Abstract
Unbalanced maternal nutrition affects fetal endocrine and cardiovascular systems, sometimes accompanied by changes in growth, although this is usually in late gestation. We determined the effect of moderate restriction for the first half of gestation of maternal dietary protein, or of total calorific intake on isolated resistance artery function of mid-gestation fetal sheep. Welsh Mountain ewes were nutritionally restricted by 30 % of the recommended nutrient intake (globally restricted) or 30 % of the recommended protein intake (protein-restricted), compared to control ewes fed 100 % of recommended nutrient intake, for ~12 days prior to conception and for the subsequent 70 days of gestation. At mid-gestation, fetal and placental weights were similar in all dietary groups. In isolated femoral arteries, the response curve to noradrenaline was reduced in protein-restricted group fetuses (P < 0.05). Maximal relaxation (P < 0.01) and sensitivity (P < 0.05) to acetylcholine were markedly reduced in protein-restricted group fetuses, and to a smaller extent in globally restricted group fetuses (response curve, P < 0.05). The dilator response (P < 0.05) and sensitivity (P < 0.05) to the α2 agonist UK14304 was lower in protein-, but not in globally restricted group fetuses. The response (P < 0.05) and sensitivity (P < 0.05) to the nitric oxide donor sodium nitroprusside were reduced in protein-restricted group fetuses compared to controls. Our data show that dietary imbalance, in particular restricted protein, of the ewe can produce blunting of endothelial-dependent and -independent relaxation in systemic arteries from the mid-gestation fetus. These changes may precede perturbed late-gestation fetal and postnatal cardiovascular control.
Many epidemiological studies now confirm and extend the concept that adult cardiovascular diseases, including coronary heart disease, stroke and hypertension, originate in part from adverse influences acting before birth (Godfrey & Barker, 2001). The association between low birth weight or ponderal index and the development of adult cardiovascular diseases has led to the hypothesis that pathological processes are initiated by poor fetal nutrition. In the face of altered nutrient supply, the fetus may programme both growth and the development and function of the cardiovascular system which, while a survival strategy, may have longer-term deleterious consequences for adult health.
Hypertensive offspring of rats fed a diet of reduced total calorific content (30–50 % restriction) have impaired vasodilator responses to sodium nitroprusside in small resistance vessels (Ozaki et al. 2001) and endothelium-dependent responses in aortic rings (Franco et al. 2002) in vitro. In offspring of rats fed a diet low in protein (50 % restriction) throughout gestation both endothelium-dependent and -independent responses are impaired (Brawley et al. 2003), and others have observed that these offspring are hypertensive (Langley-Evans et al. 1999). Alteration in maternal and fetal levels of a number of amino acids could mediate changes in physiological function following altered maternal diet (Rees et al. 1999; Kwong et al. 2000). Indeed, the hypertension in offspring of dams fed a low protein diet can be prevented by supplementation of maternal diet with glycine (Jackson et al. 2002), an amino acid needed in large amounts during fetal life. Furthermore, rat dams fed a high fat diet, designed to replicate the features of modern Western diet that are implicated in heart disease, also produced hypertensive offspring with vascular endothelial dysfunction (Khan et al. 2003). Dysfunction of the vascular endothelium could either contribute to the onset of hypertension, or develop as a consequence. Therefore, sheep models have been developed to study directly the effect of altered nutrition on cardiovascular development and function during fetal life. In sheep, a mild (15 %) reduction in maternal global nutrient intake for the first half of gestation produces low blood pressure in late gestation singleton fetuses, and postnatal hypertension (Hanson et al. 1999). A 30 % periconceptual global dietary restriction produces elevated blood pressure in the latter half of gestation in twin, but not singleton, fetuses, but with no change in fetal weight (Edwards & McMillen, 2002). The cardiovascular effects appear to be contributed to by vascular endothelial dysfunction, which can be detected at 0.9 gestation (Ozaki et al. 2000), but which possibly arises earlier in gestation. In humans, endothelial dysfunction is a risk factor for atherosclerosis (De Meyer & Herman, 1997) and signs of atheromatous lesions are apparent during fetal and early postnatal life (Palinski & Napoli, 1999). It is therefore of importance to conduct further studies of vascular programming in fetal life, especially as effects of dietary manipulation and exogenous glucocorticoids in early gestation produce long-term effects on blood pressure postnatally (Dodic et al. 1999; Kwong et al. 2000). Moreover, no study to date has addressed the effect of specific dietary protein restriction compared to total calorific restriction on fetal cardiovascular development and function.
The ‘programmability’ of the hypothalamo-pituitary-adrenal (HPA) axis during fetal life (Unno et al. 1997; see Green, 2001), the role of glucocorticoids in fetal growth (Moss et al. 2001) and their programming effect on postnatal blood pressure (Dodic et al. 1999) make the axis a strong candidate in mechanisms underlying the programming of adult cardiovascular disease. Permanent interaction, for example via DNA demethylation (Thomassin et al. 2001), of glucocorticoids with the expression of other candidate vascular genes, such as those of the renin-angiotensin system (RAS), could programme gene expression during development. In sheep, the HPA axis is programmable by altered nutrition in utero, its sensitivity to exogenous stimulation being reduced during fetal life and increased in early postnatal life (Hanson et al. 1999; Lingas et al. 1999). Moreover undernutrition has been shown to reduce the expression and activity of the enzyme 11-βhydroxysteroid dehydrogenase-2 (11βHSD-2), which converts cortisol to the inactive steroid cortisone, in the placenta, thus providing a mechanism whereby the fetus could be exposed to greater levels of circulating active glucocorticoid. Excess glucocorticoid induces vascular endothelial dysfunction in vitro (Rogers et al. 2002; Iuchi et al. 2003). Moreover the RAS is implicated in the programming of the cardiovascular system following changes in maternal nutrition (Langley-Evans et al. 1999). It is therefore possible that our observed vascular dysfunction in late gestation sheep results from persistent changes in HPA axis function and other endocrine axes.
The aim of the present study was to compare the effect of moderate (30 %) maternal dietary protein restriction with that of restriction of total calorific intake for the first half of gestation on isolated resistance artery function in mid-gestation fetal sheep. We examined the effect of vasoconstrictor and vasodilator (endothelium-dependent and -independent) agents in small arteries from the femoral bed in vitro. In addition we measured maternal and fetal plasma cortisol, angiotensin II, renin and arginine vasopressin levels.
Methods
All procedures involving animals were conducted in accordance with the UK Animals (Scientific Procedures) Act, 1986.
Animals and study design
Before conception, Welsh Mountain ewes of uniform age, weight and body condition score were randomly allocated to one of three dietary groups. Oestrous was synchronised using vaginal medroxyprogesterone acetate-impregnated sponges (Veramix, Pharmacia and Upjohn, UK) which were removed 48 h before tupping. Ewes were penned individually with wood-shavings as bedding and were fed a complete pelleted diet consisting of barley, wheat, cooked cereal meal, micronised full fat soya, grass meal, molasses, chopped straw, calcium carbonate, di-calcium phosphate, salt, and a sheep vitamin/mineral supplement. It provided 10.81 MJ (kg dry matter)−1 (metabolisable energy), 16.93 g crude protein (100 g dry matter)−1. As fed, the pelleted diet was 88.4 % dry matter. Rations were allocated according to guidelines for pregnant sheep and adjusted for gestational age (AFRC, 1993). Control animals (n = 7) were fed 100 % of the recommended nutrient intake (for all nutrients) for ≈12 days prior to conception and the subsequent 70 days of gestation. Over the same time period, nutrient-restricted animals were fed either 70 % of the recommended nutrient intake (globally restricted, n = 7) or 70 % of the recommended protein intake (11.85 g crude protein (100 g dry matter)−1) with 100 % energy requirement (protein-restricted, n = 6). Maternal body weight and condition score (Russel, 1991) were measured on a weekly basis.
Maternal jugular venous blood samples (10 ml) were taken from ewes 12.9 ± 0.1 days before conception (and prior to the introduction of the dietary regime), at 37.6 ± 0.6 and 58.6 ± 0.9 days gestation, and immediately prior to administration of terminal anaesthesia (70.3 ± 0.5 days gestation). Fetal arterial blood samples (10 ml) were taken immediately after caesarian section. Blood samples were centrifuged at 4 °C and plasma and serum stored at −80 °C.
Post-mortem procedure
At mid-gestation (70 days gestation), singleton pregnancies were confirmed by ultrasound examination and the ewes were transferred from the farm to the laboratory. Umbilical artery and fetal thoracic aorta waveforms were identified using colour Doppler and the pulsatility and resistance indices were reported separately (Kalache et al. 2001). Ewes were killed by an overdose of sodium pentobarbitone (30–40 ml Euthatal 200 mg ml−1, Rhone Merieux, Harlow, Essex, UK). Fetal biparietal diameter, chest circumference, abdominal circumference, crown-rump length, femur length, body weight and individual organ weights were recorded. Placentome weights and number were recorded. Fetal tissues were dissected and stored at −80 °C for subsequent analysis (not reported here).
Biochemical assays
Cortisol
Plasma cortisol was measured by radioimmunoassay using a method validated for sheep and described previously in detail (Giussani et al. 1994a). Briefly, duplicate 20 µl plasma samples were mixed with carbonate buffer (1.7 m) and cortisol was extracted with di-ethyl ether. Following ether evaporation, the residue was incubated with tritrated cortisol (NEN, Hounslow, UK) and anti-cortisol antiserum (Bioclinical Services, Cardiff, UK). Bound and free steroids were separated using dextran-coated charcoal. Assay recovery averaged 95 % and interassay coefficients of variation for two plasma pools (28 and 85 nm) were 9.7 and 6.3 % respectively. The lower limit of detection of the assay was 0.8 nm.
Renin
Plasma renin levels were measured using an immunoradiometric assay system purchased as a kit from Nicholls Institute Diagnostics (San Juan Capistrano, CA, USA). The renin standards used in the assay were calibrated against the WHO 2nd IRP (68/356) standard for active renin and were supplied for use dissolved in sheep serum. Plasma samples of 200 µl were used directly in the assay, therefore assay recovery was 100 %. The interassay coefficients of variation for two control values (18 and 73 µu ml−1) were 11.2 and 7.3 % respectively and the sensitivity of the assay was 2.4 µu ml−1.
AVP
Plasma arginine vasopressin (AVP) was measured using a double antibody radioimmunoassay method, validated for sheep as described previously in detail (Giussani et al. 1994b), employing reagents supplied by Mitsubishi Yuka Ltd, Japan, distributed by IDS Ltd, Bolton, Tyne and Wear, UK. Briefly, 0.5 ml plasma samples were subjected to a chromatographic extraction procedure using Sep Pak C18 cartridges. The methanol extract containing vasopressin was evaporated using an air jet and the residue was incubated with anti-rabbit AVP antiserum and iodine-labelled vasopressin followed 24 h later by a further incubation with a second antibody (anti-rabbit goat IgG serum). Recoveries exceeded 85 %. The interassay coefficients of variation for two plasma pools (12.5 and 45 pg ml−1) were 6.2 and 8.9 % respectively and the sensitivity of the assay was 1.1 pg ml−1.
Angiotensin II
Plasma angiotensin II (AngII) was measured by radioimmunoassay as described previously (Green et al. 1998) using a kit purchased from Nicholls Institute Diagnostics. Briefly, 0.5 ml plasma samples were subjected to chromatography on Sep Pak C18 columns. The AngII was eluted with methanol, the methanol evaporated and the residue incubated with anti-AngII rabbit antiserum and iodinated AngII, followed by the second antibody, which was donkey anti-rabbit antiserum. Recoveries averaged 74 %, the sensitivity of the assay was 3.8 pg ml−1 and the interassay coefficients of variation for the two control pools (23 and 65 pg ml−1) were 10.5 % and 6.6 % respectively.
Progesterone
Plasma progesterone was analysed using the Immulite 2000 Analyser (DPC Ltd, Llanberis, Gwynedd, UK). The method was an enzyme immunoassay with chemiluminescence detection of enzyme activity. The detection limit of the assay was 0.6 nmol l-1, and recovery ranged from 93 to 117 %. Interassay coefficients of variation were 10.2 % at 4.9 nm, 7.8 % at 10.8 nm and 8.0 % at 54.9 nm.
Total protein
Total protein was measured in maternal plasma (7.5 µl) using a clinical chemistry analyser (OPERA, Bayer PLC, Newbury, Berkshire, UK) and kit (Randox Laboratories Ltd, Crumlin, County Antrim, Ireland, UK). The assay used a biuret method and formation of a coloured complex (from reaction of cupric ions in an alkaline medium with protein bonds) was measured at 546 nm. The coefficients of variation for the two controls (48.8 and 89.2 g l−1) were 0.69 % and 0.55 %, respectively.
d-3-Hydroxybutyrate
Plasma d-3-hydroxybutyrate was measured using a clinical chemistry analyser (OPERA, Bayer PLC) and kit (Randox Laboratories Ltd). The assay used a kinetic enzyme method based on the oxidation of d-3-hydroxybutyrate to acetoacetate by the enzyme 3-hydroxybutyrate dehydrogenase. The concomitant oxidation of NAD+ to NADH causes a change in absorbance at 340 nm. Change in absorbance per minute for samples (9 µl) and standards was used to calculate d-3-hydroxybutyrate concentration. The coefficients of variation for the two controls (0.82 and 2.65 mm) were 5.73 % and 1.93 %, respectively.
Urea
Plasma urea was measured using a clinical chemistry analyser (OPERA, Bayer PLC) and kit (Randox Laboratories Ltd, Crumlin, County Antrim, Ireland). The assay uses an enzymatic kinetic method based on the reaction of ammonia (from hydrolysis of urea) with α-oxoglutarate and NADH in the presence of glutamate dehydrogenase to yield glutamate and NAD. The associated change in absorbance at 340 nm min−1 for samples (3 µl) and standards was used to calculate urea concentration. The coefficients of variation for the two controls (3.6 and 21.4 mm) were 6.38 % and 2.98 %, respectively.
Non esterified fatty acids (NEFA)
Plasma NEFA was measured using a clinical chemistry analyser (OPERA, Bayer PLC) and kit (Randox Laboratories Ltd) The assay used a colorimetric method based on the measurement of a purple compound formed by reaction of N-ethyl-N-(2-hydroxy-3-sulphopropyl) m-toluidine, 4-aminoantipyrine with peroxide from the oxidization of AcylCoA (a product of the combination of NEFA with ATP and CoA). The absorbance at 550 nm for samples (9 µl) and standards was used to calculate NEFA concentration.
Determination of isolated artery function
All chemicals were obtained from Sigma (Poole, UK), unless otherwise stated. Drugs were dissolved in distilled water.
Femoral arteries from the fetus (≈300 µm in diameter) were dissected and mounted on a wire myograph (Mulvany & Halpern, 1977). Arteries were bathed in physiological saline solution (PSS, pH 7.4: NaCl 119, KCl 4.7, CaCl2 2.5, MgSO4 1.17, NaHCO3 25, KH2PO4 1.7, EDTA 0.026 and glucose 5.5 mm), gassed with 5 % CO2 in air at 37 °C. After determination of passive tension and internal circumference, the arteries were set to an internal circumference equivalent to 90 % of that measured when relaxed in situ under a transmural pressure of 40 mmHg using Laplace's relationship. The vessels were subjected to a standard run-up procedure involving contractions to 1 × 10−5m noradrenaline tartrate (Winthrop, Guildford, UK), depolarising potassium solution (125 mm, KPSS, equimolar substitution of NaCl with KCl in PSS) and 1 × 10−5m noradrenaline in KPSS. Arteries which produced tension equivalent to less than 40 mmHg pressure in response to 125 mm KPSS were excluded from the study.
Vasoconstriction to cumulative concentrations of KPSS (4.7–125 × 10−3m), noradrenaline (NA) (10−8-10−4m), phenyl-ephrine (Phe) (10−8-10−4m), the thromboxane A2 mimetic U46619 (10−9-10−5m) were examined under isometric conditions. After preconstriction with U46619 (1 µm), vasorelaxation to acetylcholine (10−10-10−5m), sodium nitroprusside (SNP) (10−10-10−5m) and the α2 adrenoceptor agonist UK14304 (10−8-10−4m) were also examined. We used U46619 as a preconstrictor in order to avoid any confounding effects due to differences in the maturation of α-adrenergic constrictor responses (Nishina et al. 1999).
Statistical analysis
Myography data
Tension was expressed in mN mm−1 or as a percentage of maximal contraction to 125 mm KPSS, to correct for small differences in vessel diameter and increasing vascular smooth muscle mass with age. This allows comparison to be made between different age groups. Dose-response curves were compared by two-way repeated-measures ANOVA (Statview version 4.5, Abacus Concepts Ltd, USA) and pEC50 was calculated by Instat (GraphPAD Software Inc., San Diego, CA, USA). Values were expressed as mean ± s.e.m. and compared by one-way factorial ANOVA and Fisher's PLSD.
Fetal body weight, organ weights, dimensions, gestational age and hormone data
These were analysed by ANOVA followed by a post-hoc t test with Bonferonni correction (SPSS for Windows, v. 10). Regression analysis was carried out on post-mortem measurement of fetal body weight and gestational age.
Significance was accepted if P < 0.05.
Results
Gestational age and feto-placental measurements
There were no significant differences between the dietary groups in fetal body or organ weights, or in fetal dimensions (Table 1). There were no differences between the dietary groups in gestational age at the time of post-mortem (Table 1). However, the animals were killed over a range of gestational ages from 67 to 74 days. Examination of this revealed a significant positive correlation between fetal body weight and gestational age in all groups (control: P < 0.01, r2 = 0.79; globally restricted: P < 0.01, r2 = 0.90; low-protein: P < 0.01, r2 = 0.98). There were no significant differences between dietary groups in placentome weight, placentome number or fetal:placental weight ratio (Table 1). As previously reported, there were no significant differences between groups in Doppler-assessed pulsatility index/resistance index of the aorta or umbilical artery (Kalache et al. 2001).
Table 1.
Gestational age and feto-placental measurements at post-mortem
| Control (n = 7) | Globally restricted (n = 7) | Protein-restricted (n = 6) | |
|---|---|---|---|
| Gestational age (days) | 70 ± 1 | 70 ± 1 | 70 ± 1 |
| Mean placentome weight (g) | 7.08 ± 0.98 | 8.33 ± 1.60 | 8.36 ± 0.91 |
| Total placental weight (g) | 532.6 ± 63.5 | 526.2 ± 89.2 | 626.6 ± 60.0 |
| Placentome number | 77 ± 4 | 68 ± 6 | 76 ± 4 |
| Fetal weight (g) | 135.4 ± 10.7 | 131.7 ± 12.8 | 135.8 ± 11.9 |
| Fetal:placental weight ratio | 0.27 ± 0.02 | 0.28 ± 0.04 | 0.22 ± 0.03 |
| Crown–rump length (mm) | 180.3 ± 10.1 | 174.5 ± 5.6 (6) | 181.3 ± 6.1 |
| Abdominal circumference (mm) | 124.7 ± 2.8 | 122.5 ± 4.3 (6) | 124.4 ± 6.4 (5) |
| Biparietal diameter (mm) | 30.4 ± 0.6 | 30.0 ± 0.7 | 30.3 ± 0.6 |
| Chest circumference (mm) | 110.1 ± 3.6 | 109.3 ± 2.6 | 112.0 ± 4.5 |
| Femur length (mm) | 27.9 ± 1.3 | 27.5 ± 1.0 (6) | 28.2 ± 1.1 |
| Brain (% fetal weight) | 2.90 ± 0.37 (4) | 3.41 ± 0.13 (6) | 3.37 ± 0.11 |
| Heart (% fetal weight) | 0.98 ± 0.06 | 1.00 ± 0.08 | 1.04 ± 0.05 |
| Lung (% fetal weight) | 5.25 ± 0.37 (6) | 5.41 ± 0.31 | 5.42 ± 0.13 |
| Liver (% fetal weight) | 6.54 ± 0.47 | 7.61 ± 0.64 (6) | 7.08 ± 0.94 |
| Right kidney (% fetal weight) | 0.70 ± 0.05 | 0.64 ± 0.02 (6) | 0.70 ± 0.03 |
| Left kidney (% fetal weight) | 0.72 ± 0.05 | 0.63 ± 0.03 (6) | 0.78 ± 0.05 |
Values are means ± s.e.m.
Biochemical assays
Preconception and gestational measurements in the ewe
Maternal plasma concentration of progesterone was greater (P < 0.05), and cortisol was lower (P < 0.01), than pre-conception over the first half of gestation (36–45 and 52–64 days. Fig. 1). Plasma cortisol was greater (P < 0.01) in the globally restricted group compared to control (Fig. 1). There was no difference in progesterone between dietary groups.
Figure 1. Steroid profile of ewes.
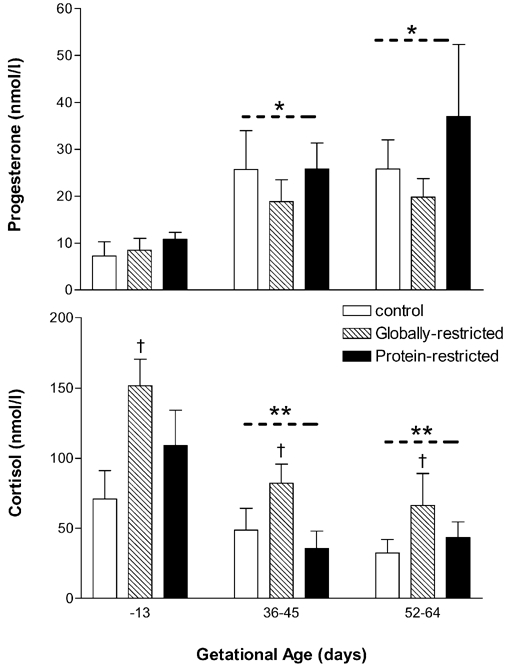
Maternal jugular vein plasma samples taken before conception (−13 days) and during the first half of gestation (36–45 and 52–64 days). Values are mean ± s.e.m.*P < 0.05 and **P < 0.01, significantly different from −13 days; †P < 0.01, globally restricted significantly different from control group (ANOVA and Bonferroni t test).
There was a small but significant decrease (P < 0.01) in total plasma protein from pre-conception during the first half of gestation (36–45 and 52–64 days. Fig. 2). Maternal plasma non-esterified-fatty-acid (NEFA) tended to rise from pre-conception values during the first half of gestation, but this was not significant (Fig. 2). d-3-hydroxybutyrate increased during pregnancy from pre-conception control values to a similar extent in the three dietary groups (Fig. 2). There were no differences in urea levels between dietary groups (Fig. 2). In all dietary groups, urea (end product of amino acid breakdown) was lower (P < 0.05) during early-gestation than pre-conception (Fig. 2).
Figure 2. Metabolic profile of ewes.
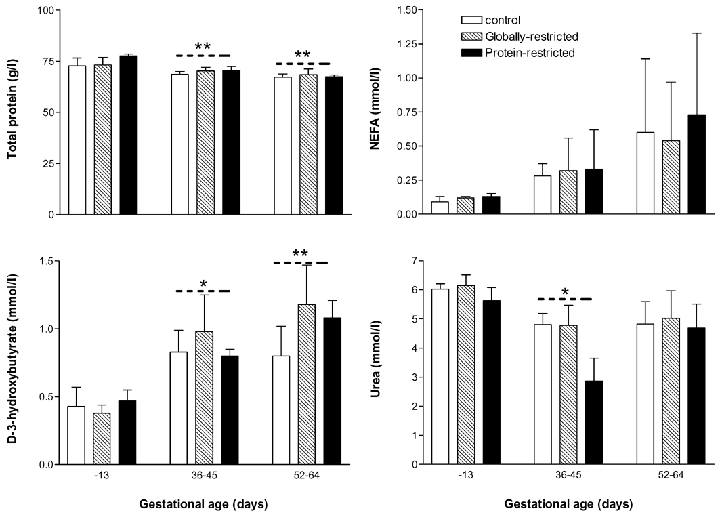
Maternal jugular vein plasma samples taken before conception (−13 days) and within two age ranges during the first half of gestation (36–45 and 52–64 days). Values are mean ± s.e.m.*P < 0.05 and **P < 0.01, significant effect of age by ANOVA and Bonferroni t test.
Post-mortem analysis in ewe and fetus
Maternal plasma cortisol was significantly greater than fetal plasma cortisol in all dietary groups (Table 2). AngII was elevated (P < 0.05) in fetal compared to maternal plasma in protein-restricted animals only, despite no difference in plasma renin levels. AVP levels tended to be higher in fetal compared to maternal plasma in all dietary groups, however this only reached significance in the globally restricted and protein-restricted animals (Table 2).
Table 2.
Post-mortem fetal and maternal plasma hormone levels at 70 days gestation
| Control (n = 7) | Globally restricted (n = 7) | Protein-restricted (n = 6) | ||
|---|---|---|---|---|
| Cortisol (nm) | Maternal | 85.7 ± 9.9 | 88.3 ± 18.1 | 66.8 ± 8.9 |
| Fetal | 26.6 ± 4.8** | 35.7 ± 7.4* | 20.6 ± 4.0** | |
| Renin (μu ml−1) | Maternal | 7.2 ± 0.6 | 8.6 ± 1.7 | 8.1 ± 1.6 |
| Fetal | 10.5 ± 1.5 | 11.5 ± 0.8 | 9.4 ± 0.5 | |
| AngII (pg ml−1) | Maternal | 25.1 ± 2.1 | 22.1 ± 1.1 | 18.1 ± 2.1 |
| Fetal | 37.6 ± 6.8 | 32.1 ± 8.5 | 39.3 ± 8.9* | |
| AVP (pg ml−1) | Maternal | 3.6 ± 2.2 | 1.4 ± 0.3 | 1.0 ± 0.2 |
| Fetal | 10.6 ± 4.3 | 3.0 ± 0.3** | 3.3 ± 0.3** |
Values are means ± s.e.m.
P < 0.05
P < 0.01, significantly different from corresponding maternal sample.
There were no effects of diet on mean fetal or maternal cortisol, renin, AVP and AngII (Table 2), or on the ratio of maternal to fetal cortisol (ewe:fetus control, 3.5 ± 0.30; globally restricted, 2.7 ± 0.5; low-protein, 3.8 ± 0.7 nm).
Isolated femoral artery function
Maximum contraction/relaxation responses and sensitivities (pEC50) and vessel lumen diameter data are shown in Table 3. There were no differences between control and restricted groups in lumen diameter.
Table 3.
Characteristics of isolated femoral artery function in 70 day gestation sheep fetuses of control and nutrient-restricted ewes
| Control (n = 7) | Globally restricted (n = 7) | Protein-restricted (n = 6) | |
|---|---|---|---|
| Lumen diameter (μm) | 391.9 ± 24.6 | 398.4 ± 16.9 | 372.5 ± 16.5 |
| Maximal contraction | |||
| 125mM K+ (mN mm−1) | 0.45 ± 0.06 | 0.44 ± 0.05 | 0.41 ± 0.04 |
| Noradrenaline (%K) | 16.13 ± 2.07 | 17.42 ± 3.40 | 15.87 ± 3.10 |
| Phenylephrine (%K) | 8.50 ± 1.05 | 7.20 ± 3.76 | 4.80 ± 1.19 |
| U46619 (%K) | 30.63 ± 2.83 | 32.3 ± 4.43 | 32.54 ± 2.95 |
| Maximum relaxation | |||
| Acetylcholine (%) | 47.09 ± 1.79 | 35.99 ± 3.56 | 4.16 ± 7.88††** |
| Sodium nitroprusside (%) | 85.73 ± 5.31 | 76.97 ± 2.22 | 74.97 ± 7.60 |
| UK14304 (%) | 46.16 ± 4.99 | 40.00 ± 2.30 | 31.96 ± 3.78 |
| pEC50(-log M) | |||
| Noradrenaline | 5.59 ± 0.17 | 5.43 ± 0.07 | 5.03 ± 0.14 |
| Phenylephrine | 5.37 ± 0.05 | 5.40 ± 0.29 | 5.38 ± 0.28 |
| U46619 | 5.84 ± 0.14 | 5.74 ± 0.25 | 5.61 ± 0.14 |
| Acetylcholine | 6.82 ± 0.10 | 6.48 ± 0.09 | 6.42 ± 0.12* |
| Sodium nitroprusside | 7.32 ± 0.13 | 7.23 ± 0.09 | 6.93 ± 0.10* |
| UK14304 | 5.87 ± 0.06 | 5.89 ± 0.06 | 5.33 ± 0.17†* |
Tension is expressed in mN mm−1, as a percentage of maximal contraction to from 125 mM K+ or as a percentage of initial pre-constriction. Values are means ± s.e.m.
P < 0.05
P significantly different from control group
P < 0.05
P < 0.01, significantly different < 0.01, significantly globally restricted group.
Vasoconstrictor responses
The whole response curve and maximal contractile response (Table 3) to 125 mm K+ was similar between groups. The overall response, maximum response and sensitivity to Phe was similar between groups (Table 3). The response curve to NA was reduced in protein-restricted compared to globally restricted group, although not to control group, fetuses (P < 0.05 ANOVA for the whole curve), however this was not reflected in changes in sensitivity (pEC50) or the maximum response (Table 3). Responses to the thromboxane mimetic U46619 were similar between the groups.
Vascular endothelium-dependent and -independent vasodilator responses
The response curve, maximal relaxation and sensitivity to ACh (endothelium-dependent) were markedly reduced in protein-restricted group fetuses (Max response P < 0.01; pEC50P < 0.05), and to a smaller extent in globally restricted group fetuses, compared to control (Table 3 and Fig. 3).
Figure 3. Vasorelaxation to acetylcholine (ACh), sodium nitroprusside (SNP) and UK14304 (α2adrenoceptor agonist) in femoral arteries of fetuses from control and nutrient restricted ewes.
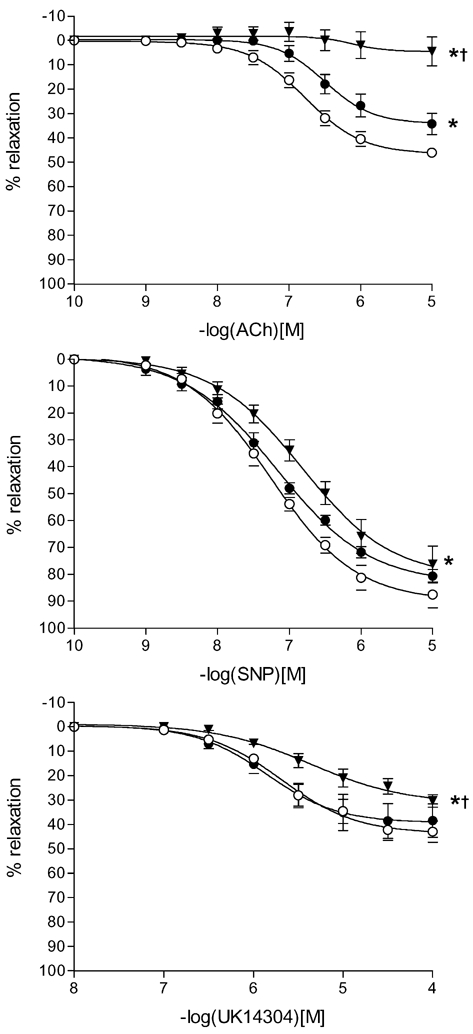
Data are expressed as a percentage of initial preconstriction. Values are mean ± s.e.m. in control (^), 70 % global (•) and 70 % protein (▾) fetuses. *P < 0.05 (ANOVA), significantly difference from control; †P < 0.05 (ANOVA), significantly different from global group.
The response curve (Fig. 3, P < 0.05) and sensitivity (Table 3, P < 0.05) to the α2 agonist UK14304 was lower in protein-restricted, but not globally restricted, group fetuses compared to control.
There was no difference between control and nutrient-restricted fetuses in maximum relaxation response to SNP, but the response curve (Fig. 3) and sensitivity (pEC50.Table 3) to SNP were reduced (P < 0.05) in protein-restricted group fetuses compared to control fetuses.
Discussion
This study has shown for the first time that moderate restriction of maternal global dietary intake in sheep for the first half of gestation markedly attenuates the fetal peripheral vascular vasodilator responses to ACh at mid-gestation. Strikingly, when specific mild restriction of maternal protein intake is employed over the same period of gestation, the ACh response is abolished. In the protein-restricted group, this finding is coupled to impaired responses to an nitric oxide (NO) donor, and α2-adrenergic responses. We speculate that such fetal vascular dysfunction at mid-gestation following early gestation maternal nutrient restriction may contribute to programming of altered blood pressure during fetal, postnatal and young adult life.
Cardiovascular disorders such as hypertension and coronary heart disease are related to low birth weight or disproportionate size at birth in a graded manner across the normal birth weight range for the population, in both developed and developing countries (Huxley et al. 2000) – thus they are not a feature of low birth weight babies only (Stein et al. 1996). The current study was carried out at mid-gestation, when placental mass is still increasing and the period of rapid fetal growth has yet to commence. At the time of post-mortem, fetal body weight was significantly correlated with gestational age in all groups. However there was no overall difference in fetal body and organ weights, fetal dimensions or placental size between control and dietary restricted groups. Our previous sheep studies have investigated the programming effects of mild early gestation restrictions in maternal diet on late gestation fetal physiology, when placental weight has reached a plateau and at the beginning of a rapid period of fetal growth (see Hanson et al. 1999). These studies also report no change in fetal weight. It is clearly possible by manipulating maternal nutrition to programme fetal physiological responses independently of overall fetal body growth. More profound restriction in maternal nutrient intake, does of course result in a decrease in fetal growth (Harding et al. 1992).
In the present study, our choice to investigate the femoral vascular bed was directed by the fact that during fetal life, femoral vascular resistance contributes significantly to total peripheral resistance which, along with combined ventricular output (CVO), determines arterial pressure. It is well established in chronically instrumented mid- to late-gestation fetal sheep that during hypoxia CVO is redistributed in favour of vital organs including the heart, and at the expense of the periphery, exemplified by a fall in femoral arterial blood flow (Giussani et al. 1994c). Preliminary evidence suggests that mild reduction in early gestation maternal nutrient intake produces a similar redistribution of fetal CVO, since we have observed a reduced fetal femoral blood flow and increased carotid blood flow during late gestation (authors’ unpublished observations) which is coupled to a lower mean arterial pressure (Hanson et al. 1999). Moreover, femoral vascular responses to an acute hypoxic challenge during late gestation are augmented in these fetuses (Hawkins et al. 2000). The results of our present study support the idea that these observations in vivo may be attributable to vascular dysfunction, producing an imbalance in the production of constrictors and dilators in the vascular bed, and that this is detectable as early as mid-gestation (≈70 days).
We did not observe any difference between the control and dietary restricted group fetuses in maximal response to 125 mm KPSS, indicating that this vascular dysfunction does not involve altered vascular smooth muscle growth or signal transduction mechanisms to a depolarising stimulus. In all dietary groups, the development of tension in response to KPSS in the femoral artery at mid-gestation was markedly lower than in other studies conducted in late-gestation fetuses (Anwar et al. 1999), probably indicative of relative immaturity of the underlying mechanisms. In addition we observed no difference in response to the thromboxane mimetic U46619 between control and dietary restricted fetuses. Therefore abnormalities in thromboxane-mediated pathways are unlikely to be programmed by such perturbations in maternal diet, at least at mid-gestation. We observed no difference between vessels from globally restricted and control group fetuses in response to noradrenaline, consistent with previous work in which neither 15 % or 50 % global restriction caused an altered response to noradrenaline at 0.9 gestation (Ozaki et al. 2000). Whilst we did observe a smaller overall response to noradrenaline in protein-restricted group fetuses in comparison to globally restricted group fetuses, this was not different from control fetuses, making difficult to interpret. It is important to note that noradrenaline acts on a range of adrenergic receptor subtypes (see below).
It is well established that the vascular endothelium plays an important part in maintaining cardiovascular homeostasis during adult and fetal life (Rubanyi, 1993; Green et al. 1996; Green, 2001) and endothelial dysfunction is likely to play a part in the development of atherosclerosis and hypertension (Busse & Fleming, 1999). The present sheep study was designed to investigate these phenomena during fetal life. Our study shows that the response to ACh is developed by mid-gestation but is less profound than previously observed in older fetuses (0.6 and 0.9 gestation) possibly due to the relative immaturity of the endothelium and nitric oxide mechanisms. (Ozaki et al. 2000). The blunting of the femoral vasodilator response to ACh in globally restricted fetuses is indicative of impaired endothelial function and is consistent with our previous study of vessels in 0.9 gestation sheep (Ozaki et al. 2000). However, the most striking aspect of our results was that the endothelium-dependent response to ACh was absent in fetuses exposed to specific maternal dietary protein restriction during early gestation, highlighting the importance of dietary balance in the programming of vascular function. In sheep, prenatal dexamethasone treatment in pregnancy alters the contribution of nitric oxide mechanisms to the fetal femoral resistance vessel response to ACh at 0.8 gestation (Molnar et al. 2002). Thus the impaired ACh response in the present study may be contributed to by reduced nitric oxide synthesis and/or release, possibly via altered maternal/fetal arginine levels, although this was not investigated in the current study. Indeed, investigations in the hypertensive offspring of rat dams fed a low protein diet during gestation have shown that impaired vascular responses to ACh and bradykinin may be attributable to reduced endothelial nitric oxide production (Brawley et al. 2003). Moreover, our finding of impaired overall response and sensitivity to the α2-adrenoceptor agonist UK14304 is also likely to result from reduced nitric oxide-mediated mechanisms (Nishina et al. 1999), and may contribute to vascular dysfunction in protein-restricted but not globally restricted group fetuses.
Our results also suggest impairment of smooth muscle dilator responses to nitric oxide in fetuses exposed to early gestation maternal protein, but not global, dietary restriction since the overall response and sensitivity to SNP was reduced. Nitrovasodilators such as SNP mediate their effects in part by the activation of guanylyl cyclase (GC) and increased intracellular cGMP levels (Waldman & Murad, 1987) and therefore impairment in this pathway might account for the blunted response to SNP in our study. In our previous sheep study a more profound (50 %) restriction of global maternal nutrient intake did produce a blunting of ACh responses coupled to blunted SNP responses in isolated fetal femoral vessels at 0.9 gestation (Ozaki et al. 2000). Differences in these findings from the present study are likely to be attributable to differences in the maturity of vascular mechanisms and the more intense (i.e. 50 % versus 30 %) dietary challenge used. The idea of programming of the GC-cGMP pathway by maternal diet is supported by demonstration that SNP sensing is altered in rat pups following gestational restriction of the dam's protein (Brawley et al. 2003) or total calorific (Ozaki et al. 2001) intake.
Thus, our results suggest that global nutrient restriction, and to a greater extent specifically protein restriction, produces an imbalance in vasodilators and vasoconstrictors. To our knowledge, this study is the first to compare the fetal programming effect of a reduction in total calorific intake with that of a specific food group, i.e. protein, on vascular function and thus to indicate the importance of dietary balance in altered vascular function versus reduction in total calorific intake per se. However it is important to stress that, whatever the mechanism which detects the change in maternal diet and initiates fetal programming, it is not linked simply to maternal diet: analysis of maternal plasma did not reveal any difference between control and restricted groups in NEFA, β-hydroxybutyrate or urea as indices of maternal metabolism. Further studies here would have to involve measurement of maternal nutrient turnover and placental function.
The rise in maternal peripheral progesterone during pregnancy, at a time when its site of production is switching from the corpus luteum to the placental trophoblast, is consistent with previous studies (Hamon & Heap, 1990) and this was unaffected by maternal diet. There is now substantial information in the literature to suggest that the fetal HPA axis is programmable by changes in the fetal environment (Unno et al. 1997; Green, 2001), including altered nutrition (see Hanson et al. 1999). Moreover, administration of synthetic glucocorticoid during critical periods of fetal life can programme postnatal hypertension (Dodic et al. 1999) and glucocorticoids are regulators of fetal growth (Moss et al. 2001) which makes the HPA a strong candidate mechanism in fetal programming (see Green, 2001). In the present study we observed a significant elevation in plasma cortisol throughout the first half of gestation in globally restricted, but not protein-restricted, group ewes compared to control, although this effect was not apparent in samples collected at the time of post-mortem.
While the precise nature of the link between altered maternal diet, the fetal HPA axis and programming of vascular function is unclear, there are numerous examples of an interaction between the glucocorticoids and other vasoactive substances, e.g. AngII, catecholamines and nitric oxide (Souness et al. 2002). Although not measured in the current study it is possible that levels of the corticosteroid inactivating enzyme 11-βhydroxysteroid dehydrogenase type 2 (11βHSD-2) in the placenta were altered preferentially by maternal dietary protein restriction as this may be affected by altered maternal peripheral cortisol levels (Clarke et al. 2002). A reduction in placental 11βHSD-2 has been proposed to elevate fetal cortisol levels and to induce fetal programming (Benediktsson et al. 1997). Indeed maternal nutrient restriction in early gestation produced a decrease in placental 11βHSD-2 at mid-gestation (Whorwood et al. 2001). However in the present study we observed no difference between dietary groups in fetal plasma cortisol, nor AVP and components of the renin-angiotensin system.
Plasma levels in themselves indicate very little about functional effects. In the rat, hypertensive offspring of dams fed a low protein diet do not have elevated plasma corticosteroids, but exhibit tissue-specific increases in glucocorticoid receptor (GR) expression and down-regulation of 11βHSD-2 activity in the placenta, kidney and adrenal gland (see Bertram et al. 2002), which will increase sensitivity and overexpose organs to glucocorticoid, respectively. Moreover, the role of low levels of fetal exposure to cortisol in producing permanent effects on the offspring has recently been questioned (Moritz et al. 2002). Furthermore, tissue-specific increases in angiotensin-type 1 receptor mRNA levels in the adrenal gland, kidney, liver and lung have been observed in sheep offspring exposed to maternal nutrient restriction in early gestation (Whorwood et al. 2001). Future studies in our model will need to measure tissue gene expression, such as components of the HPA axis and renin-angiotensin system, in relation to gestational age and growth, as well as their plasma levels.
In summary, this study has shown for the first time the importance of dietary balance, in particular restricted protein, versus restriction of total calorific intake per se in the blunting of endothelial-dependent and -independent relaxation in femoral arteries from the mid-gestation sheep fetus. We speculate that these changes may precede perturbed late-gestation fetal and postnatal cardiovascular control, as observed in similar dietary models (see Hanson et al. 1999). The molecular mechanisms underlying the perturbed vascular responses remain to be elucidated. Data from a range of studies indicate that these may be mediated in part by programming of the HPA axis (see Bertram & Hanson, 2002 for review). Further study is now essential in order to elucidate genomic mechanisms at the local vascular level and to relate them to organ growth and function.
Acknowledgments
This work was supported by the British Heart Foundation.
References
- AFRC. Energy and Protein Requirements of Ruminants. Wallingford, UK: CAB International; 1993. [Google Scholar]
- Anwar MA, Schwab M, Poston L, Nathanielsz PW. Betamethasone-mediated vascular dysfunction and changes in hematological profile in the ovine fetus. Am J Physiol. 1999;276:H1137–1143. doi: 10.1152/ajpheart.1999.276.4.H1137. [DOI] [PubMed] [Google Scholar]
- Benediktsson R, Calder AA, Edwards CR, Seckl JR. Placental 11 beta-hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure. Clin Endocrinol. 1997;46:161–166. doi: 10.1046/j.1365-2265.1997.1230939.x. [DOI] [PubMed] [Google Scholar]
- Bertram CE, Hanson MA. Prenatal programming of postnatal endocrine responses by glucocorticoids. Reproduction. 2002;124:459–467. doi: 10.1530/rep.0.1240459. [DOI] [PubMed] [Google Scholar]
- Brawley L, Poston L, Hanson MA. Mechanisms underlying the programming of small artery dysfunction: Review of the model using low protein diet in pregnancy in the rat. Arch Physiol Biochem. 2003;111:23–35. doi: 10.1076/apab.111.1.23.15138. [DOI] [PubMed] [Google Scholar]
- Busse R, Fleming I. Nitric oxide, nitric oxide synthase, and hypertensive vascular disease. Curr Hypertens Rep. 1999;1:88–95. doi: 10.1007/s11906-999-0078-6. [DOI] [PubMed] [Google Scholar]
- Clarke KA, Ward JW, Forhead AJ, Giussani DA, Fowden AL. Regulation of 11 beta-hydroxysteroid dehydrogenase type 2 activity in ovine placenta by fetal cortisol. J Endocrinol. 2002;172:527–534. doi: 10.1677/joe.0.1720527. [DOI] [PubMed] [Google Scholar]
- De Meyer GR, Herman AG. Vascular endothelial dysfunction. Prog Cardiovasc Dis. 1997;39:325–342. doi: 10.1016/s0033-0620(97)80031-x. [DOI] [PubMed] [Google Scholar]
- Dodic M, Wintour EM, Whitworth JA, Coghlan JP. Effect of steroid hormones on blood pressure. Clin Exp Pharmacol Physiol. 1999;26:550–552. doi: 10.1046/j.1440-1681.1999.03076.x. [DOI] [PubMed] [Google Scholar]
- Edwards LJ, Mcmillen IC. Periconceptional nutrition programs development of the cardiovascular system in the fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2002;283:R669–679. doi: 10.1152/ajpregu.00736.2001. [DOI] [PubMed] [Google Scholar]
- Franco M, Arruda R, Dantas A, Kawamoto E, Fortes Z, Scavone C, Carvalho M, Tostes R, Nigro D. Intrauterine undernutrition: expression and activity of the endothelial nitric oxide synthase in male and female adult offspring. Cardiovasc Res. 2002;56:145–153. doi: 10.1016/s0008-6363(02)00508-4. [DOI] [PubMed] [Google Scholar]
- Giussani DA, McGarrigle HH, Moore PJ, Bennet L, Spencer JA, Hanson MA. Carotid sinus nerve section and the increase in plasma cortisol during acute hypoxia in fetal sheep. J Physiol. 1994a;477:75–80. doi: 10.1113/jphysiol.1994.sp020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, McGarrigle HH, Spencer JA, Moore PJ, Bennet L, Hanson MA. Effect of carotid denervation on plasma vasopressin levels during acute hypoxia in the late-gestation sheep fetus. J Physiol. 1994b;477:81–87. doi: 10.1113/jphysiol.1994.sp020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, Spencer JA, Hanson MA. Fetal Cardiovascular reflex responses to hypoxaemia. Fetal Mater Med Rev. 1994c;6:17–37. [Google Scholar]
- Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutr. 2001;4:611–624. doi: 10.1079/phn2001145. [DOI] [PubMed] [Google Scholar]
- Green LR. Programming of endocrine mechanisms of cardiovascular control. J Soc Gynecol Investig. 2001;8:57–68. [PubMed] [Google Scholar]
- Green LR, Bennet L, Hanson MA. The role of nitric oxide synthesis in cardiovascular responses to acute hypoxia in the late gestation sheep fetus. J Physiol. 1996;497:271–277. doi: 10.1113/jphysiol.1996.sp021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green LR, McGarrigle HH, Bennet L, Hanson MA. Angiotensin II and cardiovascular chemoreflex responses to acute hypoxia in late gestation fetal sheep. J Physiol. 1998;507:857–867. doi: 10.1111/j.1469-7793.1998.857bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon MH, Heap RB. Progesterone and oestrogen concentrations in plasma of Barbary sheep (aoudad, Ammotragus lervia). compared with those of domestic sheep and goats during pregnancy. J Reprod Fertil. 1990;90:207–211. doi: 10.1530/jrf.0.0900207. [DOI] [PubMed] [Google Scholar]
- Hanson MA, Hawkins P, Ozaki T, Steyn C, Matthews S, Noakes DE, Poston L. Effects of experimental dietary manipulation during early pregnancy on cardiovascular and endocrine function in fetal sheep. In: O'Brien PMS, Wheeler T, Barker DJP, editors. Fetal Programming: Influences on Development and Disease in Later Life. London: RCOG Press; 1999. pp. 365–373. [Google Scholar]
- Harding J, Liu L, Evans P, Oliver M, Gluckman P. Intrauterine feeding of the growth retarded fetus: can we help? Early Hum Dev. 1992;29:193–197. doi: 10.1016/0378-3782(92)90149-b. [DOI] [PubMed] [Google Scholar]
- Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18:815–831. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- Iuchi T, Akaike M, Mitsui T, Ohshima Y, Shintani Y, Azuma H, Matsumoto T. Glucocorticoid excess induces superoxide production in vascular endothelial cells and elicits vascular endothelial dysfunction. Circ Res. 2003;92:81–87. doi: 10.1161/01.res.0000050588.35034.3c. [DOI] [PubMed] [Google Scholar]
- Jackson AA, Dunn RL, Marchand MC, Langley-Evans SC. Increased systolic blood pressure in rats induced by a maternal low-protein diet is reversed by dietary supplementation with glycine. Clin Sci. 2002;103:633–639. doi: 10.1042/cs1030633. [DOI] [PubMed] [Google Scholar]
- Kalache KD, Ojutiku D, Nishina H, Green LR, Hanson MA. Mild maternal undernutrition in the first half of ovine pregnancy influences placental morphology but not fetal Doppler flow velocity waveforms and fetal heart size. J Perinat Med. 2001;29:286–292. doi: 10.1515/JPM.2001.041. [DOI] [PubMed] [Google Scholar]
- Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension. 2003;41:168–175. doi: 10.1161/01.hyp.0000047511.97879.fc. [DOI] [PubMed] [Google Scholar]
- Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127:4195–4202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Sherman RC, Welham SJ, Nwagwu MO, Gardner DS, Jackson AA. Intrauterine programming of hypertension: the role of the renin-angiotensin system. Biochem Soc Trans. 1999;27:88–93. doi: 10.1042/bst0270088. [DOI] [PubMed] [Google Scholar]
- Lingas R, Dean F, Matthews SG. Maternal nutrient restriction (48 h) modifies brain corticosteroid receptor expression and endocrine function in the fetal guinea pig. Brain Res. 1999;846:236–242. doi: 10.1016/s0006-8993(99)02058-2. [DOI] [PubMed] [Google Scholar]
- Molnar J, Nijland MJ, Howe DC, Nathanielsz PW. Evidence for microvascular dysfunction after prenatal dexamethasone at 0. 7, 0.75, and 0.8 gestation in sheep. Am J Physiol Regul Integr Comp Physiol. 2002;283:R561–567. doi: 10.1152/ajpregu.00031.2002. [DOI] [PubMed] [Google Scholar]
- Moritz K, Butkus A, Hantzis V, Peers A, Wintour EM, Dodic M. Prolonged low-dose dexamethasone, in early gestation, has no long-term deleterious effect on normal ovine fetuses. Endocrinology. 2002;143:1159–1165. doi: 10.1210/endo.143.4.8747. [DOI] [PubMed] [Google Scholar]
- Moss TJ, Sloboda DM, Gurrin LC, Harding R, ChalliS JR, Newnham JP. Programming effects in sheep of prenatal growth restriction and glucocorticoid exposure. Am J Physiol Regul Integr Comp Physiol. 2001;281:R960–970. doi: 10.1152/ajpregu.2001.281.3.R960. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Nishina H, Ozaki T, Hanson MA, Poston L. Mechanisms of noradrenaline-induced vasorelaxation in isolated femoral arteries of the neonatal rat. Br J Pharmacol. 1999;127:809–812. doi: 10.1038/sj.bjp.0702641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki T, Hawkins P, Nishina H, Steyn C, Poston L, Hanson MA. Effects of undernutrition in early pregnancy on systemic small artery function in late-gestation fetal sheep. Am J Obstet Gynecol. 2000;183:1301–1307. doi: 10.1067/mob.2000.107463. [DOI] [PubMed] [Google Scholar]
- Ozaki T, Nishina H, Hanson MA, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol. 2001;530:141–152. doi: 10.1111/j.1469-7793.2001.0141m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinski W, Napoli C. Pathophysiological events during pregnancy influence the development of atherosclerosis in humans. Trends Cardiovasc Med. 1999;9:205–214. doi: 10.1016/s1050-1738(00)00022-0. [DOI] [PubMed] [Google Scholar]
- Rees WD, Hay SM, Buchan V, Antipatis C, Palmer RM. The effects of maternal protein restriction on the growth of the rat fetus and its amino acid supply. Br J Nutr. 1999;81:243–250. [PubMed] [Google Scholar]
- Rogers KM, Bonar CA, Estrella JL, Yang S. Inhibitory effect of glucocorticoid on coronary artery endothelial function. Am J Physiol Heart Circ Physiol. 2002;283:H1922–1928. doi: 10.1152/ajpheart.00364.2002. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM. The role of endothelium in cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol. 1993;22(suppl. 4):S1–14. doi: 10.1097/00005344-199322004-00002. [DOI] [PubMed] [Google Scholar]
- Russel A. Body condition scoring of sheep. In: Boden E, editor. Sheep and Goat Practice. London: Balliere Tindall; 1991. pp. 3–10. [Google Scholar]
- Souness GW, Brem AS, Morris DJ. 11 Beta-hydroxysteroid dehydrogenase antisense affects vascular contractile response and glucocorticoid metabolism. Steroids. 2002;67:195–201. doi: 10.1016/s0039-128x(01)00148-9. [DOI] [PubMed] [Google Scholar]
- Stein CE, Fall CH, Kumaran K, Osmond C, Cox V, Barker DJ. Fetal growth and coronary heart disease in south India. Lancet. 1996;348:1269–1273. doi: 10.1016/s0140-6736(96)04547-3. [DOI] [PubMed] [Google Scholar]
- Thomassin H, Flavin M, Espinas ML, Grange T. Glucocorticoid-induced DNA demethylation and gene memory during development. EMBO J. 2001;20:1974–1983. doi: 10.1093/emboj/20.8.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unno N, Giussani DA, Hing WK, Ding XY, Collins JH, Nathanielsz PW. Changes in adrenocorticotropin and cortisol responsiveness after repeated partial umbilical cord occlusions in the late gestation ovine fetus. Endocrinology. 1997;138:259–263. doi: 10.1210/endo.138.1.4880. [DOI] [PubMed] [Google Scholar]
- Waldman SA, Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987;39:163–196. [PubMed] [Google Scholar]
- Whorwood CB, Firth KM, Budge H, Symonds ME. Maternal undernutrition during early to midgestation programs tissue-specific alterations in the expression of the glucocorticoid receptor, 11beta-hydroxysteroid dehydrogenase isoforms, and type 1 angiotensin II receptor in neonatal sheep. Endocrinology. 2001;142:2854–2864. doi: 10.1210/endo.142.7.8264. [DOI] [PubMed] [Google Scholar]
- Hawkins P, Steyn C, McGarrigle HH, Calder NA, Saito T, Stratford LL, Noakes DE, Hanson MA. Cardiovascular and hypothalamic-pituitary-adrenal axis development in late gestation fetal sheep and young lambs following modest maternal nutrient restriction in early gestation. Reprod Fertil Dev. 2000;12:443–456. doi: 10.1071/rd99071. [DOI] [PubMed] [Google Scholar]


