Abstract
The adapter protein paxillin has been implicated in the regulation of cytoskeletal organization and cell motility. Paxillin undergoes tyrosine phosphorylation in response to the contractile stimulation of smooth muscle, and the depletion of paxillin by antisense inhibits smooth muscle contraction. In the present study, acetylcholine (ACh)-stimulation of tracheal smooth muscle tissues increased paxillin phosphorylation at tyr-31 and tyr-118 by three- to fourfold. The role of tyr-31 and tyr-118 phosphorylation of paxillin in smooth muscle was evaluated by introducing plasmids encoding wild type paxillin or paxillin mutants F31, F118 or F31/118 (phenylalanine substitution at tyrosine sites 31, 118) into tracheal smooth muscle strips by reversible permeabilization, and incubating the tissues for 2 days. The expression of recombinant proteins was confirmed by immunoblot and immunofluorescence analysis. Expression of the paxillin mutants F31, F118 or F31/118 inhibited the contractile response to ACh stimulation but did not inhibit the increase in myosin light chain phosphorylation. The expression of wild type paxillin had no significant affect on force or myosin light chain phosphorylation. ACh stimulation reduced G-actin/F-actin ratio in tissues expressing wild type paxillin; whereas the agonist-induced decrease in G-actin/F-actin was inhibited in strips expressing paxillin mutant F31/118. The paxillin mutant F31/118 showed a marked decrease in their interaction with the SH2/SH3 adaptor protein CrkII but not with vinculin or focal adhesion kinase. We conclude that paxillin phosphorylation at tyr-31 and tyr-118 regulates active tension development during contractile stimulation. Paxillin phosphorylation at these two sites may be important in regulating actin filament dynamics and organization during smooth muscle contraction.
Paxillin is a 68 kDa multidomain adapter protein that undergoes tyrosine phosphorylation in many cell types in response to extracellular stimuli. In cultured cells, the tyrosine phosphorylation of paxillin has been associated with the regulation of cytoskeletal organization, focal adhesion formation, cell migration and cell motility (Burridge & Chrzanowska-Wodnicka, 1996; Turner, 2000; Petit et al. 2000; Schaller, 2001).
The contractile activation of tracheal smooth muscle induces the tyrosine phosphorylation of paxillin concurrently with tension development (Wang et al. 1996; Tang et al. 1999; Tang & Gunst, 2001). In a previous study, we found that the depletion of paxillin by antisense oligonucleotides inhibits tension development during tracheal smooth muscle contraction without affecting intracellular Ca2+, myosin light chain phosphorylation or myosin ATPase activity, but that it alters normal actin dynamics (Tang et al. 2002). These observations demonstrate that the presence of paxillin is necessary for tension development, but that paxillin does not regulate contractile protein activation or crossbridge cycling. However, it is not known whether paxillin tyrosine phosphorylation plays a role in the regulation of smooth muscle contraction or what molecular functions of paxillin might be regulated by contractile stimulation.
The N-terminus of paxillin contains two major sites for tyrosine phosphorylation at residues 31 and 118. The phosphorylation of these sites is regulated by the focal adhesion kinase (FAK) and the FAK-related kinase, Ca2+-dependent tyrosine kinase (CADTK) also known as CAKβ, Pyk2 and RAFTK (Bellis et al. 1995; Li & Earp, 1997; Turner, 2000; Schaller, 2001). FAK also undergoes tyrosine phosphorylation during the contractile stimulation of tracheal smooth muscle (Tang et al. 1999). Both FAK and CADTK phosphorylate paxillin in vitro; however, under physiological conditions Src family kinases may bind activated FAK to catalyse the phosphorylation of paxillin at tyr-31 and tyr-118 sites (Schaller et al. 1999; Turner, 2000; Schaller, 2001). The expression of paxillin mutants in which tyr-31 and/or tyr-118 are replaced by phenylalanine results in changes in focal adhesion formation and in the organization of the actin cytoskeleton of epithelial cells (Nakamura et al. 2000), and inhibits the motility of rat bladder carcinoma cells (Petit et al. 2000). Paxillin undergoes phosphorylation on residues tyr-31 and tyr-118 during cell migration; the phosphorylation of paxillin on these two sites increases the affinity of paxillin for the SH2 domain of the adapter protein CrkII (Petit et al. 2000).
FAK binds to the N-terminus of paxillin at conserved leucine-rich sequences LD2 and LD4 (Brown et al. 1998; Turner et al. 1999; Turner, 2000); the binding of FAK to LD motifs may be important in regulating paxillin phosphorylation by FAK or other protein tyrosine kinases (Thomas et al. 1999). Vinculin also binds to N-terminal leucine rich sequences LD1, LD2 and LD4 on paxillin (Brown et al. 1996; Turner, 2000; Tumbarello et al. 2002). Vinculin is recruited to the focal adhesions of migrating cells where it may provide structural support for linkages formed between F-actin and integrin proteins (Goldmann et al. 1998).
The objective of the present study was to determine whether paxillin phosphorylation at tyr-31 and tyr-118 plays a regulatory role in smooth muscle contraction, and to evaluate the functional effects of paxillin tyrosine phosphorylation on molecular interactions that may regulate cytoskeletal functions during smooth muscle contraction. cDNA constructs encoding non-phosphorylatable paxillin proteins with phenylalanine substitutions for tyrosine at residues 31, 118 or both tyrosine 31 and 118 were expressed in smooth muscle tissues. Our results show that the expression of non-phosphorylatable paxillin mutants in tracheal smooth muscle inhibits tension generation in response to muscarinic stimulation without affecting myosin light chain phosphorylation, and that the expression of paxillin mutants also inhibits the polymerization of actin during contractile stimulation. The non-phosphorylatable paxillin mutants also show a reduced association with CrkII, but not with vinculin or FAK. These studies suggest that paxillin phosphorylation at tyr-31 and tyr-118 is critical for the regulation of tension generation in smooth muscle, and that this phosphorylation also regulates actin dynamics, perhaps through signalling pathways mediated by the adapter protein, CrkII.
METHODS
Preparation of smooth muscle tissue
Mongrel dogs (20-25 kg) were anaesthetized with pentobarbital sodium (30 mg kg−1, i.v.) and quickly killed via exsanguination. All experiments were carried out according to the guidelines of Institutional Animal Care and Use Committee, Indiana University School of Medicine. A 12-15 cm segment of extrathoracic trachea was immediately removed and immersed in physiological saline solution (PSS) at 22 °C (composition, mm: 110 NaCl, 3.4 KCl, 2.4 CaCl2, 0.8 MgSO4, 25.8 NaHCO3, 1.2 KH2PO4, and 5.6 glucose). The solution was aerated with 95 %O2-5 %CO2 to maintain a pH of 7.4. Rectangular strips of tracheal muscle 0.6-0.7 mm in diameter and 8-10 mm in length were dissected from the trachea after removal of the epithelium and connective tissue layer. The use of an appropriately sized strip was critical for maintaining muscle contractility during the incubation period and for the successful introduction of plasmids throughout the muscle strip. Each muscle strip was placed in PSS at 37 °C in a 25 ml organ bath and attached to a Grass force transducer. At the beginning of each experiment, the optimal length for muscle contraction was determined by increasing muscle length progressively until the force of active contraction in response to a contractile stimulus reached a maximum.
Plasmids encoding recombinant paxillin proteins were introduced into muscle strips according to experimental procedures described below. Muscle strips were then incubated for 2 days with plasmids in serum-free Dulbecco's modified Eagle's medium (DMEM). The strips were then returned to PSS at 37 °C in 25 ml organ baths and attached to Grass force transducers for the measurement of isometric force. For biochemical analysis, muscle strips were frozen using liquid N2-cooled tongs, and then pulverized under liquid N2 using a mortar and pestle.
Introduction of plasmids encoding recombinant paxillin into tracheal smooth muscle tissues
Site-directed mutagenesis of recombinant chicken paxillin has been previously described (Bellis et al. 1995; Petit et al. 2000). The cDNAs encoding wild type chicken paxillin and the paxillin mutants F31, F118 and F31/118 were subcloned into the mammalian expression vector pcDNA 3.1 (Invitrogen). Escherichia coli (Bluescript) transformed with these plasmids was grown in LB medium and plasmids were purified by alkaline lysis with SDS (maxipreparation) or by a kit from Invitrogen, Carlsbad, CA, USA (S.N.A.P. no. K1910-01).
Plasmids carrying wild type or mutant paxillin were introduced into the smooth muscle strips by reversible permeabilization (Tang & Gunst, 2001; Tang et al. 2002). After determination of the optimal length, muscle strips were attached to metal mounts at the appropriate length. The strips were placed in 0.5 ml tubes and incubated successively in each of the following solutions: solution 1 (at 4 °C for 120 min) containing 10 mm EGTA, 5 mm Na2ATP, 120 mm KCl, 2 mm MgCl2, and 20 mm Tes; solution 2 (at 4 °C overnight) containing 0.1 mm EGTA, 5 mm Na2ATP, 120 mm KCl, 2 mm MgCl2, 20 mm Tes and 10 µg ml−1 plasmids; solution 3 (at 4 °C for 30 min) containing 0.1 mm EGTA, 5 mm Na2ATP, 120 mm KCl, 10 mm MgCl2, 20 mm Tes; and solution 4 (at 22 °C for 60 min) containing 110 mm NaCl, 3.4 mm KCl, 0.8 mm MgSO4, 25.8 mm NaHCO3, 1.2 mm KH2PO4, and 5.6 mm dextrose. Solutions 1-3 were maintained at pH 7.1 and aerated with 100 % O2. Solution 4 was maintained at pH 7.4 and was aerated with 95 %O2-5 %CO2. After 30 min in solution 4, CaCl2 was added gradually to reach a final concentration of 2.4 mm. The strips were then incubated in a CO2 incubator at 37 °C for 2 days in DMEM containing 5 mm Na2ATP, 100 U ml−1 penicillin, 100 µg ml−1 streptomycin and 10 µg ml−1 plasmids (to compensate for the degradation of the DNA molecules by nucleases in cells) (Fisher et al. 1993; Wagner, 1994). DNA molecules can be taken up by endocytosis, maintaining appropriate DNA levels in cells (Wagner, 1994; Smyth et al. 1997). In preliminary experiments we found that the addition of plasmids to the DMEM incubation medium enhanced the expression of recombinant paxillin.
To determine the efficiency of tissue transfection, cells were dissociated from smooth muscle tissues transfected with wild type or mutant paxillin and were immunostained with anti-chicken paxillin antibody, which reacts selectively with recombinant chicken paxillin (Bellis et al. 1995; Petit et al. 2000). Tracheal muscle strips were minced and digested in a solution with the following composition (mm): 130 NaCl, 5 KCl, 1.0 CaCl2, 1.0 MgCl2, 10 Hepes, 0.25 EDTA, 10 d-glucose, 10 taurine, collagenase (400 U ml−1, type I), papain (30 U ml−1, type IV), bovine serum albumin (1 mg ml−1) and DTT (1 mm) at pH 7. The strips were triturated with a pipette to liberate individual smooth muscle cells from the tissue. The solution containing the dissociated cells was poured over glass coverslips and allowed to stand for 2 h to allow the cells to adhere to the coverslips. Cells were fixed in 4 % paraformaldehyde (v/v) for 10 min. Cells were incubated with anti-chicken paxillin antibody followed by secondary antibody conjugated to Alexa 488 fluoroprobe (Molecular Probe, Eugene, OR, USA).
Fields of dissociated cells on glass coverslips were viewed under low power using a ×10 objective with a Zeiss LSM 510 laser scanning confocal microscope. Alexa 488-labelled paxillin (green) was excited with a 488 nm argon laser light and fluorescence emissions were collected at 500-550 nm. The same field of cells was also observed using transmitted light to obtain phase-contrast images for each field. The transfection efficiency was computed as the ratio of the number of positively immunostaining cells (green fluorescence) versus total cells (observed in phase contrast images) (Fig. 3).
Figure 3. Efficiency of tissue transfection in canine tracheal smooth muscle.
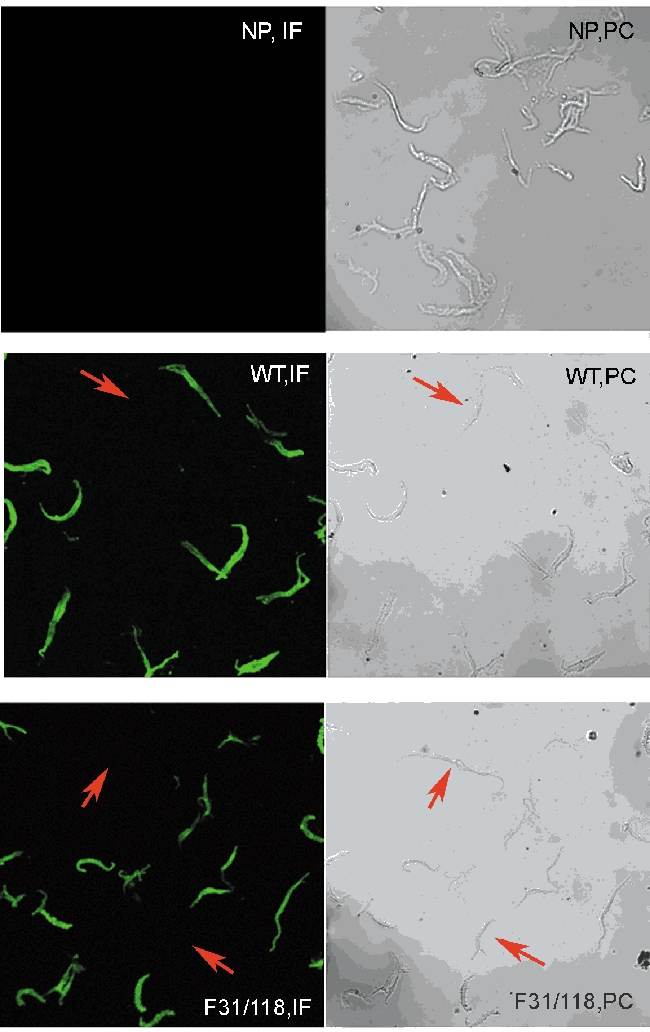
Cells freshly dissociated from muscle strips that were not treated with plasmids (NP), or that were treated with plasmids encoding wild type paxillin (WT) or F31/118 paxillin mutant (F31/118) were immunostained with anti-chicken paxillin antibody followed by secondary antibody conjugated with Alexa 488 fluoroprobe. Cells were examined under a confocal microscope to detect paxillin. Immunofluorescence (IF) images are shown on left panels; phase contrast (PC) images, right panels. The red arrows point to cells not expressing recombinant paxillin.
Analysis of protein expression
The expression of recombinant proteins and endogenous paxillin proteins were evaluated by immunoblot analysis of immunoprecipitates and homogenates from plasmid-treated muscle strips (Fig. 2A). Pulverized muscle strips were mixed with extraction buffer containing: 20 mm Tris-HCl at pH 7.4, 2 % Triton X-100, 0.2 % SDS, 2 mm EDTA, phosphatase inhibitors (2 mm sodium orthovanadate, 2 mm molybdate and 2 mm sodium pyrophosphate) and protease inhibitors (2 mm benzamidine, 0.5 mm aprotinin and 1 mm phenylmethylsulfonyl fluoride). Each sample was centrifuged for the collection of supernatant. Muscle extracts containing equal amounts of protein were precleared for 30 min with 50 µl of 10 % protein A-Sepharose (Sigma) to remove cellular proteins that associate non-specifically with protein A. The precleared extracts were centrifuged at 16 000 g for 2 min. The supernatant was then incubated with anti-chicken paxillin antibody for 90 min at 4 °C followed by the addition of 10 % protein A-Sepharose to specifically immunoprecipitate recombinant chicken paxillin. To evaluate the expression of endogenous paxillin, the supernatants of extracts that had been incubated with anti-chicken paxillin antibody were incubated with monoclonal paxillin antibody (clone 349, BD Biosciences, San Diego, CA, USA) overnight at 4 °C and then incubated with 10 % protein A-Sepharose conjugated to rabbit anti-mouse IgG for 2 h. Immunocomplexes from both the first and second immunoprecipitations were washed separately four times in Tris-buffered saline containing 0.1 % Triton X-100. The immunoprecipitates were then boiled in sample buffer (1.5 % dithiothreitol, 2 % SDS, 80 mm Tris-HCl (pH 6.8), 10 % glycerol and 0.01 % bromophenol blue) for 5 min and separated by SDS polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to nitrocellulose, after which the nitrocellulose membrane was blocked with 5 % milk for 1 h and probed with monoclonal antibody to paxillin (clone 349, BD Biosciences) followed by horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin (Ig) (Amersham Life Sciences, Arlington Heights, IL, USA). Proteins were visualized by enhanced chemiluminescence (ECL). In separate experiments, total paxillin including both recombinant and endogenous paxillin was measured in tissue homogenates by immunoblot analysis (Fig. 2A).
Figure 2. Expression of wild type and mutant paxillin proteins in smooth muscle tissues.
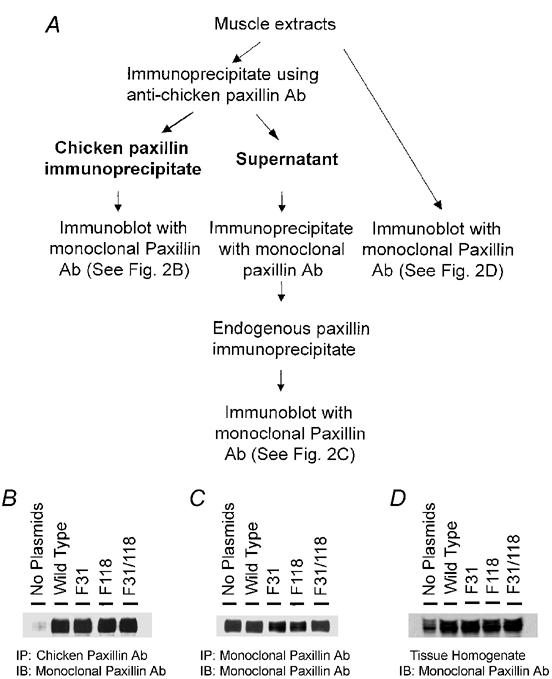
A, protocol for evaluating expression of recombinant chicken paxillin and endogenous paxillin in muscle strips. Extracts of smooth muscle strips that had been treated with plasmids encoding wild type paxillin or paxillin mutants F31, F118 or F31/118 were incubated with anti-chicken paxillin antibody to immunoprecipitate recombinant paxillin. The supernatants were then incubated with monoclonal paxillin antibody to immunoprecipitate endogenous paxillin. Immunoblots of immunoprecipitates of recombinant chicken paxillin and endogenous paxillin were blotted with monoclonal paxillin antibody. In separate experiments, homogenates of muscle strips were blotted with monoclonal paxillin antibody to evaluate the combined expression of endogenous and recombinant paxillin. B, wild type paxillin and paxillin mutants were expressed in smooth muscle tissues. The blot is representative of three experiments. C, representative immunoblot of three experiments shows that endogenous paxillin expression was not significantly different in untransfected tissues (no plasmids) and muscle strips transfected with plasmids encoding for wild type and mutant paxillin proteins. D, representative immunoblot shows that the expression of combined endogenous and recombinant paxillin in transfected tissues is higher than that in untransfected muscle strips (n = 4).
Assessment of paxillin phosphorylation at tyr-31 and tyr-118
Proteins from muscle extracts or paxillin immunoprecipitates (see above) were separated by SDS polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to nitrocellulose, after which the nitrocellulose membrane was blotted with phosphorylation site-specific, state-dependent antibodies for paxillin-tyr-31 or paxillin-tyr-118 (Biosource, Camarillo, CA, USA), stripped and reprobed with monoclonal paxillin antibody to normalize for minor differences of protein loading.
Analysis of myosin light chain phosphorylation
Muscle strips were rapidly frozen at desired time points after contractile stimulation and then immersed in acetone containing 10 % (w/v) trichloroacetic acid and 10 mm DTT (acetone- TCA- DTT) that was precooled with dry ice. Strips were thawed in acetone-TCA-DTT at room temperature and then washed four times with acetone-DTT. Proteins were extracted for 60 min in 8 m urea, 20 mm Tris base, 22 mm glycine and 10 mm DTT. Myosin light chains (MLCs) were separated by glycerol-urea polyacrylamide gel electrophoresis and transferred to nitrocellulose. The membranes were blocked with 5 % bovine serum albumin and incubated with polyclonal affinity-purified rabbit myosin light chain 20 antibody. The primary antibody was reacted with 125I-labelled recombinant protein A (New England Nuclear). Non-phosphorylated and phosphorylated bands of MLCs were detected by autoradiography. Bands were cut out and counted in a gamma counter. Background counts were subtracted, and MLC phosphorylation was calculated as the ratio of phosphorylated MLCs to total MLCs.
Analysis of G-actin/F-actin ratio
The concentrations of G-actin and F-actin in smooth muscle tissues were measured using an assay kit from Cytoskeleton Inc., Denver, Colorado, USA as previously described (Yassin et al. 1985; Rao et al. 1985; Hartwig, 1992). Briefly, each of the smooth muscle strips was homogenized in 200 µl F-actin stabilization buffer (50 mm Pipes, pH 6.9, 50 mm NaCl, 5 mm MgCl2, 5 mm EGTA, 5 % glycerol, 0.1 % Triton X-100, 0.1 % Nonidet P40, 0.1 % Tween 20, 0.1 % β-mercaptoethanol, 0.0011 % antifoam, 1 mm ATP, 1 µg ml−1 pepstatin, 1 µg ml−1 leupeptin, 10 µg ml−1 benzamidine, 500 µg ml−1 tosyl arginine methyl ester). The supernatants of protein extracts were collected after centrifugation at 100 000 g for 60 min at 30 °C. The pellets were resuspended in ice-cold distilled H2O plus 1 µm cytochalasin D and then incubated on ice for 1 h to dissociate F-actin. The resuspended pellets were gently mixed every 15 min. The supernatant of the resuspended pellets was collected after centrifugation at 2300 g, 2 min at 4 °C. Equal amounts of protein from the first supernatant (G-actin) and second supernatant (F-actin) were subjected to analysis by immunoblot using anti-actin antibody. The total amounts of G-actin and F-actin from the original soluble and insoluble fractions were calculated based on the total protein in each fraction.
Assessment of protein interactions by co-immunoprecipitation
Pulverized muscle strips were mixed with extraction buffer containing: 20 mm Tris-HCl at pH 7.4, 2 % Triton X-100, 0.2 % SDS, 2 mm EDTA, phosphatase inhibitors (2 mm sodium orthovanadate, 2 mm molybdate and 2 mm sodium pyrophosphate) and protease inhibitors (2 mm benzamidine, 0.5 mm aprotinin and 1 mm phenylmethylsulfonyl fluoride). Each sample was centrifuged for the collection of supernatant. Muscle extracts containing equal amounts of protein were precleared for 30 min with 50 µl of 10 % protein A-Sepharose. The precleared extracts were centrifuged at 16 000 g for 2 min. The extracts were incubated overnight with monoclonal antibody against paxillin to immunoprecipitate endogenous paxillin and then incubated for 2 h with 125 µl of a 10 % suspension of protein A-Sepharose beads conjugated to rabbit anti-mouse Ig. For recombinant paxillin immunoprecipitation, the extracts were incubated with anti-chicken paxillin antibody for 60 min followed by 2 h incubation with 10 % suspension of protein A-Sepharose beads. Immunocomplexes were washed four times in a buffer containing 50 mm Tris-HCl (pH 7.6), 150 mm NaCl and 0.1 % Triton X-100. All procedures of immunoprecipitation were performed at 4 °C. The immunoprecipitates of endogenous or recombinant paxillin were separated by SDS-PAGE followed by transfer to nitrocellulose membranes. The nitrocellulose membranes were divided into two parts; the lower part was probed with monoclonal antibody for CrkII (clone 22, BD Biosciences), stripped and reprobed with monoclonal paxillin antibody (clone 349, BD Biosciences). The upper part was probed with monoclonal antibody against focal adhesion kinase (clone 77, BD Biosciences), stripped and reprobed with polyclonal antibody against metavinculin/vinculin (custom-prepared by BABCO, Richmond, CA, USA). Proteins were quantified by scanning densitometry.
Statistical analysis
All statistical analysis was performed using SigmaStat software. Comparison among multiple groups was performed by one-way analysis of variance or Kruskal-Wallis one-way analysis of variance. Differences between pairs of groups were analysed by Student-Newman-Keuls test or Dunn's method. Values of n refer to the number of experiments used to obtain each value. P < 0.05 was considered to be significant.
RESULTS
Paxillin phosphorylation at tyr-31 and tyr-118 in ACh-stimulated smooth muscle strips
We evaluated the effect of contractile stimulation with ACh on the phosphorylation of tyr-31 and tyr-118 on paxillin in smooth muscle. Tracheal smooth muscle strips were stimulated with 10−4m ACh for 5 min or they were unstimulated. Site-specific tyrosine phosphorylation of paxillin from unstimulated or stimulated strips was assessed by immunoblot using phosphorylation site-specific, state-dependent antibodies for paxillin tyr-31 or tyr-118. Paxillin phosphorylation at tyr-31 and tyr-118 was higher in the ACh-stimulated muscle strips than in unstimulated strips (Fig. 1). Tyr-31 phosphorylation in the stimulated tissues was increased by 3.5 ± 0.5 times above the level in unstimulated strips (n = 4, P < 0.05), whereas paxillin phosphorylation at tyr-118 was 3.8 ± 0.6 times above the level in unstimulated strips (n = 4, P < 0.05).
Figure 1. Paxillin phosphorylation at tyr-31 and tyr-118 in ACh-stimulated smooth muscle tissues.
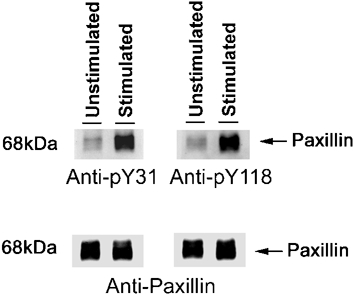
Immunoblots of protein extracts from unstimulated or stimulated tracheal smooth muscle strips (10−4m ACh, 5 min) subjected to SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blotted with phosphorylation site and state-specific antibodies for paxillin-tyr-31 (anti-pY31) or paxillin-tyr-118 (anti-pY118), stripped and reprobed with monoclonal paxillin antibody to normalize for minor differences of protein loading. Molecular mass markers (in kDa) are indicated on left. ACh stimulation increases paxillin phosphorylation at tyr-31 and tyr-118.
Expression of recombinant paxillin proteins in smooth muscle tissues
To determine whether the phosphorylation of paxillin at tyr-31 and tyr-118 plays a role in smooth muscle contraction, we introduced plasmids encoding wild type paxillin, plasmids encoding paxillin single mutants F31 or F118, or plasmids encoding the paxillin double mutant F31/118 into smooth muscle strips by reversible permeabilization. The strips were then maintained in an incubator for 2 days. Using anti-chicken paxillin antibody, recombinant chicken paxillin proteins were immunoprecipitated from extracts of muscle strips transfected with plasmids encoding wild type paxillin or various paxillin mutants (Fig. 2A). Anti-chicken paxillin antibody specifically recognizes the recombinant chicken paxillin proteins, but does not react significantly with the endogenous paxillin protein (Bellis et al. 1995; Petit et al. 2000). The immunoblots of the chicken paxillin immunoprecipitates were blotted with monoclonal paxillin antibody. Figure 2B shows that the recombinant chicken paxillin proteins were immunoprecipitated from smooth muscle tissues transfected with plasmids. There was a small amount of paxillin precipitated from untreated strips by the anti-chicken paxillin antibody, which probably represented a slight cross-reactivity of the anti-chicken antibody with endogenous paxillin (Fig. 2B). The results demonstrate that wild type and mutant recombinant paxillin proteins were expressed in the tracheal muscle tissues at similar levels.
After the recombinant paxillin proteins had been immunoprecipitated with anti-chicken paxillin antibody, the supernatant of the extract was incubated with monoclonal paxillin antibody to immunoprecipitate endogenous paxillin (Fig. 2A). As shown in Fig. 2C, the levels of endogenous paxillin were similar in tissues not treated with plasmids and in tissues that had been treated with plasmids. The results show that the expression of endogenous paxillin is not significantly affected by the expression of recombinant paxillin proteins in smooth muscle tissues.
Extracts of muscle strips transfected with recombinant paxillin were blotted with monoclonal paxillin antibody to estimate the level of combined endogenous and recombinant paxillin expression (Fig. 2D). The amount of paxillin was 2.46 ± 0.31-fold (wild type), 2.59 ± 0.26-fold (F31), 2.53 ± 0.24-fold (F118) and 2.66 ± 0.26-fold (F31/118) higher in strips expressing recombinant paxillin compared to that in untreated tissues (no plasmids) (n = 4).
To determine the efficiency of tissue transfection, cells were dissociated from smooth muscle tissues transfected with plasmids encoding wild type or mutant paxillin and were immunostained with anti-chicken paxillin antibody. The transfection efficiency was assessed by determining the number of cells that could be detected by immunofluoresence versus total cells (obtained from phase-contrast images) using a confocal microscope to obtain both fluorescence and phase-contrast images. Six fields of approximately 15-20 cells each dissociated from two tissues were analysed for each treatment group (Fig. 3). The transfection efficiencies for groups of cells dissociated from tissues that had been treated with plasmids encoding wild type paxillin, the paxillin mutants F31 and F118, and F31/118 were 90.5 ± 4.68, 86.7 ± 5.64, 91.5 ± 6.54 and 88.9 ± 4.09 % respectively. None of the cells from untransfected tissues stained positively for chicken paxillin.
Effect of paxillin mutants on paxillin phosphorylation at tyr-31 and tyr-118
We evaluated recombinant paxillin tyrosine phosphorylation and total paxillin phosphorylation (recombinant paxillin combined with endogenous paxillin) in smooth muscle tissues expressing wild type paxillin or the paxillin mutants F31, F118 or F31/118. Smooth muscle strips treated with plasmids encoding wild type paxillin, paxillin mutants, or not treated with plasmids were stimulated with 10−5m ACh for 5 min and were frozen for the analysis of paxillin phosphorylation at tyr-31 and tyr-118. To evaluate phosphorylation of the recombinant paxillin proteins, extracts of muscle strips expressing recombinant paxillin mutants were immunoprecipitated with anti chicken paxillin antibody, and immunoblots of chicken paxillin immunoprecipitates were probed using phospho-paxillin (Y31 or Y118) antibody. Phosphorylation of both endogenous and recombinant paxillin proteins (total paxillin) was determined by immunoblot analysis of paxillin immunoprecipitated using a monoclonal paxillin antibody, which reacts with both recombinant and endogenous paxillin proteins. As both force and paxillin tyrosine phosphorylation have reached a steady state by 5 min after ACh stimulation (Wang et al. 1996; Tang et al. 1999), we evaluated paxillin phosphorylation 5 min after ACh stimulation.
Immunoblots from unstimulated muscle strips expressing F31 or F31/118 paxillin mutants showed lower basal paxillin phosphorylation at tyr-31 than that in strips expressing wild type paxillin when probed with either the chicken-specific paxillin antibody or the monoclonal paxillin antibody (Fig. 4A and Fig. 5A). Similarly, basal recombinant and total paxillin phosphorylation at tyr-118 in strips expressing F118 or F31/118 paxillin mutants was lower than the level of wild-type treated strips (Fig. 4B and Fig. 5B). Basal phosphorylation of both recombinant and total paxillin proteins in strips expressing mutant paxillin proteins was significantly lower than that of strips expressing wild type proteins (n = 4, P < 0.05) (Fig. 4C and Fig. 5C).
Figure 4. Phosphorylation of recombinant paxillin at tyr-31 and tyr-118 in smooth muscle strips expressing wild type paxillin and paxillin mutants.
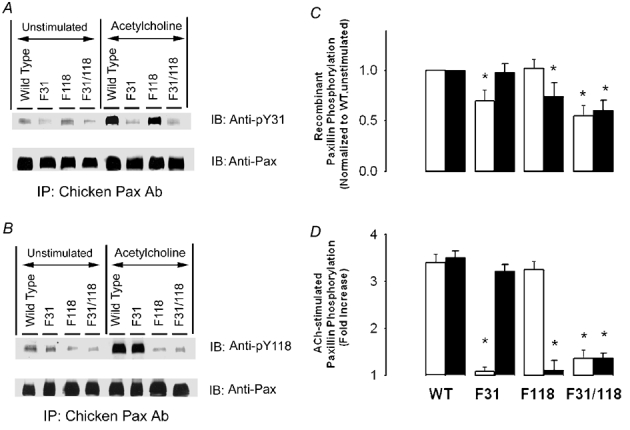
Immunoblots of anti-chicken paxillin immunoprecipitates from unstimulated or stimulated strips (10−5m ACh, 5 min) expressing wild type paxillin and mutant paxillin proteins were probed for paxillin-tyr-31 (pY31) (A) or paxillin-tyr-118 (pY118) (B) using phosphorylation-state specific antibodies. The blots were then stripped and reprobed with monoclonal anti-paxillin to normalize for minor differences in protein loading. The paxillin mutants F31, F118 and F31/118 inhibited corresponding site-specific phosphorylation of paxillin. C, mean values of paxillin phosphorylation at tyr-31 (open bars) and tyr-118 (filled bars) in unstimulated strips. The levels of paxillin phosphorylation at each site are normalized to the level of paxillin phosphorylation in unstimulated strips expressing wild type paxillin. D, mean values of paxillin phosphorylation at tyr-31 (open bars) and tyr-118 (filled bars) in strips in response to ACh stimulation. The levels of paxillin phosphorylation at each site are normalized to the level phosphorylation in corresponding unstimulated strips. Tyr-31 phosphorylation in strips expressing the F31 mutant was not significantly different from that in tissues expressing F31/118. Tyr-118 phosphorylation in strips expressing F118 mutant was not significantly different from that in tissues expressing F31/118 paxillin mutant. All values represent means ± s.e.m. (n = 4). * Significant difference from the values obtained in stimulated strips expressing wild type paxillin (P < 0.05).
Figure 5. Combined endogenous and recombinant paxillin phosphorylation at tyr-31 and tyr-118 in smooth muscle tissues expressing recombinant wild type paxillin and paxillin mutants.
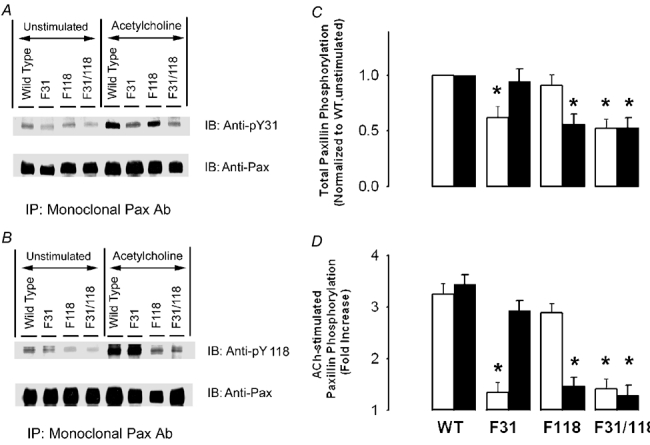
Immunoblots of monoclonal paxillin immunoprecipitates from unstimulated or stimulated strips (10−5m ACh, 5 min) expressing wild type paxillin and various mutants were probed with phosphorylation-state specific antibodies for paxillin-tyr-31 (pY31) (A) or paxillin-tyr-118 (pY118) (B). The blots were then stripped and reprobed with monoclonal anti-paxillin to normalize for minor differences of protein loading. C, the levels of unstimulated paxillin phosphorylation at tyr-31 (open bars) and tyr-118 (filled bars) are normalized to the level of unstimulated strips expressing wild type paxillin. D, the levels of ACh-induced paxillin phosphorylation at tyr-31 (open bars) and tyr-118 (filled bars) are normalized to the level of each corresponding unstimulated strip. Tyr-31 phosphorylation in strips expressing the F31 mutant was not significantly different from that in tissues expressing F31/118. Tyr-118 phosphorylation in strips expressing the F118 mutant was not significantly different from that in tissues expressing F31/118 paxillin mutant. Values represent means ± s.e.m. (n = 4). * Significant difference from values in stimulated strips expressing wild type paxillin (P < 0.05).
In muscle strips treated with plasmids encoding wild type paxillin, contractile stimulation significantly increased tyr-31 and tyr-118 phosphorylation of recombinant paxillin (Fig. 4A, B and D, n = 4, P < 0.05) and of total paxillin (Fig. 5A, B and D, n = 4, P < 0.05). The expression of the single mutant F31 paxillin protein significantly inhibited tyr-31 phosphorylation but not tyr-118 phosphorylation of recombinant paxillin (Fig. 4A and D, n = 4, P < 0.05). Total paxillin phosphorylation at this site in response to ACh stimulation was also reduced (Fig. 5A and D, n = 4, P < 0.05). In contrast, the expression of paxillin F118 depressed tyr-118 phosphorylation but not tyr-31 phosphorylation of recombinant paxillin (Fig. 4A and D, n = 4, P < 0.05). Similar effects on total paxillin phosphorylation were observed (Fig. 5A and D, n = 4, P < 0.05). The expression of the paxillin double mutant F31/118 inhibited both tyr-31 and tyr-118 phosphorylation on paxillin (Fig. 4 and Fig. 5). However, tyr-31 phosphorylation in strips transfected with plasmids encoding F31/118 paxillin double mutant was not significantly different from that in strips transfected with the F31 paxillin single mutant (P > 0.05). Likewise, paxillin phosphorylation at tyr-118 in strips transfected with double paxillin mutant F31/118 was similar to that in strips treated with F118 paxillin single mutant (P > 0.05).
Inhibition of tension development by expression of the paxillin mutants F31, F118 and F31/118
We assessed the effect of the expression of wild type paxillin or paxillin mutants on contractile force by comparing ACh-induced contraction in muscle strips transfected with plasmids encoding wild type paxillin, F31, F118 or F31/118 paxillin mutants. Force in response to 10−5m ACh was compared before and after the 2 day incubation period. In muscle strips not transfected with plasmids (no plasmids) and strips transfected with wild type recombinant paxillin, contractile force in response to stimulation with ACh was similar before and after the 2 day incubation period (Fig. 6A). In muscle strips transfected with F31or F118 paxillin mutants, isometric force in response to stimulation with ACh was 25-30 % of the preincubation force (Fig. 6B, n = 9-10, P < 0.05). In muscle tissues transfected with the paxillin double mutant F31/118, ACh-induced contraction was approximately 5 % of the preincubation force (Fig. 6B, n = 9-10), which was significantly different from the force in strips expressing the paxillin F31 or F118 mutants (P < 0.05). There were no significant differences in tension among the five groups of strips prior to contractile stimulation.
Figure 6. Effect of the expression of recombinant paxillin on contractile force and myosin light chain phosphorylation stimulated by ACh.
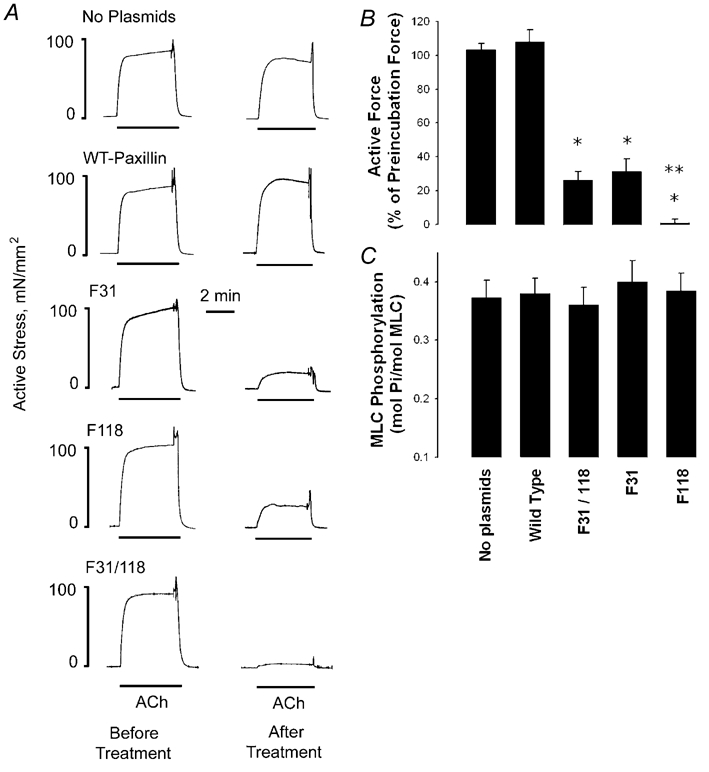
A, smooth muscle strips were contracted with 10−5m ACh before and after treatment with plasmids encoding wild type paxillin and paxillin mutants or without plasmids. Contractile force in strips expressing wild type paxillin was similar to that in strips treated without plasmids. Expression of the paxillin mutants F31, F118 and F31/118 inhibited contractile force. B, mean active force in response to 10−5m ACh was quantified as the percentage of ACh-induced force in each strip before treatment. Values are means ± s.e.m. * Significantly lower response compared with muscles without plasmids (n = 9-10, P < 0.05); ** significantly lower response compared with muscles expressing single mutant F31 or F118 (n = 9-11, P < 0.05). C, myosin light chain phosphorylation was measured in smooth muscle strips expressing wild type paxillin and paxillin mutants F31, F118 and F31/118 and in strips not treated with plasmids (No plasmids) after stimulation with 10−5m ACh for 5 min. There were no significant differences in myosin light chain phosphorylation in strips not treated with plasmids and in strips expressing wild type paxillin or paxillin mutants F31, F118 or F31/118. Values shown are means ± s.e.m. (n = 6).
Effect of paxillin mutants on myosin light chain phosphorylation
Smooth muscle strips treated with plasmids encoding wild type paxillin, paxillin mutants, or with no plasmids were frozen for the analysis of myosin light chain phosphorylation. Myosin light chain phosphorylation was determined 5 min after contractile activation. Force and myosin light chain phosphorylation in response to ACh stimulation are at a steady state by this time (Mehta et al. 1996; Tang & Gunst, 2001). Although force production was dramatically depressed (Fig. 6A and B), the increase in myosin light chain phosphorylation in strips expressing paxillin mutants F31, F118 and F31/118 was similar to that of the muscle strips not treated with plasmids. The mean increases in myosin light chain phosphorylation 5 min after ACh stimulation in the tissues not treated with plasmids, and in the muscle tissues expressing wild type paxillin, F31, F118 and F31/118 paxillin mutants were not significantly different (Fig. 6C, P > 0.05).
Effect of paxillin tyrosine phosphorylation on the association of paxillin with Crk-II, metavinculin/vinculin and FAK in smooth muscle tissues
We evaluated whether paxillin phosphorylation on tyr-31 and tyr-118 in smooth muscle in response to contractile stimulation increases the association of paxillin with CrkII, metavinculin/vinculin or FAK. Endogenous paxillin from unstimulated or ACh-stimulated muscle strips (not treated with plasmids) was immunoprecipitated with monoclonal paxillin antibody. The immunoblots of the paxillin immunoprecipitates were then probed with CrkII antibody, stripped and then reprobed with antibodies against FAK, vinculin and paxillin. As a control for non-specific protein associations with the immunocomplexes, membranes were also blotted for myosin heavy chain (MHC) and PKCα. No MHC or PKCα was found in the endogenous paxillin immunoprecipitates (Fig. 7).
Figure 7. Contractile stimulation increases the interaction of paxillin with CrkII, but not with vinculin or focal adhesion kinase (FAK).
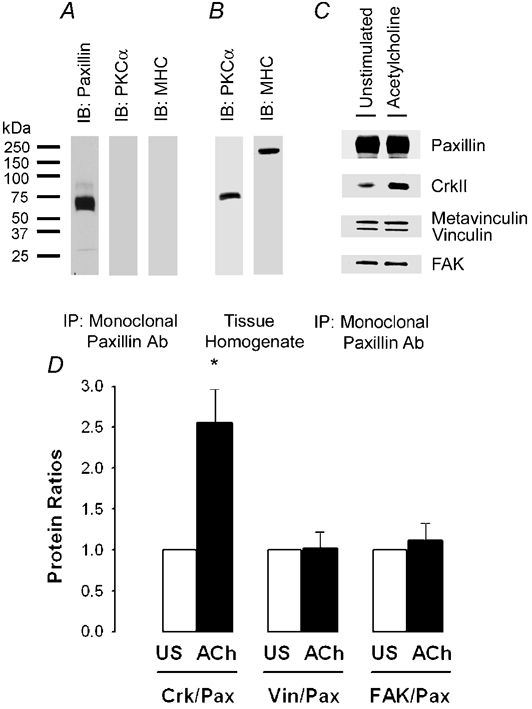
Representative immunoblots show that monoclonal paxillin antibody specifically immunoprecipitates paxillin from extracts of smooth muscle strips. A, endogenous paxillin from untransfected smooth muscle was immunoprecipitated with monoclonal paxillin antibody. Paxillin immunoprecipitates were immunoblotted with monoclonal paxillin antibody, stripped and reprobed with antibodies to myosin heavy chain (MHC) and PKCα to control for non-specific protein associations with the immunocomplexes. No MHC or PKCα was found in the paxillin immunoprecipitates. B, MHC and PKCα were detected in whole tissue homogenates but not immunoprecipitates (A), demonstrating their presence in muscle extracts prior to immunoprecipitation. C, immunoblots of endogenous paxillin immunoprecipitated from extracts of muscle tissues were blotted with antibodies against paxillin, CrkII, metavinculin/vinculin or FAK. ACh stimulation increases the interaction of paxillin with CrkII, but not with metavinculin/vinculin or FAK. D, each protein ratio was quantified as fold increase over the value obtained for the unstimulated tissues. Values are means ± s.e.m. * Significantly higher ratio compared with the unstimulated muscles (n = 4, P < 0.05).
The amount of vinculin and FAK that immunoprecipitated with paxillin was similar in muscle extracts from unstimulated and stimulated smooth muscles (Fig. 7C and D), P > 0.05, n = 4), indicating that paxillin phosphorylation does not increase the association of paxillin with metavinculin/vinculin or FAK. However, ACh stimulation of smooth muscle resulted in an increase in the amount of CrkII that co-immunoprecipitated with paxillin (Fig. 7C and D, P < 0.05, n = 4), indicating that paxillin phosphorylation enhances the affinity of paxillin for CrkII.
To determine the role of paxillin phosphorylation at tyr-31 and tyr-118 on the interaction of paxillin with Crk-II, metavinculin/vinculin and FAK, recombinant paxillin was immunoprecipitated with chicken paxillin antibody from extracts of unstimulated or stimulated smooth muscle tissues transfected with wild-type paxillin or F31/118 mutant paxillin. Immunoblots of paxillin immunoprecipitates were probed with CrkII antibody, and stripped and then reprobed with antibodies against FAK, vinculin, paxillin or MHC. In smooth muscle strips expressing wild-type paxillin, more CrkII was found in paxillin immunoprecipitates from muscle strips stimulated with ACh than in unstimulated strips, whereas the amount of metavinculin/vinculin and FAK immunoprecipitated with wild type paxillin from unstimulated or ACh-stimulated muscle extracts was not different (Fig 8, n = 4). In smooth muscle tissues expressing F31/118 paxillin mutant, the amount of metavinculin/vinculin and FAK that co-immunoprecipitated with paxillin mutant F31/118 from unstimulated muscle strips was not different from that in stimulated strips. In contrast, the amount of CrkII that precipitated with the paxillin mutant F31/118 from either stimulated or unstimulated strips was significantly less than that in muscle strips expressing wild type paxillin (Fig. 8, n = 4). The amount of CrkII that immunoprecipitated with paxillin single mutants F31 or F118 was also markedly less than that in strips expressing wild type paxillin, but somewhat more than the amount that precipitated with the paxillin mutant F31/1118 (data not shown).
Figure 8. F31/118 paxillin mutant has lower affinity for CrkII in airway smooth muscle.
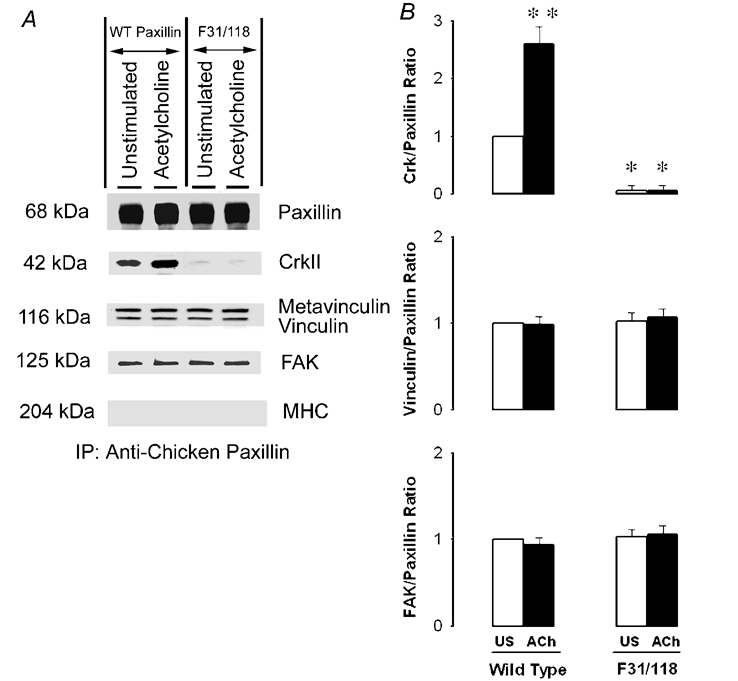
Smooth muscle strips expressing wild type paxillin or the F31/118 paxillin mutant were stimulated with 10−5m ACh for 5 min or unstimulated. Immunoblots of recombinant paxillin immunoprecipitated from extracts of these tissues were blotted with antibodies against paxillin, CrkII, metavinculin/vinculin, FAK and MHC. No MHC was found in the recombinant paxillin immunoprecipitates. A, in strips expressing wild type paxillin, ACh stimulation increases the interaction of paxillin with CrkII, but not with metavinculin/vinculin or FAK. CrkII does not co-precipitate with the F31/118 paxillin mutant, but both metavinculin/vinculin and FAK co-precipitate with the mutant F31/118 paxillin. B, each protein ratio was quantified as fold increase over the value obtained in the unstimulated tissues. Values are means ± s.e.m. **Significantly higher ratio compared with the unstimulated muscles. *Significantly lower ratio compared with corresponding strips expressing wild type paxillin (n = 4, P < 0.05).
G-actin/F-actin ratio in smooth muscle tissues expressing the paxillin mutant F31/118
We assessed the effect of paxillin phosphorylation at tyr-31 and tyr-118 on actin polymerization by determining the ratio of G(monomeric)-actin to F(filamentous)-actin in extracts from smooth muscle strips expressing the F31/118 paxillin mutant. Smooth muscle strips treated with plasmids encoding wild type paxillin and the paxillin mutant F31/118 were stimulated with 10−5m ACh for 5 min, and then homogenized and fractionated for the analysis of G-actin and F-actin. The ratio of total amounts of actin in the soluble fraction (G-actin) and insoluble fraction (F-actin) obtained from each tissue were determined. In the muscle tissues expressing wild type paxillin, the ratio of G-actin/F-actin was 0.23 ± 0.03 in unstimulated strips and 0.11 ± 0.04 in stimulated strips 5 min after stimulation with ACh (Fig. 9, P < 0.05, n = 5), indicating a decrease in G-actin and an increase in F-actin in response to contractile stimulation. However, contractile stimulation did not significantly reduce the ratio of G-actin/F-actin in strips expressing F31/118 paxillin mutant (Fig.9, P > 0.05, n = 5).
Figure 9. Effect of paxillin phosphorylation at tyr-31 and tyr-118 on G-actin/F-actin ratio in smooth muscle.
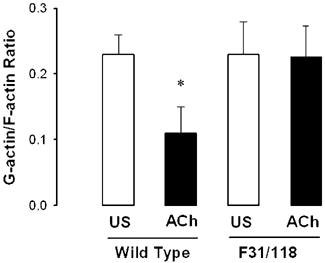
Tracheal smooth muscle strips expressing wild type paxillin or the F31/118 paxillin mutant were stimulated with 10−5m ACh for 5 min. The ratios of soluble actin (G-actin) over insoluble actin (F-actin) were determined. * G-actin/F-actin ratio in ACh-stimulated strips is significantly lower than the value for corresponding unstimulated strips (n = 4, P < 0.05).
DISCUSSION
Role of paxillin phosphorylation at tyr-31 and tyr-118 in the regulation of smooth muscle contraction
The results of the present study demonstrate that the phosphorylation of paxillin at tyr-31 and tyr-118 plays an essential role in the cellular processes that regulate active tension development in smooth muscle. Furthermore, our evidence suggests that paxillin phosphorylation regulates cytoskeletal processes that are independent of contractile protein activation. These observations provide additional evidence that the contractile stimulation of smooth muscle requires the activation of cytoskeletal processes that are separate and distinct from crossbridge activation, and that these cytoskeletal mechanisms are necessary for active tension development in smooth muscle.
We found that contractile stimulation of smooth muscle with acetylcholine induced paxillin phosphorylation at tyrosine residues 31 and 118. In order to evaluate the function of phosphorylation at these sites, we introduced plasmids encoding wild type chicken paxillin and chicken paxillin mutants with phenylalanine substitutions at tyrosine 31 and tyrosine 118 into smooth muscle tissues by a method of reversible permeabilization. The expression of these recombinant proteins by the smooth muscle tissues was verified by immunoblot analysis of chicken paxillin immunoprecipitates from plasmid-treated strips, and by dissociating cells from transfected tissues and analysing them for the expression of recombinant proteins by confocal microscopy. Recombinant paxillin proteins were expressed in transfected tissues in amounts that were equal to or greater and that of the endogenous paxillin. Immunofluorescence analysis demonstrated that 90 % of the cells in the transfected tissues express the recombinant proteins.
The phosphorylation of both recombinant and total paxillin (endogenous and recombinant paxillin proteins combined) at tyr-31 and tyr-118 induced by stimulation of the muscles with ACh was significantly lower in muscle strips expressing paxillin mutants F31, F118 or F31/118 than in muscle strips expressing wild type paxillin. Force development in tissues expressing the paxillin mutants F31, F118 or the paxillin double mutant F31/118 was also markedly inhibited, with the strongest inhibition observed in muscles expressing the F31/F118 paxillin double mutant. These data demonstrate that phosphorylation of tyr-31 and tyr-118 on paxillin plays an important regulatory role in the process of smooth muscle contraction, and that the phosphorylation of both of these tyrosine sites contributes to the regulation of tension development. Our results suggest that the paxillin mutants exert a dominant negative effect by replacing endogenous paxillin at functional sites, thereby inhibiting phosphorylation of the endogenous paxillin protein, and inhibiting the recruitment of specific signalling molecules necessary for force development.
In the present study, the expression of paxillin mutants had no effect on the increase in myosin light chain phosphorylation induced by acetylcholine stimulation. In a previous study, we found that the depletion of paxillin in tracheal smooth muscle using antisense inhibited agonist-induced tension development, without affecting agonist-induced changes in intracellular Ca2+, myosin light chain phosphorylation or myosin ATPase activity (Tang et al. 2002). Taken together, these results indicate that the regulatory role of paxillin during active contraction is not directed at processes involved in the activation of contractile proteins or in the regulation of crossbridge cycling.
Tracheal tissues expressing mutant phosphorylation-deficient paxillin proteins exhibited abnormal actin dynamics. We have previously reported that contractile activation of tracheal smooth muscle stimulates actin polymerization, and that actin polymerization is necessary for active tension development (Mehta & Gunst, 1999; Tang et al. 2002). The stimulation of tracheal muscle strips with ACh decreases the concentration of G-actin as measured by the DNase I inhibition assay, consistent with the incorporation of G-actin monomers into F-actin. Furthermore, latrunculin A, which binds to G-actin and prevents its incorporation into F-actin, inhibits tension development without inhibiting myosin light chain phosphorylation in tracheal smooth muscle (Mehta & Gunst, 1999). Actin polymerization in response to contractile stimulation has also been reported in other smooth muscle tissues and in smooth muscle cells (Cipolla & Osol, 1998; Jones et al. 1999; Emala et al. 1999; Barany et al. 2001; An et al. 2002).
We evaluated the role of paxillin tyrosine phosphorylation in regulating actin polymerization in tracheal muscle by determining the effects of the paxillin F31/F118 double mutant on changes in the ratio of G-actin to F-actin induced by ACh. Whereas, the ratio of G-actin/F-actin decreased from 0.23 ± 0.03 (unstimulated level) to 0.11 ± 0.04 in response to ACh stimulation in tissues expressing wild type paxilllin; the ratio of G-actin/F-actin did not change in tissues expressing the paxillin F31/118 double mutant. These results are consistent with our previous observations suggesting that paxillin phosphorylation regulates actin polymerization during the contractile stimulation of smooth muscle (Tang et al. 2002).
Regulation of the association of paxillin with signalling and structural proteins by site-specific tyrosine phosphorylation of paxillin
Paxillin tyrosine phosphorylation is implicated in the regulation of cell migration, actin cytoskeleton remodelling and focal adhesion formation in non-muscle cells (Turner, 2000; Petit et al. 2000; Schaller, 2001). However, the molecular mechanisms by which paxillin phosphorylation regulates these functions are not understood. Paxillin functions as a molecular adaptor or scaffolding protein that provides multiple docking sites for an array of signalling and structural proteins. The inhibitory effects of expressing paxillin phosphorylation-deficient mutants on contraction in tracheal smooth muscle may result from disruptions in the coupling of mutant paxillin with proteins involved in the regulation of downstream cytoskeletal signalling pathways.
Paxillin phosphorylation at tyrosine 31 and 118 increases its association with the SH2/SH3 adapter protein CrkII (Petit et al. 2000). CrkII has been implicated in the regulation of actin cytoskeletal remodelling and cell motility (Klemke et al. 1998; Nakashima et al. 1999; Petit et al. 2000), and the binding of CrkII to tyrosine phosphorylated paxillin is increased during the migration of bladder tumour cells (Petit et al. 2000). We assessed the possibility that paxillin tyrosine phosphorylation in response to contractile stimulation may increase the binding of CrkII to paxillin. More CrkII was found in immunoprecipitates of endogenous paxillin in stimulated muscle strips than in unstimulated muscle strips (Fig. 7). Additionally, very little CrkII was present in immunoprecipitates of the paxillin mutant F31/F118 (Fig. 8). Thus, paxillin phosphorylation at tyr-31 and tyr-118 appears to regulate paxillin-CrkII binding in tracheal smooth muscle. This interaction may be an important regulatory step in cytoskeletal signalling pathways that regulate tension generation in smooth muscle in response to contractile stimulation.
Several studies suggest that CrkII regulates the small GTPases Rac and Rho, which have been implicated in regulating the assembly and disassembly of the actin cytoskeleton in a number of non-muscle cells (Klemke et al. 1998; Altun-Gultekin et al. 1998; Nakashima et al. 1999; Ridley, 1999). Thus, it is possible that CrkII couples paxillin phosphorylation to the downstream regulation of actin polymerization and tension development during the contractile stimulation of smooth muscle.
Paxillin binds directly to FAK; and the binding of paxillin to FAK is necessary for the regulation of paxillin tyrosine phosphorylation (Thomas et al. 1999). In tracheal smooth muscle, FAK undergoes tyrosine phosphorylation coordinately with paxillin during contractile stimulation (Tang et al. 1999), and the depletion of FAK by antisense depresses tension development, intracellular Ca2+ and myosin light chain phosphorylation (Tang & Gunst, 2001). The LD2 and LD4 motifs on paxillin have been identified as critical for FAK binding (Thomas et al. 1999; Turner, 2000). These motifs are located at the N-terminus of paxillin, as are the tyr-31 and tyr-118 phosphorylation sites. Thus, we evaluated whether the association of FAK with paxillin was impaired by mutations of the tyrosine 31 or 118 residues that prevent paxillin phosphorylation. We found that the amount of FAK that co-immunoprecipitated with endogenous paxillin was similar in stimulated and unstimulated muscle strips (Fig. 7). Furthermore, FAK co-immunoprecipitated with recombinant paxillin mutant F31/118 to a similar extent as with recombinant wild type paxillin (Fig. 8), indicating that association of paxillin with FAK is not impaired by the substitution of tyrosine 31 or 118 residues with phenylalanine. Thus, paxillin phosphorylation at tyr-31 or tyr-118 did not affect the association of paxillin with FAK.
Vinculin also binds to the N-terminus of paxillin (Brown et al. 1996; Turner, 2000). Vinculin binds to actin and is believed to contribute to the formation of structural links between integrin proteins and the actin cytoskeleton (Ezzell et al. 1997; Critchley et al. 1999). The interaction between paxillin and vinculin may be important in regulating focal adhesion formation during cell migration. Moreover, vinculin co-immunoprecipitates with paxillin in tracheal smooth muscle (Fig. 7), and the depletion of paxillin from tracheal smooth muscle impairs the localization of vinculin at the cell membrane during contractile stimulation (Opazo Saez et al. 2001; Gunst et al. 2003). Vinculin binds preferentially to LD motifs 1, 2 and 4 at the N-terminus of paxillin (Turner et al. 1999). Thus, we evaluated the possibility that the interaction between vinculin and paxillin is affected by paxillin phosphorylation at tyr-31 and tyr-118. We found that the amount of metavinculin/vinculin in endogenous paxillin immunoprecipitates was not different in stimulated and unstimulated muscles (Fig. 7), and similar amounts of metavinculin/vinculin co-immunoprecipitated with the paxillin mutant F31/118 and wild type paxillin in both stimulated and unstimulated muscles (Fig. 8). Thus, our results suggest that paxillin phosphorylation at tyr-31 and tyr-118 does not affect vinculin binding to paxillin.
Conclusions
We conclude that paxillin phosphorylation plays a critical role in the regulation of active tension generation in tracheal smooth muscle. The expression of non-phosphorylatable paxillin mutants in smooth muscle tissues dramatically depresses active tension generation and inhibits actin polymerization in response to contractile stimulation without significantly inhibiting myosin light chain phosphorylation. Paxillin mutants without phosphorylatable residues at tyr-31 and tyr-118 show a marked decrease in their interaction with the SH2/SH3 adaptor protein CrkII but not with vinculin or FAK. Thus, paxillin may regulate downstream pathways involved in actin dynamics and cytoskeletal organization in smooth muscle through its interactions with the adaptor protein CrkII. Paxillin phosphorylation may be important in regulating the formation of molecular complexes that mediate cytoskeletal reorganization in smooth muscle during contractile stimulation.
Acknowledgments
We thank Mingfang Wu for his contributions to these experiments. This work was supported by a American Heart Association Scientist Development Grant, and National Heart, Lung, and Blood Institute Grants HL-29289, HL-074099 and GM47607.
REFERENCES
- Altun-Gultekin ZF, Chandriani S, Bougeret C, Ishizaki T, Narumiya S, de Graaf P, Bergen HP, Hanafusa H, Wagner JA, Birge RB. Activation of Rho-dependent cell spreading and focal adhesion biogenesis by the v-Crk adaptor protein. Mol Cell Biol. 1998;18:3044–3058. doi: 10.1128/mcb.18.5.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An SS, Laudadio RE, Lai J, Rogers RA, Fredberg JJ. Stiffness changes in cultured airway smooth muscle cells. Am J Physiol Cell Physiol. 2002;283:C792–801. doi: 10.1152/ajpcell.00425.2001. [DOI] [PubMed] [Google Scholar]
- Barany M, Barron JT, Gu L, Barany K. Exchange of the actin-bound nucleotide in intact arterial smooth muscle. J Biol Chem. 2001;276:48398–48403. doi: 10.1074/jbc.M106227200. [DOI] [PubMed] [Google Scholar]
- Bellis SL, Miller JT, Turner CE. Characterization of tyrosine phosphorylation of paxillin in vitro by focal adhesion kinase. J Biol Chem. 1995;270:17437–17441. doi: 10.1074/jbc.270.29.17437. [DOI] [PubMed] [Google Scholar]
- Brown MC, Curtis MS, Turner CE. Paxillin LD motifs may define a new family of protein recognition domains. Nat Struct Biol. 1998;5:677–678. doi: 10.1038/1370. [DOI] [PubMed] [Google Scholar]
- Brown MC, Perrotta JA, Turner CE. Identification of LIM3 as the principal determinant of paxillin focal adhesion localization and characterization of a novel motif on paxillin directing vinculin and focal adhesion kinase binding. J Cell Biol. 1996;135:1109–1123. doi: 10.1083/jcb.135.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, Osol G. Vascular smooth muscle actin cytoskeleton in cerebral artery forced dilatation. Stroke. 1998;29:1223–1228. doi: 10.1161/01.str.29.6.1223. [DOI] [PubMed] [Google Scholar]
- Critchley DR, Holt MR, Barry ST, Priddle H, Hemmings L, Norman J. Integrin-mediated cell adhesion: the cytoskeletal connection. Biochem Soc Symp. 1999;65:79–99. [PubMed] [Google Scholar]
- Emala CW, Liu F, Hirshman CA. Gialpha but not gqalpha is linked to activation of p21(ras) in human airway smooth muscle cells. Am J Physiol. 1999;276:L564–570. doi: 10.1152/ajplung.1999.276.4.L564. [DOI] [PubMed] [Google Scholar]
- Ezzell RM, Goldmann WH, Wang N, Parasharama N, Ingber DE. Vinculin promotes cell spreading by mechanically coupling integrins to the cytoskeleton. Exp Cell Res. 1997;231:14–26. doi: 10.1006/excr.1996.3451. [DOI] [PubMed] [Google Scholar]
- Fisher TL, Terhorst T, Cao X, Wagner RW. Intracellular disposition and metabolism of fluorescently-labeled unmodified and modified oligonucleotides microinjected into mammalian cells. Nucleic Acids Res. 1993;21:3857–3865. doi: 10.1093/nar/21.16.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann WH, Galneder R, Ludwig M, Xu W, Adamson ED, Wang N, Ezzell RM. Differences in elasticity of vinculin-deficient F9 cells measured by magnetometry and atomic force microscopy. Exp Cell Res. 1998;239:235–242. doi: 10.1006/excr.1997.3915. [DOI] [PubMed] [Google Scholar]
- Gunst SJ, Tang DD, Opazo Saez AM. Cytoskeletal remodeling of the airway smooth muscle cell: a mechanism for adaptation to mechanical forces in the lung. Respir Physiol Neurobiol. 2003;137:151–168. doi: 10.1016/s1569-9048(03)00144-7. [DOI] [PubMed] [Google Scholar]
- Hartwig JH. Mechanisms of actin rearrangements mediating platelet activation. J Cell Biol. 1992;118:1421–1442. doi: 10.1083/jcb.118.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KA, Perkins WJ, Lorenz RR, Prakash YS, Sieck GC, Warner DO. F-actin stabilization increases tension cost during contraction of permeabilized airway smooth muscle in dogs. J Physiol. 1999;519:527–538. doi: 10.1111/j.1469-7793.1999.0527m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, Cheresh DA. CAS/Crk coupling serves as a ‘molecular switch’ for induction of cell migration. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Earp HS. Paxillin is tyrosine-phosphorylated by and preferentially associates with the calcium-dependent tyrosine kinase in rat liver epithelial cells. J Biol Chem. 1997;272:14341–14348. doi: 10.1074/jbc.272.22.14341. [DOI] [PubMed] [Google Scholar]
- Mehta D, Gunst SJ. Actin polymerization stimulated by contractile activation regulates force development in canine tracheal smooth muscle. J Physiol. 1999;519:829–840. doi: 10.1111/j.1469-7793.1999.0829n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Wu MF, Gunst SJ. Role of contractile protein activation in the length-dependent modulation of tracheal smooth muscle force. Am J Physiol. 1996;270:C243–252. doi: 10.1152/ajpcell.1996.270.1.C243. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Yano H, Uchida H, Hashimoto S, Schaefer E, Sabe H. Tyrosine phosphorylation of paxillin alpha is involved in temporospatial regulation of paxillin-containing focal adhesion formation and F-actin organization in motile cells. J Biol Chem. 2000;275:27155–27164. doi: 10.1074/jbc.M000679200. [DOI] [PubMed] [Google Scholar]
- Nakashima N, Rose DW, Xiao S, Egawa K, Martin SS, Haruta T, Saltiel AR, Olefsky JM. The functional role of CrkII in actin cytoskeleton organization and mitogenesis. J Biol Chem. 1999;274:3001–3008. doi: 10.1074/jbc.274.5.3001. [DOI] [PubMed] [Google Scholar]
- Opazo Saez AM, Tang DD, Gunst SJ. Role of paxillin in the regulation of cytoskeletal protein relocalization during contractile activation of smooth muscle (SM) cells. Mol Biol Cell. 2001;12:301a. [Google Scholar]
- Petit V, Boyer B, Lentz D, Turner CE, Thiery JP, Valles AM. Phosphorylation of tyrosine residues 31 and 118 on paxillin regulates cell migration through an association with CRK in NBT-II cells. J Cell Biol. 2000;148:957–970. doi: 10.1083/jcb.148.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao KM, Betschart JM, Virji MA. Hormone-induced actin polymerization in rat hepatoma cells and human leucocytes. Biochem J. 1985;230:709–714. doi: 10.1042/bj2300709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ. Rho family proteins and regulation of the actin cytoskeleton. Prog Mol Subcell Biol. 1999;22:1–22. doi: 10.1007/978-3-642-58591-3_1. [DOI] [PubMed] [Google Scholar]
- Schaller MD. Paxillin: a focal adhesion-associated adaptor protein. Oncogene. 2001;20:6459–6472. doi: 10.1038/sj.onc.1204786. [DOI] [PubMed] [Google Scholar]
- Schaller MD, Hildebrand JD, Parsons JT. Complex formation with focal adhesion kinase: A mechanism to regulate activity and subcellular localization of Src kinases. Mol Biol Cell. 1999;10:3489–3505. doi: 10.1091/mbc.10.10.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth AP, Rook SL, Detmar M, Robinson GS. Antisense oligonucleotides inhibit vascular endothelial growth factor/vascular permeability factor expression in normal human epidermal keratinocytes. J Invest Dermatol. 1997;108:523–526. doi: 10.1111/1523-1747.ep12289740. [DOI] [PubMed] [Google Scholar]
- Tang D, Mehta D, Gunst SJ. Mechanosensitive tyrosine phosphorylation of paxillin and focal adhesion kinase in tracheal smooth muscle. Am J Physiol. 1999;276:C250–258. doi: 10.1152/ajpcell.1999.276.1.C250. [DOI] [PubMed] [Google Scholar]
- Tang DD, Gunst SJ. Depletion of focal adhesion kinase by antisense depresses contractile activation of smooth muscle. Am J Physiol Cell Physiol. 2001;280:C874–883. doi: 10.1152/ajpcell.2001.280.4.C874. [DOI] [PubMed] [Google Scholar]
- Tang DD, Wu MF. The focal adhesion protein paxillin regulates contraction in canine tracheal smooth muscle. J Physiol. 2002;542:501–513. doi: 10.1113/jphysiol.2002.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JW, Cooley MA. The role of focal adhesion kinase binding in the regulation of tyrosine phosphorylation of paxillin. J Biol Chem. 1999;274:36684–36692. doi: 10.1074/jbc.274.51.36684. [DOI] [PubMed] [Google Scholar]
- Tumbarello DA, Brown MC, Turner CE. The paxillin LD motifs. FEBS Lett. 2002;513:114–118. doi: 10.1016/s0014-5793(01)03244-6. [DOI] [PubMed] [Google Scholar]
- Turner CE. Paxillin and focal adhesion signalling. Nat Cell Biol. 2000;2:E231–236. doi: 10.1038/35046659. [DOI] [PubMed] [Google Scholar]
- Turner CE, Brown MC, Perrotta JA, Riedy MC, Nikolopoulos SN, McDonald AR, Bagrodia S, Thomas S, Leventhal PS. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: A role in cytoskeletal remodeling. J Cell Biol. 1999;145:851–863. doi: 10.1083/jcb.145.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner RW. Gene inhibition using antisense oligodeoxynucleotides. Nature. 1994;372:333–335. doi: 10.1038/372333a0. [DOI] [PubMed] [Google Scholar]
- Wang Z, Pavalko FM, Gunst SJ. Tyrosine phosphorylation of the dense plaque protein paxillin is regulated during smooth muscle contraction. Am J Physiol. 1996;271:C1594–1602. doi: 10.1152/ajpcell.1996.271.5.C1594. [DOI] [PubMed] [Google Scholar]
- Yassin R, Shefcyk J, White JR, Tao W, Volpi M, Molski TF, Naccache PH, Feinstein MB, Sha'afi RI. Effects of chemotactic factors and other agents on the amounts of actin and a 65,000-mol-wt protein associated with the cytoskeleton of rabbit and human neutrophils. J Cell Biol. 1985;101:182–188. doi: 10.1083/jcb.101.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]


