Abstract
Vagal mechanoreceptors to the guinea-pig oesophagus, recorded extracellularly, in vitro, fired spontaneously at 3.3 ± 0.2 Hz, (n = 75, from 57 animals), and had low thresholds to circumferential stretch. In this study, we have investigated whether mechanotransduction by intraganglionic laminar endings (IGLEs) directly relies on mechano-gated ion channels, or whether it is due to chemical activation by neurotransmitters (glutamate or ATP) released from other cells during mechanical distortion. Rapid distortion of focal transduction sites (IGLEs) evoked action potentials with a latency of < 10 ms. Antagonists to ionotropic (AP5, memantine and 6,7-dinitroquinoxaline-2,3-dione (DNQX)) and metabotropic glutamate receptors (N-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide (PHCCC) and (RS)-a-methyl-4-phosphono-phenylglycine (MPPG)) did not affect mechano-transduction. Glutamate, NMDA and the selective mGluR group II and III agonists, (2R, 4R)-APDC and l-AP4, had no effect on spontaneous or stretch-induced firing. The P2X purinoreceptor agonist, α,β-methylene ATP, caused concentration-dependent excitation of vagal mechanoreceptors (EC50 = 22.2 µm) which was blocked by the non-selective P2 antagonist PPADS (30 µm). On its own, PPADS affected neither stretch-induced firing nor spontaneous firing. Neither Ca2+-free solution (1 mm EDTA, 3.6 mm Mg2+) solution nor Cd2+ (100 µm) blocked stretch-induced firing. Thus chemical transmission is not involved in activation of vagal mechanoreceptors. The blocker of stretch-activated channels, Gd3+ (300 µm), did not inhibit stretch-induced firing. However, benzamil (100 µm) significantly inhibited spontaneous and distension-evoked firing in a stretch-dependent manner; proportionally greater inhibition was seen with larger stretches. The results suggest that IGLEs of vagal tension receptors directly transduce mechanical stimuli probably via benzamil-sensitive, Gd3+-insensitive, stretch-activated ion channels, and that chemical transmission is not involved in transduction.
Mechanosensation starts with the transduction of mechanical forces into cellular electrochemical signals in primary afferent neurones. This enables living organisms to detect touch, vibration, acceleration, proprioception (including movements of the gut) and change in cellular volume and shape. Two types of mechanism have been proposed to underlie mechanotransduction: a physical mechanism that relies on mechano-gated ion channels providing the generator (receptor) potential and chemical mechanisms (Hamill & Martinac, 2001; Ernstrom & Chalfie, 2002). Both mechano-gated channels and mechanosensitive release of transmitter (ATP) appear to be ubiquitous in eukaryotic cells (Hamill & McBride, 1996; Nakamura & Strittmatter, 1996; Chen & Grinnell, 1997; Bodin & Burnstock, 2001; Hamill & Martinac, 2001). In mammalian visceral afferent neurones, chemical activation, by substances such as ATP or glutamate released by cells in response to mechanical distortion, has been proposed to underlie mechanosensitivity of primary afferent neurones to the urinary bladder (Rong et al. 2002) or colon (McRoberts et al. 2001).
Two large superfamilies of ion channels are currently the most likely candidates for mechano-gated channels: transient receptor potential (TRP) cation channels, with six transmembrane domains, and epithelial sodium channels (ENaC/ASIC/degenerin), with two transmembrane domains (Ernstrom & Chalfie, 2002). Both ENaC and TRP channels participate in mechanosensation in nematodes and insects (Gillespie & Walker, 2001; Hamill & Martinac, 2001). Evidence has accumulated that ion channels of the ENaC/ ASIC/degenerin superfamily are involved in mechanotransduction by spinal and vagal afferent neurones in vertebrates. In BNC1 gene knock out mice there was a reduction of mechanosensitivity of rapidly adapting skin mechanoreceptors (Price et al. 2000). In addition, mRNAs encoding ENaC subunits and BNC1 have been detected in nodose and dorsal root ganglia neurones and their mechanosensory nerve terminals (Drummond et al. 2000, 2001; Price et al. 2000). Both amiloride and its analogue, benzamil, inhibited pressure-evoked nerve activity in baroreceptor vagal afferents (Drummond et al. 2001). Mechanically activated currents, depolarisation and [Ca2+]i transients have been recorded in dorsal root and nodose ganglia neurones and these were blocked by non-selective blockers of mechano-gated channels, Gd3+, amiloride and benzamil (Cunningham et al. 1995; Sharma et al. 1995; McCarter et al. 1999; Snitsarev et al. 2002). While Gd3+, amiloride and its analogues are useful tools to study mechanosensitive channels, they are unable to discriminate between ENaC and TRP family members (Hamill & Martinac, 2001; Inoue et al. 2001; Trebak et al. 2002).
Specialised intraganglionic laminar endings (IGLEs) are the mechano-transduction sites of vagal mechanoreceptors in the guinea-pig upper gut (Zagorodnyuk & Brookes, 2000; Zagorodnyuk et al. 2001). It is not known whether IGLEs directly transduce mechanical stimuli or whether they are activated indirectly by intrinsic primary afferent neurones, viscerofugal neurones (Miller & Szurszewski, 1997; Kunze et al. 2000) or by non-neuronal cells in the gut. The aim of this study was to investigate whether IGLEs in the guinea-pig oesophagus transduce mechanical stimuli directly or indirectly via chemical transmission from other cells. Preliminary accounts of this study have appeared in abstract form (Zagorodnyuk et al. 2003).
Methods
Extracellular recording
Adult guinea-pigs (total = 63 (N)), weighing between 250 and 300 g, were killed humanely by stunning and exsanguination, in a manner approved by the Animal Welfare Committee of Flinders University. The method of extracellular recordings from vagal mechanically sensitive afferents in guinea-pig oesophagus has been described previously (Zagorodnyuk & Brookes, 2000). Briefly, the distal oesophagus was opened up into a flat sheet and the mucosa then removed from a 10-mm-long preparation which was washed with Krebs solution (mm: NaCl, 118; KCl, 4.75; NaH2PO4, 1.0; NaHCO3, 25; MgSO4, 1.2; CaCl2, 2.5; glucose, 11; bubbled with 95 % O2-5 % CO2). Fine vagal nerve branches (originating from the right vagus nerve), which innervated the distal region of oesophagus, were dissected free of connective tissue and the preparation was then placed in a 5 ml organ bath. The dissected vagal trunk and a separate strand of connective tissue were pulled into a second small chamber (1 ml volume) under a partition made from a coverslip, and sealed in position with silicon grease (Ajax Chemicals, Australia). The Krebs solution in the chamber was removed and replaced with paraffin oil and extracellular recordings were made with two platinum electrodes. Signals were amplified differentially (DAM 80, WPI, USA) and recorded at 20 kHz with a MacLab 8 s (AD Instruments, Sydney, Australia) attached to a Macintosh G4 computer (Apple, Cupertino, CA, USA) using Chart 3.6.5 software (AD Instruments, Sydney, Australia). Single units were discriminated by amplitude and duration using Spike Histogram software (AD Instruments, Sydney, Australia).
An array of hooks was used to attach one edge of the preparation to an isometric force transducer (DSC no.46-1001–01, Kistler-Morse, Redmond, WA, USA) mounted on a microprocessor-controlled tissue stretcher (Brookes et al. 1999). The slack was taken up to a resting tension of 0.5–1 mN and at least 120 min of equilibration was allowed before experiments started. Oesophageal preparations were stretched by the tissue stretcher over 1–3 mm at 5 mm s−1 and held for 10 s, every 3 min. The maximum stretch of 3 mm represented an increase in diameter of about 50–60 % of the empty oesophagus and is likely to be well within the normal range of physiological distensions. To analyse firing rate of afferent units, the spontaneous firing per second was averaged for 10 s before application of stretch, the dynamic phase of firing averaged over 3 s from the onset of the stretch and the adapted phase of firing averaged over the last 3 s of the stretch. IGLEs can also be activated by light, focal compression with a von Frey hair (Zagorodnyuk & Brookes, 2000; Zagorodnyuk et al. 2001) and to determine the minimal time required for mechanotransduction, a blunt glass micropipette (100 µm tip diameter) was mounted on a piezo-electric buzzer (Radio Spares, Sydney, Australia). Voltage steps (100 V, 200 ms duration) were applied to the piezo-electric activator from a stimulator (Grass DS9A, Quincy, MA, USA), producing a rapid displacement of approximately 10 µm amplitude with a latency of less than 0.1 ms. The tip of the pipette was placed precisely above an IGLE identified and marked previously with a von Frey hair.
Anterograde labelling and immunohistochemistry
The method of anterograde labelling in vitro of vagal afferents in guinea-pig oesophagus was as follows. A drop of 5 % biotinamide (Molecular Probes, Eugene, OR, USA) in an artificial intracellular medium (150 mm monopotassium l-glutamic acid, 7 mm MgCl2, 5 mm glucose, 1 mm EGTA, 20 mm Hepes, 5 mm disodium adenosine-triphosphate, 0.02 % saponin, 1 % dimethyl sulfoxide), was placed on a fine vagal nerve trunk innervating distal oesophagus in the paraffin-filled chamber. The main chamber, containing the oesophageal preparation, was filled with sterile supplemented culture medium (DME/F12 with 10 % fetal bovine serum, 1.8 mm CaCl2, 100 i.u. ml−1 penicillin, 100 µg ml−1 streptomycin, 2.5 µg ml−1 amphotericin B, 20 µg ml−1 gentamicin (Cytosystems, NSW, Australia), pH 7.4). The tissue was incubated in humidified incubator in 5 % CO2 in air at 37 °C. After 15–20 h, preparations were fixed overnight in modified Zamboni's fixative (15 % saturated picric acid and 2 % formaldehyde in a 0.1 m phosphate buffer, pH 7.0), cleared in DMSO for 30 min and rinsed in phosphate buffered saline (PBS: 150 mm NaCl in 10 mm sodium phosphate buffer, pH 7.2). Labelled nerve fibres were visualised with streptavidin Alexa Fluor 448 (Molecular Probes, Eugene, OR, USA, 1:4000, 4 h).
Polyclonal antibodies against vesicular glutamate transporters 1 (VGluT1, 1: 1000, #135002) and 2 (VGluT2, 1: 1000, #135102) raised in the rabbit were obtained from Synaptic Systems (Göttingen, Germany). Polyclonal antibodies against synaptotagmin (S39520, 1:100) were from Transduction Laboratories (Lexington, KY, USA); a mouse anti-synaptophysin (S-5768) was obtained from Sigma Chemical Company. Polyclonal antibodies against P2X2 subunit raised in the rabbit were from Chemicon (AB5244, 1:500, Temecula, CA, USA). Tissues were incubated in primary antibodies overnight and then in secondary antibodies (donkey anti-rabbit Cy5 1:50, Jackson Immunoresearch Lab. Inc., West Grove, PA, USA, 2 h or donkey anti-mouse Cy3 1:100, Jackson Immunoresearch Lab Inc., West Grove, PA, USA, 1:50, 2 h) before mounting in bicarbonate buffered glycerol (pH 8.6). Labelled nerve fibres were analysed on an Olympus AX70 epifluorescence microscope. Images of labelled neural structures were captured via a Hamamatsu digital camera (model C4742–95, Japan) and recorded on an Apple Macintosh G3 computer using IPLab software (Scanalytics Inc., Fairfax, VA, USA). Images were combined, cropped, scaled and adjusted for brightness and contrast in Adobe Photoshop 5.5.
Drugs
l-glutamic acid, NMDA (N-methyl-d-aspartic acid), AP5 (dl-2-amino-5-phosphonopentanoic acid), DNQX (6,7-dinitroquinoxaline-2,3-dione), PHCCC (N-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide), MPPG ((RS)-a-methyl- 4- phosphono-phenylglycine), memantine, (2R,4R)-APDC ((2R,4R)- 4-aminopyrrolidine-2,4-dicarboxylate) and l-AP4 (l-(+)-2-amino-4-phosphonobutyric acid) were obtained from Tocris (Avonmouth, UK). Gadolinium chloride, cadmium chloride, EDTA, amiloride, benzamil, tetrodotoxin (TTX), PPADS (pyridoxyl 5-phosphate 6-azophenyl-2′,4′-disulfonic acid), α,β-methylene ATP (α,β-meATP) and ADPβS were obtained from Sigma Chemical Company. Gd3+ and Cd2+ were used in modified Krebs solution (mm: NaCl, 139; KCl, 4.75; Hepes, 5; MgCl2, 1.2; CaCl2, 2.5; glucose, 11; bubbled with 100 % O2).
Data analysis
Results are expressed as means ± s.e.m., with n referring to the number of units and N to the number of animals. Statistical analysis was performed by Student's two-tailed t test for paired or unpaired data or by repeated measures one-way or two-way analysis of variance using Prism 3 software (GraphPad Software, Inc., San Diego, CA, USA). Differences were considered significant if P < 0.05.
Results
The majority of vagal afferents identified as single units were spontaneously active, firing at 3.3 ± 0.2 Hz, n = 75, N = 57). They had low thresholds (less than 1 mm) to circumferential stretch, showed slow adaptation during maintained (10 s) stretch and had a silent period after removal of stretch. After an initial peak in their firing rate (dynamic phase), following the onset of step distension (at 5 mm s−1), firing adapted but remained elevated (adapted phase), compared to resting discharge, for the duration of the stimulus (10 s). After removal of stretch, a silent period was followed by recovery back to the spontaneous firing rate over a period of several seconds (Fig. 1). Stretch-induced firing was linearly related to wall tension at corresponding points of distension (r2 = 0.8, n = 16, N = 16) indicating that these mechanoreceptors function largely as tension receptors (Fig. 1).
Figure 1. Typical recordings from a mechanically-sensitive oesophageal vagal afferent and dependence of firing rate on intramural tension.
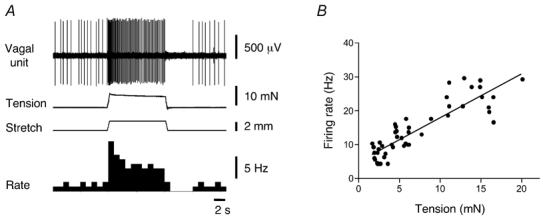
A, vagal afferent firing evoked by 2 mm circumferential stretch at 5 mm s−1, held for 10 s. B, linear dependence of the dynamic firing to the amplitude of intramural tension. Each value in B is the mean from 16 units in 16 preparations.
Latency of mechanotransduction
Focal compression of the tissue with light von Frey hairs (0.12–0.4 mN) activated stretch-sensitive vagal mechanoreceptors, but only at a few sites, which have been previously shown to correspond to intraganglionic laminar endings (IGLEs) (Zagorodnyuk & Brookes, 2000; Zagorodnyuk et al. 2001). The minimum response latency of IGLEs was determined by rapidly distorting an IGLE with the piezo-electric probe (see Methods) at 5 s intervals. In most trials, the first action potential evoked by the stimulus occurred with a short latency averaging 9.2 ± 1.6 ms (n = 4, N = 3, see Fig. 2A). The exceptions occurred when a spontaneously evoked action potential occurred within 40 ms of the mechanical stimulus. In these cases, the first evoked spike was delayed by up to 50 ms, presumably reflecting a partial refractory period in the IGLE following each action potential (see Fig. 2B). In control runs, when the probe was lifted clear of the tissue, the latency of the first spike was accurately predicted by the recency of the previous spike (r2 = 0.92, see Fig. 2C), reflecting the constant rate of spontaneous discharge. Electrical stimulation at the site of the IGLE was used to calculate the delay due to conduction, which corresponded to 3.4 ± 0.4 ms, n = 4, N = 3. Subtraction indicates that the mean delay due to mechanotransduction was less than 6 ms. This indicates that mechanotransduction must be due either to very rapid chemical transmission from other cells or by activation of mechano-gated ion channels located on IGLEs.
Figure 2. Latency of mechanotransduction in a spontaneously firing unit.
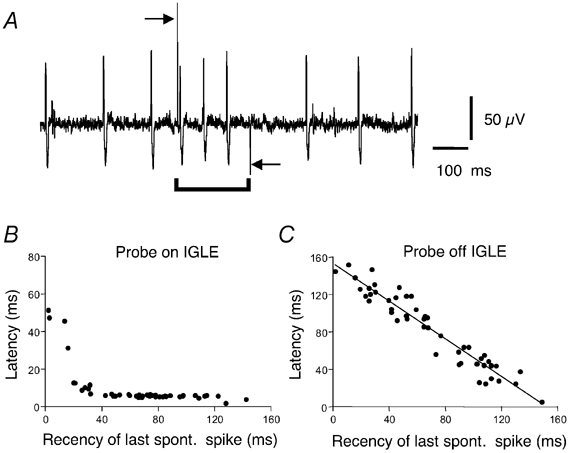
A, typical recordings of mechanically evoked firing following activation of the piezo-electric probe (indicated by bar below trace). Arrows indicate onset and removal of the piezo-electric probe. Note that the first spike was evoked with a very short latency (≈6 ms). B, dependence of the latency of the first spike after onset of the piezo-electric probe relative to the recency of the last spontaneous spike. Note that spikes were evoked with a consistent, short latency (about 6 ms in this unit) except when a spontaneous spike occurred within 40 ms of advancing the probe. C, when the piezo-electric probe was moved away from the hotspot, the latency of spikes from probe activation was predicted entirely by the recency of the last spontaneous spike.
Glutamate and mechanotransduction in vagal mechano-sensitive afferents
Since glutamate has been suggested to be involved in activation of colonic mechanoreceptors (McRoberts et al. 2001) and can mediate fast synaptic transmission, we were interested in the possible role of endogenous glutamate in activation or modulation of IGLEs. Antibodies against vesicular glutamate transporters 1 and 2 (VGluT1/2), which transport glutamate into synaptic vesicles (Takamori et al. 2000; Fremeau et al. 2001), were used to localise synaptic vesicles containing glutamate in anterogradely labelled vagal endings to the guinea-pig oesophagus. In all experiments biotinamide-filled leaf-like endings of IGLEs were strongly immunoreactive for VGluT1 and weakly immunoreactive for VGluT2 (Fig. 3A′ and B′). Biotinamide-filled varicose fibres in myenteric ganglia and muscle layers were not labelled by either VGluT1 or VGluT2, (Fig. 3C‘) while motor endplates were labelled by VGluT1 (arrow, Fig. 3C‘) but not VGluT2. No evidence was seen for VGluT-immunoreactive structures belonging to neurones or other cell types showing any close associations with IGLEs. We also investigated whether the proteins, synaptotagmin and synaptophysin, known to be involved in rapid transmitter exocytosis (Eshkind & Leube, 1995; Daly et al. 2000; Augustine, 2001) were also present in IGLEs. In each preparation, some biotinamide-filled lamellae of IGLEs were weakly immunoreactive to synaptotagmin (n = 3) and synaptophysin (n = 3); the intensity of labelling, in each case, was considerably lower than in motor endplates (Fig. 3D‘).
Figure 3. VGluT1, VGluT2 and synaptophysin immunoreactivity in myenteric ganglia and muscle layers of the guinea-pig oesophagus.
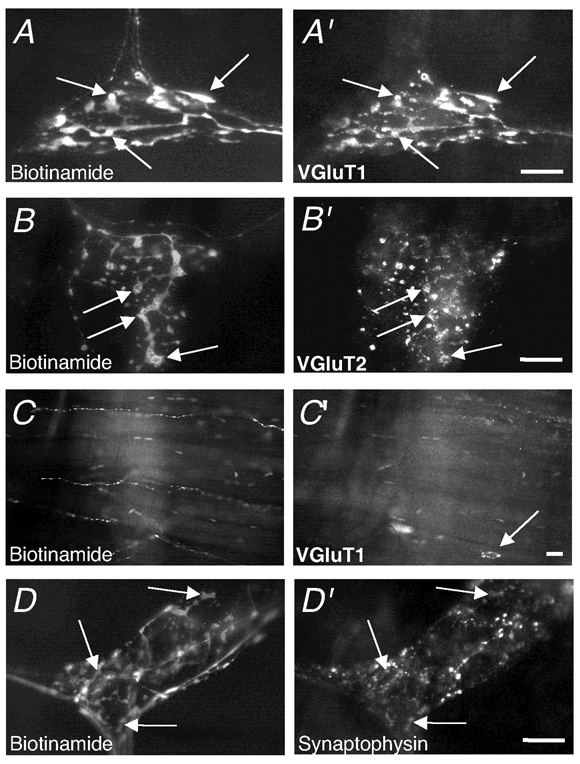
A, biotinamide-filled IGLE in a myenteric ganglion shows intense VGluT1 immunoreactivity (A‘). Note that all lamellae were immunoreactive, unlike varicose fibres. B, biotinamide-filled IGLE in myenteric ganglia showed VGluT2 immunoreactivity (B‘) which was significantly less intense than VGluT1; some lamellae were hardly visible. C, biotinamide-filled nerve fibres in the inner striated muscle layer lacked VGluT1 immunoreactivity (C‘). Note the endplate immunoreactive for VGluT1 (arrow, C‘). D, biotinamide-filled IGLE with weak synaptophysin immunoreactivity (D‘). Scale bar on all panels = 20 µm.
Since vesicular glutamate transporters and synaptic release-associated proteins are present in IGLEs we considered whether glutamate from this source might act as in an autocrine fashion to modulate afferent sensitivity, and at the same time, whether glutamate from other sources might activate IGLEs. Neither the antagonist to ionotropic NMDA receptors, AP5 (100 µm, n = 4, N = 3), nor the antagonist to non-NMDA receptors, DNQX (100 µm, n = 3, N = 3) affected significantly mechanotransduction of vagal oesophageal tension receptors (Table 1). Likewise, memantine, a non-competitive open channel blocker of NMDA receptors, (10–30 µm) did not affect spontaneous or stretch-evoked firing rate of oesophageal mechanoreceptors (Fig. 4). However at high concentrations (100 µm and 300 µm) spontaneous firing was increased by 1 ± 0.2 Hz (n = 6, N = 5, P < 0.05) and by 3.5 ± 0.3 Hz (n = 6, N = 5, P < 0.0001), respectively. Memantine (300 µm) significantly increased both dynamic (F = 19.3, P < 0.0001) and adapted stretch-evoked firing (F = 29.5, P < 0.0001) (Fig. 4). In addition, the duration of the silent period following 3 mm stretch was reduced by memantine (300 µm) by 34 ± 6 % (n = 6, N = 5, P < 0.001). These effects of high concentrations of memantine were not associated with changes in either basal or stretch-evoked intramural tension. It is worth mentioning that at the highest dose used (300 µm) memantine in all cases increased the duration (by 49 ± 18 %, n = 6, N = 5, P < 0.01) of extracellularly recorded action potentials. The group I metabotropic glutamate receptors antagonist, PHCCC (10 µm, n = 4, N = 3) and the group II and III antagonist, MPPG (100 µm, n = 4, N = 3), did not significantly change either spontaneous or stretch-evoked firing of vagal oesophageal tension receptors (Table 1).
Table 1.
Lack of effect of glutamate receptors agonists and antagonists and amiloride on spontaneous and stretch (3 mm)-evoked firing and silent period of vagal mechanoreceptors
| Drugs | Spontaneous firing (Hz) | Dynamic firing (Hz) | Adapted firing (Hz) | Silent period (s) |
|---|---|---|---|---|
| Control | 4 ± 1.5 | 20 ± 5.6 | 14 ± 5.2 | 4.3 ± 0.2 |
| Glutamate (1 mm) | 4.2 ± 1.6 | 21 ± 5.4 | 14 ± 4.7 | 4.2 ± 0.5 |
| (n = 4, N = 3) | (n = 4, N = 3) | (n = 4, N = 3) | (n = 4, N = 3) | |
| Control | 4 ± 0.8 | 14 ± 3.5 | 9.8 ± 2.6 | 2.3 ± 0.7 |
| NMDA (100 μm) | 4 ± 0.7 | 14 ± 3.5 | 10 ± 2.6 | 2.2 ± 0.6 |
| (n = 3, N = 3) | (n = 3, N = 3) | (n = 3, N = 3) | (n = 3, N = 3) | |
| Control | 3 ± 1.3 | 19 ± 0.4 | 12 ± 0.4 | 5 ± 1.7 |
| 2R, 4R-APDC (50 μm) | 3.2 ± 1.0 | 20 ± 0.6 | 12 ± 0.3 | 4.8 ± 1.5 |
| (n = 3, N = 3) | (n = 3, N = 3) | (n = 3, N = 3) | (n = 3, N = 3) | |
| Control | 2.2 ± 0.8 | 24 ± 8.5 | 14 ± 3.6 | 8.2 ± 0.8 |
| L-AP4 (50 μm) | 2.1 ± 0.7 | 24 ± 8.1 | 14 ± 3.5 | 8.7 ± 0.8 |
| (n = 6, N = 3) | (n = 6, N = 3) | (n = 6, N = 3) | (n = 6, N = 3) | |
| Control | 4 ± 1.4 | 30 ± 7.1 | 22 ± 6 | 7.3 ± 0.6 |
| AP5 (100 μm) | 4.2 ± 1.3 | 30 ± 7.6 | 23 ± 6.1 | 6.7 ± 0.7 |
| (n = 4, N = 3) | (n = 4, N = 3) | (n = 4, N = 3) | (n = 4, N = 3) | |
| Control | 2.9 ± 0.7 | 16 ± 4.1 | 9.2 ± 3.1 | 4.9 ± 2.2 |
| DNQX (100 μm) | 2.6 ± 0.8 | 16 ± 4.3 | 8.8 ± 2.9 | 5.3 ± 2.3 |
| (n = 3, N = 3) | (n = 3, N = 3) | (n = 3, N = 3) | (n = 3, N = 3) | |
| Control | 5.1 ± 0.8 | 27 ± 5.9 | 20 ± 5.1 | 5.9 ± 2.1 |
| PHCCC (10 μm) | 5 ± 0.8 | 28 ± 7.1 | 21 ± 5.8 | 5.8 ± 2 |
| (n = 4, N = 3) | (n = 4, N = 3) | (n = 4, N = 3) | (n = 4, N = 3) | |
| Control | 4.1 ± 1.4 | 22 ± 5 | 14 ± 5.1 | 4.3 ± 0.5 |
| MPPG (100 μM) | 4 ± 1.3 | 23 ± 4.8 | 16 ± 5 | 4.3 ± 0.4 |
| (n = 4, N = 3) | (n = 4, N = 3) | (n = 4, N = 3) | (n = 4, N = 3) | |
| Control | 4.2 ± 1 | 20 ± 3.1 | 15 ± 2.3 | 3.4 ± 0.8 |
| Amiloride (1 mm) | 4.6 ± 0.8 | 23 ± 4.9 | 17 ± 3.4 | 3.3 ± 0.5 |
| (n = 4, N = 3) | (n = 4, N = 3) | (n = 4, N = 3) | (n = 4, N = 3) |
Figure 4. Memantine increased stretch-evoked firing and shortened the silent period of vagal mechanoreceptor.
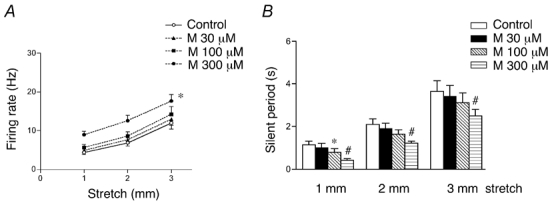
A, averaged data of the effect of memantine (M, 30–300 µm) on stretch-evoked adapted firing. B, averaged data of the effect of memantine on the duration of silent period. Each value in A and B is the mean +s.e.m. from 6 units in 5 preparations. *#P < 0.05.
Glutamate itself (100 µm-1 mm) and the selective mGluR group II and III agonists, (2R,4R)-APDC (50 µm) and l-AP4 (50 µm) did not affect spontaneous or stretch-induced firing of guinea-pig oesophageal mechanoreceptors (Table 1). Application of NMDA (100 µm, n = 3, N = 3) in Mg2+-free Krebs solution, containing the co-agonist d-serine (10 µm), also did not change spontaneous or stretch-induced firing after either 5–10 s of action or after 5–10 min of perfusion (Table 1). Taken together these results indicate that activation of glutamate receptors, from either endogenous or exogenous sources, is not involved in transduction or modulation of vagal mechanoreceptors in the guinea-pig oesophagus.
Modulation by purinergic agonists
ATP, acting via P2X3 receptors is involved in mechanosensory transduction by high threshold spinal afferents to the urinary bladder (Rong et al. 2002). In 24 of 27 single units (n = 12) the metabolically stable ATP analogue α,β-methylene ATP (α,β-meATP, a P2X-preferring agonist) caused powerful concentration-dependent excitation of vagal mechanoreceptors without affecting intramural tension (EC50 = 22.2 µm, 95 % confidence interval = 12.3–40 µm, n = 4–6, N = 5, see Fig. 5 and Fig. 6). At 30 µmα,β-meATP increased spontaneous firing by 12.2 ± 1.8 Hz (P < 0.001, n = 10, N = 7) and this effect was blocked by the non-selective P2 antagonist PPADS (30 µm for 20–25 min, see Fig. 5 and Fig. 6). Importantly, 30 µm PPADS by itself did not affect either spontaneous firing or dynamic or adapted stretch-induced firing (Fig. 6D) of vagal afferents, indicating that endogenous ATP is not involved in mechanotransduction.
Figure 5. Typical effects of α,β-meATP and PPADS on the firing rate of oesophageal afferents.
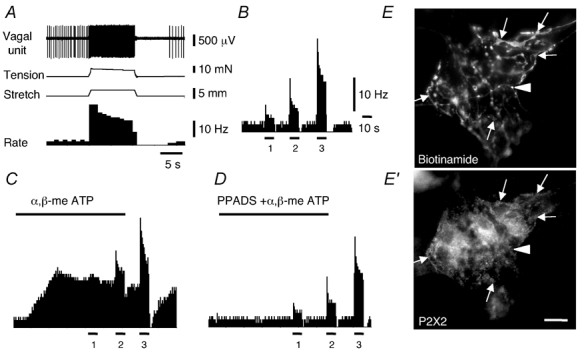
A, typical recording of firing and tension evoked by 3 mm circumferential stretch. B, spontaneous and stretch-induced firing (1, 2 and 3 mm stretches) in normal Krebs solution. C, excitatory effect α,β-meATP (30 µm for 2 min, indicated by bar) on spontaneous and stretch-induced firing. D, effect of application of α,β-meATP (30 µm for 2 min) in the presence of PPADS (30 µm for 25 min). Note the blockade of the effects of α,β-meATP on spontaneous firing, without any effect on stretch-induced firing. Traces in A-D belong to the same single unit while they are shown on different time and firing frequency scales. E, biotinamide-filled IGLE in a myenteric ganglion of the guinea-pig oesophagus showed P2X2 immunoreactivity (arrows, E′), but a varicose fibre lacked P2X2 immunoreactivity (arrowhead). Scale bar on E′= 20 µm.
Figure 6. Concentration-dependent responses to α,β-meATP and effects of PPADS.
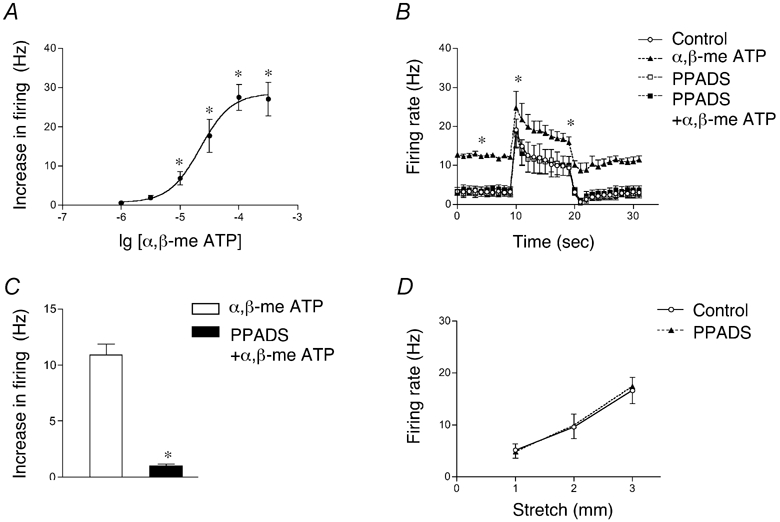
A, concentration-dependent increase of spontaneous firing by α,β-meATP (each value represents the mean ± s.e.m. of 4–6 units, n = 5). B, spontaneous and stretch (2 mm)-induced firing in control and in α,β-meATP (30 µm) with and without PPADS (30 µm, n = 7, N = 5). Note that PPADS inhibited the excitatory effects of α,β-meATP but did not affect either spontaneous or stretch-induced firing compared to controls. C, PPADS (30 µm) significantly inhibited the average increase in firing evoked by α,β-meATP (30 µm for 2 min, n = 7, N = 5). D, averaged data (from 7 units, n = 5) showing the lack of effect of PPADS alone (30 µm) on stretch-induced firing.
The P2Y-preferring agonist, ADPβS (Ralevic & Burnstock, 1998) excited vagal tension receptors, with significantly lower efficiency than α,β-meATP when studied in the same preparations. ADPβS (30 µm for 1 min) increased spontaneous firing by 1.3 ± 0.1 Hz (P < 0.001, n = 7, N = 5). The latency (44 ± 12 s) and time to peak (120 ± 20 s, respectively, n = 7, N = 5) of ADPβS effects were significantly longer than those of 30 µmα,β-meATP (18.5 ± 3 s and 66 ± 9 s respectively, n = 10, N = 7, P < 0.05 in both cases). In addition, the excitatory effect of 30 µmα,β-meATP was significantly increased (by 106 ± 41 %, n = 6, N = 4, P < 0.05) in Ca2+-free Krebs solution, while the effect of ADPβS was not significantly altered (+14 ± 14 %, n = 6, N = 5, n.s.). These data suggest that α,β-meATP and ADPβS activate different purinoreceptors (probably P2X and P2Y, respectively) on vagal mechanoreceptors in the guinea-pig oesophagus.
We tested for the presence of P2X receptor immunoreactivity on vagal tension receptor endings in the oesophagus. IGLEs were anterogradely labelled with biotinamide and examined for P2X2 immunoreactivity. In all cases, IGLEs in both the oesophagus and stomach were clearly immunoreactive for P2X2 receptor subunits, with all of the biotinamide-filled lamellae being strongly immunoreactive for P2X2 receptors, which were also visible in the unspecialised axon (Fig. 5E‘).
Effects of Ca2+-free Krebs solution and Cd2+ on mechanotransduction
In order to test whether neurotransmitter(s) other than ATP or glutamate may be involved in mechanotransduction by IGLEs, we investigated the effect of blocking rapid exocytotic release mechanisms with Ca2+-free and high Mg2+ Krebs solution. Transduction of mechanical stimuli by vagal tension receptors in the guinea-pig oesophagus persisted in Ca2+-free (1 mm EDTA, 3.6 mm Mg2+ for 30 min, n = 9, N = 6) Krebs solution (Fig. 7). In fact, Ca2+-free Krebs increased spontaneous firing significantly (by 3 ± 0.7 Hz, n = 14, N = 9, P < 0.0001) and both dynamic and adapted stretch -induced firing were slightly increased in Ca2+-free Krebs solution (Fig. 7A) although this was not significant.
Figure 7. Neither Ca2+-free Krebs solution nor Cd2+ blocked mechanotransduction by oesophageal mechanoreceptors.
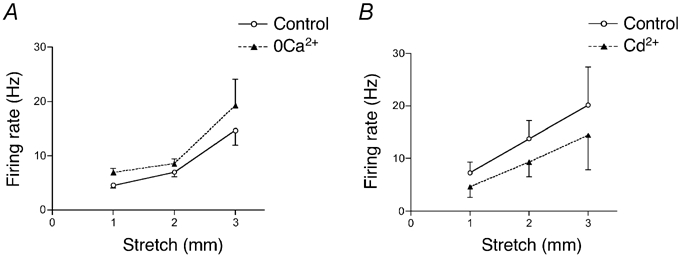
A, averaged data (n = 14, N = 9) of the effect of Ca2+-free (1 mm EDTA, 3.6 mm Mg2+) Krebs solution on stretch-evoked adapted firing. B, averaged data (n = 4–6, N = 4–5) of the effect of Cd2+ (100 µm) on stretch-evoked adapted firing. Note the small decrease in excitability, but failure to block stretch-activated increases in firing rate.
It is well established that Cd2+ (100 µm) blocks voltage-dependent Ca2+ channels (Fox et al. 1987), thus blocking Ca2+-dependent synaptic transmission. Application of Cd2+ (100 µm for 20–25 min) decreased the excitability of vagal mechanoreceptors, reducing spontaneous firing by 2.1 ± 0.5 Hz (n = 6, N = 5, P < 0.05). However it did not block stretch-induced firing, though it caused a small decrease in both dynamic and adapted stretch-evoked firing (Fig. 7B) which was not significant. Thus taken together the present data indicate that fast exocytotic release is not required for mechanosensory transduction by IGLEs.
Effect of non-selective blockers of stretch-activated channels, gadolinium and benzamil on mechanotransduction by vagal afferents
Gadolinium (300 µm for 20–25 min) significantly reduced spontaneous firing rate (by 1.9 ± 0.4 Hz, n = 6, N = 4, F = 23.5, P < 0.0001). Stretch-induced dynamic firing (1–3 mm distension) was slightly increased (by 10–30 %, n = 6, N = 4, n.s.) by 300 µm Gd3+, associated with an increase in stretch-evoked intramural tension (by 10–15 %, n = 4, n.s.), even though resting tension was unaffected. At higher concentrations, Gd3+ (1 mm) blocked spontaneous and stretch-induced firing of all vagal tension receptors studied (n = 6, N = 5) and reduced basal tension from 4.8 ± 1.8 mN to 1.6 ± 0.6 mN (n = 5, N = 5, n.s.).
Amiloride (1 mm for 25–30 min, n = 4, N = 3) did not significantly affect spontaneous or stretch-induced firing of vagal mechanoreceptors (Table 1). Its more potent analogue, benzamil (30 µm for 25–30 min) did not significantly change either spontaneous and stretch-induced firing or intramural tension. At a higher concentration, benzamil (100 µm) significantly inhibited spontaneous firing (by 1.3 ± 0.7 Hz, n = 9, N = 8, P < 0.0001) and adapted stretch-evoked firing (F = 15.2, P < 0.001, n = 9, N = 8, see Fig. 8 and Fig. 9) but did not significantly affect the dynamic firing evoked by the onset of stretch. The effect of benzamil was highly stretch-dependent (Fig. 8) with significantly greater inhibition during 3 mm stretches compared with 1 mm stretches. This interaction was significant, (F = 4.55, P < 0.05, n = 9, N = 8). Despite its inhibitory effects on firing, benzamil (100 µm) increased both resting tension (from 0.47 ± 0.1 mN to 2.57 ± 0.54 mN, n = 8, P < 0.01) and stretch-induced intramural tension (P < 0.0001, N = 8) in a stretch-dependent fashion, with bigger effect seen with larger stretches (F = 10.5, P < 0.001, N = 8, see Fig. 9B).
Figure 8. Typical effect of benzamil on spontaneous and stretch-evoked firing and intramural tension.
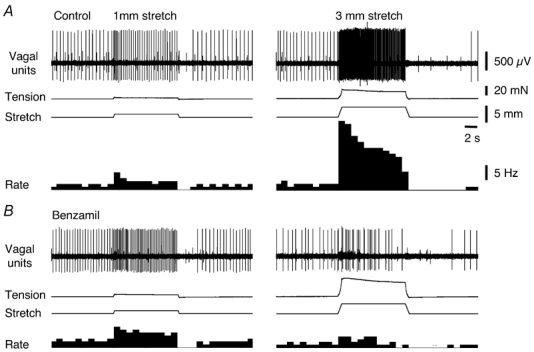
A, typical recordings of firing and tension evoked by 1 mm (left tracings) and 3 mm (right tracings) circumferential stretch in control. B, in the presence of benzamil (100 µm for 15 min) stretch-induced firing was reduced, particularly at 3 mm, while intramural tension changes were increased, demonstrating potent stretch-dependent inhibition of mechanotransduction.
Figure 9. Effects of benzamil and TTX on stretch-evoked firing and intramural tension.
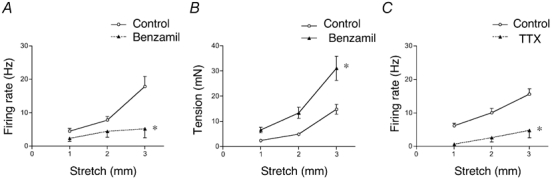
A, averaged data (n = 9, N = 8) of the effect of benzamil (100 µm) on stretch-induced adapted firing. Note the larger effect at 3 mm compared to smaller stretches. B, benzamil (100 µm) increased intramural tension evoked by circumferential stretch. C, averaged data (n = 6, N = 4) of the effect of TTX (30 nm) on stretch-induced adapted firing, showing a marked decrease in excitability that was not stretch dependent. *P < 0.05.
Some effects of benzamil (100 µm) have previously been attributed to blockade of voltage-dependent Na+ channels (Carr et al. 2001). We investigated whether a small concentration of TTX (30 nm) had similar effects to benzamil as shown for vagal neurones innervating airways (Carr et al. 2001). TTX (30 nm) blocked spontaneous firing and significantly inhibited both dynamic (F = 38.7, P < 0.0001, n = 6, N = 4) and adapted stretch-evoked firing (F = 49.1, P < 0.0001, n = 6, N = 4), but unlike benzamil, this effect was not stretch-dependent (F = 1.94, n.s., n = 6, N = 4) (Fig 9C). TTX (30 nm) did not affect resting or stretch-induced intramural tension.
Discussion
Intraganglionic laminar endings (IGLEs) are transduction sites of tension-sensitive vagal mechanoreceptors in both the stomach and oesophagus (Zagorodnyuk & Brookes, 2000; Zagorodnyuk et al. 2001). The detection of VGluT1/2 in IGLEs in the guinea-pig oesophagus confirms a recent report of VGluT2 immunoreactivity in IGLEs in the rat (Raab & Neuhuber, 2003). Vesicular glutamate transporters are responsible for accumulation of excitatory amino acids into synaptic vesicles at transmitter release sites (Takamori et al. 2000; Fremeau et al. 2001). The presence of vesicular glutamate transporters in IGLEs raises the possibility that they may release glutamate, in addition to their role in mechanotransduction. The presence of immunoreactivity for synaptotagmin and synaptophysin in some lamellae of IGLEs reinforces the suggestion that glutamate may be released via fast synaptic mechanisms from IGLEs onto enteric neurones. Coexistence of synaptophysin immunoreactivity with VGluT2 has been reported previously in IGLEs of the rat (Raab & Neuhuber, 2003), which contain small clear vesicles and make synaptic specialisations with enteric neurones (Neuhuber, 1987). Glutamate excites enteric neurones either via ionotropic AMPA and NMDA receptors (Liu et al. 1997) or via metabotropic glutamate receptors (Ren et al. 2000). However, electrical stimulation of vagal afferent nerve fibres caused very little excitation of enteric neurones, measured by Fos immunoreactivity (Zheng et al. 1997). Thus the physiological role of VGluT1/2 and synaptic release proteins in IGLEs is unclear: it may simply be a reflection of the glutamatergic release mechanisms at their central endings (Hoang & Hay, 2001; Gordon & Sved, 2002).
The experiments in this study addressed another possible association between excitatory amino acids and IGLEs. Responses of mechanosensitive sensory neurones to the colon are reduced by the NMDA channel blocker memantine, suggesting an important role for glutamate in mechanotransduction (McRoberts et al. 2001). This is unlikely to be the case for vagal mechanoreceptors, since ionotropic glutamate receptor antagonists (AP5, memantine, DNQX) did not affect responses to stretch in the current study. In fact, memantine at 100–300 µm, significantly above its IC50 for NMDA receptors (Chen & Lipton, 1997), actually had excitatory effects on vagal afferents. It increased the duration of action potentials, suggesting that it may block K+ channels, either directly, or by decreasing Ca2+ influx. Ionotropic glutamate receptor agonists (both glutamate and NMDA) had no detectable effects on vagal mechanoreceptors’ responses to stretch.
We also tested antagonists to metabotropic glutamate receptors (PHCCC and MPPG) and agonists to group II and III receptors [(2R, 4R)-APDC and l-AP4)] none of which affected either spontaneous or stretch-induced firing of vagal mechanoreceptors. In contrast, functional metabotropic glutamate receptors have been demonstrated in electrophysiological studies on rat nodose ganglion cell bodies and their central terminals (Glaum & Miller, 1992; Hay & Kunze, 1994; Hoang & Hay, 2001). Immunoreactivity for metabotropic glutamate receptors has also been detected on vagal afferent nerve cell bodies in the nodose ganglion (Li et al. 1996). Metabotropic glutamate receptors can modulate the excitability of vagal mechanoreceptors in both the ferret and mouse (Martin et al. 2001; Page et al. 2002a); this is clearly not the case in the guinea-pig. Interestingly, GABAB receptor agonists reduce the excitability of vagal mechanoreceptors in ferret oesophagus, but not in the guinea-pig (Zagorodnyuk et al. 2002b), suggesting that receptor distribution in visceral afferent neurones varies significantly between species. Overall, this study shows that glutamate is unlikely to play any significant role as a mediator of stretch, or as an autocrine or paracrine modulator of vagal mechanoreceptor firing in the guinea-pig oesophagus.
ATP is now a well-established neurotransmitter in both the central and peripheral nervous systems (Ralevic & Burnstock, 1998; Burnstock, 2001). ATP is also released from tissues during stretch, mechanical stimulation and physical damage (Nakamura & Strittmatter, 1996; Bodin & Burnstock, 2001; Vlaskovska et al. 2001; Knight et al. 2002), leading to the suggestion that purinergic transmission may play an important role in nociception in damaged or inflamed tissue (Burnstock, 2001). Both P2X and P2Y purinoreceptors are expressed on sensory neurones (Chen et al. 1995; Thomas et al. 1998; Fong et al. 2002) and ATP is involved in transduction of distension by urinary bladder afferent neurones (Rong et al. 2002). In the current study, P2X receptors were shown immunohistochemically on identified IGLEs in the guinea-pig oesophagus, extending a previous report (Castelucci et al. 2002). Consistent with this, α,β-meATP caused powerful excitation of nearly all vagal mechanoreceptors to the guinea-pig oesophagus. In contrast, fewer than 50 % of mouse vagal mechanoreceptors were excited by α,β-meATP, and in the normal ferret oesophagus, no vagal tension receptors were affected (Page et al. 2000, 2002b). These observations reinforce the extent of species differences in receptor expression in visceral afferent neurones. An advantageous feature of the preparation used in the present study is that α,β-meATP did not affect the tension of the oesophageal external muscle layers, presumably as these consist solely of striated muscle. This is in contrast to well known inhibitory and excitatory effects of purinergic agonists on gastrointestinal smooth muscle (Zagorodnyuk et al. 1996; Zagorodnyuk & Maggi, 1998; Giaroni et al. 2002) which can significantly complicate analysis of their site of action.
In the guinea-pig oesophagus, the P2Y1 receptor agonist, ADPβS (Ralevic & Burnstock, 1998) had much smaller excitatory effect on vagal tension receptors, compared to α,β-meATP on the same units. This makes it unlikely that ATP, released by stretch stimuli, activates P2Y1 receptors as a basis for mechanosensory transduction. It has been hypothesised that ATP released from mechanically stimulated cells, acting on autocrine P2Y1 receptors may underlie some vertebrate touch sensitivity (Nakamura & Strittmatter, 1996).
The effects of α,β-meATP were blocked by the P2 antagonist, PPADS. However, PPADS alone did not affect stretch-induced firing in vagal mechanoreceptors. This indicates that ATP is not involved in mechanotransduction by IGLE-bearing visceral afferents, although it may play a modulatory role under some conditions. The nature of the P2X receptors involved is not clear. Homomeric P2X2 receptors are not activated by α,β-meATP while homomeric P2X3 receptors desensitise within a few tens of milliseconds. Both homomeric P2X2, P2X3 and heteromeric P2X2/3 receptors have been localised on sensory neurones (Chen et al. 1995; Lewis et al. 1995; Thomas et al. 1998; Virginio et al. 1998). Since responses to α,β-meATP in guinea-pig oesophagus did not rapidly desensitise, they may be due to activation of heteromeric P2X2/3 receptors on IGLEs.
Since neither ATP nor glutamate is responsible for mechanotransduction in guinea-pig vagal afferent neurones, we tested whether other transmitters might be involved, by blocking synaptic transmission. Our data showed that this did not affect stretch-activated responses. In fact, reducing [Ca2+]o actually increased excitability. Several factors may contribute to the increased firing, including removal of Ca2+ block of mechano-gated channels (Hamill & McBride, 1996), changes in surface charge effects of divalent cations and, possibly, inhibition of Ca2+-dependent K+ channels (Zagorodnyuk et al. 2002a). Superfusion with 100 µm Cd2+ also failed to block stretch-activated responses, although it reduced the excitability. Cd2+ (100 µm) is widely used as a blocker of voltage-dependent Ca2+ channels (Fox et al. 1987) but also inhibits voltage-dependent sodium channels in parasympathetic cardiac axons and smooth muscle (Bowers, 1985; Yamamoto et al. 1993). Small reduction of spontaneous and stretch-induced firing by Cd2+ may have been due to blockade of sodium channels on vagal mechanoreceptors.
Mechanotransduction in IGLEs occurred with a time course of 5–10 ms, requiring either rapid chemical activation or mechanosensitive ion channels on IGLEs. Since blocking fast exocytotic transmitter release had no effect on mechanosensitive vagal afferents, chemical activation is probably not involved. Release of chemical mediators via transporters, channels or leakage are unlikely to underlie such rapid responses. Mechanosensitive ion channels belonging to the ENaC/ASIC/ degenerin and TRP families contribute to mechanosensitivity of ciliated and non-ciliated mechanoreceptors (Gillespie & Walker, 2001; Hamill & Martinac, 2001). Recent data indicate that ion channels of the ENaC superfamily are involved in mechanotransduction by extrinsic primary afferent neurones in mammals (Price et al. 2000; Drummond et al. 2001; Garcia-Anoveros et al. 2001; Welsh et al. 2002). There are no specific blockers, although Gd3+ and benzamil and its analogues can block both families (Hamill & McBride, 1996; Drummond et al. 2001; Inoue et al. 2001; Trebak et al. 2002).
In the present study, Gd3+ inhibited mechanotransduction in oesophageal mechanoreceptors only at high (1 mm) concentrations, as reported previously for gastric mechanoreceptors (Zagorodnyuk et al. 2001), but was not effective at 300 µm. It has been reported that 400 µm Gd3+ does not inhibit mechanotransduction of rat vagal aortic baroreceptors (Andresen & Yang, 1992). The effects of 1 mm Gd3+ may have been due to blockade of action potentials, since Gd3+ inhibits voltage-dependent Na+ channels (IC50≈ 70 µm (Elinder & Arhem, 1994). Amiloride did not block mechanotransduction in concentrations up to 1 mm, but its more potent analogue, benzamil (100 µm), inhibited both spontaneous and stretch-induced firing. This action was not due to indirect effects on wall tension since benzamil actually increased intramural tension. It is well established that the effective concentrations of pyrazinecarboxyamides, including amiloride and benzamil, required to block mechanically activated channels, vary widely between preparations (Hamill & McBride, 1996). It has been reported that benzamil (100 µm) inhibits voltage-dependent Na+ currents in vagal afferent neurones innervating airways (Carr et al. 2001). However, two observations suggest that this mechanism cannot entirely account for its effects on oesophageal mechanoreceptors. First, low concentrations of TTX (which should mimic effects on voltage-dependent sodium channels) caused a reduction of firing which was approximately similar for stretches of 1–3 mm. In contrast, benzamil caused a decrease in firing rate which was significantly greater at 3 mm than at 1 mm (a significant interaction between stretch and benzamil, shown by 2-way ANOVA), suggesting that its effects were stretch-dependent, possibly reflecting open-channel blockade (Hamill & McBride, 1996; Hamill & Martinac, 2001). Secondly, TTX significantly reduced both the dynamic and adapted responses to rapid stretches whereas benzamil only affected adapted responses significantly, thus demonstrating differences in the mechanisms of action of the two drugs. Nevertheless, we cannot exclude the possibility that part of the effects of benzamil may have been due to blockade of voltage-dependent Na+ channels.
The results of the present study suggest that mechanotransduction by vagal mechanoreceptors to the guinea-pig oesophagus is mediated directly by IGLEs, probably via mechanosensitive ion channels. It does not depend on neurotransmitter release from other cells and is not dependent on either glutamate or ATP release, although endogenous purines could modulate excitability. The mechanosensitive ion channels responsible for transduction remain to be identified.
Acknowledgments
This study was funded by DK56986 from the National Institute of Health (USA). S.J.H.B. is a senior research fellow of the NH&MRC of Australia.
References
- Andresen MC, Yang M. Gadolinium and mechanotransduction of rat aortic baroreceptors. Am J Physiol. 1992;262:H1415–1421. doi: 10.1152/ajpheart.1992.262.5.H1415. [DOI] [PubMed] [Google Scholar]
- Augustine GJ. How does calcium trigger neurotransmitter release? Curr Opin Neurobiol. 2001;11:320–326. doi: 10.1016/s0959-4388(00)00214-2. [DOI] [PubMed] [Google Scholar]
- Bowers CW. A cadmium-sensitive, tetrodotoxin-resistant sodium channel in bullfrog autonomic axons. Brain Res. 1985;340:143–147. doi: 10.1016/0006-8993(85)90783-8. [DOI] [PubMed] [Google Scholar]
- Brookes SJH, Chen BN, Costa M, Humphreys CMS. Initiation of peristalsis by circumferential stretch in flat sheets of guinea-pig ileum. J Physiol. 1999;516:525–538. doi: 10.1111/j.1469-7793.1999.0525v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22:182–188. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- Carr MJ, Gover TD, Weinreich D, Undem BJ. Inhibition of mechanical activation of guinea-pig airway afferent neurons by amiloride analogues. Br J Pharmacol. 2001;133:1255–1262. doi: 10.1038/sj.bjp.0704197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelucci P, Robbins HL, Poole DP, Furness JB. The distribution of purine P2X2 receptors in the guinea-pig enteric nervous system. Histochem Cell Biol. 2002;117:415–422. doi: 10.1007/s00418-002-0404-4. [DOI] [PubMed] [Google Scholar]
- Chen BM, Grinnell AD. Kinetics, Ca2+ dependence, and biophysical properties of integrin-mediated mechanical modulation of transmitter release from frog motor nerve terminals. J Neurosci. 1997;17:904–916. doi: 10.1523/JNEUROSCI.17-03-00904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- Chen HS, Lipton SA. Mechanism of memantine block of NMDA-activated channels in rat retinal ganglion cells: uncompetitive antagonism. J Physiol. 1997;499:27–46. doi: 10.1113/jphysiol.1997.sp021909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JT, Wachtel RE, Abboud FM. Mechanosensitive currents in putative aortic baroreceptor neurons in vitro. J Neurophysiol. 1995;73:2094–2098. doi: 10.1152/jn.1995.73.5.2094. [DOI] [PubMed] [Google Scholar]
- Daly C, Sugimori M, Moreira JE, Ziff EB, Llinas R. Synaptophysin regulates clathrin-independent endocytosis of synaptic vesicles. Proc Natl Acad Sci U S A. 2000;97:6120–6125. doi: 10.1073/pnas.97.11.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond HA, Abboud FM, Welsh MJ. Localization of beta and gamma subunits of ENaC in sensory nerve endings in the rat foot pad. Brain Res. 2000;884:1–12. doi: 10.1016/s0006-8993(00)02831-6. [DOI] [PubMed] [Google Scholar]
- Drummond HA, Welsh MJ, Abboud FM. ENaC subunits are molecular components of the arterial baroreceptor complex. Ann N Y Acad Sci. 2001;940:42–47. doi: 10.1111/j.1749-6632.2001.tb03665.x. [DOI] [PubMed] [Google Scholar]
- Elinder F, Arhem P. Effects of gadolinium on ion channels in the myelinated axon of Xenopus laevis: four sites of action. Biophys J. 1994;67:71–83. doi: 10.1016/S0006-3495(94)80456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernstrom GG, Chalfie M. Genetics of sensory mechanotransduction. Annu Rev Genet. 2002;36:411–453. doi: 10.1146/annurev.genet.36.061802.101708. [DOI] [PubMed] [Google Scholar]
- Eshkind LG, Leube RE. Mice lacking synaptophysin reproduce and form typical synaptic vesicles. Cell Tissue Res. 1995;282:423–433. doi: 10.1007/BF00318874. [DOI] [PubMed] [Google Scholar]
- Fong AY, Krstew EV, Barden J, Lawrence AJ. Immunoreactive localisation of P2Y1 receptors within the rat and human nodose ganglia and rat brainstem: comparison with [alpha 33P]deoxyadenosine 5′-triphosphate autoradiography. Neuroscience. 2002;113:809–823. doi: 10.1016/s0306-4522(02)00237-3. [DOI] [PubMed] [Google Scholar]
- Fox AP, Nowycky MC, Tsien RW. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Garcia-Anoveros J, Samad TA, Zuvela-Jelaska L, Woolf CJ, Corey DP. Transport and localization of the DEG/ENaC ion channel BNaC1alpha to peripheral mechanosensory terminals of dorsal root ganglia neurons. J Neurosci. 2001;21:2678–2686. doi: 10.1523/JNEUROSCI.21-08-02678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaroni C, Knight GE, Ruan HZ, Glass R, Bardini M, Lecchini S, Frigo G, Burnstock G. P2 receptors in the murine gastrointestinal tract. Neuropharmacology. 2002;43:1313–1323. doi: 10.1016/s0028-3908(02)00294-0. [DOI] [PubMed] [Google Scholar]
- Gillespie PG, Walker RG. Molecular basis of mechanosensory transduction. Nature. 2001;413:194–202. doi: 10.1038/35093011. [DOI] [PubMed] [Google Scholar]
- Glaum SR, Miller RJ. Metabotropic glutamate receptors mediate excitatory transmission in the nucleus of the solitary tract. J Neurosci. 1992;12:2251–2258. doi: 10.1523/JNEUROSCI.12-06-02251.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon FJ, Sved AF. Neurotransmitters in central cardiovascular regulation: glutamate and GABA. Clin Exp Pharmacol Physiol. 2002;29:522–524. doi: 10.1046/j.1440-1681.2002.03666.x. [DOI] [PubMed] [Google Scholar]
- Hamill OP, McBride DW., Jr The pharmacology of mechanogated membrane ion channels. Pharmacol Rev. 1996;48:231–252. [PubMed] [Google Scholar]
- Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- Hay M, Kunze DL. Glutamate metabotropic receptor inhibition of voltage-gated calcium currents in visceral sensory neurons. J Neurophysiol. 1994;72:421–430. doi: 10.1152/jn.1994.72.1.421. [DOI] [PubMed] [Google Scholar]
- Hoang CJ, Hay M. Expression of metabotropic glutamate receptors in nodose ganglia and the nucleus of the solitary tract. Am J Physiol Heart Circ Physiol. 2001;281:H457–462. doi: 10.1152/ajpheart.2001.281.1.H457. [DOI] [PubMed] [Google Scholar]
- Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular α1-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- Knight GE, Bodin P, De Groat WC, Burnstock G. ATP is released from guinea pig ureter epithelium on distension. Am J Physiol Renal Physiol. 2002;282:F281–288. doi: 10.1152/ajprenal.00293.2000. [DOI] [PubMed] [Google Scholar]
- Kunze WA, Clerc N, Furness JB, Gola M. The soma and neurites of primary afferent neurons in the guinea-pig intestine respond differentially to deformation. J Physiol. 2000;526:375–385. doi: 10.1111/j.1469-7793.2000.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- Li JL, Ohishi H, Kaneko T, Shigemoto R, Neki A, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR7, in ganglion neurons of the rat; with special reference to the presence in glutamatergic ganglion neurons. Neurosci Lett. 1996;204:9–12. doi: 10.1016/0304-3940(95)12299-0. [DOI] [PubMed] [Google Scholar]
- Liu MT, Rothstein JD, Gershon MD, Kirchgessner AL. Glutamatergic enteric neurons. J Neurosci. 1997;17:4764–4784. doi: 10.1523/JNEUROSCI.17-12-04764.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter GC, Reichling DB, Levine JD. Mechanical transduction by rat dorsal root ganglion neurons in vitro. Neurosci Lett. 1999;273:179–182. doi: 10.1016/s0304-3940(99)00665-5. [DOI] [PubMed] [Google Scholar]
- McRoberts JA, Coutinho SV, Marvizon JC, Grady EF, Tognetto M, Sengupta JN, Ennes HS, Chaban VV, Amadesi S, Creminon C, Lanthorn T, Geppetti P, Bunnett NW, Mayer EA. Role of peripheral N-methyl-d-aspartate (NMDA) receptors in visceral nociception in rats. Gastroenterology. 2001;120:1737–1748. doi: 10.1053/gast.2001.24848. [DOI] [PubMed] [Google Scholar]
- Martin CM, Page AJ, O'Donnel T, Coldwell JR, Scott C, Blackshaw LA. Metabotropic glutamate receptors inhibit vagal primary afferent mechanosensitivity. Neurogastroent Mot. 2001;13:411. [Google Scholar]
- Miller SM, Szurszewski JH. Colonic mechanosensory afferent input to neurons in the mouse superior mesenteric ganglion. Am J Physiol. 1997;272:G357–366. doi: 10.1152/ajpgi.1997.272.2.G357. [DOI] [PubMed] [Google Scholar]
- Nakamura F, Strittmatter SM. P2Y1 purinergic receptors in sensory neurons: contribution to touch-induced impulse generation. Proc Natl Acad Sci U S A. 1996;93:10465–10470. doi: 10.1073/pnas.93.19.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhuber WL. Sensory vagal innervation of the rat esophagus and cardia: a light and electron microscopic anterograde tracing study. J Auton Nerv Syst. 1987;20:243–255. doi: 10.1016/0165-1838(87)90153-6. [DOI] [PubMed] [Google Scholar]
- Page AJ, Martin CM, Blackshaw LA. Group II and III metabotropic glutamate receptors inhibit mechnotransduction in mouse primary vagal afferents. 2002a. Am Gastroenterological Ass, DDW, 58.
- Page AJ, Martin CM, Blackshaw LA. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J Neurophysiol. 2002b;87:2095–2103. doi: 10.1152/jn.00785.2001. [DOI] [PubMed] [Google Scholar]
- Page AJ, O'Donnell TA, Blackshaw LA. P2X purinoceptor-induced sensitization of ferret vagal mechanoreceptors in oesophageal inflammation. J Physiol. 2000;523:403–411. doi: 10.1111/j.1469-7793.2000.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, Qiao J, Benson CJ, Tarr DE, Hrstka RF, Yang B, Williamson RA, Welsh MJ. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature. 2000;407:1007–1011. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- Raab M, Neuhuber WL. Vesicular glutamate transporter 2 immunoreactivity in putative vagal mechanosensor terminals of mouse and rat esophagus: indication of a local effector function? Cell Tissue Res. 2003;312:141–148. doi: 10.1007/s00441-003-0721-5. [DOI] [PubMed] [Google Scholar]
- Ren J, Hu HZ, Liu S, Xia Y, Wood JD. Glutamate receptors in the enteric nervous system: ionotropic or metabotropic? Neurogastroenterol Motil. 2000;12:257–264. doi: 10.1046/j.1365-2982.2000.00207.x. [DOI] [PubMed] [Google Scholar]
- Sharma RV, Chapleau MW, Hajduczok G, Wachtel RE, Waite LJ, Bhalla RC, Abboud FM. Mechanical stimulation increases intracellular calcium concentration in nodose sensory neurons. Neuroscience. 1995;66:433–441. doi: 10.1016/0306-4522(94)00560-r. [DOI] [PubMed] [Google Scholar]
- Snitsarev V, Whiteis CA, Abboud FM, Chapleau MW. Mechanosensory transduction of vagal and baroreceptor afferents revealed by study of isolated nodose neurons in culture. Auton Neurosci. 2002;98:59–63. doi: 10.1016/s1566-0702(02)00033-4. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Thomas S, Virginio C, North RA, Surprenant A. The antagonist trinitrophenyl-ATP reveals co-existence of distinct P2X receptor channels in rat nodose neurones. J Physiol. 1998;509:411–417. doi: 10.1111/j.1469-7793.1998.411bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebak M, Bird GS, McKay RR, Putney JW., Jr Comparison of human TRPC3 channels in receptor-activated and store-operated modes. Differential sensitivity to channel blockers suggests fundamental differences in channel composition. J Biol Chem. 2002;277:21617–21623. doi: 10.1074/jbc.M202549200. [DOI] [PubMed] [Google Scholar]
- Virginio C, North RA, Surprenant A. Calcium permeability and block at homomeric and heteromeric P2X2 and P2X3 receptors, and P2X receptors in rat nodose neurones. J Physiol. 1998;510:27–35. doi: 10.1111/j.1469-7793.1998.027bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford AP, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci. 2001;21:5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh MJ, Price MP, Xie J. Biochemical basis of touch perception: mechanosensory function of degenerin/epithelial Na+ channels. J Biol Chem. 2002;277:2369–2372. doi: 10.1074/jbc.R100060200. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Fukuta H, Suzuki H. Blockade of sodium channels by divalent cations in rat gastric smooth muscle. Jpn J Physiol. 1993;43:785–796. doi: 10.2170/jjphysiol.43.785. [DOI] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Brookes SJH. Transduction sites of vagal mechanoreceptors in the guinea pig esophagus. J Neurosci. 2000;20:6249–6255. doi: 10.1523/JNEUROSCI.20-16-06249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Brookes SJ. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol. 2001;534:255–268. doi: 10.1111/j.1469-7793.2001.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Costa M, Brookes SJ. 4-Aminopyridine- and dendrotoxin-sensitive potassium channels influence excitability of vagal mechano-sensitive endings in guinea-pig oesophagus. Br J Pharmacol. 2002a;137:1195–1206. doi: 10.1038/sj.bjp.0704964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Costa M, Brookes SJH. Mechano-gated channels of intraganglionic laminar endings (IGLEs) transduce mechanical stimuli in oesophagus. Proc Au Neurosci Soc. 2003;14 Or0205. [Google Scholar]
- Zagorodnyuk VP, D'Antona G, Brookes SJ, Costa M. Functional GABAB receptors are present in guinea pig nodose ganglion cell bodies but not in peripheral mechanosensitive endings. Auton Neurosci. 2002b;102:20–29. doi: 10.1016/s1566-0702(02)00183-2. [DOI] [PubMed] [Google Scholar]
- Zagorodnyuk V, Maggi CA. Pharmacological evidence for the existence of multiple P2 receptors in the circular muscle of guinea-pig colon. Br J Pharmacol. 1998;123:122–128. doi: 10.1038/sj.bjp.0701558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk V, Santicioli P, Maggi CA, Giachetti A. The possible role of ATP and PACAP as mediators of apaminsensitive NANC inhibitory junction potentials in circular muscle of guinea-pig colon. Br J Pharmacol. 1996;119:779–786. doi: 10.1111/j.1476-5381.1996.tb15740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Lauve A, Patterson LM, Berthoud HR. Limited excitatory local effector function of gastric vagal afferent intraganglionic terminals in rats. AmJ Physiol. 1997;273:G661–669. doi: 10.1152/ajpgi.1997.273.3.G661. [DOI] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res. 2001;26:959–969. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Rong W, Spyer KM, Burnstock G. Activation and sensitisation of low and high threshold afferent fibres mediated by P2X receptors in the mouse urinary bladder. J Physiol. 2002;541:591–600. doi: 10.1113/jphysiol.2001.013469. [DOI] [PMC free article] [PubMed] [Google Scholar]


