Abstract
Both stable isotope methodology and fluorescence microscopy were applied to define the use of intramuscular triglyceride (IMTG) stores as a substrate source during exercise on a whole-body as well as on a fibre type-specific intramyocellular level in trained male cyclists. Following an overnight fast, eight subjects were studied at rest, during 120 min of moderate intensity exercise (60 % maximal oxygen uptake capacity (V̇O2,max)) and 120 min of post-exercise recovery. Continuous infusions of [U-13C]palmitate and [6,6-2H2]glucose were administered at rest and during subsequent exercise to quantify whole-body plasma free fatty acid (FFA) and glucose oxidation rates and the contribution of other fat sources (sum of muscle- plus lipoprotein-derived TG) and muscle glycogen to total energy expenditure. Fibre type-specific intramyocellular lipid content was determined in muscle biopsy samples collected before, immediately after and 2 h after exercise. At rest, fat oxidation provided 66 ± 5 % of total energy expenditure, with FFA and other fat sources contributing 48 ± 6 and 17 ± 3 %, respectively. FFA oxidation rates increased during exercise, and correlated well with the change in plasma FFA concentrations. Both the use of other fat sources and muscle glycogen declined with the duration of exercise, whereas plasma glucose production and utilisation increased (P < 0.001). On average, FFA, other fat sources, plasma glucose and muscle glycogen contributed 28 ± 3, 15 ± 2, 12 ± 1 and 45 ± 4 % to total energy expenditure during exercise, respectively. Fluorescence microscopy revealed a 62 ± 7 % net decline in muscle lipid content following exercise in the type I fibres only, with no subsequent change during recovery. We conclude that IMTG stores form an important substrate source during moderate intensity exercise in endurance-trained male athletes following an overnight fast, with the oxidation rate of muscle- plus lipoprotein-derived TG being decreased with the duration of exercise.
Fat and carbohydrate are the principal substrates that fuel aerobic ATP synthesis in skeletal muscle. Endogenous carbohydrates, mainly stored as muscle and liver glycogen, represent less than 5 % of total energy storage in an average man. The vast majority of our energy reserves is stored as fat, mainly deposited as triacylglycerol (TG) in subcutaneous and deep visceral adipose tissue. Smaller quantities of TG are present in circulating lipoprotein particles and in lipid droplets inside the muscle fibres, intramyocellular triacylglycerol (IMTG; Hoppeler et al. 1985). The latter has recently regained much attention due to the proposed functional relationship between IMTG accumulation and the development of insulin resistance (Boden et al. 2001). It is speculated that elevated free fatty acid (FFA) delivery and/or impaired FA oxidation result in intramyocellular accumulation of TG and FA metabolites, which could induce defects in the insulin signalling cascade, causing skeletal muscle insulin resistance. The progressive accumulation of IMTG in sedentary, obese and/or type 2 diabetes patients should therefore form a major therapeutic target and efforts should be made to develop interventions that prevent excess IMTG accretion by stimulating their rate of oxidation. However, the latter is complicated by the fact that information on the regulation of IMTG metabolism is scarce.
Several studies applying FA isotope tracers have shown that during moderate intensity exercise ≈40–60 % of total fat oxidation is accounted for by plasma derived FFA oxidation in endurance trained male subjects following an overnight fast (Romijn et al. 1993; Sidossis et al. 1998; Coyle et al. 2001; van Loon et al. 2001). This implies that other fat sources can contribute substantially to total fat oxidation during exercise. However, the relative contribution of these other fat sources to energy expenditure has been shown to depend on exercise intensity (Romijn et al. 1993; van Loon et al. 2001), exercise duration (Romijn et al. 1993), training status (Martin et al. 1993; Phillips et al. 1996a; Sidossis et al. 1998; Schrauwen et al. 2002) and diet (Schrauwen et al. 2000; Coyle et al. 2001) and is much lower in obese and/or type 2 diabetes patients (Blaak et al. 2000; Borghouts et al. 2002) compared with trained subjects. It is generally assumed that both muscle- and lipoprotein-derived TG contribute to the oxidation rate of these other fat sources (Havel et al. 1967; Oscai et al. 1990; Frayn et al. 1996). Though the oxidation rate of IMTG plus lipoprotein-derived TG appears to be most pronounced in highly trained endurance athletes exercising at a moderate intensity workload following an overnight fast (van Loon et al. 2001), recent findings indicate that only 3 months of low-intensity endurance training are needed to achieve a 2- to 3-fold increase in the capacity of sedentary subjects to use these TG sources during moderate intensity exercise (Schrauwen et al. 2002).
Not all expert laboratories agree with the contention that IMTG stores are oxidised during exercise (Watt et al. 2002b). For example, studies applying direct muscle TG extraction analysis on muscle samples collected before and after moderate intensity exercise in both trained and untrained subjects have provided contradictory findings (Watt et al. 2002b). For example, it has recently been suggested that IMTG stores do not play a significant role during moderate intensity exercise in trained men as opposed to trained women (Roepstorff et al. 2002; Steffensen et al. 2002), but rather form an important substrate source during post-exercise recovery (Kiens & Richter, 1998). Watt et al. (2002b) suggested that the apparent discrepancy between the many published studies is largely due to the high between biopsy variability when using the muscle TG extraction technique to estimate net IMTG use. The latter could probably be explained by the presence of extramyocellular fat and/or differences in fibre-type composition in the obtained muscle samples. Studies using magnetic resonance spectroscopy (MRS) to quantify both intra- and extramyocellular lipid content all support the contention that substantial net decreases in intramyocellular TG content (≈20–40 %) occur following prolonged endurance exercise in both trained men and women (Boesch et al. 1997, 1999; Krssak et al. 2000; Rico-Sanz et al. 2000; Brechtel et al. 2001; Decombaz et al. 2001; Larson-Meyer et al. 2002; van Loon et al. 2003b).
None of the techniques mentioned so far discriminate between muscle fibre type-specific IMTG content. The latter could be of importance as muscle fibre-type recruitment during endurance-type exercise tasks mainly relies on the use of type I muscle fibres (Hultman, 1995), which have been shown to contain ≈3- to 4-fold more lipid than type II fibres (Essen et al. 1975; Malenfant et al. 2001). Therefore, we hypothesised that any net decreases in muscle TG content after prolonged moderate intensity exercise would be more pronounced in type I versus type II muscle fibres. To enable a direct and selective quantification of muscle TG content on a fibre type-specific intramyocellular level, we recently optimised the combined use of oil red O staining of muscle cross-sections with (immuno)fluorescence microscopy (Koopman et al. 2001).
Due to the apparent discrepancy in the existing literature on the capacity of human skeletal muscle to oxidise IMTG we investigated a number of novel aspects on the role of IMTG as a substrate source during moderate intensity exercise (≈60 % V̇O2,max) in endurance-trained male cyclists following an overnight fast. These conditions were selected as they most probably lead to the highest absolute total fat and/or IMTG oxidation rates, and as such provide the best opportunity to determine whether IMTG stores can be used as a substrate source during exercise. Our main aim was to determine whether fibre type-specific changes in muscle lipid content occur following prolonged endurance exercise and/or short-term recovery. The latter was performed by applying fluorescence microscopy on oil red O-stained muscle cross-sections, prepared from biopsy samples taken before, immediately after and 2 h after exercise. Simultaneously, using stable isotope tracer methodology, we investigated the time course of the contribution of muscle- plus lipoprotein-derived TG oxidation to whole-body energy expenditure during the entire 2 h exercise trial.
Methods
Subjects
Eight male cyclists (age: 22.8 ± 0.8 years; height: 1.85 ± 0.03 m; body weight: 76.5 ± 3.8 kg; fat-free mass: 66.1 ± 2.7 kg; maximal power output (Wmax): 391 ± 14 W; and maximal oxygen uptake capacity (V̇O2,max): 60.5 ± 2.3 ml (kg body weight)−1 min−1) were selected to participate in this study. Subjects were informed about the nature and risks of the experimental procedures before their written informed consent was obtained. This study was approved by the medical ethical committee of the Maastricht Academic Hospital (AZM) and it conforms to the standards set by the Declaration of Helsinki.
Pre-testing
Wmax and V̇O2,max were measured on an electronically braked cycle ergometer (Lode Excalibur, Groningen, The Netherlands) during an incremental exhaustive exercise test (Kuipers et al. 1985) 1 week before the first experimental trial. Body composition was assessed using the hydrostatic weighing method in the morning after an overnight fast. Simultaneously, residual lung volume was measured by the helium-dilution technique using a spirometer (Volugraph 2000, Mijnhardt, Bunnik, The Netherlands). Body weight was measured with a digital balance with an accuracy of 0.001 kg (E1200, August Sauter GmbH, Albstadt, Germany). Body fat percentage was calculated using Siri's equation (Siri, 1956). Fat-free mass (FFM) was calculated by subtracting fat mass (FM) from total body weight.
Diet and activity prior to testing
All subjects were instructed to refrain from heavy physical labour and exercise training for at least 3 days prior to each trial. They were also instructed to fill out a food intake diary for 2 days prior to the first exercise trial to keep their dietary intake as identical as possible prior to the other trial. The evening before each trial, subjects received the same standardised meal (41.2 kJ (kg body weight)−1; consisting of 72 Energy % (En %) carbohydrate, 11 En % fat and 17 En % protein).
Experimental trials
Each subject performed two similar trials, separated by at least 1 week. Each trial consisted of 90 min of resting measurements, followed by 120 min of cycling exercise (50 % Wmax) and a subsequent 120 min recovery period. In the main trial, a [U-13C] palmitate and [6,6-2H2]glucose tracer were infused continuously at rest and during exercise with breath, blood and muscle samples collected to quantify the use of different substrate sources and to determine changes in intramyocellular lipid content. The other trial was performed to determine the acetate recovery factor to correct [U-13C]palmitate oxidation rates for the loss of label by way of isotopic exchange reactions in the TCA cycle (Sidossis et al. 1995; van Loon et al. 2003a). During this trial a [1,2-13C]acetate tracer was infused continuously at rest and during exercise and only breath samples were collected.
Protocol
After an overnight fast, subjects arrived at the laboratory at 08.00 h by car or public transportation. After 30 min of supine rest, a percutaneous muscle biopsy sample (Bergstrom, 1975) was taken from the vastus lateralis muscle. A Teflon catheter (Baxter BV, Utrecht, The Netherlands) was inserted into an antecubital vein of one arm for blood sampling and another catheter was inserted in the contralateral arm for isotope infusion. Thereafter, a resting blood sample was taken and an expired breath sample was collected into a vacutainer tube (Becton Dickinson, France). Subsequently, subjects were administered a single intravenous dose of NaH13CO3 (0.06375 mg kg−1), to prime the bicarbonate pool(s), followed by a [6,6-2H2]glucose prime (13.5 µmol kg−1). Thereafter, a continuous infusion of [6,6-2H2]glucose (0.3 µmol kg−1 min−1) and [U-13C]palmitate (0.01 µmol kg−1 min−1) (or [1,2-13C]acetate in the acetate recovery trial) was started (t = 0) via a calibrated IVAC pump (IVAC 560, San Diego, CA, USA) and continued for 210 min until cessation of exercise. At t = 90 min subjects started to exercise on a cycle ergometer at a workload of 50 % Wmax for a 2 h period. Whilst at rest, oxygen uptake (V̇O2) and carbon dioxide production (V̇CO2) were measured continuously (Oxycon-β, Mijnhardt, The Netherlands). During exercise, V̇O2 and V̇CO2 were measured for 5 min every 15 min before sampling of blood and expired breath. Immediately after cessation of exercise, a second muscle biopsy sample was taken, after which subjects rested supine for 2 h during which V̇O2 and V̇CO2 were measured continuously. After 2 h of post-exercise recovery a third muscle biopsy sample was taken. Muscle biopsy samples were collected from both legs. The first two biopsy samples were taken from the same incision in one leg, the third was collected from another incision in the contralateral leg. When biopsy samples were taken from the same incision, the first sample was taken from different fibres (distal of the incision, with the needle pointing inwards) than the second (proximal with the needle pointing outwards). Breath and blood samples were collected at t = 0, 30, 60, 75 and 90 min (at rest) and at t = 105, 120, 135, 150, 165, 180, 195 and 210 min (during exercise). In addition, blood samples were collected at t = 225, 240, 270, 300, 315 and 330 min (during post-exercise recovery).
Tracer infusion
Stable isotope tracers were infused at rest, before onset of exercise, and continued until cessation of exercise. Infusion rates of [U-13C]palmitate and [6,6-2H2]glucose averaged 8.8 ± 0.2 and 281.3± 8.9 nmol kg−1 min−1, respectively. At the onset of exercise [U-13C]palmitate infusion rates were doubled (17.6 ± 0.4 nmol kg−1 min−1). In the acetate recovery trial, a corresponding amount of 13C was infused, resulting in average [1,2-13C]acetate infusion rates of 70.3 ± 2.0 and 139.9 ± 3.9 nmol kg−1 min−1 at rest and during exercise, respectively. The palmitate tracer (99 % enriched, Cambridge Isotope Laboratories, Andover, MA, USA) was dissolved in heated sterile water and passed through a 0.2 µm filter into 5 % warm human serum albumin to make a 1.00 mm solution. Both the glucose and acetate tracer (99 % enriched, Cambridge Isotope Laboratories) were dissolved in 0.9 % saline. Palmitate, glucose and acetate tracer concentrations in the infusates averaged 1.00 ± 0.02, 22.1 ± 0.9 and 4.58 ± 0.13 mmol l−1, respectively.
Blood and breath sample analysis
Blood samples (7 ml) were collected in EDTA-containing tubes and centrifuged at 1000 g at +4 °C for 10 min. Aliquots of plasma were frozen immediately in liquid nitrogen and stored at −80 °C. Plasma glucose (Uni Kit III, Roche, Basel, Switzerland), lactate (Gutmann & Wahlefeld, 1974), FFA (Wako NEFA-C test kit, Wako Chemicals, Neuss, Germany), glycerol (148270, Roche Diagnostics, Indianapolis, IN, USA) and triglyceride (GPO-trinder 337B, Sigma Diagnostics, St Louis, MO, USA) concentrations were analysed with a COBAS FARA semi-automatic analyser (Roche).
For determination of plasma palmitate concentration and enrichment, palmitate was first extracted from plasma. Briefly, heptadecanoic acid (100 µl) was added to 400 µl of plasma after which samples were deproteinised by adding 3.7 ml methanol. After centrifugation, lipids were extracted by adding 3.9 ml chloroform and 1.7 ml saline (17 mm). After centrifugation the chloroform fraction was collected. A second extraction was performed on the water fraction, by adding 2 ml chloroform/methanol/water (85:14:1). After centrifugation, both chloroform fractions were combined and evaporated under N2. The different lipid classes were isolated by thin-layer chromatography (TLC). The evaporated chloroform fractions were dissolved in 100 µl chloroform/methanol (1:1), transferred onto the TLC plates (113894, Merck, Germany), developed in chloroform/ methanol/acetic acid/water (100:100:10:10) and, after drying (N2 at 50 °C), in petroleum benzine/diethyl ether/acetic acid (120:25:1.5). After development of the plates the FFA band was isolated. Following the addition of 2 ml methanol/iso-octane (4:1) and 0.2 ml acetylchloride, the mixture was incubated for 1 h at 100 °C. Thereafter, the methylesters were extracted by adding 5 ml of 6 % potassium carbonate. After mixing and centrifugation, the iso-octane fraction was evaporated under N2, re-dissolved in 25 µl iso-octane and measured on an analytical gas chromatograph (GC; Autosystem XL, Perkin Elmer, USA) with flame ionisation detection using heptadecanoic acid as an internal standard. The palmitate concentration was determined by injecting 2 µl in the split mode (1/20), onto a 50 m Chrompack capillary column (cp-sil88 tailor FAME made, i.d. 0.25 mm, 2 µm film thickness; Varian), injector and detector temperature at 300 °C, with helium carrier gas at 130 kPa. The instrument was controlled and the palmitate concentration automatically calculated from palmitic and heptadecanoic acid standard curves. Plasma palmitate comprised on average 25.2 ± 0.6 % of total FFA. Isotope tracer/tracee ratio (TTR) of [U-13C]palmitate was determined using GC-combustion-isotope ratio mass spectrometry (GC-C-IRMS; Varian-Finnigan GC combustion II, Finnigan MAT 252, Bremen, Germany). The palmitate enrichment was determined by 2 µl split injection on a 25 m capillary fused-silica column (Ultra1, HP, USA) with a splitflow of 30 ml min−1. The methyl derivative of palmitate contains 17 carbons of which 16 are palmitate, and thus the TTR of palmitate was corrected by a factor 17/16. Plasma glucose was first extracted with chloroform-methanol-water and derivatisation was performed with butylboronic acid and acetic anhydride as described previously (Pickert et al. 1991). Following derivatisation, plasma [6,6-2H2]glucose enrichment was determined by electron ionisation GC-MS (Finnigan INCOS-XL). Both glucose (Uni Kit III, 0736724, Roche) and acetate (Kit 148261, Boehringer) concentrations in the infusates were determined with the COBAS FARA semi-automatic analyser.
Calculations
From respiratory measurements, total fat and carbohydrate oxidation rates were calculated using the non-protein respiratory quotient (Peronnet & Massicotte, 1991).
| (1) |
| (2) |
V̇O2 and V̇CO2 are measured in l min−1 and oxidation rates are measured in g min−1. Breath and plasma enrichments are expressed as tracer/tracee ratios (TTR):
| (3) |
where Sa indicates sample and Bk indicates background value. As plasma glucose and palmitate concentrations and/or enrichments gradually changed over time (Fig. 2A and Fig. 6), non-steady-state Steele equations (Steele, 1959) were applied to calculate tracer kinetics. Rate of appearance (Ra) and rate of disappearance (Rd) of palmitate and glucose were calculated using the single-pool Steele equations adapted for stable isotope methodology as described elsewhere (Wolfe & Jahoor, 1990).
Figure 2. Plasma and breath enrichment data.
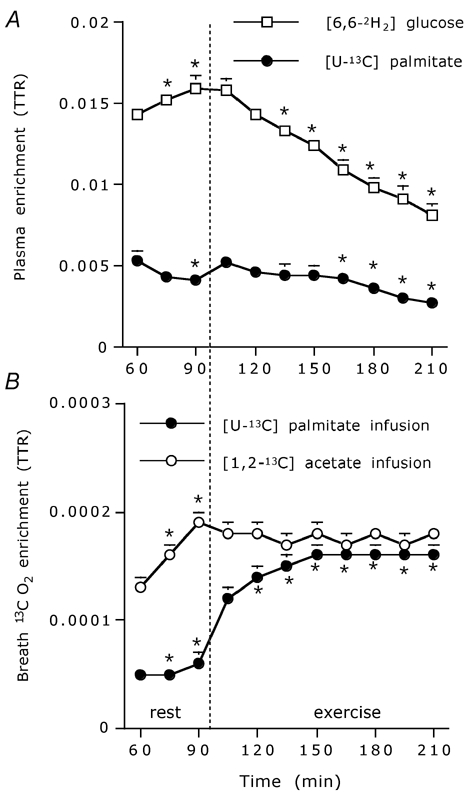
Plasma [6,6-2H2]glucose and [U-13C]palmitate enrichment (expressed in tracer/tracee ratio; TTR) at rest and during exercise (A) and 13CO2 enrichment in the expired breath (expressed in TTR) following [U-13C]palmitate infusion and following [1,2-13C] acetate infusion in the acetate recovery trial (B). Data are means ± s.e.m.; * significantly different from values at t = 60 or 105 during the resting period and the exercise trial, respectively (P < 0.05).
Figure 6. Plasma metabolite concentrations at rest, during prolonged submaximal exercise and during 2 h of post-exercise recovery.
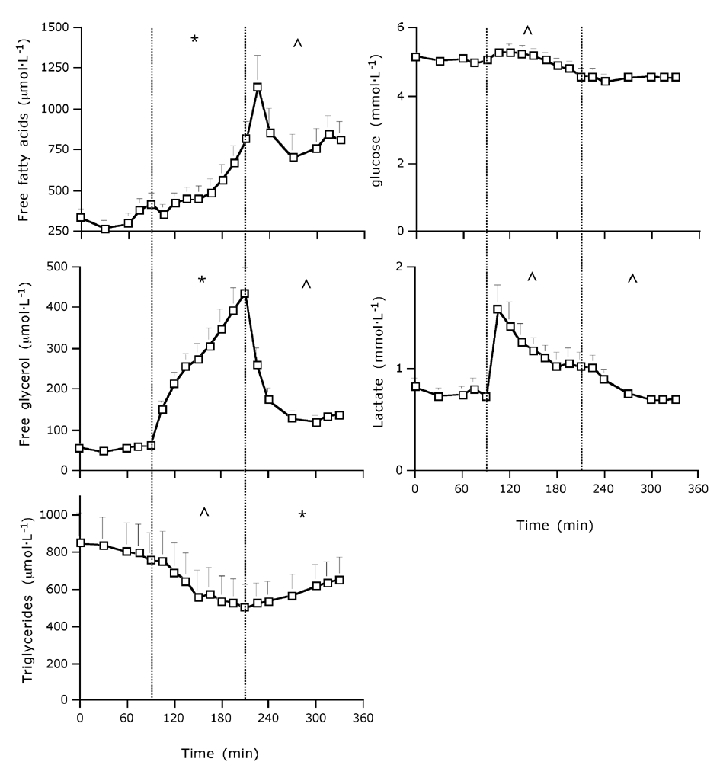
Data provided are means ± s.e.m.; * significant increase over time within the resting, exercise and/or post-exercise recovery period; ∧ significant decrease over time within the resting, exercise and/or post-exercise recovery period (P < 0.05).
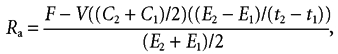 |
(4) |
| (5) |
where F is the infusion rate (in µmol kg−1 min−1); V is the distribution volume for palmitate or glucose (40 and 160 ml kg−1, respectively); C1 and C2 are the palmitate or glucose concentrations (in mmol l−1) at time 1 (t1) and time 2 (t2), respectively, and E2 and E1 are the plasma palmitate or glucose enrichments (TTR) at times 1 and 2, respectively. The rate of 13CO2 production (Pr13CO2; measured in mol min−1) from the infused palmitate tracer was calculated as:
| (6) |
where TTRCO2 is the breath 13C/12C ratio at a given time point, V̇CO2 is the rate of carbon dioxide production (in l min−1), k is the volume of 1 mol CO2 (22.4 l mol−1); and Ar is the fractional 13C label recovery in breath CO2, observed after the infusion of labelled acetate (Sidossis et al. 1995; Schrauwen et al. 1998; van Loon et al. 2003a) and calculated as:
| (7) |
where F is the infusion rate of [1,2-13C] acetate (in mol min−1). The rate of plasma palmitate oxidation (Rox) (in mol min−1) can subsequently be calculated as:
| (8) |
where Rd(palmitate) is the rate of disappearance of palmitate (mol min−1); F is the palmitate infusion rate (mol min−1) and 16 is the number of labelled carbon atoms in palmitate. Total plasma FFA oxidation was calculated by dividing palmitate oxidation rates by the fractional contribution of plasma palmitate to total plasma FFA concentration. The contribution of plasma FFA oxidation to total fat oxidation was calculated by assuming that triacylglycerol molecular mass equals 860 g mol−1 and that every triacylglycerol molecule contains three fatty acids. The contribution of fat sources other than plasma FFA was calculated by subtracting plasma FFA oxidation from total fat oxidation.
In a previous study, where we applied both [U-13C] and [6,6-2H2]glucose tracers (Jeukendrup et al. 1999) during moderate intensity exercise, it was shown that the percentage of plasma glucose Rd that was oxidised was between 96–100 %. Therefore, plasma glucose oxidation rate during exercise was calculated as:
| (9) |
Whole-body muscle glycogen use was calculated by subtracting plasma glucose oxidation from total carbohydrate oxidation. As plasma glucose Rd does not match Rox during resting conditions (Trimmer et al. 2001), plasma glucose oxidation rates cannot be accurately calculated at rest when using a [6,6-2H2]glucose tracer.
Muscle sample analysis
Muscle samples were dissected carefully, freed from any visible non-muscle material, rapidly frozen in liquid nitrogen-cooled isopentane and embedded in Tissue-Tek (Sakura Finetek, Zoeterwoude, The Netherlands). Multiple serial sections (5 µm) from biopsy samples collected before, immediately after and 2 h after exercise were thaw-mounted together on uncoated, pre-cleaned glass slides for each subject. To permit quantification of intramyocellular lipid stained by oil red O together with immunolabelled cellular constituents we used the protocol as described by Koopman et al. (2001). Briefly, cryosections were fixed in 3.7 % formaldehyde for 1 h. Slides were rinsed with deionised water, treated with 0.5 % Triton X-100 in PBS, and washed with PBS. Thereafter, sections were incubated with antibodies against human laminin (polyclonal rabbit antibody, Sigma Diagnostics, Steinheim, Germany) and human myosin heavy chain (A4.840), developed by Dr Blau (Cho et al. 1993), enabling us to visualise individual cell membranes and to determine muscle fibre-type (I or II), respectively. Incubation was followed by washes in PBS, after which the appropriate conjugated antibodies GARIgGAlexa350 and GAMIgMAlexa488 (Molecular Probes, Leiden, The Netherlands) were applied. After several washes with PBS, glass slides were immersed in the oil red O working solution. Oil red O stock solution was prepared by adding 500 mg oil red O (Fluka Chemie, Buchs, Switzerland) to 100 ml 60 % triethylphosphate. Prior to staining, a 36 % triethylphosphate working solution, containing 12 ml oil red O stock solution and 8 ml deionised water, was prepared and filtered to remove crystallised oil red O. After 30 min of oil red O immersion, slides were rinsed with deionised water followed by a 10 min wash with tap water. Stained sections were embedded in Mowiol and covered with a coverslip.
After 24 h, glass slides were examined using a Nikon E800 fluorescence microscope (Uvikon, Bunnik, The Netherlands) coupled to a Basler A113 C progressive scan colour CCD camera, with a Bayer colour filter. Epifluorescence signal was recorded using a Texas red excitation filter (540–580 nm) for oil red O, a fluorescein isothiocyanate (FITC) excitation filter (465–495 nm) for muscle fibre-type, and a 4′,6-diamidino-2-phenylindole (DAPI) UV excitation filter (340–380 nm) for laminin. Digitally captured images (×240 magnification; Fig. 1), at least five fields-of-view per muscle cross-section (with an average of 10 ± 0.3 fibres per field-of-view), were processed and analysed using Lucia 6.01 software (Nikon, Düsseldorf, Germany). The oil red O epifluorescence signal was quantified for each muscle fibre, resulting in a total of 56 ± 3 muscle fibres analysed for each muscle cross-section (32 ± 2 type I and 24 ± 2 type II muscle fibres). An intensity threshold representing minimal intensity values corresponding to lipid droplets was set manually and uniformly used for all images. Total area measured and the area as well as the number of objects emitting oil red O epifluorescence signal were recorded. Fibre type-specific IMTG content was expressed as the percentage of the measured area that was stained with oil red O. In two subjects, two different muscle samples were collected and analysed; the coefficient of variance averaged 10.5 and 38.6 % for the type I and type II fibres, respectively. Average lipid droplet size was calculated by dividing the total area lipid stained by total number of droplets. Lipid droplet density was calculated by dividing the total number of droplets by the total area measured.
Figure 1. Immunohistochemistry.
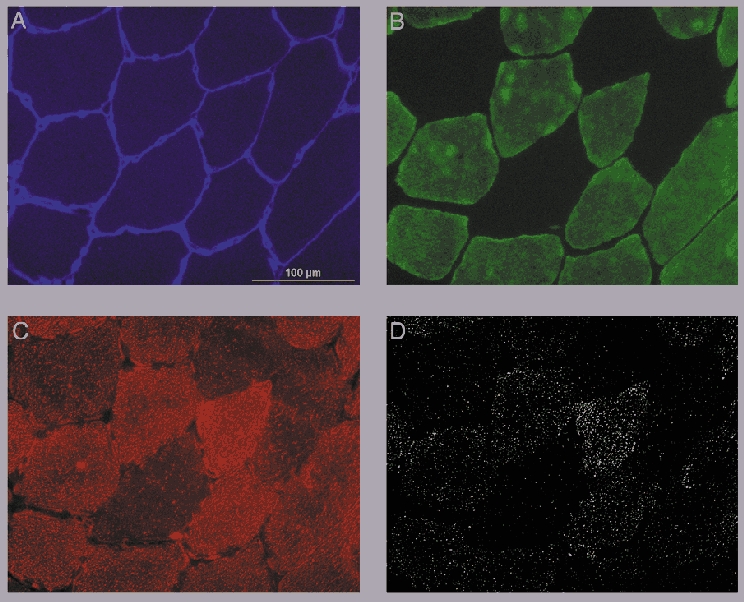
Digitally captured images of one single field-of-view (×240 magnification) taken from a muscle cross-section obtained from a pre-exercise muscle biopsy sample showing the epifluorescence signal as recorded using a DAPI UV excitation filter for laminin (showing the cell membranes in blue; A), a fluorescein isothiocyanate (FITC) excitation filter for myosin heavy chains (showing the type I muscle fibres in green; B) and a Texas red excitation filter for the oil red O signal (showing the intramyocellular lipid droplets in red; C). D, the oil red O signal obtained in Fig. 1C after applying the intensity threshold (showing the lipid droplets in white), which was subsequently used for data processing and quantitative analysis.
Statistics
All data are expressed as means ± s.e.m. To compare tracer kinetics, substrate utilisation rates, IMTG contents and/or plasma metabolite concentrations over time, a repeated measures analysis of variance (ANOVA) was applied. A Scheffépost hoc test was applied in case of a significant F-ratio to locate specific differences. For non-time-dependent variables, Student's t test for paired observations was used. Simple linear regression was used to investigate specific correlations. Significance was set at the 0.05 level of confidence.
Results
Tracer kinetics
Plasma glucose and palmitate enrichments are shown in Fig. 2A. Both Ra and Rd of glucose were significantly higher during exercise compared to pre-exercise resting values (Table 2 and Fig. 3A), with plasma glucose Ra and Rd increasing over time during the exercise trial (Fig. 3A; P < 0.001). Breath 13CO2 enrichments at rest and during exercise are shown in Fig. 2B. Ra, Rd and Rox of palmitate were significantly higher during exercise compared to resting values (Table 2 and Fig. 3B), with plasma palmitate Ra, Rd and Rox increasing over time during exercise (Fig. 3B; P < 0.001). Fractional acetate label recovery was significantly higher during exercise compared to resting values (Table 2).
Table 2.
Tracer kinetics at rest and during exercise
| Rest | Exercise | ||
|---|---|---|---|
| Ra | Palmitate | 2.18 ± 0.24 | 5.09 ± 0.62* |
| Rd | Palmitate | 2.14 ± 0.24 | 5.04 ± 0.62* |
| Ac | Recovery | 0.14 ± 0.01 | 0.85 ± 0.03* |
| Rox | Palmitate | 0.77 ± 0.11 | 4.67 ± 0.56* |
| %Ra,ox | Palmitate | 34.8 ± 2.18 | 91.4 ± 3.65* |
| Ra | Glucose | 16.0 ± 1.2 | 30.3 ± 1.7* |
| Rd | Glucose | 16.2 ± 1.3 | 31.4 ± 1.8* |
Tracer kinetics as calculated at rest and averaged during 2h of exercise at 50% Wmax. Ra, rate of appearance; Rd, rate of disappearance; Rox, rate of oxidation (μmol kg−1 min−1); %Raox, percentage of Ra palmitate oxidised (%); Ac recovery, fractional [1,2-13C]acetate label recovery in expired CO2. Values are expressed as means ± s.e.m. (n = 8).
Significantly different from resting values (P < 0.01).
Figure 3. Plasma glucose and palmitate kinetics.
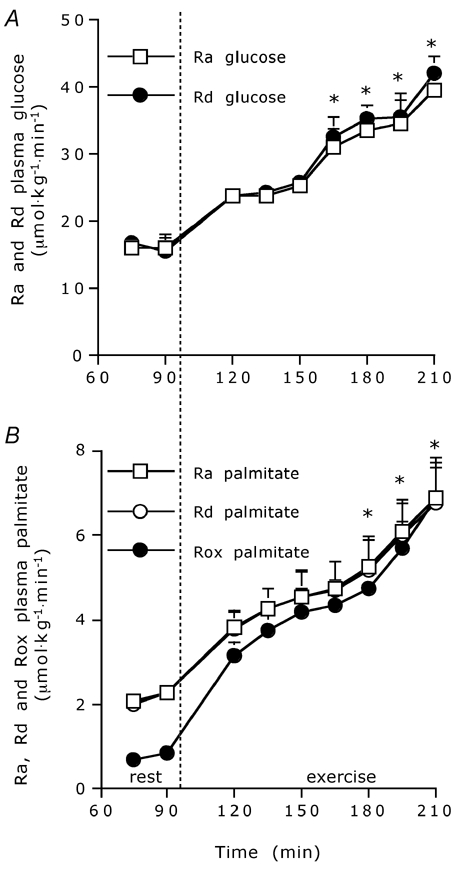
Plasma glucose rate of appearance (Ra) and disappearance (Rd) (A) and plasma palmitate Ra, Rd and rate of oxidation (Rox) (B) during prolonged submaximal endurance cycling exercise (expressed in µmol kg−1 min−1). Plasma glucose Ra and Rd and palmitate Ra, Rd and Rox substantially increased during exercise (P < 0.001). Data are means ± s.e.m.; * significantly higher than value at t = 120 min (P < 0.05).
Substrate utilisation
Results of the gas exchange measurements are provided in Table 1. Energy expenditure and average utilisation rates of the different endogenous substrate sources at rest, during 2 h of submaximal exercise and during 2 h of post-exercise recovery are summarised in Table 3. Changes in substrate utilisation rates over time during exercise are illustrated in Fig. 4. At rest, fat oxidation rates averaged 0.09 ± 0.01 g min−1, contributing 66 ± 5 % to total energy expenditure (Table 3). An average of 48 ± 6 % of total energy expenditure was accounted for by plasma FFA oxidation, with the use of other fat sources contributing 17 ± 3 %. Carbohydrate oxidation rates averaged 0.12 ± 0.02 g min−1, representing the remaining 34 ± 5 % of total energy expenditure.
Table 1.
Gas exchange and ergometry measurements
| Gas exchange | Rest | Exercise | Recovery |
|---|---|---|---|
| V̇O2(1 min−1) | 0.28 ± 0.01 | 2.75 ± 0.09 * | 0.31 ± 0.01 * |
| V̇CO2 (1 min−1) | 0.22 ± 0.01 | 2.38 ± 0.08 * | 0.24 ± 0.01 * |
| RER | 0.80 ± 0.02 | 0.87 ± 0.01 * | 0.77 ± 0.01 * |
| Exercise (min) | 15 | 30 | 45 | 60 | 75 | 90 | 105 | 120 |
|---|---|---|---|---|---|---|---|---|
| V̇O2 (1 min−1) | 2.70 | 2.69 | 2.72 | 2.73 | 2.78 | 2.76 | 2.79 | 2.80 |
| V̇CO2 (1 min−1) | 2.43 | 2.38 | 2.37 | 2.36 | 2.39 | 2.37 | 2.41 | 2.38 |
| RER | 0.90 | 0.88 | 0.87 | 0.87 | 0.86 | 0.86 | 0.85 | 0.85 † |
| Ergometry | |
|---|---|
| 50% Wmax workload (W) | 196 ± 7 |
| Relative workload (%V̇O2,max) | 60.1 ± 1.0 |
| Average heart rate (beats min−1) | 149 ± 7 |
Average oxygen uptake (V̇O2), carbon dioxide production (V̇CO2) and respiratory exchange ratio (RER) as measured at rest, during exercise and during 2h of post-exercise recovery. Average V̇O2, V̇CO2, and RER are also measured during exercise at 15 min intervals. In addition, the absolute workload corresponding to the 50% maximal power output (50% Wmax) setting and the corresponding relative workload and average heart rate during exercise are provided. Values are expressed as means ± s.e.m. (n = 8).
Significantly different from resting values (P < 0.01)
significant decrease over time (P < 0.01).
Table 3.
Contribution of substrates to total energy expenditure
| Substrate | Rest | Exercise | 2 h recovery |
|---|---|---|---|
| Fat | 0.09 ± 0.01 (66%) | 0.61 ± 0.06 (43%)* | 0.12 ± 0.01 (75%)* |
| Free fatty acids | 0.07 ± 0.008 (48%) | 0.40 ± 0.05 (28%)* | — |
| Other fat sources | 0.02 ± 0.004 (17%) | 0.22 ± 0.03 (15%)* | — |
| Carbohydrate | 0.12 ± 0.02 (34%) | 2.05 ± 0.15 (57%)* | 0.10 ± 0.02 (25%)* |
| Plasma glucose | — | 0.43 ± 0.03 (12%) | — |
| Muscle glycogen | — | 1.61 ± 0.16(45%) | — |
| Energy expenditure | 5.76 ± 0.22 | 58.05 ± 0.61* | 6.39 ± 0.27* |
Energy expenditure (kJ min−1) and substrate use at rest, during exercise and during post-exercise recovery; expressed as average oxidation rate (g min−1) as well as relative contribution to total energy expenditure (%). Values are means ± s.e.m. (n = 8)
significantly different from pre-exercise resting values (P < 0.05).
Figure 4. Substrate source utilisation during exercise.
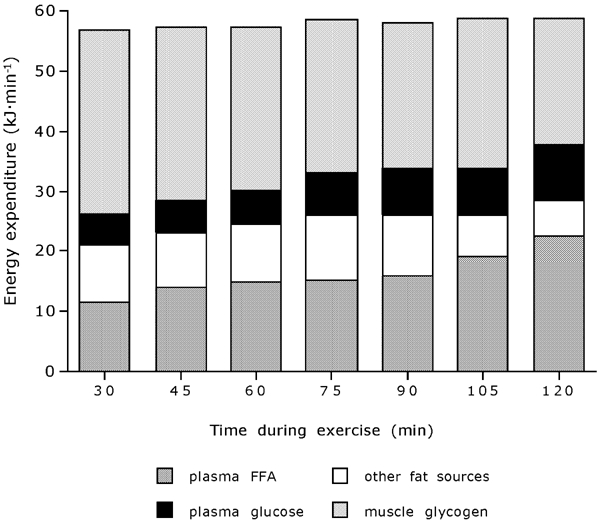
Substrate utilisation (expressed in kJ min−1) over time during prolonged submaximal exercise. Plasma FFA and glucose oxidation rates significantly increased over time, with the oxidation rates of other fat sources (sum of muscle- and lipoprotein-derived TG) and muscle glycogen being decreased over time (P < 0.001).
In the subsequent exercise trial, the 50 % Wmax workload applied represented 196 ± 7 W on average, which corresponded to 60.1 ± 1.0 % V̇O2,max (Table 1). During exercise, total fat oxidation rates significantly increased over time, with a concomitant decrease in total carbohydrate oxidation rates (P < 0.001). The increase in total fat oxidation rate during exercise was accounted for by a substantial increase in plasma FFA oxidation rate, whereas the oxidation rate of other fat sources (muscle- and lipoprotein-derived TG) was reduced over time (P < 0.001). The latter decline became most evident during the second hour of exercise. Calculated over the entire 2 h exercise period, fat oxidation averaged 0.61 ± 0.06 g min−1 (43 ± 4 % of total energy expenditure), with plasma FFA oxidation being the main fat source representing 28 ± 3 % of total energy expenditure, and the utilisation of other fat sources contributing the remaining 15 ± 2 %. The decrease in total carbohydrate oxidation rate during exercise was accounted for by a substantial decrease in the use of muscle glycogen, whereas plasma glucose oxidation increased progressively over time (P < 0.001). Calculated over the entire 2 h exercise period, carbohydrate oxidation rates averaged 2.05 ± 0.15 g min−1 (57 ± 4 %), with muscle glycogen being the main carbohydrate source representing 45 ± 4 % of total energy expenditure and plasma glucose utilisation contributing 12 ± 1 %.
In the post-exercise recovery period fat oxidation averaged 0.12 ± 0.01 g min−1, which was significantly higher compared to pre-exercise resting values (P < 0.001). Concomitantly, carbohydrate oxidation rates averaged 0.10 ± 0.02 g min−1, which was significantly lower compared to pre-exercise resting values (P < 0.001). Though the increased fat utilisation rate observed in the recovery period was partly attributed to the higher energy expenditure during post-exercise recovery compared to pre-exercise resting values (6.39 ± 0.3 vs. 5.76 ± 0.22 kJ min−1; P < 0.01), the relative contribution of fat oxidation to total energy expenditure was also significantly increased in the post-exercise recovery period (75 ± 5 vs. 66 ± 5 %; P < 0.001).
Muscle tissue analysis
Muscle tissue analysis for IMTG content using fluorescence microscopy on oil red O stained muscle cross-sections showed a substantial 62 ± 7 % decrease in lipid content in the type I fibres only (P < 0.01), with IMTG content in the type I muscle fibres being significantly lower both immediately post-exercise as well as following 2 h of post-exercise recovery compared to pre-exercise values (Fig. 5A). The latter was observed in each individual subject (Fig. 5B). The observed decrease in IMTG content was accounted for by a significant decrease in both lipid droplet size (0.74 ± 0.06, 0.49 ± 0.06 and 0.51 ± 0.05 µm2) as well as lipid droplet density (0.07 ± 0.004, 0.03 ± 0.004 and 0.04 ± 0.007 droplets µm−2) as determined before and immediately after exercise and after 2 h of post-exercise recovery, respectively (P < 0.01). In the type II muscle fibres no significant changes were observed.
Figure 5. Intramyocellular lipid content.
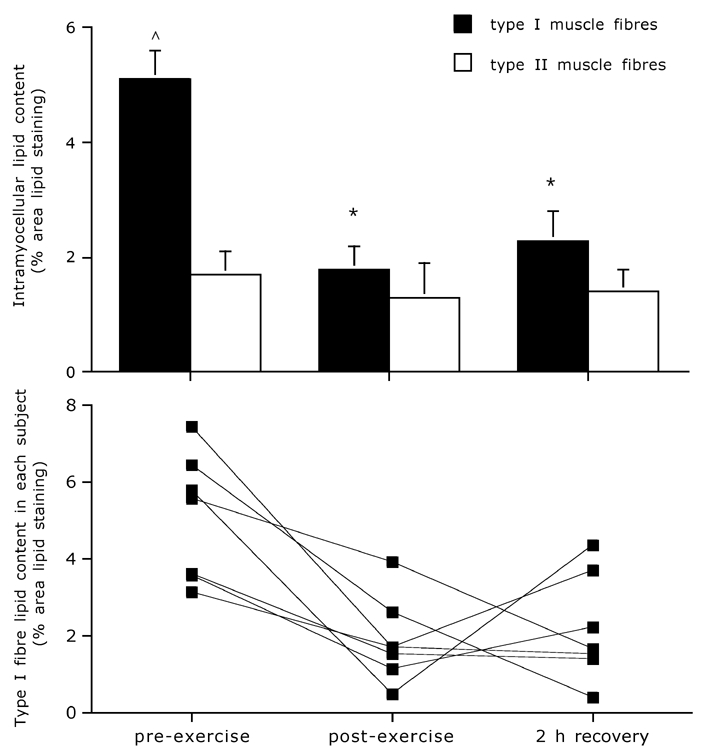
Mean fibre type-specific intramyocellular lipid content (expressed as percentage of area lipid stained) before exercise, immediately after exercise and following 2 h of post-exercise recovery as determined by quantitative fluorescence microscopy on oil red O stained muscle cross-sections (A). Data provided are means ± s.e.m.; * significantly lower than pre-exercise values; ∧ significantly higher than type II muscle fibres (P < 0.05). Individual results showing average intramyocellular lipid content in the type I muscle fibres before, after and 2 h after exercise (B).
Comparisons between muscle fibre types showed significantly higher IMTG content in the type I compared to the type II fibres before exercise (Fig. 1 and Fig. 5), which was accounted for by a significantly greater lipid droplet size (0.74 ± 0.06 vs. 0.54 ± 0.06 µm2) as well as a higher lipid droplet density (0.07 ± 0.004 vs. 0.03 ± 0.004 droplets µm−2) in the type I vs. type II muscle fibres, respectively. Immediately after exercise as well as after 2 h of recovery, lipid content was not different between muscle fibre types (Fig. 5). In accordance, average lipid droplet size was similar between muscle fibre types immediately post-exercise as well as after 2 h of recovery; 0.49 ± 0.06 and 0.51 ± 0.05 vs. 0.51 ± 0.11 and 0.47 ± 0.04, in the type I and II muscle fibres, respectively. Lipid droplet density remained significantly higher in the type I compared to the type II muscle fibres; 0.03 ± 0.004 and 0.04 ± 0.007 vs. 0.02 ± 0.004 and 0.03 ± 0.007 droplets µm−2, respectively.
Plasma metabolite concentrations
Plasma FFA, glycerol, TG, glucose and lactate concentrations are shown in Fig. 6. Following the onset of exercise, plasma FFA concentrations increased continuously over time reaching peak levels 15 min after cessation of exercise. Thereafter, plasma FFA concentrations declined during recovery, remaining well above pre-exercise resting levels. Plasma glycerol concentrations significantly increased during exercise and immediately decreased following the cessation of exercise. Plasma TG concentrations gradually decreased during exercise, and subsequently increased during recovery. Plasma glucose concentrations showed a gradual decline with the duration of exercise, with no significant changes during the recovery period. Within the first 15 min after the onset of exercise plasma lactate concentrations had increased well above pre-exercise resting levels, after which concentrations declined during both the exercise and recovery stages.
Correlations
The intramyocellular lipid content in the type I muscle fibres revealed a positive correlation with the lipid content in the type II fibres (R = 0.80; P < 0.05). Pre-exercise intramyocellular lipid content in both the type I and type II muscle fibres revealed a positive correlation with Wmax (R = 0.81 and 0.77 respectively; P < 0.05) but not with V̇O2,max. Pre-exercise IMTG content in the both the type I and II muscle fibres showed a positive correlation with the net decrease in intramyocellular lipid content during the exercise trial. In accordance, the average oxidation rate of muscle- and lipoprotein-derived TG revealed a significant positive correlation with pre-exercise IMTG content in the type I fibres (R = 0.76; P = 0.03). However, the average oxidation rate of muscle- and lipoprotein-derived TG during exercise (using stable isotope methodology) did not correlate with the net decrease in IMTG content in the type I and/or II muscle fibres (using immunofluorescence microscopy).
Discussion
The oil red O fluorescence data in the present study provide convincing evidence that IMTG is rapidly hydrolysed during moderate intensity exercise in trained male athletes following an overnight fast. Two hours of moderate intensity exercise reduced intramyocellular lipid content by more than 62 ± 7 % in the type I muscle fibres, whereas no significant changes were observed in the type II fibres. In addition, on a whole-body level, we show that the sum of muscle- plus lipoprotein-derived TG oxidation contributes substantially to total energy expenditure at rest and during moderate intensity exercise. Furthermore, this study shows that the oxidation rate of these TG sources gradually declines with exercise duration, whereas plasma FFA oxidation rates increase. Together, our data suggest that IMTG forms an important fuel source for the exercising muscle in endurance-trained subjects in an overnight fasted state.
The present study shows that plasma-derived FFA provide the majority of fat as a substrate source, with the use of other fat sources contributing as much as 28 ± 5 and 36 ± 4 % of total fat oxidation at rest and during moderate intensity exercise, respectively (Table 3 and Fig. 4). Though these other fat sources have often been assumed to reflect muscle-derived TG, the applied stable isotope methodology does not allow differentiation between muscle- and lipoprotein-derived TG use. At rest, plasma palmitate Rd by far exceeded Rox (Rox was 36 ± 2 % of Rd; Table 2). The latter is in accordance with the contention that a substantial part of plasma FFA uptake is routed towards re-esterification into IMTG under resting conditions (van Hall et al. 2002) as well as during exercise in non-contracting muscle (Sacchetti et al. 2002). In the present study, we measured plasma [U-13C]palmitate oxidation rates from the excretion of 13CO2 in the expired breath. To correct for 13C label retention in the bicarbonate pool and for incorporation of label into TCA cycle intermediates, an acetate correction factor was applied (van Loon et al. 2003a). Omission to apply such a correction factor would have led to a substantial underestimation of plasma FFA oxidation rates. As the oxidation of [U-13C]palmitate generates [1,2-13C]acetyl-CoA, we applied a [1,2-13C]acetate correction factor to correct for 13C label retention. The latter has been shown to generate valid and reproducible estimates of plasma [U-13C]palmitate oxidation rates (Schrauwen et al. 1998). Trimmer et al. (2001) recently suggested that the use of a bicarbonate recovery factor would be preferred over an acetate recovery factor in a study measuring [1-13C]glucose oxidation rates. Though this suggestion is unlikely to apply to estimates of [U-13C]palmitate oxidation, it should be noted that the use of a bicarbonate recovery factor would have resulted in a slightly lower estimate of plasma FFA oxidation rate, and therefore an even larger estimate of IMTG plus lipoprotein TG oxidation.
During exercise, indirect calorimetry revealed a time-dependent increase in total fat oxidation rate with a concomitant decline in total carbohydrate use (Fig. 4). The observed decrease in total carbohydrate oxidation rate during exercise was entirely accounted for by a progressive decrease in muscle glycogen use, whereas the rates of plasma glucose production (Ra) and utilisation (Rd) gradually increased (Fig. 3A). Similar shifts in endogenous carbohydrate source utilisation have been described before (Romijn et al. 1993; Phillips et al. 1996a). In accordance with a progressive increase in peripheral lipolytic rate during prolonged moderate intensity exercise (Turcotte et al. 1992; Romijn et al. 1993; Phillips et al. 1996a), we observed a substantial increase in palmitate Ra (Fig. 3) and a concomitant increase in plasma palmitate, total FFA and glycerol concentrations (Fig. 6) during exercise. In line with the increase in palmitate Ra, both Rd and Rox showed similar increases (Fig. 3). After the onset of exercise the proportion of palmitate Rd that was oxidised increased substantially, with Rox representing 92 ± 4 % of Rd during exercise. The latter confirms that muscle contraction strongly increases the proportion of FFA uptake that is routed towards oxidation, at the expense of re-esterification and storage in muscle TG stores (Sacchetti et al. 2002). Though re-esterification rates of FA into the IMTG pool are particularly low during exercise (Guo et al. 2000), it should be noted that minor re-esterification of labelled palmitate into the IMTG pool, followed by subsequent lipolysis and oxidation, would induce a slight overestimation of plasma FFA oxidation rates and would, therefore, lead to an underestimation of TG use. The latter implies that these stable isotope estimates of muscle plus lipoprotein TG oxidation rates could actually be considered minimal estimates.
The observed increase in plasma FFA oxidation rate during exercise strongly correlated with the increase in FFA concentration within each individual subject (R = 0.88± 0.02; P < 0.05). The latter agrees with the contention that plasma FFA availability forms one of the factors that determine FFA oxidation during low-to-moderate intensity exercise (Randle et al. 1963; Romijn et al. 1995; Dyck et al. 1996). Whereas plasma FFA oxidation rates substantially increased during exercise, the rate of muscle- plus lipoprotein-derived TG oxidation declined during the second hour of exercise, concomitant with the increase in plasma FFA Ra, Rd and Rox (Fig. 3). These findings confirm earlier estimations by Romijn et al. (1993, 1995) and more recent suggestions by Watt et al. (2002a). and support the contention that a progressive increase in peripheral lipolytic rate and subsequent increase in plasma FFA concentrations can suppress IMTG hydrolysis and its subsequent rate of oxidation. With hormone-sensitive lipase (HSL; Langfort et al. 1998) as a likely site at which IMTG hydrolysis and/or oxidation is regulated, it has been speculated that HSL is not only stimulated by muscle contraction and increased plasma adrenaline concentrations (Langfort et al. 1999, 2000) but may also be inhibited by an increase in intramuscular long-chain fatty acyl-CoA concentrations, secondary to an increase in plasma FFA uptake. Indirect evidence for such a mechanism has been provided by the in vitro observation that HSL is inhibited in a non-competitive manner by oleoyl-CoA in bovine adipose tissue (Jepson & Yeaman, 1992). However, other factors such as fibre type-specific intramyocellular TG depletion could also contribute to the observed decline in muscle derived TG use during the latter stages of prolonged exercise (Fig. 5). Clearly, more research is warranted to investigate the potential reciprocal relationship between FFA uptake and muscle TG utilisation. The latter could be clinically relevant, as elevated FFA concentrations in obese and/or type 2 diabetes patients could be (partly) responsible for their reduced capacity to utilise muscle- plus lipoprotein-derived TG during exercise (Blaak et al. 2000; Borghouts et al. 2002).
In the present study, stable isotope methodology resulted in an estimated average muscle- plus lipoprotein-derived TG oxidation rate of 0.22 ± 0.03 g min−1, which represented a substantial contribution to total energy expenditure (Table 3 and Fig. 4). As such, our data confirm earlier reports (Martin et al. 1993; Romijn et al. 1993; Phillips et al. 1996a; Sidossis et al. 1998; Coyle et al. 2000; van Loon et al. 2001) and extend their findings by the observed increase in plasma FFA oxidation rates and the concomitant decrease in the use of TG sources with the duration of exercise. However, measurements over the working limb by Bergman et al. (1999) showed respiratory quotients (RQ) close to 1.0 during moderate intensity exercise. Since whole-body respiratory exchange ratios (RER) values were observed to be lower, Bergman and co-workers speculated that the majority of fat oxidation occurs in inactive tissue and that total fat and/or IMTG oxidation in active muscle is minimal. However, in the present study, whole-body fat oxidation rates were shown to increase ≈7-fold from rest to exercise, which is unlikely to be explained by an increase in inactive tissue fat use. In addition, the observed net reduction in lipid content in the active vastus lateralis muscle also argues against this suggestion. The apparent discrepancy is likely to be explained by the fact that Bergman et al. (1999) tested subjects after receiving a standardised breakfast, whereas in the present study subjects were studied after an overnight fast. In addition, Bergman et al. (1999) studied untrained subjects before and after 9 weeks of endurance training, whereas in the present study trained cyclists with a training history of over 5 years were included. Such differences are likely to explain the much lower RER values reported in the present study compared with those reported by Bergman et al. (1990) (Table 1). In accordance, other studies measuring FA oxidation over the working leg have reported much lower values for leg RQ as well as RER during exercise at similar workloads (see van Hall et al. 1999). Recent data by van Hall et al. (2002) show considerable FA oxidation rates over the working leg during 2 h of one-leg knee-extensor exercise at 65 % of maximal power output. In line with our observations, they reported 68 % of total fat oxidation over the leg accounted for by plasma FFA oxidation during the first 30–60 min of exercise, leaving a considerable contribution for the use of other fat sources.
Though most stable isotope studies suggest that IMTG- and/or lipoprotein-derived TG can be used as a substrate source during prolonged moderate intensity exercise, studies measuring net changes in muscle TG content by applying muscle TG extraction analysis on muscle samples collected before and after exercise have reported contradicting findings (Watt et al. 2002b). Whereas several studies have reported a significant net decrease in muscle TG content following prolonged exercise (Carlson et al. 1971; Froberg & Mossfeldt, 1971; Bergstrom et al. 1973; Essen et al. 1977; Hurley et al. 1986; Cleroux et al. 1989; Phillips et al. 1996b; Sacchetti et al. 2002; Watt et al. 2002a) others did not observe such a decline (Kiens et al. 1993; Wendling et al. 1996; Starling et al. 1997; Kiens & Richter, 1998; Bergman et al. 1999; Guo et al. 2000). In addition, others have reported a significant decline in IMTG content after prolonged exercise in female but not in male subjects (Roepstorff et al. 2002; Steffensen et al. 2002). This apparent discrepancy in the literature according to Watt et al. (2002b) can be explained by the high between-biopsy variability in muscle TG content when using the biochemical TG extraction method. The latter could be caused by large differences in the amount of extramyocellular fat and/or fibre type composition of the muscle samples (Wendling et al. 1996; Watt et al. 2002a). Studies applying MRS to quantify both extra- and intramyocellular TG content have all reported significant (≈20–40 %) decreases in mixed muscle IMTG content following prolonged endurance exercise in both trained men and women (Boesch et al. 1997, 1999; Krssak et al. 2000; Rico-Sanz et al. 2000; Brechtel et al. 2001; Decombaz et al. 2001; Larson-Meyer et al. 2002; van Loon et al. 2003b).
Both the TG extraction method and the use of MRS are restricted to the quantification of muscle TG content in mixed muscle samples and do not differentiate for fibre-type specific IMTG content. As muscle fibre type recruitment during endurance type exercise tasks predominantly relies on the use of type I muscle fibres (Hultman, 1995), potential net changes in IMTG content following exercise were hypothesised to be most predominant in the type I fibres. Therefore, in the present study, oil red O staining of muscle cross-sections in combination with (immuno)fluorescence microscopy (Koopman et al. 2001) was applied to enable selective quantification of type I and type II muscle fibre lipid content. Though we recently showed a good correlation between IMTG content as determined by MRS and fluorescence microscopy (van Loon et al. 2003b), the latter technique does not eliminate the problems associated with the heterogeneity of repeat muscle biopsy samples (Wendling et al. 1996; Watt et al. 2002a). Nonetheless, methodological limitations of the TG extraction method, due to contamination of muscle biopsy samples with extramyocellular fat and/or differences in muscle fibre-type content, are excluded. We observed a ≈3-fold greater lipid content in the type I vs. type II muscle fibres (Fig. 1 and Fig. 5), which is in agreement with others reporting similar findings following fibre type-specific quantification of lipid content by biochemical TG extraction (Essen et al. 1975) as well as histochemical oil red O staining using conventional light microscopy (Malenfant et al. 2001). In line with the hypothesis that IMTG depletion would be most pronounced in the type I fibres, we observed a 62 ± 7 % net reduction in lipid content in the type I muscle fibres after exercise (Fig. 5). This observed decline in intramyocellular lipid content was accounted for by a decrease in lipid droplet size as well as droplet density. Assuming the average IMTG content to range between 3–6 mmol (kg wet muscle)−1 (Guo, 2001), the observed 62 % decline in type I muscle fibre lipid content represents a TG consumption between 1.5 and 3.0 mmol (kg mixed muscle)−1. The latter seems in accordance with the observed average TG oxidation rate of 0.22 ± 0.03 g min−1, which would translate into an average reduction of ≈1.8 mmol TG (kg mixed muscle)−1 over the entire exercise trial (assuming active muscle mass ≈15 kg).
Interestingly, pre-exercise intramyocellular lipid content in both the type I and type II fibres showed a significant correlation with individual Wmax. Though the latter is not the best indicator of endurance training status, these findings agree with the contention that endurance training enhances skeletal muscle lipid storage and/or utilisation (Hoppeler et al. 1985; Martin et al. 1993; Phillips et al. 1996a; Goodpaster et al. 2001). The latter is substantiated by the observation that net intramyocellular lipid depletion (fluorescence microscopy) and the average IMTG plus lipoprotein TG oxidation rate (stable isotope methodology) both correlated with pre-exercise intramyocellular lipid content. This implies that in endurance-trained athletes the capacity to utilise IMTG is indeed linked with increased IMTG storage. We did not observe a significant correlation between the net decline in intramyocellular lipid content (in the type I and/or type II fibres) and the IMTG- plus lipoprotein-derived TG oxidation rate. The latter is not surprising, as the local decline in absolute muscle lipid content in the vastus lateralis is unlikely to represent whole-body TG oxidation. Besides large inter-individual differences in contracting muscle mass and muscle recruitment, substantial differences in IMTG content and fat oxidative capacity have also been reported between various muscle groups (Boesch et al. 1999; Hwang et al. 2001). Furthermore, a substantial use of lipoprotein-derived TG could also contribute to the absence of such a direct correlation.
It has been suggested that IMTG stores could play an important role as a preferred substrate source during post-exercise recovery in male subjects (Kiens et al. 1993, 1998). We did not observe a net change in intramyocellular lipid content in the type I or type II muscle fibres following 2 h of post-exercise recovery (Fig. 5). As we did not continue isotope infusion during recovery, we can only assume that the increased total fat oxidation rate during post-exercise recovery period is accounted for by increased plasma FFA oxidation rate secondary to the increase in plasma FFA concentration when compared to pre-exercise resting conditions (Fig. 6). The latter would be in accordance with van Hall et al. (2002) who observed a substantial increase in total fat oxidation during recovery from exercise, which was entirely accounted for by an increase in plasma FFA oxidation.
In conclusion, the present study shows that prolonged moderate intensity exercise substantially reduces intramyocellular lipid content in the type I muscle fibres of endurance trained male athletes following an overnight fast. On a whole-body level, we show that the sum of muscle- and lipoprotein-derived TG oxidation contributes substantially to total energy expenditure both at rest and during prolonged exercise. The oxidation rate of these TG sources declines in the second hour of exercise, whereas plasma FFA oxidation rate increases in time. Combined these data suggest that IMTG forms an important fuel source for the exercising muscle in endurance-trained subjects in an overnight fasted state.
Acknowledgments
We gratefully acknowledge the enthusiastic support of the subjects who volunteered to participate in these trials and the experimental assistance of Sabine Snieders, Luke Schoenmakers and Joan Senden. The monoclonal antibody A4.840 developed by Dr Blau was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Science, Iowa City, IA 52242, USA.
References
- Bergman BC, Butterfield GE, Wolfel EE, Casazza GA, Lopaschuk GD, Brooks GA. Evaluation of exercise and training on muscle lipid metabolism. Am J Physiol. 1999;276:E106–117. doi: 10.1152/ajpendo.1999.276.1.E106. [DOI] [PubMed] [Google Scholar]
- Bergstrom J, Hultman E, Saltin B. Muscle glycogen consumption during cross-country skiing (the Vasa ski race) Int Z Angew Physiol. 1973;31:71–75. doi: 10.1007/BF00693727. [DOI] [PubMed] [Google Scholar]
- Blaak EE, van Aggel-Leijssen DPC, Wagenmakers AJM, Saris WHM, van Baak MA. Impaired oxidation of plasma-derived fatty acids in type 2 diabetic subjects during moderate-intensity exercise. Diabetes. 2000;49:2105–2107. doi: 10.2337/diabetes.49.12.2102. [DOI] [PubMed] [Google Scholar]
- Boden G, Lebed B, Schatz M, Homko C, Lemieux S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50:1612–1617. doi: 10.2337/diabetes.50.7.1612. [DOI] [PubMed] [Google Scholar]
- Boesch C, Decombaz J, Slotboom J, Kreis R. Observation of intramyocellular lipids by means of 1H magnetic resonance spectroscopy. Proc Nutr Soc. 1999;58:841–850. doi: 10.1017/s0029665199001147. [DOI] [PubMed] [Google Scholar]
- Boesch C, Slotboom J, Hoppeler H, Kreis R. In vivo determination of intra-myocellular lipids in human muscle by means of localized 1H-MR-spectroscopy. Magn Reson Med. 1997;37:484–493. doi: 10.1002/mrm.1910370403. [DOI] [PubMed] [Google Scholar]
- Borghouts LB, Wagenmakers AJM, Goyens PL, Keizer HA. Substrate utilization in non-obese Type II diabetic patients at rest and during exercise. Clin Sci. 2002;103:559–566. doi: 10.1042/cs1030559. [DOI] [PubMed] [Google Scholar]
- Brechtel K, Niess AM, Machann J, Rett K, Schick F, Claussen CD, Dickhuth HH, Haering HU, Jacob S. Utilisation of intramyocellular lipids (IMCLs) during exercise as assessed by proton magnetic resonance spectroscopy (1H-MRS) Horm Metab Res. 2001;33:63–66. doi: 10.1055/s-2001-12407. [DOI] [PubMed] [Google Scholar]
- Carlson LA, Ekelund LG, Froberg SO. Concentration of triglycerides, phospholipids and glycogen in skeletal muscle and of free fatty acids and beta-hydroxybutyric acid in blood in man in response to exercise. Eur J Clin Invest. 1971;1:248–254. doi: 10.1111/eci.1971.1.4.248. [DOI] [PubMed] [Google Scholar]
- Cho M, Webster SG, Blau HM. Evidence for myoblast-extrinsic regulation of slow myosin heavy chain expression during muscle fiber formation in embryonic development. J Cell Biol. 1993;121:795–810. doi: 10.1083/jcb.121.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleroux J, Van Nguyen P, Taylor AW, Leenen FH. Effects of beta 1- vs. beta 1 + beta 2-blockade on exercise endurance and muscle metabolism in humans. J Appl Physiol. 1989;66:548–554. doi: 10.1152/jappl.1989.66.2.548. [DOI] [PubMed] [Google Scholar]
- Coyle EF, Jeukendrup AE, Oseto MC, Hodgkinson BJ, Zderic TW. Low-fat diet alters intramuscular substrates and reduces lipolysis and fat oxidation during exercise. Am J Physiol Endocrinol Metab. 2001;280:E391–398. doi: 10.1152/ajpendo.2001.280.3.E391. [DOI] [PubMed] [Google Scholar]
- Decombaz J, Schmitt B, Ith M, Decarli B, Diem P, Kreis R, Hoppeler H, Boesch C. Postexercise fat intake repletes intramyocellular lipids but no faster in trained than in sedentary subjects. Am J Physiol Regul Integr Comp Physiol. 2001;281:R760–769. doi: 10.1152/ajpregu.2001.281.3.R760. [DOI] [PubMed] [Google Scholar]
- Dyck DJ, Peters SJ, Wendling PS, Chesley A, Hultman E, Spriet LL. Regulation of muscle glycogen phosphorylase activity during intense aerobic cycling with elevated FFA. Am J Physiol. 1996;270:E116–125. doi: 10.1152/ajpendo.1996.270.1.E116. [DOI] [PubMed] [Google Scholar]
- Essen B, Hagenfeldt L, Kaijser L. Utilization of blood-borne and intra-muscular substrates during continuous and intermittent exercise in man. J Physiol. 1977;265:489–506. doi: 10.1113/jphysiol.1977.sp011726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essen B, Jansson E, Henriksson J, Taylor AW, Saltin B. Metabolic characteristics of fibre types in human skeletal muscle. Acta Physiol Scand. 1975;95:153–165. doi: 10.1111/j.1748-1716.1975.tb10038.x. [DOI] [PubMed] [Google Scholar]
- Frayn KN, Hodgetts V, Griffiths AJ. Mobilization and clearance of fat in exercising humans studies by regional venous catheterization. In: Maughan RJ, Shirreffs SM, editors. Biochemistry of Exercise IX. Leeds: Human Kinetics Publishers; 1996. pp. 73–88. [Google Scholar]
- Froberg SO, Mossfeldt F. Effect of prolonged strenuous exercise on the concentration of triglycerides, phospholipids and glycogen in muscle of man. Acta Physiol Scand. 1971;82:167–171. doi: 10.1111/j.1748-1716.1971.tb04955.x. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86:5755–5761. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- Guo Z. Triglyceride content in skeletal muscle: variability and the source. Anal Biochem. 2001;296:1–8. doi: 10.1006/abio.2001.5233. [DOI] [PubMed] [Google Scholar]
- Guo Z, Burguera B, Jensen MD. Kinetics of intramuscular triglyceride fatty acids in exercising humans. J Appl Physiol. 2000;89:2057–2064. doi: 10.1152/jappl.2000.89.5.2057. [DOI] [PubMed] [Google Scholar]
- Gutmann I, Wahlefeld AW. L-(+)-Lactate, determination with lactate dehydrogenase and NAD. In: Bergmeyer HU, editor. Methods in Enzymatic Analysis. New York: Academic Press; 1974. pp. 1464–1468. [Google Scholar]
- Havel RJ, Pernow B, Jones NL. Uptake and release of free fatty acids and other metabolites in the legs of exercising men. J Appl Physiol. 1967;23:90–99. doi: 10.1152/jappl.1967.23.1.90. [DOI] [PubMed] [Google Scholar]
- Hoppeler H, Howald H, Conley K, Lindstedt SL, Claassen H, Vock P, Weibel ER. Endurance training in humans: aerobic capacity and structure of skeletal muscle. J Appl Physiol. 1985;59:320–327. doi: 10.1152/jappl.1985.59.2.320. [DOI] [PubMed] [Google Scholar]
- Hultman E. Fuel selection, muscle fibre. Proc Nutr Soc. 1995;54:107–121. doi: 10.1079/pns19950041. [DOI] [PubMed] [Google Scholar]
- Hurley BF, Nemeth PM, Martin WHD, Hagberg JM, Dalsky GP, Holloszy JO. Muscle triglyceride utilization during exercise: effect of training. J Appl Physiol. 1986;60:562–567. doi: 10.1152/jappl.1986.60.2.562. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Pan JW, Heydari S, Hetherington HP, Stein DT. Regional differences in intramyocellular lipids in humans observed by in vivo 1H-MR spectroscopic imaging. J Appl Physiol. 2001;90:1267–1274. doi: 10.1152/jappl.2001.90.4.1267. [DOI] [PubMed] [Google Scholar]
- Jepson CA, Yeaman SJ. Inhibition of hormone-sensitive lipase by inter-mediary lipid metabolites. FEBS Lett. 1992;310:197–200. doi: 10.1016/0014-5793(92)81328-j. [DOI] [PubMed] [Google Scholar]
- Jeukendrup AE, Raben A, Gijsen A, Stegen JH, Brouns F, Saris WHM, Wagenmakers AJM. Glucose kinetics during prolonged exercise in highly trained human subjects: effect of glucose ingestion. J Physiol. 1999;515:579–589. doi: 10.1111/j.1469-7793.1999.579ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiens B, Essen-Gustavsson B, Christensen NJ, Saltin B. Skeletal muscle substrate utilization during submaximal exercise in man: effect of endurance training. J Physiol. 1993;469:459–478. doi: 10.1113/jphysiol.1993.sp019823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiens B, Richter EA. Utilization of skeletal muscle triacylglycerol during post-exercise recovery in humans. Am J Physiol. 1998;38:E332–337. doi: 10.1152/ajpendo.1998.275.2.E332. [DOI] [PubMed] [Google Scholar]
- Koopman R, Schaart G, Hesselink MK. Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochem Cell Biol. 2001;116:63–68. doi: 10.1007/s004180100297. [DOI] [PubMed] [Google Scholar]
- Krssak M, Petersen KF, Bergeron R, Price T, Laurent D, Rothman DL, Roden M, Shulman GI. Intramuscular glycogen and intramyocellular lipid utilization during prolonged exercise and recovery in man: a 13C and 1H nuclear magnetic resonance spectroscopy study. J Clin Endocrinol Metab. 2000;85:748–754. doi: 10.1210/jcem.85.2.6354. [DOI] [PubMed] [Google Scholar]
- Kuipers H, Verstappen FT, Keizer HA, Geurten P, van Kranenburg G. Variability of aerobic performance in the laboratory and its physiologic correlates. Int J Sports Med. 1985;6:197–201. doi: 10.1055/s-2008-1025839. [DOI] [PubMed] [Google Scholar]
- Langfort J, Ploug T, Ihlemann J, Enevoldsen LH, Stallknecht B, Saldo M, Kjaer M, Holm C, Galbo H. Hormone, sensitive lipase (HSL) expression and regulation in skeletal muscle. Adv Exp Med Biol. 1998;441:219–228. doi: 10.1007/978-1-4899-1928-1_20. [DOI] [PubMed] [Google Scholar]
- Langfort J, Ploug T, Ihlemann J, Holm C, Galbo H. Stimulation of hormone-sensitive lipase activity by contractions in rat skeletal muscle. Biochem J. 2000;351:207–214. doi: 10.1042/0264-6021:3510207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfort J, Ploug T, Ihlemann J, Saldo M, Holm C, Galbo H. Expression of hormone-sensitive lipase and its regulation by adrenaline in skeletal muscle. Biochem J. 1999;340:459–465. [PMC free article] [PubMed] [Google Scholar]
- Larson-Meyer DE, Newcomer BR, Hunter GR. Influence of endurance running and recovery diet on intramyocellular lipid content in women: a 1H NMR study. Am J Physiol. 2002;282:E95–106. doi: 10.1152/ajpendo.2002.282.1.E95. [DOI] [PubMed] [Google Scholar]
- Malenfant P, Joanisse DR, Theriault R, Goodpaster BH, Kelley DE, Simoneau JA. Fat content in individual muscle fibers of lean and obese subjects. Int J Obes Relat Metab Disord. 2001;25:1316–1321. doi: 10.1038/sj.ijo.0801733. [DOI] [PubMed] [Google Scholar]
- Martin WH, Dalsky GP, Hurley BF, Matthews DE, Bier DM, Hagberg JM, Rogers MA, King DS, Holloszy JO. Effect of endurance training on plasma FFA turnover and oxidation during exercise. Am J Physiol. 1993;265:E708–714. doi: 10.1152/ajpendo.1993.265.5.E708. [DOI] [PubMed] [Google Scholar]
- Oscai LB, Essig DA, Palmer WK. Lipase regulation of muscle triglyceride hydrolysis. J Appl Physiol. 1990;69:1571–1577. doi: 10.1152/jappl.1990.69.5.1571. [DOI] [PubMed] [Google Scholar]
- Peronnet F, Massicotte D. Table of non-protein respiratory quotient: an up-date. Can J Sport Sci. 1991;16:23–29. [PubMed] [Google Scholar]
- Phillips SM, Green HJ, Tarnopolsky MA, Heigenhauser GF, Hill RE, Grant SM. Effects of training duration on substrate turnover and oxidation during exercise. J Appl Physiol. 1996a;81:2182–2191. doi: 10.1152/jappl.1996.81.5.2182. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Green HJ, Tarnopolsky MA, Heigenhauser GJ, Grant SM. Progressive effect of endurance training on metabolic adaptations in working skeletal muscle. Am J Physiol. 1996b;270:E265–272. doi: 10.1152/ajpendo.1996.270.2.E265. [DOI] [PubMed] [Google Scholar]
- Pickert A, Overkamp D, Renn W, Liebich H, Eggstein M. Selected ion monitoring gas chromatography/mass spectrometry using uniformly labelled (13C)-glucose for determination of glucose turnover in man. Biol Mass Spectrom. 1991;20:203–209. doi: 10.1002/bms.1200200408. [DOI] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose-fatty acid cycle: its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Rico Sanz J, Moosavi M, Thomas EL, McCarthy J, Coutts GA, Saeed N, Bell JD. In vivo evaluation of the effects of continuous exercise on skeletal muscle triglycerides in trained humans. Lipids. 2000;35:1313–1318. doi: 10.1007/s11745-000-0647-2. [DOI] [PubMed] [Google Scholar]
- Roepstorff C, Steffensen CH, Madsen M, Stallknecht B, Kanstrup IL, Richter EA, Kiens B. Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. Am J Physiol Endocrinol Metab. 2002;282:E435–447. doi: 10.1152/ajpendo.00266.2001. [DOI] [PubMed] [Google Scholar]
- Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. 1993;265:E380–391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- Romijn JA, Coyle EF, Sidossis LS, Zhang XJ, Wolfe RR. Relationship between fatty acid delivery and fatty acid oxidation during strenuous exercise. J Appl Physiol. 1995;79:1939–1945. doi: 10.1152/jappl.1995.79.6.1939. [DOI] [PubMed] [Google Scholar]
- Sacchetti M, Saltin B, Osada T, van Hall G. Intramuscular fatty acid meta-bolism in contracting and non-contracting human skeletal muscle. J Physiol. 2002;540:387–395. doi: 10.1113/jphysiol.2001.013912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauwen P, van Aggel-Leijssen DPC, van Marken Lichtenbelt WD, van Baak MA, Gijsen AP, Wagenmakers AJM. Validation of the [1,2-13C]acetate recovery factor for correction of [U-13C]palmitate oxidation. J Physiol. 1998;513:215–223. doi: 10.1111/j.1469-7793.1998.215by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauwen P, van Aggel-Leijssen DP, Hul G, Wagenmakers AJM, Vidal H, Saris WHM, van Baak MA. The effect of a 3-month low-intensity endurance training program on fat oxidation and acetyl-CoA carboxylase-2 expression. Diabetes. 2002;51:2220–2226. doi: 10.2337/diabetes.51.7.2220. [DOI] [PubMed] [Google Scholar]
- Schrauwen P, Wagenmakers AJM, van Marken Lichtenbelt WD, Saris WHM, Westerterp KR. Increase in fat oxidation on a high-fat diet is accompanied by an increase in triglyceride-derived fatty acid oxidation. Diabetes. 2000;49:640–646. doi: 10.2337/diabetes.49.4.640. [DOI] [PubMed] [Google Scholar]
- Sidossis LS, Coggan AR, Gastaldelli A, Wolfe RR. A new correction factor for use in tracer estimations of plasma fatty acid oxidation. Am J Physiol. 1995;269:E649–656. doi: 10.1152/ajpendo.1995.269.4.E649. [DOI] [PubMed] [Google Scholar]
- Sidossis LS, Wolfe RR, Coggan AR. Regulation of fatty acid oxidation in untrained vs. trained men during exercise. Am J Physiol. 1998;274:E510–515. doi: 10.1152/ajpendo.1998.274.3.E510. [DOI] [PubMed] [Google Scholar]
- Siri WE. The gross composition of the body. Adv Biol Med Physiol. 1956;4:238–280. doi: 10.1016/b978-1-4832-3110-5.50011-x. [DOI] [PubMed] [Google Scholar]
- Starling RD, Trappe TA, Parcell AC, Kerr CG, Fink WJ, Costill DL. Effects of diet on muscle triglyceride and endurance performance. J Appl Physiol. 1997;82:1185–1189. doi: 10.1152/jappl.1997.82.4.1185. [DOI] [PubMed] [Google Scholar]
- Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann New York Acad Sci. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Steffensen CH, Roepstorff C, Madsen M, Kiens B. Myocellular triacyl-glycerol breakdown in females but not in males during exercise. Am J Physiol Endocrinol Metab. 2002;282:E634–642. doi: 10.1152/ajpendo.00078.2001. [DOI] [PubMed] [Google Scholar]
- Trimmer JK, Casazza GA, Horning MA, Brooks GA. Recovery of 13CO2 during rest and exercise after [1-13C]acetate, [2-13C]acetate, and NaH13CO3 in-fusions. Am J Physiol Endocrinol Metab. 2001;281:E683–692. doi: 10.1152/ajpendo.2001.281.4.E683. [DOI] [PubMed] [Google Scholar]
- Turcotte LP, Richter EA, Kiens B. Increased plasma FFA uptake and oxidation during prolonged exercise in trained vs. untrained humans. Am J Physiol. 1992;262:E791–799. doi: 10.1152/ajpendo.1992.262.6.E791. [DOI] [PubMed] [Google Scholar]
- van Hall G, Gonzalez-Alonso J, Sacchetti M, Saltin B. Skeletal muscle substrate metabolism during exercise: methodological considerations. Proc Nutr Soc. 1999;58:899–912. doi: 10.1017/s0029665199001202. [DOI] [PubMed] [Google Scholar]
- van Hall G, Sacchetti M, Radegran G, Saltin B. Human skeletal muscle fatty acid and glycerol metabolism during rest, exercise and recovery. J Physiol. 2002;543:1047–1058. doi: 10.1113/jphysiol.2002.023796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon LJC, Greenhaff PL, Constantin-Teodosiu D, Saris WHM, Wagenmakers AJM. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol. 2001;536:295–304. doi: 10.1111/j.1469-7793.2001.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon LJC, Koopman R, Schrauwen P, Stegen J, Wagenmakers AJM. The use of the [1,2-13C]acetate recovery factor in metabolic research. Eur J Appl Physiol. 2003a;89:377–383. doi: 10.1007/s00421-003-0810-x. [DOI] [PubMed] [Google Scholar]
- van Loon LJC, Schrauwen-Hinderling VB, Koopman R, Wagenmakers AJM, Hesselink MK, Schaart G, Kooi ME, Saris WHM. Influence of prolonged endurance cycling and recovery diet on intramuscular triglyceride content in trained males. Am J Physiol Endocrinol Metab. 2003b;285:E804–811. doi: 10.1152/ajpendo.00112.2003. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Heigenhauser GJ, Dyck DJ, Spriet LL. Intramuscular triacylglycerol, glycogen and acetyl group metabolism during 4 h of moderate exercise in man. J Physiol. 2002a;541:969–978. doi: 10.1113/jphysiol.2002.018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt MJ, Heigenhauser GJ, Spriet LL. Intramuscular triacylglycerol utilization in human skeletal muscle during exercise: is there a controversy? J Appl Physiol. 2002b;93:1185–1195. doi: 10.1152/japplphysiol.00197.2002. [DOI] [PubMed] [Google Scholar]
- Wolfe RR, Jahoor F. Recovery of labeled CO2 during the infusion of C-1- vs. C-2-labeled acetate: implications for tracer studies of substrate oxidation. Am J Clin Nutr. 1990;51:248–252. doi: 10.1093/ajcn/51.2.248. [DOI] [PubMed] [Google Scholar]
- Wendling PS, Peters SJ, Heigenhauser GJ, Spriet LL. Variability of triacyl-glycerol content in human skeletal muscle biopsy samples. J Appl Physiol. 1996;81:1150–1155. doi: 10.1152/jappl.1996.81.3.1150. [DOI] [PubMed] [Google Scholar]


