Abstract
Transcranial direct current stimulation (tDCS) of the human motor cortex results in polarity-specific shifts of cortical excitability during and after stimulation. Anodal tDCS enhances and cathodal stimulation reduces excitability. Animal experiments have demonstrated that the effect of anodal tDCS is caused by neuronal depolarisation, while cathodal tDCS hyperpolarises cortical neurones. However, not much is known about the ion channels and receptors involved in these effects. Thus, the impact of the sodium channel blocker carbamazepine, the calcium channel blocker flunarizine and the NMDA receptor antagonist dextromethorphane on tDCS-elicited motor cortical excitability changes of healthy human subjects were tested. tDCS-protocols inducing excitability alterations (1) only during tDCS and (2) eliciting long-lasting after-effects were applied after drug administration. Carbamazepine selectively eliminated the excitability enhancement induced by anodal stimulation during and after tDCS. Flunarizine resulted in similar changes. Antagonising NMDA receptors did not alter current-generated excitability changes during a short stimulation, which elicits no after-effects, but prevented the induction of long-lasting after-effects independent of their direction. These results suggest that, like in other animals, cortical excitability shifts induced during tDCS in humans also depend on membrane polarisation, thus modulating the conductance of sodium and calcium channels. Moreover, they suggest that the after-effects may be NMDA receptor dependent. Since NMDA receptors are involved in neuroplastic changes, the results suggest a possible application of tDCS in the modulation or induction of these processes in a clinical setting. The selective elimination of tDCS-driven excitability enhancements by carbamazepine proposes a role for this drug in focussing the effects of cathodal tDCS, which may have important future clinical applications.
The transcranial application of weak direct currents (transcranial direct current stimulation, tDCS) to the human primary motor cortex is capable of eliciting intracortical excitability changes. The direction of these modulations depends on stimulation polarity: Anodal stimulation increases excitability, while cathodal stimulation diminishes it (Nitsche & Paulus, 2000). The respective changes evolve during the stimulation but remain, so far, for up to 1 h after the end of stimulation, given sufficiently long stimulation duration (Nitsche & Paulus, 2000, 2001; Nitsche et al. 2003a). Beyond these electrophysiological changes, which were assessed by transcranial magnetic stimulation (TMS), a functional magnetic resonance imaging study has revealed similarly directed tDCS-elicited changes in motor cortical activity (Baudewig et al. 2001). The efficacy of tDCS is not restricted to the motor cortex: Stimulation of the visual cortex has been shown to modulate contrast and phosphene thresholds (Antal et al. 2001, 2003). Functionally, tDCS modulates use-dependent neuroplasticity as well as implicit motor learning (Rosenkranz et al. 2000; Nitsche et al. 2003b).
Little is known about the mechanisms underlying the effects of tDCS in the human. In other animals, during anodal stimulation cortical neurones are depolarised at a subthreshold level, while they are hyperpolarised by cathodal DCS (Purpura & McMurtry, 1965). The respective membrane potential changes could serve as a precondition for the induction of after-effects in these animals (Bindman et al. 1964; Frègnac et al. 1990; Tsumoto, 1993; Froc et al. 2000). The fact that the voltage-dependent sodium channel blocker carbamazepine (CBZ) eliminates the short-lasting after-effects induced by anodal, but not by cathodal stimulation indicates that this could be similar in the human (Liebetanz et al. 2002). However, the involvement of sodium channels in the effects of tDCS during stimulation has not been tested so far. Moreover, it is unknown whether additional ion channels participate in tDCS-elicited excitability changes. Calcium channels are likely candidates, since in the animal, intracellular calcium levels are increased after anodal DCS (Islam et al. 1995) and changes in intracellular calcium level are important for the induction of neuroplasticity (Bennett, 2000). Moreover, modulation of calcium-channel activity could change the amount of transmitter release and thus modify cortical excitability. At the receptor level, NMDA-receptor modulation seems to be involved in the induction of the short-lasting after-effects of tDCS in humans (Liebetanz et al. 2002), which is of special importance because these are important for the induction of neuroplastic mechanisms (Bennett, 2000). However, so far it is not known whether NMDA receptors are modulated even during short-lasting DCS, which does not per se induce after-effects, and whether they are of importance for the induction of the long-lasting after-effects elicited by prolonged tDCS.
Therefore, in the present study we tested (1) the dependence of intracurrent excitability modifications on changes of ion-channel conductivity by applying the sodium channel blocker CBZ and the calcium channel blocker flunarizine (FLU), (2) the involvement of NMDA receptors in the generation of intracurrent effects by antagonising these receptors with dextromethorphane (DMO) and (3) the dependence of long-lasting tDCS-induced after-effects on sodium and calcium channel activity as well as NMDA receptor modulation by applying CBZ, FLU and DMO prior to tDCS protocols that are known to elicit long-lasting after-effects.
It has already been shown that the long-lasting after-effects of tDCS are localised intracortically (Nitsche & Paulus, 2001; Nitsche et al. 2003a). However, since this has not yet been studied for the intra-tDCS excitability shifts, we conducted a control experiment comparing the effects of tDCS on TMS-generated motor-evoked potentials (MEPs) and F-waves. Whereas the former results primarily in an indirect excitation of pyramidal tract neurones through activation of intracortical neurones (Edgley et al. 1997), F-waves reflect the excitability of the second motor neurone.
METHODS
Subjects
Eleven to fourteen healthy subjects were included in each main experiment (for details see Table 1). All gave their written informed consent to participate. The investigation was approved by the ethics committee of the University of Goettingen, and conformed with the Declaration of Helsinki.
Table 1.
Study and subject characteristics
| tDCS condition | Drug | Dosage (mg) | Drug intake before tDCS (h) | TMS stimulation intensity* (% of maximum stimulator output) | MEP amplitude* (μV) | tDCS stimulation duration per cycle | Number of subjects | Age of subjects * (years) | Sex of subjects (f = female, m = male) |
|---|---|---|---|---|---|---|---|---|---|
| Intra-tDCS | CBZ | 600 | 12–16/2 | 52.455 (9.512) | a: 1025.515 (91.450) | 4 s a/c | 12 | 25.067 (3.535) | f = 6; m = 6 |
| c: 991.202 (54.102) | |||||||||
| FLU | 10 | 2 | 48.182 (9.693) | a: 1005.935 (45.379) | 4 s a/c | 11 | 25.636 (3.749) | f = 5; m = 6 | |
| c: 1005.504 (45.475) | |||||||||
| DMO | 150 | 2 | 49.273 (9.253) | a: 988.433(57.194) | 4 s a/c | 12 | 25.067(3.535) | f = 6; m = 6 | |
| c: 1001.038 (66.044) | |||||||||
| PLC | 40 | 2 | FLU: 42.8 (13.580) | FLU: | 4 s a/c | FLU: 11 | FLU: 25.636 | FLU: f = 5; m = 6 | |
| Other: 49.545 (8.054) | a: 1027.303 (68.519) | Other: 12 | (3.749) | Other: f = 6; m = 6 | |||||
| c: 1039.78 (66.987) | Other: 25.067 | ||||||||
| Other: | (3.535) | ||||||||
| a: 964.023 (144.350) | |||||||||
| c: 982.620 (127.630) | |||||||||
| After-effects | CBZ | 600 | 12–16/2 | a: 41.500 (8.751) | a: 1043. 825 (103.198) | 11 min a | 10 | 26.000(2.900) | f = 2; m = 8 |
| c: 42.875 (8.323) | c: 1120.293 (140.869) | 9 min c | |||||||
| FLU | 10 | 2 | a: 44.929 (10.118) | a: 1013.445 (40.213) | 13 min a | 14 | 25.000 (4.057) | f = 7; m = 7 | |
| c: 44.429 (9.027) | c: 1016.015 (28.251) | 9 min c | |||||||
| DMO | 150 | 2 | a: 38.875 (9.015) | a:1022.356 (131.836) | 11 min a | 10 | 26.000(2.900) | f = 2; m = 8 | |
| c: 38.375 (9.070) | c: 1029.932 (149.038) | 9 min c | |||||||
| PLC | 40 | 2 | FLU: | FLU: | 11/13 min a | FLU: 14 | FLU: 25.000 | FLU: f = 7; m = 7 | |
| a: 45.308 (10.053) | a: 1019.463 (84.489) | 9 min c | Other: 10 | (4.057) | Other: f = 2; m = 8 | ||||
| c: 45.385 (9.269) | c: 1007.275 (75.679) | Other 26.000 | |||||||
| Other: | Other: | (2.900) | |||||||
| a: 40.250 (9.223) | a: 942.868 (111.506) | ||||||||
| c: 38.375 (9.591) | c: 1016.287 (132.407) |
This table illustrates the drugs applied, drug dosages, time-point of drug application relative to tDCS, TMS stimulation intensity required to achieve a baseline/non-current stimulation MEP amplitude of 1 mV, achieved baseline/non-current MEP amplitudes, tDCS duration, and number, age and sex of the subjects used for the intra- and after-effect parts of the study.
Values are means ± s.d. a = anodal tDCS, c = cathodal tDCS.
tDCS of the motor cortex
Current was induced by a pair of saline-soaked surface sponge electrodes (35 cm2). The DC currents were generated by a specially developed, battery-driven constant-current stimulator (Schneider Electronic, Gleichen, Germany) with a maximum output of 1 mA. The motor-cortical electrode was fixed over the representational field of the right abductor digiti minimi muscle (ADM), as identified by TMS; the other electrode was placed contralaterally above the right orbit. In the experiments, the currents flowed continuously for either 4 s, 9 min (cathodal tDCS) or 11-13 min (anodal tDCS) at an intensity of 1.0 mA. These stimulation durations were chosen because 4 s of tDCS results in an excitability change during stimulation without producing after-effects, while a stimulation duration of 9-13 min generates after-effects that last for about 1 h after the end of stimulation (Nitsche & Paulus 2000, 2001; Nitsche et al. 2003a). Constant current flow and the imposed voltage were monitored during tDCS. Most subjects were able to feel the current flow, at least initially, as a slight itching sensation at both the anodal and cathodal electrode contact points, and/or by perceiving light flashes when the current was turned on and off.
Pharmacological interventions
Two hours before the start of each experimental session, 150 mg DMO, 10 mg FLU or equivalent placebo drugs (PLC) were administered orally to the subjects. The oral intake of CBZ was split into two doses to minimise the side effects of drug intake: The first dose (300 mg) was given the evening before the day that the experimental session took place. Another 300 mg was given 2 h before the start of the experiment. It is well known with these drugs that a sufficient plasma level is achieved 2 h after oral intake (Geradin et al. 1976; Pynnonen, 1979; Holmes et al. 1984; Silvasti et al. 1987), and that the respective doses are sufficient to elicit prominent effects in the central nervous system (Louis & Spierings, 1982; Stoica & Enulescu, 1993; Ziemann et al. 1996, 1998). To avoid cumulative drug effects, each experimental session was separated by at least 1 week, or 2 weeks in the case of CBZ and FLU. The subjects and the person conducting the experiment were blinded to the respective pharmacological condition.
Measurement of motor-system excitability
To detect current-driven changes of cortical excitability, MEPs of the right ADM were recorded following TMS of its motor-cortical representational field. These were elicited by single-pulse TMS using a Magstim 200 magnetic stimulator (Magstim, Whiteland, Dyfed, UK) and a figure-of-eight magnetic coil (diameter of one winding = 70 mm, peak magnetic field = 2.2 T). The coil was held tangentially to the skull, with the handle pointing backwards and laterally at 45 ° from midline. The optimal position was defined as the site where stimulation resulted consistently in the largest MEP. The surface EMG was recorded from the right ADM. The signals were amplified and filtered with a time constant of 10 ms and a low-pass filter of 2.5 kHz. Signals were then digitised at an analog-to-digital rate of 5 kHz, and further relayed into a laboratory computer using the Neuroscan software collection (Neuroscan, Herndon, VA, USA) and conventional averaging software.
Experimental procedures
Each experiment was conducted using a repeated-measurement design. The subjects were seated in a reclining chair. First, the left motor-cortical representational field of the right ADM was identified with the aid of TMS (the coil position that leads to the largest MEPs of the ADM). Then one tDCS electrode, which will be referred to henceforth as presenting either cathodal or anodal stimulation, was fixed at this position, the other one was mounted on the contralateral forehead above the orbit.
Experiment 1 (intracurrent excitability changes)
A randomised series (0.1 Hz) of 15 TMS-evoked MEPs elicited (1) immediately before the end of a 4 s-long current stimulation or (2) without preceding current stimulation was recorded. Anodal and cathodal tDCS were carried out in one session in randomised order.
Experiment 2 (after-effects of long-lasting tDCS)
First, a baseline of TMS-evoked MEPs (20 stimuli) was recorded at 0.25 Hz, after which tDCS was applied. Anodal tDCS was performed for 11 or 13 min, and cathodal tDCS for 9 min (for details see Table 1). Immediately after tDCS, 15 MEPs were recorded every 5th min at 0.25 Hz for 30 min. After this, 15 MEPs were recorded at the same frequency every 30 min until 90 min after the end of tDCS. Anodal and cathodal stimulation were separated by at least 1 week to avoid cumulative effects of tDCS.
Calculations and statistics
MEP amplitude means were calculated in experiment 1 for the current and non-current conditions (15 stimuli each), and in experiment 2 for each time bin covering baseline (20 stimuli) and poststimulation time-points (15 stimuli). These were normalised in each experiment; they are given as a quotient of the without-current (experiment 1) or pre-current (experiment 2) baseline.
For experiment 1, a repeated-measures ANOVA was calculated with the independent variables drug condition, current flow and the dependent variable MEP amplitude. Then Student's t tests (paired samples, two-tailed, P < 0.05) were performed to test whether the values of the current and non-current conditions differed and if those differences depended on drug condition. Since different groups of subjects were measured for the FLU condition on the one hand and CBZ and DMO on the other, separate ANOVAs were calculated for both.
For experiment 2, repeated-measures ANOVAs (independent variables time course, current stimulation, drug condition, dependent variable MEP-amplitude) were calculated, then Student's t tests (paired samples, two-tailed, level of significance P < 0.05) were performed to test whether the MEP amplitudes before and after tDCS differed in each condition, and if these differences were dependent on the drug condition. Because different groups of subjects were measured for the FLU condition on the one hand and CBZ and DMO on the other, separate ANOVAs were calculated for both.
Student's t tests were used to compare the amplitudes of non-tDCS (experiment 1) and baseline MEPs (experiment 2) between the different pharmacological conditions to control for a priori differences, which could have influenced the results systematically.
Post hoc t tests were not adjusted for multiple comparisons according to Perneger (1998).
F-waves
Using F-waves, we investigated the effect of tDCS applied to the left M1 on spinal excitability in four subjects (three male, mean ± s.e.m. age 31.5 ± 5.7 years). TMS to the left M1 and ulnar nerve stimulation (UNS), each given alone or preceded by 4 s of tDCS to M1, were applied in a randomised order at 0.1 Hz. For UNS, electrical stimuli (200-300 V) were delivered by a Digitimer D185 MultiPulse stimulator (Digitimer, Welwyn Garden City, Hertfordshire, UK) to the right ulnar nerve at the wrist through a pair of surface Ag/AgCl electrodes in order to elicit F-waves in the right ADM muscle. TMS, tDCS, surface EMG recording and data acquisition were conducted as mentioned above. Ten stimuli were applied for each condition (TMS with/without tDCS, UNS with/without tDCS) and in two separate sessions in order to study the effects of anodal and cathodal tDCS.
Mean peak-to-peak amplitudes and areas of MEP and F-waves were evaluated and a ratio of those values obtained with tDCS and those obtained without tDCS was calculated. These ratios were then entered in a three-factorial ANOVA with inner-subject factors ‘tDCS’ (anodal, cathodal), ‘stimulation’ (MEP, F-wave) and ‘measure’ (peak-to-peak amplitude, area). When significant findings were revealed using the ANOVA, paired-sample two-tailed t tests were performed to determine whether the ratios for MEP and F-waves of the anodal tDCS and cathodal tDCS conditions differed. A P level of < 0.05 was considered significant in all tests.
RESULTS
Baseline and without-tDCS MEP amplitudes did not differ between the respective pharmacological conditions (P > 0.05, Table 2).
Table 2.
Results of the ANOVAs
| tDCS | d.f. | F | P | |
|---|---|---|---|---|
| Intra-tDCS | tDCS | 1 | 77.383 | < 0.001* |
| (CBZ, DMO, PLC) | Drug | 2 | 6.228 | 0.007* |
| tDCS × drug | 2 | 8.345 | 0.002* | |
| Intra-tDCS | tDCS | 1 | 64.249 | < 0.001* |
| (FLU, PLC) | Drug | 1 | 4.234 | 0.067 |
| tDCS × drug | 1 | 4.185 | 0.068 | |
| After-effects | tDCS | 1 | 199.371 | < 0.001* |
| (CBZ, DMO, PLC) | Drug | 2 | 39.105 | < 0.001* |
| Time course | 9 | 2.806 | 0.007* | |
| tDCS × drug | 2 | 49.810 | < 0.001* | |
| tDCS × time course | 9 | 14.785 | < 0.001* | |
| Drug × time course | 18 | 1.899 | 0.020* | |
| tDCS × drug × time course | 18 | 7.164 | < 0.001* | |
| After-effects | tDCS | 1 | 158.595 | < 0.001* |
| (FLU, PLC) | Drug | 1 | 50.782 | < 0.001* |
| Time course | 9 | 1.309 | 0.240 | |
| tDCS × drug | 1 | 40.202 | < 0.001* | |
| tDCS × time course | 9 | 34.557 | < 0.001* | |
| Drug × time course | 9 | 9.500 | < 0.001* | |
| tDCS × drug × time course | 9 | 6.592 | < 0.001* |
Repeated-measure ANOVAs were calculated for the intra-tDCS and the after-effect part of the study. Notice that separate ANOVAs were calculated for the CBZ/DMO data and for the FLU data, because of different subjects and different stimulation durations (after-effects of anodal condition only). Asterisks indicate significant results (P < 0.05).
Excitability shifts during short-lasting tDCS (experiment 1)
For the intra-tDCS effects, the ANOVAs revealed a significant effect of the drug and tDCS condition as well as a significant interaction with CBZ/DMO, whereas in the FLU condition only the main effect of tDCS was significant (Table 2). As revealed by the t tests, this is due to different effects of tDCS in the various drug conditions: in the PLC and DMO conditions, a significant enhancement and reduction of excitability was revealed during anodal and cathodal tDCS, respectively. In contrast, the anodal tDCS-elicited excitability enhancement was eliminated by CBZ and was significantly reduced by FLU. The cathodal tDCS-induced excitability reduction was not modulated by any of the drugs (Fig. 1).
Figure 1. Drug-induced modulation of tDCS-driven cortical excitability changes during stimulation.
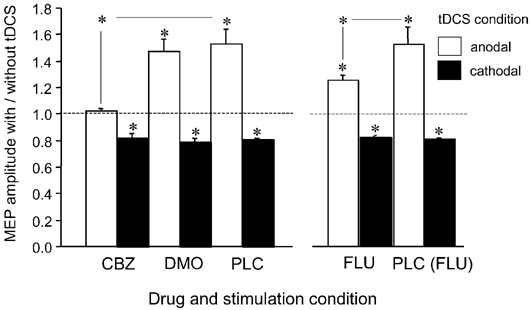
As the results show, the enhancement in anodal tDCS-generated cortical excitability is eliminated by CBZ and reduced by FLU, but not modulated by DMO. Conversely, none of the applied drugs changed the cathodal stimulation-elicited cortical excitability reduction. Due to different subject groups, PLC values differ from FLU values on the one hand and DMO/CBZ values on the other. Asterisks indicate significant deviations of the current from the non-current conditions and differences between the drug conditions regarding identical current conditions (Student's t test, two-tailed, paired samples, P < 0.05). Error bars indicate s.e.m.
Figure 2 shows the effects of anodal and cathodal tDCS on MEP and F-waves. The ANOVA performed on MEP and F-wave ratios revealed a significant effect for ‘tDCS’ (df = 1, F = 18.79, P = 0.023) and for an interaction between ‘tDCS’ and ‘stimulation’ (df = 1, F = 20.6, P = 0.02), but not for ‘stimulation’ alone (df = 1, F = 0.172, P = 0.706). Post hoc analysis showed that MEP ratios of the anodal and the cathodal tDCS conditions were significantly different for both types of measure (amplitude: t test P = 0.024; area: t test P = 0.016). The mean MEP amplitude increased during anodal tDCS by 22 % (area by 19 %) and decreased during cathodal tDCS by 15 % (area by 13 %). This was clearly not the case for F-waves. The mean change in F-waves was in the range of ± 2 %, but this was not significant (amplitude: t test P = 0.368; area: t test P = 0.234).
Figure 2. The neuronal excitability shifts during tDCS are localised proximal to the spinal motor neurone.
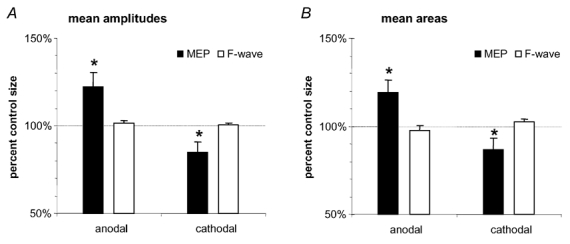
TMS-generated MEPs and F-waves were elicited during short-lasting tDCS, which elicits no after-effects. Whereas anodal tDCS diminished the MEP amplitudes and area under the curve significantly, and cathodal stimulation resulted in reverse effects, F-waves were not changed by tDCS. Asterisks indicate significant deviations of the current from the respective non-current conditions (Student's t test, two-tailed, repeated measures, P < 0.05). Error bars indicate s.e.m.
Excitability shifts after long-lasting tDCS (experiment 2)
For the long-lasting after-effects, the ANOVAs revealed significant main effects for drug, tDCS and time course (CBZ/DMO condition only) as well as significant interactions of these variables (Table 2). This is due to the following pattern of results: in the PLC condition, anodal tDCS enhanced excitability significantly, whereas cathodal tDCS led to a reduction of excitability. Both effects were present for up to 1 h after the end of tDCS (Figs 3-5). In contrast, the application of CBZ and FLU selectively prevented the generation of the after-effects of anodal stimulation (Fig. 3 and Fig. 4), whereas DMO application eliminated the anodal as well the cathodal tDCS-generated after-effects (Fig. 4). For the CBZ condition, a trend for anodal tDCS to reduce instead of enhance cortical excitability as well as to increase the efficacy of cathodal tDCS was observed. These differences were not, however, significant.
Figure 3. Sodium channel blockade selectively prevents the anodal stimulation after-effect.
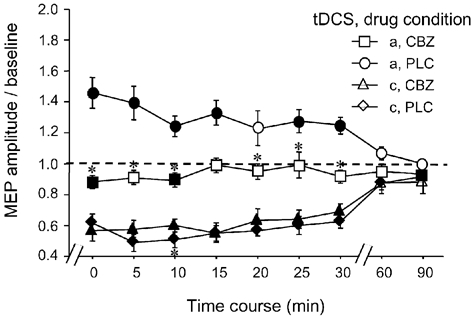
Blocking sodium channels with the aid of the voltage-dependently acting CBZ resulted in complete abolition of the prolonged excitability enhancement caused by anodal tDCS in the PLC condition. Asterisks indicate significant differences between the drug conditions regarding identical current conditions and time points, filled symbols represent significant deviations from baseline in regard to each drug condition (Student's t test, two-tailed, repeated measures, P < 0.05). a, anodal; c, cathodal. Error bars indicate s.e.m.
Figure 5. The NMDA receptor antagonist DMO eliminates the after-effects induced by both anodal and cathodal tDCS.
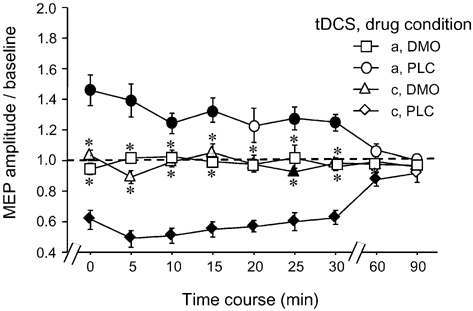
Blocking NMDA receptors abolished any after-effect caused by prolonged tDCS, thus favouring a prominent role of this receptor in the evolvement of neuroplastic effects induced by this kind of stimulation. Asterisks indicate significant differences between the drug conditions regarding identical current conditions and time points, filled symbols significant deviations from baseline in regard to each drug condition (Student's t test, two-tailed, repeated measures, P < 0.05). Error bars indicate s.e.m.
Figure 4. Calcium channel blockade eliminates only the anodal tDCS-induced after-effects on cortical excitability.
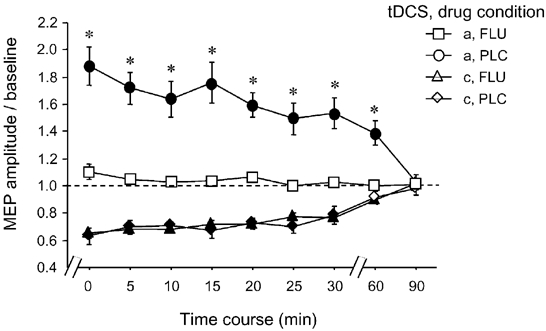
Blocking calcium channels with the aid of FLU resulted in complete abolition of the prolonged excitability enhancement caused by anodal tDCS in the PLC condition. Asterisks indicate significant differences between the drug conditions regarding identical current conditions and time points, filled symbols signify significant deviations from baseline in regard to each drug condition (Student's t test, two-tailed, repeated measures, P < 0.05). Error bars indicate s.e.m.
The intensity of the TMS had to be increased in order to achieve an MEP amplitude of 1 mV in the CBZ condition as compared to PLC, because of the elevation of motor threshold known to be induced by the former drug (Ziemann et al. 1996), but not in the other pharmacological conditions.
Most subjects reported minor side-effects of the medication such as tiredness and ataxia, with all the drugs except PLC. None of the subjects terminated their participation in the experiment as a result of side-effects.
DISCUSSION
In this study we tested the involvement of sodium and calcium channel conductivity as well as NMDA receptors in the cortical excitability modulations elicited during and after tDCS of the human primary motor cortex by applying the voltage-dependent sodium channel blocker CBZ, the calcium channel blocker FLU and the NMDA receptor antagonist DMO, prior to tDCS.
Short-lasting tDCS (experiment 1)
With regard to the excitability changes observed during short-lasting tDCS, which does not elicit any after-effects (Nitsche & Paulus 2000), the results are compatible with a dependency on membrane polarisation: blocking voltage-dependent sodium channels eliminates completely the excitability enhancement that is observed during anodal stimulation, and blocking calcium channels diminishes it. This pattern of results is in accordance with animal experiments (Purpura & McMurtry, 1965) in which it was found that anodal tDCS causes neuronal depolarisation. On the other hand, the reduction in excitability caused by cathodal tDCS is not changed by ion channel blockade. Because the activity of both channels is voltage dependent (Holmes et al. 1984; McLean & McDonald, 1986), this result could be due to cathodal tDCS-generated neuronal hyperpolarisation, which in the non-human animal represents the main effect of cathodal stimulation (Purpura & McMurtry 1965). Neuronal hyperpolarisation would inactivate the respective sodium and calcium channels, and thus administration of the voltage-dependently acting ion channel blockers CBZ and FLU would be without any effect. Since F-waves did not change during tDCS, these effects are localised proximal to the spinal motor neurone.
It should be noticed that the evidence from these experiments for tDCS-generated changes in neuronal polarisation during current flow is indirect; additional experiments are needed to confirm the results.
The lower efficacy of FLU as compared to CBZ may be caused by the predominance of sodium channels over calcium channels in controlling neuronal membrane potential changes. Alternatively, a relatively low dose of this drug could have limited its efficacy. However, because of the prominent influence on the after-effects of tDCS (see below), the latter explanation seems unlikely.
In contrast, it appears that the intracurrent excitability changes during short-lasting tDCS do not depend on changes of NMDA-receptor efficacy, as shown by the lack of any effect of antagonising these receptors with DMO in these conditions.
Long-lasting tDCS (experiment 2)
For long-lasting after-effects, the situation is different. DMO-induced NMDA receptor inhibition completely prevents the poststimulation excitability enhancement. Moreover, NMDA receptor blockade abolishes the excitability diminution caused by cathodal tDCS. This suggests that this receptor is of some importance in the development of tDCS-induced after-effects. This is particularly interesting since NMDA receptors are known to be involved in cortical neuroplastic mechanisms like long-term potentiation and depression (Bennett, 2000).
Similar to the intrastimulation effects, blocking sodium or calcium channels prevents only the excitability enhancement caused by anodal tDCS. If tDCS acts in humans similarly to other animals by depolarising or hyperpolarising neuronal membranes in a polarity-dependent manner, this suggests an important function of these membrane potential shifts not only for the tDCS-induced excitability changes during stimulation, but also for the induction of after-effects.
General remarks
From a functional perspective, this pattern of results could be explained as follows. The long-lasting after-effects of tDCS may be due to an enhanced efficacy of NMDA receptors in the case of anodal stimulation and a reduced receptor efficacy in the case of cathodal stimulation. These efficacy changes may be induced by an intrastimulation polarity-specific neuronal depolarisation or hyperpolarisation. With regard to likely mechanisms ‘translating’ the changes of membrane polarisation into changes of receptor efficacy, at least two possibilities must be taken into account, which may act in combination. On the one hand, high-frequency presynaptic activity, which would be caused by anodal tDCS-induced subthreshold membrane depolarisation (Bindman et al. 1964), combined with postsynaptic subthreshold membrane depolarisation enhances NMDA receptor efficacy, whilst a reduction in presynaptic discharge rate, which is induced by cathodal tDCS (Bindman et al. 1964), combined with postsynaptic hyperpolarisation decreases it (Frégnac et al. 1990; Froc et al. 2000). On the other hand, it is known that NMDA receptor efficacy depends on intracellular calcium level (Bennett, 2000); a prolonged calcium increase enhances NMDA receptor efficacy, while a low calcium level reduces it. During tDCS, calcium channel activity seems to be modified, and blocking calcium channels by FLU and thus diminishing intracellular calcium levels eliminates the anodal but not the cathodal after-effects. The presumed changes in intracellular calcium concentration may contribute to the receptor modifications probably elicited by prolonged tDCS. However, it cannot be ruled out that a decrease in synaptic glutamate release, which is also caused by blocking calcium channels, also contributes to the effect. Additional experiments are needed to test these hypotheses.
Since the effects are highly specific to the drugs used and were all fully reversible in a much shorter time course than the half-life of the substances used (Pyonnen, 1979; Holmes et al. 1984; Silvasti et al. 1987), it seems unlikely that unspecific drug effects such as tiredness or dizziness, although present, contributed to the results achieved. However, most of the drugs used in this study are not ‘pure’ substances, to some extent they influence, at least at higher concentrations, other ion channels and receptors than those mentioned (Netzer et al. 1993; Okada et al. 1997; Mizuno et al. 2000). Animal experiments are needed to confirm the results of this study. That the efficacy of anodal tDCS was somewhat less in some experiments but not in all compared to former studies (Nitsche & Paulus 2001) may be due to group specifics. The tendency of CBZ to increase the cathodal after-effects and to cause a trend towards a small excitability decrease in cases of anodal tDCS, could be due to subject tiredness. However, this did not occur in the DMO condition, which caused similar side-effects. Thus this pattern of results may have other underlying mechanisms: CBZ, since it acts voltage dependently, may have reduced the anodal tDCS-generated depolarisation, as shown by the intra-tDCS results. It is known from brain slice experiments that a neuronal membrane depolarisation that is not sufficient to induce enhancement of long-term excitability can nonetheless result in reductions of long-term excitability due to minor calcium influx (Malenka & Nicoll, 1999; Lisman 2001). This could have been the case in our experiments. Moreover, the effects of tDCS depend critically on the direction of current flow through neurones (Purpura & McMurtry 1965), and thus small populations of neurones oriented differently to those causing the net effects may be inhibited by anodal tDCS. The inhibitory action of anodal tDCS on this neuronal population could be revealed in the CBZ condition, which eliminates all current-generated excitability enhancements. Conversely, these neurones would be depolarised by cathodal stimulation, and again these after-effects would be inhibited by applying CBZ. Although this study does not allow a final decision to be made between the possible explanations of the results, the current flow hypothesis offers a possible role for CBZ in focussing the effects of cathodal tDCS, which could be important for future clinical applications (e.g. diminishing cortical excitability in epilepsy or dystony) where a maximum pure excitability reduction would be favourable.
Taken together, the results of this study suggest that short-lasting tDCS of the human motor cortex, which causes no after-effects, acts via a polarity-specific neuronal membrane depolarisation or hyperpolarisation. Long-lasting after-effects may reflect a change of NMDA receptor efficacy. The latter may be caused, at least partially, by prolonged membrane polarisation-induced modifications in intracellular calcium concentration. Because NMDA receptors are involved in neuroplastic changes, the results suggest a possible application of tDCS in the modulation or induction of these processes in a clinical setting (e.g. rehabilitation). For healthy human subjects, it has already been shown that tDCS can modulate neuroplastic changes (Rosenkranz et al. 2000; Nitsche et al. 2003b). However, pharmacological modulations in humans can only deliver indirect evidence for the mode of action of tDCS. Thus, additional animal and human experiments have to be performed to confirm these results.
REFERENCES
- Antal A, Kincses TZ, Nitsche MA, Paulus W. Manipulation of phosphene thresholds by transcranial direct current stimulation in man. Exp Brain Res. 2003;150:375–378. doi: 10.1007/s00221-003-1459-8. [DOI] [PubMed] [Google Scholar]
- Antal A, Nitsche MA, Paulus W. External modulation of visual perception in humans. Neuroreport. 2001;12:3553–3555. doi: 10.1097/00001756-200111160-00036. [DOI] [PubMed] [Google Scholar]
- Baudewig J, Nitsche MA, Paulus W, Frahm J. Regional modulation of BOLD MRI responses to human sensorimotor activation by transcranial direct current stimulation. Magn Reson Med. 2001;45:196–201. doi: 10.1002/1522-2594(200102)45:2<196::aid-mrm1026>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Bennett MR. The concept of long term potentiation of transmission at synapses. Progr Neurobiol. 2000;60:109–137. doi: 10.1016/s0301-0082(99)00006-4. [DOI] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OC, Redfearn JW. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol. 1964;172:369–382. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Eyre JA, Lemon RN, Miller S. Comparison of activation of corticospinal neurons and spinal motor neurons by magnetic and electrical transcranial stimulation in the lumbosacral cord of the anaesthetized monkey. Brain. 1997;120:839–853. doi: 10.1093/brain/120.5.839. [DOI] [PubMed] [Google Scholar]
- Frégnac Y, Smith D, Friedlander MJ. Postsynaptic membrane potential regulates synaptic potentiation and depression in visual cortical neurons [abstract] Soc Neurosci Abstr. 1990;16:798. [Google Scholar]
- Froc DJ, Chapman CA, Trepel C, Racine RJ. Long-term depression and depotentiation in the sensorimotor cortex of the freely moving rat. J Neurosci. 2000;20:438–445. doi: 10.1523/JNEUROSCI.20-01-00438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geradin AP, Abadie FV, Campestrini JA, Theobald W. Pharmacokinetics of carbamazepine in normal humans after single and repeated oral doses. J Pharmacokinet Biopharm. 1976;4:521–535. doi: 10.1007/BF01064556. [DOI] [PubMed] [Google Scholar]
- Holmes B, Brogden RN, Heel RC, Speight TM, Avery GS. Flunarizine. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use. Drugs. 1984;27:6–44. doi: 10.2165/00003495-198427010-00002. [DOI] [PubMed] [Google Scholar]
- Islam N, Aftabuddin M, Moriwaki A, Hattori Y, Hori Y. Increase in the calcium level following anodal polarization in the rat brain. Brain Res. 1995;684:206–208. doi: 10.1016/0006-8993(95)00434-r. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to synaptic and membrane mechanisms of DC-induced neuroplasticity in man. Brain. 2002;125:2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Three Ca2+ levels affect plasticity differently: the LTP zone, the LTD zone and no man's land. J Physiol. 2001;532:285. doi: 10.1111/j.1469-7793.2001.0285f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P, Spierings EL. Comparison of flunarizine (Sibelium) and pizotifen (Sandomigran) in migraine treatment: a double-blind study. Cephalgia. 1982;2:197–203. doi: 10.1046/j.1468-2982.1982.0204197.x. [DOI] [PubMed] [Google Scholar]
- McLean MJ, MacDonald RL. Carbamazepine and 10, 11-epoxycarbamazepine produce use- and voltage-dependent limitation of rapidly firing action potentials of mouse central neurons in cell culture. J Pharmacol Exp Ther. 1986;238:727–738. [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation - a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Okada M, Murakami T, Kamata A, Zhu G, Kawata Y, Wada K, Kaneko S. Effects of carbamazepine on acetylcholine release and metabolism. Epilepsy Res. 2000;40:187–195. doi: 10.1016/s0920-1211(00)00129-7. [DOI] [PubMed] [Google Scholar]
- Netzer R, Pflimlin P, Trube G. Dextromethorphan blocks N-methyl-d-aspartate-induced currents and voltage-operated inward currents in cultured cortical neurons. Eur J Pharmacol. 1993;238:209–216. doi: 10.1016/0014-2999(93)90849-d. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol. 2003a;114:600–604. doi: 10.1016/s1388-2457(02)00412-1. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, Tergau F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cog Neurosci. 2003b;15:619–626. doi: 10.1162/089892903321662994. [DOI] [PubMed] [Google Scholar]
- Okada M, Kiryu K, Kawata Y, Mizuno K, Wada K, Tasaki H, Kaneko S. Determination of the effects of caffeine and carbamazepine on striatal dopamine release by in vivo microdialysis. Eur J Pharmacol. 1997;321:181–188. doi: 10.1016/s0014-2999(96)00938-7. [DOI] [PubMed] [Google Scholar]
- Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316:1235–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol. 1965;28:166–185. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- Pynnonen S. Pharmacokinetics of carbamazepine in man: a review. Ther Drug Monit. 1979;1:409–431. doi: 10.1097/00007691-197901030-00014. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Nitsche MA, Tergau F, Paulus W. Diminution of training-induced transient motor cortex plasticity by weak transcranial direct current stimulation in the human. Neurosci Lett. 2000;296:61–63. doi: 10.1016/s0304-3940(00)01621-9. [DOI] [PubMed] [Google Scholar]
- Silvasti M, Karttunen P, Tukiainen H, Kokkonen P, Hanninen U, Nykanen S. Pharmacokinetics of dextromethorphan and dextrorphan: a single dose comparison of three preparations in human volunteers. Int J Clin Pharmacol Ther Toxicol. 1987;25:493–497. [PubMed] [Google Scholar]
- Stoica E, Enulescu O. The influence of amitriptyline and flunarizine on catecholamine response to light in patients with migraine. Rom J Neurol Psychiatry. 1993;31:11–19. [PubMed] [Google Scholar]
- Tsumoto T. Long-term depression in the cerebral cortex: a possible substrate of ‘forgetting’ that should not be forgotten. Neurosci Res. 1993;16:263–270. doi: 10.1016/0168-0102(93)90036-p. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Chen R, Cohen LG, Hallett M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology. 1998;51:1320–1324. doi: 10.1212/wnl.51.5.1320. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]


