Abstract
The rat SK1 gene (rSK1) does not form functional Ca2+-activated potassium channels when expressed alone in mammalian cell lines. Using a selective antibody to the rSK1 subunit and a yellow fluorescent protein (YFP) tag we have discovered that rSK1 expression produces protein that remains largely at intracellular locations. We tested the idea that rSK1 may need an expression partner, rSK2, in order to form functional channels. When rSK1 was co-expressed with rSK2 in HEK 293 cells it increased the current magnitude by 77 ± 34 % (as compared with cells expressing rSK2 alone). Co-expression of rSK1 with rSK2 also changed the channel pharmacology. The sensitivity of SK current to block by apamin was reduced ∼16-fold from an IC50 of 94 pm (for SK2 alone) to 1.4 nm (for SK2 and SK1 together). The sensitivity to block by UCL 1848 (a potent small molecule blocker of SK channels) was similarly reduced, ∼26-fold, from an IC50 of 110 pm to 2.9 nm. These data clearly demonstrate that rSK1 and rSK2 subunits interact. The most likely explanation for this is that the subunits are able to form heteromeric assemblies.
Small conductance calcium-activated potassium channels (SK channels) are widely distributed throughout the body, occurring in neuronal and non-neuronal tissues. In many neurones SK channels underlie components of the post-spike afterhyperpolarization (AHP) (reviewed in Sah & Faber, 2002). Native SK channels can be identified by their characteristic sensitivity to block by the bee venom peptide toxin apamin. These apamin-sensitive channels can also be inhibited by several small molecule blockers, such as UCL 1848, which, like apamin, are active in nanomolar or sub-nanomolar concentrations (Benton et al. 1999; Chen et al. 2000; Shah & Haylett, 2000; Faber & Sah, 2002).
Three genes, SK1, SK2 and SK3, code for SK channel α subunits (Kohler et al. 1996; Joiner et al. 1997; Chandy et al. 1998). The rat homologues of SK2 and SK3 (rSK2 and rSK3, respectively) produce functional SK channels in heterologous expression systems (Kohler et al. 1996; Strobaek et al. 2000; Hosseini et al. 2001). However, the rat SK1 gene (rSK1) has been reported not to produce detectable SK current in transfected cells (Bowden et al. 2001). Information about SK1 has, therefore, been obtained from the human homologue of SK1 (hSK1). However, work with this gene produces results that vary with the expression system used. In Xenopus oocytes most hSK1 channels are insensitive to apamin at concentrations up to 100 nm (Kohler et al. 1996), although a small apamin-sensitive component exists (Grunnet et al. 2001). In mammalian cell lines, hSK1 produces channels that are mostly apamin sensitive (IC50 3-12 nm) (Shah & Haylett, 2000; Strobaek et al. 2000), although occasionally the apamin concentration-inhibition curve also contains an insensitive component (Shah & Haylett, 2000). The pharmacology of native SK1 channels is therefore uncertain.
Since most data concerning native SK channels come from rat tissues, and the behaviour of the rat gene differs from that of the human, it seemed important to re-examine the properties of rSK1.
A preliminary account of this work has been reported to The Physiological Society (Benton et al. 2003).
METHODS
Constructs
The rat SK1 and SK2 genes, subcloned into the pTracer or pcDNA3 mammalian expression vector, were a generous gift of Drs Len Kaczmarek and William Joiner (Yale University). The rat SK1 clone is recorded under Genbank accession number AF000973 and was re-engineered to introduce an optimal Kozak sequence just prior to the start methionine. The rat SK1 construct was tagged with YFP by subcloning it into the pEYFP vector (Clontech). Constructs were sequenced on an ABI 377 sequencer using the Big Dye II sequencing kit. Plasmid DNA for transfection was purified using Maxi Prep or Midi Prep kits (Qiagen).
SK1 antibody production
The peptide ‘KLPPPWPGPSHLTAA’, corresponding to a unique sequence in the C-terminal region of rSK1, was synthesized (Alta Bioscience, Birmingham, UK) and coupled to Keyhole limpet hemacyanin before being used for an initial rabbit immunization (Cocalico, PA, USA). Three subsequent boosts 1 month apart were then administered. Rabbit sera were assayed by ELISA and the IgG fraction of the serum was eventually purified on a protein A Sepharose affinity column prior to use in immunohistochemistry.
Maintenance and transient transfection of cell lines
HEK 293 and rat H4 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10 % fetal calf serum, 2 mml-glutamine, 100 units ml−1 penicillin and 100 µg ml−1 streptomycin. Cells were plated onto either 35 mm culture dishes for electrophysiology or 18 mm glass coverslips for immunohistochemistry. Transfections were carried out using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. For initial studies of the pharmacology of homomeric SK channels we used 1 or 2 µg of channel plasmid. For co-expression of rSK1 with rSK2 we used 2 µg of the rSK1 construct and 1 µg of rSK2. In addition, cells were co-transfected with 1 µg of QBI plasmid DNA (Qbiogene) which expresses green fluorescent protein (GFP), allowing identification of transfected cells.
Immunohistochemistry
Immunostaining of transfected cells plated on glass coverslips was carried out as described previously (Hosseini et al. 2001). Briefly, cells were fixed, permeabilized and blocked, then incubated for 4 h with primary rabbit polyclonal anti-rSK1 antibody (UCL 56) at a concentration of 20 µg ml−1. The cells were washed, then incubated in a 1:200 dilution of a Cy3-conjugated goat anti-rabbit secondary antibody (Chemicon) for 1 h. All antibodies were diluted in blocking buffer. Following a final wash step, the coverslips were mounted onto slides using a small drop of antifade mount (Vector Laboratories Inc.). All staining operations were carried out at room temperature (≈22 °C). Stained cells were viewed with a Leica TCS confocal microscope.
Electrophysiology
Currents were recorded from HEK 293 cells using conventional whole-cell voltage clamp methods as previously described (Hosseini et al. 2001). The bathing solution contained (mm): NaCl 140, KCl 5, MgCl2 1, CaCl2 2, glucose 10, Hepes 10, pH 7.4 with NaOH. The pipette filling solution contained (mm): KCl 130, Hepes 10, K2HEDTA 5 and CaCl2 1.2 (free Ca2+ 1 µm), pH 7.2 with KOH. The pipettes had resistances of 2-4 MΩ when filled with pipette solution. Experiments were conducted at room temperature (20-25 °C).
Membrane currents were recorded with either a List EPC7 amplifier using a Digidata 1320A interface and pCLAMP 8.2 software (Axon Instruments) for acquisition, or a HEKA EPC9 patch clamp amplifier under control of Pulse (HEKA). Data were filtered at 1 kHz and digitized at 5 kHz. Acquired current traces were analysed with either Clampfit 8.2 or HEKA Pulsefit.
Data analysis
For comparison of current levels in cells expressing rSK2 alone or in combination with rSK1 the current at -20 mV was normalized against whole-cell capacitance and expressed as current density.
The effect of blocking agents was expressed as the current recorded at -20 mV in the presence of blocker as a percentage of that in its absence. The resulting concentration-inhibition curves were fitted by the Hill equation in the form:
where y is the current in the presence of blocker as a percentage of the control, [I] is the concentration of inhibitor, nH is the Hill coefficient and IC50 is the concentration of blocker that reduces the current to 50 % of the control value.
(A potential source of error in our measurement of current inhibition is that the period of exposure of the cells to blockers (3 or 4 min for UCL1848 or apamin, respectively) might be too short for equilibrium to be reached at the lowest concentrations used (30 and 100 pm). Taking published values of k+1 and k-1 for apamin binding to SK2 (Strobaek et al. 2000), it can be shown that at our lowest concentration (30 pm) this error is likely to be less than 10 % and thus any effect on our estimates of the IC50 and nH value will be small. No kinetic data are available for UCL 1848. The onset and offset of the action of this compound are, however, faster than for apamin and thus errors will be smaller still.)
Curve fitting was performed by the method of least squares with data points weighted by the inverse of their variance, using the curve-fitting routine of Origin 5.0 (Microcal). Where appropriate other values are quoted as the mean ± s.e.m. The significance of differences between means was assessed using Student's unpaired t tests.
Drugs and reagents
All materials used for tissue culture were obtained from Invitrogen. UCL 1848 (8,14-diaza-1,7(1,4)-diquinolinacyclotetradecaphanedium ditrifluoroacetate) was synthesized under the supervision of Professor C. R. Ganellin, in the Department of Chemistry, UCL, as previously described (Chen et al. 2000). Apamin was purchased from Sigma. Hepes and HEDTA were from Calbiochem. All other reagents were of Analar quality and obtained from VWR. Horse serum, BSA and paraformaldehyde were obtained from Sigma.
RESULTS
Expression of rSK1
HEK 293 cells, when transfected with rSK1 alone, did not exhibit Ca2+-activated K+ currents whereas transfection with rSK2 produced a substantial Ca2+-activated K+ conductance (Fig. 1A and B). To verify that rSK1 expression produces a protein in these cells we used an anti-rSK1 antibody. To confirm the antibody's selectivity we expressed a YFP-tagged SK1 subunit and then stained transfected cells with the SK1 antibody. A bright, punctate staining pattern was produced by the antibody fluorescence that closely matched fluorescence from the YFP tag (Fig. 2A and B). This shows that SK1 protein is made and is specifically recognized by the antibody. Interestingly, this staining (which was not altered when untagged subunits were expressed) did not extend evenly around the circumference of the cell, suggesting that the protein remains primarily in intracellular compartments.
Figure 1. Expression of rSK1 and rSK2 alone or together in HEK 293 cells.
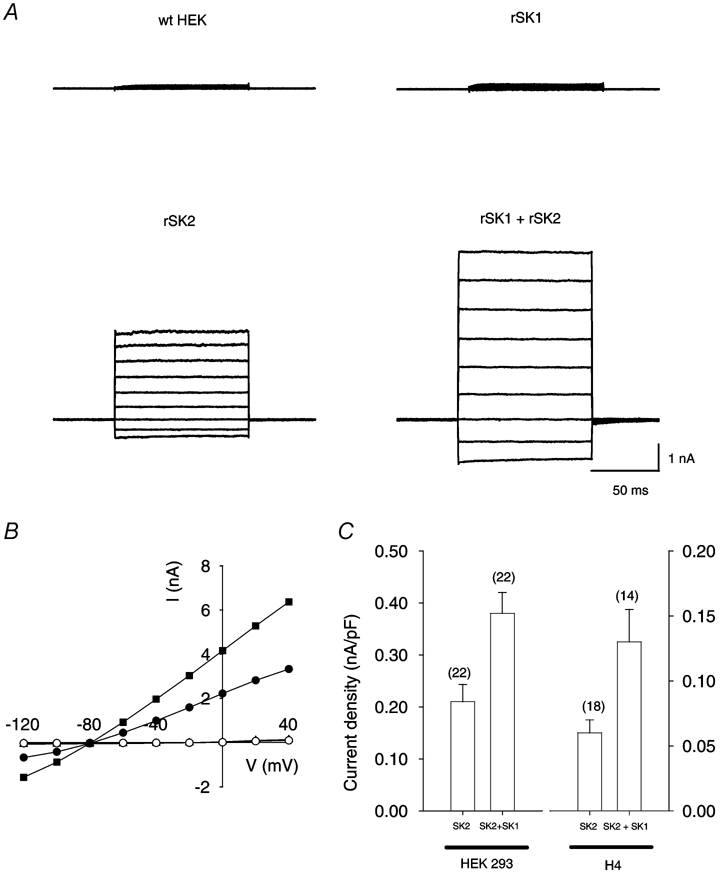
A, typical whole-cell current records obtained from an untransfected (wild-type, wt) HEK 293 cell (top left) and from cells transfected with rSK1 (top right), rSK2 (bottom left) or rSK1 together with rSK2 (bottom right). The cells were held at −80 mV and 100 ms steps applied from −120 to +40 mV. In all cases, the pipette solution contained 1 µm free calcium to activate SK currents. Currents were recorded 1-2 min after patch rupture. B, current-voltage relationships for the currents shown in A. ○, wild-type HEK; ▴, rSK1 (obscured beneath wt data); •, rSK2; ▪, rSK1 and rSK2. Currents recorded from wild-type cells or cells transfected with rSK1 were essentially identical and reversed between −20 and −10 mV. In contrast cells transfected with rSK2 or a combination of rSK1 and rSK2 exhibited large currents with an approximately linear current-voltage relationship, which reversed at approximately −80 mV, close to the predicted value of EK (−85 mV). C, comparison of SK current density in HEK 293 (left) or H4 (right) cells transfected with rSK2 either alone or with a 2-fold excess of rSK1. Histograms represent the mean current density at −20 mV, with the number of cells for each condition shown in parentheses. In both cases co-transfection with rSK1 caused a significant (P < 0.05) increase in current. Error bars indicate s.e.m.
Figure 2. Distribution of rSK1 protein in HEK 293 cells.
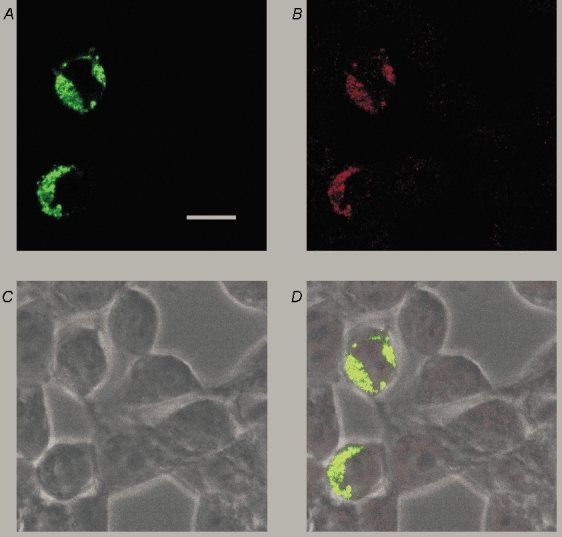
A, confocal image of HEK 293 cells transiently transfected with a YFP-tagged rSK1 construct (green filter) showing that the expressed protein is unevenly distributed around the cell and has a punctate staining pattern. B, the staining seen with rSK1-specific antibody UCL 56. Comparison with A shows that it recognizes the channel protein because the staining coincides well with YFP fluorescence. C, bright field image of cells depicted in A and B. D, overlay of images A, B and C. Scale bar is 20 µm.
Many ion channel α subunits are known to require other subunits to form functional channels (Krapivinsky et al. 1995; Post et al. 1996; Ottschytsch et al. 2002). Moreover, hSK1 subunits have been shown to co-assemble with SK2 subunits in oocytes (Ishii et al. 1997), suggesting that rSK1 may also be able to co-assemble with rSK2 to form functional SK channels.
Co-expression of rSK1 and rSK2 leads to a higher current density
Currents in HEK 293 cells transfected with rSK2 alone or with the same quantity of rSK2 and a 2-fold excess of rSK1 were qualitatively similar, having the same reversal potential and an approximately linear current-voltage relationship (Fig. 1A and B). Currents in co-transfected cells were, however, significantly greater than in cells expressing rSK2 alone: the mean current at -20 mV was 1.9 ± 0.2 nA in cells transfected with rSK2 and 3.1 ± 0.3 nA in cells transfected with both rSK1 and rSK2 (n = 22 for each condition, P < 0.05). When expressed as a current density this corresponds to a 77 ± 34 % increase (Fig. 1C). A similar result was obtained using the rat cell line H4; rSK2 expression produced SK currents while rSK1 expression did not, and co-expression of rSK1 with rSK2 produced a 123 ± 59 % increase in current density (Fig. 1C). If this change in current magnitude occurs because rSK1 and rSK2 form a functional heteromeric channel complex, then the SK channel pharmacology might change. Given the known pharmacology of hSK1, even in its ‘apamin-sensitive’ form, one would predict that the formation of SK1-SK2 complexes would lead to a reduction in apamin and UCL 1848 sensitivity (Ishii et al. 1997; Shah & Haylett, 2000; Strobaek et al. 2000).
We thus compared the effects of two selective SK channel blockers, apamin and UCL 1848, on the currents produced by transfection of rSK2 alone or by co-transfection of rSK1 with rSK2.
Co-expression of rSK1 and rSK2 results in the formation of channels with a novel pharmacology
In control experiments, on homomeric rSK2 channels, the apamin dose-response curve was well fitted by the Hill equation with an IC50 of 95 ± 8 pm and a Hill coefficient (nH) of 0.80 ± 0.06 (Fig. 3A and C). This IC50 for apamin is very close to those previously obtained for inhibition of SK2 current in Xenopus oocytes (Kohler et al. 1996) and HEK 293 cells (Strobaek et al. 2000). In contrast, cells co-transfected with rSK1 produced a concentration- inhibition curve that was shifted significantly to the right, yielding an IC50 of 1.4 ± 0.3 nm and nH value of 0.6 ± 0.1 (Fig.3B and C). Thus co-expression of rSK1 with rSK2 results in the formation of channels with ≈15-fold lower sensitivity to apamin.
Figure 3. Apamin block of current in cells expressing rSK2 alone or rSK1 and rSK2.
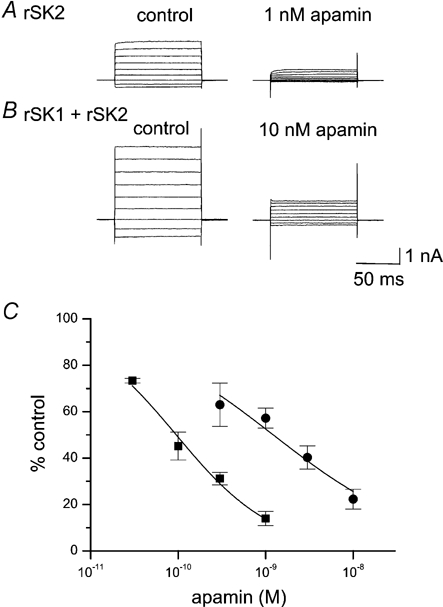
A, typical current traces showing the effect of 1 nm apamin on homomeric rSK2 currents. B, typical current traces showing the effect of 10 nm apamin on a cell co-transfected with rSK1 and rSK2. In both A and B, currents were recorded immediately before and after 4 min exposure to apamin. The scale bar applies to both A and B. C, apamin concentration-inhibition curves for cells transfected with rSK2 alone (▪) or rSK1 and rSK2 (•). Continuous lines are fits of the Hill equation to the data, yielding estimates of IC50 of 95 ± 8 pm (nH = 0.80 ± 0.06) and 1.4 ± 0.3 nm (nH = 0.6 ± 0.1) for rSK2 and rSK1 with rSK2, respectively. Each point is the mean of 4–9 observations and the error bars indicate s.e.m.
We next tested UCL 1848, which has a similar affinity for SK2 to apamin but the advantage of rapid reversibility. For cells expressing rSK2 alone, UCL 1848 blocked SK current with an IC50 of 110 ± 26 pm (nH value of 0.7 ± 0.1), which is very close to the value of 120 pm previously reported (Hosseini et al. 2001). When rSK2 was co-expressed with rSK1 the dose-response curve was best fitted with an IC50 of 2.9 ± 0.3 nm (nH value of 0.49 ± 0.04) as shown in Fig. 4C. This is qualitatively similar to the result obtained with apamin, although the shift in IC50 was rather greater with UCL 1848, a factor of 26. In addition to a change in the IC50, if co-assembly is occurring, then one might expect the dose-response curve to become slightly shallower because it could reflect a number of different populations where various stoichiometries of SK1 and SK2 subunits have formed. Although it is difficult to estimate nH values accurately, for both UCL 1848 and apamin, in our co-expression experiments, concentration-inhibition curves were best fitted using smaller values of the Hill coefficient. (Our fitting of a single component Hill equation to the SK1-2 data is, therefore, only to aid comparison with the SK2 homomeric expression data.) Taken together, these data show that rSK1 and rSK2 subunits can interact, possibly directly, to form functional channels, with a unique pharmacology.
Figure 4. UCL 1848 block of current in cells expressing rSK2 alone or rSK1 and rSK2.
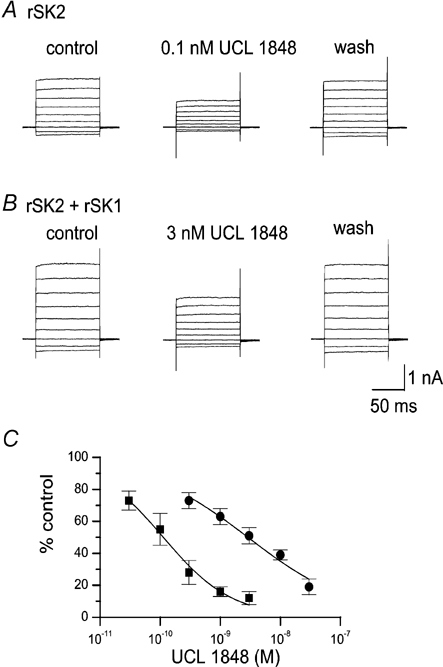
A, effect of 0.1 nm UCL 1848 on rSK2 currents. B, effect of 3 nm UCL 1848 on a cell co-transfected with rSK1 and rSK2. In A and B currents were recorded before and after 3 min exposure to UCL 1848, and 3 min after washout. The scale bar applies to both A and B. C, UCL 1848 concentration-inhibition curves for cells transfected with rSK2 alone (▪) or rSK1 and rSK2 (•). Continuous lines show fits of the Hill equation to the data, yielding estimates of IC50 of 110 ± 26 pm (nH = 0.7 ± 0.1) and 2.9 ± 0.3 nm (nH = 0.49 ± 0.04) for rSK2 and rSK1 with rSK2, respectively. Each point is the mean of 3-5 observations. Error bars indicate s.e.m.
DISCUSSION
We have confirmed that the rat SK1 gene does not produce functional channels when expressed alone in either human (HEK 293) or rat (H4) cell lines. However, our results with an anti-SK1 antibody and the YFP-tagged rSK1 construct indicate that a protein is synthesized.
Many potassium channel genes produce ‘silent’ subunits. These proteins do not form functional channels alone, but can form functional heteromeric channels when co-expressed with related subunits (see for example Krapivinsky et al. 1995; Post et al. 1996; Ottschytsch et al. 2002). The most obvious interpretation of our findings is that rSK1 has a similar function because co-expression of rSK1 with rSK2 caused two important changes. Firstly, the currents in cells where rSK1 was co-expressed with rSK2 were substantially greater than when rSK2 was expressed alone. This increase in current was seen in both a human (HEK 293) and a rat (H4) cell line, suggesting that this result is not related to our choice of expression system. Secondly, co-expression of rSK1 with rSK2 altered the pharmacology, producing SK currents that were ≈15-fold less sensitive to apamin and ≈26-fold less sensitive to UCL 1848.
Our observations clearly indicate an important interaction between rSK1 and rSK2. The simplest explanation for this interaction is that rSK1 and rSK2 can form a functional heteromeric complex. Unfortunately, our attempts to confirm this idea by co-immunoprecipitation failed because tagged channel constructs suitable for this purpose did not display normal channel function (data not shown). Thus, an indirect interaction between rSK1 and rSK2 cannot be ruled out. However, it is tempting to speculate that since both human and rat SK1 subunits interact with SK2 and both also interact with SK3 (Ishii et al. 1997; Benton et al. 2003), the only functional difference between hSK1 and rSK1 may be that the human gene can produce a functional channel when expressed alone.
The interaction between rSK1 and rSK2 demonstrated here in vitro is particularly intriguing in the light of the reported distribution of rSK1 and rSK2 mRNA in vivo. A number of in situ hybridization studies of the rat brain have revealed that the presence of SK1 mRNA is nearly always accompanied by SK2 mRNA (Kohler et al. 1996; Stocker et al. 1999; Stocker & Pedarzani, 2000). Whether SK1 requires SK2 to produce functional channels in vivo, however, remains an open question because recent immunohistochemical data show strong staining for the SK1 subunit where there is only a low level of SK2 protein (Sailer et al. 2002).
Interestingly, Stocker, Pedarzani and colleagues (1999) have reported an IC50 of 480 pm for apamin inhibition of the current underlying a medium duration AHP (mAHP) in CA1 hippocampal neurons. They point out that this value is considerably higher than would be expected for homomeric SK2 channels and suggest a possible role for SK1-2 heteromers (Stocker & Pedarzani, 2000). (Our IC50 of 1.4 nm for apamin block of SK currents produced when SK1 and SK2 are co-expressed is somewhat higher but it must be remembered that this value probably depends on the ratio of SK1:SK2 mRNAs, which are not controlled to be at the ratio of ≈1:1, found in vivo.) Pedarzani, Stocker and colleagues (2002) have similarly reported that the IC50 for block of hippocampal apamin-sensitive channels by the peptide toxin tamapin is ≈10-fold higher than expected for homomeric SK2 channels (Pedarzani et al. 2002). Again this suggests that the currents may not be carried by homomeric SK2 alone. Based on immunohistochemical experiments, however, others have suggested that the hippocampal apamin-sensitive currents are formed by homomeric SK2 channels (Sailer et al. 2002). This conclusion was reached because of a correlation observed between the size of the apamin-sensitive mAHP current and the intensity of SK2, rather than SK1, protein staining. Our data might reconcile these apparently discrepant immunohistochemical and pharmacological findings because if SK1 interacts with SK2 in vivo, then the supply of SK2 would be a ‘rate-limiting step’ in the production of apamin-sensitive channels.
Our data are also interesting with regard to the suggestion that rSK1 subunits are involved in the formation of the channels underlying the apamin-insensitive slow afterhyperpolarization (sAHP) in rat hippocampal pyramidal neurons (Bowden et al. 2001). In the present study we have shown that rSK1 does not form a functional channel by itself and that the channels formed when it is co-expressed with SK2 are still apamin sensitive (albeit less so than when SK2 is expressed alone). These findings would seem to weaken the argument that rSK1 forms the sAHP channel by itself.
More work is needed to clarify these issues and to better understand the physiological role(s) of SK1, but our data provide a starting point for examining the functional properties of the rat SK1 gene, and for comparisons with native tissues.
Acknowledgments
This work was supported by the Wellcome Trust and UK Medical Research Council. P. K. Bahia is the recipient of an MRC/GSK collaborative research studentship. We are grateful to Professor L. Kaczmarek and Dr W. J. Joiner for the supply of rSK2 and rSK1 clones and to Professor D. H. Jenkinson for many helpful discussions.
REFERENCES
- Benton DCH, Dunn PM, Chen JQ, Galanakis D, Ganellin CR, Malik-Hall M, Shah M, Haylett DG, Jenkinson DH. UCL 1848: a novel bis-quinolinium cyclophane which blocks apamin-sensitive K+ channels with nanomolar affinity. Br J Pharmacol. 1999;128:39P. [Google Scholar]
- Benton DCH, Monaghan AS, Hosseini R, Bahia PK, Shah Y, Haylett DG, Moss GWJ. Interactions between rat SK channel subunits expressed in HEK 293 cells. J Physiol. 2003;551.P:C43. doi: 10.1113/jphysiol.2003.054551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden SE, Fletcher S, Loane DJ, Marrion NV. Somatic colocalization of rat SK1 and D class (Cav1. 2) L-type calcium channels in rat CA1 hippocampal pyramidal neurons. J Neurosci. 2001;21:RC175. doi: 10.1523/JNEUROSCI.21-20-j0006.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandy KG, Fantino E, Wittekindt O, Kalman K, Tong LL, Ho TH, Gutman GA, Crocq MA, Ganguli R, Nimgaonkar V, Morris-Rosendahl DJ, Gargus JJ. Isolation of a novel potassium channel gene hSKCa3 containing a polymorphic CAG repeat: a candidate for schizophrenia and bipolar disorder? Mol Psychiatry. 1998;3:32–37. doi: 10.1038/sj.mp.4000353. [DOI] [PubMed] [Google Scholar]
- Chen JQ, Galanakis D, Ganellin CR, Dunn PM, Jenkinson DH. bis-Quinolinium cyclophanes: 8, 14-diaza-1,7 (1,4)-diquinolinacyclotetradecaphane (UCL 1848), a highly potent and selective, nonpeptidic blocker of the apamin-sensitive Ca2+-activated K+ channel. J Med Chem. 2000;43:3478–3481. doi: 10.1021/jm000904v. [DOI] [PubMed] [Google Scholar]
- Faber ES, Sah P. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J Neurosci. 2002;22:1618–1628. doi: 10.1523/JNEUROSCI.22-05-01618.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunnet M, Jensen BS, Olesen SP, Klaerke DA. Apamin interacts with all subtypes of cloned small-conductance Ca2+-activated K+ channels. Pflugers Arch. 2001;441:544–550. doi: 10.1007/s004240000447. [DOI] [PubMed] [Google Scholar]
- Hosseini R, Benton DCH, Dunn PM. SK3 is an important component of K+ channels mediating the afterhyperpolarization in cultured rat SCG neurones. J Physiol. 2001;535:323–334. doi: 10.1111/j.1469-7793.2001.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii TM, Maylie J, Adelman JP. Determinants of apamin and d-tubocurarine block in SK potassium channels. J Biol Chem. 1997;272:23195–23200. doi: 10.1074/jbc.272.37.23195. [DOI] [PubMed] [Google Scholar]
- Joiner WJ, Wang LY, Tang MD, Kaczmarek LK. hSK4, a member of a novel subfamily of calcium-activated potassium channels. Proc Natl Acad Sci U S A. 1997;94:11013–11018. doi: 10.1073/pnas.94.20.11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, Clapham DE. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature. 1995;374:135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- Ottschytsch N, Raes A. Obligatory heterotetramerization of three previously uncharacterized Kv channel alpha-subunits identified in the human genome. Proc Natl Acad Sci U S A. 2002;99:7986–7991. doi: 10.1073/pnas.122617999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedarzani P, D'hoedt D, Doorty KB, Wadsworth JD, Joseph JS, Jeyaseelan K, Kini RM, Gadre SV, Sapatnekar SM, Stocker M, Strong PN. Tamapin, a venom peptide from the Indian red scorpion (Mesobuthus tamulus) that targets small conductance Ca2+-activated K+ channels and afterhyperpolarization currents in central neurons. J Biol Chem. 2002;277:46101–46109. doi: 10.1074/jbc.M206465200. [DOI] [PubMed] [Google Scholar]
- Post MA, Kirsch GE, Brown AM. Kv2. 1 and electrically silent Kv6.1 potassium channel subunits combine and express a novel current. FEBS Lett. 1996;399:177–182. doi: 10.1016/s0014-5793(96)01316-6. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol. 2002;66:345–353. doi: 10.1016/s0301-0082(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Sailer CA, Hu H, Kaufmann WA, Trieb M, Schwarzer C, Storm JF, Knaus HG. Regional differences in distribution and functional expression of small-conductance Ca2+-activated K+ channels in rat brain. J Neurosci. 2002;22:9698–9707. doi: 10.1523/JNEUROSCI.22-22-09698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M, Haylett DG. The pharmacology of hSK1 Ca2+-activated K+ channels expressed in mammalian cell lines. Br J Pharmacol. 2000;129:627–630. doi: 10.1038/sj.bjp.0703111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 1999;96:4662–4667. doi: 10.1073/pnas.96.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M, Pedarzani P. Differential distribution of three Ca2+-activated K+ channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol Cell Neurosci. 2000;15:476–493. doi: 10.1006/mcne.2000.0842. [DOI] [PubMed] [Google Scholar]
- Strobaek D, Jorgensen TD, Christophersen P, Ahring PK, Olesen SP. Pharmacological characterization of small-conductance Ca2+-activated K+ channels stably expressed in HEK 293 cells. Br J Pharmacol. 2000;129:991–999. doi: 10.1038/sj.bjp.0703120. [DOI] [PMC free article] [PubMed] [Google Scholar]


