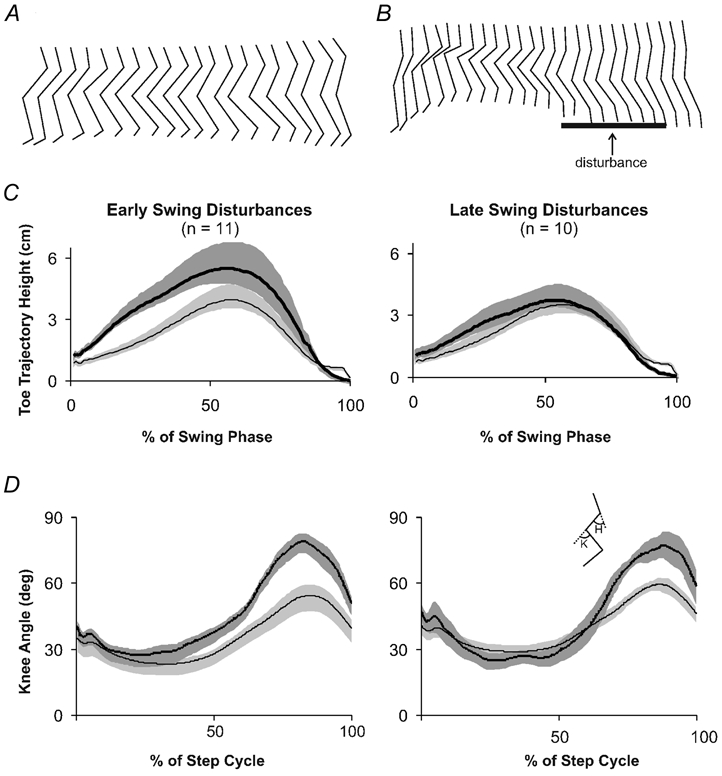
A, stick figure of an infant's leg during the swing phase of an undisturbed step. B, stick figure of the same infant's leg during a disturbed step. Thick horizontal line represents the presence of the mechanical disturbance. In A and B, the time between each stick figure is 33 ms. Stick figures are equally spaced for clarity. C, toe trajectory during the swing phase of steps with early (left panel) or late (right panel) swing disturbances. D, knee angle during steps with early swing disturbances (left panel) and late swing disturbances (right panel). In both C and D, thick black lines represent the average angle during disturbed steps and thin black lines represent the average angle from control steps. Shaded areas represent 1 s.e.m. Inset stick figure represents the measured angles (K, knee; H, hip). Upward deflections are flexion; n, the number of infants contributing data to each figure.
After late stance disturbances, no significant change in toe trajectory or knee flexion angle was detected in the subsequent swing phase. Late stance phase disturbances caused a small but significant increase in the subsequent swing phase duration (Fig. 1D). The absence of a measurable change in the kinematics of the subsequent swing phase suggest that this short prolongation may not reflect an actual functional or meaningful change to the locomotor pattern.
Early stance disturbances resulted in a prolongation of the ongoing stance phase (Fig. 1C). Corresponding to this was the finding of a small but insignificant increase in maximum hip extension angle at the end of the stance phase after stance phase disturbances (paired t test, P > 0.05). Hip angle was measured by the video data in these infants (see Methods). During undisturbed stepping, the average (±s.e.m.) angle of the hip at the end of stance was 28 (±4) deg while after stance phase disturbances, hip extension reached 21 (±4) deg (see inset stick figure in Fig. 2 for how hip angle was measured). This corresponds with the finding that stance phase duration tended to be increased with stance phase disturbances.
EMG data
Representative muscle activity patterns are illustrated in Fig. 3. Figure 3A illustrates raw, rectified EMG activity from the TA, Sol, Quad and Hams muscles as well as the knee angle measured from the goniometer in a single subject. The disturbance (represented by the thick horizontal bar) occurred during the early swing phase and resulted in a clear augmentation of TA muscle activity. Averaged EMG signals during undisturbed and disturbed (early swing) steps from the same infant are illustrated in Fig. 3B. Averages are aligned to foot contact for each trace. Because the averages are aligned to foot contact, the onset time of the disturbance is distributed in the averaged record. This is indicated by the grey shaded vertical bar. Note the increase in TA burst after the disturbance was applied while there was little response observed in the other muscles.
Figure 3. Sample raw and averaged EMG and goniometer signals from a single subject.
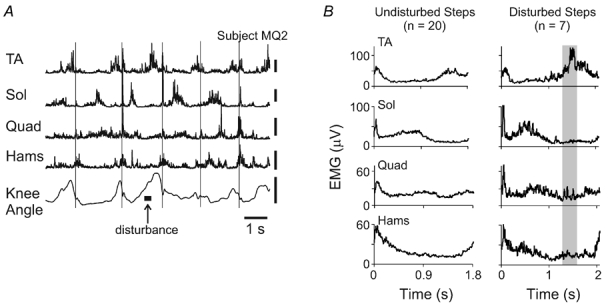
A, EMG and goniometer signals from a single trial. Thin vertical lines delineate foot contact. Vertical calibration bars for EMG signals represent 100 µV. Vertical calibration bar for the goniometer signal represents 50 ° and upward deflections are flexion. B, averaged EMG signals from the same infant in A during undisturbed and disturbed (early swing) steps. Steps are aligned with foot contact for both undisturbed and disturbed steps. The shaded area represents the average timing of the onset of the early swing disturbances.
Figure 4 illustrates the subtracted EMG response from the tibialis anterior muscle during steps with early swing (Fig. 4A), late swing (Fig. 4B), early stance (Fig. 4C) and late stance (Fig. 4D) disturbances. Each subtracted record is an average across all subjects of the EMG amplitude within a 200 ms window centred on the peak of the force signal from the baton (indicated by the grey bar in each panel). The average force signal from each type of disturbance is positioned below its respective subtracted EMG trace and plotted with the same time scale. Note that the force measured from the baton was slower to decline during the swing phase disturbances compared to the stance phase disturbances. This is due to the fact that the foot pushes against the baton after the onset of the swing phase disturbances, but not during stance phase disturbances. The average change in EMG amplitude in all four muscles recorded across all infants is represented in the bar graphs in each panel of Fig. 4. Note that due to noise in some EMG channels, the number of infants in each group is different. This number is indicated under each muscle in each bar graph. With early and late swing phase disturbances, there was an excitatory response in the tibialis anterior muscle activity (paired t test, P < 0.05, Fig. 4A and B). Of the 11 infants in the early swing disturbance group, 10 had an augmentation in TA activity with the disturbances. Of the 10 infants in the late swing disturbance group, all showed an excitatory TA response with the disturbances.
Figure 4. Averaged EMG responses during swing and stance phase disturbances.
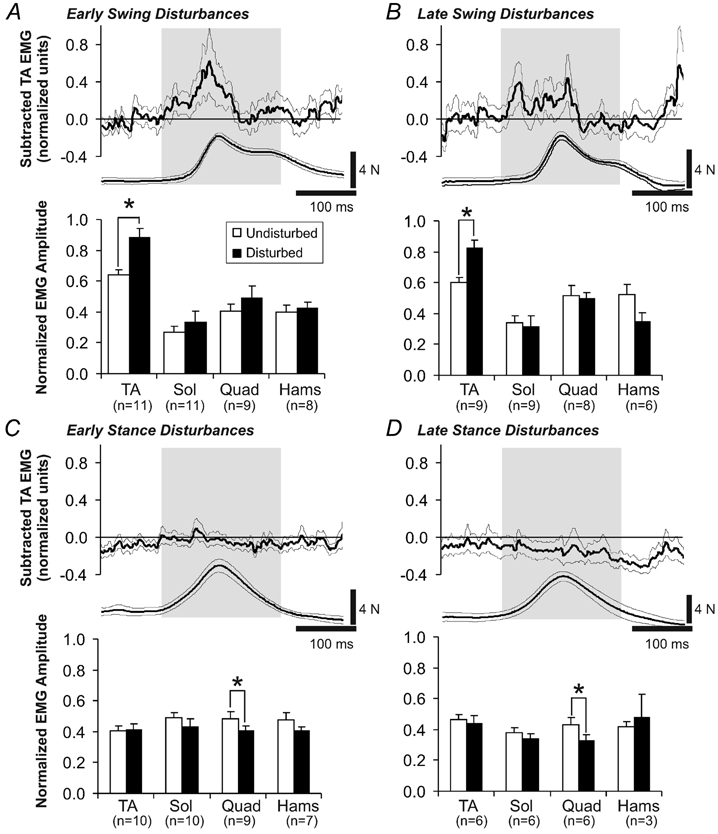
Average subtracted EMG activity from the tibialis anterior muscle after early swing, late swing, early stance, and late stance disturbances are shown in the top panels of A, B, C and D, respectively. Averages are taken across all infants in each group. The sample size of each group is indicated by n. The average force and duration of the mechanical disturbance for each disturbance is shown underneath the subtracted EMG record. Surrounding thin lines represent 1 s.e.m. For each type of disturbance, the EMG amplitude averaged within a 200 ms window centred around the peak of the force signal was calculated (indicated by shading over subtracted EMG and force signal traces). These data are presented in the bar graphs for each disturbance. Error bars represent 1 s.e.m. * P < 0.05.
Early and late stance disturbances yielded a significant reduction in ongoing quadriceps activity (paired t test, P < 0.05, Fig. 4C and D) and no effect in the other muscles. Eight out of the nine infants contributing quadriceps data after early stance disturbances showed a reduction in quadriceps EMG. Of the six infants providing quadriceps data after late stance disturbances, five showed a reduction in quadriceps EMG. Responses in the soleus muscle to stance phase disturbances tended to be variable. For example, three infants had a significant reduction in soleus EMG activity after early stance disturbances while four other infants showed an increase (non-significant) in soleus EMG. Overall, there was no significant change in the soleus EMG amplitude during the stance phase disturbances (Fig. 4C).
Section 2. Location-specific responses to touch
To determine whether the responses to the disturbance depended on the location of the stimulus, we compared the response to disturbances applied to the medial or lateral aspects (side) of the foot with those applied to the dorsum of the foot. Data from eight infants are presented in this part of the study.
Both side and dorsal disturbances were comparable in terms of their onset times (paired t test, P > 0.05). On average (±s.e.m.), dorsal disturbances occurred 117.5 (±16.4) ms after the start of the swing phase, which corresponds to 21 % of the swing phase duration. Side disturbances occurred 103.1 (±11.9) ms after the start of the swing phase, corresponding to 18 % of the swing phase duration. In addition, there was no overall difference in the amount of force applied to the foot in either type of disturbance (paired t test, P > 0.05). On average (±s.e.m.), the force of the dorsal disturbances was 4.3 (±0.5) N and that of the side disturbances was 3.9 (±0.5) N.
Disturbances applied to the side of foot did not result in any effect to the locomotor rhythm, unlike those applied to the dorsum of the foot. Figure 5A illustrates a bar graph comparing the effects of side and dorsal disturbances on step cycle duration. While dorsal disturbances during the early swing phase resulted in a prolongation of step cycle duration compared to control (paired t test, P < 0.05, Fig. 5A), side disturbances at the same time resulted in no effect on step cycle duration. Disturbances to the side of the foot also did not result in any difference in the overall stepping pattern. Figure 5B illustrates the average knee and hip angle during steps with early swing disturbances to the side of the foot (thin black lines) compared to control steps (grey line) and early swing disturbances to the foot dorsum (thick black lines). Neither peak toe trajectory (data not shown) nor peak hip or knee angles were significantly different from control as a result of early swing disturbances to the side of the foot (ANOVA, P > 0.05, Fig. 5B). Note that this group of infants did show an increase in hip and knee flexion with disturbances to the dorsal surface of the foot, similar to that observed in section 1 of the study (Fig. 5B, thick black lines cf. grey lines). However, only the increase in peak hip flexion angle was statistically significant in this group of infants (ANOVA, P < 0.05, Fig. 5C).
Figure 5. Comparison of disturbances to the dorsum of the foot compared with the side of the foot during forwards stepping.
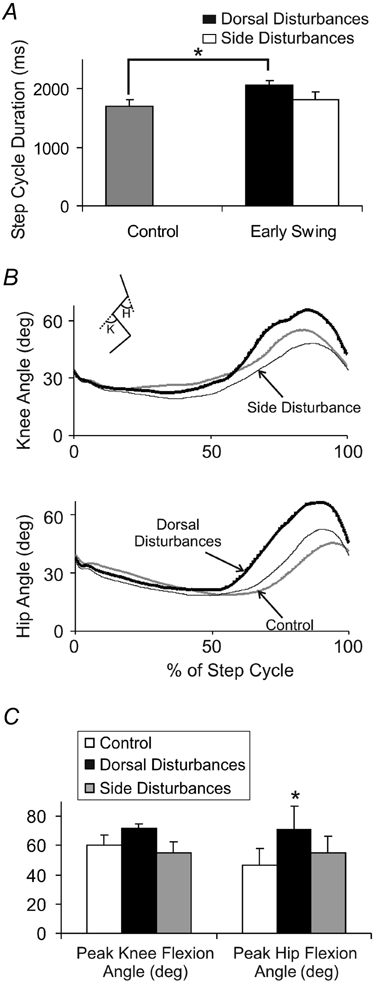
A, average step cycle duration during steps with disturbances to the dorsum of the foot (black bar) and disturbances to the side of the foot (white bar) compared to control (grey bar). The significant prolongation of step cycle duration seen with early swing disturbances to the dorsum of the foot (indicated by asterisk) was not observed with early swing disturbances to the side of the foot. Error bars represent 1 s.e.m.B, knee angle (top panel) and hip angle (bottom panel) during steps with side disturbances (thin lines) compared to steps with dorsal disturbances (thick lines) and control steps (grey lines). Traces are aligned to foot contact. Inset stick figure represents the measured angles (K, knee; H, hip). Upward deflections are flexion. C, average peak knee flexion angle (left panel) and average peak hip flexion angle (right panel) during the swing phase. * P < 0.05. All data in this figure represent the average values across the 8 infants in section 2.
Figure 6 illustrates the changes in EMG activity during dorsal and side disturbances. Raw, rectified EMG activity from a single subject is shown in Fig. 6A and B. In Fig. 6A, a disturbance to the dorsal surface of the foot was applied during the early swing phase. The typical augmentation of TA EMG activity was observed. Figure 6B illustrates raw, rectified EMG signals (from the same infant in Fig. 6A) during a stepping sequence where a disturbance to the side of the foot was applied during the early swing phase. In this case, there appears to be little change in the activation pattern of any of the muscles.
Figure 6. Comparison of disturbances to the dorsum of the foot compared with the side of the foot during forwards stepping.
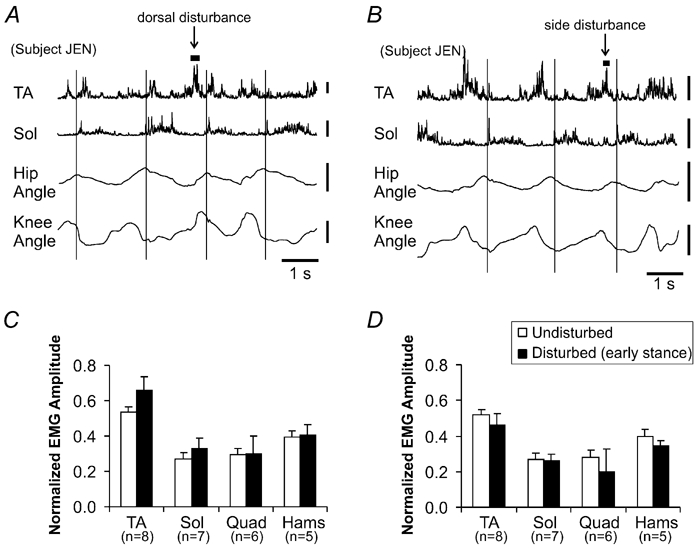
Raw, rectified EMG signals from a single trial showing the response to dorsal foot disturbances (A) and side disturbances (B). The examples in A and B are taken from the same infant. Vertical calibration bars for EMG represent 100 µV. Vertical calibration bar for the goniometer signal represents 30 ° and upward deflections are flexion. Thin vertical lines delineate foot contact. For both dorsal disturbances (C) and side disturbances (D), the EMG amplitude averaged within a 200 ms window centred around the peak of the force signal was calculated and averaged across infants in section 2 (sample size for each muscle group indicated by n). These data are presented in the bar graphs for each disturbance. Error bars represent 1 s.e.m. * P < 0.05.
The bottom half of Fig. 6 illustrates the average EMG amplitude within a 200 ms window centred around the peak of the force signal for both dorsal (Fig. 6C) and side disturbances (Fig. 6D). Averaged values were calculated across all eight infants used in this section of the study. None of the changes was statistically significant. Of note, however, was that the TA amplitude tended to be higher than control after dorsal disturbances, similar to that shown in Fig. 4, while it tended to be inhibited or unchanged after side disturbances. Five of the eight infants showed an increase in TA amplitude after dorsal disturbances and only three of the eight infants showed an increase in TA after side disturbances. While the group of infants tested in section 2 had less of an increase in TA amplitude after early swing disturbances compared with those in section 1 (23 % increase compared with 40 % increase in section 1, Fig. 4), there was no significant difference in the percent change in TA amplitude between the two groups of infants (Student's t test, P > 0.05).
Section 3. Task-specific responses to touch
To examine whether infants also show task-specific responses to touches to the foot, we compared the response to lateral touches during the early swing phase of forwards stepping to those during sideways stepping in 13 infants. Disturbances applied during the different directions of walking were comparable (paired t test, P > 0.05). On average (±s.e.m.), disturbances during forwards stepping occurred 98.1 (±11.7) ms after the start of the swing phase, which corresponds to 20 % of the swing phase duration. Disturbances during sideways stepping started 114.2 (±7.4) ms after the start of the swing phase, corresponding to 26 % of the swing phase duration. In addition, the amount of force used to apply the early swing disturbances during forwards and sideways stepping was also similar (paired t test, P > 0.05). On average (±s.e.m.), the force of disturbances applied during forward stepping was 5.0 (±0.4) N and that during sideways stepping was 5.7 (±0.5) N.
Disturbances to the lateral aspect of the foot during forwards stepping resulted in a slight increase in the height of the foot trajectory (Fig. 7A). The maximum height of the toe during the swing phase was, on average, 1 cm higher during disturbed steps compared to control steps. This slight increase was found to be significant (paired t test, P < 0.05). The same type of disturbances resulted in a higher foot trajectory (measured from the heel) during sideways stepping (Fig. 7B). The maximum heel trajectory height during the swing phase after the disturbances was, on average, 3.3 cm higher (paired t test, P < 0.05).
Figure 7. Kinematic comparison of stepping after disturbances to the lateral aspect of the foot during forwards and sideways stepping.
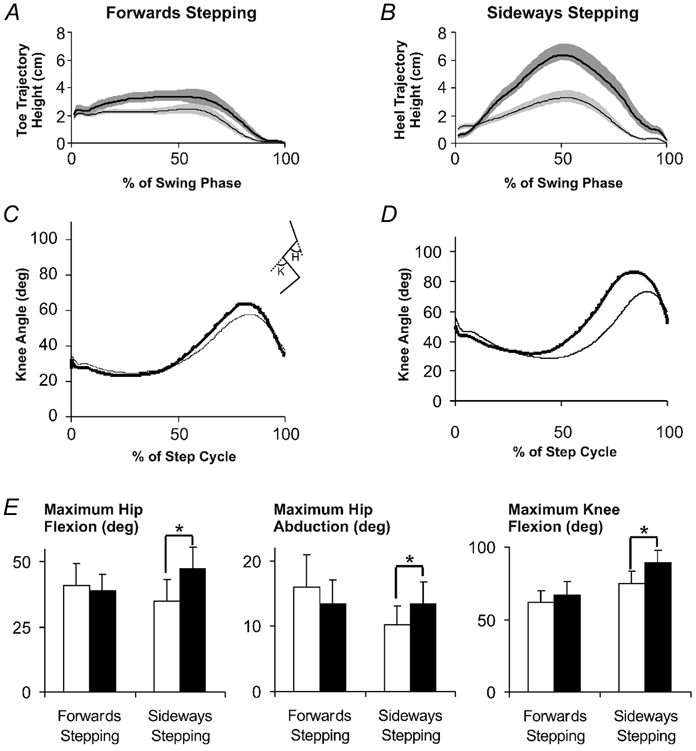
A, averaged toe trajectory from forwards stepping, and B, averaged heel trajectory from sideways stepping during the swing phase of steps with early swing disturbances (thick black line) compared with control (thin black line). Shaded areas represent the s.e.m. Knee angle during forward stepping (C) and during sideways stepping (D) after early swing disturbances (thick black line) compared with control (thin black line). Inset stick figure between C and D represents the measured angles (K, knee; H, hip). Upward deflections are flexion. E, maximum hip flexion (left panel), hip abduction (middle panel) and knee flexion (right panel) angles of undisturbed steps (white bars) and disturbed steps (grey bars). Error bars represent s.e.m. * P < 0.05. All data represent averaged values across the 13 subjects in section 3.
During forwards stepping, lateral disturbances to the foot did not result in any change in knee angle (Fig. 7C). However, during sideways stepping, the same disturbance resulted in an increase in knee flexion angle during the swing phase (Fig. 7D). The increase in knee flexion angle during sideways stepping was significant (paired t test, P < 0.05, Fig. 7E, right panel). Similarly, the maximum hip flexion and hip abduction angles measured after lateral disturbances during sideways stepping was significantly increased compared to control (paired t test, P < 0.05, Fig. 7E, left and middle panels). Corresponding with this was an increase in quadriceps EMG amplitude, although this was not statistically significant (data not shown). No difference in the maximum hip flexion, hip abduction, or knee flexion angles was observed after lateral disturbances to the foot during forwards stepping (Fig. 7E).
DISCUSSION
Disturbances applied to the infants’ foot resulted in phase-dependent reflex responses during treadmill-elicited stepping. None of the infants were independently walking, yet well-organized responses to a touch to the foot were observed. Touches to the dorsum of the foot during the swing phase resulted in a recruitment of additional TA muscle activity, accompanied by an increase in toe trajectory height and knee and hip flexion during the swing phase and a significant prolongation of the swing phase and step cycle duration. Touches to the dorsum of the foot during the stance phase resulted in little change to the locomotor rhythm or to muscle activity. Thus, infants show phase-dependent corrective responses to mechanical disturbances to the foot. While swing phase disturbances to the dorsal surface of the foot resulted in flexor responses, the same disturbance to the medial or lateral surface of the foot during forward walking resulted in no effect on the locomotor rhythm or pattern, demonstrating the characteristic of location-specificity. Also, while disturbances to the side of the foot during sideways stepping resulted in a flexor response, the same disturbance during forwards stepping resulted in no response, demonstrating the characteristic of task-specificity.
Methodological considerations
Disturbances to the foot were applied using a foam-padded baton instrumented with a force transducer. While mechanical disturbances applied in this way are subject to more variability in duration and amount of force provided compared with electrical disturbances, they are certainly more realistic than disturbances applied by electrical stimulation. Differences between mechanical and electrical stimuli to elicit stumbling corrective responses have been discussed at length elsewhere (Buford & Smith 1993). We minimized the variability in the amount and duration of applied force by monitoring the disturbance during the recording session. The force applied during the stance phase had slower rates of rise compared with those applied during the swing phase. This is likely to be due to the fact that during the swing phase, the foot pushes against the baton. Nevertheless, comparable amounts of force were applied in all conditions, thus similar receptors were likely to be activated in all types of disturbances. In sections 2 and 3 of the study, an examination of the timing of the stimuli relative to the onset of the step cycle and the amount of force applied showed that we were able to deliver them consistently. It is worth noting that during the pilot stages of this study, we attempted a variety of approaches to delivering consistent cutaneous disturbances to the foot during stepping. Electrical stimulation of cutaneous nerves was attempted, but was found to be too uncomfortable for the infants. A small vibrator affixed to the top of the foot was also attempted; however we found that the responses to this stimulus were not very robust and it was difficult to ascertain the amount of stimulation.
Both cutaneous as well as proprioceptive receptors were likely to be activated with the mechanical disturbances used here. Previous investigations have reported similar phase-dependent responses with disturbances applied by either electrical stimulation of cutaneous nerves or mechanically applied disturbances. However, the responses to mechanically applied disturbance, while qualitatively similar to electrically stimulated responses, tend to be more robust and complex (Wand et al. 1980; Drew & Rossignol 1987; Buford & Smith 1993; Schillings et al. 1996, 2000; Zehr et al. 1997).
The possibility that touching the foot during stepping would elicit a startle response is unlikely. The presence of phase-dependent responses is not consistent with a general startle response. In addition, touches to different areas of the foot (dorsum vs. side of the foot) elicited different responses as did touches applied during different directions of stepping. For example, touches to the side of the foot during the swing phase did not elicit any change to the stepping pattern while touches to the top of the foot during the swing phase elicited a very clear augmentation of flexor activity. If a startle response was involved, then one would expect uniform responses to touches to various locations of the foot and during various times of the locomotor cycle.
Infants show phase-dependent responses
Forssberg et al. (1975) first described a phase-dependent reflex response to cutaneous stimuli during walking in chronic spinal cats. Tactile stimulation or electrical stimulation of cutaneous nerves of the paw dorsum during the swing phase of cat locomotion elicited an enhancement of flexion while such stimulation during the stance phase resulted in a brief enhancement of extension (Forssberg et al. 1975). Since then, numerous investigators have reported similar responses to cutaneous stimuli in spinal, decerebrate and intact cats as well as adult humans (e.g. Miller et al. 1977; Prochazka et al. 1978; Forssberg, 1979; Drew & Rossignol 1987; Zehr et al. 1997, 1998; Schillings et al. 2000).
In this study, we show that there is a phase-dependent reflex response to mechanical disturbances to the foot during stepping in human infants. When the dorsum of the foot was touched with the foam-padded baton, different responses were elicited depending on the timing of the disturbance in the locomotor cycle. Touches to the dorsum of the foot during the swing phase resulted in an enhancement of flexor activity. This excitatory effect on flexor activity with swing phase disturbances has been a consistent finding across preparations and species with either stimulation of the plantar or dorsal surface of the paw, from chronic spinal cats (Forssberg et al. 1975, 1977), decerebrate cats (Duysens & Pearson 1976; Duysens, 1977; Miller et al. 1977) and intact cats (Duysens & Stein 1978; Prochazka et al. 1978; Forssberg, 1979; Drew & Rossignol 1987). In the cats, an additional brief, short-latency activation of the ankle extensor muscles is also seen with mechanical swing phase disturbances (Prochazka et al. 1978; Forssberg, 1979; Wand et al. 1980; Buford & Smith, 1993). In adult humans, there is also an indication of such facilitation in the ankle extensor muscles after early swing disturbances after electrical stimulation, but this facilitation is quite small (Zehr et al. 1997). Whether there is a facilitation in ankle extensor muscles with mechanical disturbances is unclear (Eng et al. 1994; Schillings et al. 2000). This brief activation of the ankle extensor muscles after swing phase disturbances was generally not observed in the infants in the present study. Since the data from adult humans is inconclusive, it is difficult to determine whether the absence of ankle extensor responses after swing phase disturbances is a function of differences in species (i.e. cat vs. human) or nervous system maturation (i.e. infant vs. adult).
Touches to the dorsum of the foot during the stance phase tended to prolong the stance phase (Fig. 1C and D) and resulted in a statistically significant reduction in the quadriceps EMG amplitude (Fig. 4C and D). Overall, however, stance phase responses were variable. Previous investigations show that effects on stance phase activity with stance phase disturbances are more variable and dependent on the nature of the disturbance and the preparation used (reviewed in Rossignol, 1996). For example, in chronic spinal cats, there is an excitation in quadriceps muscle activity after electrical (Forssberg et al. 1975, 1977) or mechanical stimuli (Forssberg et al. 1977) given during the stance phase. In intact walking cats, mechanical or electrical stimuli to the dorsum of the paw during the stance phase yields either an inhibitory or an excitatory effect on the extensor muscles (Wand et al. 1978; Forssberg et al. 1979). In adult humans, stimulation of the superficial peroneal nerve (which supplies the skin of the dorsum of the foot) during the stance phase of walking produces little effect on the locomotor pattern (Zehr et al. 1997). In the sample of infants studied here, we observed a variety of responses to stance phase disturbances consistent with results in the existing literature.
Infants show location- and task-specific reflex responses
Sherrington (1947) used the term ‘local sign’ to refer to the observation that the response to a given disturbance is dependent on the area of the limb that is stimulated. Location-specific effects are a feature of cutaneous reflex responses during locomotion in humans (van Wezel et al. 1997) as well as in cats (Duysens & Loeb, 1980). In the present study, the characteristic of ‘local sign’ (location-specificity) is demonstrated by the fact that touches to the dorsum of the foot resulted in different responses compared to touches to the side of the foot at the same time in the locomotor cycle during forward stepping. We show that the infants studied in section 2 of the study were comparable to those studied in section 1. Both groups of infants showed a flexor response, both in terms of EMG responses and kinematic responses, to touches to the dorsum of the foot during early swing of forwards stepping (cf. Fig. 1A and Fig. 5A; Fig. 2D and Fig. 5B; Fig. 4A and Fig. 6C). However, no such flexor responses were observed after touches to the side of the foot during the early swing phase of forwards stepping (Figs 5, 6B and 6D).
We also found task-specific responses to touches of the foot dependent on the direction of walking. Similar findings have been reported by others (Buford & Smith, 1993; Duysens et al. 1996). Disturbances that normally impede the progression of the limb and elicit flexion responses during forward walking (i.e. touches to the top of the foot) do not cause any response during backwards walking in intact cats (Buford & Smith, 1993). In adult humans, Duysens et al. (1996) reported that the modulation pattern of reflex responses to sural nerve stimulation was different depending on the direction of walking (forwards vs. backwards). We now show a similar task-specific response to tripping disturbances in infants. Disturbances to the side of the foot, which would impede the progression of the swinging limb during sideways stepping, caused a high-stepping response that would enable clearance of the foot over an obstacle. The same disturbance during forward stepping did not result in any significant change to the limb's trajectory (Fig. 7).
Conclusions
The results of the present study demonstrate that infants can appropriately compensate for mechanical disturbances analogous to a tripping stimulus during locomotion. Infants show well-organized phase-dependent, location-specific and task-dependent reflex responses to mechanical disturbances applied to the foot during treadmill-elicited stepping. Some characteristics of the response are similar to results obtained from human adults as well as from spinal, decerebrate and intact cats. In addition to the results of the present study, previous results from this laboratory have shown that information about limb loading and hip position are powerful signals for regulating the stepping pattern in human infants (Pang & Yang, 2000), in much the same way as that reported in decerebrate and spinal cats (Grillner & Rossignol, 1978; Duysens & Pearson, 1980; Hiebert et al. 1996). All of these responses were robust across all infants and provide supporting evidence for the concept that the locomotor system in infants possesses the sophistication necessary for producing appropriate responses to a variety of sensory inputs during different walking tasks.
Why then, do these infants not walk independently? We do not have direct evidence to answer this question yet, but there are a number of possibilities. Volitional control of the lower limbs, as seen in the ability to kick a ball, appears around the same time as independent walking (Gallahue & Ozmun, 1995). Since descending control from the brain plays an important role in human walking (Schubert et al. 1997; Capaday et al. 1999; Petersen et al. 2001), maturation of this control may be necessary. Alternatively, the control of equilibrium may be a limiting factor. Equilibrium is especially challenging for bipeds. Infants start to exhibit consistent, adult-like responses to disturbances of the support surface in standing around the time they start to walk independently (Sveistrup & Woollacott, 1996). Newly walking infants also depend heavily on visual input (Sveistrup & Woollacott, 1998), suggesting the importance of equilibrium control for walking. Perhaps a critical level of control in equilibrium is necessary before independent walking is possible. Future studies will address these intriguing possibilities.
Acknowledgments
This study was supported by operating grants from the Canadian Institutes of Health Research and the Natural Sciences and Engineering Council of Canada to J.F.Y. M.L. was supported by a grant from the University of Nijmegen. M.Y.C.P. was supported by the Alberta Heritage Foundation for Medical Research. We thank Eliza Walters for technical assistance.
REFERENCES
- Buford JA, Smith JL. Adaptive control for backward quadrupedal walking. III. Stumbling corrective reactions and cutaneous reflex sensitivity. J Neurophysiol. 1993;70:1102–1114. doi: 10.1152/jn.1993.70.3.1102. [DOI] [PubMed] [Google Scholar]
- Capaday C, Lavoie BA, Barbeau H, Schneider C, Bonnard M. Studies on the corticospinal control of human walking. I. Responses to focal transcranial magnetic stimulation of the motor cortex. J Neurophysiol. 1999;81:129–139. doi: 10.1152/jn.1999.81.1.129. [DOI] [PubMed] [Google Scholar]
- Drew T, Rossignol S. A kinematic and electromyographic study of cutaneous reflexes evoked from the forelimb of unrestrained walking cats. J Neurophysiol. 1987;57:1160–1184. doi: 10.1152/jn.1987.57.4.1160. [DOI] [PubMed] [Google Scholar]
- Duysens J. Reflex control of locomotion as revealed by stimulation of cutaneous afferents in spontaneously walking premammillary cats. J Neurophysiol. 1977;40:737–751. doi: 10.1152/jn.1977.40.4.737. [DOI] [PubMed] [Google Scholar]
- Duysens J, Loeb GE. Modulation of ipsi- and contralateral reflex responses in unrestrained walking cats. J Neurophysiol. 1980;44:1024–1037. doi: 10.1152/jn.1980.44.5.1024. [DOI] [PubMed] [Google Scholar]
- Duysens J, Pearson KG. The role of cutaneous afferents from the distal hindlimb in the regulation of the step cycle of thalamic cats. Exp Brain Res. 1976;26:245–255. doi: 10.1007/BF00235013. [DOI] [PubMed] [Google Scholar]
- Duysens J, Pearson KG. Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Res. 1980;187:321–332. doi: 10.1016/0006-8993(80)90206-1. [DOI] [PubMed] [Google Scholar]
- Duysens J, Stein RB. Reflexes induced by nerve stimulation in walking cats with implanted cuff electrodes. Exp Brain Res. 1978;32:213–224. doi: 10.1007/BF00239728. [DOI] [PubMed] [Google Scholar]
- Duysens J, Tax AA, Trippel M, Dietz V. Phase-dependent reversal of reflexly induced movements during human gait. Exp Brain Res. 1996;90:404–414. doi: 10.1007/BF00227255. [DOI] [PubMed] [Google Scholar]
- Eng JJ, Winter DA, Patla AE. Strategies for recovery from a trip in early and late swing during human walking. Exp Brain Res. 1994;102:339–349. doi: 10.1007/BF00227520. [DOI] [PubMed] [Google Scholar]
- Eyre JA, Miller S, Ramesh V. Constancy of central conduction delays during development in man: investigation of motor and somatosensory pathways. J Physiol. 1991;434:441–452. doi: 10.1113/jphysiol.1991.sp018479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssberg H. Stumbling corrective reaction: a phase-dependent compensatory reaction during locomotion. J Neurophysiol. 1979;42:936–953. doi: 10.1152/jn.1979.42.4.936. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Rossignol S. Phase dependent reflex reversal during walking in chronic spinal cats. Brain Res. 1975;85:103–107. doi: 10.1016/0006-8993(75)91013-6. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Rossignol S. Phasic gain control of reflexes from the dorsum of the paw during spinal locomotion. Brain Res. 1977;132:121–139. doi: 10.1016/0006-8993(77)90710-7. [DOI] [PubMed] [Google Scholar]
- Gallahue KL, Ozmun JC. Understanding Motor Development: Infants, Children, Adolescents. Madison, Wisconsin: Benchmark Press; 1995. [Google Scholar]
- Grillner S, Rossignol S. Contralateral reflex reversal controlled by limb position in the acute spinal cat injected with clonidine i.v. Brain Res. 1978;144:411–414. doi: 10.1016/0006-8993(78)90169-5. [DOI] [PubMed] [Google Scholar]
- Hiebert GW, Whelan PJ, Prochazka A, Pearson KG. Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J Neurophysiol. 1996;75:1126–1137. doi: 10.1152/jn.1996.75.3.1126. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Zehr EP, Stein RB. Absence of nerve specificity in human cutaneous reflexes during standing. Exp Brain Res. 2000;133:267–272. doi: 10.1007/s002210000411. [DOI] [PubMed] [Google Scholar]
- Miller S, Ruit JB, van der Meche FG. Reversal of sign of long spinal reflexes dependent on the phase of the step cycle in the high decerebrate cat. Brain Res. 1977;128:447–459. doi: 10.1016/0006-8993(77)90170-6. [DOI] [PubMed] [Google Scholar]
- Müller K, Hömberg V, Lenard HG. Magnetic stimulation of motor cortex and nerve roots in children. Maturation of cortico-motoneuronal projections. Electroencephalogr Clin Neurophysiol. 1991;81:63–70. doi: 10.1016/0168-5597(91)90105-7. [DOI] [PubMed] [Google Scholar]
- Pang MYC, Yang JF. The initiation of the swing phase in human infant stepping: importance of hip position and leg loading. J Physiol. 2000;528:389–404. doi: 10.1111/j.1469-7793.2000.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen NT, Butler JE, Marchand-Pauvert V, Fisher R, Ledebt A, Pyndt HS, Hansen NL, Nielsen JB. Suppression of EMG activity by transcranial magnetic stimulation in human subjects during walking. J Physiol. 2001;537:651–656. doi: 10.1111/j.1469-7793.2001.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A, Sontag K-H, Wand P. Motor reactions to perturbations of gait: proprioceptive and somesthetic involvement. Neurosci Lett. 1978;7:35–39. doi: 10.1016/0304-3940(78)90109-x. [DOI] [PubMed] [Google Scholar]
- Rossignol S. Neural control of stereotypic limb movements. In: Rowell LB, Sheperd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. Bethesda: American Physiological Society; 1996. pp. 173–216. [Google Scholar]
- Schillings AM, van Wezel BM, Duysens J. Mechanically induced stumbling during human treadmill walking. J Neurosci Methods. 1996;67:11–17. doi: 10.1016/0165-0270(95)00149-2. [DOI] [PubMed] [Google Scholar]
- Schillings AM, van Wezel BM, Mulder T, Duysens J. Muscular responses and movement strategies during stumbling over obstacles. J Neurophysiol. 2000;83:2093–2102. doi: 10.1152/jn.2000.83.4.2093. [DOI] [PubMed] [Google Scholar]
- Schubert M, Curt A, Jensen L, Dietz V. Corticospinal input in human gait: modulation of magnetically evoked motor responses. Exp Brain Res. 1997;115:234–246. doi: 10.1007/pl00005693. [DOI] [PubMed] [Google Scholar]
- Sherrington . Integrative Actions of the Nervous System. Cambridge, UK: Cambridge University Press; 1947. [Google Scholar]
- Sveistrup H, Woollacott MH. Longitudinal development of automatic postural response in infants. J Mot Behav. 1996;28:58–70. doi: 10.1080/00222895.1996.9941734. [DOI] [PubMed] [Google Scholar]
- Sveistrup H, Woollacott MH. The influence of vision on the automatic postural muscle responses of newly standing and newly walking infants. Exp Brain Res. 1998;120:537–540. doi: 10.1007/s002210050429. [DOI] [PubMed] [Google Scholar]
- van Wezel BM, Ottenhoff FA, Duysens J. Dynamic control of location-specific information in tactile cutaneous reflexes from the foot during human walking. J Neurosci. 1997;17:3804–3814. doi: 10.1523/JNEUROSCI.17-10-03804.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand P, Prochazka A, Sontag KH. Neuromuscular responses to gait perturbations in freely moving cats. Exp Brain Res. 1980;38:109–114. doi: 10.1007/BF00237937. [DOI] [PubMed] [Google Scholar]
- Winter DA. Biomechanics and Motor Control of Human Movement. New York: Wiley; 1990. [Google Scholar]
- Yakovlev PI, Lecours A-R. Regional Development of the Brain in Early Life. Oxford: Blackwell; 1967. The myelogenetic cycles of regional maturation of the brain; pp. 3–70. [Google Scholar]
- Yang JF, Stephens MJ, Vishram R. Infant stepping: a method to study the sensory control of human walking. J Physiol. 1998;507:927–937. doi: 10.1111/j.1469-7793.1998.927bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr EP, Komiyama T, Stein RB. Cutaneous reflexes during human gait: electromyographic and kinematic responses to electrical stimulation. J Neurophysiol. 1997;77:3311–3325. doi: 10.1152/jn.1997.77.6.3311. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Stein RB, Komiyama T. Function of sural nerve reflexes during human walking. J Physiol. 1998;507:305–314. doi: 10.1111/j.1469-7793.1998.305bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


