Abstract
Experimental denervation in animals has shown that carotid baro- and chemoreceptors play an eminent role in maintaining blood pressure and blood gas homeostasis. Denervation of carotid sinus baro- and chemoreceptors in humans may occur as a complication of invasive interventions on the neck or after experimental surgical treatment in asthma. In this topical review, the short- and long-term effects of carotid baro- and chemoreceptor denervation on the control of circulation and ventilation in humans are discussed. Carotid baroreceptor denervation in humans causes a persistent decrease in vagal and sympathetic baroreflex sensitivity and an increase in blood pressure variability; however, carotid denervation does not lead to chronic hypertension. Therefore, although carotid baroreceptors contribute to short-term blood pressure control, other receptors are able to maintain normal chronic blood pressure levels in the absence of carotid baroreceptors. Conversely, carotid chemoreceptor denervation leads to permanent abolition of normocapnic ventilatory responses to hypoxia and reduced ventilatory responses to hypercapnia.
Hering (1927) and Koch (1931) were the first to recognize the reflex nature of changes in heart rate and blood pressure evoked by external massage of the neck. The afferents were tracked as nerve endings at the carotid bifurcation. At about the same time, Heymans et al. (1930) unequivocally demonstrated chemoreceptor activity of the carotid bodies. With their experiments in the 1920s/1930s, these investigators inaugurated the modern era of baro- and chemoreflex research.
The arterial baroreflex buffers abrupt transients of blood pressure and originates from stretch-sensitive receptors in the arterial wall of the carotid sinus and the aortic arch and the large vessels of the thorax (Fig. 1) (Persson & Kirchheim, 1991; Eckberg & Sleight, 1992). Afferent fibres from carotid sinus baroreceptors join the glossopharyngeal nerve (ninth cranial nerve) and project to the nucleus tractus solitarii in the dorsal medulla, which is under cortical command and in turn projects to efferent cardiovascular neurones in the medulla and spinal cord. Extra-carotid baroreceptors consist of arterial baroreceptors in the aortic arch as well as stretch-sensitive receptors in the heart and pulmonary vessels, the latter two being lumped together as ‘cardiopulmonary’ receptors despite their distinctive properties (Hainsworth, 1991). The extra-carotid baroreceptors transmit their afferent information along with the vagus nerves to the same brainstem nuclei. The efferent limbs of the baroreflex loop consist of sympathetic and parasympathetic fibres to the heart as well as to smooth muscles in the peripheral blood vessels.
Figure 1. Arterial baro- and chemoreceptors.
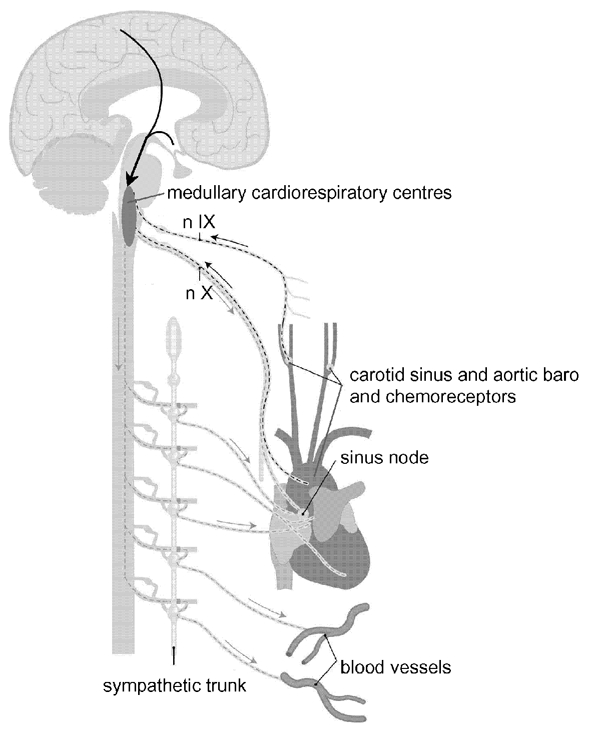
Arterial baroreflex loops: (1) carotid sinus baroreceptors, nIX, medullary centres, sympathetic and parasympathetic fibres to heart and blood vessels; (2) aortic baroreceptors, nX, medullary centres, sympathetic and parasympathetic fibres to heart and blood vessels. Peripheral chemoreflex loops: (1) carotid body chemoreceptors, nIX, medullary respiratory centres, motor nerves to respiratory muscles; (2) aortic body chemoreceptors, nX, medullary respiratory centres, motor nerves to respiratory muscles. nIX = ninth cranial nerve (glossopharyngeal nerve), nX = tenth cranial nerve (vagus nerve); the arrows coming from the cortex signify the modulation of brainstem nuclei by higher, cortical centres. Adapted with permission from Timmers et al. 2001b.
Adjustment of respiration in response to alterations in levels of oxygen, carbon dioxide and hydrogen ions in the body fluids are mediated by a complex interplay between central and peripheral chemoreceptors (O'Regan & Majcherczyk, 1982). The peripheral arterial chemoreceptors, located in the carotid and aortic bodies, are responsible for the immediate ventilatory and arterial pressure increments during acute hypoxia (Heymans et al. 1930) (Fig. 1). Carotid and aortic bodies contain glomus (type I) cells, which release neurotransmitters in response to hypoxia, causing depolarization of nearby afferent nerve endings (Prabhakar, 2000). Apart from hypoxaemia, peripheral chemoreceptors play a minor role in the sensing of changes in arterial carbon dioxide tension (PCO2) and pH. Other glomus tissues (glomus jugulare, trigeminale, pulmonare etc.) are not relevant to chemoreflex function in humans. Carotid and aortic bodies are supplied with sensory fibres, which course through carotid sinus/glossopharyngeal and vagus nerve respectively towards medullary centres, including the nucleus tractus solitarii (Felder & Mifflin, 2003). Central chemoreceptive areas located at the rostral ventrolateral medulla respond to changes in the hydrogen ion concentration in the interstitial fluid in the brain and are chiefly responsible for ventilatory and circulatory adjustments during hypercapnia and chronic disturbances of acid-base balance.
The relative contribution of carotid receptors to baro- and chemoreflex function as well as the compensation after functional loss of these receptors has been investigated extensively by well-controlled denervation studies in experimental animals. For obvious reasons, no human counterparts for the controlled prospective denervation studies in animals are available. Information on the impact of carotid sinus denervation in humans is limited and largely relies on investigations following iatrogenic damage to the carotid sinus as a complication of medical interventions like carotid body tumour surgery, jugular radiotherapy and carotid endarterectomy. Interpretation of these human studies is hampered by uncertainty regarding the completeness of denervation, differences in acute (surgical) versus gradual (radiation) denervation, additional changes in the mechanical properties of the carotid artery wall or surrounding tissue due to the interventions, and the lack of prospective studies. Most studies consist of retrospective, post-intervention assessment of reflex function in small numbers of patients and matched (healthy) control subjects.
We review the short- and long-term effects of carotid baro- and chemoreceptor denervation on the control of circulation and ventilation respectively. Whereas findings in animal studies are briefly mentioned, this review is focused on data obtained from investigations in humans, including recent studies in this field by the authors. Although the effects of carotid baroreceptor and chemoreceptor denervation will be presented separately, the two conditions arise in parallel in most instances, due to the underlying anatomy.
Carotid baroreceptor denervation
Animal studies
Arterial baroreceptors provide a tonic inhibitory influence on sympathetic tone, thus controlling peripheral vasoconstriction and cardiac output (Persson & Kirchheim, 1991; Eckberg & Sleight, 1992). Therefore, baroreceptor denervation would be expected to result in a sustained increase in sympathetic tone and, as a consequence, a sustained increase in blood pressure. The chronic effects of carotid and extra-carotid baroreceptor denervation on blood pressure control have been studied extensively in animals and have been reviewed by others (Shade et al. 1991; Eckberg & Sleight, 1992; Persson, 1996). After selective carotid baroreceptor denervation, both blood pressure level and variability increased markedly but returned to intact levels within 7 to 14 days in dogs and baboons respectively (Ito & Scher, 1978; Shade et al. 1990). Selective aortic baroreceptor denervation in baboons causes a mild temporary increase in blood pressure, whereas blood pressure variability was unchanged (Bishop et al. 1986). Combined sino-aortic baroreceptor denervation in dogs produced an increase in blood pressure and heart rate in the acute phase, whereas permanent elevation of blood pressure was either present (Cowley et al. 1973; Ito & Scher, 1981) or absent (Persson et al. 1988). Sino-aortic denervation in baboons - the investigated species that is closest to humans - resulted in a persistent increase in blood pressure level and variability and a decrease in heart rate variability (Shade et al. 1990). Combined sino-aortic, cardiac and pulmonary baroreceptor denervation in dogs produced a persistent increase in blood pressure level and variability (Persson et al. 1988).
These animal studies show, that extra-carotid baroreceptor areas have a large ability to compensate for the loss of carotid baroreceptors. In some species, chronic hypertension is evoked by combined sino-aortic and cardiopulmonary baroreceptor denervation and by sino-aortic denervation but not by selective carotid denervation.
Human studies
Acute unilateral carotid baroreceptor denervation
The first report on baroreceptor denervation in humans appeared in the 1930s (Bucy, 1936). Unilateral section of the glossopharyngeal nerve in five patients with glossopharyngeal neuralgia produced a prompt and pronounced rise in blood pressure in four out of five patients, which lasted from 5 to 12 days. This phenomenon was recognized as an effect of disruption of ‘nervous impulses from the carotid sinus which have a reducing effect on blood pressure’. In 1956 a patient died from a fatal hypertensive crisis following unilateral carotid sinus denervation, which had been performed for the relief of recurrent syncope due to a hypersensitive carotid sinus syndrome (Ford, 1956).
Lability of blood pressure in the hours following unilateral carotid endarterectomy for symptomatic carotid stenosis has been attributed to carotid baroreflex dysfunction (Ille et al. 1995; Ejaz & Meschia, 1999). However, in the acute phase following carotid endarterectomy, baroreflex sensitivity has been reported to be increased, decreased or unaltered (Tyden et al. 1980; Hirschl et al. 1991; Landesberg et al. 1998). Apart from trauma to the carotid sinus, baroreceptors or to the carotid sinus nerve (Angell-James & Lumley, 1974), removal of an atherosclerotic plaque may have a beneficial effect on baroreflex function by means of changes in the mechanical properties of the carotid sinus arterial wall and re-integration of baroreceptor areas into circulatory regulation (Angell-James & Lumley, 1974). In addition, the effect of unilateral carotid endarterectomy depends on the compensatory ability of the contralateral baroreceptor integrity. This may be limited by atherosclerotic changes in the non-operated carotid artery, since in atherosclerosis distensibility of the carotid sinus vessel wall and sensitivity of baroreceptors are reduced (Sleight, 1976; Randall et al. 1976).
Although evidence for lateralization of certain human autonomic control functions has been published (Hilz et al. 2001), a differential effect of left- versus right-sided deafferentiation of carotid baroreceptors has not been reported.
Acute bilateral carotid baroreceptor denervation
Bilateral anaesthetic injections in the regions of the carotid sinuses in patients with malignant hypertension were shown to elevate blood pressures to even higher levels (Lampen et al. 1949). Paroxysms of severe hypertension and tachycardia were reported following bilateral carotid body resection as an experimental treatment of asthma (Holton & Wood, 1965), carotid paraganglioma resection (Robertson et al. 1993; De Toma et al. 2000), carotid endartectomy (Ille et al. 1995; Boyle et al. 1995) and trauma of the neck (Robertson et al. 1993).
Long-term effects of unilateral carotid baroreceptor denervation
In a prospective study, the effects of unilateral carotid endartectomy on carotid sinus baroreflex function were measured in 25 patients (Dehn & Angell-James, 1987). Six months after surgery, no overall change in blood pressure was found. Baroreflex sensitivity decreased in 2, remained unchanged in 15 and increased in 8 patients. Thus, similar to the findings in the acute phase following surgery, the long-term effects on baroreflex function were heterogeneous among individuals.
In a retrospective study on the effects of unilateral carotid endarterectomy (Timmers et al. 2001a), at a median interval of 4.3 years after surgery, baroreflex sensitivity was significantly lower in endarterectomized patients than in patients with an untreated uni-/bilateral carotid stenosis and healthy controls. So in these patients an unfavourable effect on baroreflex sensitivity prevailed. Despite this, ambulatory blood pressure level and variability did not differ between groups.
Long-term effects of bilateral carotid baroreceptor denervation
In 1993, chronic failure of the baroreflex due to bilateral carotid denervation was described as a separate clinical syndrome, characterized by a limited blood pressure buffering capacity against excessive rises or falls in response to emotional and physical stimuli (Robertson et al. 1993) (Fig. 2A). Symptoms and signs included headache, palpitations, diaphoresis and pale flushing. They bear a strong resemblance to those of a phaeochromocytoma. In baroreflex failure, desinhibition of central activation of efferent sympathetic pathways arises from the absence of tonic inhibitory baroreceptor input to the vasomotor centres of the brainstem (Robertson et al. 1993; Persson, 1996). Apart from volatile hypertension, which is most common, baroreflex failure has a broad spectrum of other clinical presentations including predominant hypotension, orthostatic tachycardia and intolerance and malignant vagotonia with severe bradycardia, depending on the extent of baroreceptor denervation and concomitant destruction of autonomic structures (Kochar et al. 1984; Jordan et al. 1997; Ketch et al. 2002). Centrally acting sympatholytic agents like clonidine may reduce the frequency and severity of the attacks (Robertson et al. 1993; Biaggioni et al. 1994; Ejaz & Meschia, 1999).
Figure 2. Blood pressure variability following carotid sinus baroreceptor denervation.
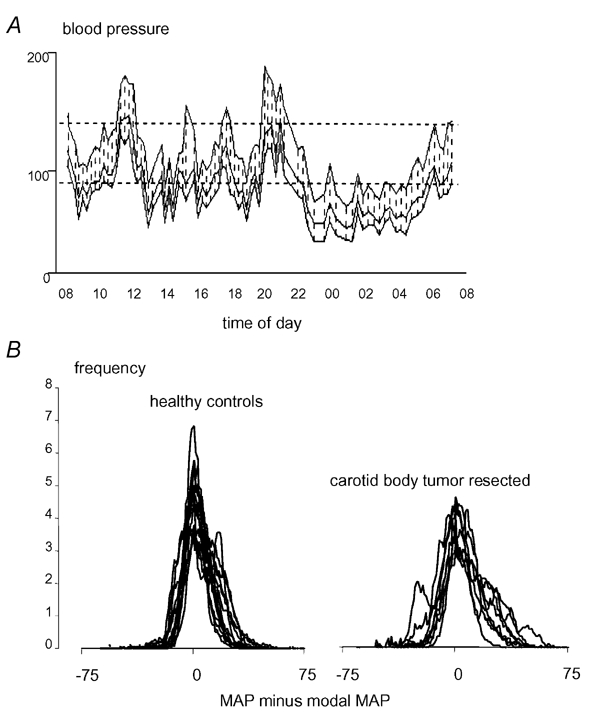
A, 24 h ambulatory blood pressure profile during normal daily activities characterized by labile hyper- and hypotension in a patient with baroreflex failure due to radiotherapy of the neck for nasopharyngeal carcinoma (Timmers et al. 1999). Dotted lines indicate upper levels for diastolic (< 90 mmHg) and sytolic (< 140 mmHg) normotension. B, individual frequency histograms of blood pressure calculated from 5 h ambulatory beat-by-beat recordings in 12 healthy controls (bottom left) and 8 patients after bilateral carotid body tumour resection (bottom right). x-axis: MAP = mean arterial pressure, y-axis: frequency of MAP level as percentage of total number of frequencies. Carotid body resected patients exhibit a broader distribution of MAP, indicating higher blood pressure variability. ‘Modal MAP’ refers to the MAP with the highest frequency during the individual blood pressure tracing.
Long-term effects of bilateral baroreceptor denervation have been investigated in patients who had suffered from acute baroreflex failure following bilateral carotid body tumour resection (Smit et al. 2002). Ambulatory blood pressure level was found to remain slightly elevated as compared to pre-operative values. Overt hypertension, however, appears to be limited to the days and months following surgical carotid baroreceptor denervation, whereas episodic surges of hyper- as well as hypotension may persist for a longer period (Holton & Wood, 1965; Robertson et al. 1993; De Toma et al. 2000).
Retrospective studies were performed in patients who had undergone similar surgery, with a mean interval between the second (i.e. contralateral) operation and the study of 3.4 years (Timmers et al. 2003a). At the time of the study, at least 1 year after surgery, ambulatory blood pressure levels were normal in all patients. In the absence of chronic clinically overt baroreflex failure, vagal baroreflex sensitivity, calculated from the reflex changes in RR interval to phenylephrine injections (Smyth et al. 1969), was approximately 50 % lower in these patients than in healthy age-matched controls. The subnormal vagal baroreflex gain also emerged from minimal reciprocal heart rate changes during phase II blood pressure decrease and phase IV blood pressure overshoot during Valsalva's manoeuvre (Goldstein et al. 1982; Wieling & Karemaker, 1999). In addition, microneurography recording in a subgroup of five carotid body resected patients showed, that the baroreflex modulation of muscle sympathetic nerve activity (MSNA) was profoundly affected as well (Timmers et al. 2003b). These studies indicate that bilateral carotid sinus trauma causes permanent impairment of vagal as well as sympathetic baroreflex sensitivity. In these patients, the additional finding of an increased ambulatory blood pressure variability was explained by a lower baroreflex sensitivity (Fig. 2B). In hypertensives, baroreflex sensitivity has been shown to be negatively correlated with blood pressure variability (Mancia et al. 1986).
Chronic baroreflex failure has been reported as a late complication of radiotherapy of the neck (Robertson et al. 1983; Robertson et al. 1993; Timmers et al. 1999). Changes in carotid sinus baroreceptor function may be induced by irradiation damage to the carotid sinus and/or the glossopharyngeal nerves, although cranial nerve palsies are uncommon complications after radiotherapy to the neck (Cheng & Schultz, 1975). Alternatively, arterial baroreflex function may have been altered by structural changes of the internal carotid artery wall. Irradiation-induced atherosclerosis (Cheng et al. 1999) and fibrosis (Zidar et al. 1997) may result in a decreased distensibility of the carotid sinus and thereby may reduce stretch-induced afferent carotid sinus nerve activity (Angell-James, 1974).
The impact of neck irradiation for laryngeal or pharyngeal carcinoma on baroreflex function was retrospectively studied in 12 patients who had undergone bilateral radiation therapy for locally advanced laryngeal or pharyngeal cancer (Timmers et al. 2002). Irradiation fields included the carotid sinus area and the median interval between completion of radiotherapy and time of investigation was 3.3 years. Baroreflex sensitivity was 45 % lower in patients than in matched healthy controls. Ambulatory blood pressure variability was not different from matched control subjects. Although baroreflex sensitivity was decreased after neck irradiation, blood pressure buffering was unaffected.
In summary, labile hypertension due to baroreflex failure may arise from both uni- and bilateral carotid baroreceptor denervation. The incidence of this syndrome following carotid body tumour surgery, radiotherapy of the neck and carotid endarterectomy is low. Baroreflex dysfunction after unilateral denervation is usually mild and transient. In the long term following bilateral carotid denervation, the expression of baroreflex dysfunction is heterogeneous. Bilateral carotid denervation in humans does not elicit chronic hypertension, but in contrast to other investigated species, it causes a long-term increase of blood pressure variability. In humans, carotid baroreceptors are more important for dynamic than static blood pressure control. A chronic decrease in blood pressure buffering following carotid denervation suggests that humans have less potent compensatory mechanisms for loss of carotid baroreflex function than other investigated species. This may be due to our upright position, whereby tonic sympathoinhibitory influences from cardiac and pulmonary baroreceptors have become less than in quadruped species (Shade et al. 1991). As a consequence of a minor role for cardiac and pulmonary baroreceptors, loss of arterial baroreceptor function in humans may have a larger impact on blood pressure homeostasis.
Previous studies on the relative importance of carotid versus aortic baroreceptors in intact humans have yielded contrasting results. Experiments on selective (un)loading of aortic baroreceptors by simultaneous infusion of vasoactive substances and application of neck suction/pressure in order to maintain a stable carotid sinus transmural pressure, indicated that aortic baroreceptors are dominant in the baroreflex control of heart rate, with the carotid baroreceptors contributing only about 30 % (Mancia et al. 1977; Ferguson et al. 1985). In line with these observations, baroreflex control of heart rate is more importantly determined by the distensibility of the aortic arch than of the carotid sinus (Lenard et al. 2001). In contrast, combined neck suction/pressure with non-pharmacological (de)loading of aortic baroreceptors, indicate, that carotid baroreceptors are the principal contributors to baroreflex control of heart rate (Fadel et al. 2003). Our review of studies on iatrogenic denervation is in agreement with the latter study. These studies in intact humans should be interpreted with caution, however, since the baroreceptors respond to stretch and not pressure. The stimulus to be measured should be the diameter of the arteries and not blood pressure. Changes of dimensions of the baroreceptive arteries during the several interventions were not measured.
Denervation of carotid chemoreceptors
Animal studies
In general, acute effects of carotid body chemoreceptor denervation in experimental animals include hypoventilation, apnoea, a variable decrease in hypoxic ventilatory responsiveness and attenuation of CO2 sensitivity. The occurrence of (partial) restoration of chemoreflex function varies among species, but is more likely in neonatal than in adult animals and effects are more marked following bilateral than after unilateral denervation (Forster et al. 2000). In carotid body denervated rats, hypoxic responsiveness is first abolished, but returns to about half of normal within weeks (Martin-Body et al. 1986). Compensation was stated to result from inputs from either aortic or abdominal chemoreceptors or from central mechanisms. Superimposed aortic denervation had no effect in these animals, suggesting that the aortic body has little chemoreceptor function. In carotid body denervated dogs, hypoventilation and CO2 hyposensitivity persisted through the three-week follow-up period (Rodman et al. 2001), whereas in goats, there was a near normalization of breathing and CO2 sensitivity within days to weeks (Pan et al. 1998). In carotid sinus denervated ponies, arterial CO2 levels did not normalize until two years after denervation (Bisgard et al. 1980). In these ponies, but also in cats (Smith & Mills, 1980), partial regain of hypoxic ventilatory responsiveness was attributed to aortic body chemoreceptor function. Subsequent aortic denervation resulted in loss of chemoreflex function. However, this denervation was not necessarily aorta specific and may have also affected cardiac chemoreceptors (Forster et al. 2000).
These studies indicate, that most mammals show a considerable ability to compensate for the loss of carotid chemoreflex function. Compensatory mechanisms on a peripheral and/or central level remain largely unclarified.
Human studies
Studies on the effect of peripheral chemoreceptor removal or denervation on human ventilatory control are limited. Experimental anaesthetic blockade of the glossopharyngeal and vagus nerves in healthy subjects was shown to result in abolition of the ventilatory response to hypoxia, without any depression of resting ventilation (Guz et al. 1966a,b). Information on selective abolition of carotid body chemoreflex function is mainly derived from studies in small numbers of patients who underwent bilateral resection of healthy carotid bodies as an experimental treatment of bronchial asthma or chronic obstructive pulmonary disease (Holton & Wood, 1965; Lugliani et al. 1971; Honda et al. 1979; Vermeire et al. 1987; Whipp & Ward, 1992). Baroreflex function was presumed to be unaffected by this procedure. These subjects exhibit an on-average limited hypoxaemia and hypercapnia response, with a large interindividual variability (Vermeire et al. 1987; Whipp, 1994). They do not hyperventilate in response to sustained or progressive hypoxaemia either at rest or during exercise (Holton & Wood, 1965; Lugliani et al. 1971). In addition, they do not show a decline in ventilation following the abrupt and surreptitious administration of 100 % oxygen against a hypoxic background (Lugliani et al. 1971). Abnormalities were shown to persist in the long term after removal of carotid bodies and were more severe after bilateral than unilateral carotid body removal (Honda et al. 1979). In response to muscle exercise, there was a slower compensatory hyperpnoea, resulting in more profound hypoxia and metabolic acidosis (Wasserman et al. 1975). Sleep structure and frequency of nocturnal haemoglobin desaturation were found to be unaltered as shown by polysomnographic studies (Vermeire et al. 1987).
However, all of these observations are hampered by the possible confounding chronic pulmonary disease, which itself alters chemoreflex function (Godfrey et al. 1971). Peripheral chemoreflex function was assessed in eight patients who had undergone bilateral carotid body tumour resection and were free of pulmonary disease (Timmers et al. 2003a). The ventilatory response to hypoxia was assessed by a rebreathing method. Peripheral oxygen desaturation was reduced to a level of 80 % within 3-4 min while alveolar PCO2 was kept constant. The ventilatory increase relative to the decrease in oxygen saturation was taken as a measure of hypoxic responsiveness. Hypoxic responsiveness was assessed at two constant levels of clamped alveolar PCO2: normocapnia and 1 kPa above normocapnia. At baseline, oxygen saturation and ventilation did not differ between patients and controls. A slightly higher resting alveolar PCO2 in patients than in controls however, suggested mild chronic hypoventilation. Whether this is due to the absence of carotid bodies is uncertain. Subjects with severe chronic obstructive pulmonary disease who have undergone bilateral carotid body resection show a further hypoxaemia and hypercapnia that is consistent with the removal of an ongoing hypoxic drive as result of the surgery (Vermeire et al. 1987; Whipp & Ward, 1992). Long-term hypoventilation with increased levels of arterial PCO2 were also demonstrated in patients with inadvertent denervation of carotid chemoreceptors when undergoing carotid endarterectomy (Wade et al. 1970). On the other hand, normoventilation with no effect on arterial blood gas was found by others (Lugliani et al. 1971; Honda et al. 1979).
Complete abolition of normocapnic hypoxive responsiveness was observed in all carotid body tumour resected patients. Two of eight patients exhibited a slight ventilatory response to hypoxia under hypercapnic conditions. An increased arterial PCO2 enhances peripheral hypoxic chemosensitivity. In line with our observations, a small component of hypoxic ventilatory drive during simultaneous hypercapnia in patients after carotid body resection for chronic pulmonary disease was demonstrated (Swanson et al. 1978; O'Regan & Majcherczyk, 1982). Residual responsiveness to hypoxaemia in these patients may originate from the aortic bodies, which have a minor role in the modulation of spontaneous respiratory activity, but generate a discernible response when their gain is increased by hypercapnia (Whipp, 1994). Alternative explanations for residual chemoreflex function include incomplete carotid body resection and regeneration of carotid chemosensitivity. The latter has been demonstrated in cats (Mitchell et al. 1972), but not in humans.
Carotid body resection was also shown to decrease the steady-state ventilatory response to hypercapnia, independently of the degree of concomitant hypoxaemia (Lugliani et al. 1971; Bellville et al. 1979). In normoxia, carotid chemoreceptors were estimated to modulate 20-30 % of the ventilatory drive to hypercapnia. In our study, a 1 kPa rise in PCO2 induced an increase in ventilation of 6.4 ± 5.2 l min−1 in patients versus 9.2 ± 3.4 l min−1 in controls (means ± s.d.; n.s.). Taking into account the large standard deviation and small sample size, a blunted CO2 response due to loss of carotid body function may well be present. Evaluation of chemoreflex function in patients who had undergone radiation therapy for laryngeal or pharyngeal cancer showed no abnormalities (Timmers et al. 2002).
In contrast to baroreceptors, stimulation of peripheral chemoreceptors has a sympatho-excitatory effect in humans (Wallin & Fagius, 1988). Activation of peripheral chemoreceptors by hypoxaemia accounts for the strong increase in blood pressure and MSNA that is observed during prolonged voluntary apnoea in awake healthy humans (Fagius & Sundlof, 1986; van den Aardweg & Karemaker, 1992; Hardy et al. 1994). In patients lacking carotid bodies, breath-hold time was appreciably longer than in either healthy subjects or asthmatic controls (Davidson et al. 1974). Carotid bodies contribute to the sensation of breathlessness that results in the resumption of breathing. In these patients, hypoxia induced a decrease in blood pressure, which was ascribed to lack of chemoreceptor-dependent sympathetic activation (Wade et al. 1970; Lugliani et al. 1971). However, direct evidence from MSNA recordings during apnoea was lacking. Our preliminary microneurography studies in paraganglioma resected patients show that increases in MSNA during apnoea occur despite the absence of carotid bodies. Therefore, carotid chemoreceptors do not seem to be the sole determinant of sympatho-excitation during voluntary apnoea.
In summary, bilateral denervation or removal of carotid body chemoreceptors causes a permanent abolition of ventilatory responsiveness to hypoxia under normocapnic conditions. A small residual hypoxic response may be present during simultaneous hypercapnia. In addition, the condition causes a 20-30 % decrease in CO2 sensitivity. Long-term resting hypoventilation and hypercapnia may occur. The impairment of chemoreflex function is less severe following unilateral than after bilateral carotid body resection. These observations emphasize the importance of carotid relative to aortic chemoreceptor function in humans. The aortic bodies have a minor role in the modulation of spontaneous respiratory activity, but may generate a discernible response when their gain is amplified by hypercapnia. In comparison to other species, compensation for the loss of carotid body chemoreceptor function is limited in humans. The impact of this chronic loss of chemoreflex function on the control of blood gas and acid-base status in response to chemoreflex challenges like sleep, exercise (Whipp & Wasserman, 1980) and chronic hypoxia at high altitudes, needs further investigation in subjects that lack the possible confounder of pulmonary disease. Carotid paraganglioma resection offers a unique opportunity for research in the field of chemoreceptor physiology in humans.
Conclusions
Inadvertent denervation of carotid sinus baro- and chemoreceptors in humans may occur as a complication of invasive interventions on the neck like carotid body tumour surgery, radiotherapy and endarterectomy. Carotid baroreceptor denervation in humans causes a persistent decrease in baroreflex sensitivity and an increase in blood pressure variability; however, carotid denervation does not lead to chronic hypertension. Therefore, although carotid baroreceptors contribute to short-term blood pressure control, other receptors can maintain normal chronic blood pressure levels, in the absence of carotid baroreceptors. Conversely, carotid chemoreceptor denervation leads to permanent abolition of normocapnic ventilatory responses to hypoxia and reduced ventilatory responses to hypercapnia.
Acknowledgments
This project was supported by a grant from the Netherlands Heart Foundation (grant no. 97.069).
REFERENCES
- Angell-James JE. Arterial baroreceptor activity in rabbits with experimental atherosclerosis. Circ Res. 1974;40:27–39. doi: 10.1161/01.res.40.4.27. [DOI] [PubMed] [Google Scholar]
- Angell-James JE, Lumley JS. The effects of carotid endarterectomy on the mechanical properties of the carotid sinus and carotid sinus nerve activity in atherosclerotic patients. Br J Surg. 1974;61:805–810. doi: 10.1002/bjs.1800611014. [DOI] [PubMed] [Google Scholar]
- Bellville JW, Whipp BJ, Kaufman RD, Swanson GD, Aqleh KA, Wiberg DM. Central and peripheral chemoreflex loop gain in normal and carotid body- resected subjects. J Appl Physiol. 1979;46:843–853. doi: 10.1152/jappl.1979.46.4.843. [DOI] [PubMed] [Google Scholar]
- Biaggioni I, Whetsell WO, Jobe J, Nadeau JH. Baroreflex failure in a patient with central nervous system lesions involving the nucleus tractus solitarii. Hypertension. 1994;23:491–495. doi: 10.1161/01.hyp.23.4.491. [DOI] [PubMed] [Google Scholar]
- Bisgard GE, Forster HV, Klein JP. Recovery of peripheral chemoreceptor function after denervation in ponies. J Appl Physiol. 1980;49:964–970. doi: 10.1152/jappl.1980.49.6.964. [DOI] [PubMed] [Google Scholar]
- Bishop VS, Haywood JR, Shade RE, Siegel M, Hamm C. Aortic baroreceptor deafferentation in the baboon. J Appl Physiol. 1986;60:798–801. doi: 10.1152/jappl.1986.60.3.798. [DOI] [PubMed] [Google Scholar]
- Boyle JR, London NJ, Tan SG, Thurston H, Bell PR. Labile blood pressure after bilateral carotid body tumour surgery. Eur J Vasc Endovasc Surg. 1995;9:346–348. doi: 10.1016/s1078-5884(05)80142-x. [DOI] [PubMed] [Google Scholar]
- Bucy PC. The carotid sinus nerve in man. Arch Int Med. 1936;58:418–432. [Google Scholar]
- Cheng SW, Wu LL, Ting AC, Lau H, Lam LK, Wei WI. Irradiation-induced extracranial carotid stenosis in patients with head and neck malignancies. Am J Surg. 1999;178:323–328. doi: 10.1016/s0002-9610(99)00184-1. [DOI] [PubMed] [Google Scholar]
- Cheng VS, Schultz MD. Unilateral hypoglossal nerve atrophy as a late complication of radiation therapy of head and neck carcinoma: a report of four cases and a review of the literature on peripheral and cranial nerve damages after radiation therapy. Cancer. 1975;35:1537–1544. doi: 10.1002/1097-0142(197506)35:6<1537::aid-cncr2820350610>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Cowley AW, Jr, Liard JF, Guyton AC. Role of baroreceptor reflex in daily control of arterial blood pressure and other variables in dogs. Circ Res. 1973;32:564–576. doi: 10.1161/01.res.32.5.564. [DOI] [PubMed] [Google Scholar]
- Davidson JT, Whipp BJ, Wasserman K, Koyal SN, Lugliani R. Role of the carotid bodies in breath-holding. N Engl J Med. 1974;290:819–822. doi: 10.1056/NEJM197404112901502. [DOI] [PubMed] [Google Scholar]
- De Toma G, Nicolanti V, Plocco M, Cavallaro G, Letizia C, Piccirillo G, Cavallaro A. Baroreflex failure syndrome after bilateral excision of carotid body tumors: an underestimated problem. J Vasc Surg. 2000;31:806–810. doi: 10.1067/mva.2000.103789. [DOI] [PubMed] [Google Scholar]
- Dehn TC, Angell-James JE. Long-term effect of carotid endarterectomy on carotid sinus baroreceptor function and blood pressure control. Br J Surg. 1987;74:997–1000. doi: 10.1002/bjs.1800741113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL, Sleight P. Human Baroreflexes in Health and Disease. Oxford: Clarendon Press; 1992. Hypertension; pp. 327–345. [Google Scholar]
- Ejaz AA, Meschia JF. Thalamic hemorrhage following carotid endarterectomy-induced labile blood pressure: controlling the liability with clonidine - a case report. Angiology. 1999;50:327–330. doi: 10.1177/000331979905000409. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Stromstad M, Wray DW, Smith SA, Raven PB, Secher NH. New insights into differential baroreflex control of heart rate in humans. Am J Physiol Heart Circ Physiol. 2003;284:H735–743. doi: 10.1152/ajpheart.00246.2002. [DOI] [PubMed] [Google Scholar]
- Fagius J, Sundlof G. The diving response in man: effects on sympathetic activity in muscle and skin nerve fascicles. J Physiol. 1986;377:429–443. doi: 10.1113/jphysiol.1986.sp016196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagius J, Wallin BG, Sundlof G, Nerhed C, Englesson S. Sympathetic outflow in man after anaesthesia of the glossopharyngeal and vagus nerves. Brain. 1985;108:423–438. doi: 10.1093/brain/108.2.423. [DOI] [PubMed] [Google Scholar]
- Felder RB, Mifflin SB. Baroreceptor and chemoreceptor afferent processing in the solitary tract nucleus. In: Barraco IRA, editor. Nucleus of the Solitary Tract. Boca Raton: CRC Press; 2003. pp. 169–185. [Google Scholar]
- Ferguson DW, Abboud FM, Mark AL. Relative contribution of aortic and carotid baroreflexes to heart rate control in man during steady state and dynamic increases in arterial pressure. J Clin Invest. 1985;76:2265–2274. doi: 10.1172/JCI112236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford FR. Fatal hypertensive crisis following denervation of the carotid sinus for the relief of repeated attacks of syncope. John Hopkins Med J. 1956;100:14–16. [PubMed] [Google Scholar]
- Forster HV, Pan LG, Lowry TF, Serra A, Wenninger J, Martino P. Important role of carotid chemoreceptor afferents in control of breathing of adult and neonatal mammals. Respir Physiol. 2000;119:199–208. doi: 10.1016/s0034-5687(99)00115-2. [DOI] [PubMed] [Google Scholar]
- Godfrey S, Edwards RH T, Copland GM, Gross PL. Chemosensitivity in normal subjects, athletes, and patients with chronic airways obstruction. J Appl Physiol. 1971;30:193–199. [Google Scholar]
- Goldstein DS, Horwitz D, Keiser HR. Comparison of techniques for measuring baroreflex sensitivity in man. Circulation. 1982;66:432–439. doi: 10.1161/01.cir.66.2.432. [DOI] [PubMed] [Google Scholar]
- Guz A, Noble MI, Widdicombe JG, Trenchard D, Mushin WW. Peripheral chemoreceptor block in man. Respir Physiol. 1966a;1:38–40. doi: 10.1016/0034-5687(66)90027-2. [DOI] [PubMed] [Google Scholar]
- Guz A, Noble MI, Widdicombe JG, Trenchard D, Mushin WW, Makey AR. The role of vagal and glossopharyngeal afferent nerves in respiratory sensation, control of breathing and arterial pressure regulation in conscious man. Clin Sci. 1966b;30:161–170. [PubMed] [Google Scholar]
- Hainsworth R. Reflexes from the heart. Physiol Rev. 1991;71:617–658. doi: 10.1152/physrev.1991.71.3.617. [DOI] [PubMed] [Google Scholar]
- Hardy JC, Gray K, Whisler S, Leuenberger U. Sympathetic and blood pressure responses to voluntary apnea are augmented by hypoxemia. J Appl Physiol. 1994;77:2360–2365. doi: 10.1152/jappl.1994.77.5.2360. [DOI] [PubMed] [Google Scholar]
- Hering HE. Die Karotissinusreflexe auf Herz und Gefasse. Dresden: Steinkopff; 1927. [Google Scholar]
- Heymans C, Bouckhaert JJ, Dautrebande L. Sinus carotidien et reflexes respiratoires II. Influences respiratoires reflexes de l'acidose, de l'alcalose, de l'anhydride carbonique, de l'ion hydrogene et de l'anoxeme. Sinus carotidiens et echanges respiratores dans les poumons et au dela des poumons. Arch Intern Pharmacodyn. 1930;39:400–448. [Google Scholar]
- Hilz MJ, Dutsch M, Perrine K, Nelson PK, Rauhut U, Devinsky O. Hemispheric influence on autonomic modulation and baroreflex sensitivity. Ann Neurol. 2001;49:575–584. [PubMed] [Google Scholar]
- Hirschl M, Hirschl MM, Magometschnigg D, Liebisch B, Wagner O, Fux B, Kundi M. Arterial baroreflex sensitivity and blood pressure variabilities before and after carotid surgery. Klin Wochenschr. 1991;69:763–768. doi: 10.1007/BF01797615. [DOI] [PubMed] [Google Scholar]
- Holton P, Wood JB. The effects of bilateral removal of the carotid bodies and denervation of the carotid sinuses in two human subjects. J Physiol. 1965;181:365–378. doi: 10.1113/jphysiol.1965.sp007767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y, Watanabe S, Hashizume I, Satomura Y, Hata N, Sakakibara Y, Severinghaus JW. Hypoxic chemosensitivity in asthmatic patients two decades after carotid body resection. J Appl Physiol. 1979;46:632–638. doi: 10.1152/jappl.1979.46.4.632. [DOI] [PubMed] [Google Scholar]
- Ille O, Woimant F, Pruna A, Corabianu O, Idatte JM, Haguenau M. Hypertensive encephalopathy after bilateral carotid endarterectomy. Stroke. 1995;26:488–491. doi: 10.1161/01.str.26.3.488. [DOI] [PubMed] [Google Scholar]
- Ito CS, Scher AM. Regulation of arterial blood pressure by aortic baroreceptors in the unanesthetized dog. Circ Res. 1978;42:230–236. doi: 10.1161/01.res.42.2.230. [DOI] [PubMed] [Google Scholar]
- Ito CS, Scher AM. Hypertension following arterial baroreceptor denervation in the unanesthetized dog. Circ Res. 1981;48:576–591. doi: 10.1161/01.res.48.4.576. [DOI] [PubMed] [Google Scholar]
- Jordan J, Shannon JR, Black BK, Costa F, Ertl AC, Furlan R, Biaggioni I, Robertson D. Malignant vagotonia due to selective baroreflex failure. Hypertension. 1997;30:1072–1077. doi: 10.1161/01.hyp.30.5.1072. [DOI] [PubMed] [Google Scholar]
- Ketch T, Biaggioni I, Robertson R, Robertson D. Four faces of baroreflex failure: hypertensive crisis, volatile hypertension, orthostatic tachycardia, and malignant vagotonia. Circulation. 2002;105:2518–2523. doi: 10.1161/01.cir.0000017186.52382.f4. [DOI] [PubMed] [Google Scholar]
- Koch E. Die reflektorische Selbststeuerung des Kreislaufs. Dresden: Steinkopff; 1931. [Google Scholar]
- Kochar MS, Ebert TJ, Kotrly KJ. Primary dysfunction of the afferent limb of the arterial baroreceptor reflex system in a patient with severe supine hypertension and orthostatic hypotension. J Am Coll Cardiol. 1984;4:802–805. doi: 10.1016/s0735-1097(84)80409-x. [DOI] [PubMed] [Google Scholar]
- Lampen H, Kezdi P, Koppermann E, Kaufmann L. Experimenteller Entzugelungshochdruck bei arterieller Hypertonie. Zeitschrift fur Kreislaufforschung. 1949;38:577–592. [Google Scholar]
- Landesberg G, Adam D, Berlatzky Y, Akselrod S. Step baroreflex response in awake patients undergoing carotid surgery: time- and frequency-domain analysis. Am J Physiol. 1998;274:H1590–1597. doi: 10.1152/ajpheart.1998.274.5.H1590. [DOI] [PubMed] [Google Scholar]
- Lenard Z, Studinger P, Kovats Z, Reneman R, Kollai M. Comparison of aortic arch and carotid sinus distensibility in humans-relation to baroreflex sensitivity. Auton Neurosci. 2001;92:92–99. doi: 10.1016/S1566-0702(01)00309-5. [DOI] [PubMed] [Google Scholar]
- Lugliani R, Whipp BJ, Seard C, Wasserman K. Effect of bilateral carotid-body resection on ventilatory control at rest and during exercise in man. N Engl J Med. 1971;285:1105–1111. doi: 10.1056/NEJM197111112852002. [DOI] [PubMed] [Google Scholar]
- Mancia G, Ferrari A, Gregorini L, Valentini R, Ludbrook J, Zanchetti A. Circulatory reflexes from carotid and extracarotid baroreceptor areas in man. Circ Res. 1977;41:309–315. doi: 10.1161/01.res.41.3.309. [DOI] [PubMed] [Google Scholar]
- Mancia G, Parati G, Pomidossi G, Casadei R, Di Rienzo M, Zanchetti A. Arterial baroreflexes and blood pressure and heart rate variabilities in humans. Hypertension. 1986;8:147–153. doi: 10.1161/01.hyp.8.2.147. [DOI] [PubMed] [Google Scholar]
- Martin-Body RL, Robson GJ, Sinclair JD. Restoration of hypoxic respiratory responses in the awake rat after carotid body denervation by sinus nerve section. J Physiol. 1986;380:61–73. doi: 10.1113/jphysiol.1986.sp016272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RA, Sinha AK, Mcdonald DM. Chemoreceptive properties of regenerated endings of the carotid sinus nerve. Brain Res. 1972;43:681–685. doi: 10.1016/0006-8993(72)90430-1. [DOI] [PubMed] [Google Scholar]
- O'Regan RG, Majcherczyk S. Role of peripheral chemoreceptors and central chemosensitivity in the regulation of respiration and circulation. J Exp Biol. 1982;100:23–40. doi: 10.1242/jeb.100.1.23. [DOI] [PubMed] [Google Scholar]
- Pan LG, Forster HV, Martino P, Strecker PJ, Beales J, Serra A, Lowry TF, Forster MM, Forster AL. Important role of carotid afferents in control of breathing. J Appl Physiol. 1998;85:1299–1306. doi: 10.1152/jappl.1998.85.4.1299. [DOI] [PubMed] [Google Scholar]
- Persson PB. Modulation of cardiovascular control mechanisms and their interaction. Physiol Rev. 1996;76:193–244. doi: 10.1152/physrev.1996.76.1.193. [DOI] [PubMed] [Google Scholar]
- Persson P, Ehmke H, Kirchheim H, Seller H. Effect of sino-aortic denervation in comparison to cardiopulmonary deafferentiation on long-term blood pressure in conscious dogs. Pflugers Arch. 1988;411:160–166. doi: 10.1007/BF00582309. [DOI] [PubMed] [Google Scholar]
- Persson PB, Kirchheim HR. In: Baroreceptor Reflexes: Integrative Functions and Clinical Aspects. Persson PB, Kirchheim HR, editors. Berlin: Springer-Verlag; 1991. [Google Scholar]
- Prabhakar NR. Oxygen sensing by the carotid body chemoreceptors. J Appl Physiol. 2000;88:2287–2295. doi: 10.1152/jappl.2000.88.6.2287. [DOI] [PubMed] [Google Scholar]
- Randall OS, Esler MD, Bulloch EG, Maisel AS, Ellis CN, Zweifler AJ, Julius S. Relationship of age and blood pressure to baroreflex sensitivity and arterial compliance in man. Clin Sci Mol Med. 1976;(suppl 3):357–360s. doi: 10.1042/cs051357s. [DOI] [PubMed] [Google Scholar]
- Robertson D, Goldberg MR, Hollister AS, Wade D, Robertson RM. Clonidine raises blood pressure in severe idiopathic orthostatic hypotension. Am J Med. 1983;74:193–200. doi: 10.1016/0002-9343(83)90607-1. [DOI] [PubMed] [Google Scholar]
- Robertson D, Hollister AS, Biaggioni I, Netterville JL, Mosqueda Garcia R, Robertson RM. The diagnosis and treatment of baroreflex failure. N Engl J Med. 1993;329:1449–1455. doi: 10.1056/NEJM199311113292003. [DOI] [PubMed] [Google Scholar]
- Rodman JR, Curran AK, Henderson KS, Dempsey JA, Smith CA. Carotid body denervation in dogs: eupnea and the ventilatory response to hyperoxic hypercapnia. J Appl Physiol. 2001;91:328–335. doi: 10.1152/jappl.2001.91.1.328. [DOI] [PubMed] [Google Scholar]
- Shade RE, Bishop VS, Haywood JR, Hamm CK. Cardiovascular and neuroendocrine responses to baroreceptor denervation in baboons. Am J Physiol. 1990;258:R930–938. doi: 10.1152/ajpregu.1990.258.4.R930. [DOI] [PubMed] [Google Scholar]
- Shade RE, Haywood JR, Bishop VS. Effects of arterial baroreceptor denervation on long-term regulation of arterial blood pressure. In: Persson CG, editor. Baroreceptor Reflexes. Integrative Functions and Clinical Aspects. Berlin: Springer Verlag; 1991. pp. 209–225. [Google Scholar]
- Sleight P. Neurophysiology of the carotid sinus receptors in normal and hypertensive animals and man. Cardiology. 1976;61(suppl. 1):31–45. doi: 10.1159/000169790. [DOI] [PubMed] [Google Scholar]
- Smit AJ, Timmers HJ L M, Wieling W, Wagenaar M, Marres HAM, Lenders JW, van Montfrans GA, Karemaker JM. Long-term effects of carotid sinus denervation on arterial blood pressure in humans. Circulation. 2002;105:1329–1335. doi: 10.1161/hc1102.105744. [DOI] [PubMed] [Google Scholar]
- Smith PG, Mills E. Restoration of reflex ventilatory response to hypoxia after removal of carotid bodies in the cat. Neuroscience. 1980;5:573–580. doi: 10.1016/0306-4522(80)90054-8. [DOI] [PubMed] [Google Scholar]
- Smyth HS, Phil D, Sleight P, Pickering GW. Reflex regulation of arterial pressure during sleep in man. Circ Res. 1969;24:109–121. doi: 10.1161/01.res.24.1.109. [DOI] [PubMed] [Google Scholar]
- Swanson GD, Whipp BJ, Kaufman RD, Aqleh KA, Winter B, Bellville JW. Effect of hypercapnia on hypoxic ventilatory drive in carotid body-resected man. J Appl Physiol. 1978;45:871–877. doi: 10.1152/jappl.1978.45.6.971. [DOI] [PubMed] [Google Scholar]
- Timmers HJ, Karemaker JM, Lenders JW, Wieling W. Baroreflex failure following radiation therapy for nasopharyngeal carcinoma. Clin Auton Res. 1999;9:317–324. doi: 10.1007/BF02318378. [DOI] [PubMed] [Google Scholar]
- Timmers HJ, Karemaker JM, Wieling W, Buskens FG, Lenders JW. Arterial barroreflex function after unilateral carotid endarterectomy. Clin Auton Res. 2001a;11:188–189. doi: 10.1007/s10286-004-0165-3. abstract. [DOI] [PubMed] [Google Scholar]
- Timmers HJ, Karemaker JM, Wieling W, Kaanders JHAM, Folgering HThM, Marres HA, Lenders JW. Arterial baroreflex and peripheral chemoreflex function after radiotherapy for laryngeal or pharyngeal cancer. Int J Radiat Oncol Biol Phys. 2002;53:1203–1210. doi: 10.1016/s0360-3016(02)02827-4. [DOI] [PubMed] [Google Scholar]
- Timmers HJ LM, Karemaker JM, Wieling W, Marres HA, Folgering HThM, Lenders JW. Baroreflex and chemoreflex function after bilateral carotid body tumor resection. J Hypertens. 2003a;21:591–599. doi: 10.1097/00004872-200303000-00026. [DOI] [PubMed] [Google Scholar]
- Timmers HJ, Karemaker JM, Wieling W, Marres HA, Lenders JW. Baroreflex control of muscle sympathetic nerve activity following carotid body tumor resection. Hypertension. 2003b;42:143–149. doi: 10.1161/01.HYP.0000080495.07301.31. [DOI] [PubMed] [Google Scholar]
- Timmers HJ, Wieling W, Karemaker JM, Marres HA, Lenders JW. Labiele hypertensie door iatrogene denervatie van de sinus caroticus. [Labile hypertension due to iatrogenic denervation of the carotid sinus] Ned Tijdschr Geneeskd. 2001b;145:1413–1416. [PubMed] [Google Scholar]
- Tyden G, Samnegard H, Thulin L, Muhrbeck O. Effect of carotid endarterectomy on baroreflex sensitivity in man. Intraoperative studies. Acta Chir Scand Suppl. 1980;500:67–69. [PubMed] [Google Scholar]
- van den Aardweg JG, Karemaker JM. Repetitive apneas induce periodic hypertension in normal subjects through hypoxia. J Appl Physiol. 1992;72:821–827. doi: 10.1152/jappl.1992.72.3.821. [DOI] [PubMed] [Google Scholar]
- Vermeire P, de Backer W, van Maele R, Bal J, van Kerckhoven W. Carotid body resection in patients with severe chronic airflow limitation. Bull Eur Physiopathol Respir. 1987;23(suppl 11):165–166. [PubMed] [Google Scholar]
- Wade JG, Larson CP, Jr, Hickey RF, Ehrenfeld WK, Severinghaus JW. Effect of carotid endarterectomy on carotid chemoreceptor and baroreceptor function in man. N Engl J Med. 1970;282:823–829. doi: 10.1056/NEJM197004092821501. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Fagius J. Peripheral sympathetic neural activity in conscious humans. Annu Rev Physiol. 1988;50:565–576. doi: 10.1146/annurev.ph.50.030188.003025. [DOI] [PubMed] [Google Scholar]
- Wasserman K, Whipp BJ, Koyal SN, Cleary MG. Effect of carotid body resection on ventilatory and acid-base control during exercise. J Appl Physiol. 1975;39:354–358. doi: 10.1152/jappl.1975.39.3.354. [DOI] [PubMed] [Google Scholar]
- Whipp BJ. Carotid bodies and breathing in humans [editorial] Thorax. 1994;49:1081–1084. doi: 10.1136/thx.49.11.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipp BJ, Ward SA. Physiologic changes following bilateral carotid-body resection in patients with chronic obstructive pulmonary disease. Chest. 1992;101:656–661. doi: 10.1378/chest.101.3.656. [DOI] [PubMed] [Google Scholar]
- Whipp BJ, Wasserman K. Carotid bodies and ventilatory control dynamics in man. Fed Proc. 1980;39:2668–2673. [PubMed] [Google Scholar]
- Wieling W, Karemaker JM. Measurement of heart rate and blood pressure to evaluate disturbances in neurocardiovascular control. In: Mathias CJ, Bannister R, editors. Autonomic Failure, a Textbook of Clinical Disorders of the Autonomic Nervous System. Oxford: Oxford University Press; 1999. pp. 196–210. [Google Scholar]
- Zidar N, Ferluga D, Hvala A, Popovic M, Soba E. Contribution to the pathogenesis of radiation-induced injury to large arteries. J Laryngol Otol. 1997;111:988–990. doi: 10.1017/s0022215100139167. [DOI] [PubMed] [Google Scholar]


